Abstract
Aims
We aimed to evaluate the frequency, clinical features, and prognostic implications of cardiac arrest (CA) in takotsubo syndrome (TTS).
Methods and results
We reviewed the records of patients with CA and known heart rhythm from the International Takotsubo Registry. The main outcomes were 60-day and 5-year mortality. In addition, predictors of mortality and predictors of CA during the acute TTS phase were assessed. Of 2098 patients, 103 patients with CA and known heart rhythm during CA were included. Compared with patients without CA, CA patients were more likely to be younger, male, and have apical TTS, atrial fibrillation (AF), neurologic comorbidities, physical triggers, and longer corrected QT-interval and lower left ventricular ejection fraction on admission. In all, 57.1% of patients with CA at admission had ventricular fibrillation/tachycardia, while 73.7% of patients with CA in the acute phase had asystole/pulseless electrical activity. Patients with CA showed higher 60-day (40.3% vs. 4.0%, P < 0.001) and 5-year mortality (68.9% vs. 16.7%, P < 0.001) than patients without CA. T-wave inversion and intracranial haemorrhage were independently associated with higher 60-day mortality after CA, whereas female gender was associated with lower 60-day mortality. In the acute phase, CA occurred less frequently in females and more frequently in patients with AF, ST-segment elevation, and higher C-reactive protein on admission.
Conclusions
Cardiac arrest is relatively frequent in TTS and is associated with higher short- and long-term mortality. Clinical and electrocardiographic parameters independently predicted mortality after CA.
Keywords: Takotsubo syndrome, Broken heart syndrome, Cardiac arrest, Acute heart failure, Outcome
Introduction
Takotsubo syndrome (TTS) has long been considered a benign disorder.1 However, in recent years it has been uncovered that TTS can be associated with different complications including cardiogenic shock and life-threatening arrhythmias.2,3 Furthermore, sudden death due to cardiac arrest (CA) is an underestimated threat in TTS. Although 9% of TTS patients require resuscitation,4 CA in TTS has not been comprehensively investigated.5 Life-threatening arrhythmias, including bradyarrhythmias, ventricular fibrillation (VF), and sustained/non-sustained ventricular tachycardia (VT) occur in 3.4–12.2% of TTS patients.6–8 However, these reports are based on small scale studies and do not directly explore CA in TTS. Indeed, TTS may be the cause or the consequence of CA.9 The hyperadrenergic milieu (endogenous or iatrogenic) associated with CA may trigger TTS, and TTS features like corrected QT-interval (QTc) prolongation and diffuse myocardial oedema could lead to life-threatening arrhythmias.10,11 Moreover, the long-term implications of CA in TTS remain unclear. Although TTS is characterized by recovery of wall motion abnormality and left ventricular ejection fraction (LVEF), it is unclear whether successfully resuscitated TTS patients with CA subsequently experience adverse outcomes. We therefore aimed to investigate the incidence and impact of CA in TTS.
Methods
Study population
The International Takotsubo Registry (InterTAK Registry, www.tako-tsubo-registry.com) is a multicentre prospective and retrospective registry13 encompassing 35 centres from 11 countries. Data for this study were collected in accordance with institutional review board regulations. From 2011 to 2017, a core team of investigators from University Hospital Zurich reviewed the medical records of patients diagnosed with TTS between 1998 and 2017. The methods have been extensively reported in a previous study.4, 13
In brief, TTS was diagnosed based on modified Mayo Clinic Diagnostic Criteria4,14: (i) transient abnormal left ventricular wall motion beyond the perfusion territory of a single epicardial coronary artery; (ii) no obstructive coronary artery disease or acute plaque rupture on angiography; (iii) new electrocardiographic abnormalities or elevated cardiac troponins; and (iv) no myocarditis. Exceptions included i) patients with coexisting coronary artery disease that can not explain the wall motion abnormalities ii) patients with wall motion abnormalities restricted to a single coronary artery territory but matching all other criteria iii) patients who died during the acute phase before recovery of wall motion abnormality was documented. In patients with uncertain TTS diagnoses, the medical records were reviewed by all core-team members, and a decision was reached based on consensus.
The data of all TTS patients who underwent cardiopulmonary resuscitation (CPR) were reviewed, and those with confirmed CA and known underlying heart rhythm were included in the present analysis. Cardiac arrest was defined as the absence of a palpable pulse in an unconscious patient requiring CPR to restore or attempt to restore systemic circulation.15 Ventricular fibrillation, pulseless VT, or organized electric activity other than VT [pulseless electrical activity (PEA) or asystole] on the electrocardiogram registered during the acute event were recorded.16 Patients were divided into those presenting with CA (i.e. CA as the first manifestation) and those subsequently developing CA during the acute phase of TTS (i.e. no CA at presentation). A subgroup analysis was also conducted including out-of-hospital vs. in-hospital CA. Information regarding implantable/wearable cardioverter defibrillators and pacemakers was recorded.
Study outcomes
Data regarding in-hospital complications and management were collected. The main outcomes were 60-day and 5-year mortality. Independent predictors of mortality and CA during the acute phase were investigated. A 5-year landmark survival analysis was conducted for patients who survived the first 60 days after TTS.
Statistical analysis
Continuous variables are presented as means and standard deviations or medians and inter-quartile ranges (IQRs). Categorical variables are provided with percentages. Continuous variables were compared using the Mann–Whitney U-test, whereas categorical variables were compared using the Pearson χ2 test or Fisher exact test. Kaplan–Meier curves were established to provide survival estimates, and group differences were assessed using the log-rank test. A univariable Cox regression analysis was performed to identify predictors of 60-day mortality in patients with CA. Covariates with P < 0.05 were included in a multivariable Cox regression model to identify independent predictors of 60-day mortality; missing values were replaced by multiple regression imputation. Another univariable Cox regression analysis was performed to identify predictors of CA in the acute phase among patients without CA at presentation. A two-sided P-value <0.05 was considered statistically significant. Hazard ratios are reported with 95% confidence intervals. All analyses were performed with SPSS v23.0 (IBM Corp., Armonk, NY, USA), and graphs were compiled with Prism 7 (GraphPad, La Jolla, CA, USA). The study is registered on clinicaltrials.gov (NCT01947621).
