Abstract
Traumatic brain injury is a leading cause of cognitive and behavioral deficits in children in the US each year. There is an increasing interest in both clinical and pre-clinical studies to discover biomarkers to accurately diagnose traumatic brain injury (TBI), predict its outcomes, and monitor its progression especially in the developing brain. In humans, the heterogeneity of TBI in terms of clinical presentation, injury causation, and mechanism has contributed to the many challenges associated with finding unifying diagnosis, treatment, and management practices. In addition, findings from adult human research may have little application to pediatric TBI, as age and maturation levels affect the injury biomechanics and neurophysiological consequences of injury. Animal models of TBI are vital to address the variability and heterogeneity of TBI seen in human by isolating the causation and mechanism of injury in reproducible manner. However, a gap between the pre-clinical findings and clinical applications remains in TBI research today. To take a step toward bridging this gap, we reviewed several potential TBI tools such as biofluid biomarkers, electroencephalography (EEG), actigraphy, eye responses, and balance that have been explored in both clinical and pre-clinical studies and have shown potential diagnostic, prognostic, or monitoring utility for TBI. Each of these tools measures specific deficits following TBI, is easily accessible, non/minimally invasive, and is potentially highly translatable between animals and human outcomes because they involve effort-independent and non-verbal tasks. Especially conspicuous is the fact that these biomarkers and techniques can be tailored for infants and toddlers. However, translation of preclinical outcomes to clinical applications of these tools necessitates addressing several challenges. Among the challenges are the heterogeneity of clinical TBI, age dependency of some of the biomarkers, different brain structure, life span, and possible variation between temporal profiles of biomarkers in human and animals. Conducting parallel clinical and pre-clinical research, in addition to the integration of findings across species from several pre-clinical models to generate a spectrum of TBI mechanisms and severities is a path toward overcoming some of these challenges. This effort is possible through large scale collaborative research and data sharing across multiple centers. In addition, TBI causes dynamic deficits in multiple domains, and thus, a panel of biomarkers combining these measures to consider different deficits is more promising than a single biomarker for TBI. In this review, each of these tools are presented along with the clinical and pre-clinical findings, advantages, challenges and prospects of translating the pre-clinical knowledge into the human clinical setting.
Keywords: Pediatric traumatic brain injury, Translational research, Serum biomarkers, Electroencephalography (EEG), Actigraphy, Eye tracking, Balance assessment, Multi-domain deficits, Panel of biomarkers, TBI heterogeneity
1. Introduction
In the United States, children ages 0–4 years had the highest estimated annual rates of TBI-related emergency room visits, followed by adolescents ages 15–19 years of age (Faul et al., 2010). The most common causes of TBI are falls and assaults in young children and motor vehicle accidents and sport related injuries in elementary school children and adolescents (Faul et al., 2010). Among pediatric and adolescent, most TBIs are classified as mild, however, there are still many TBI hospitalizations categorized as moderate to severe TBI (Rivara et al., 2011; Asemota et al., 2013). TBI has devastating acute effects and in many cases seems to initiate long-term neurodegeneration (Johnson et al., 2010). It is estimated that 145,000 children and adolescents (ages 0–19 years) are living with lasting cognitive, physical, or behavioral effects of TBI (Zaloshnja et al., 2008). Due to the long-term and potentially detrimental effects of TBI on the young brain, there is increasing interest in both pre-clinical and clinical studies to discover diagnostic tools to accurately identify TBI especially concussion/mild TBI in the developing brain. The nature of TBI is complex, often a number of injury mechanisms are simultaneously at play presenting diverse spatial and temporal pathophysiology and injury severities. The heterogeneity in human TBI is an important factor from the clinical perspective in predicting outcomes and injury trajectories which has led to the absence of relevant and validated outcome measures in TBI and are the primary reason for discouraging results from neuroprotective and therapeutic clinical trials over the last four decades (Yue et al., 2013).
Animal models of TBI mimic the neurological deficits of human TBI and offer a unique opportunity to reduce the heterogeneity seen in humans. As such animal studies allow researchers to isolate and investigate the pathological and behavioral changes associated with brain injury in a consistent and reproducible manner. The use of animal models provides a means to explore the underlying reasons for a given outcome, to accelerate preclinical therapeutic findings, and evaluate the efficacy of clinical treatment and management of TBI. In addition, animal models can help to improve correlations with different severities of TBI and to refine the mechanisms of injury involved to develop reliable, efficient and valid classification systems to link specific patterns of brain and neurovascular injury with the appropriate therapeutic interventions. However, there is still a gap between preclinical findings and clinical applications. To take a step toward bridging this gap, in this paper we first present several validated animal models of TBI. We then discuss translatable metrics as potential TBI diagnostic tools including biofluid biomarkers, electroencephalography (EEG), actigraphy, eye tracking, and balance tests. We include metrics that have been explored in both clinical and laboratory studies and can be translated between humans and animals. We will also detail the challenges and research opportunities toward development and evaluation of effort-independent and clinically translatable TBI metrics to advance our understanding and management of TBI in the developing brain.
2. Pre-clinical animal models of TBI
Animal models have been developed that produce different types of TBI such as contusion, diffuse or focal axonal injury, hematomas, and subarachnoid hemorrhage(Pitt and Leung, 2015). Utilizing idealized animal models make it possible to conduct a thorough investigation of pathophysiological mechanisms of TBI, the validation and prognostic value of TBI biomarkers, and the assessment of treatments in a setting of a reproducible phenotype with a known pre-injury exposure. Here we briefly present four specific models that are widely used in animal TBI research: controlled cortical impact (CCI) injury, rapid non-impact rotational injury (RNR), weight-drop impact injury (WDI), and fluid percussion injury (FPI) mainly focusing on pediatric models.
2.1. Controlled cortical impact TBI model
Controlled cortical impact (CCI) TBI models are developed to produce a purely focal cortical/subcortical contusion injury with direct focal damage to the exposed, open or intact dura of the subject and no inertial motion of the head. Contusion injury is one of the most common types of brain injury in children caused by events such as falls, vehicular accident, sport injuries and child abuse (Graham et al., 1989). The CCI model utilizes a blunt indentation device driven by either a loaded spring, a pneumatic piston, or an electromagnetic coil to create a rapid displacement of the cortical surface (Margulies et al., 2015). The severity and functional deficits of CCI injury in animal models can be adjusted by the impact velocity, duration of impact, depth of deformation, and size of the impactor tip. CCI models of infant/pediatric TBI have been developed in the rat (Raghupathi and Huh, 2007; Robinson et al., 2016; Schober et al., 2014), mice (Mannix et al., 2011), rabbit (Zhang et al., 2015), and piglet (Baker et al. (2018); Duhaime et al., 2000; Missios et al., 2009) typically focusing on moderate to severe injury outcomes.
2.2. Rapid non-impact head rotation TBI model
Rapid non-impact head rotation (RNR) TBI models produce a purely inertial head movement with no head contact, similar to levels experienced in motor vehicle or high velocity trauma that result in unconsciousness, sustained cognitive dysfunction, bilateral diffuse axonal and hemorrhagic injury (Margulies et al., 2015). RNR consists of a pneumatic device that moves a thrust column at controlled acceleration and deceleration levels employing a shaft that is externally coupled to a custom-built linkage assembly to produce the desired kinematics (often rotational motion). The RNR injury model has been mostly studied on pigs (Kilbaugh et al., 2015; Margulies et al., 2015) and primates (Gennarelli et al., 1982), however, there have been limited studies on rats (Xiao-Sheng et al., 2000) and rabbits (Gutierrez et al., 2001). Among those, only the pig studies (Kilbaugh et al., 2015; Margulies et al., 2015) were focused on infant/pediatric TBI.
2.3. Weight drop impact TBI model
Weight drop impact (WDI) injury model consists of a falling weight on the skull to induce focal cerebral contusion and diffuse axonal injury and used to simulate concussion to severe TBI. The diameter, velocity and dwell time of the impactor are adjustable to alter the severity of injury. In Marmarou’s WDM (Marmarou et al., 1994), which mainly results in diffuse TBI, a stainless steel disc is mounted to the midline the animal’s skull to distribute the force of the impact and prevent skull fracture. Later, WDI was modified to produce disruption in the blood–brain barrier, cerebral edema, and neuronal cell death below the contusion site and remotely in the hippocampus by dropping the weight onto one side of an unprotected skull while resting on a hard surface (Shohami et al., 1988). Another modification to WDI was to support the animal on a fragile aluminum foil support which allows post impact rapid acceleration of the free-moving head and torso (Kane et al., 2012). WDI has been utilized in small animals such as rat (Mychasiuk et al., 2014; Semple et al., 2016) and mouse (Adelson et al., 1996; Chhor et al., 2017) to model pediatric TBI.
2.4. Fluid percussion injury TBI model
The fluid percussion injury (FPI) model employs a rapid fluid pressure pulse delivered to the open dura of an animal causing graded severities of brain injury including contusions, intracranial hemorrhages, brain swelling, grey matter damage, concussion and traumatic axonal injury (McIntosh et al., 1989; Xiong et al., 2013). Injury severity is controlled by the magnitude of the fluid pressure pulse and the location of the craniotomy determines the clinical and pathological effects of injury (O’Connor et al., 2011). Due to the nature of FPI models requiring an opening in the skull, translation of mechanisms and interventions to human head injury is often difficult because the majority of human head injuries involve closed head injury mechanisms (O’Connor et al., 2011). FPI model of TBI have been primarily studied in animals, such as the cat (Stalhammar et al., 1987), sheep (Millen et al., 1985), swine (Fritz et al., 2005), mice (Carbonell et al., 1998; Folweiler et al., 2018; Ogino et al., 2018), and rat (Gorse and Lafrenaye, 2018; Katz and Molina, 2018; McIntosh et al., 1987). Many of these studies typically represent the adult brain, however those employing rats (Prins and Hovda, 2003) and piglets (Fritz et al., 2005; Lafrenaye et al., 2015) have been used to model the newborn and juvenile brain.
3. Translatable metrics
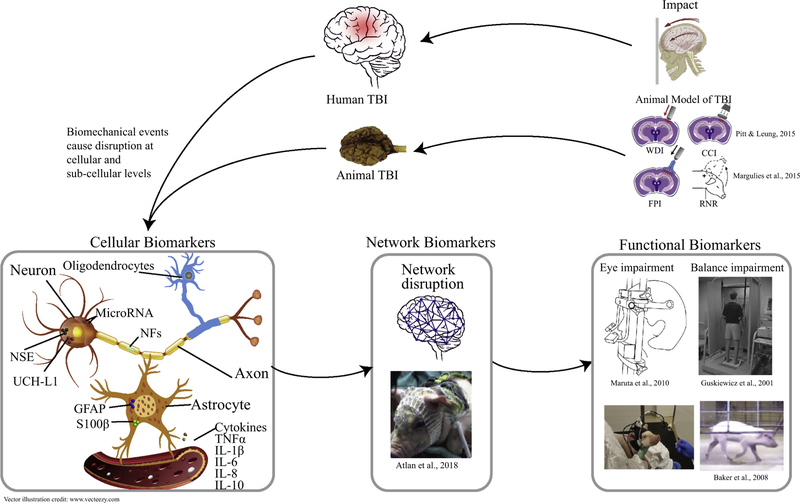
In this section, we reviewed metrics including biofluid biomarkers, electroencephalography (EEG), actigraphy, eye response, and balance tests that have been explored in both clinical and preclinical studies and can be translated between humans and animals. The possible cellular origin, causation and interactions of these biomarkers are illustrated in Fig. 1. In addition, a summary of pediatric clinical and pre-clinical TBI studies that examined the diagnostic and prognostic utility of these translatable biomarkers for TBI is given in Table 1. The main focus of this table was to summarize the literature on the utility of a single or a panel of biomarkers to: (1) detect intracranial lesions to identify patients in need of computed tomography (CT) scan; (2) assess injury severity and prognosis, (3) predict short- or long-term neurological outcomes of TBI; (4) diagnose injured patients (especially concussion/mild TBI) from healthy or non-TBI trauma controls; and/or (5) distinguish between mechanisms of injury, for example, differentiating between inflicted (iTBI) from non-inflicted (nTBI) where abusive head trauma is prevalent in infants and toddlers.
Fig. 1.
Schematic of cellular origins and causations of biomarkers and relation between biomechanical event and biomarkers at cellular, neuronal network and functional levels.
Table 1.
A summary of pediatric clinical and preclinical studies for TBI biomarkers.
| Study | Biomarker(s) | Sample size | Causes and severity of TBIs | Age range | Sampling time | Key findings (e.g. patients vs control, correlation with severity or abnormal CT) | Recommended for diagnostic and prognostic |
|---|---|---|---|---|---|---|---|
| Clinical studies – biofluid biomarkers | |||||||
| Fridriksson et al. (2000) | Serum NSE | 50 | Mild/moderate/severe (n = 50), no control | 2 months-16 years | Within 10 h post injury (except one data in 23 h) | – Correlation between NSE level and severity – Serum NSE is capable of predicting ICI with sensitivity of 77% and specificity of 52%, however 25% of ICI patients would have been missed using solely NSE – Used NSE cut-off level of 15 ng/mL |
– Recommended serum NSE as a potential diagnostic tool for predicting ICL in children with blunt head trauma but not to be used as a sole marker |
| Berger et al. (2002) | Serum S100β | 61 | Mild (n = 27), moderate (n = 6), and severe (n = 12), control (n = 16) | 0–13 years | At 0.5–15.25 h post injury and every 12 h for up to 5 days | – Higher serum S100β (H1 > avg. + 2SD of control) after TBI in half of TBI patients (any severity) but the increase lasted > 12 h after injury only in severe TBI patients. – Marginal correlation between serum S100β concentration and GSC score (higher serum S100Β for lower GSC) – No relationship between serum S100Β elevation and the presence of an abnormal CT – Used S100β cut-off value of 0.25 μg/L |
N/A |
| Spinella et al. (2003) | Serum S100β | 163 | Mild/moderate/severe (n = 27), control (n = 136) | 0–18 years | Within 12 h post injury | – Higher S100β serum levels (≥2.0 μg/L) in TBI patient with poor 6-months post-injury outcome – Found a mean S100β level of 0.3 μg/L (0.03–1.47 μg/L) for healthy children |
Recommended serum S100β as a potential prognostic tool as its level seems to be associated with long-term outcome |
| Chiaretti et al. (2005) | Serum and CSD IL-6, IL-1β | 26 | Severe TBI (n = 14), control (n = 12, obstructive hydrocephalus) | 3 months–16 years | 2 h and 24 h post-TBI | – IL-1βl was significantly lower than the IL-6 level both in the CSF and serum – Significant correlation between increase of IL-6 and IL-1β in both CSF and serum from 2 h to 24 h post TBI and severity of TBI and 6 months post-TBI outcomes |
– Recommended IL-6 and IL-1β as potential prognostic biomarkers of severe TBI in children |
| Bandyopadhyay et al. (2005) | Serum NSE | 86 | Mild (n = 76), moderate (n = 6), severe (n = 4), no control | 11 months-18 years | Within 0.4–14.8 h post injury | – Found higher NSE serum levels (mean = 46.4 ng/mL) in TBI patients with poor short-term functional outcomes – Used NSE cut-off level of 21.2 ng/mL |
– Recommended serum NSE as a potential prognostic tool as its level seems to be associated with short-term outcomes and maybe useful early predictor of disability in children following closed TBI |
| Berger et al. (2005) | Serum S100β, NSE (and MBP) | 164 | TBI (n = 100, including 56 nTB and 44 iTBI), control (n = 64) | 0–13 years | Within 12 h post injury, another with 12–24 h, for severe TBI daily up to 5 days | – No difference between serum NSE, S100β and MBP between children with iTBI and nTBI, however iTBI patients had a later peak of all three biomarkers compared with nTBI patients – Higher initial serum NSE and S100β levels for TBI than control |
– Recommended time to peak serum NSE, S100β and MBP as a potential tool to discriminate iTBI from nTBI |
| Berger and Kochanek (2006) | Serum and urine S100β | 29 | TBI (n = 111, including 105 without ICI and 6 with ICI), no control | < 13 years | Every 12 h for 3 days post TBI | – Urinary S100β peak later (55 h post TBI) than serum S100β concentrations (14 h post TBI) – Urine S100β was detectable in 80% of TBI patients and 0% of control – TBI patients with undetectable S100β urine as well as TBI patients with normal serum S100β levels were more likely to have good outcomes |
– Recommended serum and/or urine S100β as a potential prognostic tool to assist in the prediction of outcome after pediatric TBI |
| Berger et al. (2007) | Serum S100β, NSE (and MBP) | 159 | TBI (n = 159), no control | 0–13 years | Within 12 h post-TBI, 12–24 h, for severe TBI daily to 5 days | – Correlation between serum S100β, NSE, MBP at all time points and long-term outcomes – Initial and peak NSE concentrations and initial MBP levels more strongly correlated with outcome in children ≤4 years compared with those 4 years |
– Recommended serum and/or urine S100β as a potential prognostic tool to assist in the prediction of outcome after pediatric TBI |
| Beers et al. (2007) | Serum S100β, NSE (and MBP) | 30 | TBI (n = 30, including15 nTB and 15 iTBI), no control | 0–13 years | Within 12 h post injury, every12hrs up to 5 days | – Significant difference between time to peak serum NSE, S100β and MBP between children with iTBI and nTBI – Correlation between time to peak serum NSE, S100Β and MBP and 6-months post TBI outcomes |
– Recommended time course of serum NSE, S100β and MBP as a potential prognostic tool for long-term outcomes of TBI |
| Piazza et al. (2007) | Serum S100β | 15 | 8 accidental falls, 7 road accidents, mild (n = 9), moderate (n = 2), severe (n = 4), no control | 1–15 years | At admission to ER (within 12 h post injury) and after 48 h | – A trend toward correlation between the severity of TBI and serum S100β increase (higher serum S100Β for lower GCS score at admission). – CT scans were performed; no data was reported – Used S100Β cut-off value of 0.3 μg/L |
– Did not recommend serum S100β to be used as a prognostic tool, particularly when TBI is associated with extracranial lesions |
| Buttram et al. (2007) | CSF cytokine including IL-6, IL-8, Ilβ, IL-10 (, MIP-1α) | 46 | Severe TBI (n = 36), control (n = 10) | N/A | Serial sampling up to 72 h | – Moderate therapeutic hypothermia did not reduce the elevation of CSF cytokine levels in children after TBI compared to normothermia – Severe TBI in infants and children induces increases in pro- and anti-inflammatory cytokines |
none |
| Pickering et al. (2008) | Urine S100β | 35 | Mild TBI (n = 17), moderate TBI (n = 1), severe TBI (n = 2),control with limb/fracture injury (n = 15) | < 13 years | Within 12 h post injury, | – Detect S100β in urine in 50% of TBI (0.02–0.07 μg/L) and all extracranial trauma (0.02–0.09 μg/L) patients | Did not recommend urine S100β as an early diagnostic biomarker of TBI |
| Castellani et al. (2009) | Serum S100β | 109 | Mild (n = 109), no control | 0–18 years | Within 6 h post injury | – Higher serum S100β for patients with positive abnormal CT scans – Used S100β cut-off value 0.16 μg/L |
– Recommended serum S100β as a potential diagnostic tool with high sensitivity but low specificity for ICI in mild TBI patients as evidenced by CT scans (raised limitation for children ≤3 years as S100β varied by age a lot at this period) |
| Geyer et al. (2009) | Serum S100β and NSE | 148 | Mild (n = 148), 53 asymptomatic and 95 symptomatic mTBI | 0.5–15 years | Within 6 h post injury | – Did not find serum S100β and NSE capable of discriminating between symptomatic and asymptomatic pediatric mTBI – Correlation between S100Β and NSE – Negative correlation between both S100β and NSE and age |
none |
