Abstract
Background:
Opioid use disorder (OUD) is highly prevalent among justice-involved individuals. While risk for overdose and other adverse consequences of opioid use are heightened among this population, most justice-involved individuals and other high-risk groups experience multiple barriers to engagement in opioid agonist treatment.
Methods:
This paper describes the development of Project Connections at Re-Entry (PCARE), a low-threshold buprenorphine treatment program that engages vulnerable patients in care through a mobile van parked directly outside the Baltimore City Jail. Patients are referred by jail staff or can walk in from the street. The clinical team includes an experienced primary care physician who prescribes buprenorphine, a nurse, and a peer recovery coach. The team initiates treatment for those with OUD and refers those with other needs to appropriate providers. Once stabilized, patients are transitioned to longer-term treatment programs or primary care for buprenorphine maintenance. This paper describes the process of developing this program, patient characteristics and initial outcomes for the first year of the program, and implications for public health practice.
Results:
From November 15, 2017 through November 30, 2018, 220 people inquired about treatment services and completed an intake interview, and 190 began treatment with a buprenorphine/naloxone prescription. Those who initiated buprenorphine were primarily male (80.1%), African American (85.1%), had a mean age of 44.1 (SD = 12.2), and a mean of 24.0 (SD = 13.6) years of opioid use. The majority of patients (94.4%) had previous criminal justice involvement, were unemployed (72.9%) and were unstably housed (70.8%). Over a third (32.1%) of patients had previously overdosed. Of those who began treatment, 67.9% returned for a second visit or more, and 31.6% percent were still involved in treatment after 30 days. Of those who initiated care, 20.5% have been transferred to continue buprenorphine treatment at a partnering site.
Conclusions:
The PCARE program illustrates the potential for low-threshold buprenorphine treatment to engage populations who are justice-involved and largely disconnected from care. While more work is needed to improve treatment retention among vulnerable patients and engaging persons in care directly after release from detention, offering on-demand, flexible and de-stigmatizing treatment may serve as a first point to connect high-risk populations with the healthcare system and interventions that reduce risk for overdose and related harms.
Keywords: Buprenorphine Low threshold Harm reduction Opioid use disorder Vulnerable populations Criminal justice
1. Introduction
The opioid crisis continues to be a leading cause of suffering and mortality across the U.S. (Rudd, Aleshire, Zibbell, & Matthew Gladden, 2016). As illicit opioids have become more potent and widely available, deaths have continued to increase in many parts of the country. Deaths from fentanyl and other synthetic opioids have doubled from 2015 to 2016 alone (Hedegaard, Warner, & Minino, 2017). While this crisis has impacted all sectors of society, historically marginalized groups, including those who are involved in the criminal justice system, continue to suffer disproportionately from opioid use disorder (OUD) and its consequences. Having justice involvement, along with other vulnerabilities such as having mental health conditions or experiencing poverty and homelessness, increases risk for overdose (Feng, Iser, & Yang, 2016; Hasegawa, Brown, Tsugawa, & Camargo, 2014) and exacerbates other negative outcomes such as risk of infectious disease (Galea & Vlahov, 2002). Fortunately, treatment with opioid agonist medications, such as buprenorphine and methadone, has been shown to significantly reduce illicit opioid use and many adverse correlates of its use, such as overdose and HIV transmission (Connery, 2015; Veilleux, Colvin, Anderson, York, & Heinz, 2010). Despite notable efforts to expand the availability of these medications, particularly, buprenorphine, across the country (Jones, Campopiano, Baldwin, & McCance-Katz, 2015), justice-involved and other vulnerable populations continue to experience multiple barriers to receiving this treatment (Fox et al, 2015).
1.1. Barriers to medication treatment
Many individuals who experience the most severe opioid problems are involved in the criminal justice system (Winkelman, Chang, & Binswanger, 2018). In the United States, there are over 1.5 million state and federal inmates (Kaeble & Cowhig, 2018), of whom an estimated 26–28% have histories of opioid use (Bronson, Stroop, Zimmer, & Berzofsky, 2017). Scarce resources are provided for corrections-based substance use treatment, and many inmates with OUDs remain untreated (Aronowitz & Laurent, 2016; Kinlock, Gordon, & Schwartz, 2011). In addition, the risk of opioid overdose and death is greatest in the period following release from jail or prison (Binswanger et al., 2007; Merrall et al., 2010; Ranapurwala et al., 2018). A 2009 study found that most state prison systems offered either no or very limited medication treatment (Nunn et al., 2009) and a more recent analysis found that only one state’s correctional facilities allow access to all three types of FDA-approved medications for OUD (buprenorphine, methadone, & naltrexone) (Lopez, 2018). This means that those who already receive medication treatment/pharmacotherapy in the community often have to come off their medication while incarcerated, which places them at greater risk for overdose upon release (Cornish, Macleod, Strang, Vickerman, & Hickman, 2010). Moreover, it has been shown that only 5% of those referred to specialty treatment for OUD from criminal justice sources (such as parole or probation) receive any medication treatment as part of their care (Krawczyk, Picher, Feder, & Saloner, 2017).
Even when no longer incarcerated, justice-involved and other marginalized groups often experience difficulty engaging in and remaining in care (Fox et al, 2015; Perreault et al., 2015). While specific barriers to treatment among this population are multifactorial, program and system-level barriers such as requirements for admission and obligations of treatment compliance may be especially burdensome to patients with multiple vulnerabilities. For example, many medication treatment programs have stringent requirements for enrollment and continued treatment such as participation in a specific type of counseling service in order to remain in treatment, despite inconclusive evidence about the impact of behavioral therapies on opioid treatment outcomes (Carroll & Weiss, 2017; Schwartz, Kelly, O’Grady, Gandhi, & JafFe, 2012), and some patients may not be interested or able to attend counseling. Indeed, prior research with buprenorphine patients in primary care found that patients were more satisfied with treatment when they had fewer medication appointments and less counseling requirements (Barry et al., 2007). Furthermore, many programs will discontinue medication if a person continues to engage in illicit drug use during treatment (Bentzley, Barth, Back, & Book, 2015). This may be especially difficult for persons with more severe or longer-term opioid and polysubstance use, whom may not be ready to stop using all illicit substances despite a genuine desire for treatment.