Results
Clinical characteristics
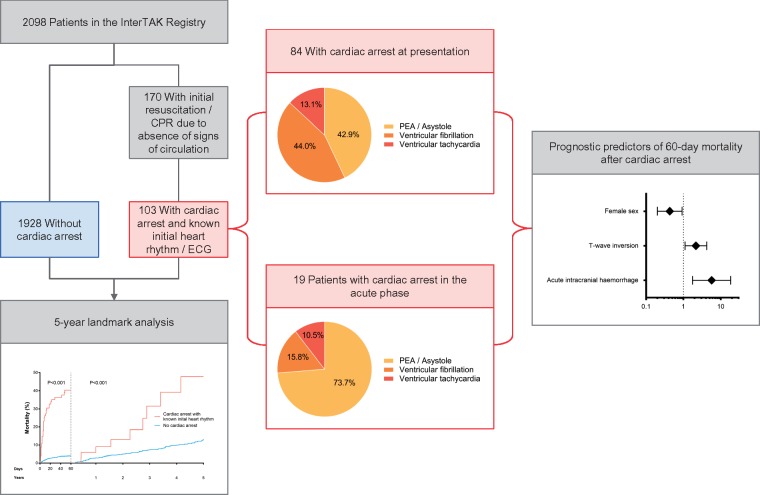
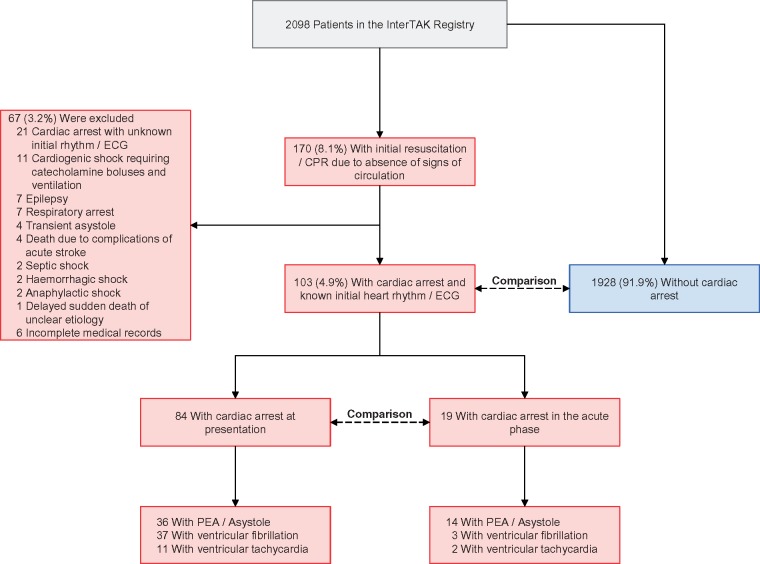
During the study period, 2098 patients were included in the InterTAK Registry (Figure 1 and Take home figure). Of the 170 (8.1%) patients who received CPR, 46 patients without documented CA were excluded. Of the remaining 124 patients with documented CA (5.9% of overall cohort, 72.9% of patients requiring CPR), 103 had data on the heart rhythm during CA (Figure 1).
Figure 1.
Study design. CPR, cardiopulmonary resuscitation; ECG, electrocardiogram; PEA, pulseless electrical activity.
Take home figure.
Cardiac arrest affects a relevant number of patients presenting with takotsubo syndrome and is associated with higher short and long-term mortality. Although typically occurring at presentation, cardiac arrest can also occur in the subsequent acute phase in patients with takotsubo syndrome. Among patients with cardiac arrest and takotsubo syndrome, simple clinical and electrocardiographic parameters may help to identify those at higher risk of death.
Compared with patients without CA, those with CA were younger and more frequently male (Table 1). Cardiac arrest patients had higher heart rate, lower systolic blood pressure, longer QTc on admission, higher maximal creatine kinase, and lower LVEF. They more frequently had atrial fibrillation (AF: 21.1% vs. 6.4%, P < 0.001) and apical TTS.
Table 1.
