Abstract
Background:
The human brain remains highly plastic for a protracted developmental period. Thus, although early caregiving adversities that alter amygdala development can result in enduring emotion regulation difficulties, these trajectories should respond to subsequent enriched caregiving. Exposure to high quality parenting can regulate (e.g., decrease) children’s amygdala reactivity, a process that, over the long-term, is hypothesized to enhance emotion regulation. We tested the hypothesis that, even following adversity, the parent-child relationship would be associated with amygdala decreases to parent cues, which would in turn predict lower future anxiety.
Methods:
Participants were 102 children (6–10 years old) and adolescents (11–17 years old), with one or two data points, that had experienced institutional care before adoption (PI; n=45) or lived with their biological parents (Comparison; n=57). We examined how amygdala reactivity to visual cues of the parent at Time 1 predicted longitudinal change (Time 1 to 2) in parent reported child anxiety across 3 years.
Results:
At Time 1, on average, amygdala reactivity decrements to parent cues were not seen in children that were PI but were seen in Comparison children. However, some children that were PI did show decreased amygdala to parent cues (~40%), which was associated with greater child-reported feelings of security with their parent. Amygdala decreases at Time 1 were followed by steeper anxiety reductions from Time 1 – 2 (i.e., 3 years).
Conclusions:
These data provide a neurobiological mechanism by which the parent-child relationship can increase resilience, even in children at significant risk for anxiety symptoms.
Keywords: Parental-deprivation, amygdala, buffering, parent, previously institutionalized, fMRI
Introduction
Neuro-affective processes contribute to mental health across the lifespan, and parental caregiving lays the foundation for their construction. Whereas stability, warmth, and support promote emotion regulation development [1], caregiving adversities are risk factors, contributing to over a third of mental illnesses [e.g., anxiety and mood disorders; 2, 3] and increasing developmental risk to associated neurobiology [e.g., amygdala-cortical circuitry; 4, 5].
Across many species, high-quality parenting has a powerful regulatory effect on offspring’s stress and emotional reactivity, particularly during the juvenile period. Parents reduce distress, block stress hormone release, and modulate emotional behavior, effects collectively known as ‘parental buffering’ [6–13]. Rodent models implicate the amygdala as one part of the complex neurobiology involved in such buffering effects [see 14 for a review of extra-amygdala regions involved in social buffering effects]. That is, in the presence of parental cues (visual, tactile, or olfactory), the amygdala often exhibits decreased activation. For example, parental presence (or a learned maternal odor cue) blocks glucocorticoid elevations and decreases amygdala reactivity in rat pups, thereby decreasing aversive learning [15, 16]. In humans too, amygdala reactivity is decreased by parental cues (i.e., parent photographs) during childhood [13], suggesting that the amygdala may be part of the mechanism for parental regulation of child emotions also. Childhood has been posited as a ‘sensitive period’ for parental influence on the developing amygdala, when parents both attenuate amygdala reactivity, and at the same time, help to shape the strength and nature of amygdala-cortical connectivity development, which supports future affective self-regulation [17, 18]. Hence, amygdala reactivity to parent cues during childhood is a strong candidate mechanism for linking early caregiving experiences with long-term mental health.
Parental buffering of stress responses can be compromised by early adversity exposure. For example, parental buffering of cortisol responsivity to a social stressor was weaker in children exposed to early caregiver deprivation [i.e., those with a history of previous institutional [PI] care; 18]. Similarly, in animal models, parental presence has been shown to be less effective in buffering fear/stress reactivity in offspring exposed to adversity [16, 19], suggesting that early adversity can lead to a loss of neoteny (and associated plasticity) in amygdala function [16]. We recently hypothesized that early parental care, brain development, and behavior, come together to form a ‘Neuroenvironmental Loop’, which scaffolds the maturation of emotion regulation circuitry [17]. According to this model, there is an intimate and dynamic association between parental stimuli and the development of amygdala-related circuitry. Specifically, normally-occurring developmental plasticity of the amygdala allows for parental influence over amygdala function (e.g., decreased reactivity); this influence of the parent is hypothesized to exert enduring effects on amygdala circuitry and associated emotional reactivity. However, the amygdala and its connections are highly susceptible to alterations following adverse care experiences [4, 5, 16, 20–31]. Thus, parental cues, which might be most effective in regulating amygdala activity in childhood [13], may have less influence on amygdala function following adversity exposure in some children. However, as there is high heterogeneity in these amygdala-related outcomes, we would anticipate that children who exhibit amygdala buffering by parental cues, despite early adversity, would be protected against future psychopathology (e.g., anxiety), as predicted by the ‘Neuroenvironmental Loop’ model. This finding would provide a social-neural mechanism for resilience in this high-risk group.
