Abstract
Aims
Haemodynamic load induces cardiac remodelling via mechano-transduction pathways, which can further trigger inflammatory responses. We hypothesized that particularly in an inflammatory disorder such as myocarditis, a therapeutic strategy is required which, in addition to providing adequate circulatory support, unloads the left ventricle, decreases cardiac wall stress, and mitigates inflammatory responses.
Methods and results
Axial flow pumps such as the Impella systems comply with these requirements. Here, we report a potential mode-of-action of prolonged Impella support (PROPELLA concept) in fulminant myocarditis, including a decrease in cardiac immune cell presence, and integrin α1, α5, α6, α10 and β6 expression during unloading.
Conclusion
PROPELLA may provide benefits beyond its primary function of mechanical circulatory support in the form of additional disease-altering effects, which may contribute to enhanced myocardial recovery/remission in patients with chronic fulminant myocarditis.
Keywords: Mechanical unloading, Fulminant myocarditis, Endomyocardial biopsies, Integrins
Introduction
Patients with fulminant myocarditis presented with profound cardiogenic shock often need immediate mechanical circulatory support (MCS) to treat the haemodynamic compromise, enabling time for accurate diagnosis and for initiating anti-inflammatory strategies, when indicated.1 Several registries have so far demonstrated that among the available short-term MCS options, fulminant myocarditis patients are most frequently treated with veno-arterial extracorporeal life support (ECLS).2 This preferential use is based on the fact that ECLS can be implanted in fulminant myocarditis patients of all ages, including especially children, as well as in hearts with small cavity volumes due to hypertrophy or oedema of the myocardium. However, the use of ECLS increases the afterload of the left ventricle (LV), which, in the absence of additional left ventricular venting, can also cause left ventricular distention and worsening of pulmonary oedema. Such elevations in load also lead to increased systolic myocardial wall stress. Less well appreciated is the fact that these increases in stress induce further activation of cardiac mechano-transduction pathways3 which, over time, trigger inflammatory reactions.4 The combination of increased load and inflammation promotes unfavourable cardiac remodelling (Figure 1A). If not interrupted, either by the body’s intrinsic mechanisms or by therapeutic interventions, these processes can ultimately lead to a chronic dilated cardiomyopathy. This suggests, particularly in an inflammatory disorder such as myocarditis, that a therapeutic strategy is required that provides adequate circulatory support, unloads the LV, decreases cardiac wall stress, and subsequently mitigates inflammatory responses.5 All these requirements can be achieved via transcutaneously deployed axial flow pumps such as the Impella systems (2.5, CP, 5.0), which directly unload the LV throughout the cardiac cycle, decreasing total mechanical work and myocardial oxygen demand, while lowering wall stress an improving subendocardial coronary blood flow.
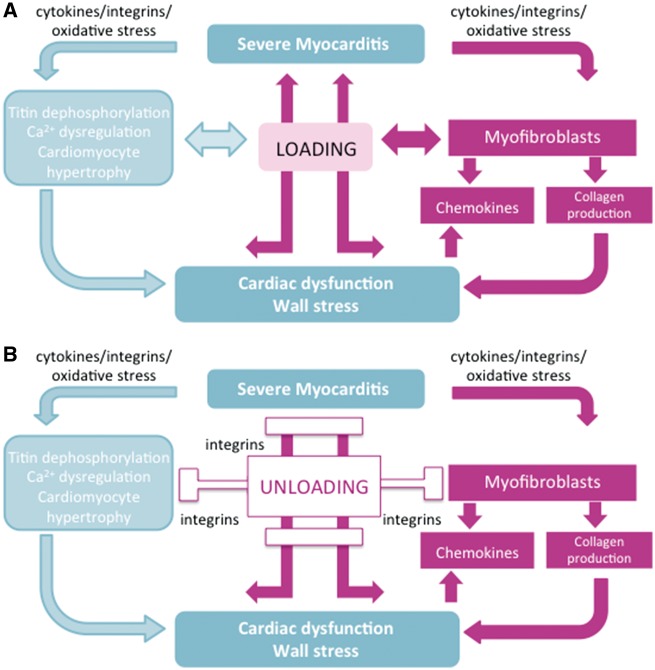
Figure 1.