Characteristics of takotsubo patients with and without cardiac arrest
| TTS with CA and known initial heart rhythm | TTS without CA | ||
|---|---|---|---|
| Characteristic | N = 103 | N = 1928 | P-value |
| Demographics | |||
| Female sex, no./total no. (%) | 85/103 (82.5) | 1752/1928 (90.9) | 0.005 |
| Age (years) | 61.9 ± 14.8 (N = 103) | 67.3 ± 12.6 (N = 1928) | <0.001 |
| Symptoms and triggers, no./total no. (%) | |||
| Chest pain | 27/75 (36.0) | 1345/1759 (76.5) | <0.001 |
| Dyspnoea | 29/75 (38.7) | 816/1759 (46.4) | 0.23 |
| Physical trigger | 62/103 (60.2) | 652/1928 (33.8) | <0.001 |
| Emotional trigger | 13/103 (12.6) | 599/1928 (31.1) | <0.001 |
| ECG on admission | |||
| Sinus rhythm, no./total no. (%) | 71/90 (78.9) | 1590/1698 (93.6) | <0.001 |
| ST-segment elevation, no./total no. (%) | 38/90 (42.2) | 728/1698 (42.9) | 0.90 |
| ST-segment depression, no./total no. (%) | 12/90 (13.3) | 125/1698 (7.4) | 0.038 |
| T-wave inversion, no./total no. (%) | 32/90 (35.6) | 714/1698 (42.0) | 0.22 |
| Corrected QT (ms) | 474.6 ± 46.8 (N = 81) | 457.7 ± 47.3 (N = 1305) | 0.001 |
| Imaging and haemodynamic findings | |||
| Apical type, no./total no. (%) | 84/103 (81.6) | 1367/1928 (70.9) | 0.020 |
| LVEF (%)a | 33.5 ± 9.8 (N = 96) | 41.3 ± 11.4 (N = 1695) | <0.001 |
| Heart rate (beats/min) | 91.2 ± 22.2 (N = 61) | 87.2 ± 21.2 (N = 1503) | 0.09 |
| Systolic blood pressure (mmHg) | 113.6 ± 25.0 (N = 62) | 131.8 ± 28.4 (N = 1531) | <0.001 |
| LVEDP (mmHg) | 21.2 ± 8.1 (N = 68) | 21.7 ± 8.3 (N = 1102) | 0.60 |
| Neurologic and psychiatric disorders, no./total no. (%) | |||
| Acute neurologic disorders | 13/97 (13.4) | 149/1730 (8.6) | 0.10 |
| Acute intracranial bleeding | 4/97 (4.1) | 34/1730 (2.0) | 0.15 |
| Acute stroke/TIA | 3/97 (3.1) | 39/1730 (2.3) | 0.59 |
| Acute seizure | 3/97 (3.1) | 50/1730 (2.9) | 0.91 |
| Past or chronic neurologic disorders | 20/94 (21.3) | 314/1712 (18.3) | 0.48 |
| Acute psychiatric disorders | 8/97 (8.2) | 161/1727 (9.3) | 0.72 |
| Past or chronic psychiatric disorders | 31/94 (33.0) | 468/1711 (27.4) | 0.24 |
| Cardiac biomarkers, median (IQR) | |||
| Troponin admission, factor increase in ULNb | 6.70 (2.00–19.64) N = 79 | 8.20 (2.68–23.61) N = 1466 | 0.20 |
| Troponin maximum, factor increase in ULNb | 12.07 (5.11–43.23) N = 84 | 14.04 (4.99–35.00) N = 1588 | 0.49 |
| Creatine kinase admission, factor increase in ULN | 1.00 (0.48–1.67) N = 76 | 0.87 (0.54–1.45) N = 1279 | 0.51 |
| Creatine kinase maximum, factor increase in ULN | 1.95 (0.73–4.42) N = 81 | 1.10 (0.65–1.87) N = 1319 | <0.001 |
| BNP admission, factor increase in ULNc | 3.71 (1.33–10.15) N = 29 | 6.66 (2.35–18.22) N = 499 | 0.10 |
| BNP maximum, factor increase in ULNc | 7.51 (3.82–28.07) N = 39 | 10.22 (4.36–24.64) N = 640 | 0.61 |
| In-hospital complications and management, no./total no. (%) | |||
| Cardiogenic shock | 70/103 (68.0) | 90/1908 (4.7) | <0.001 |
| Death | 36/103 (35.0) | 46/1928 (2.4) | <0.001 |
| Catecholamine administration | 83/103 (80.6) | 137/1911 (7.2) | <0.001 |
| Invasive or non-invasive ventilation | 93/103 (90.3) | 205/1911 (10.7) | <0.001 |
Left ventricular ejection fraction (%): information from catheterization or echocardiography, if both available: catheterization.
Including upper limits of the normal range for Troponin T, high sensitivity Troponin T, and Troponin I.
Including upper limits of the normal range for brain natriuretic peptide and the N-terminal of prohormone brain natriuretic peptide.
BNP, brain natriuretic peptide; CA, cardiac arrest; ECG, electrocardiogram; IQR, inter-quartile range; LVEDP, left ventricular end-diastolic pressure; LVEF, left ventricular ejection fraction; TIA, transient ischaemic attack; TTS, takotsubo syndrome; ULN, upper limit of the normal range.
Cardiac arrest in relation to TTS onset
Eighty-four (81.6%) patients had CA at presentation and 19 (18.4%) developed CA in the acute phase, as shown in Figure 1. Median onset time of CA was 1 day (IQR 0–3 days) after TTS. The incidence of CA in those without CA at presentation was 1.0% (19/1947). Compared with patients with CA at presentation, those with CA in the acute phase had higher peak brain natriuretic peptide (BNP) values, ST-segment elevation (Table 2) and PEA or asystole (73.7% vs. 42.9%; Figure 1 and Take home figure) more frequently. Atrial fibrillation, ST-segment elevation, and high C-reactive protein on admission were associated with a higher risk of CA in the acute phase, whereas female sex was associated with a lower risk (see Supplementary material online, Figure S1).
Table 2.
