Abstract
The influence of emotion on association-memory is often attributed to arousal, but negative stimuli are typically used to test for these effects. While prior studies of negative emotion on association-memory have found impairments, theories suggest that positive emotion may have a distinct effect on memory, and may lead to enhanced association-memory. Here we tested participants’ memory for pairs of positive and neutral words using cued recall, supplemented with a mathematical modeling approach designed to disentangle item-versus association-memory effects that may otherwise confound cued-recall performance. We consistently found enhanced association-memory due to positive emotion. These results provide further evidence that positive information is processed differently than negative and that, when examining association formation, valence as well as arousal must be considered.
Keywords: valence, association-memory, emotion, arousal, positive emotion, memory
Introduction
Emotion, the internal state associated with our experience of affect, has an impact on our cognitive processes, behavior, and memory. Emotion is typically described as being composed of two orthogonal dimensions: valence, ranging from pleasant to unpleasant, and arousal, ranging from calm to excited (Russell, 1980, 2003), though other prevalent theories of emotion also exist (Ekman, 1992; Plutchik, 1980). Within this view of emotion being comprised of two dimensions, many theories regarding the influence of emotion on memory have focused on the contributions of the arousal dimension (e.g., Christianson, 1992; Easterbrook, 1959; Mather & Sutherland, 2011), with substantially less research being conducted on the influence of valence (but see Kensinger, 2009; Sakaki, Fryer, & Madan, 2014; Bowen, Kark, Kensinger, in press). Previous research suggests that positive and negative emotion can have distinct effects on cognitive processes. For instance, valence has been shown to influence the scope of perceptual processing: Positive emotion has been shown to lead to greater global perceptual processing in a global-local focus test, whereas negative emotion led to greater local processing (Basso, Schefft, Ris, & Dember, 1996; Fredrickson & Branigan, 2005; Gasper & Clore, 2002). Fredrickson’s (1998, 2001) broaden-and-build theory suggests that cognitive broadening and increased attentional scope due to positive emotion may serve an adaptive function by enabling us to build both physical and intellectual resources.
Prior work has suggested that arousal may impair associative binding. This account is supported by a variety of paradigms, with arousal impairing memory for associations between pairs of pictures (Bisby & Burgess, 2014; Bisby, Horner, Hørlyck, & Burgess, 2016; Madan, Fujiwara, Caplan, & Sommer, 2017), scenes with objects (Bisby, Horner, Bush & Burgess, 2018; Touryan, Marian, & Shimamura, 2007; Rimmele, Davachi, Petrov, Dougal, & Phelps, 2011), and pairs of words (Madan, Caplan, Lau, & Fujiwara, 2012). This reduced association-memory has been proposed to reflect the differences between the types of mnemonic processes supported by the amygdala and the hippocampus. In particular, the amygdala may support memory for emotion-related item features, while the hippocampus may bind associations (Madan et al., 2017; Ritchey, Libby, & Ranganath, 2015; Yonelinas & Ritchey, 2015). Arousal is proposed to shift the balance from hippocampal-driven mnemonic processes to amygdala-driven mnemonic processes, thus impairing associative memories (Madan et al., 2017; Roozendaal, McEwen, & Chattarji, 2009; Tejeda & O’Donnell, 2014; Williams et al., 2001), reminiscent of theories describing opposing ‘hot’ and ‘cold’ systems (Kerr & Zelazo, 2004; Metcalfe & Mischel, 1999; Dolcos, Iordan, & Dolcos, 2011; Prencipe et al., 2011). Note, however, that sometimes arousal has been found to enhance association-memory (e.g., Anderson & Shimamura, 2005; Doerksen & Shimamura, 2001; Mickley Steinmetz, Knight, & Kensinger, 2016). An important difference between these studies and the focus of the current work is the nature of the association, e.g., if both items are similar types of items (words, pictures, videos, etc.) and how distinct the two to-be-associated items are (see Mather, 2007, for a review).
A limitation of the perspective presented in prior work, however, is the ubiquitous focus on negatively valenced emotional stimuli (or high-arousal stimuli of mixed valence, e.g., taboo words [Madan et al., 2012]). If arousal is the principal factor that influences association-memory, then association-memory should be similarly affected when the to-be-associated content is positive in valence as when it is negative in valence (arousal hypothesis). There is an alternate possibility, however, which is that impaired association memory may not generalize to the case of positive stimuli. One prior study of association-memory used pairs of positive words, along with pairs of negative and neutral words, Zimmerman and Kelley (2010). This study found that pairs consisting of two positive words were recalled better in cued recall than pairs consisting of two neutral words—potentially an enhancement of association-memory due to positive emotion (valence hypothesis). It is ambiguous, however, whether this enhanced cued recall performance is due to item-memory effects (e.g., enhanced probe effectiveness and/or target retrievability) or enhanced association-memory, an ambiguity that the current study was designed to resolve.