Patients may also experience logistical barriers to medication treatment. Many persons, especially those who are unstably housed or who have recently been incarcerated, may not possess photo identification, and as a result can be turned away from clinics or pharmacies (Edidin, Ganim, Hunter, & Karnik, 2012; National Alliance for Model State Drug Laws, 2013). Others may not be insured, even when eligible (Tsai, Rosenheck, Culhane, & Artiga, 2013), and unlikely to have the means to pay out of pocket for medication or even cover small copayments. Moreover, many treatment programs require daily attendance at a clinic to receive medication. While in the case of methadone this is mandated by law due to federal regulations, a significant number of buprenorphine programs also require daily attendance to monitor treatment compliance, especially in early stages of care. Such policies may deter patients who are not ready or able to attend a clinic daily due to work or family obligations or chaotic living and financial conditions. A previous study of methadone patients, for example, reported logistical concerns, including the challenge of attending a clinic daily, as one of the primary challenges to continuing care (Reisinger et al., 2009). Lastly, persons with OUD from marginalized groups who have experienced trauma and stigma in many aspects of their lives, including in health care settings (van Boekel, Brouwers, van Weeghel, & Garretsen, 2013) may be hesitant to trust or engage with more formal clinical settings.
1.2. The case for expanding mobile buprenorphine
Improving access to effective medication treatments for OUD for vulnerable and high-risk patients is fundamental to addressing the opioid crisis. While improving accessibility among justice-involved individuals detained in correctional facilities continues to be a long-term policy goal (Wakeman & Rich, 2015), bridging other barriers and shortfalls in access to medications are urgently needed. One modality used to deliver treatment to hard-to-reach populations are mobile treatment units, which are designed to eliminate barriers to care by bringing services to underserved communities and meeting patients where they are. This has been especially notable in the case of syringe service programs that deliver harm reduction services, and which have been widely successful at reducing the burden of HIV and other harms (Bramson et al., 2015). Mobile treatment clinics have also played a role in overdose prevention through naloxone distribution and education services (Maxwell, Bigg, Stanczykiewicz, & Carlberg-Racich, 2006), as well as in expanding general primary care and prevention services to communities largely disconnected from care (Song, Hill, Bennet, Vavasis, & Oriol, 2013).
Mobile treatment clinics have been similarly used to improve access to medications for OUD for vulnerable patients. Compared to fixed site programs, mobile methadone treatment programs have been found to enroll a greater proportion of African-American, homeless, and uninsured individuals than fixed-site methadone clinics (Hall et al., 2014) and to retain vulnerable patients in treatment for longer (median 15.5 months versus 5 months; (Greenfield, Brady, Besteman, & De Smet, 1996)). The less stringent regulation of buprenorphine as a take home medication (Vestal, 2018) has highlighted the potential of buprenorphine, in particular, to provide flexible and convenient care to hard-to-engage patients (Sullivan et al., 2006).
Given the growing lethality of opioid drugs, and the demand for many jurisdictions to address their local opioid crises, expanding mobile buprenorphine can be a critical strategy for reaching persons most in need that remain underserved. This paper describes one mobile and low-threshold buprenorphine program, “Project Connections at Re-Entry” that is located directly outside of a local jail and works to engage vulnerable adults who have been recently released from jail. We discuss the process of developing this program, self-reported patient characteristics and initial outcomes, and implications for public health practice.
2. Material and methods
2.1. Program design and planning
“Project Connections at Re-Entry” (PCARE) was instituted in 2017 as an extension of the Project Connections Buprenorphine Program, a multi-site project operated since 2001 by the Behavioral Health Leadership Institute (BHLI), a local non-profit organization in Baltimore city. The Project Connections program, described previously (Daniels, Salisbury-Afshar, Hoffberg, Agus, & Fingerhood, 2014), works with local community partners in Baltimore to deliver low-threshold buprenorphine in non-traditional settings to adults who are otherwise disconnected from treatment and health services.
The PCARE van was established with the cooperation of the Maryland Department of Public Safety and Correctional Services (DPSCS) Division of Pretrial Detention and Services, who recognized a need to provide services for persons with substance use and other behavioral health disorders who were cycling in and out of jail. The Baltimore City jail, similar to other large metropolitan jails in the US, has a large proportion of inmates with substance use disorders. Yet, most don’t receive any treatment for OUD or other mental health disorders while incarcerated (Walsh, 2010), placing them at very high risk of overdose and death following release (Maryland Department of Health and Mental Hygiene, 2014). PCARE provides immediate access to treatment for persons leaving jail by providing treatment services directly outside of the discharge area. Support from local correctional administrators both during the planning stage and throughout implementation was an essential component of ensuring smooth coordination of services (Gordon, Kinlock, & Miller, 2011). BHLI held multiple planning meetings with jail leadership and led a training session on behavioral health for jail staff in advance of launching the van. This training allowed correctional staff to share their insights about challenges they face managing behavioral health conditions in the jail and learn about the evidence behind pharmacotherapy treatment and the impact on health and criminal justice outcomes. This training component was essential to build a relationship for communication, especially given that many criminal justice staff had reservations and misconceptions about pharmacotherapy for OUD (Doernberg, Krawczyk, Agus, & Fingerhood, 2019; Friedmann et al., 2012; Matusow et al, 2013).
2.2. Treatment model and protocol
The PCARE program operates four mornings a week and is staffed with an experienced, buprenorphine-waivered primary-care provider (either a physician or nurse practitioner, hereinafter referred to as provider), a nurse, a driver/site manager, and a peer recovery specialist. During the first year of operation, a provider was on-site two mornings per week (4 h), and a peer recovery specialist was on-site two mornings per week (8h). The van has an open-door policy in which persons interested in services can knock on the door and speak with a clinician at any time during hours of operation. The program primarily targets persons who are exiting the jail or who have been recently incarcerated. Therefore, corrections staff at different stages of the jail intake process from booking through discharge are asked to inform individuals about the availability of treatment for opioid use upon release. Though persons recently discharged are a primary target population, the van accepts and works with any patient interested in buprenorphine or being referred to other behavioral health services.
During intake, patients meet with the nurse to do an initial assessment, after which they meet with a provider to determine eligibility and initiate buprenorphine treatment. Persons who are interested in another treatment regimen (either methadone, counseling services, or other types of care for behavioral health disorders) are linked with established partner organizations that can provide these services. Naloxone prescriptions (and free supply of intranasal naloxone when available) and overdose prevention counseling are also provided to persons who visit the van. Those who are interested in buprenorphine but prefer to begin treatment elsewhere can be referred to another Project Connections site or a primary care partner that can initiate buprenorphine immediately. All referrals and follow up appointments are coordinated by nurses and peer recovery specialists, who follow up with patients through phone calls. Patient information is stored in an internal and secure electronic database, which allows for coordination of care among the clinical team and for monitoring outcomes.