Characteristics of takotsubo patients with cardiac arrest
| Patients with CA at initial presentation | Patients with CA during the acute phase | ||
|---|---|---|---|
| Characteristic | N = 84 | N = 19 | P-value |
| Demographics | |||
| Female sex, no./total no. (%) | 71/84 (84.5) | 14/19 (73.7) | 0.26 |
| Age (years) | 61.3 ± 15.3 (N = 84) | 64.9 ± 12.4 (N = 19) | 0.35 |
| Symptoms and triggers, no./total no. (%) | |||
| Chest pain | 19/59 (32.2) | 8/16 (50.0) | 0.19 |
| Dyspnoea | 22/60 (36.7) | 7/14 (50.0) | 0.36 |
| Physical trigger | 52/84 (61.9) | 10/19 (52.6) | 0.46 |
| Emotional trigger | 9/84 (10.7) | 4/19 (21.1) | 0.22 |
| ECG findings | |||
| Sinus rhythm on admission, no./total no. (%) | 56/71 (78.9) | 15/19 (78.9) | 0.99 |
| ST-segment elevation on admission, no./total no. (%) | 24/71 (33.8) | 14/19 (73.7) | 0.003 |
| ST-segment depression on admission, no./total no. (%) | 10/71 (14.1) | 2/19 (10.5) | 0.69 |
| T-wave inversion on admission, no./total no. (%) | 25/71 (35.2) | 7/19 (36.8) | 0.90 |
| Corrected QT on admission (ms) | 478.7 ± 48.3 (N = 63) | 460.6 ± 39.2 (N = 18) | 0.11 |
| Corrected QT at the event (ms) | 478.7 ± 48.3 (N = 63) | 468.6 ± 39.2 (N = 18) | 0.28 |
| Imaging and haemodynamic findings | |||
| Apical type, no./total no. (%) | 67/84 (79.8) | 17/19 (89.5) | 0.32 |
| LVEF (%)a | 33.7 ± 9.7 (N = 78) | 32.8 ± 10.3 (N = 18) | 0.60 |
| Heart rate (beats/min) | 90.6 ± 22.8 (N = 50) | 94.2 ± 20.2 (N = 11) | 0.49 |
| Systolic blood pressure (mmHg) | 113.3 ± 25.3 (N = 50) | 114.9 ± 24.9 (N = 12) | 0.73 |
| LVEDP (mmHg) | 20.6 ± 8.1 (N = 60) | 25.5 ± 7.2 (N = 8) | 0.15 |
| Neurologic and psychiatric disorders, no./total no. (%) | |||
| Acute neurologic disorders | 11/80 (13.8) | 2/17 (11.8) | 0.83 |
| Past or chronic neurologic disorders | 17/77 (22.1) | 3/17 (17.6) | 0.69 |
| Acute psychiatric disorders | 6/80 (7.5) | 2/17 (11.8) | 0.56 |
| Past or chronic psychiatric disorders | 28/77 (36.4) | 3/17 (17.6) | 0.17 |
| Cardiac biomarkers, median (IQR) | |||
| Troponin admission, factor increase in ULNb | 5.94 (1.97–15.65) N = 64 | 8.20 (3.10–37.07) N = 15 | 0.38 |
| Troponin maximum, factor increase in ULNb | 11.22 (5.03–46.06) N = 68 | 27.90 (9.13–43.23) N = 16 | 0.31 |
| Creatine kinase admission, factor increase in ULN | 0.85 (0.45–1.47) N = 62 | 1.49 (0.82–3.20) N = 14 | 0.06 |
| Creatine kinase maximum, factor increase in ULN | 1.71 (0.70–5.44) N = 66 | 2.09 (0.99–3.26) N = 15 | 0.82 |
| BNP admission, factor increase in ULNc | 3.68 (1.05–5.40) N = 21 | 28.57 (1.71–95.20) N = 8 | 0.11 |
| BNP maximum, factor increase in ULNc | 5.25 (3.07–10.69) N = 29 | 41.11 (10.55–160.51) N = 10 | <0.001 |
| In-hospital complications and management, no./total no. (%) | |||
| Cardiogenic shock | 59/84 (70.2) | 11/19 (57.9) | 0.30 |
| Death | 27/84 (32.1) | 9/19 (47.4) | 0.21 |
| Catecholamine administration | 68/84 (81.0) | 15/19 (78.9) | 0.84 |
| Invasive or non-invasive ventilation | 77/84 (91.7) | 16/19 (84.2) | 0.32 |
Left ventricular ejection fraction (%): information from catheterization or echocardiography, if both available: catheterization.
Including upper limits of the normal range for Troponin T, high sensitivity Troponin T, and Troponin I.
Including upper limits of the normal for brain natriuretic peptide and the N-terminal of prohormone brain natriuretic peptide.
BNP, brain natriuretic peptide; CA, cardiac arrest; ECG, electrocardiogram; IQR, inter-quartile range; LVEDP, left ventricular end-diastolic pressure; LVEF, left ventricular ejection fraction; ULN, upper limit of the normal range.
Outcomes
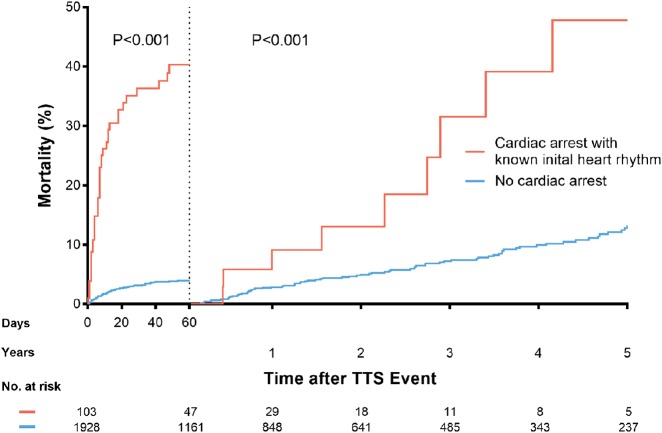
Cardiac arrest was associated with higher rates of in-hospital mortality, cardiogenic shock, catecholamine administration, and mechanical ventilation (Table 1). Compared with patients without CA, those with CA had higher 60-day mortality (40.3% vs. 4.0%, P < 0.001; Figure 2) and 5-year mortality (68.9% vs. 16.7%, P < 0.001; Supplementary material online, Figure S2). A 5-year outcome analysis with a landmark set at 60 days after TTS index showed that patients surviving CA had higher mortality than patients without CA (44.4% vs. 13.3%, P < 0.001; Figure 2, and Take home figure). Among the CA patients who survived the first 60 days, nine deaths occurred within 5 years: two (22.2%) cardiovascular deaths, three (33.3%) non-cardiovascular deaths, and four (44.4%) deaths from unknown causes. Among patients without CA, there were 10 (12.7%) cardiovascular deaths, 47 (59.5%) non-cardiovascular deaths, and 22 (27.8%) deaths from unknown causes.