| Lo et al. (2009) | Serum S100β, NSE, IL-6, IL-8, IL-10, (SICAM), L-selectin, and endothelin) | 28 | Mild TBI (n = 2), moderate TBI (n = 9), severe TBI (n = 17) (19 diffuse and 9 focal TBI) | 4 months–14 years | At 24 h post-TBI | – 8 biomarkers were measured, and 20 combination of 2-biomarkers were examined, and 5 paired showed high degree of outcome predictability compared to any single biomarker | – Recommended combination of S100β with IL-6, L-selectin, or NSE as a potential prognostic biomarker for predicting poor 6-month post-TBI outcome which outperformed any individual marker |
| Hallén et al. (2010) | Serum and urine S100β | 111 | TBI (n = 111, including 105 without ICI and 6 with ICI), no control | < 13 years | Within 6 h post injury, and 6 h later | – Significant higher serum S100β in TBI patients with ICI (as evidenced by CT scans) than the ones without ICI – Did not find any difference between urine S100β between group with and without ICI |
– Recommended serum (but not urine) S100β as a diagnostic tool for TBI addition to those used in clinical practice today but not as a sole tool |
| Fraser et al. (2011) | Serum (and CSF) GFAP | 27 | Severe TBI (n = 27), no control | 2–17 years | Serums were collected daily for 10 days post TBI | – Serum GFAP on day 1 correlated with 6 months post TBI outcomes but not correlated w/injury severity or CT results – GFAP was dramatically elevated in CSF and serum in children after severe TBI – Hypothermia therapy did not alter serum GFAP levels compared with normothermia after severe TBI in children |
– Recommended serum GFAP concentration as a prognostic tool and treatment efficacy assessment for TBI in combined with other biomarkers |
| Bouvier et al. (2012) | Serum S100β | 466 | Mild TBI (n = 424 including 183 with GSC = 15 and no symptoms), moderate/ severe (n = 22),no control | 0–16 years | Within 3 h post injury | – Found serum S100β to be capable of discriminating between patients with normal and abnormal CT scans with high sensitivity (100%) and relatively low (33%) specificity – S100β cut off values were used: 0.35 μg/L for age 0–9 months,0.23 μg/ L for age 10–24 months, and 0.18 μg/L for age > 24 months |
– Recommended serum S100β as potential diagnostic tool to reduce the number of required CT scans |
| Berger et al. (2012) | Serum S100β, NSE, MBP, UCH-L1 | 49 | Mild (n = 11), moderate (n = 12), severe (n = 16), control (n = 10) | 1 week-12.4 years (4.1 ± 3.8 years) | Within 24 h of hospital admission | – Correlation between serum UCH-L1 (not NSE, MBP, S100β) and GSC scores, suggesting it may have potential in predicting injury severity and outcomes | – Recommended serum UCH-L1 as potential prognostic tool, may be useful in assessing outcome after moderate and severe pediatric TBI |
| Žurek and Fedora (2012) | Serum S100β, NSE, GFAP, NF-H (Hsp70) | 63 | TBI (n = 63), no control | 0–19 years | Within 12 h post injury, daily up to 6 days | – NF-H in significant amounts in the blood of children with TBI and grew significantly faster in patients who had worse outcomes or died – Higher initial serum NSE, S100β, GFAP levels in patients who died or had worse outcomes – 2nd peak for GFAP 3–4 days post TBI – Studied temporal profile by serial sampling & found S100β, GFAP peaked within 12 h,NSE peaked later within1–2 days post-TBI and NFH continued to increase even 6 days post TBI |
– Recommended these serum biomarker as potential prognostic tools to predict outcomes of TBI in children as their serum levels seems to correlate with mortality |
| Babcock et al. (2012) | Serum S100β | 109 | Mild (n = 94), moderate/severe (n = 15), no control | < 19 years (14.6 ± 4.0) | Within 6 h post injury | – Higher serum S100β for TBI patients with abnormal CT scans than with normal CT – Found serum S100β levels to correlate with TBI severity in children. – S100β was a fair predictor of abnormal cranial CT results for children with all severities of TBI except children with mTBI and a GCS of 15 – Determined S100β cut-off value of 0.006 μg/L |
– Recommended serum S100β to be used as diagnostic tool for predicting abnormal cranial CTs in children with TBI except for mTBI with GSC = 15 |
| Babcock et al. (2013) | Serum S100β | 76 | Mild (n = 76), no control | ≥ 5–18 years | Within 6 h post injury | – Did not find an association between S100β levels and post-concussion syndrome (PCS) for children with mTBI | – Did not recommend serum S100β to be used as a prognostic tool for children with mTBI |
| Mannix et al. (2014) | Serum GFAP | 13 | Concussion (n = 13), no control | 11–17 years | One within 24 h and one withig 24–72 h post-TBI | – Initial GFAP levels (within 24 h) to be correlated with initial and follow-up symptom burden up to 1-month post TBI – Did not find follow-up GFAP levels (within 24–72 h) to be correlated with outcomes – Average GFAP within 24 h post-concussion 0.12 ng/mL and 0.04 ng/mL within 24–72 h post-concussion (no ICI when imaging was obtained) |
– Recommended serum GFAP as a potential prognostic tool for objective measure of injury and recovery after pediatric concussion |
| Manzano et al. (2016) | Serum S100β | 73 | Mild (n = 73), no control | n = 9 < 2 years, n = 64 2–16 years | Within 6 h post injury | – Found higher sensitivity for serum S100β in predicting abnormal CT scans in children > 2 years in comparison to children < 2 years – Found higher serum S100β for patients with intracranial injury (ICI) as evidenced abnormal CT scans than without ICI – Used S100β cut-off value of 0.14 μg/L |
– Recommended serum S100β to be used as diagnostic tool with high sensitivity and poor specificityfor abnormal CT, helpful to rule out CTs but not to be used as a sole marker due to poor specificity performance |
| Papa et al. (2015) | Serum GFAP | 257 | Mild (n = 254), moderate (n = 3), trauma control without head trauma (n = 60) | 2 weeks-21 years (n = 56 < 5 years) | Within 6 h post injury | – Found serum GFAP (6 h post-TBI) correlated with injury severity and CT results – Determined GFAP cutoff level of 0.15 ng/mL for detecting ICI – Found higher GFAP serum levels in subjects with ICI on CT scans compared to those without ICI (with TBI-no ICI or with other trauma) |
– Recommended serum GFAP as a diagnsotic tool for detecting ICI as even for infants and toddlers (< 5 years) and mTBI with GSC = 15 |
| Papa et al. (2016) | Serum GFAP & S100β | 155 | Mild (n = 112), moderate (n = 2), trauma control without head trauma (n = 41) | 6 months-21 years (n = 56 < 5 years) | Within 6 h post injury | – GFAP performed better than S100β in diagnosis of TBI and predicting intracranial lesions on head CT specially for children < 10 years and even better for children < 5 years – Determined GFAP cutoff level of 0.15 ng/mL and S100β cutoff level of 0.020 ng/mL for detecting ICI |
– Recommended serum GFAP over S100β as diagnsotic tool of TBI particullarly for young children |
| Rhine et al. (2016) | Serum GFAP & UCH-L1 | 45 | Mild TBI (n = 25), orthopedic injury (OI)(n = 20) | 11–16 years | Within 6 h post-TBI & 3 more times over 1 moth post-TBI | – GFAP but not UCH-L1 to be significantly higher acutely following mTBI compared to orthopedic injury – Did not find GFAP or UCH-L1 capable of predicting 1 month post-TBI outcomes |
– Recommended GFAP (but not UCH-L1 specially in presence of non-TBI trauma) as a potential diagnostic tool for children with mild TB |
| Mondello et al. (2016b) | Serum S100β, GFAP, UCH-L1 (and MBP) | 85 | Mild (n = 20), moderate (n = 6), severe (n = 19), control (n = 40) | < 15 years (3.8 ± 3.7 years) | 0.5–20.6 h post injury | – Serum UCH-L1 and GFAP performed better than S100β and MBP in prediction of 6-months post-injury outcomes – Found higher GFAP and UCH-L1 serum levels in TBI patient compared with controls – Correlations between serum UCH-L1 and GFAP levels and severity of injury – GFAP and UCH-L1capable of discrimiate mild TBI from control – UCH-L1 but not GFAP capable of discriminating patients with and without ICI – Determined UCH-L1 cut-off value of 0.09 ng/mL for ICI detection |
– Recommended serum UCH-L1 and GFAP as diagnostic tools to discriminate between TBI vs contol and as potential prognostic tools for predicting long-term outcome – Recomended serum UCH-L1 as a diagnostic tool to detect ICI |
| Papa et al. (2017) | Serum UCH-L1 | 256 | Mild (n = 253), moderate (n = 3), control (n = 60) | 2 weeks-21 years (n = 56 < 5 years) | Within 6 h post injury | – Higher UCH-L1 serum levels in subjects with ICI on CT scans compared to those without ICI (with TBI-no ICI or with other trauma), no significant difference between mild TBI without ICI and non-TBI trauma control – Determined UCH-L1 cut-off level of 0.18 ng/mL for detecting ICI |
– Recommended serum UCH-L1 as a diagnsotic tool for detecting ICI as evidanced by CT scans, even for infants and toddlers (< 5 years) and mTBI with GSC = 15 |
| Johnson et al. (2018) | Saliva microRNA | 59 | Concussion (n = 59) | 7–21 years | Within 14 days | – Found 5 miRNAs (miR-320c-1, miR-133a-5p, miR-769–5p, let-7a-3p, and miR-1307–3p) levels in saliva capable of identifying concussion prolonged symptom | – Recommended salivary microRNA as a potential tool to identify to identify prolonged concussion symptoms |
| Park et al. (2018) | Serum S100β and NSE | 10 | mild/moderate TBI (n = 5), moderate/severe TBI (n = 5), no control | 6–18 years | With in 6 h post injury, and 1-week post injury | – Elevated serum S100β and NSE levels within 6 h after TBI decreased at 1 week after trauma – Found serum S100β level at 1 week after TBI to be correlated to the severity of brain damage |
– Recommended S100β and NSE serum biomarker as potential peognostic tools to predict and monitor outcomes of TBI in children |
| Park and Hwang (2018) | Serum S100β, NSE, and IL-6 | 15 | TBI (n = 15), no control | 4–18 years | Within 6 h post injury, and 1-week post-injury | – Levels of S100β and NSEwithin 6 h post TBI were related to injury severity and at 1 week after TBI were related to 6-month post TBI outcomes – Elevated serum S100β, NSE, and IL-6 levels within 6 h after TBI decreased at 1 week after trauma |
– Recommended serial sampling of serum S100β and NSE (but not IL-6) as potential prognotic tools for assessing injury severity and predicting long-term outcomes in pediatric TBI |
| Hicks et al. (2018) | Saliva and CSF microRNA | 129 | Severe TBI (n = 8 for CSF), mild TBI (n = 61, for saliva), control (n = 60, for saliva) | 4–21 years | Saliva sampled within 14 days post-TBI, CSF sampled: 1, 4–7, & 8–17 days post TBI | – Six miRNAs showed parallel alternation in saliva after mild TBI and in CSF after severe TBI (miR-182–5p, miR-221–3p, mir-26b-5p, miR-320c, miR-29c-3p, miR-30e-5p) – microRNA in saliva were capable of distinguish mild TBI from control – Found 135 microRNA in saliva after mild TBI |
– Recommended Salivary microRNA as a potential diagnostic biomarker for TBI but more studies are required |
| Clinical studies – EEG | |||||||
| Mizrahi and Kellaway (1984) | EEG measures | 660 | Concussion with LOC | 3 mos.–15 years | At least 6 months post injury | Resting EEG abnormalities correlated with severity of concussion | – Recommend the use of EEG as a tool to measure and monitor pediatric concussion |
| Gaetz et al. (2000) | EEG measures | 60 | Sport related concussion (n = 15 one concusison, n = 15 two concussions, n = 15 with 3+ concussions), Control (n = 15) | 16–21years | At least 6 months post injury | Longer P3 latency for 3+ concussion groups in comparison to control for visuall oddball task | – Recommend P3 as an EEG feature of increased severity of injury |
| Kiefer et al. (2015) | EEG measures | 1 | Sport related concussion | 15 years | 36 h before injury, 18 h, 21d, 50d, 116d | Brain Network Activation algorithm demonstrated improved scores over time and after subject returned to play during auditory oddball task | – Recommend multivariate EEG measures as a potential tool to evaluate brain function over time |
| Moore et al. (2015) | EEG measures | 30 | Sport related concussion (n = 15), Control (n = 15) | 8–10 years | At least 6 months post injury | Decreased N1 amplitude and prolonged N2 latency for Switch Task, decreased P3 amplitude and prolonged N2 for Go-NoGo Using a visual Go/No-Go | – Recommend N1, N2, and P3 features for EEG analysis of concussed children |
| Howell et al. (2018) | EEG, SCAT-3, Reaction time | 364 | Sport related concussion | 8–18 years | 7 ± 2.5 days after injury | Using a visual Go/No-Go, Brain Network Activation score for relative time between visual events found to be moderately correlated with average walking speed | – Recommend analyzing EEG with dual task gait schemes |
| Jacquin et al. (2018) | EEG, SCAT-3, Reaction time | 364 | Sport related concussion (n = 177), Control (n = 187) | 13–25 years | Day 0 (time of injury), Day 5, Return To Play date, 45 days after RTP | Multimodal Brain Function Index (eBFI) able to detect differences between control and concussed group during resting EEG | – Recommend multivariate, multimodal analysis combining EEG and other measures as a potential tool to detect and monitor concussion |
| Baillargeon et al. (2012) | EEG measures | 96 | Sport related concussion (n = 48), Control (n = 48) | 9–27 years | Within 1 year after injury | No differences in P3a amplitude and latency. Main effect of age on amplitude and latency for P3b for visual oddball task | – Recommend age specific P3b as a potential measure for concussion detection and monitoring |
| Clinical studies – actigraphic measures, gait and eye response | |||||||
| Kaufman et al. (2001) | Actigraphic measures | 34 | Mild head injury (n = 19), Control (n = 15) | 10–17 years | 4 years post injury | Actigraphic recordings were able to detect poorer sleep quality, lower sleep efficiency, and more time awake for the injured group | – Recommend the use of actigraphy as an indicator of sleep quality after concussion |
| Milroy et al. (2008) | Actigraphic measures | 48 | Concussion (n = 18), Orthopedic Control (n = 30) | 7–12 years | At least 6 months post injury | No significant differences were reported for sleep disturbances between groups | – Actigraphy measures may not be specific to brain injury, and polytrauma may be a cofounding factor when interpreting data |
| Katz-Leurer et al. (2008) | Gait parameters | 48 | TBI (n = 24), control (n = 24) | 7–14 years (8.7 ± 3.5 years) | 3–12 months | – Significantly shorter step length, longer step time, higher hip abductor and knee extensor strength values and higher variability of step length and time post-TBI | None |
| Tham et al. (2015) | Actigraphic measures | 100 | Concussion (n = 50), Control (n = 50) | 12–18 years | At least 12 months post injury | – Actigraphy measures were able to detect poorer sleep quality, shorter sleep durations, and more active minutes during the night for injured group | – Recommend the use of actigraphy |
| DiCesare et al. (2017) | Eye response | 49 | Sport related TBI (n = 17 recent and n = 15 days after injury) Control(n = 17) | 16.8 ± 1.2 years | 7.67 days after injury | – Significant change in saccade latency and fixation error and initial fixation error | – Recommended the eye-tracking analysis scheme can be used for accurate diagnosis and prognosis |
| Preclinical studies – biofluid biomarkers | |||||||
| Robinson et al. (2016) | Serum GFAP, serum cytokines including TNFα, IL-1β, IL-6, IL-10, gait parameters | 125 | CCI TBI model (n = 76), control/sham (n = 49) | P12 rat equivalent to several-month-old infant human age stage | 3 days post-TBI or 13–16 days post-TBI (chronic group) | – Functional outcomes post TBI were reflected in serum and imaging biomarkers using P12 rat CCI model of infant TBI – Significant elevation of serum GFAP and TNFα 3 days post-TBI |
– Recommended combination of imaging and serum biomarker using pre-clinical models as a potential tool for therapeutic interventions and efficacy assessment tool |
| Hajiaghamemar et al. (2017) | Serum amino acids (17 amino acids) | 25 | CCI TBI (n = 12), RNR (n = 13), pre-injury as control (n = 25) | 4 weeks piglets equivalent to toddler human stage | Pre injury, at 24 h or 4 days post-TBI | – Combination of Glycine, Taurine, and Ornithine aminoacids as optimal TBI diagnosis for focal-diffuse TBI | – Recommended a panel of biomarker rather than single biomarker as a potential diagnostic tool for TBI |
| Preclinical studies – EEG | |||||||
| Ibrahim et al. (2010) | EEG measures | 5 | Low and moderate level axial plane rapid non impact rotation (RNR), | 4 weeks piglets equivalent to toddler human stage | 6 h post injury | Suppression of resting EEG activity after moderate injury and remained up to 6 h post injury | – Recommend EEG as a tool to monitor concussion in the piglet model |
| Atlan et al. (2018) | EEG measures | 22 | Rapid non impact rotation (RNR), Controlled cortical injury (CCI) | 4 weeks piglets equivalent to toddler human stage | Pre, 1, 4 or 5, 7 days | Decreased N40 and P60 amplitude and longer latency on days post injury in comparsion to pre injury day for auditory oddball paradigm | – Recommend auditory oddball paradigm for use to study concussion in piglet model of brain injury |
| Sabir et al. (2015) | Electrocorticoraphic measures | 14 | Weight drop injury (n = 7), Sham (n = 7) | 10–21 weeks mice equivalent to toddler human stage | Day 1 and 2 post injury | Brain injured mice had a decreased ability to stay awake and were reflected in spectral activity measures | – Electrocorticographic measures are sensitivity to sleep disruptions post-concussion, however implanted electrodes are invasive |
| Preclinical studies – actigraphic measures, gait and eye response | |||||||
| Jaber et al. (2015) | Balance parameters | 25 | CCI and RNR piglets | 4 weeks piglets equivalent to toddler human stage | pre,1–6, 24 h | – Significant increase in the root mean square acceleration in the anterior- posterior and medial-lateral directions | – Recommended bispectral index and postural sway as diagnostic tools to assess brain injury deficits in a piglet model of TBI |
| Olson et al. (2016) | Actigraphic measures | 30 | CCI (n = 8), RNR (n = 9), Sham (n = 6), Naïve (n = 7) | 3–4 weeks piglets equivalent to toddler human stage | Day 4–6 post injury | Injured piglets had greater periods of inactivity during the day and greater active periods during the night | – Recommend the use of accelerometer based actigraphic measures as an indicator of sleep quality for concussion in piglets |
| Baker et al. (2018) | Balance parameters | 16 | CCI piglets | 3- & 6-weeks piglets equivalent to toddler human stage | Pre, 1, 3, 7 days | Significant decrease in stride velocity and 2-limb support | – Recommended gait parameters as diagnostic tool |
3.1. Biofluid biomarkers
TBI can cause disruption at cellular and sub-cellular levels such as neurons, astrocyte and microglial cells, vasculature and extracellular matrix. These disruptions can initiate a variety of neurotransmitter, metabolomic, mitochondrial, and ionic dysregulations which have been shown to be reflected in affected brain tissues as well as biofluids such as serum, cerebrospinal fluid, saliva and urine (Baker et al. (2018); Gazzolo et al., 2003; Giza and Hovda, 2014; Kilbaugh et al., 2016; Margulies et al., 2015; Smith et al., 2013). The reflections in different biofluids showed to be dynamic, interactive, and dependent on type, severity, and progression of injury. Therefore, biofluids are treasure trove of injury related information and are valuable surrogates to be used as potential diagnostic, prognostic, or monitoring, and even therapeutic efficacy assessment tools for TBI. Biofluid biomarkers are non/minimally invasive and effort-independent techniques that can be studied in both animals and humans, and thus, they are of great value to serve as a bridge between pre-clinical and clinical findings and advance diagnosis, management and treatment of TBI. Biomarker discovery are particularly invaluable for pediatric and especially neonatal population as radiation from routine CT imaging can be harmful for this group (Brenner and Hall, 2007; Thelin et al., 2017b). Although CSF is in direct contact with the extracellular space of the brain and can more prominently reflect biochemical alternations in brain due to TBI, blood-based biomarkers are less invasive, cost effective, and more feasible and acceptable for patients especially in the pediatric population. Therefore, serum TBI biomarkers have been studied more extensively than CSF biomarkers. There are also limited studies available on the utility of biomarkers in urine (Pickering et al., 2008) and saliva (Hicks et al., 2018) for pediatric TBI. However, it is challenging to utilize urine and saliva to diagnose TBI due to the fact that biomarkers have to cross a number of barriers to appear in these biofluids. In this section, we will mainly focus on TBI biomarkers in serum and briefly review TBI biomarkers in urine and saliva.