Here, we tested the group-level hypothesis that previous institutional caregiving would be followed by a relative absence of amygdala reactivity decreases to parent cues in childhood. Also, we tested two within-subjects hypotheses that decreased amygdala reactivity to parental cues (even following adversity) would (1) mitigate future anxiety levels, and (2) be associated with the child’s reported security in the attachment relationship for youth that were previously institutionalized; these hypotheses are based on findings that placement in a stable family has proven benefits for children’s anxiety [32, 33], particularly for those who establish a secure attachment. To address these hypotheses, we examined differences in amygdala reactivity to visual cues of the parent versus stranger during functional magnetic resonance imaging (fMRI), parent report of child/adolescent anxiety, and child report of their relationship with the parent (see Figure 1). We focused on anxiety, rather than depression, due to the associations of amygdala reactivity with anxiety symptoms [34].
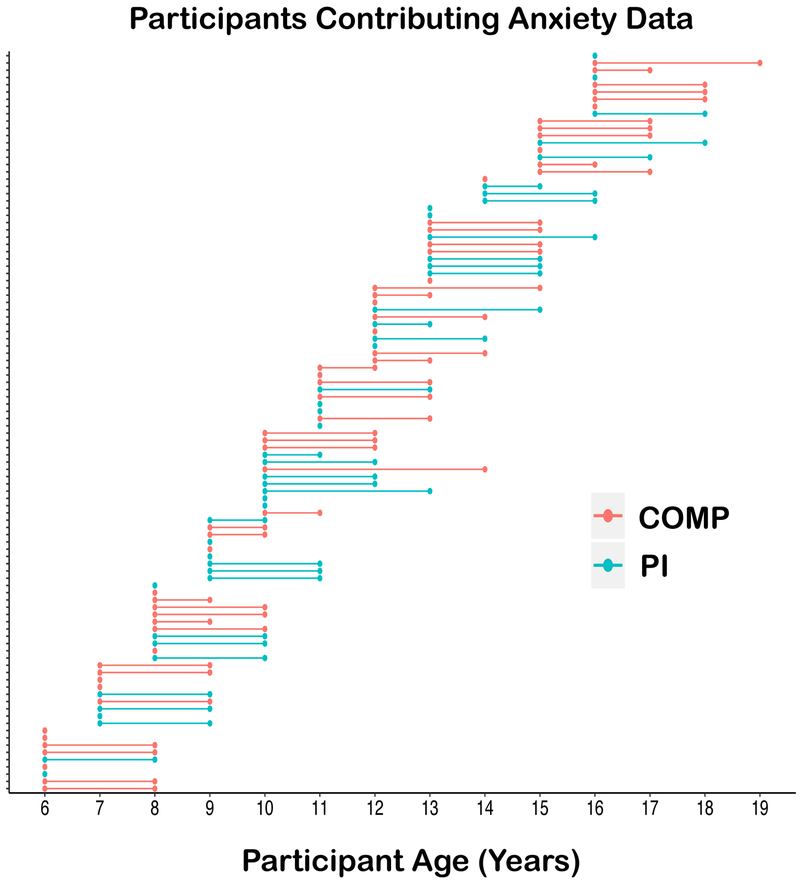
Figure 1.
Follow-up period for each participant that contributed MRI and anxiety data. Lines represent the length of follow up period in the longitudinal analysis; dots represent the anxiety assessments for each individual (fMRI collected at first dot (i.e., Time 1)).