Hypothetical schemes illustrating the impact of (A) loading and (B) unloading in the pathogenesis of myocarditis. (A) Myocarditis is characterized by cardiac immune cell infiltration and inflammation that can trigger cardiac fibrosis, dysregulation of calcium (Ca2+) homeostasis and titin function, and cardiomyocyte hypertrophy. These cellular and molecular changes subsequently lead to left ventricular overload and myocyte and left ventricular dysfunction. Increased left ventricular load in turn further provokes cardiomyocyte hypertrophy, affects titin phosphorylation, Ca2+ homeostasis, and myofibroblast activation thus initiating a vicious circle of progressive left ventricular dysfunction. (B) Unloading involving mechanotransduction via integrins may reduce these processes and consequently improve cardiac dysfunction.
The well-established cardioprotective effects of prolonged unloading via durable left ventricular assist devices6 in chronic heart failure and the link between mechano-transduction and inflammation4 form the rationale to support patients with fulminant myocarditis for a prolonged time period of several weeks even when left ventricular function is already improving and circulatory support per se is not the focus of treatment. Prolonged left ventricular unloading via a subclavicular/axillary-implanted axial flow pump allows a patient to be mobilized and awake (described and illustrated in detail in Supplementary material online, Movie) during prolonged periods of support. The strategy of prolonged Impella support (the PROPELLA concept) in fulminant myocarditis7 belongs to a newly established programme in our clinic and has consistently been shown to be a viable treatment option for patients with severe heart failure due to fulminant myocarditis in the setting of bridge-to-transplant8 or bridge-to-recovery.7 The mechanisms underlying this approach are previously unknown. Based on endomyocardial biopsy (EMB) findings, this report provides further insights into the mode-of-action and disease-modifying effects that may contribute to enhanced myocardial recovery/remission during PROPELLA support.
For reasons of diagnosis and in order to gain insights into the mode-of-action of PROPELLA, EMB9,10 were obtained from fulminant myocarditis patients before, during and after PROPELLA. Detailed patient characteristics, medications and procedures regarding EMB analysis are described in detail in the Supplementary material online.
As recommended by the European Society of Cardiology,9 immunosuppressive therapy was started in a patient who was presented with a fulminant EMB-proven lymphocytic viral-negative myocarditis (T0, Figure 2A) who required catecholamine support due to severely impaired ejection fraction (EF). For further haemodynamic stabilization and to reduce the need of pro-inflammatory catecholamines, MCS was added via an Impella 5.0 axial flow pump. Impella 5.0 support was performed for a total of 39 days, resulting in an improvement of left ventricular EF to 62% after successful weaning from MCS.
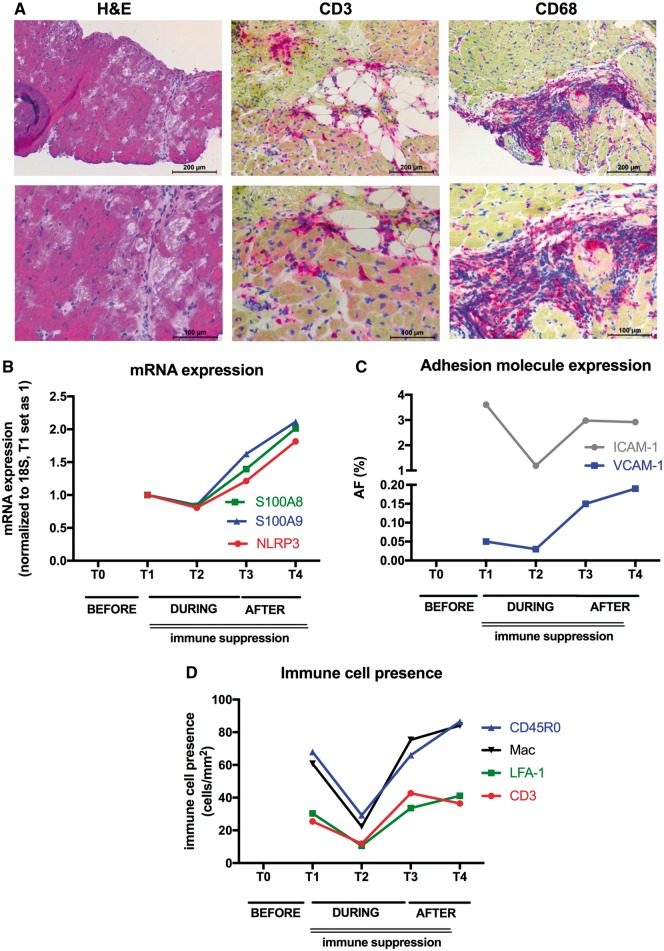
Figure 2.