To date, numerous clinical and pre-clinical TBI studies have examined several brain-related and injury sensitive biofluid biomarkers that are linked to dynamic changes in metabolism, extracellular matrix, glial, axonal or neuronal damages, and/or neuroinflammation over time following TBI. Among those are S100β and glial fibrillary acidic protein (GFAP) that are related to glial damage; Hyperphosphorylated neurofilaments (NFs: NF-L and NF-H) and Tau that are related to axonal damage; Ubiquitin Carboxy-terminal Hydrolase L1 (UCH-L1) and Neuron-Specific Enolase (NSE) that are related to neuronal cell damage; Interleukins (IL-1β, IL-6, IL-8, IL-10) and Tumor Necrosis Factor-α (TNFα) that are related to inflammation, and amino acids and other metabolites that are related to energy deficits following TBI.
3.1.1. S100β
S100β is a small calcium binding protein expressed mainly in astrocytes and certain neuronal cell types and is the most frequently explored biomarker for TBI diagnosis (Babcock et al., 2012; Bouvier et al., 2012; Castellani et al., 2009; Hallén et al., 2010; Manzano et al., 2016; Pickering et al., 2008) and prognosis (Babcock et al., 2013; Park et al., 2018; Park and Hwang, 2018; Piazza et al., 2007; Spinella et al., 2003; Žurek and Fedora, 2012) in pediatric clinical studies (Table 1). Serial serum sampling in clinical studies showed that S100β elevates and declines quickly following TBI and is present for a short time in serum with a half-life of an hour to a day (Park et al., 2018; Thelin et al., 2017b; Žurek and Fedora, 2012). Pre-clinical studies using different models of TBI such as CCI (Cardinell, 2017), WDI (Ma et al., 2018), and blast (Ahmed et al., 2015) also reported significant alternation of S100β serum concentration following TBI. These studies focused on adult animals and, to our knowledge, none of the pre-clinical studies investigating S100β focused on infant/pediatric TBI.
For diagnostic purposes, most pediatric studies (Table 1) focused on investigating the capability of S100β to detect intracranial lesions as evidenced by abnormal CT scans. Higher serum concentration of S100β were found in patients with abnormal CT scans compared to control cohorts or patients with normal CT. Therefore, it is suggested that serum S100β is a potential diagnostic tool for predicting intracranial lesion and abnormal CT in children with TBI (Babcock et al., 2012; Bouvier et al., 2012; Castellani et al., 2009; Hallén et al., 2010; Manzano et al., 2016). Although S100β serum concentration is limitedly in clinical use for adults as a general screening tool to identify those in need of CT scanning (Thelin et al., 2017b), it has not yet been studied and validated extensively enough to be recommended for clinical use in the pediatric population (Lumba-Brown et al., 2018). The literature on S100β serum biomarker in pediatric TBI is not as rich as it is for adult and the cut-off values for abnormal S100β are diverse in the pediatric literature (0.006–0.35 μg/L, Table 1). These diverse results may be explained by the fact that brain tissue and biofluid expression of S100β are highly age-dependent, particularly in the very young age due to the ongoing central neurodevelopmental process (Modi and Kanungo, 2010; Park et al., 2018; Portela et al., 2002). The large age variation in most current pediatric TBI studies (Babcock et al., 2012; Castellani et al., 2009; Geyer et al., 2009; Lugones et al., 2018; Manzano et al., 2016) (Table 1) is another factor that contributes to the diverse cut-off value results in the literature. Another explanation is that S100β is not a brain-specific biomarker and may be derived from extracranial sources such as musculoskeletal injury and bone fracture (Agoston et al., 2017; Rothermundt et al., 2003). Some of the pediatric TBI studies used healthy subjects as control while some others used patients with limb or fracture injury as control cohort. The S100β threshold value associated with TBI detection is still debated even for adults (Kövesdi et al., 2010) in whom S100β were shown to be less sensitive with age (Modi and Kanungo, 2010). Non-brain specificity and high age dependency of S100β in the young brain make it difficult to determine an optimal cut-off value sensitive and specific enough to reliably diagnose TBI in infant and pediatric populations. The utility of S100β serum biomarker to distinguish iTBI from nTBI has also been investigated in a few pediatric studies (Beers et al., 2007; Berger et al., 2005) and did not find significant difference between peak serum concentration of S100β or NSE in children from either cause. However, Beers et al. (2007) and Berger et al. (2005) found significant longer time to peak for iTBI patients compared to nTBI patients and therefore, suggested time to peak of S100β and NSE as potential tools for discriminating iTBI from nTBI in pediatrics.
For prognostic purposes, many pediatric studies investigated the peak or temporal profile of S100β concentration following TBI to determine the severity of injury and/or predict short- or long-term outcomes of TBI (Babcock et al., 2013; Berger and Kochanek, 2006; Park et al., 2018; Park and Hwang, 2018; Piazza et al., 2007; Spinella et al., 2003; Žurek and Fedora, 2012) (Table 1). The results of these studies are sometimes conflicting, with some showing correlation between S100β levels early after TBI (within a day) (Berger and Kochanek, 2006; Spinella et al., 2003; Žurek and Fedora, 2012) or 1-week post-TBI (Park et al., 2018; Park and Hwang, 2018) and the long-term TBI outcomes while others did not find any correlation especially in the presence of extracranial injury (Babcock et al., 2013; Piazza et al., 2007). The conflicting results may be explained by the fact that S100β is not a brain specific marker. The elevation of serum S100β following TBI has been shown to be an indication of blood-brain barrier (BBB) disruption, which has a critical role in pathophysiology outcomes of TBI even years later (Blyth et al., 2009; Kanner et al., 2003) that may explain the correlation of serum S100β and the long-term post-TBI outcomes. Studies demonstrated that the rate of decline of serum S100β inversely correlates with severity of TBI, with a slower decline for more severe TBI in both pediatrics (Žurek and Fedora, 2012) and adults (Thelin et al., 2017a).
In addition to serum, S100β urine concentrations has been investigated as diagnostic and/or prognostic TBI biomarker in a few pediatric studies (Berger and Kochanek, 2006; Hallén et al., 2010) but the results are inconclusive. Hallén et al. (2010) found no significant difference in urine S100β concentrations between pediatric TBI patients with and without intracranial complications while they found a significant difference in serum S100β concentrations between these two groups. Contrarily, Berger and Kochanek (2006) found an increase in both urinary and serum S100β concentrations in children with TBI. They reported that peak urinary S100β concentrations occurred significantly later than peak serum S100β concentrations.
In summary, although S100β is the most widely studied biomarker in pediatric TBI, the non-brain specificity and high age-dependency attributes in the young brain suggest that S100β is not an appropriate biomarker to be used as a sole tool for diagnosis of TBI in pediatric population. However, due to its rapid release into blood following TBI, it is a valuable biomarker to be combined with other biomarkers and tools in clinical practice today for early diagnosis of TBI especially in the absence of extracranial injuries.
3.1.2. Glial fibrillary acidic protein (GFAP)
GFAP, a marker of astroglial injury, is another well studied TBI biomarkers explored in many pediatric clinical studies (Fraser et al., 2011; Mannix et al., 2014; Mondello et al., 2016b; Rhine et al., 2016; Žurek and Fedora, 2012) as well as infant/pediatric pre-clinical studies (Robinson et al., 2016) (Table 1) and has been shown to be dramatically elevated in serum following TBI. GFAP showed a rapid influx, but slower than S100β, in serum following TBI and remained elevated for a longer time in comparison to S100β (Thelin et al., 2017b; Žurek and Fedora, 2012), which provides a wider time window for this biomarker to diagnose TBI, and thus, reduces the probability of missing detection due to late blood sampling. Although serum GFAP and S100β are both linked to glial injury, GFAP performs better than S100β in detecting head trauma and predicting intracranial lesions on head CT in pediatric TBI especially in young children (< 5 years) and in presence of extracranial injuries (Papa et al., 2016). This enhanced predictive performance may be attributed to the fact that GFAP, in contrast to S100β, is a CNS specific protein (Mondello et al., 2016b; Papa et al., 2015). Several studies recommended serum GFAP to be used as a diagnostic marker of TBI specially for detecting intracranial hemorrhage (Kou et al., 2013; Mondello et al., 2011; Mondello et al., 2016b). Serum GFAP also found to be capable of discriminating between focal and diffuse TBI in adults as its elevation was shown to be significantly higher in focal and/or hemorrhagic injury than in non-hemorrhagic and/or diffuse brain injury (Kou et al., 2013; Mondello et al., 2011).
On the prognostic applications, serum GFAP concentration was shown to be predictive of TBI-induced brain pathology (Huang et al., 2015; Mondello et al., 2016a), injury severity and poor long-term post-TBI outcomes (Fraser et al., 2011; Mondello et al., 2016b). All the pediatric studies explored GFAP (Table 1) except one (Rhine et al., 2016) reported that GFAP peak values within 24 h correlated with severity of injury and long-term post-TBI outcomes. Although both acute disintegration of astrocytes and reactive astrogliosis underlie circulating GFAP in serum and CSF after TBI, tissue pathology assessment of pre-clinical TBI models revealed that the acute disintegration of astrocytes is the dominant source of GFAP elevation in serum and CSF following TBI (Huang et al., 2015). This mechanism is consistent with the rapid and high elevation of serum GFAP which peaks within hours to a day following TBI (Fraser et al., 2011; Luoto et al., 2017) and has been attributed to early astrocyte damage that occurs within hours after injury (Huang et al., 2015; Zhao et al., 2003), whereas reactive astrogliosis occurs days post-TBI (Hellewell et al., 2010). A secondary peak in serum GFAP that has been observed a few days post-TBI (Fraser et al., 2011; Žurek and Fedora, 2012) may be due to the increase in reactive microglia.
The brain specificity of GFAP, its rapid and high elevation in serum following TBI, and the consistency of the finding in the literature (Table 1) suggest that serum GFAP is a suitable biomarker to be used as a TBI diagnostic and/or prognostic tool in pediatric population, especially if it combines with other biomarkers with different cellular origins and temporal profiles. However, more studies need to be done to better determine its predictability and characteristics to support the use of it in clinical practice.
3.1.3. Ubiquitin carboxy-terminal hydrolase L1 (UCH-L1)
UCH-L1 is a neuronal injury marker and similar to S100β and GFAP showed a rapid influx and short time availability following TBI; however, UCH-L1 has a faster decline in comparison to GFAP (Huang et al., 2015; Kou et al., 2013; Mondello et al., 2011; Thelin et al., 2017a). Serum UCH-L1 has been explored in several pediatric clinical studies (Berger et al., 2012; Mondello et al., 2016b; Papa et al., 2017; Rhine et al., 2016) (Table 1) and adult pre-clinical studies (Huang et al., 2015; Mondello et al., 2016a) as a potential biomarker of TBI. Mondello et al. (2016b) and Papa et al. (2017) found serum UCH-L1 capable of identifying TBI patients with intracranial lesions as evidenced by abnormal CT from TBI patients with normal CT, uninjured (Mondello et al., 2016b) or non-TBI trauma control subjects for mild to severe TBI and suggested serum UCH-L1 as a potential diagnosis biomarker of intracranial lesions. However, Rhine et al. (2016) and Berger et al. (2012) did not find significant difference in serum UCH-L1 levels between pediatric patients with mild TBI and uninjured/non-TBI trauma controls.
On the prognostic application, Mondello et al. (2016b) and (Berger et al., 2012) found correlation between early elevation of UCH-L1 in serum and injury severity and long-term post-TBI outcomes. Pre-clinical studies using different models of TBI also showed direct relation between lesion volume, neuronal degeneration and astrocyte damage of brain tissue, and temporal profile of GFAP and UCH-L1 biomarkers in serum and CSF post-TBI (Huang et al., 2015). Studies that compared GFAP and UCH-L1 and/or S100β showed that GFAP is a better diagnostic biomarker for TBI in comparison to UCH-L1 and S100β especially in the presence of extracranial lesions as it is a CNS specific protein while UCH-L1 and S100β are not (Huang et al., 2015; Kou et al., 2013; Lewis et al., 2017; Papa et al., 2016; Rhine et al., 2016). For example, serum UCH-L1 showed to be elevated for sham-TBI animals in the presence of anesthesia and surgical procedure while no elevation was observed for GFAP (Huang et al., 2015). However, GFAP and UCH-L1, have different temporal profiles and cellular origins, therefore, multivariate analysis may enhance the predictability of each of them individually.
The utility of UCH-L1 in urine as a biomarker of brain injury has been also explored in adult TBI and no significant difference between controls and patients with white matter lesions in UCH-L1 levels in urine were reported (Li et al., 2015). To date, no study has been explored urine UCH-L1 for pediatric TBI.
3.1.4. Neuron-specific enolase (NSE)
NSE is a glycolytic enzyme which is localized predominantly in the cytoplasm of neurons. Following TBI, NSE releases passively into the extracellular compartments under pathological conditions during neuronal cell destruction (Kövesdi et al., 2010) and then leaks into CSF and serum following neuronal cell death secondary to traumatic injury (Berger et al., 2002; Žurek and Fedora, 2012). This mechanism makes serum NSE a great biomarker candidate for monitoring ongoing injury after TBI (Park et al., 2018) and have been examined as TBI biomarker in many pediatric (Beers et al., 2007; Berger et al., 2005; Berger et al., 2007; Berger et al., 2012; Fridriksson et al., 2000; Park et al., 2018; Park and Hwang, 2018) and adult clinical studies (reviewed in (Thelin et al., 2017b)) as well as adult pre-clinical studies (Gyorgy et al., 2011). NSE like GFAP has a prolonged serum elevation post-TBI, however, serum NSE elevation has a longer time to peak and slower decline profile especially for more severe cases or cases with poor outcomes (Park et al., 2018; Thelin et al., 2017b; Žurek and Fedora, 2012). NSE with a serum half-life of 24–72 h has a longer temporal profile in comparison to S100β, UCH-L1 and GFAP (Beers et al., 2007; Thelin et al., 2017b; Žurek and Fedora, 2012) which makes it a better prognostic biomarker for possibly predicting outcomes and monitoring treatment effects. Slower elevation to peak NSE values, and appearance of a secondary peak in NSE temporal profiles in patients with progressing injury may also be attributed to delayed neuronal death following TBI (Bandyopadhyay et al., 2005; Park et al., 2018; Žurek and Fedora, 2012). Serial sampling in several pediatric studies showed that peak and time to peak of NSE concentrations correlate with short and long-term post-TBI outcomes in children with varying severity (Bandyopadhyay et al., 2005; Beers et al., 2007; Berger et al., 2007; Park et al., 2018; Žurek and Fedora, 2012). One of these studies showed stronger correlation of peak NSE concentration with outcome in children < 4 years of age (Berger et al., 2007). Many pediatric studies demonstrated the capability of NSE as predictor of intracranial injury and poor outcome following TBI (Bandyopadhyay et al., 2005; Beers et al., 2007; Berger et al., 2007; Park et al., 2018). Like UCH-L1 and S100β, NSE is not CNS specific and is also present in red blood cells and platelets which limits its utility as a predictor of TBI in multi-trauma cases (Johnsson et al., 2000). Although NSE is a marker of neuronal injury, histopathology of damaged brain tissue showed that NSE can also be an effective marker of axonal injury in its early stages (Ogata and Tsuganezawa, 1999). It is reported that after axonal injury, NSE is upregulated to maintain homeostasis, and thus, NSE can be detected in cytoplasm surrounding the disrupted axons (Ogata and Tsuganezawa, 1999; Yokobori et al., 2013). NSE serum levels in children with diffuse brain injury were twice as high as those with focal brain injury (Lo et al., 2009), and thus, this biomarker can discriminate these two types of TBIs. NSE has been shown to be age-independent in the pediatric population (Berger et al., 2005). Žurek and Fedora (2012) reported that NSE serum concentration was much higher and remain elevated for longer time after severe TBI in children compared to adults which may be an indication of more neuronal cell death and higher susceptibility of developing brains to TBI in compare to adults.