Patients who are interested in initiating treatment at the van complete an initial medical assessment with the provider, which initially includes a cheek swab drug test and a review of the Prescription Drug Monitoring database to determine any other substances and medications the patient is using to assure safety of medication initiation. Patients are considered eligible for treatment if they meet criteria for opioid use disorder, are not actively receiving treatment elsewhere and had not used methadone or long-acting prescription opioids in the last few days. Patients are then counseled on buprenorphine use, including risk of precipitated withdrawal, and the home initiation process (Lee, Grossman, DiRocco, & Gourevitch, 2009) and are given a prescription for buprenorphine/naloxone for an initial number of days determined by the patient and physician (initial prescriptions range from 2 to 7 days). Those not eligible or appropriate for buprenorphine treatment for any reason are referred to partner services programs that can provide other needed types of care. This included patients actively receiving buprenorphine or methadone from another program, or who plan to remain on opioids for pain management.
For patients who require a co-payment for the prescription or are uninsured, an agreement with a local pharmacy (located one mile away) allows patients arriving from this program to receive the medication free of charge, with the full cost or co-payment billed directly to the non-profit that funds the program. This agreement, originally established a decade ago between BHLI’s other low-threshold buprenorphine programs and a family-owned pharmacy in Baltimore, was sustained even when a large national pharmacy chain took over the local store and was even expanded to other branches in the area. As many patients are homeless or recently incarcerated and do not possess official photo identification, program staff take a photo of each patient using polaroid film and attach it to a document from BHLI to take with them to the pharmacy that indicates that the patient sought treatment on the van and that the accompanying script was prescribed to them. This is critical given many states and individual pharmacy retailers have policies that require photo identification before dispensing controlled substances (National Alliance for Model State Drug Laws, 2013). A peer recovery specialist is also available two mornings a week to work with patients to address needs such as acquiring proper identification, enrolling in insurance, and connecting with housing through linkage to partnering programs in the city of Baltimore such as ID clinics and short and long-term shelter and housing programs. The peer meets with patients during the intake process and later on a walk-in basis or by appointment to follow up on specific patient needs.
For each patient, a care plan is devised with input from the patient, provider, nurse and peer based on patient desires and needs. Follow-up appointments to return for subsequent prescriptions are then determined based on individual circumstances, with most patients returning once a week. The protocol is purposefully flexible to maximize engagement and retention in care for patients who have varied needs and preferences, especially at initial stages of treatment when patients are most vulnerable to leaving care and/or relapsing. The clinical team works to keep patients engaged by focusing on harm reduction and compassionate care. Patients are asked to provide cheek drug tests weekly and are expected to be taking medication as prescribed. The maximum buprenorphine dose prescribed was 16 mg daily, given increased risk of diversion at higher doses. Based on insurance formularies, most patients received buprenorphine-naloxone 8–2 mg sublingual films. Patients who have more than two weeks in which toxicology exams were buprenorphine negative or methadone positive are not eligible for further prescriptions and are referred to a partnering program such as an opioid treatment program that can provide methadone or buprenorphine on site or other substance use services that may be more appropriate. However, patients are not discharged from the program for continued use of other illicit substances (including opioids and benzodiazepines), as long as they continue to come to appointments and drug tests show presence of buprenorphine. Instead, drug tests are used as a tool to help clinicians discuss patient progress and provide counseling on their health and safety. Patients are not required to attend psychosocial counseling to remain in the program but are given the option to attend such services at a partnering organization when they express interest in doing so. The peer and nurses are available to provide support during visits and over phone calls as necessary.
Patients are asked to return weekly but are not removed from the program for missing appointments. Patients who have not returned to treatment for four continuous weeks are considered no longer active in the program but can reinitiate care at a later time. Patients are generally considered stable and doing well in their buprenorphine treatment if they are continuously attending appointments and report decreased illicit opioid use, taking buprenorphine as prescribed (as evidenced by buprenorphine-positive toxicology), and report satisfaction in treatment and/or improvement in other health and psychosocial needs that were indicated as individualized treatment plan goals. When the patient feels ready, the clinical team works with the patient to refer them to another buprenorphine site or eventually transition to a primary care provider that can continue to provide buprenorphine maintenance and address other co-occurring health conditions.
2.3. Initial outcome reporting
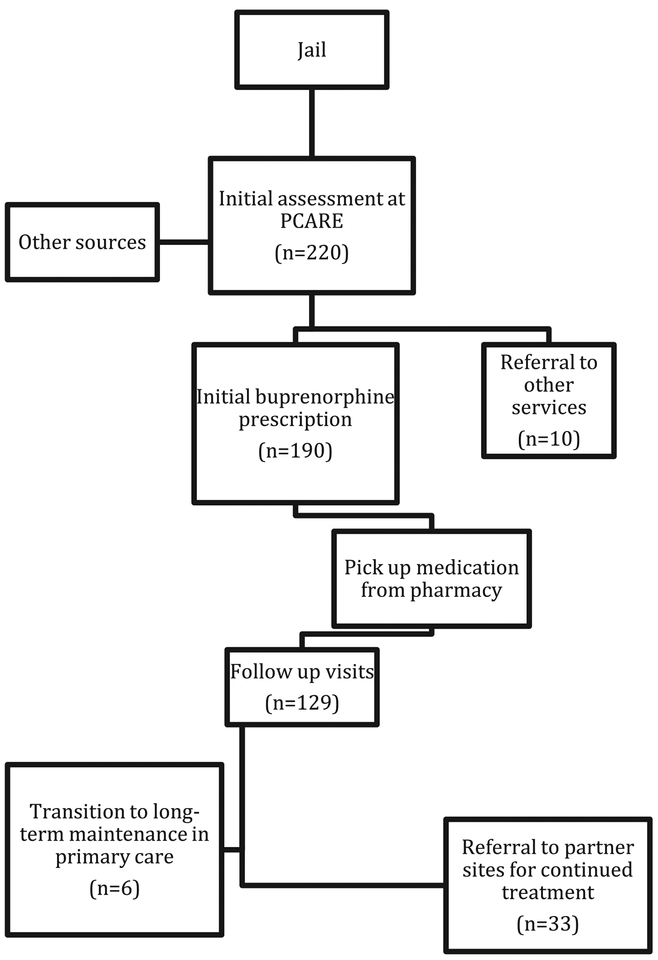
A consort-diagram of the PCARE process of patient initiation, stabilization, and referral to continued care, with the number of patients who made it to each step, is illustrated in Fig. 1. We report demographic, clinical and criminal justice characteristics among patients who initiated buprenorphine based on information collected at intake into the program. Initial outcomes data are reported to illustrate some measures of patient engagement and retention over the first year of program operation. We report the number of patients served, the proportion who returned for at least one visit after initial buprenorphine prescription and their average length of care, the proportion who engaged in care for at least 30 days (measured in days between first buprenorphine prescription and latest patient visit). We also report the number of persons who have been referred elsewhere to continue buprenorphine treatment or to other types of care as well as the types of settings to which they were referred. Lastly, we conducted Pearson Chi2 tests to evaluate any significant sociodemographic differences (from those presented in Table 1) among persons who left treatment before 30 days and those who remained in care.