Although associative memory can be tested in many ways – using recognition or recall – not all methods allow contributions of association-memory to be distinguished from contributions of item-memory (Madan, Glaholt, & Caplan, 2010). For instance, if some items are more easily retrieved from memory, these items will be recalled more often in response to a cue, even if they were not better bound to the cue word. When associative recognition paradigms are used to test associative memory (e.g., Pierce & Kensinger, 2011; Onoda et al., 2009), it is not possible to disentangle item- and association-memory contributions. When cued recall is used, mathematical modeling can be used to disentangle these effects of item- and association-memory (Madan et al., 2010, 2012; Madan, 2014). This modeling approach is based on the assumption that three separable components are involved in successful cued recall performance and can be influenced by item properties: probe effectiveness, relationship strength, and target retrievability. Madan et al. (2012) revealed that this modeling approach could help to resolve seemingly discrepant findings with regard to the effects of arousal on associative memory (e.g., Guillet and Arndt, 2009; Zimmerman and Kelley, 2010) and further revealed that association-memory was impaired with negative stimuli, while item-memory was enhanced. This pattern of results was replicated by Bisby and Burgess (2014) and Madan et al. (2017). In Madan et al. (2012), the modeling findings indicated that negative stimuli enhanced target retrievability, but simultaneously impaired relationship strength—i.e., the formation of associations. More broadly, this modeling approach, In earlier work, this modeling approach was used to clarify how word imageability and frequency differentially influenced cued recall performance (Madan et al., 2010); imageability was found to enhance relationship strength, whereas word frequency primarily influenced target retrievability.
In the present study, we define association-memory as memory for unique pairs of items, such as word-word pairs, each emphasized equivalently during encoding. We test associative memory using cued recall, following the modeling approach of Madan and colleagues (2010, 2012) to separate association-memory effects from item-memory effects. If the principal influence of emotion on association-memory is based on arousal, we expect that association-memory should be impaired for positive high-arousal stimuli, as has been previously found with negative stimuli (e.g., Bisby & Burgess, 2014; Madan et al., 2012, 2017) (arousal hypothesis). Alternatively, if positive emotion results in a broadening of attention as demonstrated in perceptual studies, we may instead observe an enhancement of association-memory due to positive emotion (valence hypothesis).
Methods
Participants
Participants included 60 young adults (53 females), ranging from 18 to 28 years old (M=20.20, SD=2.33), pre-screened to exclude individuals with a history of psychiatric or neurological disorder. Informed written consent was obtained from all participants prior to beginning the study, which was approved by the Boston College Institutional Review Board. No individual participated in more than one experiment.
Materials
Word pairs were constructed using two pools of words: positive and neutral. All words and normative ratings of arousal, valence, and dominance were obtained from Warriner, Kuperman, and Brysbaert (2013). Ratings for imageability, word frequency, and familiarity, as well as the number of syllables and letters were obtained from the MRC Psycholinguistic Database (Wilson, 1988). Number of orthographic neighbors (number of words of the same length that differ in only one letter) and average word frequency of orthographic neighbors (per million words) were calculated with MCWord (Medler & Binder, 2005) based on the CELEX Lexical Database (Baayen, Piepenbrock, & Gulikers, 1995). Words were selected such that both words in both pools would be equivalent for all item properties except for arousal and valence. Each of the final word pools consisted of 64 words, and statistically differed in valence, arousal, and dominance ratings, but not on any of the other measures [valence: t(126)=36.47, p<.001; arousal: t(126)=3.42, p<.001; dominance: t(126)=6.45, p<.001]. See Table 1 for the word pool statistics.
Table 1.
Word property statistics for Experiment 1 based on normative ratings from Warriner et al (2013), Wilson (1988), Medler and Binder (2005), and Landauer and Dumais (1997).ON = Orthographic Neighborhood. Mean ratings are shown with standard deviation in parentheses.
| Positive | neutral | t | |
|---|---|---|---|
| Valence | 7.12 (0.35) | 5.06 (0.28) | 36.47 *** |
| Arousal | 4.42 (0.94) | 3.92 (0.70) | 3.42 *** |
| Dominance | 6.17 (0.77) | 5.37 (0.62) | 6.45 *** |
| Familiarity | 524.69 (54.93) | 519.34 (39.99) | 0.63 |
| Imageability | 514.27 (96.48) | 499.02 (95.74) | 0.90 |
| Word Frequency | 42.48 (47.10) | 45.20 (53.16 ) | 0.31 |
| N. of Letters | 6.53 (0.50) | 6.41 (0.50) | 1.42 |
| N. of Syllables | 2.00 (0.00) | 2.00 (0.00) | 0.00 |
| LSA cos(θ) | 0.14 (0.11) | 0.08 (0.08) | 0.71 |
See main text for details on the word databases used.
p < .10
p < .05
p < .01
p < .001.
We also calculated LSA cos(θ) as a measure of within-pool word similarity (Landauer & Dumais, 1997). LSA cos(θ) for each word pool is as follows (M±SD): positive (0.14±0.11) and neutral (0.08±0.08). Independent-sample t-tests (with df adjusted based on the effective number of independent comparisons) of the LSA cos(θ) values suggest that both pools were similar in their semantic cohesiveness [t(126)=0.71].