Materials and Methods
Participants
Functional Magnetic Resonance Imaging (fMRI) data were collected from 109 youths. The final sample that provided usable data were N=102 participants (M=10.25 years, 5–16 years old; see Supplemental Table S1 and Supplemental Figure 1 for demographics and exclusion criteria). Age was grouped into children versus adolescents because there was no expectation of linear age-related changes in amygdala response to parent cues, and our previous publication showed that typically raised children, but not adolescents, showed decreased amygdala reactivity to parent cues [13]. That prior finding, as well as the changing dynamic of the parent-child relationship from childhood to adolescence (increased independence from the parent [35]), guided the current hypothesis regarding PI youth. These two age groups also differed in pubertal development as measured by Testosterone levels (see supplemental results).
Comparison youths always lived with their biological parents. Upon enrollment, their parents reported no child/adolescent psychiatric diagnoses, and as a group they scored in the average range on the Child Behavior Checklist (CBCL [36], M=45.58, SD=1.42, range=23–66). PI youths had a history of institutional care before international adoption into the United States (see Supplemental Table S2 for adoption related information). The University of California, Los Angeles Institutional Review Board, approved the protocol. Parents provided informed consent.
Procedure
Data were collected over two separate waves (Time 1=Year 1, Time 2=Year 3; see Figure 1) using an accelerated longitudinal design. At Time 1, youth were acclimatized to the scanner environment using an MRI replica and completed fMRI scanning within 3.91 months (SD=4.07; range 0–20 months). Also at Time 1, questionnaire data were collected (N=99 parents, N=89 children). Parents (N=72) completed questionnaires again at Time 2 (Year 3; mean (SD) interval=2.43 (.58) years). Attrition was not associated with variables of interest (see supplemental data).
Questionnaires
Revised Child Anxiety and Depression Scale Parent Version (RCADS-P).
Youth anxiety was measured using the Revised Child Anxiety and Depression Scale: [RCADS-P; 37], which has good internal consistency [Cronbach’s α = .84; 37] and requires parents to rate the frequency with which their child displays specific emotional behaviors. The scale has been validated for childhood and adolescence, including youth with a history of institutional care [38]. We focused on total anxiety symptoms across all categories.
The Security Scale.
The Security Scale [39], which has good internal consistency [Chronbach’s α = .93; 39], assesses the child/adolescent’s reported security in the parent-child relationship in the domains of parent responsivity/availability, reliability during times of stress, and interest in communicating with the parent; higher scores indicate greater feelings of security in the parent-child relationship. This measure was collected at Time 1 only (Comp Mean (SD)=3.16 (.58), PI Mean (SD)=3.15 (.44)), and was not different between groups, t(87)=.06, p=.951. Prior studies have reported high convergent validity of this measure with other observed and reported measures of attachment security from infancy [40, 41].
Parent/Stranger fMRI Task.
In the scanner, participants were presented with 8 alternating blocks (28s each, total time=4.34m) of color photographs of their parent or another child’s parent (stranger; ethnicity- and sex-matched) with smiling and neutral facial expressions (see Supplemental methods for more details). Parent sex was mostly female (82–93% female across groups) and parent sex was not associated with amygdala buffering, t(99)=−.33, p=.745. This design was intended to provide participants with blocks of their parents image alternating with blocks of the stranger. The happy and neutral expressions were included to provide a behavioral task to ensure attention, but there was no expectation that this behavior would be meaningfully related to buffering (nonetheless, see supplemental results for analyses of these behavioral data).
Image Acquisition.
Images were acquired with a Siemens Trio 3-T fMRI scanner (Erlangen, Germany). A whole brain, high resolution, T1-weighted anatomical scan (MPRAGE; 256×256 in-plane resolution, 256mm FOV, 192mmx1mm sagittal slices), was used for transformation and localization of each subject’s functional data into Talairach space [42]. For the functional task, T2*-weighted echo-planar images (34 slices) were acquired using an oblique angle of approximately 30° from each subject’s position, 4-mm slice thickness (skip=0), repetition time=2000ms, echo time=30ms, flip=90°, matrix 64×64.
fMRI Preprocessing.
Functional imaging data were pre-processed and analyzed with the Analysis of Functional NeuroImages (AFNI, version 16.1.28) software package [43]. Volumes with excessive absolute motion (>.5 voxel from reference volume) were censored. Preprocessing steps included slice-timing correction, image registration to the first volume, smoothing with an anisotropic 6mm Gaussian kernel (FWHM), time series normalization, and transformation into Talairach space (see Supplemental Methods for more details).
Statistical Analysis
Right Amygdala ROI.