Figure 2.
Landmark survival analysis showing a significantly higher mortality rate in patients with cardiac arrest during the first 60 days (P < 0.001) and during the 5-year follow-up (P < 0.001). TTS, takotsubo syndrome.
In-hospital outcomes did not differ between patients with CA at presentation or in the acute phase (Table 2), though the latter showed a trend towards higher 60-day mortality (33.3% vs. 52.6%, P = 0.12). Of the 12 CA patients who required implantable/wearable devices (implantable cardioverter defibrillator, 9; pacemaker, 2; and wearable cardioverter defibrillator, 1), 2 died during the 5-year follow-up. Of the 58 CA patients discharged without a device, 10 died during the 5-year follow-up. Among the 67 patients who underwent CPR but had unconfirmed CA or unknown heart rhythm, 60-day mortality was 17.5%. Patients with out-of-hospital and in-hospital CA had similar 60-day mortality (44% vs. 37%, P = 0.29).
Predictors of outcomes
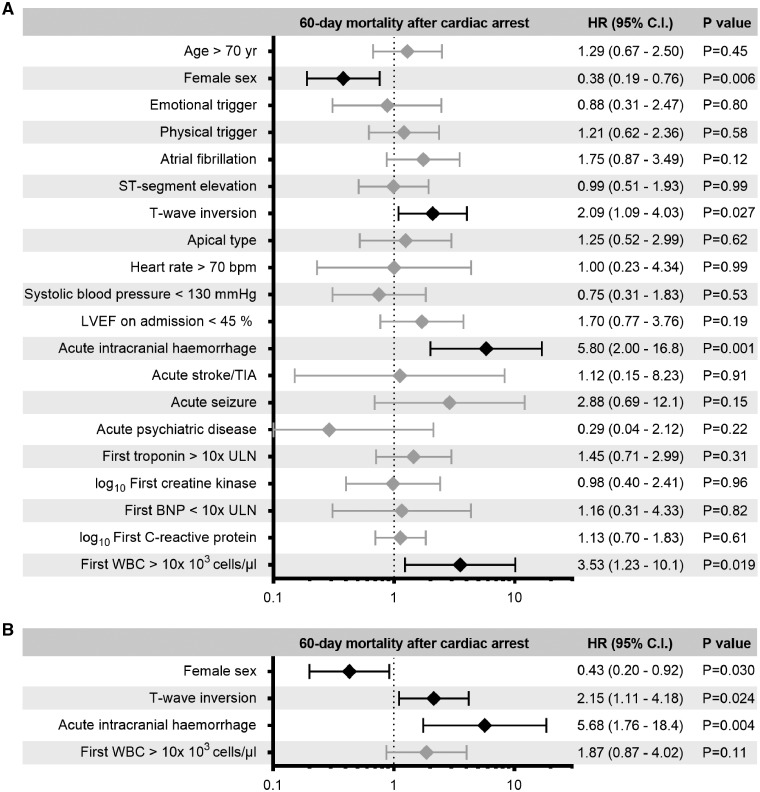
On univariable analysis, acute intracranial haemorrhage, T-wave inversion on admission, and white blood cell count >10 × 103 cells/µL were associated with higher 60-day mortality in patients with CA than in patients without CA (Figure 3A). After adjusting for potential confounders, T-wave inversion on admission and acute intracranial haemorrhage were independently associated with higher 60-day mortality after CA (Figure 3B). Female sex was associated with lower mortality both on univariable and multivariable analysis. The results remained unchanged even after excluding patients with intracranial haemorrhage.
Figure 3.
Univariable (A) and multivariable (B) predictors of 60-day mortality in patients with cardiac arrest (CA) and known initial heart rhythm. Acute intracranial haemorrhage and T-wave inversion are independently associated with an increased risk of 60-day mortality after CA, whereas female sex is associated with a decreased risk. Black: statistically significant predictors; grey: not significant. BNP, brain natriuretic peptide; CI, confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction; TIA, transient ischaemic attack; ULN, upper limit of normal range; WBC, white blood cell count.
Discussion
This study found that (i) CA occurred in 5.9% of TTS patients; (ii) CA was associated with a six-fold increase in short- and long-term mortality; (iii) T-wave inversion on admission and acute intracranial haemorrhage were independently associated with 60-day mortality following CA; (iv) in-hospital outcomes did not differ between patients with CA at presentation and during the acute phase; and (v) AF, ST-segment elevation, and high C-reactive protein on admission were associated with a higher frequency of CA during the acute phase, whereas female sex was associated with a lower frequency (Take home figure).