Procedure
The paired-associate task consisted of eight repetitions through study, distractor, and cued recall phases, with one preceding practice study set which was not included in the data analysis. After completion of the eight repetitions, participants were given a final free recall task. The session concluded with a word ratings task.
Paired-associate task.
Words were presented in a white “Courier New” font, which ensured fixed letter width, in the center of a black screen. Words were presented sequentially, for 3,000 ms each, with a 50 ms inter-stimulus interval within pairs and a 4,000 ms inter-pair interval. During these intervals, a fixation cross, “+”, was displayed in the center of the screen. During the study phase, participants were presented with eight word pairs that they were instructed to study in preparation for a later memory test. Each study set consisted of positive-positive, positive-neutral, neutral-positive, and neutral-neutral pairs, with two pairs of each type presented. Word pairings, word membership by pair type, order of pairs, and order of pair types were all randomized across participants.
The distractor task included four arithmetic trials, in the form of A + B + C = _______, where A, B, and C were randomly selected digits between two and eight. Each problem remained in the center of the screen for 5,000 ms. The participant was asked to type the correct answer during this fixed interval. The inter-trial interval was 200 ms.
During the cued recall task, a probe word was presented next to a blank line. Participants were asked to type the word that was paired with the probe word during the study phase. If the blank line was presented on the right, the target word was the second item of the pair (“forward direction”). If the blank line was presented on the left of the probe word, the target word was the first item of the pair (“backward direction”). Within each study set, half the pairs of each pair type were tested in the forward direction and half were in the backward direction. Participants had a maximum of 15,000 ms to respond, with an inter-trial interval of 250 ms. If participants could not recall a target word for the probe word, they were instructed to type “PASS”.
Final free recall task.
Participants had five minutes to recall as many words as they could remember from the experiment. Participants typed in a word and pressed the “Enter” key. When they pressed the “Enter” key, the screen cleared and the participant was allowed to type in another word. Repeated responses were only counted once.
Word ratings task.
Participants rated all of the words first for arousal and then for valence. Words were presented one at a time on the computer screen, along with a 5-point version of the respective Self-Assessment Manikin diagram (SAM; Bradley & Lang, 1994). In the arousal rating task, ‘1’ corresponded to excited and ‘5’ corresponded to calm. In the valence rating task, ‘1’ corresponded to pleasant and ‘5’ corresponded to unpleasant. Presentation order of words was randomized in each rating task. Note that the Warriner et al. (2013) normative study used a 9-point scale (see Table 1), whereas we used a 5-point scale in our ratings task.
Data analysis
Effects were considered significant based on an alpha level of 0.05. In the final free recall task, participants who recalled fewer than two positive and two neutral words were excluded (N=4).
Model-based estimation of cued recall accuracy
To quantify the relative effects of positive emotion on item-vs. association-memory, we fit a probabilistic “item-relationship” model (Madan et al., 2010, 2012; Madan, 2014) to the mean accuracy data. This model assumes that successful cued recall relies on three separable and independent processes, each with a probability of being completed successfully: probe effectiveness (Probei), association strength (Relatj), and target retrievability (Targetk). Successful cued recall [Acc(Probe,Target)] can therefore be defined as:
where P(Probei) and P(Targetk) denote the probabilities of effectively cueing memory with the probe item and effectively retrieving the target item from memory, respectively, where i={P, n} and k={P, n}, denoting positive and neutral words. P(Relatj) denotes the probability of retrieving the pair depending on the relationship between the two items, where j={PP, mixed, nn}. PP denotes positive-positive pairs; nn denotes neutral-neutral pairs; mixed denotes pairs consisting of one positive word and one neutral word. By this logic, the probability that all three processes will be successful, resulting in successful cued recall, is the result of multiplying the probabilities from the three processes together. This general equation can thus be expanded as:
By testing all combinations of probe and target, we are able to determine the relative effect of positive emotion on each process. This relative effect is implemented as a ratio, where each process is assigned a parameter.
Each parameter represents the relative effect of positive emotion on that particular process: probe effectiveness (p), association strength (r1, r2), and target retrievability (t). In relation to behavior, the parameters represent separable component in the cued recall process. Target retrievability represents how easily it is for an item to be retrieved from memory and output, sometimes referred to as redintegration (e.g., Lewandowsky & Farrell, 2000), and is exemplified as items differing in word frequency (Madan et al., 2010; also see Criss, Aue, & Smith, 2011). Relationship strength represents the association between the probe and target, best corresponding to association-memory, and is exemplified by items differing in imageability (Madan et al., 2010). Probe effectiveness represents how well an item can cue the specific episodic association, and is exemplified by items differing in contextual distinctiveness (Criss, Aue, & Smith, 2011; also see McDonald & Shillcock, 2001).