Based on prior findings [13], we had an a priori hypothesis that we would see changes in the right anatomical amygdala (Talairach & Tournoux Atlas in AFNI) to pictures of the parent in children (but see supplemental material for right and left amygdala signal broken down by sub nuclei (central medial, superficial, and basolateral).
A 2×2 Analysis of Covariance (ANCOVA) was performed in SPSS to test for effects of age group (children vs. adolescents) and caregiving group (Comparison vs. PI) on right amygdala β weights (for the parent–stranger contrast; see supplemental results for the ANOVA outcomes where age was treated continuously). As there was a slight overrepresentation of females in the PI group (see Supplemental Table S1), participant sex was included in all analyses.
Longitudinal associations between decreased amygdala reactivity to parent and anxiety symptoms across time, were performed using a linear mixed model in SPSS with maximum likelihood estimation to accommodate the nested structure of the data (individual change in anxiety symptoms from Time 1 to Time 2). This method captures individual variance while allowing for missing data points, thus dealing with the attrition we had at Time 2 (but see supplemental data for a model which includes only participants the contributed two data points).
We used separate linear regressions to assess associations between amygdala buffering and age of adoption on felt security in the attachment relationship, controlling for age and sex. The alpha value was set at .05 for all analyses and, unless specified, two-sided tests were used.
Results
Response in right anatomical amygdala region of interest (parent–stranger contrast).
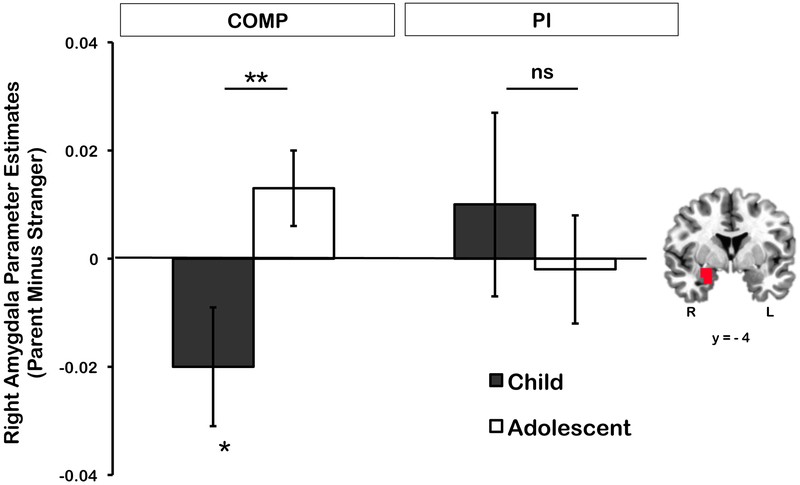
There was a significant Caregiving Group X Age Group interaction, F(1,93)=5.63, p=.020, η2p=.06 (Figure 2, see Supplemental Figure 2 for the effect broken down into parent and stranger contrasts). Post hoc t-tests showed that children in the PI group did not exhibit decreased amygdala to pictures of the parent, t(22)=.61, p=.726, and Bayesian analyses (1-sided, one sample t-test) in JASP [44] indicated that the data from the PI children were 6.82 times more likely to be observed under the null hypothesis (i.e., no difference in amygdala response to parent vs. stranger pictures) than under the alternative hypothesis (i.e., lower amygdala reactivity to parent pictures relative to stranger pictures). In contrast, children in the comparison group showed lower amygdala reactivity to parent than stranger pictures, t(26)=1.84, p=.039 (1-sided t-test) as shown previously [13], but Comparison adolescents did not, ps>.05 (see Supplemental data for post hoc Bayesian analyses in the Comparison youth and PI adolescents). Post-hoc tests of the simple effects indicated no age-related change in amygdala responses to parent cues in the PI group, F(1,93)=.40, p=.529, η2p=.004, whereas age related change was seen in the Comparisons (i.e., decreases in amygdala reactivity to parent cues for children, not adolescents), F(1,93)=8, p=.005, η2p=.08. There were no other main effects or interactions, largest F(1,93)=.693, p=.407, η2p=.01.
Figure 2.