Fig. 1.
Consort diagram for patient initiation, stabilization, and transition to long-term care with buprenorphine among patients who initiated care in first twelve months of PCARE program operation.
Table 1.
Characteristics of patients initiating buprenorphine Nov 2017–Nov 2018 (N = 190).
| Client characteristics | n (%)a |
|---|---|
| Age (mean (SD)) | 45.1 (12.2) |
| Sex | |
| Male | 146 (80.7) |
| Female | 35 (19.34) |
| Race | |
| African American | 154 (85.1) |
| White | 21 (11.6) |
| Mixed Race | 5 (2.8) |
| Other | 1 (0.55) |
| Employment | |
| Employed (full or part time) | 11 (6.1) |
| Unemployed | 132 (72.9) |
| Disabled | 36 (19.9) |
| Other | 2 (1.0) |
| Living arrangement | |
| Rent or own home | 36 (20.2) |
| With family or friends | 74 (41.6) |
| Street/shelter | 52 (29.2) |
| Recovery house | 4 (2.25) |
| Other | 12 (6.7) |
| Insurance | |
| Medicaid | 120 (66.6) |
| Medicare/Medicaid and Medicare | 27 (15.0) |
| None | 28 (15.6) |
| Other | 5 (2.8) |
| Years of opioid use (mean (SD)) | 24.0 (13.6) |
| Regular mode of opioid use (not mutually exclusive) | |
| Smoked | 14 (7.4) |
| Oral | 17 (9.0) |
| Intranasal | 133 (70.0) |
| Injection | 39 (20.5) |
| Number of previous overdoses (mean (range)) | 1.4 (0–40) |
| Substances use regularly (not mutually exclusive) | |
| Heroin | 166 (87.4) |
| Fentanyl | 48 (25.3) |
| Opioid pain relievers | 25 (13.2) |
| Alcohol | 35 (18.4) |
| Marijuana | 53 (27.9) |
| Cocaine | 78 (41.0) |
| Benzodiazepines | 21 (11.1) |
| Tobacco | 82 (43.1) |
| Had previous buprenorphine from treatment program | 70 (36.8) |
| Had previous buprenorphine from street | 63 (33.2) |
| Had primary care visit in past 12 months | 50 (26.3) |
| Had been to ER or hospital in past 12 months | 65 (34.2) |
| Comorbid mental health condition | 93 (52.5) |
| Comorbid chronic health condition | 91 (52.9) |
| HIV positive | 6 (3.4) |
| HCV positive | 33 (18.9) |
Percentages are valid percentages (percent of respondents who reported each field) rounded to 1 decimal pt
3. Results
3.1. Patient characteristics
The PCARE pilot program was launched in November of 2017. In its first 12 months of operation, from November 15, 2017 to November 30 of 2018, a total of 220 people inquired about treatment services and completed an intake interview, and 190 people received a buprenorphine prescription and began treatment on the van. Of those who completed an intake but did not receive buprenorphine, 10 were referred to other services and 20 never stayed or returned to complete a physician assessment for buprenorphine initiation. Detailed characteristics of the 190 patients that initiated buprenorphine are summarized in Table 1. Patients who initiated buprenorphine were primarily male (n = 146; 80.1%), African American (n = 154; 85.1%), unemployed (n =132; 72.9%), and had a mean age of 45.1 (SD = 12.2). Most did not have stable housing: 41.6% (n = 74) reported living with family/ friends and 29.2% (n = 52) reported living on the street or in a shelter. Two-thirds of patients had Medicaid (n = 120; 66.6%), and 15.6% (n = 28) of patients indicated having no insurance.
Patients who initiated treatment had a mean of 23.9 years of opioid use (SD = 13.6) and the majority were primarily heroin users (n = 166; 87.4%). Most patients used opioids intranasally (n = 133; 70.0%), or by injection (n = 39; 20.5%). Many patients also reported regularly using other substances (See Table 1). As many as 32.1% (n = 61) of patients reported having overdosed at least once, with the number of previous overdoses ranging from 0 to 40. Over a third of patients (n = 70; 36.8%) had previous treatment with buprenorphine from a medical provider, and a third had also reported previously using buprenorphine bought on the street (n = 66; 33.2%). Over half of patients as 51.91% (n = 91) reported having a primary care provider at time of intake, but only 26.3% (n = 50) had visited a primary care provider in the past year. Many patients indicated having comorbid mental and physical health conditions and having had an emergency room or hospital encounter in the past year (See Table 1).
Of patients who began treatment, 169 (94.4%) indicated having previous justice-involvement, 53 (38.4%) indicated they were released from incarceration in the last 3 months, 20 (14.5%) in the past week, and 10 (5.3%) within the same or previous day. Patients with justice involvement had a median of 10 previous arrests and 3 previous incarcerations and a third (n =47; 29.0%) indicated being currently on probation or parole. When asked how they learned about the van, 10 (5.3%) indicated they saw the van upon discharge from the jail, 39 (20.5%) learned about it from criminal justice staff or resources, and 121 (63.7%) came by the van on their own or were referred by a non-criminal justice source.
3.2. Initial outcomes
Of the 190 patients that received an initial buprenorphine prescription, 129 (67.9%) returned for a second visit or more, and 60 (31.6%) were still engaged in buprenorphine treatment 30 days after initiation. A Pearson Chi2comparison of persons who remained in care after 30 days and those who left care beforehand found that those who left earlier than 30 days were more likely to be female (23.88% vs. 10.34%; χ2 (df, N = 129) = 4.42, p = 0.04) and with borderline statistical significance more likely to have hepatitis C (25.53% vs. 8.93%; χ2 (df, N = 129) = 5.31, p = 0.07). On average, patients who returned for a second visit or more were involved in treatment for 8 weeks. During the first year, a total of 39 of patients (20.5%) have already been transferred to continue buprenorphine treatment at a partnering site, including 17 who were transferred to another Project Connections site, 16 to an external buprenorphine treatment provider, and 6 to a primary care provider. 11 patients were referred to a higher level of substance use treatment (such as opioid treatment program for methadone or consideration of higher-dose buprenorphine) due to buprenorphine negative toxicology or not complying with treatment requirements.