For each of these four parameters, a ratio value greater than 1 represents an enhancement of that process due to positive emotion (e.g., t>1 suggests greater target retrievability for positive than neutral words), a value less than 1 represents an impairment for positive relative to neutral words, and a value equal to 1 represents a null effect. The relationship strength process comprises two parameters, r1 and r2, for the ratios between (a) positive-positive pairs relative to mixed pairs, and (b) mixed pairs relative to neutral-neutral pairs, respectively. In other words, we do not assume that these two ratios are identical, and instead fit them independently. An additional scaling parameter (c) is also fit to scale the ratios to the behavioural data. For example, accuracy on a neutral-neutral pair would be equivalent to simply c; however, accuracy on a positive-positive pair would be equivalent to c × p × r1 × r2 × t. Accuracy for a pair with a neutral probe and a positive target would be equivalent c × r2 × t.
Importantly, our item-relationship model is underdetermined, i.e., there are multiple ways to explain the data using various combinations of parameters. For this reason, we only used further-constrained model variants wherein a subset of the parameters p, r1, r2, and t was fixed to 1 and the remaining parameters were free to vary (as we have done previously; Madan et al., 2010, 2012). After constraining the model, the model can be fit to each participant and parameter values and model fits be summarized across participants. To compare the relative fits of the model variants, we used BIC (Bayesian Information Criterion), which takes into account the number of free parameters. By convention, if the difference between two model fits is less than two, neither of the models’ fit to the data is significantly better – thus we report all scores as ΔBIC relative to the best-fitting model.
Results
Confirmatory analyses
Confirming our word selection criteria, participants rated the positive words as more positive than the neutral words, as well as higher in arousal [valence: t(59)=21.76, p<.001, d=3.04; arousal: t(59) = 6.04, p<.001, d=0.95]. As expected, participants recalled more positive than neutral words in the final free recall task [proportion recalled (M±SEM): Positive=.29±.01, neutral=.22±.01; t(55)=7.16, p<.001, d=0.65].
Cued recall
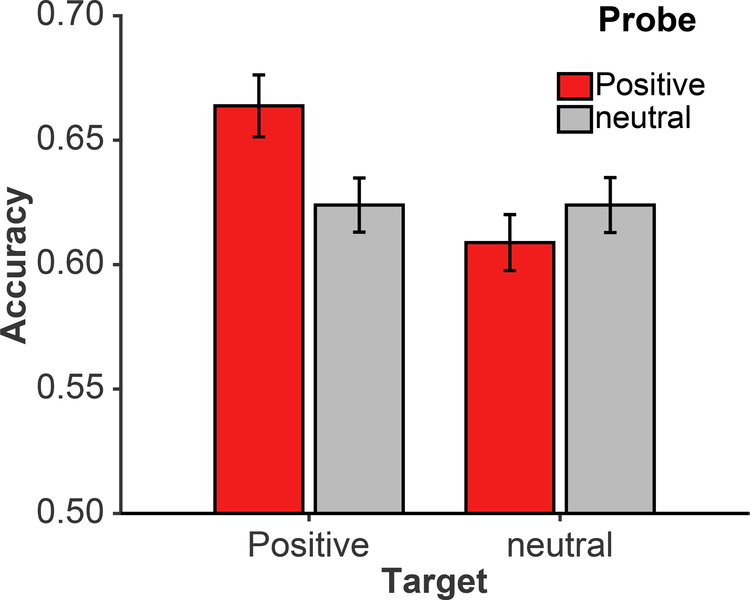
We conducted a PROBE [2: positive, neutral] x TARGET [2: positive, neutral] x TEST DIRECTION [2: forward, backward] repeated-measures ANOVA. There was a significant interaction of PROBE and TARGET [F(1,59)=4.18, p=.045, ηp2=.066], with better cued recall for pairs where both the probe and target were positive words [M=.64], relative to the other three pair types (see Figure 1A). Post-hoc t-tests indicated that pairs with both the probe and target as positive words resulted in significantly better cued recall performance than all other pair types: versus positive probe and a neutral target [t(59)=2.59, p=.012, Cohen’s d=0.33], versus neutral probe and a positive target [t(59)=2.10, p=.040, d=0.27], and versus neutral probe and a neutral target [t(59)=2.00, p=.050, d=0.26]. The main effect of TEST DIRECTION was also significant [F(1,59)=4.06, p=.048, ηp2=.064], with better cued recall in the backward direction [M=.62] than in the forward direction [M=.60]. No other main effects or interactions were significant. While the ANOVA demonstrates that there is an effect of positive emotion on cued recall accuracy, it is not sufficient in disentangling the effects of positive emotion on item-versus association-memory – for this, we utilized the cued recall modeling approach (Madan et al., 2010, 2012).
Figure 1. Cued recall accuracy from the main experiment, by probe and target type.
Error bars are standard error of the mean, corrected for inter-individual differences.
Before fitting the model to the cued recall data, we wanted to ensure that the differences in cued recall accuracy could not be explained by variability in LSA cos(θ) (i.e., semantic similarity), despite there being no significant difference in LSA cos(θ) between the word pools (see Methods and Table 1). Thus, we calculated the correlation between cued recall accuracy and pairwise LSA cos(θ) for each participant and averaged these correlations across participants using Fisher’s r-to-Z transform. Critically, this correlation was not significant, allowing us to rule out this potential confound [rpop(63, N=60)=.008].
Model fits
Model fitness and best-fitting parameters for all of the model variants are listed in Table 2.