3.1.5. Neurofilaments (NFs)
NFs are the most abundant protein components of the axonal cytoskeleton and consists of three subunits NF-L (light), NF-M (medium), and NF-H (heavy) containing 543, 916 and 1020 amino acids, respectively (Petzold, 2005). NFs play an important role in the maintenance of axon caliber, growth of axons during development, and conducting of electrical impulses along axons (Yuan et al., 2012). NFs are proved markers of axonal injury that have been shown to accumulate in discrete regions of the axon following cytoskeleton damage resulting in swollen bulbs, disconnection and additional neuropathologic changes (Smith and Meaney, 2000). However, there are presently a limited number of studies that have examined serum level of NFs as biomarkers of TBI in adult (Al Nimer et al., 2015) and pediatric clinical studies (Žurek and Fedora, 2012) as well as adult rat pre-clinical studies (Anderson et al., 2008; Shaw et al., 2005). Serum NFs remained elevated days after TBI in mild to severe cases but rose faster in severe cases or those with poor outcomes, suggesting that NFs may be of good prognostic value (Anderson et al., 2008; Žurek and Fedora, 2012). More studies are needed to evaluate the utility of NFs as biomarkers of TBI and to characterize their temporal profile.
3.1.6. Neuroinflammatory and metabolomic biomarkers
Besides biomarkers such as S100β, GFAP, UCH-L1, NSE, NF-L, NF-H that are linked to primary injury because of neuronal, glial, and/or axonal damages, there are biomarkers that are linked to the sequalae of metabolomic and inflammatory events following TBI which lead to energy deficits, brain tissue damages and atrophy evolving over hours, days, months, and even years. Therefore, metabolomic and neuroinflammatory biomarkers are linked to secondary pathology after TBI and underlie some of the acute and chronic neuropathological outcomes of TBI (Nizamutdinov and Shapiro, 2017).
Among neuroinflammatory biomarkers are cytokines that can be released by a wide variety of cells such as microglia, macrophages, and endothelial cells and play an important role in repair and maintenance of brain function after TBI, and thus, influence the secondary injury (Sordillo et al., 2016). Cytokines including TNFα, IL-1β, IL-6, IL-8, IL-10, and L-selectin are some of the common neuroinflammatory biomarkers that explored in a few pediatric clinical TBI studies (Buttram et al., 2007; Chiaretti et al., 2005; Park and Hwang, 2018) and pediatric pre-clinical studies (Robinson et al., 2016). These cytokines showed to be elevated in injured brain tissue, CSF and serum following TBI. IL-1β and IL-6 (Lo et al., 2009) and combination of S100β and IL-6 or L-selectin (Castellani et al., 2009) were found to be corelated with severity of injury and was predictive of poor long-term outcomes. From a therapeutic point of view, pre-clinical studies have illustrated that blockade of these cytokines can reduce brain injury (Sordillo et al., 2016). Given the role of neuroinflammatory cytokines in the secondary injury and long-term pathological outcomes of TBI, more studies are needed to evaluate these biomarkers for pediatric TBI and assess their possible age dependency.
Serum metabolite and metabolomic pathways have also shown to be significantly altered after TBI (Bahado-Singh et al., 2016; Hajiaghamemar et al., 2017; Louin et al., 2007; Yi et al., 2016), and their alternations are shown to be correlated with post-TBI neurodegeneration, neurological and cognitive impairments (Louin et al., 2007; Yi et al., 2016). Among metabolites, amino acids are shown to have an important role in neuronal survival, growth and differentiation as well as neuronal circuitry development and maintenance (Kurbat and Lelevich, 2009). Their role is even more significant in the developing brain as they are crucial to provide energy for all cellular processes required for brain development and function. Hence, the additional high metabolic demands following TBI have a synergistic effect and can intensify the outcomes of TBI in the pediatric population (McKenna et al., 2015). Despite the important role of metabolite biomarkers on post-TBI outcomes in pediatric, to our knowledge, these biomarkers have not yet investigated in any pediatric clinical TBI study. In adult TBI population, Yi et al. (2016) investigated the utility of metabolite biomarkers for diagnosis of TBI and identified a panel of nine serum metabolite biomarkers (serine, pyroglutamic acid, phenylalanine, galactose, palmitic acid, arachidonic acid, linoleic acid, citric acid, and 2,3,4-trihydroxybutyrate) capable of discriminating between TBI patients with and without cognitive impairment, and healthy controls. In a pediatric pre-clinical study, Hajiaghamemar et al. (2017) also found a panel of three amino acids capable of diagnosis of focal and disuse TBI with high sensitivity and specificity. In an adult pre-clinical study, plasma concentrations of the amino acid proline were found to be correlated with post-TBI neurological deficit as a sign of brain damage severity, and thus, it has been suggested as a potential TBI monitoring biomarker candidate (Louin et al., 2007). The variety of metabolomic biomarkers and their key roles in dysregulating the normal neuronal developmental process after TBI emphasize their prognostic potentials to predict possible long-term neuronal deficits post-TBI, however more studies to be done in pediatric TBI.
3.1.7. microRNA
MicroRNAs are short non-coding endogenous RNA molecules that play key roles in the regulation of cellular processes such as cell signaling, proliferation, differentiation, survival, and death post-trauma. MicroRNAs recently became of great interest in the biomarker field due to their stability and abundancy in biofluids and their tissue-specific expression patterns. So far over 2000 microRNAs have been identified in the human and many studies have used microRNA profiling in different tissues and biofluids with the purpose of biomarker discovery for trauma and disease such as cancer, cardiovascular diseases, diabetes, nervous system disorders, and TBI (Di Pietro et al., 2018). Utility of microRNAs as TBI biomarkers is at very early stage. There are only handful of studies that have examined serum microRNA signatures as biomarkers of TBI and none of them are specific to children (Bhomia et al., 2016; Di Pietro et al., 2017; Redell et al., 2010; Yang et al., 2016). In addition, a couple of studies have recently explored microRNAs in saliva as TBI biomarkers for pediatric (Hicks et al., 2018; Johnson et al., 2018) and adult (Di Pietro et al., 2018). Hicks et al. (2018) examined alternations of CSF and salivary microRNAs after severe and mild TBIs. They found six microRNAs, functionally related to neuronal development, including miR-182–5p, miR-221–3p, mir-26b-5p, miR-320c, miR-29c-3p, and miR-30e-5p, that demonstrate similar alternation trends in CSF after severe TBI and in saliva after mild TBI. They reported that downregulation of miR-320c were directly correlated with attention difficulty post-TBI and it increased to baseline over time after injury. In the other pediatric study, Johnson and collaborators found concentrations of five salivary microRNAs including miR-320c-1, miR-133a-5p, miR-769–5p, let-7a-3p, and miR-1307–3p capable of distinguishing patients with prolonged symptoms of concussion. They also reported that concentrations of miR-320c-1 were correlated with memory difficulty 4 weeks post injury (Johnson et al., 2018). In another study, 21 microRNAs in saliva including two brain specific microRNAs (miR-27b and miR-142–3p) were reported that can discriminate between concussed and non-concussed adult athletes (Di Pietro et al., 2018). There are some pre-clinical studies that explored microRNA expressions in different brain regions after TBI (Di Pietro et al., 2018). Due to the variety of microRNAs that are expressed uniquely within specific brain regions and cell types and their stability and richness in biofluids, microRNAs have the potential to be ideal biomarkers for TBI. With additional pre-clinical and clinical investigations, biofluid microRNAs may be potential biomarkers for diagnosis and prognosis after TBI.
3.1.8. Biofluid biomarker considerations: clinical and preclinical
One of the challenges in clinical and translational studies of serum biomarkers is the development of baseline levels that represent the normal healthy population and the determination of a cut-off value for TBI prediction. The baseline and cut-off values will be even more difficult to determine when the biomarker is age-dependent. Many brain-related biomarkers such as S100β, GFAP and UCH-L1 are shown to be age dependent in the pediatric population and are significantly higher in younger children specially during the first year of age (Mondello et al., 2016b; Sabbatini et al., 1999). Conversely, NSE serum biomarkers did not show age dependency in children (Berger et al., 2006). Most current TBI pediatric studies were conducted over wide range of ages (e.g. up to 18 years) which may explain the diverse serum cut-off values of age-dependent biomarkers such as S100β reported in the literature. Cut-off values of age-insensitive biomarkers such as NSE is more consistent in the pediatric TBI literature, probably due to age insensitivity of this biomarker (Papa et al., 2015). Overall, age-independent biomarkers have superiority over age dependent ones for pediatric population. For age-dependent biomarkers, normal serum concentrations and TBI cut-off values needs to be determined over a narrow age range to minimize age variability. Age dependency of some serum biomarkers also emphasizes the importance of using age appropriate pre-clinical models in translational biomarker pediatric TBI research to enable discovering compatible and sensitive biomarkers. Within this context, one of important challenges for successful translation of serum biomarkers to clinical diagnostics is difference of the concentration levels and validity of biomarkers cross-species which can be overcome through a direct comparison between animal models and human data (Mondello et al., 2016a).
Another challenge with TBI biomarkers is that many of them such as S100β, UCH-L1, NSE, amino acids and many others are not brain specific. These biomarkers are expressed in other organs and cell types such as endocrine system, endothelial cells, smooth muscle cells, erythrocytes, and peripheral nerves (Agoston et al., 2017) that limits the utility of them as TBI biomarkers in multi-trauma scenarios (Castellani et al., 2009; Geyer et al., 2009; Lugones et al., 2018). In that regard, brain specific serum biomarkers such as NF-L, NF-H and GFAP may perform better in multi-injury events.
All biomarkers discussed in this section showed to pose a distinct temporal profile following TBI which is also dependent on injury type, mechanism and severity. Moreover, many of the biomarkers explored in the literature are not brain specific, however, the temporal profile of these biofluid biomarkers may be different after TBI and injury to other organs. For example, although biofluid S100β elevation was observed in injuries other than TBI, S100β released from an extracerebral origin showed a shorter temporal profile than S100β released due to TBI (Savola et al., 2004). Therefore, coupling the biofluid concentration of these biomarkers with their temporal profiles may enhance the capability of these biomarkers in diagnosing TBI and monitoring the progression of TBI. For example, characteristics of temporal profiles of NSE and S100β serum concentrations such as time to peak and possible secondary peak after TBI were shown to be able to distinguish between children with iTBI and nTBI in mild to severe TBIs (Beers et al., 2007; Berger et al., 2005; Berger et al., 2006). Frequency and time domain of blood sampling have a critical role in determining the accuracy of temporal signatures of biomarkers, especially in translational research where different life spans of animal models and humans need to be taken into consideration.
Unfortunately, longitudinal studies assessing biofluid biomarkers of clinical and pre-clinical pediatric TBI are severely limited, also the sampling frequency and the time points are dispersed among studies (Beers et al., 2007; Robinson et al., 2016; Žurek and Fedora, 2012). Late blood sampling and differences in the time point and frequency of sampling, in part, may explain some of the conflicting results of biomarkers between studies. To overcome these limitations, more longitudinal studies are needed with high frequency sampling in order to characterize the temporal profile of biomarkers so that the underlying molecular and pathological events following pediatric TBI can be elucidated.
3.2. Electroencephalography (EEG)
TBI can disrupt the functional neural processes of the brain resulting in altered electrophysiological states (Rapp et al., 2015). Electroencephalography (EEG) provides a measure of the electrical activity of the brain which can help to monitor the changes in cognitive processing over the course of the TBI, informing on differences between healthy and diseased populations and the time course of recovery (Schmitt and Dichter, 2015). EEG in humans is typically collected non-invasively using surface scalp electrodes capturing the summation of synchronous activity of millions of post-synaptic potentials of the cerebral cortex (Luck, 2014; Rapp et al., 2015). The electrical potential difference between two electrodes establishes a channel. These channels are arranged in a montage that display patterns of electrical activity across the scalp indicative of regional activity or inactivity (Rapp et al., 2015). Due to the lack of structural abnormalities associated with mild traumatic brain injury, EEG provides a non-invasive indicator of brain functional activity on the millisecond scale. EEG research employs the recording of spontaneous activity or evoked potentials in response to an external stimulus. Event related potentials (ERPs) are segments of the continuous EEG signal that are time-locked with an external event stimulus and have been considered a sensitive measure of brain activity after a concussion (Gosselin et al., 2006).
Typical measurement variables associated with EEG are power frequency bands from continuous measurement and amplitude and latency characteristics associated with ERP waveforms. Power frequency bands include: Delta: 0.5–4 Hz, Theta: 4 – 8 Hz, Alpha: 8–12 Hz, Beta: 12–20 Hz, Gamma: 20–80 Hz, where common findings associated with concussion are increased alpha, increased delta, and decreased theta (Ianof and Anghinah, 2017; Kenzie et al., 2017; Nuwer et al., 2005; Oster et al., 2010; Sandsmark et al., 2017). Common ERP components that have been studied for sports-related concussions in human subjects include: N2, found at 200–350 ms with a frontal central distribution on the scalp (Broglio et al., 2009; Gaetz and Weinberg, 2000; Ledwidge and Molfese, 2016; Moore et al., 2015); the P3, found at 300–800 ms with a midline parietal distribution (Baillargeon et al., 2012; De Beaumont et al., 2009; Dupuis et al., 2000; Gaetz et al., 2000; Gosselin et al., 2006; Lavoie et al., 2004; Moore et al., 2016; Moore et al., 2014; Moore et al., 2017; Nandrajog et al., 2017; Ozen et al., 2013; Parks et al., 2015; Theriault et al., 2009); the error-related negativity (ERN) (De Beaumont et al., 2009; Pontifex et al., 2009), typically occurring at 50–100 ms at the midline frontal and central scalp sites; and the error positivity (eP), which occurs at 200–500 ms following the ERN, typically observed in the midline central and parietal scalp areas (Brush et al., 2018; Lesiakowski et al., 2018). General trends across research studies denote a decrease in amplitude and increase in latency for ERP components associated with concussed cohorts in comparison with healthy controls, furthermore the P3 or P300 is the most widely used for injury (Brush et al., 2018). A few studies have suggested the use of a panel of EEG features as part of a multi-modal analysis of concussion that include behavioral measures such as balance and gait to detect and monitor concussion (Howell et al., 2018; Jacquin et al., 2018); however, more evidence is needed to evaluate the robustness of including a collection of measures. The diverse findings on ERP related components are attributed to variability in the type of cognitive tasks used to elicit the ERP responses (i.e., auditory or visual stimuli). Due to the non-uniformity of tests run across studies, a direct comparison is difficult. Small and heterogeneous study samples are common across research studies, often age, injury mechanism, and outcomes are factors that result in underpowered sample sizes that are susceptible to bias, false and inflated effects (Brush et al., 2018).
To control for the variability in subject sample and characteristics, animal models of TBI are a promising avenue to study the mechanisms of concussive injury from injury causation to changes to neural function, intracranial injury mechanisms, and histopathological changes post mortem. Research employing animal models of TBI combined with EEG measures have primarily used rats under lateral fluid percussion injury (Biswas et al., 2018), projectile concussive impact (Leung et al., 2014; Mountney et al., 2017), high deceleration impact system (Napoli et al., 2012), a weight drop model (Ucar et al., 2006), and mice under blast loading (Liu et al., 2017) and also using the weight drop model (Sabir et al., 2015). While differences observed in the power frequency bands between injured and non-injured animals were demonstrated, the measurement of EEG in these studies involved opening up the cranial vault and implanting electrodes directly on the brain. Due to the smaller size of the rat and mouse brain and the invasive nature of electrode implantation in these studies, translation to human TBI is difficult. In addition, EEG research employing rodent models of TBI are not specific to the pediatric age range. Research employing a piglet model of pediatric concussion has demonstrated promise in the utility of this model for clinical measures of concussion using EEG. Large animal models better resemble the biomechanics of brain tissue injury because the gross neuroanatomy and relative composition of white and grey matter tissues are similar between the human and pig brain (Cullen et al., 2016). Atlan et al. (2018) conducted a pilot study on 4-week-old piglets subject to two injury conditions (CCI and RNR in the sagittal and coronal directions). EEG was captured using a non-invasive 32 electrode array placed on the surface of the scalp of each animal to measure responses from an auditory oddball paradigm. Unlike previous animal work employing invasive measurement techniques for studying TBI, the piglet model provides a novel means to capture EEG responses with potential translation to pediatric concussion. Atlan et al. (2018) reported a reduction in the P60 amplitude and an increase in the N40 latency were associated with injured animals.
These studies demonstrate the efficacy of EEG as a diagnostic tool able to detect differences between injured and healthy brains in both human and animals. There is still a need to identify relevant features of the EEG signal that consistently indicates an injured brain in addition to those that are sensitive to differing severities and symptoms across species. Human studies provide a subset of potentially relevant features with which to guide and evaluate the experimental animal work, such as the N1 and P3. However, homologous EEG features that represent similar cognitive processes and functioning between the human and animal remains elusive. Such works that have attempted to identify homologues between human and pigs were examined by Arnfred et al. (2004); Arnfred et al. (2003). These authors conducted auditory odd-ball paradigms to adult male pigs, attempting to describe ERP homologues to human ERPs. The authors concluded that the pig P30 is equivalent to the human P300a in distribution and latency, showing promise of the pig model of TBI for studying injury mechanisms in the human.
3.3. Actigraphy
Mild traumatic brain injury has been documented to result in abnormal sleep and activity patterns in humans and animals (Sandsmark et al., 2017). In the pediatric population, disruptions in the sleeping and waking hours can lead to problems with cognition, chronic pain, and psychological distress, normal neurocognitive development and impaired learning (Tham et al., 2015). The gold standard for measuring sleep quality is through the use of polysomnography (PSG). Patients are often required to stay at a sleep laboratory fitted with a series of electrodes (EEG, electrocardiographic and video) to record physiological patterns during sleep. While these studies provide the ideal laboratory environment with which to observe and take measurements of the patient throughout the stages of sleep, it takes place in an unfamiliar environment (not at home in their own bed), often requiring sophisticated and expensive equipment. A promising alternative to polysomnography is actigraphy to capture sleep disturbances inferred from changes in activity. Actigraphy employs an accelerometer-based device (usually in the form of a watch worn on the non-dominant wrist) to capture time-stamped perturbations in the acceleration signal (Allan et al., 2017).
Kaufman et al. (2001) compared sleep and activity in adolescent subjects whom sustained a mild TBI approximately 4 years prior in comparison with healthy controls. Polysomnographic recordings taken in a sleep laboratory for one night, followed by actigraphic recordings and a sleep diary at home for 5 nights. Both the PSG techniques and the actigraphic recordings combined with sleep diaries were able to detect that the injured group had poorer sleep quality, including lower sleep efficiency and more time spent awake (Kaufman et al., 2001). Furthermore, this study demonstrates persistent sleep disturbances in adolescent children years after head injury. In one study by Milroy et al. (2008) whom compared actigraphic data in a sample of children with mild TBI (n = 18) in comparison with a non-head injured, orthopedic injury control group (n = 30) using an actigraphic watch for 5 nights. These authors reported no significant differences in sleep disturbances between groups, however, Tham et al. (2015), using larger sample sizes of children with mild TBI (n = 50) and healthy controls (n = 50) found that actigraphy was able to detect poorer sleep quality including shorter sleep durations and more active minutes during the night for the injured group.