4. Discussion
The PCARE mobile buprenorphine pilot program illustrates the need for and potential role of low-threshold programs that cater to patients that have justice involvement and other vulnerabilities that make it challenging for them to engage in traditional substance use treatment. Most patients who arrived at the van were long-term and poly-drug users, had multiple comorbidities, were unstably housed, were often uninsured, and had extensive prior or ongoing justice involvement. These findings demonstrate that this program reached its target population of persons who are often hardest to engage in care. Given the nature of this patient population, the large number of persons who initiated treatment and returned for follow up visits illustrates that providing buprenorphine initiation and stabilization in a low-threshold treatment setting may be a strategy to engage patients who have a range of psychosocial vulnerabilities. The large number of persons who chose to inquire and initiate treatment on the van indicates that there is still a large unmet demand for buprenorphine, even in a city such as Baltimore that has a relatively large number of traditional outpatient treatment programs or OTPs that deliver buprenorphine treatment (Oros & Welsh, 2013).
While more research is needed to understand specific barriers that patients experience seeking and attending care at other clinical settings, flexible, on-demand and low-threshold care attracted patients who, for many different reasons, wanted treatment but were not actively involved in care. Unique aspects of the program that allowed patients to avoid copays, acquire a prescription without a photo ID, and begin treatment while not yet enrolled in insurance may have played a role for those who may have not been otherwise able to begin treatment. Thus, buprenorphine programs may benefit from addressing logistical and financial barriers that often prevent the most vulnerable from receiving treatment. Starting patients on buprenorphine treatment on a same or next-day basis was critical for engaging patients at the time they were ready to begin care. Indeed, longer wait times to initiate treatment have been noted as a common barrier to engaging and keeping patients in care (Peles, Schreiber, & Adelson, 2013; Sigmon et al., 2016).
The success of this program in engaging marginalized populations points to the potential role of buprenorphine treatment programs that are based on a harm-reduction model focused on recovery rather than on abstinence and that provides treatment without mandating behavioral therapy or other ancillary conditions of care. While there is a clear value to counseling or other types of behavioral therapies for persons with substance use and mental health needs, the field has yet to produce clear indication on what specific type of psychosocial care is most effective for OUD and for whom (Dugosh et al., 2016). Furthermore, there is evidence that medication treatment can be effective in treating OUD even in the absence of behavioral therapy (Carroll & Weiss, 2017; Timko, Schultz, Cucciare, Vittorio, & Garrison-Diehn, 2016) and has a high safety profile compared to other opioids (Walsh, 2010). Minimizing requirements for the receipt of medications for OUD has been a growing topic of discussion internationally, with lower threshold and harm-reduction approaches being recognized for their potential to minimize harms and engage high-risk patients (Kourounis et al., 2016; Rogers & Ruefli, 2004). Expanding these discussions to similar settings in the U.S., especially in the current state of the opioid crisis, is critical given the high risk of overdose and death among this population (a third of patients had previously overdosed), and the proven effectiveness of buprenorphine in reducing overdose risk (Sordo et al., 2017), even during continued illicit substance use (Gjersing & Bretteville-Jensen, 2013).
Opioid use was just one of many challenges experienced by patients who sought services on the van. Most had a range of medical and psychiatric comorbidities and were lacking basic necessities such as stable housing. The open-door policy of the treatment van, including provision of care by experienced primary care physicians and nursing staff may help patients connect with needed services beyond buprenorphine. The eventual transition of patients to a primary care practice for ongoing treatment and the gradual work of peers to link patients with other needed health and social services are key components of this program. However, for many persons with severe OUD, addressing symptoms of withdrawal and stabilization with buprenorphine may be a necessary first step to address co-occurring needs that often take a back seat during active addiction (De Hert, Vancampfort, & Detraux, 2015; Dickey, Noimand, Weiss, Drake, & Azeni, 2002). Finally, the welcoming and flexible atmosphere of the van and the dedication to a harm reduction approach by a compassionate team of clinicians may have appealed to patients who often experience discrimination in clinical settings. The ability to begin treatment for OUD in a setting that is sensitive to needs and respectful of patients may help de-stigmatize treatment and create trust and a gateway for entry into comprehensive health care to address multiple health needs.
At the same time, the experiences of the first year of the PCARE mobile treatment van point to many challenges that remain to be addressed among this highly vulnerable population. For one, only a third of patients who initiated buprenorphine were actively engaged in treatment after 30 days. While challenges to retention are not unexpected given the high rates of justice involvement, homelessness, and other vulnerabilities of this patient population (Kertesz, Horton, Friedmann, Saitz, & Samet, 2003), previous research with mobile opioid agonist treatment suggests the potential for longer-term retention even among patients with high levels of vulnerability (Greenfield et al, 1996). Finding ways to better support patients with complex needs and improve adherence and retention in care without imposing restrictive requirements should be a continued focus of research and practice efforts. Interestingly, the only sociodemographic characteristics that were found to be more prevalent among those who left treatment before 30 days were being female and reporting having hepatitis C. This implies that efforts to specifically attend to the needs of women and persons suffering from comorbid chronic conditions may be especially relevant to helping persons remain in care.
Another important challenge and limitation of this program was the low proportion of patients who were engaged in treatment immediately following release from detention. While almost all patients had histories of criminal justice-involvement and a third were actively under community supervision, less than half had actually been incarcerated in the last three months, and less than 15% had been released from detention in the week prior to starting treatment. Given that the days and weeks immediately following release from detention carry the highest risk of overdose death (Binswanger et al., 2007; Maryland Department of Health and Mental Hygiene, 2014), it is important that efforts continue to be made to engage persons in treatment at the time they are exiting detention. This challenge likely resulted in part from the chaotic process of discharge from the jail, where persons can be released 24 h a day, seven days a week often without prior notice and without a discharge plan. Indeed, only 20% of patients reported hearing about the van from jail staff or resources. Better engaging with the jail discharge process so that persons learn about the program before exiting detention may be important to increasing engagement among detainees exiting the jail. In attempts to begin to address this gap, a video is currently being designed by BHLI to inform people about evidence-based treatment for opioid use disorder and the services available on the van. The Baltimore jail has agreed to screen this video in the discharge area of the jail so that inmates can be informed about available services before leaving detention.