Results for the right amygdala response analysis. The graph shows the mean extracted β weights from the right amygdala in the parent relative to the stranger condition across both caregiving groups (COMP and PI) and age groups (children and adolescents). The double asterisk (**) indicates a significant difference between groups (p<.05), and the single asterisk (*) indicates a significant difference from zero. Error bars show ±1 SEM. The image of the brain represents the region of interest from which the β weight values were extracted in each subject (Montreal Neurological Institute coordinate y=−4; R=right; L=left).
Amygdala decreases to parent cues predicting future anxiety symptoms.
Although at the group level, PI children did not exhibit lower amygdala reactivity to parent than stranger cues, 43% of PI children and 36% of PI adolescents (compared to 55% of Comp children and 33% of Comp adolescents) did show decreased amygdala to parental cues, which could have important implications for long-term anxiety. There were no differences in baseline anxiety (i.e., Time 1) between participants that showed amygdala decreases to parent cues and those that did not (controlling for participant age, sex, and caregiving group), β=2.17, p=.332, d=.19.
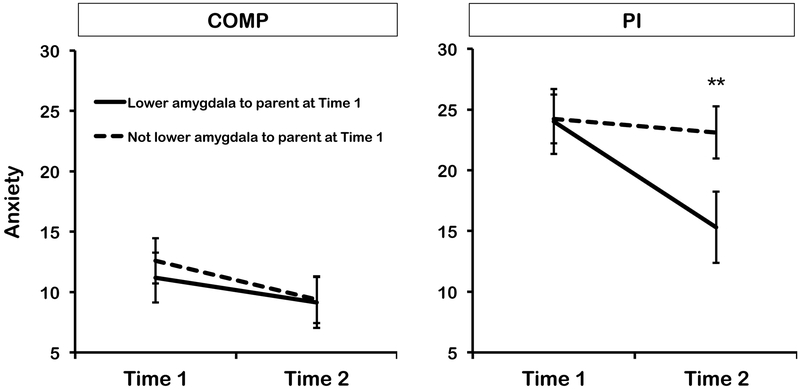
We tested the change in anxiety symptoms across time (Time 1 to Time 2) as a function of amygdala decreases to parental images in PI and Comparison youth using a mixed linear model, with Caregiving condition (Comparison vs. PI), age group (child vs. adolescent), sex (male vs. female), time (Time 1 vs. Time 2), and amygdala response status (categorical: where amygdala betas that were lower to pictures of parent than stranger were considered as ‘decreased to parent’) as fixed effects predictors of RCADS scores, with random slope and intercept between individuals (total N=101 with anxiety data at either Time 1, or Time 2, or both; Time 1: n=99; Time 1 and Time 2: n=70, Time 2 only: n=2). As hypothesized, there was a significant Time × Caregiving Group × Amygdala Signal interaction, F(1,75.67)=5.90, p=.018, η2p=.07, whereby PI youth (both children and adolescents) that exhibited amygdala decreases to parental stimuli at Time 1 showed a sharper decline in anxiety symptoms between Time 1 and Time 2 than PI youth who did not exhibit such amygdala decreases, and comparison youth (Figure 3). Post-hoc tests on the estimated marginal means from the model showed that those PI youth who did and those who did not exhibit decreased amygdala to parent cues did not significantly differ in anxiety symptoms at Time 1, F(1,89.44)=.006, p=.937, η2p=.00, but scores did differ at Time 2, F(1,97.72)=4.53, p=.036, η2p=.04. Comparison youth that did, and did not, exhibit lower amygdala to parent than stranger cues did not differ from each other at either Time 1, F(1,100.25)=.25, p=.618, η2p=.00, or Time 2, F(1,101.17)=.004, p=.951, η2p=.00. See supplemental results for remaining main effects and interactions.
Figure 3.
Mean parent-rated youth anxiety scores on the RCADS in comparison (COMP) and previously institutionalized (PI) groups at Time 1 and Time 2 (2 years after the Time 1 assessment) assessments as a function of whether participants exhibited amygdala buffering or not in response to parent versus stranger stimuli at Time 1. Plot represents the estimated marginal means from the mixed linear model for the effect of Time × Caregiving Group × Buffering interaction, estimated at the mean level of the covariate of participant average motion in scanner).
Associations with age of adoption, time with adoptive family, and child reported security in the attachment relationship.