Animal research examining the relationship between brain injury with activity patterns and sleep disruptions have primarily used invasive techniques with EEG data captured from an implanted electrode on the surface of the animal brain (Petraglia et al., 2014; Sabir et al., 2015; Sandsmark et al., 2017). Research employing non-invasive techniques used an instrumented cage system that measured pressure on the cage floor to infer on activity in rodents (Rowe et al., 2014a; Rowe et al., 2014b), as well as an accelerometer imbedded harness in piglets (Olson et al., 2016). The animal TBI research employing rats and mice mentioned above often involved invasive techniques either from the injury model itself (FPI and CCI) or from the implanted EEG electrodes in addition to the cage system. In the piglet model of TBI, Olson et al. (2016) instrumented 4-week old piglets with an accelerometer embedded harness jacket to monitor the animal’s day and night time activity 4 days after both CCI and RNR. In comparison with naïve piglets, brain injured animals demonstrated longer periods of inactivity during the day time and increased activity during the night time. Overall, animal models of TBI have indicated altered sleep and wake disturbances that are like those reported in humans with TBI (Wickwire et al., 2016).
Actigraphic measurements are a promising tool to non-invasively monitor active and inactive periods in both humans and animals. Actigraphy provides an inexpensive alternative to PSG, and while lacks specificity, it permits an understanding of the behavioral responses affected in mild BTI in comparison to healthy controls. Future research efforts employing actigraphy in an animal model of TBI includes increasing the time points studied to provide an idea of the progression of sleep disturbances over time and their resolution. Correlation of actigraphy with other measures of cognitive functional and behavioral tests, serum biomarkers and EEG, can provide further insight on the interrelation between sleep quality with on-going symptoms and behaviour states. A link between these objective measures and subjective symptoms can inform clinical management and decisions on prognosis with regarding rest and recovery after TBI.
3.4. Gait and balance assessments
Increase in postural instability and vestibulomotor impairment are frequently reported as outcomes of TBI. Reliable balance assessments are essential to identifying balance problems for both diagnostic and rehabilitation purposes. Several balance assessment techniques have been introduced and their reliability have been investigated in the literature. Among those are the Balance Error Scoring System (BESS) which is a low-cost balance assessment that consists of single leg, double leg, and tandem stances on a firm and on a foam surface while hands are on the hips. Errors are tallied to calculate a BESS score. Errors include opening eyes, hands lifted off iliac crest, step, stumble, fall, moving hips > 30 degrees abduction, lifting forefoot or hill and remaining out of test position > 5 s. The BESS has been utilized in several studies as a diagnostic tool for TBI in pediatrics and adults and has shown to detect significant difference between concussed and control groups. (Cushman et al., 2018; Guskiewicz et al., 2001; Quatman-Yates et al., 2014; Riemann and Guskiewicz, 2000). Later, BESS was modified to improve its reliability to three stances on a hard surface only. Modified BESS has also shown significant changes in postural stability for a TBI group (Muir et al., 2014). In a few studies, it was shown that instrumenting BESS with measurement tools such as accelerometers (King et al., 2014) and force plates (Chang et al., 2014) can increase its reliability to evaluate balance improvements (Shetty et al., 2018). The Berg Balance Scale and the Pediatric Balance Scale are other common balance assessments that can be utilized for school-aged children (Franjoine et al., 2003). These assessments consist of 14 items such as the time for sitting to stand, standing with eyes closed, standing with feet together, turning 360 degrees, and reaching forward with outstretched arm. There are also two simple balance performance assessments: the functional reach test (FRT) that evaluates the reaching ability while standing and Timed Up and Go (TUG) that is the recorded time for a task of standing from a chair, walking 3 m, turning around, walking back and sitting down. Lower FRT values and higher TUG values in children post-TBI in comparison with the controls were reported in the literature (Katz-Leurer et al., 2008). Ghent Developmental Balance Test (GDBT) was developed for younger children from the moment of independent walking until the age of 5 and it consists of 35 balance items such as the time and the ability of bipedal standing, standing on a balance pad, kicking a ball, and standing on a line with eyes closed. Each item is scored from 0, for the case that the child cannot attempt the items, to 2 for the successful performance. The sum of the scores on all items can be converted to a percentile score based on GDBT manual for standardization (De Kegel et al., 2012). Use of force plates and wearable sensors (e.g. accelerometer) have become a key advancement to develop more quantitative balance assessment techniques. In one of the first studies, Lehmann et al. (1990) utilized force plate to assess balance and found significant differences in all balance parameters such as postural sway between TBI patients and controls. Computerized posturography testing (CPT) is a more advance quantitative technique to assess balance in human and has become the gold standard of balance assessment. CPT utilizes moving force plate and provides 3 test protocols including: 1) “Sensory Organization Test” that assesses the patient’s ability to make effective use of visual, vestibular, and proprioceptive information under three visual conditions (eyes open, eyes closed, sway-referenced visual surrounding); 2) “Motor Control Test” that assesses the patient’s ability to quickly and automatically recover from unexpected external provocations; and 3) “Adaptation Test” that assesses the patient’s ability to modify motor reactions and minimize sway when the support moves unpredictably in the toes-up or toes-down direction. Eye closed tests in CPT assessments showed that patients with severe TBI have more difficulty processing information from their vestibular system to maintain balance in compare to controls (Pickett, 2007) that may be attributable to the fact that TBI subjects are often over reliant on visual compensatory rather than vestibular system strategies to compensate for vestibular dysfunction and maintain balance. Wearable sensors have also been used to quantify clinical balance test results. Gera et al. (2018) quantified postural sway of 38 athletes who had sustained a mild TBI and 81 control athletes using a commercially available wearable inertial sensor that contains a tri-axial accelerometer, a tri-axial gyroscope, and a tri-axial magnetometer. They observed higher postural sway and increased postural sway in the mild TBI group compared to the control group due to a higher dependence on visual cues to maintain balance.
Several studies have explored balance deficits in more dynamic settings (i.e. gait analysis using motion capture systems). Katz-Leurer et al. (2008) performed gait analysis on children diagnosed with severe closed head injury and healthy controls and found significantly shorter step length, longer step time, higher hip abductor and knee extensor strength values as well as higher variability of step length and time in children post-TBI in comparison to controls. In another study, Basford et al. (2003) performed gait analysis using a motion capture system and found lower walking velocity and stride length in adult TBI subjects with complaints of instability in compare to healthy controls.
In pre-clinical research, several techniques have been developed to assess balance in animals. One of the most common assessments of the vestibulomotor system in mice is with the use of a beam balance. Mice are placed on a narrow beam and balance is scored from 0, for no attempt to keep balance, to 5 for a steady posture. Another common balance test used in mice and rats is the rotarod (Hamm et al., 1994). The rotarod consists of a motorized rotating rod and two large plexiglas discs positioned on each side. The mice walk on the rotating disks at various speeds and the latency until the animal falls is an indicator of balance performance. Inclined plane is another assessment that measures ability of rats to maintain its position at a given angle (Hamm, 2001). There are limited studies in large animal balance assessment for TBI diagnosis. In one study an accelerometer and video camera were used to measure postural sway of piglets subject to sham, CCI and RNR TBI. A significant increase in the root mean square acceleration in the anterior-posterior and medial-lateral directions in both CCI and RNR models in comparison to sham animals (Jaber et al., 2015). In another study, gait parameters of sham and CCI injured piglets were investigated using video analysis. A significant increase in percent stance time, a significant decrease in stride velocity and 2-limb support were reported in the more severe CCI injured animals (Baker et al. (2018)). By reviewing the literature, it is evident that quantitative balance and gait assessments are reliable low-cost tools for TBI diagnosis. However, most of these assessment methods rely on instructional-based assessments rather than non-instructional involuntary movements. A challenge in balance assessment that needs to be addressed is the development of methods that rely on non-instructional involuntary movements that can be implemented for non-verbal populations including animal models and young children.
3.5. Eye response
The cognitive symptoms such as deficits associated with concentration, attention, memory, planning and decision making are associated with microstructural changes in the frontal white matter in TBI (Maruta et al., 2010). These deficits may result from disruption of cerebellar-cortical tracts due to diffuse axonal injury (Suh et al., 2006). On the other hand, measuring eye response is a prominent, cost effective, quantitative diagnostic tool with high reliability. Eye response assessments include a variety of measures such as pupil response to light, fixation, smooth pursuit eye movements, saccades (rapid shifting of gaze to a new area of interest), measuring the point of gaze and visual tracking. Among these measures, visual tracking performance which is tracking of a moving target requires integration of attention and planning. This measure includes smooth and saccadic elements of eye movement (Heitger et al., 2009) that have been widely used to study the cognitive symptoms associated with TBI in pediatrics (Ellis et al., 2015)and adults (Caplan et al., 2015; Heitger et al., 2009; Maruta et al., 2010; Samadani et al., 2016; Samadani et al., 2015). All eye tracking systems either measure the position of the eye relative to the head or the orientation of the eye in space (Duchowski, 2007). In general, eye movement techniques can be categorized into three groups: (1) Electro-OculoGraphy which is based on the electric potential differences of the skin around the eye; (2) scleral contact lens that relies on a search coil embedded in contact lenses; and (3) video-based methods that utilize combined pupil and corneal reflection of the light source (typically infrared) and image processing (Duchowski, 2007). There are numerous studies that have been conducted to investigate the utility of these techniques to diagnose TBI in pediatrics and adults. Significant change in saccade latency and fixation error and initial fixation error have been reported in eye response in pediatric TBI (DiCesare et al., 2017). In an adult TBI studty, Caplan et al. (2015) captured horizontal and vertical binocular gaze data for subjects with post concussive syndrome (n = 60) and asymptomatic control subjects (n = 26). They reported significant differences in a number of eye tracking components including saccades and smooth pursuit eye movements for subjects with symptomatic mild TBI which can discriminate between individuals with mild TBI and the control group. Similar findings were observed in another study (Suh et al., 2006) on adult subjects with mild TBI (20 chronic and 6 acute) and controls (n = 26) using a circular pursuit target-blanking paradigm. Increased oculomotor deficits during target blanking were reported for the TBI subjects, indicated by earlier generation of saccades, increased oculomotor error, and increased intra-individual variability compared to controls. In another mild TBI study, eye movement function with sensitivity and specificity of nearly 100% was reported as the most effective metric in identifying adult patients with post-concussion syndrome (Heitger et al., 2008). Samadani et al. (2016) developed an eye tracking algorithm to assess eye movements of the adult subjects watching a video clip rather than following instruction. They investigated 89 eye tracking measures and suggested a subset as the best predictor of brain injured subjects. They also reported that the severity of disconjugate gaze in TBI and concussion patients were detectable with their algorithm and was proportionate to the severity of concussion symptoms.
On the pre-clinical side, several eye tracking systems have been developed for animals for different purposes such as peahens to study the process of mate choice (Yorzinski et al., 2013), dogs to measure canine visual behavior (Williams et al., 2011) and rats to study the response to sensory stimuli (Schriver et al., 2018). However, there is a paucity of preclinical studies using eye response in animals as a TBI diagnostic biomarker which is a critical gap in the literature worthy of future consideration in TBI pre-clinical studies. Another area that has not been adequately studied is the effect of TBI on non-voluntary eye response that brings insight to potential biomarkers based on non-voluntary movement and non-instructional cognitive tasks. Therefore, the pursuit of non-voluntary eye response measures of particular interest in translational TBI research.
4. Translational challenges and pathways for exploration
TBI biomarkers are valuable tools for diagnosis of TBI and monitoring its progression as well as evaluation of therapeutic efficacy in TBI patients. In the process of biomarker discovery, animal models are extremely valuable as they can provide unique opportunities to closely explore molecular and cellular pathology in the brain tissue, which is not easily possible in the clinical setting. TBI biomarker studies in animals also play an important role in the development of new therapies, and to quantitate the therapeutic efficiency. However, translation of preclinical outcomes to clinical applications face several challenges. In this section, we review the advantages, challenges and prospects of translating pre-clinical knowledge into the human clinical setting.
4.1. Heterogeneity
Heterogeneity remains the main challenge in the field of clinical TBI. Mechanisms of injury, injury severity, spatial and temporal pathophysiology, and clinical outcomes are just a few examples contributing to variability in clinical data. One approach to address the heterogeneity of human TBI into pre-clinical studies is to integrate several preclinical models to find the biomarkers that are applicable to a spectrum of TBI mechanisms and severities (Hajiaghamemar et al., 2017; Margulies et al., 2015; Shultz et al., 2017). Conducting studies across species and over variety of TBI models is also an important step toward identifying preclinical-clinical compatible panel of biomarkers which can help to achieve more translational success. This effort would be possible through large scale collaborative research initiatives between multiple centers.
4.2. Age equivalency of humans and animals
The selection of an appropriately aged animal model to reflect the desired human population under study is based on the stages of development of the animal that closely resembles the human stage of maturation and is not simply based on age after birth (Finnie, 2012). Researchers have proposed that rodent models at 7 days old, 7–11 days, 17–21 days, and adult can be used to represent human birth, infants, and toddlers, and adults respectively (Hagberg et al., 2002; Kochanek et al., 2017). While rodents tends to be the most commonly used species in research with a large number of published studies available regarding pathophysiology, functional and behavior outcomes, these animals have smaller, lissencephalic brains, composed of less white matter tissue than grey matter than those typically seen in the human brain (Cernak, 2005; Finnie, 2001; Vink, 2018). Adult humans have gryencephalic brains with a 2–4 mm thick grey matter ribbon enclosing the white matter (Dahnke and Gaser, 2018). These characteristics play an important role affecting the movement of the brain within the skull and subsequent patterns of localized mechanical stress culminating on the neural tissues (Duhaime, 2006; Ho and Kleiven, 2009; Xiong et al., 2013). Larger animal models, such as sheep and pigs have distributions of grey matter and white matter tissues that are more similar to the human brain. Additionally, the presence of well-formed gryi and sulci in these large animal models better resemble the pathology of white matter injuries (Duhaime, 2006; Finnie, 2001). More recent efforts employing magnetic resonance imaging techniques for longitudinal assessment of white matter composition of the 3-month old piglet brain demonstrated similar trajectories to the human adolescent brain (ages 10–19 years old), which further supports the piglet model for studying pediatric concussion (Ryan et al., 2018a; Ryan et al., 2018b). Newborn piglets, piglets at 1–5 days, and 3–4 weeks old resemble the newborn human, infant and children brain maturations (Hagberg et al., 2002; Kochanek et al., 2017).
The age-dependency of some of the TBI biomarkers discussed in this review also emphasize the importance of using age appropriate animal models in TBI studies especially with the purpose to translate to the pediatric clinical setting. In addition, utilizing age-independent metrics and/or narrow age groups in study designs are a good path to minimize age dependency of tests and establish robust biomarkers.
4.3. Temporal profile
One of the challenges that prevents successful translation of clinical and pre-clinical studies is the neglect of distinct biomarker temporal profile in human and animal models due to different life span and time scale of biological process, metabolomic rate, pharmacokinetic, pathophysiological responses. The proper selection of the sequential time points is necessary to capture the dynamic progression of biomarkers. This selection is achievable through parallel clinical/pre-clinical studies as well as more precisely characterizing the temporal pattern of biomarkers by more frequent serial sampling. Direct comparison of the temporal profile in pathophysiology response in animals and humans can help to identify appropriate translating time scaling factors, which is an important parameter for the successful translation of biomarkers. On the other hand, most animal TBI models have focused on acute outcomes but the majority of clinical studies concentrate on long term outcomes of TBI. This misalignment is a clear deficiency in the literature and more work needs to be done to assess long term outcomes after TBI in preclinical studies. One of the challenges with animal TBI studies is the difficulty to maintain and house severely injured animals post TBI to mimic the intensive care settings of humans. Often, a human subject diagnosed with TBI receives medication that can affect the assay and trajectory of serum biomarkers as well as long-term outcomes of TBI measured using other indicators (Oli et al., 2009). Overall, increasing the number of studies employing parallel clinical/pre-clinical longitudinal research designs provides the opportunity to directly compare biomarker occurrence between preclinical models and human data and is a promising path toward overcoming some of the challenges in translation of animal data into clinical practice.
4.4. Panel of multi-domain translatable metrics
Many TBI studies in the literature have assessed TBI diagnostic and prognostic capability of one or a few targeted biomarkers individually. However, brain injury is a multifactorial process involving complex linkage between metabolic evolution, molecular interaction between brain tissue and biofluids, and neuropathological sequelae of injury that can lead to many deficits such as neurodegeneration, neurological, functional and cognitive impairments and many more. Given all the complexity and variation of TBI especially in pediatric population, it is unlikely that a single biomarker will be able to predict TBI with enough sensitivity and specificity. A panel of biomarkers considering different deficits of TBI seems to be more promising to diagnose the diversity of injury and monitor its progression. For example, in biofluid biomarkers, a panel of multiple glial, neuronal, metabolism, inflammatory, and microRNA biomarkers seems to be a more appropriate approach to identify and assess the severity, timing and pattern of injury. The concept of using multivariate biofluid biomarkers for TBI is growing and several clinical studies showed superiority of multivariate biofluid biomarker over single biomarker for TBI (Beers et al., 2007; Diaz-Arrastia et al., 2014; Hajiaghamemar et al., 2017; Lo et al., 2009; Peacock et al., 2017). In addition, a panel of different EEG features or scores have shown promise as a potential tool to detect and monitor concussion (Jacquin et al., 2018; Kiefer et al., 2015).
Ideal translatable biomarkers should be accessible, non-invasive, nonverbal, effort-independent, and rely on objective rather than subjective measures. For example, serum biomarkers are minimally invasive and relatively objective measures and several biofluid biomarkers showed good potential to be used as diagnostic and prognostic tools for TBI. Actigraphy and EEG metrics are also non-invasive, both measure passive responses, and thus require minimal effort from the studied subject. In addition, as discussed in this review, both actigraphy and EEG measures have demonstrated promise in differentiating between healthy controls and brain injured cohorts. Similarly, balance and eye response assessment techniques that are based on involuntary movements also have a high potential of translatability between animals and human outcomes. Especial, conspicuous is the fact that these preclinical biomarkers and techniques can be tailored for nonverbal infants and toddlers. Combining these clinical/pre-clinical translatable measures considering their distinct temporal profiles seems to be a path toward development of comprehensive and unbiased panel of biomarkers capable of diagnosis and monitoring the progression of TBI from different deficit aspects. All of the reviewed metrics showed the capability to be implemented in clinical as well as preclinical studies especially using large animal models of TBI. Measuring multi-domain biomarkers at uniform time points across clinical and preclinical allows for relationships to be established that relate cellular, functional and behavioral processes and identify markers that are more sensitive and specific at different post injury time windows that can relate to prognosis.