Despite many of these limitations, the PCARE program illustrates the willingness of correctional agencies to partner with evidence-based treatment programs geared towards this population. While the resistance to medications in criminal justice settings has been well-documented (Lopez, 2018), correctional staff and leaders at the Baltimore jail recognized the unmet need to address substance use disorders, and many were open to supporting novel interventions that both reduce health harms and minimize burden on the criminal justice system. Assuring success of such collaborations, such as improving referral and linkage from the jail to the treatment van, require continued persistence and buy-in from correctional staff. Working towards a system in which community services and diversion programs prevent initial incarceration of persons with behavioral use disorders, and in which prisons and jails offer buprenorphine and other medication treatment alternatives to inmates while incarcerated and upon release is an ideal goal. In fact, recent data from Rhode Island show that implementing medications for addiction treatment in the correctional system was associated with a 60% decrease in overdose mortality among persons who were recently incarcerated (Green et al., 2018). Although medication treatment in jails and prisons is receiving greater attention (Fiscella, Wakeman, & Beletsky, 2018), implementing such changes across the country will likely be a slow moving process both politically and logistically. Many institutions continue to resist buprenorphine, specifically, due to concerns about diversion (Doemberg et al, 2019; Wish et al., 2012). Even when the institutions want to participate in providing medication treatment, it is difficult to do so effectively for detainees who are pretrial due to the rapid turnover and lack of time for discharge planning or for providing stability in treatment over the critical first few weeks. Thus, as we work more broadly to implement effective treatment strategies across health care and criminal justice systems, it is important to create viable, low threshold community programs located in proximity to the release door that partner with criminal justice entities. These programs are able to work with corrections staff to provide whatever is needed to create continuity of care and provide a partner for continued treatment as people are released and for those for whom there is insufficient time to effectively initiate treatment.
5. Conclusions
As we work to address the opioid crisis, it is critical that we include those who are most in need and yet often the most underserved. States across the country are working to strengthen their local responses to opioid use and overdose, improve their substance use treatment systems, and better integrate behavioral health care into their larger health and criminal justice systems. A comprehensive behavioral health system requires that treatment be available for each stage of need, from those who require low-threshold care to those who are ready to begin more structured treatment. The PCARE program is a pilot demonstrating the potential that low-threshold mobile treatment programs have to reach patients with justice involvement and other vulnerabilities that are often harder to engage in care, although long-term retention in buprenorphine treatment remains an ongoing challenge. The current paper presents an initial description and pilot data from the program, and further research is needed to determine effectiveness, including impact on post-release overdose mortality, and analysis of cost-effectiveness of this model. Such studies should include following up with patients even once they are transitioned to ongoing care with other providers. Ensuring the availability of care that is welcoming and accessible to the most marginalized and hard-to-reach is key to achieving successful recovery and a true reduction in overdose deaths and related suffering in our communities.
Acknowledgments
The authors would like to thank all PCARE staff members as well as Commissioner Michael Resnick of the Baltimore Division of Pretrial Detention and Services for their continued dedication to the PCARE model and its mission.
Funding
PCARE van operations were supported by grants from The Jacob and Hilda Blaustein Foundation, The Krieger Fund, The Open Society Institute-Baltimore, The Leonard and Helen R. Stulman Foundation, and the Abell Foundation. Noa Krawczyk was also supported by the National Institute On Drug Abuse of the National Institutes of Health under Award Number F31DA047021.
References
- Aronowitz SV, & Laurent J (2016). Screaming behind a door: The experiences of individuals incarcerated without medication-assisted treatment. Journal of Correctional Health Care, 22(2), 98–108 (SAGE Publications Sage CA: Los Angeles, CA). [DOI] [PubMed] [Google Scholar]
- Barry DT, Moore BA, Pantalon MV, Chawarski MC, Sullivan LE, O’Connor PG, Schottenfeld RS, et al. (2007). Patient satisfaction with primary care office-based buprenorphine/naloxone treatment. Journal of General Internal Medicine, 22(2), 242–245. 10.1007/sll606-006-0050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Barth KS, Back SE, & Book SW (2015). Discontinuation of buprenorphine maintenance therapy: Perspectives and outcomes. Journal of Substance Abuse Treatment, 52, 48–57 (Elsevier). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binswanger IA, Stem MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, & Koepsell TD (2007). Release from prison—A high risk of death for former inmates. New England Journal of Medicine, 356(2), 157–165. 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramson H, Des Jarlais DC, Arasteh K, Nugent A, Guardino V, Feelemyer J, & Hodel D (2015). State laws, syringe exchange, and HIV among persons who inject drugs in the United States: History and effectiveness. Journal of Public Health Policy, 36(2), 212–230. Palgrave Macmillan UK 10.1057/jphp.2014.54. [DOI] [PubMed] [Google Scholar]
- Bronson J, Stroop J, Zimmer S, & Berzofsky M (2017). Drug use, dependence, and abuse among state prisoners and jail inmates, 2007–2009. Source: Bureau of Justice Statistics. Retrieved from http://www.ilcoe.us/wp-content/uploads/2017/04/dudaspji0709.pdf. [Google Scholar]
- Carroll KM, & Weiss RD (2017). The role of behavioral interventions in buprenorphine maintenance treatment: A review. American Journal of Psychiatry, 174(8), 738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connery HS (2015). Medication-assisted treatment of opioid use disorder. Harvard Review of Psychiatry, 23(2), 63–75. 10.1097/HRP.0000000000000075. [DOI] [PubMed] [Google Scholar]
- Cornish R, Macleod J, Strang J, Vickerman P, & Hickman M (2010). Risk of death during and after opiate substitution treatment in primary care: Prospective observational study in UK General Practice Research Database. BMJ, 341, c5475 (Clinical research ed., School of Social and Community Medicine, University of Bristol, Bristol BS8 2PS, UK) 10.1136/bmj.c5475 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels AM, Salisbuiy-Afshar E, Hoffberg A, Agus D, and Fingerhood MI (2014). A novel community-based buprenoiphine program: Client description and initial outcomes. Journal of Addiction Medicine, 8(1), 40–46. 10.1097/ADM.0000000000000004 [doi]. [DOI] [PubMed] [Google Scholar]
- De Hert M, Vancampfort D, & Detraux J (2015). Somatic problems and dual disorder patients Co-occurring addictive and psychiatric disorders (pp. 349–361). Springer. [Google Scholar]