The finding that CA is relatively common in TTS is important, as TTS has historically been regarded as a benign condition. The American Heart Association acknowledges the proarrhythmic risk of TTS and includes TTS among the potential indications for wearable cardioverter defibrillators. In contrast, TTS is not mentioned in the current European Society of Cardiology guidelines for the management of ventricular arrhythmias.17,18 The CA incidence (5.9%) in this study is consistent with previous reports.7,8,19 Only Pant et al.20 reported a lower incidence rate, but their diagnoses were based on ICD-9 and not reviews of individual medical records. Compared with patients without CA, those with CA were younger, and less frequently female. Patients with CA have lower LVEF, longer QTc, higher cardiac biomarker values, and higher prevalence of apical TTS and AF. These findings suggest that more severe cardiac dysfunction accompanies CA, which is in agreement with the high prevalence of cardiogenic shock in these patients. These differences in patients’ characteristics suggest that different pathophysiological mechanisms are involved in patients with and without CA.21 Cardiac arrest was more common among TTS patients with physical triggers rather than emotional triggers, which is consistent with previous observations.4 Cardiogenic shock, though a consequence of CA,22 may also be a determinant of CA, as hypotension is an important mechanism of CA in TTS.5 QTc prolongation in CA may be partly attributable to the drugs administered, particularly amiodarone.23
The higher 5-year mortality after CA was driven by not only a higher 60-day mortality but also a steady increase in mortality after the acute phase, as shown by the 5-year landmark analysis. This might suggest that other factors beyond the acute myocardial dysfunction are detrimental in these patients even after acute recovery. This is consistent with a previous report linking life-threatening arrhythmias during the index hospitalization with worse 1-year survival.12 In contrast, another study found no differences in 1-year survival between patients with and without life-threatening arrhythmias who survived hospitalization.8
The use of devices (e.g. pacemakers) did not yield a substantial survival benefit, although our study was not designed to assess these treatments. Stiermaier et al. have also not identified a prognostic advantage of implantable cardioverter defibrillators in TTS patients with life-threatening arrhythmias, although they indicated a potential advantage of pacemakers in patients with bradyarrhythmias in the acute phase.7
Multivariable analysis of the predictors of mortality after CA showed that acute intracranial haemorrhage was independently associated with unfavourable outcomes, while female sex was associated with a more benign clinical course. Another study has reported a higher risk of CA in male TTS patients, but a clear-cut explanation for this finding is lacking.20 T-wave inversion on admission was associated with increased mortality following CA. As T-wave inversion has been associated with myocardial oedema,10,24 the early appearance of T-wave inversion may imply earlier or more extended oedema in the acute phase. Myocardial oedema is also associated with QTc prolongation, which is associated with adverse events (e.g. ventricular arrhythmias, mortality) in not only TTS but also other conditions like coronary artery disease and dilated cardiomyopathy.10,25–28 Polymorphic VT is a potential mechanism of CA in TTS patients with QTc prolongation.5,12,26 Thus, myocardial oedema may be the pathophysiological mechanism underlying increased mortality after CA in the presence of T-wave inversion.29
In patients with CA at TTS onset, TTS might be a consequence of the stress associated with CA and/or CPR.30,31 Left ventricular dysfunction can occur after CA; however, global hypokinesia is the more frequent alteration, and TTS-like patterns occur in few CA patients.32,33 Based on the current data, it is not possible to distinguish whether TTS is the cause or the consequence of CA, although the former explanation is more likely.34,35 Conditions other than TTS that could explain CA were not identifiable in the current analysis, but a systematic screening for rare causes, such as channelopathies, was not performed.36 Hence, it is likely that a substantial proportion of these events was caused by TTS, as a causal relationship between TTS and life-threatening arrhythmias has already been established. Most of our patients had CA at presentation, which is consistent with previous reports.5,8 A potential explanation for the relationship between TTS and CA is that TTS onset is associated with massive increases in circulating catecholamine levels, which together with myocardial stunning and oedema, may lead to life-threatening arrhythmias.37 Compared with patients with CA at presentation, those with CA during the acute phase were more likely to have PEA/asystole, higher BNP values, and ST-segment elevation on admission. This suggests that CA may develop in the acute phase due to severely compromised heart function, whereas a more purely arrhythmic substrate may lead to CA at presentation, which was often associated with VF/pulseless VT. Another study has reported a similar distribution of CA at presentation vs. during the acute phase (60 vs. 17 patients) and more frequent ventricular tachyarrhythmias rather than PEA/asystole in CA at presentation.5 However, that study was a systematic review of case reports, and hence, relevant reporting and selection biases should be acknowledged. In our study, patients with CA during the acute phase showed a trend towards higher mortality compared with patients with CA at initial presentation. Out-of-hospital CA was not associated with higher mortality than in-hospital CA. These interesting results should be investigated in future studies, even though mortality is probably underestimated in patients with CA at initial presentation. This is because TTS can only be diagnosed in patients arriving alive and in condition to undergo imaging exams in the emergency department. Cardiac arrest patients who die before undergoing testing could never receive a diagnosis of TTS.
In the small subset of 19 patients who developed CA after initial presentation, we identified that AF on admission, ST-segment elevation, and high C-reactive protein were univariably associated with higher CA frequency, whereas female sex was associated with lower CA frequency. ST-segment elevation might be associated with electrical instability and may indicate the transmural extension of myocardial oedema. Thus, TTS patients with these features at admission merit intensive monitoring for about 48–72 h, given that the median time to CA was 1 day (IQR, 0–3 days).
Study limitations
The study involved a partly retrospective analysis from a multicentre registry. Therefore, potential confounding and selection biases must be considered when evaluating the results. Given the low number of absolute events, the multivariable model for predictors of 60-day mortality was based on few covariates that might have an impact on the outcome. Data on left ventricular outflow tract obstruction,38 time to return of spontaneous circulation, and neurologic outcomes after CPR were unavailable.
Conclusions
Cardiac arrest is relatively common in TTS and is associated with worse outcomes. Cardiac arrest typically occurs at presentation, but may occur in the subsequent acute phase. Clinical and electrocardiographic parameters may independently predict mortality after CA and identify patients at a higher risk for developing CA in the acute phase.
Funding
C.T. has been supported by the H.H. Sheikh Khalifa bin Hamad Al-Thani Research Programme and the Swiss Heart Foundation. The InterTAK Registry is supported by the Biss Davies Charitable Trust. The funding source had no role in the study design and execution; data collection, management, analysis, and interpretation; manuscript preparation, review, and approval; or decision to submit the manuscript for publication.
Conflict of interest: Disclosures are reported by individual authors in the ICMJE form as submitted to the editorial office.
Supplementary Material
This CLP has been handled by our Guest Editor Prof. Anthony DeMaria.