5. Conclusion
A large proportion of TBI related emergency department visits are from young children and adolescents where falls, assaults, motor vehicle accidents, and sport related injuries are among the top events leading to injury. The acute and potential long-term effects include cognitive, physical, and behavioral deficits, which may be exacerbated in the developing brain affecting their productivity in academics, contributions to society, and life at home with close family and friends. The nature of TBI is complex, with diverse clinical presentations, causes and mechanisms of injury contributing to challenges associated with diagnosis, treatment, and management practices. In efforts to minimize the detrimental long-terms effects of TBI, an increase in pre-clinical and clinical research have sought to determine translatable diagnostic and prognostic measures of TBI and especially concussion in order to expedite injury resolution and reduce long term risks associated with TBI. Animal models of TBI are necessary in addressing the heterogeneity of TBI by isolating the mechanisms of injury in a reproducible manner, however translation to humans is difficult due to inter-species differences such as brain anatomy, behavior, life-span and time scale of biological process, metabolomic rate, pharmacokinetic, pathophysiological responses. In this review, we presented the findings, advantages, challenges and prospects of biofluid biomarkers, EEG, actigraphy, eye responses, and balance as potential tools for diagnosis, prognosis and TBI monitoring. These tools are valuable for bridging the gap between animal and human TBI outcomes because they involve effort-independent and non-verbal tasks capturing specific deficits. In addition, they are easily accessible and are non/minimally invasive. TBI causes dynamic, age dependent deficits in multiple domains, and thus, combining a number of biomarkers into a panel may be more suitable to detect these subtleties in deficits across age groups. These techniques can be used in parallel clinical and pre-clinical studies involving both human and animals and help to overcome some of the challenges associated with translation. Direct comparison of the temporal profile in different pathophysiological responses and behavioral and functional deficits in animals and humans can help to identify appropriate translating time scaling factors to enhance translation of biomarkers. Multicenter collaborations and large-scale data sharing will help to close this gap and promote advancements in therapies and interventions.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) grant R01NS097549 and the Georgia Research Alliance.
References
- Adelson PD, Robichaud P, Hamilton RL, Kochanek PM, 1996. A model of diffuse traumatic brain injury in the immature rat. J. Neurosurg 85, 877–884. [DOI] [PubMed] [Google Scholar]
- Agoston DV, Shutes-David A, Peskind ER, 2017. Biofluid biomarkers of traumatic brain injury. Brain Inj 31, 1195–1203. [DOI] [PubMed] [Google Scholar]
- Ahmed F, Plantman S, Cernak I, Agoston DV, 2015. The temporal pattern of changes in serum biomarker levels reveals complex and dynamically changing pathologies after exposure to a single low-intensity blast in mice. Front. Neurol 6, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Nimer F, Thelin E, Nyström H, Dring AM, Svenningsson A, Piehl F, Nelson DW, Bellander B-M, 2015. Comparative assessment of the prognostic value of biomarkers in traumatic brain injury reveals an independent role for serum levels of neurofilament light. PLoS One 10, e0132177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan AC, Edmed SL, Sullivan KA, Karlsson LJE, Lange RT, Smith SS, 2017. Actigraphically measured sleep-wake behavior after mild traumatic brain injury: a case-control study. J. Head Trauma Rehabil 32, E35–E45. [DOI] [PubMed] [Google Scholar]
- Anderson KJ, Scheff SW, Miller KM, Roberts KN, Gilmer LK, Yang C, Shaw G, 2008. The phosphorylated axonal form of the neurofilament subunit NF-H (pNF-H) as a blood biomarker of traumatic brain injury. J. Neurotrauma 25, 1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnfred SM, Lind NM, Moustgaard A, Hansen AK, Gjedde A, 2003. Minipig negative slow wave demonstrates target/nontarget differences in P300 paradigm. NeuroImage 20, 587–590. [DOI] [PubMed] [Google Scholar]
- Arnfred SM, Lind NM, Gjedde A, Hansen AK, 2004. Scalp recordings of mid-latency AEP and auditory gating in the Göttingen minipig: a new animal model in information processing research. Int. J. Psychophysiol 52, 267–275. [DOI] [PubMed] [Google Scholar]
- Asemota AO, George BP, Bowman SM, Haider AH, Schneider EB, 2013. Causes and trends in traumatic brain injury for United States adolescents. J. Neurotrauma 30, 67–75. [DOI] [PubMed] [Google Scholar]
- Atlan LS, Lan IS, Smith C, Margulies SS, 2018. Changes in event-related potential functional networks predict traumatic brain injury in piglets. Clin. Biomech 64, 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock L, Byczkowski T, Mookerjee S, Bazarian JJ, 2012. Ability of S100B to predict severity and cranial CT results in children with TBI. Brain Inj 26, 1372–1380. [DOI] [PubMed] [Google Scholar]
- Babcock L, Byczkowski T, Wade SL, Ho M, Bazarian JJ, 2013. Inability of S100B to predict post-concussion syndrome in children who present to the emergency department with mild traumatic brain injury: a brief report. Pediatr. Emerg. Care 29, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahado-Singh RO, Graham SF, Han B, Turkoglu O, Ziadeh J, Mandal R, Er A, Wishart DS, Stahel PL, 2016. Serum metabolomic markers for traumatic brain injury: a mouse model. Metabolomics 12, 100. [Google Scholar]
- Baillargeon A, Lassonde M, Leclerc S, Ellemberg D, 2012. Neuropsychological and neurophysiological assessment of sport concussion in children, adolescents and adults. Brain Inj 26, 211–220. [DOI] [PubMed] [Google Scholar]
- Baker EW, Kinder HA, Hutcheson JM, Duberstein KJJ, Platt SR, Howerth EW, West FD, 2018. Controlled cortical impact severity results in graded cellular, tissue, and functional responses in a piglet traumatic brain injury model. J. Neurotrauma 36, 61–73. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Hennes H, Gorelick MH, Wells RG, Walsh-Kelly CM, 2005. Serum neuron-specific enolase as a predictor of short-term outcome in children with closed traumatic brain injury. Acad. Emerg. Med 12, 732–738. [DOI] [PubMed] [Google Scholar]
- Basford JR, Chou L-S, Kaufman KR, Brey RH, Walker A, Malec JF, Moessner AM, Brown AW, 2003. An assessment of gait and balance deficits after traumatic brain injury. Arch. Phys. Med. Rehabil 84, 343–349. [DOI] [PubMed] [Google Scholar]
- Beers SR, Berger RP, Adelson PD, 2007. Neurocognitive outcome and serum biomarkers in inflicted versus non-inflicted traumatic brain injury in young children. J. Neurotrauma 24, 97–105. [DOI] [PubMed] [Google Scholar]
- Berger RP, Kochanek PM, 2006. Urinary S100B concentrations are increased after brain injury in children: a preliminary study. Pediatr. Crit. Care Med 7, 557–561. [DOI] [PubMed] [Google Scholar]
- Berger RP, Pierce MC, Wisniewski SR, Adelson PD, Clark RS, Ruppel RA, Kochanek PM, 2002. Neuron-specific enolase and S100B in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatrics 109, e31. [DOI] [PubMed] [Google Scholar]
- Berger RP, Adelson PD, Pierce MC, Dulani T, Cassidy LD, Kochanek PM, 2005. Serum neuron-specific enolase, S100B, and myelin basic protein concentrations after inflicted and noninflicted traumatic brain injury in children. J. Neurosurg. Pediatr 103, 61–68. [DOI] [PubMed] [Google Scholar]
- Berger RP, Dulani T, Adelson PD, Leventhal JM, Richichi R, Kochanek PM, 2006. Identification of inflicted traumatic brain injury in well-appearing infants using serum and cerebrospinal markers: a possible screening tool. Pediatrics 117, 325–332. [DOI] [PubMed] [Google Scholar]
- Berger RP, Beers SR, Richichi R, Wiesman D, Adelson PD, 2007. Serum biomarker concentrations and outcome after pediatric traumatic brain injury. J. Neurotrauma 24, 1793–1801. [DOI] [PubMed] [Google Scholar]
- Berger RP, Hayes RL, Richichi R, Beers SR, Wang KK, 2012. Serum concentrations of ubiquitin C-terminal hydrolase-L1 and αII-spectrin breakdown product 145 kDa correlate with outcome after pediatric TBI. J. Neurotrauma 29, 162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhomia M, Balakathiresan NS, Wang KK, Papa L, Maheshwari RK, 2016. A panel of serum MiRNA biomarkers for the diagnosis of severe to mild traumatic brain injury in humans. Sci. Rep 6, 28148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas C, Markovic D, Giza CC, 2018. Alterations in mesoscopic oscillations affecting episodic memory following developmental traumatic brain injury. Exp. Neurol 300, 259–273. [DOI] [PubMed] [Google Scholar]
- Blyth BJ, Farhavar A, Gee C, Hawthorn B, He H, Nayak A, Stocklein V, Bazarian JJ, 2009. Validation of serum markers for blood-brain barrier disruption in traumatic brain injury. J. Neurotrauma 26, 1497–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier D, Fournier M, Dauphin J-B, Amat F, Ughetto S, Labbé A, Sapin V, 2012. Serum S100B determination in the management of pediatric mild traumatic brain injury. Clin. Chem 58, 1116–1122. [DOI] [PubMed] [Google Scholar]
- Brenner DJ, Hall EJ, 2007. Computed tomography—an increasing source of radiation exposure. N. Engl. J. Med 357, 2277–2284. [DOI] [PubMed] [Google Scholar]
- Broglio SP, Pontifex MB, O’Connor P, Hillman CH, 2009. The persistent effects of concussion on neuroelectric indices of attention. J. Neurotrauma 26, 1463–1470. [DOI] [PubMed] [Google Scholar]
- Brush CJ, Ehmann PJ, Olson RL, Bixby WR, Alderman BL, 2018. Do sport-related concussions result in long-term cognitive impairment? A review of event-related potential research. Int. J. Psychophysiol 132, 124–134. [DOI] [PubMed] [Google Scholar]
- Buttram SD, Wisniewski SR, Jackson EK, Adelson PD, Feldman K, Bayir H, Berger RP, Clark RS, Kochanek PM, 2007. Multiplex assessment of cytokine and chemokine levels in cerebrospinal fluid following severe pediatric traumatic brain injury: effects of moderate hypothermia. J. Neurotrauma 24, 1707–1717. [DOI] [PubMed] [Google Scholar]
- Caplan B, Bogner J, Brenner L, Cifu DX, Wares JR, Hoke KW, Wetzel PA, Gitchel G, Carne W, 2015. Differential eye movements in mild traumatic brain injury versus normal controls. J. Head Trauma Rehabil 30, 21–28. [DOI] [PubMed] [Google Scholar]
- Carbonell WS, Maris DO, McCall T, Grady MS, 1998. Adaptation of the fluid percussion injury model to the mouse. J. Neurotrauma 15, 217–229. [DOI] [PubMed] [Google Scholar]
- Cardinell B, 2017. Towards a Hand-Held Multi-Biomarker Point-of-Care Diagnostic to Quantify Traumatic Brain Injur
- Castellani C, Bimbashi P, Ruttenstock E, Sacherer P, Stojakovic T, Weinberg A-M, 2009. Neuroprotein s-100B – a useful parameter in paediatric patients with mild traumatic brain injury? Acta Paediatr 98, 1607–1612. [DOI] [PubMed] [Google Scholar]
- Cernak I, 2005. Animal models of head trauma. NeuroRx 2, 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JO, Levy SS, Seay SW, Goble DJ, 2014. An alternative to the balance error scoring system: using a low-cost balance board to improve the validity/reliability of sports-related concussion balance testing. Clin. J. Sport Med 24, 256–262. [DOI] [PubMed] [Google Scholar]
- Chhor V, Moretti R, Le Charpentier T, Sigaut S, Lebon S, Schwendimann L, Oré M-V, Zuiani C, Milan V, Josserand J, 2017. Role of microglia in a mouse model of paediatric traumatic brain injury. Brain Behav. Immun 63, 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaretti A, Genovese O, Aloe L, Antonelli A, Piastra M, Polidori G, Di Rocco C, 2005. Interleukin 1β and interleukin 6 relationship with paediatric head trauma severity and outcome. Childs Nerv. Syst 21, 185–193. [DOI] [PubMed] [Google Scholar]
- Cullen DK, Harris JP, Browne KD, Wolf JA, Duda JE, Meaney DF, Margulies SS, Smith DH, 2016. A porcine model of traumatic brain injury via head rotational acceleration. Methods Mol. Biol 289–324. [DOI] [PMC free article] [PubMed]
- Cushman D, Hendrick J, Teramoto M, Fogg B, Bradley S, Hansen C, 2018. Reliability of the balance error scoring system in a population with protracted recovery from mild traumatic brain injury. Brain Inj 32, 569–574. [DOI] [PubMed] [Google Scholar]
- Dahnke R, Gaser C, 2018. Surface and Shape Analysis, Brain Morphometry Springer, pp. 51–73. [Google Scholar]
- De Beaumont L, Thoret H, Mongeon D, Messier J, Leclerc S, Tremblay S, Ellemberg D, Lassonde M, 2009. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain 132, 695–708. [DOI] [PubMed] [Google Scholar]
- De Kegel A, Baetens T, Peersman W, Maes L, Dhooge I, Van Waelvelde H, 2012. Ghent developmental balance test: a new tool to evaluate balance performance in toddlers and preschool children. Phys. Ther 92, 841–852. [DOI] [PubMed] [Google Scholar]
- Di Pietro V, Ragusa M, Davies D, Su Z, Hazeldine J, Lazzarino G, Hill LJ, Crombie N, Foster M, Purrello M, 2017. MicroRNAs as novel biomarkers for the diagnosis and prognosis of mild and severe traumatic brain injury. J. Neurotrauma 34, 1948–1956. [DOI] [PubMed] [Google Scholar]
- Di Pietro V, Porto E, Ragusa M, Barbagallo C, Davies D, Forcione M, Logan A, Di Pietro C, Purrello M, Grey M, 2018. Salivary MicroRNAs: diagnostic markers of mild traumatic brain injury in contact-sport. Front. Mol. Neurosci 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Arrastia R, Wang KK, Papa L, Sorani MD, Yue JK, Puccio AM, McMahon PJ, Inoue T, Yuh EL, Lingsma HF, Maas AI, Valadka AB, Okonkwo DO, Manley GT, 2014. Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J. Neurotrauma 31, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCesare CA, Kiefer AW, Nalepka P, Myer GD, 2017. Quantification and analysis of saccadic and smooth pursuit eye movements and fixations to detect oculomotor deficits. Behav. Res. Methods 49, 258–266. [DOI] [PubMed] [Google Scholar]
- Duchowski AT, 2007. Eye tracking methodology. Theory Pract 328. [Google Scholar]
- Duhaime AC, 2006. Large animal models of traumatic injury to the immature brain. Dev. Neurosci 28, 380–387. [DOI] [PubMed] [Google Scholar]
- Duhaime A-C, Margulies SS, Durham SR, O’Rourke MM, Golden JA, Marwaha S, Raghupathi R, 2000. Maturation-dependent response of the piglet brain to scaled cortical impact. J. Neurosurg 93, 455–462. [DOI] [PubMed] [Google Scholar]
- Dupuis F, Johnston KM, Lavoie M, Lepore F, Lassonde M, 2000. Concussions in athletes produce brain dysfunction as revealed by event-related potentials. Neuroreport 11, 4087–4092. [DOI] [PubMed] [Google Scholar]
- Ellis MJ, Cordingley D, Vis S, Reimer K, Leiter J, Russell K, 2015. Vestibuloocular dysfunction in pediatric sports-related concussion. J. Neurosurg. Pediatr 16, 248–255. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG, 2010. Traumatic Brain Injury in the United States. Emergency Department Visits, Hospitalizations, and Deaths, 2010
- Finnie JW, 2001. Animal models of traumatic brain injury: a review. Aust. Vet. J 79, 628–633. [DOI] [PubMed] [Google Scholar]
- Finnie J, 2012. Comparative approach to understanding traumatic injury in the immature, postnatal brain of domestic animals. Aust. Vet. J 90, 301–307. [DOI] [PubMed] [Google Scholar]
- Folweiler K, Samuel S, Metheny H, Cohen AS, 2018. Diminished dentate gyrus filtering of cortical input leads to enhanced area Ca3 excitability after mild traumatic brain injury. J. Neurotrauma 35, 1304–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franjoine MR, Gunther JS, Taylor MJ, 2003. Pediatric balance scale: a modified version of the berg balance scale for the school-age child with mild to moderate motor impairment. Pediatr. Phys. Ther 15, 114–128. [DOI] [PubMed] [Google Scholar]
- Fraser DD, Close TE, Rose KL, Ward R, Mehl M, Farrell C, Lacroix J, Creery D, Kesselman M, Stanimirovic D, 2011. Severe traumatic brain injury in children elevates glial fibrillary acidic protein in cerebrospinal fluid and serum. Pediatr. Crit. Care Med 12, 319–324. [DOI] [PubMed] [Google Scholar]
- Fridriksson T, Kini N, Walsh-Kelly C, Hennes H, 2000. Serum neuron-specific enolase as a predictor of intracranial lesions in children with head trauma: a pilot study. Acad. Emerg. Med 7, 816–820. [DOI] [PubMed] [Google Scholar]
- Fritz HG, Walter B, Holzmayr M, Brodhun M, Patt S, Bauer R, 2005. A pig model with secondary increase of intracranial pressure after severe traumatic brain injury and temporary blood loss. J. Neurotrauma 22, 807–821. [DOI] [PubMed] [Google Scholar]
- Gaetz M, Weinberg H, 2000. Electrophysiological indices of persistent post-concussion symptoms. Brain Inj 14, 815–832. [DOI] [PubMed] [Google Scholar]
- Gaetz M, Goodman D, Weinberg H, 2000. Electrophysiological evidence for the cumulative effects of concussion. Brain Inj 14, 1077–1088. [DOI] [PubMed] [Google Scholar]
- Gazzolo D, Marinoni E, Di Iorio R, Bruschettini M, Kornacka M, Lituania M, Majewska U, Serra G, Michetti F, 2003. Measurement of urinary S100B protein concentrations for the early identification of brain damage in asphyxiated full-term infants. Arch. Pediatr. Adolesc. Med 157, 1163–1168. [DOI] [PubMed] [Google Scholar]
- Gennarelli TA, Thibault LE, Adams JH, Graham DI, Thompson CJ, Marcincin RP, 1982. Diffuse axonal injury and traumatic coma in the primate. Ann. Neurol 12, 564–574. [DOI] [PubMed] [Google Scholar]
- Gera G, Chesnutt J, Mancini M, Horak FB, King LA, 2018. Inertial sensor-based assessment of central sensory integration for balance after mild traumatic brain injury. Mil. Med 183, 327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer C, Ulrich A, Gräfe G, Stach B, Till H, 2009. Diagnostic value of S100B and neuron-specific enolase in mild pediatric traumatic brain injury. J. Neurosurg. Pediatr 4, 339–344. [DOI] [PubMed] [Google Scholar]
- Giza CC, Hovda DA, 2014. The new neurometabolic cascade of concussion. Neurosurgery 75, S24–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorse KM, Lafrenaye AD, 2018. The importance of inter-species variation in traumatic brain injury-induced alterations of microglial-axonal interactions. Front. Neurol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin N, Thériault M, Leclerc S, Montplaisir J, Lassonde M, 2006. Neurophysiological anomalies in symptomatic and asymptomatic concussed athletes. Neurosurgery 58, 1151–1160. [DOI] [PubMed] [Google Scholar]