Impact of prolonged mechanical unloading (PROPELLA approach) on cardiac innate immunity, adhesion molecule expression, and immune cell infiltration in chronic inflammatory cardiomyopathy. (A) Upper (100× magnification) and lower (200× magnification) panels depict haematoxylin and eosin-stained (left), CD3-stained (middle), and CD68-stained (right) sections of EMB isolated before combined unloading and immune suppression (T0), illustrating cardiomyocyte damage and massive infiltration of CD3 and CD68 cells. Graphs represent (B) mRNA expression of S100A8, S100A9, and NLRP3, (C) adhesion molecule expression depicted as area fraction (AF; %) (ICAM-1: grey and VCAM-1: blue) and (D) immune cell presence depicted as cells/mm2 (CD3: red; LFA-1: green; CD45RO: blue; Mac: black) in EMB isolated during combined unloading and immune suppression (T1, T2), after Impella explantation (T3), and after Impella explantation and termination of immunosuppressive therapy. Unloading combined with immunosuppressive therapy decreases alarmin S100A8, S100A9, and NLRP3 mRNA expression, adhesion molecule expression and immune cell infiltration (T1, T2). However, after explantation of the Impella, mRNA expression of the innate immunity members S100A8, S100A9, and NLRP3, adhesion molecule expression and immune cell infiltration rise again (T3), which is even more pronounced after termination of immunosuppressive therapy (T4).
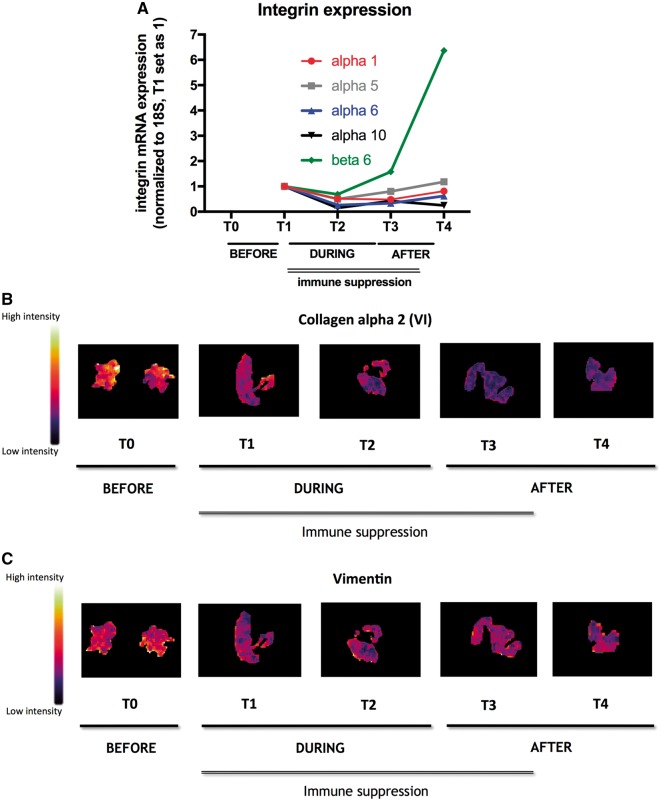
mRNA expression of innate immunity members, alarmins S100A8, S100A9, and the NLRP3 inflammasome, known to be up-regulated in myocarditis11,12 decreased during MCS and immunosuppressive therapy (between T1 and T2) (Figure 2B). However, this effect was abrogated after removal of the Impella support despite continuation of immunotherapy (T3), suggesting a primary unloading-dependent mechanism. Evaluation of adhesion molecule expression via immunohistochemistry on cryosections demonstrated that the expression of ICAM-1 and VCAM-1 followed a pattern similar to the alarmins S100A8, S100A9, and NLRP3 over time (Figure 2C). Concomitant with the course of adhesion molecule expression, immune cell (CD45RO, Mac, LFA-1, and CD3) infiltration reduced during PROPELLA and immunotherapy. However, immune cell infiltration increased again after withdrawal of Impella despite continuation of immunosuppressive therapy (T3), supporting the notion that myocardial unloading is primarily responsible for reduction of adhesion molecule expression and immune cell infiltration (Figure 2D). Integrins, a family of heterodimeric proteins expressed by cardiac fibroblasts and cardiomyocytes that provide critical adhesive and signalling functions through their interactions with the extracellular matrix and the actin cytoskeleton, are important factors in mechano-transduction and are known to be up-regulated in heart failure patients. Interestingly, the expression patterns of the integrins α1, 5, 6, 10, and ß6 over time were similar to those of the adhesion molecules and the presence of immune cells. During unloading, integrin expression fell, whereas after explantation of the Impella, their expression rose again (Figure 3A). As with immune cell infiltration, this effect was present despite continuation of immunosuppressive therapy suggesting that the expression of these integrins was also load-dependent. Hypothesis-free analysis via matrix-assisted laser desorption/ionization (MALDI) imaging mass spectrometry next demonstrated that the protein expression of collagen alpha 2 (VI) and vimentin, proteins which are important for mechanical support and integrin adhesiveness, decreased over time during PROPELLA (Figure 3B and C). However, in contrast to the expression of adhesion molecules, integrins and cell infiltration, this effect was durable after Impella explantation. This implies a different mechanism of collagen and vimentin regulation in comparison to adhesion molecules and integrins, which might be explained by differences between RNA and protein kinetics as well as differences in the time course of protein turnover. MALDI-imaging mass spectrometry analysis further revealed that the combination of Impella and immune suppression decreased cardiac titin expression over time (Supplementary material