Table 2.
Model fits for cued recall accuracy in the main experiment. All model variants are shown, with the exception of the full model as it is underdetermined by the data). All free parameter fits are presented as 95% confidence intervals. Note that the “Relationship & Target” and the “Relationship & Probe” models algebraically produce identical fits due to model mimicry, although their best-fitting parameters are not equivalent.
| ΔBIC | ΔBIC | p | r1 | r2 | t | |
|---|---|---|---|---|---|---|
| with r1-only model included | ||||||
| Target-only | 0.00 | 1.90 | 1 | 1 | 1 | [1.00 1.09] |
| Probe-only | 0.83 | 2.74 | [0.98, 1.07] | 1 | 1 | 1 |
| Relationship-only | 0.10 | 2.00 | 1 | [1.02 1.13] | [0.95, 1.04] | 1 |
| Probe & Target | 2.93 | 4.84 | [0.98, 1.07] | 1 | 1 | [1.00 1.09] |
| Relationship & Target | 1.95 | 3.85 | 1 | [1.01, 1.13] | [0.93, 1.04] | [0.96, 1.08] |
| Relationship & Probe | 1.95 | 3.85 | [0.93, 1.04] | [1.02, 1.16] | [0.95, 1.06] | 1 |
| r1-only * | -- | 0 | 1 | [1.02, 1.13] | 1 | 1 |
denotes the best-fitting models according to our model-fitness measure (ΔBIC) and additional converging evidence.
Almost all models were found to have ΔBIC values of less than 2, i.e., the models explained the data nearly equally well. However, it became apparent all models that allowed r1 to vary, the parameter was found to be significantly greater than 1. Because of this we made the post-hoc decision to include one additional model: an r1-only model. After re-calculating ΔBIC to include this new model within the set of possible model variants, it significantly out-performed almost all other models. In the r1-only model, r1 was significantly greater than 1, indicating that positive emotion enhanced association-memory. However, since r1, but not r2, was greater than 1, it is possible that this enhancement only occurs when there is a sufficient amount of positive emotion, rather than occurring as an incremental enhancement. In other words, this enhancement may only occur when both words are positive, rather than a ‘dose-dependent’ enhancement in relation to the number of positive words in the pair. As r2 is constrained to 1, this model assumes equivalent performance on the mixed and pure neutral pairs.
Earlier we described how item-properties could influence cued recall accuracy, and we found that positive words had higher free recall performance than neutral words, i.e., an emotional enhancement of item-memory. In the modeling, this free recall effect could have materialized in the t parameter. However, as the best-fitting model did not make use of the t parameter being different than 1, this free recall effect did not end up influencing the modeling interpretation in the current dataset. As neither the t (nor p) parameter was influenced by positive emotion, we can conclude that the difference between cued recall accuracy for the pure positive and pure neutral pairs is due to an effect on association-memory, not item-memory.
General Discussion
Our results reveal no evidence of an impairment of association-memory for positive words, supported by both the cued recall accuracy itself and the additional mathematical modeling. This result is counter to the arousal hypothesis, that arousal impairs association-memory (e.g., Bisby & Burgess, 2014; Madan et al., 2012, 2017). Instead, the results suggest that positive valence exerts an enhancing influence on association-memory, distinct from the often-impairing effects of negative valence (valence hypothesis). Across a number of experiments (see supplementary material), we consistently found enhanced association-memory due to positive emotion. While this finding has previously been reported by Zimmerman and Kelley (2010), our results rule out the important potential confound of the effects of item-memory on cued recall, through the use of all possible pair types and the modeling approach (Madan et al., 2010, 2012). These results are also generally supportive of the findings of Pierce and Kensinger (2011), insofar as they revealed that, after a short (15 min) retention delay, negative valence led to poorer association-memory than positive. They concluded that valence plays an important role in association-memory, in addition to arousal. However, they used an associative-recognition task, where it is unclear how item-memory effects contribute to performance. Thus, the current results are the first to demonstrate an effect of valence on association-memory, while ruling out possible confounds with item-memory effects.
As outlined in the Introduction, the majority of the literature surrounding association-memory has emphasized the influence of arousal, with less focus on the role of valence. There is good reason to be focused on the effects of arousal on memory, with extensive literature in human and non-human animals demonstrating the strong modulatory influence of arousal (e.g., LeDoux, 2000; Mather & Sutherland, 2011; McGaugh, 2018; Sutherland & Mather, in press). Yet there is increasing evidence that these modulatory influences of arousal cannot explain all emotional influences on memory (e.g., Talmi, 2013), and one possible reason for this focus on arousal in the literature is that the majority of studies investigating emotional influences on memory have compared negative and neutral stimuli, and have not also included positive stimuli.