- Graham D, Ford I, Adams JH, Doyle D, Lawrence A, McLellan D, Ng H, 1989. Fatal head injury in children. J. Clin. Pathol 42, 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskiewicz KM, Ross SE, Marshall SW, 2001. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J. Athl. Train 36, 263. [PMC free article] [PubMed] [Google Scholar]
- Gutierrez E, Huang Y, Haglid K, Bao F, Hansson H-A, Hamberger A, Viano D, 2001. A new model for diffuse brain injury by rotational acceleration: I. Model, gross appearance, and astrocytosis. J. Neurotrauma 18, 247–257. [DOI] [PubMed] [Google Scholar]
- Gyorgy A, Ling G, Wingo D, Walker J, Tong L, Parks S, Januszkiewicz A, Baumann R, Agoston DV, 2011. Time-dependent changes in serum biomarker levels after blast traumatic brain injury. J. Neurotrauma 28, 1121–1126. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Ichord R, Palmer C, Yager JY, Vannucci SJ, 2002. Animal models of developmental brain injury: relevance to human disease. Dev. Neurosci 24, 364–366. [DOI] [PubMed] [Google Scholar]
- Hajiaghamemar M, Kilbaugh T, Margulies S, 2017. Identification of amino acid panel as a biomarker for traumatic brain injury. J. Neurotrauma 34, A25. [Google Scholar]
- Hallén M, Karlsson M, Carlhed R, Hallgren T, Bergenheim M, 2010. S-100B in serum and urine after traumatic head injury in children. J. Trauma Acute Care Surg 69, 284–289. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, 2001. Neurobehavioral assessment of outcome following traumatic brain injury in rats: an evaluation of selected measures. J. Neurotrauma 18, 1207–1216. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Pike BR, O’Dell DM, Lyeth BG, Jenkins LW, 1994. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J. Neurotrauma 11, 187–196. [DOI] [PubMed] [Google Scholar]
- Heitger MH, Jones RD, Anderson TJ, 2008. A new approach to predicting post-concussion syndrome after mild traumatic brain injury based upon eye movement function. In: Engineering in Medicine and Biology Society, 2008. EMBS 2008. 30th Annual International Conference of the IEEE IEEE, pp. 3570–3573. [DOI] [PubMed] [Google Scholar]
- Heitger MH, Jones RD, Macleod A, Snell DL, Frampton CM, Anderson TJ, 2009. Impaired eye movements in post-concussion syndrome indicate suboptimal brain function beyond the influence of depression, malingering or intellectual ability. Brain 132, 2850–2870. [DOI] [PubMed] [Google Scholar]
- Hellewell SC, Yan EB, Agyapomaa DA, Bye N, Morganti-Kossmann MC, 2010. Post-traumatic hypoxia exacerbates brain tissue damage: analysis of axonal injury and glial responses. J. Neurotrauma 27, 1997–2010. [DOI] [PubMed] [Google Scholar]
- Hicks SD, Johnson J, Carney MC, Bramley H, Olympia RP, Loeffert AC, Thomas NJ, 2018. Overlapping microRNA expression in saliva and cerebrospinal fluid accurately identifies pediatric traumatic brain injury. J. Neurotrauma 35, 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J, Kleiven S, 2009. Can sulci protect the brain from traumatic injury? J. Biomech 42, 2074–2080. [DOI] [PubMed] [Google Scholar]
- Howell DR, Meehan WP III, Barber Foss KD, Reches A, Weiss M, Myer GD, 2018. Reduced dual-task gait speed is associated with visual go/no-go brain network activation in children and adolescents with concussion. Brain Inj 32, 1129–1134. [DOI] [PubMed] [Google Scholar]
- Huang XJ, Glushakova O, Mondello S, Van K, Hayes RL, Lyeth BG, 2015. Acute temporal profiles of serum levels of UCH-L1 and GFAP and relationships to neuronal and Astroglial pathology following traumatic brain injury in rats. J. Neurotrauma 32, 1179–1189. [DOI] [PubMed] [Google Scholar]
- Ianof JN, Anghinah R, 2017. Traumatic brain injury: an EEG point of view. Dement. Neuropsychol 11, 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim NG, Natesh R, Szczesny SE, Ryall K, Eucker SA, Coats B, Margulies SS, 2010. In situ deformations in the immature brain during rapid rotations. J. Biomech. Eng 132, 044501. [DOI] [PubMed] [Google Scholar]
- Jaber SM, Sullivan S, Margulies SS, 2015. Noninvasive metrics for identification of brain injury deficits in piglets. Dev. Neuropsychol 40, 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquin A, Kanakia S, Oberly D, Prichep LS, 2018. A multimodal biomarker for concussion identification, prognosis and management. Comput. Biol. Med 102, 95–103. [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH, 2010. Traumatic brain injury and amyloid-β pathology: a link to Alzheimer’s disease? Nat. Rev. Neurosci 11, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JJ, Loeffert AC, Stokes J, Olympia RP, Bramley H, Hicks SD, 2018. Association of salivary microRNA changes with prolonged concussion symptoms. JAMA Pediatr 172, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson P, Blomquist S, Lührs C, Malmkvist G, Alling C, Solem J-O, Ståhl E, 2000. Neuron-specific enolase increases in plasma during and immediately after extracorporeal circulation. Ann. Thorac. Surg 69, 750–754. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Angoa-Pérez M, Briggs DI, Viano DC, Kreipke CW, Kuhn DM, 2012. A mouse model of human repetitive mild traumatic brain injury. J. Neurosci. Methods 203, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner AA, Marchi N, Fazio V, Mayberg MR, Koltz MT, Siomin V, Stevens GH, Masaryk T, Aumayr B, Vogelbaum MA, Barnett GH, Janigro D, 2003. Serum S100beta: a noninvasive marker of blood-brain barrier function and brain lesions. Cancer 97, 2806–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz PS, Molina PE, 2018. A lateral fluid percussion injury model for studying traumatic brain injury in rats. In: Tharakan B (Ed.), Traumatic and Ischemic Injury: Methods and Protocols Springer; New York, New York, NY, pp. 27–36. [DOI] [PubMed] [Google Scholar]
- Katz-Leurer M, Rotem H, Lewitus H, Keren O, Meyer S, 2008. Relationship between balance abilities and gait characteristics in children with post-traumatic brain injury. Brain Inj 22, 153–159. [DOI] [PubMed] [Google Scholar]
- Kaufman Y, Tzischinsky O, Epstein R, Etzioni A, Lavie P, Pillar G, 2001. Long-term sleep disturbances in adolescents after minor head injury. Pediatr. Neurol 24, 129–134. [DOI] [PubMed] [Google Scholar]
- Kenzie ES, Parks EL, Bigler ED, Lim MM, Chesnutt JC, Wakeland W, 2017. Concussion as a multi-scale complex system: an interdisciplinary synthesis of current knowledge. Front. Neurol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer AW, Barber Foss K, Reches A, Gadd B, Gordon M, Rushford K, Laufer I, Weiss M, Myer GD, 2015. Brain network activation as a novel biomarker for the return-to-play pathway following sport-related brain injury. Front. Neurol 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbaugh TJ, Lvova M, Karlsson M, Zhang Z, Leipzig J, Wallace DC, Margulies SS, 2015. Peripheral blood mitochondrial DNA as a biomarker of cerebral mitochondrial dysfunction following traumatic brain injury in a porcine model. PLoS One 10, e0130927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbaugh TJ, Karlsson M, Duhaime A-C, Hansson MJ, Elmer E, Margulies SS, 2016. Mitochondrial response in a toddler-aged swine model following diffuse non-impact traumatic brain injury. Mitochondrion 26, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LA, Horak FB, Mancini M, Pierce D, Priest KC, Chesnutt J, Sullivan P, Chapman JC, 2014. Instrumenting the balance error scoring system for use with patients reporting persistent balance problems after mild traumatic brain injury. Arch. Phys. Med. Rehabil 95, 353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek PM, Wallisch JS, Bayır H, Clark RSB, 2017. Pre-clinical models in pediatric traumatic brain injury—challenges and lessons learned. Childs Nerv. Syst 33, 1693–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou Z, Gattu R, Kobeissy F, Welch RD, O’Neil BJ, Woodard JL, Ayaz SI, Kulek A, Kas-Shamoun R, Mika V, 2013. Combining biochemical and imaging markers to improve diagnosis and characterization of mild traumatic brain injury in the acute setting: results from a pilot study. PLoS One 8, e80296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kövesdi E, Lückl J, Bukovics P, Farkas O, Pál J, Czeiter E, Szellár D, Dóczi T, Komoly S, Büki A, 2010. Update on protein biomarkers in traumatic brain injury with emphasis on clinical use in adults and pediatrics. Acta Neurochir 152, 1–17. [DOI] [PubMed] [Google Scholar]
- Kurbat M, Lelevich V, 2009. Metabolism of amino acids in the brain. Neurochem. J 3, 23–28. [Google Scholar]
- Lafrenaye AD, Todani M, Walker SA, Povlishock JT, 2015. Microglia processes associate with diffusely injured axons following mild traumatic brain injury in the micro pig. J. Neuroinflammation 12, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie ME, Dupuis F, Johnston KM, Leclerc S, Lassonde M, 2004. Visual p300 effects beyond symptoms in concussed college athletes. J. Clin. Exp. Neuropsychol 26, 55–73. [DOI] [PubMed] [Google Scholar]
- Ledwidge PS, Molfese DL, 2016. Long-term effects of concussion on electrophysiological indices of attention in varsity college athletes: an event-related potential and standardized low-resolution brain electromagnetic tomography approach. J. Neurotrauma 33, 2081–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Boswell S, Price R, Burleigh A, Jaffe K, Hertling D, 1990. Quantitative evaluation of sway as an indicator of functional balance in post-traumatic brain injury. Arch. Phys. Med. Rehabil 71, 955–962. [PubMed] [Google Scholar]
- Lesiakowski P, Lubiński W, Zwierko T, 2018. Evoked potentials in diagnosis of visual dysfunction in amateur boxers. Phys. Sportsmed 46 (4), 449–459. [DOI] [PubMed] [Google Scholar]
- Leung LY, Larimore Z, Holmes L, Cartagena C, Mountney A, Deng-Bryant Y, Schmid K, Shear D, Tortella F, 2014. The WRAIR projectile concussive impact model of mild traumatic brain injury: re-design, testing and preclinical validation. Ann. Biomed. Eng 42, 1618–1630. [DOI] [PubMed] [Google Scholar]
- Lewis LM, Schloemann DT, Papa L, Fucetola RP, Bazarian J, Lindburg M, Welch RD, 2017. Utility of serum biomarkers in the diagnosis and stratification of mild traumatic brain injury. Acad. Emerg. Med 24, 710–720. [DOI] [PubMed] [Google Scholar]
- Li Y, Sun Y, Li J, Wang Z, Lin Y, Tang L, Xia D, Zheng T, Yang X, Sha L, 2015. Changes of ubiquitin C-terminal hydrolase-L1 levels in serum and urine of patients with white matter lesions. J. Neurol. Sci 357, 215–221. [DOI] [PubMed] [Google Scholar]
- Liu Y, McAfee SS, Guley NM, Del Mar N, Bu W, Heldt SA, Honig MG, Moore BM 2nd, Reiner A, Heck DH, 2017. Abnormalities in dynamic brain activity caused by mild traumatic brain injury are partially rescued by the cannabinoid Type-2 receptor inverse agonist SMM-189. eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo T-YM, Jones PA, Minns RA, 2009. Pediatric brain trauma outcome prediction using paired serum levels of inflammatory mediators and brain-specific proteins. J. Neurotrauma 26, 1479–1487. [DOI] [PubMed] [Google Scholar]
- Louin G, Neveux N, Cynober L, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M, 2007. Plasma concentrations of arginine and related amino acids following traumatic brain injury: proline as a promising biomarker of brain damage severity. Nitric Oxide 17, 91–97. [DOI] [PubMed] [Google Scholar]
- Luck SJ, 2014. An Introduction to the Event-Related Potential Technique MIT Press. [Google Scholar]
- Lugones M, Parkin G, Bjelosevic S, Takagi M, Clarke C, Anderson V, Ignjatovic V, 2018. Blood biomarkers in paediatric mild traumatic brain injury: a systematic review. Neurosci. Biobehav. Rev 87, 206–217. [DOI] [PubMed] [Google Scholar]
- Lumba-Brown A, Yeates K, Sarmiento K, et al. , 2018. Centers for disease control and prevention guideline on the diagnosis and management of mild traumatic brain injury among children. JAMA Pediatr 172, e182853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoto TM, Raj R, Posti JP, Gardner AJ, Panenka WJ, Iverson GL, 2017. A systematic review of the usefulness of glial fibrillary acidic protein for predicting acute intracranial lesions following head trauma. Front. Neurol 8, 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Wang N, Fan X, Zhang L, Luo Y, Huang R, Zhang L, Li Y, Zhao G, Li L, 2018. Protective effects of cornel iridoid glycoside in rats after traumatic brain injury. Neurochem. Res 43, 959–971. [DOI] [PubMed] [Google Scholar]
- Mannix RC, Zhang J, Park J, Zhang X, Bilal K, Walker K, Tanzi RE, Tesco G, Whalen MJ, 2011. Age-dependent effect of apolipoprotein E4 on functional outcome after controlled cortical impact in mice. J. Cereb. Blood Flow Metab 31, 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannix R, Eisenberg M, Berry M, Meehan WP III, Hayes RL, 2014. Serum biomarkers predict acute symptom burden in children after concussion: a preliminary study. J. Neurotrauma 31, 1072–1075. [DOI] [PubMed] [Google Scholar]
- Manzano S, Holzinger IB, Kellenberger CJ, Lacroix L, Klima-Lange D, Hersberger M, La Scala G, Altermatt S, Staubli G, 2016. Diagnostic performance of S100B protein serum measurement in detecting intracranial injury in children with mild head trauma. Emerg. Med. J 33, 42–46. [DOI] [PubMed] [Google Scholar]
- Margulies SS, Kilbaugh T, Sullivan S, Smith C, Propert K, Byro M, Saliga K, Costine BA, Duhaime AC, 2015. Establishing a clinically relevant large animal model platform for TBI therapy development: using C yclosporin a as a case study. Brain Pathol 25, 289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmarou A, Foda MAA-E, Brink W.v.d., Campbell J, Kita H, Demetriadou K, 1994. A new model of diffuse brain injury in rats: part I: pathophysiology and biomechanics. J. Neurosurg 80, 291–300. [DOI] [PubMed] [Google Scholar]
- Maruta J, Suh M, Niogi SN, Mukherjee P, Ghajar J, 2010. Visual tracking synchronization as a metric for concussion screening. J. Head Trauma Rehabil 25, 293–305. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Noble L, Andrews B, Faden AI, 1987. Traumatic brain injury in the rat: characterization of a midline fluid-percussion model. Cent. Nerv. Syst. Trauma 4, 119–134. [DOI] [PubMed] [Google Scholar]
- McIntosh T, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden A, 1989. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience 28, 233–244. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Scafidi S, Robertson CL, 2015. Metabolic alterations in developing brain after injury: knowns and unknowns. Neurochem. Res 40, 2527–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millen JE, Glauser FL, Fairman RP, 1985. A comparison of physiological responses to percussive brain trauma in dogs and sheep. J. Neurosurg 62, 587–591. [DOI] [PubMed] [Google Scholar]
- Milroy G, Dorris L, McMillan TM, 2008. Brief report: sleep disturbances following mild traumatic brain injury in childhood. J. Pediatr. Psychol 33, 242–247. [DOI] [PubMed] [Google Scholar]
- Missios S, Harris BT, Dodge CP, Simoni MK, Costine BA, Lee Y-L, Quebada PB, Hillier SC, Adams LB, Duhaime A-C, 2009. Scaled cortical impact in immature swine: effect of age and gender on lesion volume. J. Neurotrauma 26, 1943–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi EM, Kellaway P, 1984. Cerebral concussion in children: assessment of injury by electroencephalography. Pediatrics 73, 419–425. [PubMed] [Google Scholar]
- Modi PK, Kanungo M, 2010. Age-dependent expression of S100β in the brain of mice. Cell. Mol. Neurobiol 30, 709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondello S, Papa L, Buki A, Bullock MR, Czeiter E, Tortella FC, Wang KK, Hayes RL, 2011. Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: a case control study. Crit. Care 15, R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondello S, Shear DA, Bramlett HM, Dixon CE, Schmid KE, Dietrich WD, Wang KK, Hayes RL, Glushakova O, Catania M, Richieri SP, Povlishock JT, Tortella FC, Kochanek PM, 2016a. Insight into pre-clinical models of traumatic brain injury using circulating brain damage biomarkers: operation brain trauma therapy. J. Neurotrauma 33, 595–605. [DOI] [PubMed] [Google Scholar]
- Mondello S, Kobeissy F, Vestri A, Hayes RL, Kochanek PM, Berger RP, 2016b. Serum concentrations of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein after pediatric traumatic brain injury. Sci. Rep 6, 28203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RD, Hillman CH, Broglio SP, 2014. The persistent influence of concussive injuries on cognitive control and neuroelectric function. J. Athl. Train 49, 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RD, Pindus DM, Drolette ES, Scudder MR, Raine LB, Hillman CH, 2015. The persistent influence of pediatric concussion on attention and cognitive control during flanker performance. Biol. Psychol 109, 93–102. [DOI] [PubMed] [Google Scholar]
- Moore DR, Pindus DM, Raine LB, Drollette ES, Scudder MR, Ellemberg D, Hillman CH, 2016. The persistent influence of concussion on attention, executive control and neuroelectric function in preadolescent children. Int. J. Psychophysiol 99, 85–95. [DOI] [PubMed] [Google Scholar]
- Moore RD, Lepine J, Ellemberg D, 2017. The independent influence of concussive and sub-concussive impacts on soccer players’ neurophysiological and neuropsychological function. Int. J. Psychophysiol 112, 22–30. [DOI] [PubMed] [Google Scholar]
- Mountney A, Boutte AM, Cartagena CM, Flerlage WF, Johnson WD, Rho C, Lu XC, Yarnell A, Marcsisin S, Sousa J, Vuong C, Zottig V, Leung LY, Deng-Bryant Y, Gilsdorf J, Tortella FC, Shear DA, 2017. Functional and molecular correlates after single and repeated rat closed-head concussion: indices of vulnerability after brain injury. J. Neurotrauma 34, 2768–2789. [DOI] [PubMed] [Google Scholar]
- Muir B, Lynn A, Maguire M, Ryan B, Calow D, Duffy M, Souckey Z, 2014. A pilot study of postural stability testing using controls: the modified BESS protocol integrated with an H-pattern visual screen and fixed gaze coupled with cervical range of motion. J. Can. Chiropr. Assoc 58, 361. [PMC free article] [PubMed] [Google Scholar]
- Mychasiuk R, Farran A, Esser MJ, 2014. Assessment of an experimental rodent model of pediatric mild traumatic brain injury. J. Neurotrauma 31, 749–757. [DOI] [PubMed] [Google Scholar]