- Dickey B, Normand S-LT, Weiss RD, Drake RE, & Azeni H (2002). Medical morbidity, mental illness, and substance use disorders. Psychiatric Services, 53(7), 861–867 (Am Psychiatric Assoc). [DOI] [PubMed] [Google Scholar]
- Doemberg M, Krawczyk N, Agus D, & Fingerhood MI (2019). Demystifying buprenorphine misuse: Has fear of diversion gotten in the way of addressing the opioid crisis? Substance Abuse, 22,1–6 (Apr). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugosh K, Abraham A, Seymour B, McLoyd K, Chalk M, & Festinger D (2016). A systematic review on the use of psychosocial interventions in conjunction with medications for the treatment of opioid addiction. Journal of Addiction Medicine,10(2), 93–103. Treatment Research Institute, Philadelphia, PA (KD, AA, BS, KML, MC, DF); Department of Health Policy and Management, University of Georgia, Athens, GA (AA) 10.1097/ADM.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin JP, Ganim Z, Hunter SJ, & Kamik NS (2012). The mental and physical health of homeless youth: A literature review. Child Psychiatry & Human Development, 43(3), 354–375. Springer US 10.1007/sl0578-011-0270-l. [DOI] [PubMed] [Google Scholar]
- Feng J, Iser JP, & Yang W (2016). Medical encounters for opioid-related intoxications in southern Nevada: Sociodemographic and clinical correlates. BMC Health Services Research, 16(1), 438 BioMed Central 10.1186/sl2913-016-1692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiscella K, Wakeman SE, & Beletsky L (2018). Implementing opioid agonist treatment in correctional facilities. JAMA Internal Medicine, 178(9), 1153–1154. [DOI] [PubMed] [Google Scholar]
- Fox AD, Maradiaga J, Weiss L, Sanchez J, Starrels JL, & Cunningham CO (2015). Release from incarceration, relapse to opioid use and the potential for buprenorphine maintenance treatment: A qualitative study of the perceptions of former inmates with opioid use disorder. Addiction Science & Clinical Practice, 10(1), 2 (BioMed Central). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann PD, Hoskinson R Jr, Gordon M, Schwartz R, Kinlock T, Knight K, Flynn PM, et al. (2012). Medication-assisted treatment in criminal justice agencies affiliated with the criminal justice-drug abuse treatment studies (CJ-DATS): Availability, barriers, and intentions. Substance Abuse, 33(1), 9–18. Taylor & Francis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea S, & Vlahov D (2002). Social determinants and the health of drug users: Socioeconomic status, homelessness, and incarceration. Public Health Reports, 117 suppl 1(Suppl. 1), S135–S145 (Washington, D.C.: 1974, SAGE Publications. Retrieved from http.y/www.ncbi.nlm.nih.gov/pubmed/12435837. [PMC free article] [PubMed] [Google Scholar]
- Gjersing L, & Bretteville-Jensen AL (2013). Is opioid substitution treatment beneficial if injecting behaviour continues? Drug and Alcohol Dependence, 133(1), 121–126. Elsevier https://doi.Org/10.1016/J.DRUGALCDEP.2013.05.022. [DOI] [PubMed] [Google Scholar]
- Gordon MS, Kinlock TW, & Miller PM (2011). Medication-assisted treatment research with criminal justice populations: Challenges of implementation. Behavioral Sciences & the Law, 29(6), 829–845. 10.1002/bsl.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TC, Clarke J, Brinkley-Rubinstein L, Marshall BD 1., Alexander-Scott N, Boss R, & Rich JD (2018). Postincarceration fatal overdoses after implementing medications for addiction treatment in a statewide correctional system. JAMA Psychiatry,75(4), 405–407. 10.1001/jamapsychiatry.2017.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield L, Brady JV, Besteman KJ, & De Smet A (1996). Patient retention in mobile and fixed-site methadone maintenance treatment. Drug and Alcohol Dependence, 42(2), 125–131. Elsevier https://doi.org/’l0.1016/0376-8716(96)01273-2. [DOI] [PubMed] [Google Scholar]
- Hall G, Neighbors CJ, Iheoma J, Dauber S, Adams MB, Culleton R, Muench F, et al. (2014). Mobile opioid agonist treatment and public funding expands treatment for disenfranchised opioid-dependent individuals. Journal of Substance Abuse Treatment, 46(4),511–515. Elsevier Inc. https://doi.Org/10.1016/j.jsat.2013.ll.002. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Brown DFM, Tsugawa Y, & Camargo CA (2014). Epidemiology of emergency department visits for opioid overdose: A population-based study. Mayo Clinic Proceedings, 89(4), 462–471. [DOI] [PubMed] [Google Scholar]
- Hedegaard H, Warner M, & Minino AM (2017). Drug overdose deaths in the United States, 1999–2015. NCHS Data Brief, 273. [PubMed] [Google Scholar]
- Jones CM, Campopiano M, Baldwin G, & McCance-Katz E (2015). National and state treatment need and capacity for opioid agonist medication-assisted treatment. Journal Information, 105(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeble D, & Cowhig M (2018). Correctional populations in the United States, 2016. Retrieved from https://www.bjs.gov/content/pub/pdf/cpusl6.pdf.
- Kertesz SG, Horton NJ, Friedmann PD, Saitz R, & Samet JH (2003). Slowing the revolving door: Stabilization programs reduce homeless persons1 substance use after detoxification. Journal of Substance Abuse Treatment, 24(3), 197–207. Pergamon 10.1016/50740-5472(03)00026-6. [DOI] [PubMed] [Google Scholar]
- Kinlock TW, Gordon MS, & Schwartz RP (2011). In carcerated populations In Ruiz P, & Strain E (Eds.). Lowinson & Ruiz’s substance abuse: A comprehensive textbook (pp. 881–891). (Fifth). Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Kourounis G, Richards BDW, Kyprianou E, Symeonidou E, Malliori M-M, & Samartzis L (2016). Opioid substitution therapy: Lowering the treatment thresholds. Drug and Alcohol Dependence, 161,1–8. https://doi.Org/10.1016/j.drugalcdep.2015.12.021. [DOI] [PubMed] [Google Scholar]
- Krawczyk N, Picher CE, Feder KA, & Saloner B (2017). Only one in twenty’justice-referred adults in specialty treatment for opioid use receive methadone or buprenorphine. Health Affairs, 36(12), 2046–2053 (Project Hope). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Grossman E, DiRocco D, & Gourevitch M (2009). Home buprenorphine/na-loxone induction in primary’ care. Journal of General Internal Medicine, 24(2), 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez G (2018). How America’s prisons are fueling the opioid qpidemic. Vox. Retrieved from https://www.vox.com/policy-and-politics/2018/3/13/17020002/prison-opioid-epidemic-medications-addiction. [Google Scholar]
- Maryland Department of Health and Mental Hygiene (2014). Risk of overdose death following release from prison or jail. Retrieved from https://bha.health.maryland.gov/OVERDOSE_PREVENTION/Documents/correctionsbrief_V3.pdf.
- Matusow H, Dickman SL, Rich JD, Fong C, Dumont DM, Hardin C, Marlowe D, et al. (2013). Medication assisted treatment in US drug courts: Results from a nationwide survey of avaQability, barriers and attitudes. Journal of Substance Abuse Treatment, 44(5),473–480 (Elsevier). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell S, Bigg D, Stanczykiewicz K, & Carlberg-Racich S (2006). Prescribing naloxone to actively injecting heroin users. Journal of Addictive Diseases, 25(3), 89–96. 10.1300/J069v25n03_11. [DOI] [PubMed] [Google Scholar]
- Meirall ELC, Kariminia A, Binswanger IA, Hobbs MS, Farrell M, Marsden J, Hutchinson SJ, et al. (2010). Meta-analysis of drug-related deaths soon after release from prison. Addiction, 105(9), 1545–1554 (Wiley Online Library). [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Alliance for Model State Drug Laws (2013). States that require an ID from a recipient prior to dispensing prescriptions. Retrieved from http://wvw.namsdl.org/library,/856CB9AF-19B9-ElC5-317AD4BE6BA7D309/.