Furthermore, to directly measure effects of emotion on memory, other item properties need to be controlled for, such as semantic cohesiveness (e.g., Buchanan, Etzel, Adolphs, & Tranel, 2006; Talmi & Moscovitch, 2004), imageability (e.g., Altarriba, Bauer, & Benvenuto, 1999; Warriner et al., 2013), and word frequency (e.g., Warriner et al., 2013) (also see Bennion, Ford, Murray, & Kensinger, 2013). Focusing on association-memory specifically, it is important to consider that item-memory effects can influence cued recall performance, in addition to effects of association-memory (Madan et al., 2010, 2012, Madan, 2014). Controlling for these properties is particularly important in studies of emotional memory, as it is known that some item properties that co-vary with emotion can also influence association-memory (e.g., imageability, word frequency; Madan et al., 2010).
Positive emotion and cognitive scope
Positive emotion has been shown to broaden perceptual and cognitive scope, relative to neutral or negative states (Fredrickson, 1998, 2001). For instance, several studies have shown that individuals in a positive mood are more likely to focus on global features of visual stimuli, whereas negative emotion promotes a more narrowed, local focus (Gasper & Clore, 2002; Fredrickson & Branigan, 2005; Basso et al., 1996). Additionally, positive emotion can increase cognitive scope, as demonstrated through improved ability to see connections between weakly-related concepts (Isen, Johnson, Mertz, and Robinson, 1985). Brunye and colleagues (2013) showed that this effect might also occur in the reverse direction, where expanding the breadth of word associations may improve emotion. Other studies suggest that cognitive broadening caused by positive emotion can facilitate creative problem-solving (see Isen, 1999, for a review). Thus, it is possible that the influence of positive emotion on the ability to think broadly and creatively may be linked to the increased ability to form associations. Indeed, Zimmerman and Kelley (2010) suggested cognitive broadening due to positive emotion as a possible mechanism for valence effects on association-memory.
Influence of positive versus negative emotion on association-memory
The enhancing effect of positive emotion on association-memory contrasts with the association-memory impairments often observed for negative words. As previously discussed, negative words, used in prior investigations of arousal on association-memory, have been shown to either impair or enhance memory for associations; however, these discrepant results could be attributed to confounding factors (see Madan et al., 2012, for a detailed discussion). By using a probabilistic model of cued recall designed specifically to disentangle item-versus association-memory and by matching the stimuli for various item properties (e.g., imageability, word frequency, semantic cohesiveness), Madan et al. (2012) found that negative emotion impaired association-memory, despite increased item-memory. This result was recently replicated by Bisby and Burgess (2014) and Madan et al. (2017) using sufficiently different procedures. Here, when using positive stimuli in the same paradigm as Madan et al. (2012), we showed a markedly different pattern of association-memory effects, suggesting a distinct influence of positive (main experiment) versus negative valenced emotion (Supplementary Experiment 1). Indeed, even when positive and negative stimuli were closely matched for arousal, positive emotion continued to have a larger enhancing effect on associative memory (Supplementary Experiment 3). Taken together, these studies lend support to the valence hypothesis for associative memory.
Two results from the current experiments will require additional follow-up for explication. First, although positive valence reliably enhanced associative memory, negative valence led to more variable effects (see supplementary material), and this variability did not appear to be fully explained by the types of differences (e.g., contributions of associative and item memory, or stimulus characteristics) previously considered (Madan et al., 2012). Future research will do well to examine whether there may be other combinations of stimulus or task design features (e.g., whether positive and negative valenced words appear within-subject) that make it more likely that negative valence will impair associative memory. Second, our results suggest that the effect of positive and negative emotion on association-memory may differ in their effect on mixed versus pure pairs. Specifically, here we found that positive emotion only enhanced association-memory when both pair constituents were positive, but not when only one item was positive (r1>1, r2=1). The replicability and mechanism underlying this unexpected valence difference remains to be elucidated.
In summary, while effects of emotion on association-memory are typically described as impairments and attributed to the influence of arousal, here we found that positive emotion enhanced association-memory. The results reveal that emotion does not always impair association-memory and suggest that valence should be considered in future theories proposed to explain the influence of emotion on association-memory.
Supplementary Material
Acknowledgements
This research was supported by a grant from the National Institute of Health [MH080833] to EAK and a Canadian Graduate Scholarship (doctoral-level) from the Natural Sciences and Engineering Research Council (NSERC) of Canada to CRM. The authors have no conflicts of interest.
We would like to thank Anthony Singhal, Chris Donoff, and Kathryn Lambert for assisting with data collection at the University of Alberta. We would also like to thank Colleen Kelley for providing the word lists from Zimmerman and Kelley (2010).