online, Figure S1A). Detailed evaluation of the titin isoforms further defined a time-dependent reduction in the stiff N2B isoform, which was paralleled by a rise in the compliant N2BA isoform (Supplementary material online, Figure S1B). In addition, prolonged unloading combined with immune suppression led to an increase in titin phosphorylation (Supplementary material online, Figure S1C) and protein kinase A and G activity (Supplementary material online, Figure S1D) over time. These findings suggest that Impella support combined with immune suppression may increase myocardial and left ventricular compliance (i.e. decreases myocardial and left ventricular stiffness) effects, which were durable even after device explantation and termination of immunosuppressive therapy.
Figure 3.
Impact of prolonged mechanical unloading (PROPELLA approach) on cardiac mechano-transduction pathways in chronic inflammatory cardiomyopathy. (A) Graphs represent integrin mRNA expression, normalized to 18S with T1 set as 1 (α1: red, α5: grey, α6: blue, α10: black, and ß6: green) in EMB isolated during combined unloading and immune suppression (T1, T2), after Impella explantation (T3), and after Impella explantation and termination of immunosuppressive therapy (T4). Unloading combined with immunosuppressive therapy (T1, T2) decreases integrin expression. However, after explantation of the Impella, integrin expression increases again (T3), which is for α1, 5, 6, and ß6 also further pronounced after termination of immunosuppressive therapy (T4). The intensity distribution of (B) collagen alpha-2 (VI) (1215 Da) and (C) vimentin (1271 Da) is significantly decreased during combined unloading and immune suppression (T1, T2) compared with before unloading (T0). In contrast to integrin mRNA expression, this effect is further present post-explantation (T3) and termination of immunosuppressive therapy (T4).
The PROPELLA-concept and mode-of-action were further confirmed in an HIV-positive patient in cardiogenic shock due to an EMB-proven viral-negative fulminant myocarditis. MCS and unloading were performed via an Impella-CP in the absence of immunosuppressive therapy due to preexisting AIDS. Starting with an initial echocardiographic left ventricular EF of <10% during catecholamine treatment, Impella-CP support for a total of 25 days resulted in an improvement of left ventricular EF up to 40% after successful weaning from MCS. Furthermore, Impella-CP support in the absence of immunosuppressive support led to 11% and 93% drops in ICAM-1 and VCAM-1 expression, respectively, which was paralleled by 18%, 19%, 32%, and 3% decreases in CD45RO, Mac, LFA-1, and CD3 cells and 54%, 71%, and 69% downregulations of S100A8, S100A9, NLRP3 mRNA expression. Concomitantly, mRNA expression of the integrins α1, 5, 6, 10, and ß6 fell by 59%, 68%, 75%, 58%, and 82%, respectively. In addition, a 15% shift towards the compliant N2BA titin isoform, and 24%, 100%, and 222% increases in titin phosphorylation, protein kinase G and A activity were observed.
Conclusion
Mechanistic data concerning the effect of left ventricular unloading in fulminant myocarditis have heretofore been lacking. We describe here insights illustrating that PROPELLA may provide benefits beyond its primary function of MCS in the form of additional disease-altering effects (Figure 1B) that can be important for enhancing myocardial recovery/remission in patients with chronic fulminant myocarditis. Since the rate of spontaneous full recovery of fulminant myocarditis is on the order of approximately 50%, the insights gained through the current results may provide clues as to how more complete and higher rates of restoration of cardiac structure and function can be achieved in this condition. Clinical trials, preferably with EMB analysis, are required to validate this promising PROPELLA concept and the significance of our proposed mode-of-action.
Human subjects/informed consent statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Supplementary Material
Acknowledgements
We thank Annika Koschel and Kerstin Puhl (in alphabetical order) for excellent technical support. Moreover, we thank the total clinical station team (doctors and nursing) of the cardiology intensive care unit W47i, CVK, Charité.
