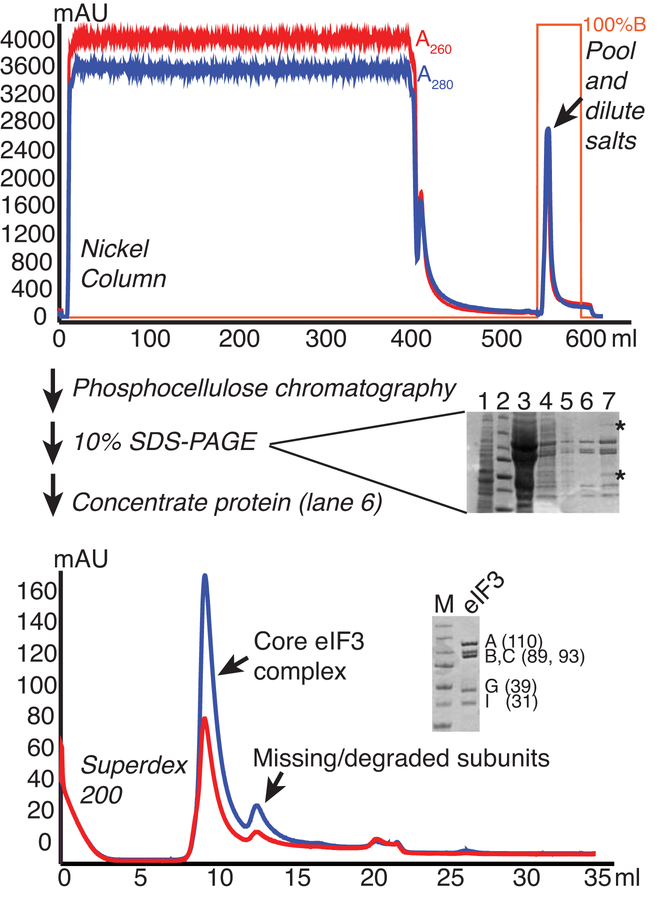
Figure 1.
eIF3 purification from yeast. Lysate from cells overexpressing His-tagged eIF3B were fractionated on Nickel, Phosphocellulose (P11), and Superdex 200 columns to purify the core eIF3 complex. Chromatograms are shown for Nickel and Superdex columns, with A260 and A280 traces shown in red and blue, respectively and percentage buffer B (high imidazole) in orange. 10% SDS-PAGE gels stained with Coomassie are shown. The gel run following P11 (center) shows 1) clarified lysate, 2) MW marker, 3) P11 bound, 4) Ni eluate/P11 load, 5) P11 flowthrough, 6) P11-B200 eluate, 7) P11-B350 eluate. Asterisks mark contaminants that are consistently found in B350/1000 fractions. The larger contaminant is associated with RNase activity and the lower is a similar size to eIF3 ligands eIF5 and eIF2-gamma. The lower gel shows the final purified protein next to MW marker (Precision plus, Biorad: 250, 150, 100, 75, 50, 37, and 25 kDa bands), with the subunits and approximate MWs indicated.