References
- Altarriba J, Bauer LM, Benvenuto C (1999). Concreteness, context availablility, and imageability ratings and word associations for abstract, concrete, and emotion words. Behavior Research Methods, Instruments, & Computers, 31, 578–602. [DOI] [PubMed] [Google Scholar]
- Anderson L, & Shimamura AP (2005). Influences of emotion on context memory while viewing film clips. American Journal of Psychology, 118, 323–337. [PubMed] [Google Scholar]
- Baayen R, Piepenbrock R, & Gulikers L (1995). The CELEX lexical database (Release 2) [CD-ROM] Philadelphia, PA: Linguistic Data Consortium, University of Pennsylvania (Distributor). [Google Scholar]
- Basso MR, Schefft BK, Ris MD, & Dember WN (1996). Mood and global local visual processing. Journal of the International Neuropsychological Society, 2, 249–255. [DOI] [PubMed] [Google Scholar]
- Bennion KA, Ford JH, Murray BD, Kensinger EA (2013). Oversimplification in the study of emotional memory. Journal of the International Neuropsychological Society 19, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisby JA, & Burgess N (2014). Negative affect impairs associative memory but not item memory. Learning and Memory, 21, 760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisby JA, Horner AJ, Bush D, & Burgess N (2018). Negative emotional content disrupts the coherence of episodic memories. Journal of Experimental Psychology: General, 147, 243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisby JA, Horner AJ, Hørlyck LD, & Burgess N (2016). Opposing effects of negative emotion on amygdalar and hippocampal memory for items and associations. Social Cognitive and Affective Neuroscience, 11, 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen HJ, Kark SM, & Kensinger EA (in press). NEVER forget: negative emotional valence enhances recapitulation. Psychonomic Bulletin & Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ (1994). Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry, 25, 49–59. [DOI] [PubMed] [Google Scholar]
- Brunyé TT, Gagnon SA, Paczynski M, Shenhav A, Mahoney CR, Taylor HA (2013). Happiness by association: Breadth of free association influences affective states. Cognition, 127, 93–98. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Etzel JA, Adolphs R, & Tranel D (2006). The influence of autonomic arousal and semantic relatedness on memory for emotional words. International Journal of Psychophysiology, 61, 26–33. [DOI] [PubMed] [Google Scholar]
- Christianson SA (1992). Emotional stress and eyewitness testimony: A critical review. Psychological Bulletin, 112, 284–309. [DOI] [PubMed] [Google Scholar]
- Criss AH, Aue WR, & Smith L (2011). The effects of word frequency and context variability in cued recall. Journal of Memory and Language, 64, 119–132. [Google Scholar]
- Doerksen S, & Shimamura AP (2001). Source memory enhancement for emotional words. Emotion, 1, 5–11. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Iordan AD, & Dolcos S (2011). Neural correlates of emotion–cognition interactions: A review of evidence from brain imaging investigations. Journal of Cognitive Psychology, 23, 669–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterbrook JA (1959). The effects of emotion on cue utilization and the organization of behavior. Psychological Review, 66, 183–201. [DOI] [PubMed] [Google Scholar]
- Ekman P (1992). An argument for basic emotions. Cognition & Emotion, 6, 169–200. [Google Scholar]
- Fredrickson B (1998). What good are positive emotions? Review of General Psychology, 2, 300–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson B (2001). The role of positive emotions in positive psychology: The broaden-and-build theory of positive emotions. American Psychologist, 56, 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, & Branigan C (2005). Positive emotions broaden the scope of attention and thought‐action repertoires. Cognition & Emotion, 19, 313–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasper K, & Clore GL (2002). Attending to the big picture: Mood and global versus local processing of visual information. Psychological Science, 13, 34–40. [DOI] [PubMed] [Google Scholar]
- Guillet R, & Arndt J (2009). Taboo words: The effect of emotion on memory for peripheral information. Memory and Cognition 37, 866–879. [DOI] [PubMed] [Google Scholar]
- Isen AM (1999). On the relationship between affect and creative problem solving In Russ SW (Ed.), Affect, creative experience, and psychological adjustment. (pp. 3–17). Philadelphia: Brunner/Mazel. [Google Scholar]
- Isen AM, Johnson MMS, Mertz E, & Robinson GF (1985). The influence of positive affect on the unusualness of word associations. Journal of Personality and Social Psychology, 48, 1413–1426. [DOI] [PubMed] [Google Scholar]
- Kensinger EA (2009). Remembering the details: Effects of emotion. Emotion Review, 1, 99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr A, & Zelazo PD (2004). Development of “hot” executive function: The children’s gambling task. Brain and Cognition, 55, 148–157. [DOI] [PubMed] [Google Scholar]
- Landauer TK, & Dumais ST (1997). A solution to Plato’s problem: The latent semantic analysis theory of acquisition, induction, and representation of knowledge. Psychological Review, 104, 211–240. [Google Scholar]
- LeDoux JE (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23, 155–184. [DOI] [PubMed] [Google Scholar]
- Lewandowsky S, & Farrell S (2000). A redintegration account of the effects of speech rate, lexicality, and word frequency in immediate serial recall. Psychological Research, 63, 163–173. [DOI] [PubMed] [Google Scholar]