See page 2152 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz324)
References
- 1. Sato HTH, Uchida T, Dote K, Ishihara M.. Tako-tsubo-like left ventricular dysfunction due to multivessel coronary spasm In: Kodama K, Haze K, Hori M, eds. Clinical Aspect of Myocardial Injury: From Ischemia to Heart Failure. Tokyo: Kagakuhyoronsha Publishing; 1990. p56–64. (In Japanese.) [Google Scholar]
- 2. Di Vece D, Citro R, Cammann VL, Kato K, Gili S, Szawan KA, Micek J, Jurisic S, Ding KJ, Bacchi B, Schwyzer M, Candreva A, Bossone E, D'Ascenzo F, Sarcon A, Franke J, Napp LC, Jaguszewski M, Noutsias M, Münzel T, Knorr M, Heiner S, Katus HA, Burgdorf C, Schunkert H, Thiele H, Bauersachs J, Tschöpe C, Pieske BM, Rajan L, Michels G, Pfister R, Cuneo A, Jacobshagen C, Hasenfuβ G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Banning A, Cuculi F, Kobza R, Fischer TA, Vasankari T, Airaksinen KEJ, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Empen K, Felix SB, Delmas C, Lairez O, El-Battrawy I, Akin I, Borggrefe M, Gilyarova E, Shilova A, Gilyarov M, Horowitz J, Kozel M, Tousek P, Widimský P, Winchester DE, Ukena C, Di Mario C, Prasad A, Böhm M, Bax JJ, Lüscher TF, Ruschitzka F, Ghadri JR, Templin C.. Outcomes associated with cardiogenic shock intakotsubosyndrome. Circulation 2019;139:413–415. [DOI] [PubMed] [Google Scholar]
- 3. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, Y-Hassan S, Migliore F, Horowitz JD, Shimokawa H, Lüscher TF, Templin C.. International expert consensus document on takotsubo syndrome (part II): diagnostic workup, outcome, and management. Eur Heart J 2018;39:2047–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschöpe C, Schultheiss HP, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Böhm M, Erbel R, Cuneo A, Kuck KH, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KE, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Lüscher TF.. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med 2015;373:929–938. [DOI] [PubMed] [Google Scholar]
- 5. Singh K, Carson K, Hibbert B, Le May M.. Natural history of cardiac arrest in patients with takotsubo cardiomyopathy. Am J Cardiol 2015;115:1466–1472. [DOI] [PubMed] [Google Scholar]
- 6. Syed FF, Asirvatham SJ, Francis J.. Arrhythmia occurrence with takotsubo cardiomyopathy: a literature review. Europace 2011;13:780–788. [DOI] [PubMed] [Google Scholar]
- 7. Stiermaier T, Rommel KP, Eitel C, Möller C, Graf T, Desch S, Thiele H, Eitel I.. Management of arrhythmias in patients with takotsubo cardiomyopathy: is the implantation of permanent devices necessary? Heart Rhythm 2016;13:1979–1986. [DOI] [PubMed] [Google Scholar]
- 8. El-Battrawy I, Lang S, Ansari U, Tülümen E, Schramm K, Fastner C, Zhou X, Hoffmann U, Borggrefe M, Akin I.. Prevalence of malignant arrhythmia and sudden cardiac death in takotsubo syndrome and its management. Europace 2018;20:843–850. [DOI] [PubMed] [Google Scholar]
- 9. Madias JE. Cardiac arrest-triggered takotsubo syndrome vs. takotsubo syndrome complicated by cardiac arrest. Int J Cardiol 2016;225:142–143. [DOI] [PubMed] [Google Scholar]
- 10. Madias C, Fitzgibbons TP, Alsheikh-Ali AA, Bouchard JL, Kalsmith B, Garlitski AC, Tighe DA, Estes NA 3rd, Aurigemma GP, Link MS.. Acquired long QT syndrome from stress cardiomyopathy is associated with ventricular arrhythmias and torsades de pointes. Heart Rhythm 2011;8:555–561. [DOI] [PubMed] [Google Scholar]
- 11. Migliore F, Zorzi A, Perazzolo Marra M, Iliceto S, Corrado D.. Myocardial edema as a substrate of electrocardiographic abnormalities and life-threatening arrhythmias in reversible ventricular dysfunction of takotsubo cardiomypopathy: imaging evidence, presumed mechanisms, and implications for therapy. Heart Rhythm 2015;12:1867–1877. [DOI] [PubMed] [Google Scholar]
- 12. Stiermaier T, Eitel C, Denef S, Desch S, Schuler G, Thiele H, Eitel I.. Prevalence and clinical significance of life-threatening arrhythmias in takotsubo cardiomyopathy. J Am Coll Cardiol 2015;65:2148–2150. [DOI] [PubMed] [Google Scholar]
- 13. Ghadri JR, Cammann VL, Templin C.. The International Takotsubo Registry: rationale, design, objectives, and first results. Heart Fail Clin 2016;12:597–603. [DOI] [PubMed] [Google Scholar]
- 14. Prasad A, Lerman A, Rihal CS.. Apical ballooning syndrome (tako-tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J 2008;155:408–417. [DOI] [PubMed] [Google Scholar]
- 15. Kleinman ME, Brennan EE, Goldberger ZD, Swor RA, Terry M, Bobrow BJ, Gazmuri RJ, Travers AH, Rea T.. Part 5: adult basic life support and cardiopulmonary resuscitation quality: 2015 American Heart Association Guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015;132:S414–S435. [DOI] [PubMed] [Google Scholar]
- 16. Myerburg RJ, Halperin H, Egan DA, Boineau R, Chugh SS, Gillis AM, Goldhaber JI, Lathrop DA, Liu P, Niemann JT, Ornato JP, Sopko G, Van Eyk JE, Walcott GP, Weisfeldt ML, Wright JD, Zipes DP.. Pulseless electric activity: definition, causes, mechanisms, management, and research priorities for the next decade: report from a National Heart, Lung, and Blood Institute workshop. Circulation 2013;128:2532–2541. [DOI] [PubMed] [Google Scholar]