- Nunn A, Zaller N, Dickman S, Trimbur C, Nijhawan A, & Rich JD (2009). Methadone and buprenorphine prescribing and referral practices in US prison systems: Results from a nationwide survey. Drug and Alcohol Dependence, 105(1), 83–88 (Elsevier). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oros M, & Welsh C (2013). The Baltimore buprenorphine initiative: Clinical guidelines for buprenorphine treatment of opioid dependence in the Baltimore buprenorphine initiative. Baltimore. Retrieved from http://wvw.bhsbaltimore.org/site/wp-content/uploads/2013/09/BBI-Clinical-Guidelines-Revised-2013.pdf.
- Peles E, Schreiber S, & Adelson M (2013). Opiate-dependent patients on a waiting list for methadone maintenance treatment are at high risk for mortality until treatment entry. Journal of Addiction Medicine, 7(3), 177–182. Retrieved from https://joumals.lww.com/joumaladdictionmedicine/Abstract/2013/05000/Opiate_Dependent_Patients_on_a_Waiting_List_for.4.aspx. [DOI] [PubMed] [Google Scholar]
- Perreault M, Julien D, White ND, Rabouin D, Lauzon P, & Milton D (2015). Psychological predictors of retention in a low-threshold methadone maintenance treatment for opioid addicts: A 1-year follow-up study. Substance Use & Misuse, 50(1), 24–31. 10.3109/10826084.2014.957769. [DOI] [PubMed] [Google Scholar]
- Ranapurwala SI, Shanahan ME, Alexandridis AA, Proescholdbell SK, Naumann RB, Edwards D, & Marshall SW (2018). Opioid overdose mortality among former North Carolina inmates: 2000–2015. American Journal of Public Health, el–e7. American Public Health Association; 10.2105/AJPH.2018.304514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisinger HS, Schwartz RP, Mitchell SG, Peterson JA, Kelly SM, O’Grady KE, Marrari EA, et al. (2009). Premature discharge from methadone treatment: Patient perspectives. Journal of Psychoactive Drugs, 41(3), 285–296. 10.1080/02791072.2009.10400539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, & Ruefli T (2004). Does harm reduction programming make a difference in the lives of highly marginalized, at-risk drug users? Harm Reduction Journal J (1), 7 BioMed Central 10.1186/1477-7517-l-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RA, Aleshire N, Zibbell JE, & Matthew Gladden R. (2016). Increases in drug and opioid overdose deaths—United States, 2000–2014. American Journal of Transplantation, 16(4), 1323–1327 (Wiley Online Library). [Google Scholar]
- Schwartz RP, Kelly SM, O’Grady KE, Gandhi D, & Jaffe JH (2012). Randomized trial of standard methadone treatment compared to initiating methadone without counseling: 12-month findings. Addiction, 107(5), 943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmon SC, Ochalek TA, Meyer AC, Hruska B, Heil SH, Badger GJ, Rose G, et al. (2016). Interim buprenorphine vs. waiting list for opioid dependence. New England Journal of Medicine, 375(25), 2504–2505. 10.1056/NEJMC1610047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Hill C, Bennet J, Vavasis A, & Oriol NE (2013). MobQe clinic in Massachusetts associated with cost savings from lowering blood pressure and emergency department use. Health Affairs, 32(1), 36–44. Health Affairs 10.1377/hlthaff.2011.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, Ferri M, et al. (2017). Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ, 357, jl550 (Clinical research ed., National Centre for Epidemiology, Carlos III Institute of Health, Madrid, Spain.; Consortium for Biomedical Research in Epidemiology’ and Public Health (CIBERESP), Madrid, Spain.; Department of Preventive Medicine and Public Health, Faculty of Me(TRUNCATED)) 10.1136/bmj.jl550[doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LE, Bruce RD, Haltiwanger D, L. GM, Eldred L, Finkelstein R, Fiellin D, et al. (2006). Initial strategies for integrating buprenorphine into HIV care settings in the United States. Clinical Infectious Diseases, 43(Supplement_4), S191–S196. [DOI] [PubMed] [Google Scholar]
- Timko C, Schultz NR, Cueciare MA, Vittorio L, & Garrison-Diehn C (2016). Retention in medication-assisted treatment for opiate dependence: A systematic review. Journal of Addictive Diseases, 35(1), 22–35. 10.1080/10550887.2016.1100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J, Rosenheck RA, Culhane DP, & Artiga S (2013). Medicaid expansion: Chronically homeless adults will need targeted enrollment and access to a broad range of services. Health Affairs, 32(9), 1552–1559. 10.1377/hlthaff.2013.0228. [DOI] [PubMed] [Google Scholar]
- van Boekel LC, Brouwers EPM, van Weeghel J, & Garretsen HFL (2013). Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: Systematic review. Drug and Alcohol Dependence,131(1–2), 23–35. Elsevier https://doi.0rg/lO.lOl6/J.DRUGALCDEP.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Veilleux JC, Colvin PJ, Anderson J, York C, & Heinz AJ (2010). A review of opioid dependence treatment: Pharmacological and psychosocial interventions to treat opioid addiction. Clinical Psychology Revim, 30(2), 155–166 (Elsevier). [DOI] [PubMed] [Google Scholar]
- Vestal C (2018, March). Federal ban on methadone vans seen as barrier to treatment. Pew Charitable Trusts. [Google Scholar]
- Wakeman SE, & Rich JD (2015). Addiction treatment within U.S. correctional facilities: Bridging the gap between current practice and evidence-based care. Journal of Addictive Diseases, 34(2–3), 220–225. Routledge 10.1080/10550887.2015.1059217. [DOI] [PubMed] [Google Scholar]
- Walsh N (2010). Baltimore Behind Bars, (June). Retrieved from http://www.justicepolicy.org/images/upload/10-06_rep_baltbehindbars_md-ps-ac-rd.pdf. [Google Scholar]
- Wihkelman TNA, Chang VW, & Binswanger IA (2018). Health, polysubstance use, and criminal justice involvement among adults with varying levels of opioid use. JAMA Network Open, 1(3), el80558 American Medical Association 10.1001/jamanetworkopen.2018.0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wish ED, Artigiani E, Billing A, Hauser W, Hemberg J, Shiplet M, & DuPont RL (2012). The emerging buprenorphine epidemic in the United States. Journal of Addictive Diseases, 31(1), 3–7. Taylor & Francis Group 10.1080/10550887.2011.642757. [DOI] [PubMed] [Google Scholar]