- Madan CR (2014). Manipulability impairs association-memory: Revisiting effects of incidental motor processing on verbal paired-associates. Acta Psychologica, 149, 45–51. [DOI] [PubMed] [Google Scholar]
- Madan CR, Fujiwara E, Caplan JB, & Sommer T (2017). Emotional arousal impairs association-memory: Roles of amygdala and hippocampus. NeuroImage, 156, 14–28. [DOI] [PubMed] [Google Scholar]
- Madan CR, Caplan JB, Lau CSM, Fujiwara E, (2012). Emotional arousal does not enhance association-memory. Journal of Memory and Language, 66, 695–716. [Google Scholar]
- Madan CR, Glaholt MG, Caplan JB (2010). The influence of item properties on association-memory. Journal of Memory and Language, 63, 46–63. [Google Scholar]
- Mather M (2007). Emotional arousal and memory binding. Perspectives on Psychological Science, 2, 33–52. [DOI] [PubMed] [Google Scholar]
- Mather M, & Sutherland MR (2011). Arousal-biased competition in perception and memory. Perspectives on Psychological Science, 6, 114–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald SA, & Shillcock RC (2001). Rethinking the word frequency effect: The neglected role of distributional information in lexical processing. Language and Speech, 44, 295–323. [DOI] [PubMed] [Google Scholar]
- McGaugh JL (2018). Emotional arousal regulation of memory consolidation. Current Opinions in Behavioral Sciences, 19, 55–60. [Google Scholar]
- Medler DA, & Binder JR (2005). MCWord: An On-Line Orthographic Database of the English Language. http://www.neuro.mcw.edu/mcword/
- Metcalfe J, & Mischel W (1999). A hot/cool-system analysis of delay of gratification: Dynamics of willpower. Psychological Review, 106, 3–19. [DOI] [PubMed] [Google Scholar]
- Mickley Steinmetz KR, Knight AG, & Kensinger EA (2016). Neutral details associated with emotional events are encoded: evidence from a cued recall paradigm. Cognition and Emotion, 30, 1352–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, & Yamawaki S (2009). Neural correlates of associative memory: The effects of negative emotion. Neuroscience Research, 64, 50–55. [DOI] [PubMed] [Google Scholar]
- Pierce BH, & Kensinger EA (2011). Effects of emotion on associative recognition: Valence and retention interval matter. Emotion, 11, 139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plutchik R (1980), Emotion: Theory, research, and experience: Vol. 1 Theories of emotion. New York: Academic Press. [Google Scholar]
- Prencipe A, Kesek A, Cohen J, Lamm C, Lewis MD, & Zelazo PD (2011). Development of hot and cool executive function during the transition to adolescence. Journal of Experimental Child Psychology, 108, 621–637. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Libby LA, & Ranganath C (2015). Cortico-hippocampal systems involved in memory and cognition: the PMAT framework. Progress in Brain Research, 219, 45–64. [DOI] [PubMed] [Google Scholar]
- Rimmele U, Davachi L, Petrov R, Dougal S, Phelps EA, 2011. Emotion enhances the subjective feeling of remembering, despite lower accuracy for contextual details. Emotion 11, 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, & Chattarji S (2009). Stress, memory and the amygdala. Nature Reviews Neuroscience, 10, 423–433. [DOI] [PubMed] [Google Scholar]
- Russell JA (1980). A circumplex model of affect. Journal of Personality and Social Psychology, 39, 1161–1178. [DOI] [PubMed] [Google Scholar]
- Russell JA (2003). Core affect and the psychological construction of emotion. Psychological Review, 110, 145–172. [DOI] [PubMed] [Google Scholar]
- Sakaki M, Fryer K, & Mather M (2014). Emotion strengthens high-priority memory traces but weakens low-priority memory traces. Psychological Science, 25, 387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MR, & Mather M (in press). Arousal (but not valence) amplifies the impact of salience. Cognition and Emotion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmi D (2013). Enhanced emotional memory: Cognitive and neural mechanisms. Current Directions in Psychological Science, 22, 430–436. [Google Scholar]
- Talmi D, & Moscovitch M, (2004). Can semantic relatedness explain the enhancement of memory for emotional words? Memory & Cognition, 32, 742–751. [DOI] [PubMed] [Google Scholar]
- Tejeda HA, & O’Donnell P (2014). Amygdala inputs to the prefrontal cortex elicit heterosynaptic suppression of hippocampal inputs. Journal of Neuroscience, 34, 14365–14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touryan SR, Marian DE, & Shimamura AP (2007). Effect of negative emotional pictures on associative memory for peripheral information. Memory, 15, 154–166. [DOI] [PubMed] [Google Scholar]
- Warriner AB, Kuperman V, & Brysbaert M (2013). Norms of valence, arousal, and dominance for 13,915 English lemmas. Behavior Research Methods, 45, 1191–1207. [DOI] [PubMed] [Google Scholar]
- Williams LM, Phillips ML, Brammer MJ, Skerrett D, Lagopoulos J, Rennie C, … Gordon E (2001). Arousal dissociates amygdala and hippocampal fear responses: evidence from simultaneous fMRI and skin conductance recording. NeuroImage, 14, 1070–1079. [DOI] [PubMed] [Google Scholar]
- Wilson MD (1988). The MRC psycholinguistic database: Machine readable dictionary, version 2. Behavior Research Methods, Instruments, & Computers, 20, 6–11. [Google Scholar]
- Yonelinas AP, & Ritchey M (2015). The slow forgetting of emotional episodic memories: an emotional binding account. Trends in Cognitive Sciences, 19, 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman CA, & Kelley CM (2010). “I’ll remember this!” Effects of emotionality on memory predictions versus memory performance. Journal of Memory and Language, 62, 240–253. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.