Conflict of interest: F.S. receives lecturing fees from Abiomed. S.A. reports grants and personal fees from Vifor, grants from AV, personal fees from Bayer, BI, Novartis, V-Wave, and Servier, outside the submitted work. E.V.P. reports other from proctoring by Abiomed, Abbott and Medtronic, institutional travel grants from Abbott and Medtronic, outside the submitted work. D.B. is recipient of an institutional unrestricted educational grant from Abiomed, outside the submitted work. B.P. reports lecture fees and steering committee honoraria from Novartis, MSD, Bayer Healthcare, Servier, BMS, Daiichi-Sanky, Stealth Peptides, and Vifor/Fresenius Healthcare. C.T. reports lecturing fees from Abiomed, Astra, Bayer Healthcare, Novartis, and Pfizer, and consulting and lecturing fees from Dynamics. Other authors report no conflict of interest.
References
- 1. Heymans S, Eriksson U, Lehtonen J, Cooper LT Jr.. The quest for new approaches in myocarditis and inflammatory cardiomyopathy. J Am Coll Cardiol 2016;68:2348–2364. [DOI] [PubMed] [Google Scholar]
- 2. den Uil CA, Akin S, Jewbali LS, Dos Reis Miranda D, Brugts JJ, Constantinescu AA, Kappetein AP, Caliskan K.. Short-term mechanical circulatory support as a bridge to durable left ventricular assist device implantation in refractory cardiogenic shock: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2017;52:14–25. [DOI] [PubMed] [Google Scholar]
- 3. Chen C, Li R, Ross RS, Manso AM.. Integrins and integrin-related proteins in cardiac fibrosis. J Mol Cell Cardiol 2016;93:162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lindner D, Zietsch C, Tank J, Sossalla S, Fluschnik N, Hinrichs S, Maier L, Poller W, Blankenberg S, Schultheiss HP, Tschope C, Westermann D.. Cardiac fibroblasts support cardiac inflammation in heart failure. Basic Res Cardiol 2014;109:428. [DOI] [PubMed] [Google Scholar]
- 5. Van Linthout S, Tschöpe C.. Inflammation—cause or consequence of heart failure or both? Curr Heart Fail Rep 2017;14:251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levin HR, Oz MC, Chen JM, Packer M, Rose EA, Burkhoff D.. Reversal of chronic ventricular dilation in patients with end-stage cardiomyopathy by prolonged mechanical unloading. Circulation 1995;91:2717–2720. [DOI] [PubMed] [Google Scholar]
- 7. Tschöpe C, Van Linthout S, Klein O, Mairinger T, Krackhardt F, Potapov E, Schmidt G, Burkhoff D, Pieske B, Spillmann F.. Mechanical unloading by fulminant myocarditis: LV-IMPELLA, ECMELLA, BI-PELLA and PROPELLA concepts. J Cardiovasc Transl Res 2018. doi: 10.1007/s12265-018-9820-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perry P, David E, Atkins B, Raff G.. Novel application of a percutaneous left ventricular assist device as a bridge to transplant in a paediatric patient with severe heart failure due to viral myocarditis. Interact Cardiovasc Thorac Surg 2017;24:474–476. [DOI] [PubMed] [Google Scholar]
- 9. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648, 2648a–2648d. [DOI] [PubMed] [Google Scholar]
- 10. Savvatis K, Schultheiss HP, Tschope C.. Endomyocardial biopsy and ultrastructural changes in dilated cardiomyopathy: taking a ‘deeper’ look into patients' prognosis. Eur Heart J 2015;36:708–710. [DOI] [PubMed] [Google Scholar]
- 11. Tschope C, Muller I, Xia Y, Savvatis K, Pappritz K, Pinkert S, Lassner D, Heimesaat MM, Spillmann F, Miteva K, Bereswill S, Schultheiss HP, Fechner H, Pieske B, Kuhl U, Van Linthout S.. NOD2 (Nucleotide-Binding Oligomerization Domain 2) is a major pathogenic mediator of coxsackievirus B3-induced myocarditis. Circ Heart Fail 2017;10:e003870. [DOI] [PubMed] [Google Scholar]
- 12. Muller I, Vogl T, Pappritz K, Miteva K, Savvatis K, Rohde D, Most P, Lassner D, Pieske B, Kuhl U, Van Linthout S, Tschope C.. Pathogenic role of the damage-associated molecular patterns S100A8 and S100A9 in coxsackievirus B3-induced myocarditis. Circ Heart Fail 2017;10:e004125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.