- Nandrajog P, Idris Z, Azlen WN, Liyana A, Abdullah JM, 2017. The use of event-related potential (P300) and neuropsychological testing to evaluate cognitive impairment in mild traumatic brain injury patients. Asian J. Neurosurg 12, 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli A, Barbe M, Darvish K, Obeid I, 2012. Assessing traumatic brain injuries using EEG power spectral analysis and instantaneous phase. In: Conference Proceedings : … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference 2012, pp. 4692–4695. [DOI] [PubMed] [Google Scholar]
- Nizamutdinov D, Shapiro L, 2017. Overview of traumatic brain injury: an immunological context. Brain Sci 7, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuwer MR, Hovda DA, Schrader LM, Vespa PM, 2005. Routine and quantitative EEG in mild traumatic brain injury. Clin. Neurophysiol 116, 2001–2025. [DOI] [PubMed] [Google Scholar]
- O’Connor WT, Smyth A, Gilchrist MD, 2011. Animal models of traumatic brain injury: a critical evaluation. Pharmacol. Ther 130, 106–113. [DOI] [PubMed] [Google Scholar]
- Ogata M, Tsuganezawa O, 1999. Neuron-specific enolase as an effective immunohistochemical marker for injured axons after fatal brain injury. Int. J. Legal Med 113, 19–25. [DOI] [PubMed] [Google Scholar]
- Ogino Y, Vascak M, Povlishock JT, 2018. Intensity specific repetitive mild traumatic brain injury evokes an exacerbated burden of neocortical axonal injury. J. Neuropathol. Exp. Neurol 77, 782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oli MW, Hayes RL, Robinson G, Wang KK, 2009. Traumatic brain injury biomarkers: from pipeline to diagnostic assay development. Neuroproteomics 293–302 Springer. [DOI] [PubMed] [Google Scholar]
- Olson E, Badder C, Sullivan S, Smith C, Propert K, Margulies SS, 2016. Alterations in daytime and nighttime activity in piglets after focal and diffuse brain injury. J. Neurotrauma 33, 734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster I, Shamdeen GM, Gottschling S, Gortner L, Meyer S, 2010. Electroencephalogram in children with minor traumatic brain injury. J. Paediatr. Child Health 46, 373–377. [DOI] [PubMed] [Google Scholar]
- Ozen LJ, Itier RJ, Preston FF, Fernandes MA, 2013. Long-term working memory deficits after concussion: electrophysiological evidence. Brain Inj 27, 1244–1255. [DOI] [PubMed] [Google Scholar]
- Papa L, Ramia MM, Edwards D, Johnson BD, Slobounov SM, 2015. Systematic review of clinical studies examining biomarkers of brain injury in athletes after sports-related concussion. J. Neurotrauma 32, 661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa L, Mittal MK, Ramirez J, Ramia M, Kirby S, Silvestri S, Giordano P, Weber K, Braga CF, Tan CN, 2016. In children and youth with mild and moderate traumatic brain injury, glial fibrillary acidic protein out-performs S100β in detecting traumatic intracranial lesions on computed tomography. J. Neurotrauma 33, 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa L, Mittal MK, Ramirez J, Silvestri S, Giordano P, Braga CF, Tan CN, Ameli NJ, Lopez MA, Haeussler CA, Mendez Giordano D, Zonfrillo MR, 2017. Neuronal biomarker ubiquitin C-terminal hydrolase detects traumatic intracranial lesions on computed tomography in children and youth with mild traumatic brain injury. J. Neurotrauma 34, 2132–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-H, Hwang S-K, 2018. Prognostic value of serum levels of S100 calcium-binding protein B, neuron-specific enolase, and Interleukin-6 in pediatric patients with traumatic brain injury. World Neurosurg 118, e534–e542. [DOI] [PubMed] [Google Scholar]
- Park D-W, Park S-H, Hwang S-K, 2018. Serial measurement of S100B and NSE in pediatric traumatic brain injury. Childs Nerv. Syst 1–6. [DOI] [PubMed]
- Parks AC, Moore RD, Wu CT, Broglio SP, Covassin T, Hillman CH, Pontifex MB, 2015. The association between a history of concussion and variability in behavioral and neuroelectric indices of cognition. Int. J. Psychophysiol 98, 426–434. [DOI] [PubMed] [Google Scholar]
- Peacock WFT, Van Meter TE, Mirshahi N, Ferber K, Gerwien R, Rao V, Sair HI, Diaz-Arrastia R, Korley FK, 2017. Derivation of a three biomarker panel to improve diagnosis in patients with mild traumatic brain injury. Front. Neurol 8, 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraglia AL, Plog BA, Dayawansa S, Chen M, Dashnaw ML, Czerniecka K, Walker CT, Viterise T, Hyrien O, Iliff JJ, Deane R, Nedergaard M, Huang JH, 2014. The spectrum of neurobehavioral sequelae after repetitive mild traumatic brain injury: a novel mouse model of chronic traumatic encephalopathy. J. Neurotrauma 31, 1211–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold A, 2005. Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J. Neurol. Sci 233, 183–198. [DOI] [PubMed] [Google Scholar]
- Piazza O, Storti M, Cotena S, Stoppa F, Perrotta D, Esposito G, Pirozzi N, Tufano R, 2007. S100B is not a reliable prognostic index in paediatric TBI. Pediatr. Neurosurg 43, 258–264. [DOI] [PubMed] [Google Scholar]
- Pickering A, Carter J, Hanning I, Townend W, 2008. Emergency department measurement of urinary S100B in children following head injury: can extracranial injury confound findings? Emerg. Med. J 25, 88–89. [DOI] [PubMed] [Google Scholar]
- Pickett TC, 2007. Objectively assessing balance deficits after TBI: role of computerized posturography. J. Rehabil. Res. Dev 44, 983–990. [DOI] [PubMed] [Google Scholar]
- Pitt J, Leung Y, 2015. Clinical and cellular aspects of traumatic brain injury. In: Handbook of Toxicology of Chemical Warfare Agents Elsevier, pp. 691–708. [Google Scholar]
- Pontifex MB, O’Connor PM, Broglio SP, Hillman CH, 2009. The association between mild traumatic brain injury history and cognitive control. Neuropsychologia 47, 3210–3216. [DOI] [PubMed] [Google Scholar]
- Portela LV, Tort AB, Schaf DV, Ribeiro L, Nora DB, Walz R, Rotta LN, Silva CT, Busnello JV, Kapczinski F, 2002. The serum S100B concentration is age dependent. Clin. Chem 48, 950–952. [PubMed] [Google Scholar]
- Prins ML, Hovda DA, 2003. Developing experimental models to address traumatic brain injury in children. J. Neurotrauma 20, 123–137. [DOI] [PubMed] [Google Scholar]
- Quatman-Yates C, Hugentobler J, Ammon R, Mwase N, Kurowski B, Myer GD, 2014. The utility of the balance error scoring system for mild brain injury assessments in children and adolescents. Phys. Sportsmed 42, 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghupathi R, Huh JW, 2007. Diffuse brain injury in the immature rat: evidence for an age-at-injury effect on cognitive function and histopathologic damage. J. Neurotrauma 24, 1596–1608. [DOI] [PubMed] [Google Scholar]
- Rapp PE, Keyser DO, Albano A, Hernandez R, Gibson DB, Zambon RA, David Hairston W, Hughes JD, Krystal A, Nichols AS, 2015. Traumatic brain injury detection using electrophysiological methods. Front. Hum. Neurosci 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redell JB, Moore AN, Ward NH III, Hergenroeder GW, Dash PK, 2010. Human traumatic brain injury alters plasma microRNA levels. J. Neurotrauma 27, 2147–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhine T, Babcock L, Zhang N, Leach J, Wade SL, 2016. Are UCH-L1 and GFAP promising biomarkers for children with mild traumatic brain injury? Brain Inj 30, 1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann BL, Guskiewicz KM, 2000. Effects of mild head injury on postural stability as measured through clinical balance testing. J. Athl. Train 35, 19. [PMC free article] [PubMed] [Google Scholar]
- Rivara FP, Koepsell TD, Wang J, Temkin N, Dorsch A, Vavilala MS, Durbin D, Jaffe KM, 2011. Disability 3, 12, and 24 months after traumatic brain injury among children and adolescents. Pediatr. Engl. Ed 128, e1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Winer JL, Berkner J, Chan LA, Denson JL, Maxwell JR, Yang Y, Sillerud LO, Tasker RC, Meehan WP III, Mannix R, Jantzie LL, 2016. Imaging and serum biomarkers reflecting the functional efficacy of extended erythropoietin treatment in rats following infantile traumatic brain injury. J. Neurosurg. Pediatr 17, 739–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermundt M, Peters M, Prehn JH, Arolt V, 2003. S100B in brain damage and neurodegeneration. Microsc. Res. Tech 60, 614–632. [DOI] [PubMed] [Google Scholar]
- Rowe RK, Harrison JL, O’Hara BF, Lifshitz J, 2014a. Recovery of neurological function despite immediate sleep disruption following diffuse brain injury in the mouse: clinical relevance to medically untreated concussion. Sleep 37, 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe RK, Striz M, Bachstetter AD, Van Eldik LJ, Donohue KD, O’Hara BF, Lifshitz J, 2014b. Diffuse brain injury induces acute post-traumatic sleep. PLoS One 9, e82507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MC, Kochunov P, Sherman PM, Rowland LM, Wijtenburg SA, Acheson A, Hong LE, Sladky J, McGuire S, 2018a. Miniature pig magnetic resonance spectroscopy model of normal adolescent brain development. J. Neurosci. Methods 308, 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MC, Sherman P, Rowland LM, Wijtenburg SA, Acheson A, Fieremans E, Veraart J, Novikov DS, Hong LE, Sladky J, Peralta PD, Kochunov P, McGuire SA, 2018b. Miniature pig model of human adolescent brain white matter development. J. Neurosci. Methods 296, 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbatini M, Barili P, Bronzetti E, Zaccheo D, Amenta F, 1999. Age-related changes of glial fibrillary acidic protein immunoreactive astrocytes in the rat cerebellar cortex. Mech. Ageing Dev 108, 165–172. [DOI] [PubMed] [Google Scholar]
- Sabir M, Gaudreault P-O, Freyburger M, Massart R, Blanchet-Cohen A, Jaber M, Gosselin N, Mongrain V, 2015. Impact of traumatic brain injury on sleep structure, electrocorticographic activity and transcriptome in mice. Brain Behav. Immun 47, 118–130. [DOI] [PubMed] [Google Scholar]
- Samadani U, Ritlop R, Reyes M, Nehrbass E, Li M, Lamm E, Schneider J, Shimunov D, Sava M, Kolecki R, 2015. Eye tracking detects disconjugate eye movements associated with structural traumatic brain injury and concussion. J. Neurotrauma 32, 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadani U, Li M, Qian M, Laska E, Ritlop R, Kolecki R, Reyes M, Altomare L, Sone JY, Adem A, 2016. Sensitivity and specificity of an eye movement tracking-based biomarker for concussion. Concussion 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandsmark DK, Elliott JE, Lim MM, 2017. Sleep-wake disturbances after traumatic brain injury: synthesis of human and animal studies. Sleep 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savola O, Pyhtinen J, Leino TK, Siitonen S, Niemela O, Hillbom M, 2004. Effects of head and extracranial injuries on serum protein S100B levels in trauma patients. J. Trauma 56, 1229–1234 (discussion 1234). [DOI] [PubMed] [Google Scholar]
- Schmitt S, Dichter MA, 2015. Electrophysiologic recordings in traumatic brain injury. In: Handbook of Clinical Neurology Elsevier, pp. 319–339. [DOI] [PubMed] [Google Scholar]
- Schober ME, Requena DF, Block B, Davis LJ, Rodesch C, Casper TC, Juul SE, Kesner RP, Lane RH, 2014. Erythropoietin improved cognitive function and decreased hippocampal caspase activity in rat pups after traumatic brain injury. J. Neurotrauma 31, 358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriver BJ, Bagdasarov S, Wang Q, 2018. Pupil-linked arousal modulates behavior in rats performing a whisker deflection direction discrimination task. J. Neurophysiol 120, 1655–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Carlson J, Noble-Haeusslein LJ, 2016. Pediatric rodent models of traumatic brain injury. In: Injury Models of the Central Nervous System Springer, pp. 325–343. [DOI] [PubMed] [Google Scholar]
- Shaw G, Yang C, Ellis R, Anderson K, Mickle JP, Scheff S, Pike B, Anderson DK, Howland DR, 2005. Hyperphosphorylated neurofilament NF-H is a serum biomarker of axonal injury. Biochem. Biophys. Res. Commun 336, 1268–1277. [DOI] [PubMed] [Google Scholar]
- Shetty T, Nguyen JT, Cogsil T, Tsiouris AJ, Niogi SN, Kim EU, Dalal A, Halvorsen K, Cummings K, Zhang T, 2018. Clinical findings in a multicenter MRI study of mild TBI. Front. Neurol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohami E, Shapira Y, Cotev S, 1988. Experimental closed head injury in rats: pros-taglandin production in a noninjured zone. Neurosurgery 22, 859–863. [PubMed] [Google Scholar]
- Shultz SR, McDonald SJ, Haar CV, Meconi A, Vink R, van Donkelaar P, Taneja C, Iverson GL, Christie BR, 2017. The potential for animal models to provide insight into mild traumatic brain injury: translational challenges and strategies. Neurosci. Biobehav. Rev 76, 396–414. [DOI] [PubMed] [Google Scholar]
- Smith DH, Meaney DF, 2000. Axonal damage in traumatic brain injury. Neuroscientist 6, 483–495. [Google Scholar]
- Smith DH, Hicks R, Povlishock JT, 2013. Therapy development for diffuse axonal injury. J. Neurotrauma 30, 307–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordillo PP, Sordillo LA, Helson L, 2016. Bifunctional role of pro-inflammatory cytokines after traumatic brain injury. Brain Inj 30, 1043–1053. [DOI] [PubMed] [Google Scholar]
- Spinella PC, Dominguez T, Drott HR, Huh J, McCormick L, Rajendra A, Argon J, McIntosh T, Helfaer M, 2003. S-100β protein–serum levels in healthy children and its association with outcome in pediatric traumatic brain injury. Crit. Care Med 31, 939–945. [DOI] [PubMed] [Google Scholar]
- Stalhammar D, Galinat BJ, Allen AM, Becker DP, Stonnington HH, Hayes RL, 1987. A new model of concussive brain injury in the cat produced by extradural fluid volume loading: I. Biomechanical properties. Brain Inj 1, 73–91. [DOI] [PubMed] [Google Scholar]
- Suh M, Basu S, Kolster R, Sarkar R, McCandliss B, Ghajar J, Cognitive, Consortium, N.R, 2006. Increased oculomotor deficits during target blanking as an indicator of mild traumatic brain injury. Neurosci. Lett 410, 203–207. [DOI] [PubMed] [Google Scholar]
- Tham SW, Fales J, Palermo TM, 2015. Subjective and objective assessment of sleep in adolescents with mild traumatic brain injury. J. Neurotrauma 32, 847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelin EP, Nelson DW, Bellander B-M, 2017a. A review of the clinical utility of serum S100B protein levels in the assessment of traumatic brain injury. Acta Neurochir 159, 209–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelin EP, Zeiler FA, Ercole A, Mondello S, Büki A, Bellander B-M, Helmy A, Menon DK, Nelson DW, 2017b. Serial sampling of serum protein biomarkers for monitoring human traumatic brain injury dynamics: a systematic review. Front. Neurol 8, 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriault M, De Beaumont L, Gosselin N, Filipinni M, Lassonde M, 2009. Electrophysiological abnormalities in well functioning multiple concussed athletes. Brain Inj 23, 899–906. [DOI] [PubMed] [Google Scholar]
- Ucar T, Tanriover G, Gurer I, Onal MZ, Kazan S, 2006. Modified experimental mild traumatic brain injury model. J. Trauma 60, 558–565. [DOI] [PubMed] [Google Scholar]
- Vink R, 2018. Large animal models of traumatic brain injury. J. Neurosci. Res 96, 527–535. [DOI] [PubMed] [Google Scholar]
- Wickwire EM, Williams SG, Roth T, Capaldi VF, Jaffe M, Moline M, Motamedi GK, Morgan GW, Mysliwiec V, Germain A, Pazdan RM, Ferziger R, Balkin TJ, MacDonald ME, Macek TA, Yochelson MR, Scharf SM, Lettieri CJ, 2016. Sleep, sleep disorders, and mild traumatic brain injury. What we know and what we need to know: findings from a National Working Group. Neurotherapeutics 13, 403–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams FJ, Mills DS, Guo K, 2011. Development of a head-mounted, eye-tracking system for dogs. J. Neurosci. Methods 194, 259–265. [DOI] [PubMed] [Google Scholar]
- Xiao-Sheng H, Sheng-Yu Y, Xiang Z, Zhou F, Jian-Ning Z, Li-Sun Y, 2000. Diffuse axonal injury due to lateral head rotation in a rat model. J. Neurosurg 93, 626–633. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M, 2013. Animal models of traumatic brain injury. Nat. Rev. Neurosci 14, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Song J, Bu X, Wang C, Wu J, Cai J, Wan S, Fan C, Zhang C, Wang J, 2016. Elevated serum miR-93, miR-191, and miR-499 are noninvasive biomarkers for the presence and progression of traumatic brain injury. J. Neurochem 137, 122–129. [DOI] [PubMed] [Google Scholar]
- Yi L, Shi S, Wang Y, Huang W, Xia Z. a., Xing Z, Peng W, Wang Z, 2016. Serum metabolic profiling reveals altered metabolic pathways in patients with post-traumatic cognitive impairments. Sci. Rep 6, 21320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokobori S, Hosein K, Burks S, Sharma I, Gajavelli S, Bullock R, 2013. Biomarkers for the clinical differential diagnosis in traumatic brain injury—a systematic review. CNS Neurosci. Ther 19, 556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorzinski JL, Patricelli GL, Babcock JS, Pearson JM, Platt ML, 2013. Through their eyes: selective attention in peahens during courtship. J. Exp. Biol 216, 3035–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A, Rao MV, Nixon RA, 2012. Neurofilaments at a Glance The Company of Biologists Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue JK, Vassar MJ, Lingsma HF, Cooper SR, Okonkwo DO, Valadka AB, Gordon WA, Maas AI, Mukherjee P, Yuh EL, 2013. Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. J. Neurotrauma 30, 1831–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaloshnja E, Miller T, Langlois JA, Selassie AW, 2008. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J. Head Trauma Rehabil 23, 394–400. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Saraswati M, Koehler RC, Robertson C, Kannan S, 2015. A new rabbit model of pediatric traumatic brain injury. J. Neurotrauma 32, 1369–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Ahram A, Berman RF, Muizelaar JP, Lyeth BG, 2003. Early loss of astrocytes after experimental traumatic brain injury. Glia 44, 140–152. [DOI] [PubMed] [Google Scholar]
- Žurek J, Fedora M, 2012. The usefulness of S100B, NSE, GFAP, NF-H, secretagogin and Hsp70 as a predictive biomarker of outcome in children with traumatic brain injury. Acta Neurochir 154, 93–103. [DOI] [PubMed] [Google Scholar]