- 17. Piccini JP, Allen LA, Kudenchuk PJ, Page RL, Patel MR, Turakhia MP; American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing. Wearable cardioverter-defibrillator therapy for the prevention of sudden cardiac death: a science advisory from the American heart association. Circulation 2016;133:1715–1727. [DOI] [PubMed] [Google Scholar]
- 18. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ.. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 19. Wang Y, Xia L, Shen X, Han G, Feng D, Xiao H, Zhai Y, Chen X, Miao Y, Zhao C, Wang Y, Guo M, Li T, Zhu HY.. A new insight in sudden cardiac death in young people—a systematic review of cases of takotsubo cardiomyopathy. Medicine 2015;94:e1174.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pant S, Deshmukh A, Mehta K, Badheka AO, Tuliani T, Patel NJ, Dabhadkar K, Prasad A, Paydak H.. Burden of arrhythmias in patients with takotsuo cardiomyopathy (Apical Ballooning Syndrome). Int J Cardiol 2013;170:64–68. [DOI] [PubMed] [Google Scholar]
- 21. Ancona F, Bertoldi LF, Ruggieri F, Cerri M, Magnoni M, Beretta L, Cianflone D, Camici PG.. Takotsubo cardiomyopathy and neurogenic stunned myocardium: similar albeit different. Eur Heart J 2016;7:2830–2832. [DOI] [PubMed] [Google Scholar]
- 22. Napp LC, Kühn C, Bauersachs J.. ECMO in cardiac arrest and cardiogenic shock. Herz 2017;42:27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yap YG, Camm AJ.. Drug induced QT prolongation and torsades de pointes. Heart 2003;89:1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zorzi A, Perazzolo Marra M, Migliore F, De Lazzari M, Tarantini G, Iliceto S, Corrado D.. Relationship between repolarization abnormalities and myocardial edema in atypical tako-tsubo syndrome. J Electrocardiol 2013;46:348–351. [DOI] [PubMed] [Google Scholar]
- 25. Imran TF, Rahman I, Dikdan S, Shah R, Niazi OT, Thirunahari N, Alhaj E, Klapholz M, Gaziano JM, Djousse L.. QT prolongation and clinical outcomes in patients with takotsubo cardiomyopathy. Pacing Clin Electrophysiol 2016;39:607–611. [DOI] [PubMed] [Google Scholar]
- 26. Samuelov-Kinori L, Kinori M, Kogan Y, Swartzon M, Shalev H, Guy D, Ferenidou F, Mashav N, Sadeh B, Atzmony L, Kliuk-Ben-Basat O, Steinvil A, Justo D.. Takotsubo cardiomyopathy and QT interval prolongation: who are the patients at risk for torsades de pointes? J Electrocardiol 2009;42:353–357. [DOI] [PubMed] [Google Scholar]
- 27. Williams ES, Thomas KL, Broderick S, Shaw LK, Velazquez EJ, Al-Khatib SM, Daubert JP.. Race and gender variation in the QT interval and its association with mortality in patients with coronary artery disease: results from the Duke Databank for Cardiovascular Disease (DDCD). Am Heart J 2012;164:434–441. [DOI] [PubMed] [Google Scholar]
- 28. Karatolios K, Holzendorf V, Richter A, Schieffer B, Pankuweit S; Competence Network Heart Failure Germany. Long-term outcome and predictors of outcome in patients with non-ischemic dilated cardiomyopathy. Int J Cardiol 2016;220:608–612. [DOI] [PubMed] [Google Scholar]
- 29. Madias JE. Takotsubo syndrome/QTc interval prolongation/myocardial edema/cardiac sympathetic denervation/cardiac arrhythmias: a quintet needing exploration. Int J Cardiol 2016;203:259–261. [DOI] [PubMed] [Google Scholar]
- 30. Wortsman J, Frank S, Cryer PE.. Adrenomedullary response to maximal stress in humans. Am J Med 1984;77:779–784. [DOI] [PubMed] [Google Scholar]
- 31. Paradis NA, Martin GB, Rosenberg J, Rivers EP, Goetting MG, Appleton TJ, Feingold M, Cryer PE, Wortsman J, Nowak RM.. The effect of standard- and high-dose epinephrine on coronary perfusion pressure during prolonged cardiopulmonary resuscitation. JAMA 1991;265:1139–1144. [PubMed] [Google Scholar]
- 32. Kern KB, Hilwig RW, Rhee KH, Berg RA.. Myocardial dysfunction after resuscitation from cardiac arrest: an example of global myocardial stunning. J Am Coll Cardiol 1996;28:232–240. [DOI] [PubMed] [Google Scholar]
- 33. Cha KC, Kim HI, Kim OH, Cha YS, Kim H, Lee KH, Hwang SO.. Echocardiographic patterns of postresuscitation myocardial dysfunction. Resuscitation 2018;124:90–95. [DOI] [PubMed] [Google Scholar]
- 34. Madias JE. Cardiac arrest in patients before and after the inception of takotsubo syndrome. Am J Cardiol 2015;115:1785.. [DOI] [PubMed] [Google Scholar]
- 35. Liang JJ, Cha YM, Oh JK, Prasad A.. Sudden cardiac death: an increasingly recognized presentation of apical ballooning syndrome (takotsubo cardiomyopathy). Heart Lung 2013;42:270–272. [DOI] [PubMed] [Google Scholar]
- 36. Prystowsky EN, Padanilam BJ, Joshi S, Fogel RI.. Ventricular arrhythmias in the absence of structural heart disease. J Am Coll Cardiol 2012;59:1733–1744. [DOI] [PubMed] [Google Scholar]
- 37. Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC.. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005;352:539–548. [DOI] [PubMed] [Google Scholar]
- 38. Templin C, Ghadri JR, Napp LC.. Takotsubo (stress) cardiomyopathy. N Engl J Med 2015;373:2689–2691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.