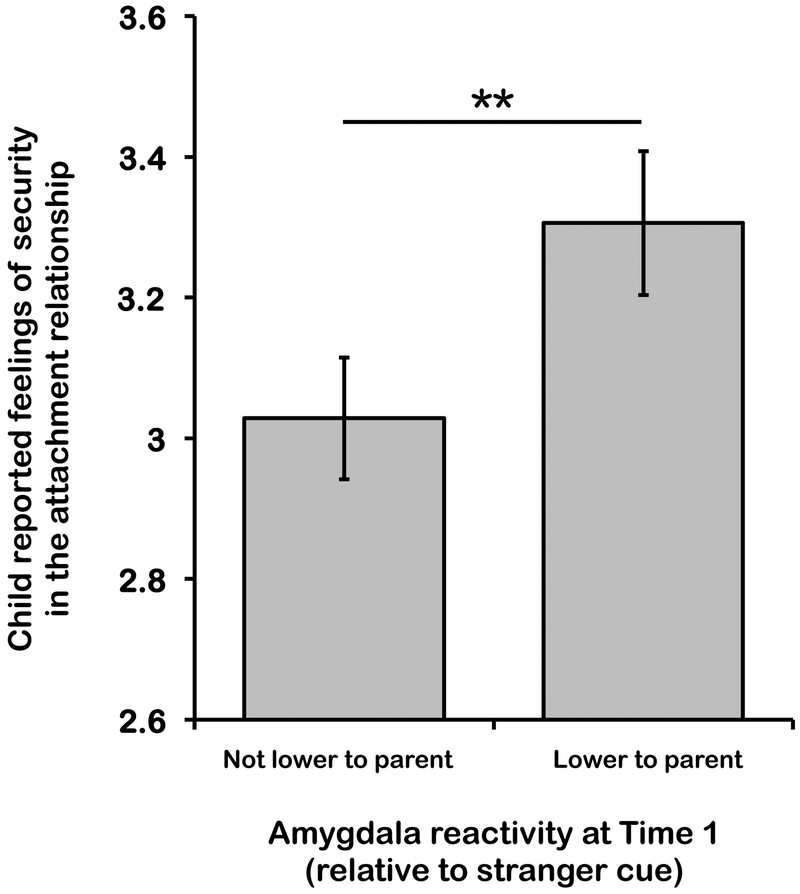
Within the PI group, 15 children were adopted before 12 months of age, and 29 children were adopted after 1 year of age (data were missing for one child). There was no association between age at adoption and amygdala responses (controlling for age at scan and sex) in PI children and adolescents, β=.003, t(40)=1.02, p=.316, d=.14. Decreased amygdala to parent cues was also not associated with the amount of time that PI youth had spent with their adoptive family (controlling for age group and sex), t(40)=.58, p=.563, d=.18, suggesting that caregiving group differences in amygdala response to parent cues were not related to familiarity with the parent stimulus. However, child/adolescent reported security with the adoptive parent did predict whether amygdala decreases occurred, with higher security scores being associated with lower amygdala reactivity to parent than stranger cues, β=.415, t(38)=2.44, p=.019, d=.38 (Figure 4; see supplemental results for child reported security in the attachment relationship and amygdala responses in the Comparison children).
Figure 4.
Child-reported security in the attachment relationship with parent in PI youth that did, and did not, exhibit amygdala buffering to parent cues. Within the PI group, amygdala buffering to parent cues was associated with higher youth-perceived security in the attachment relationship.
Discussion
Here we tested three hypotheses generated from the Neuroenvironmental Loop model [17]. First, we tested whether, on average, early caregiver deprivation would reduce the likelihood of children’s right amygdala showing decreased reactivity to parental stimuli. Second, we examined within the PI group, if more secure parent-child relationships (characterized by higher child-reported feelings of security in the attachment relationship) were associated with amygdala decreases to parent cues. Finally, we asked whether individual differences in amygdala response to parent cues predicted anxiety across time. We found support for all of these hypotheses. First, we found that children from the PI group were less likely to show decreased amygdala reactivity to parent cues, exhibiting amygdala responses that on average paralleled those seen in adolescents. This was unlike the comparison children who exhibited a relative decrease in amygdala activity when viewing pictures of their parent than when viewing images of a stranger, as we have previously shown [13]. Secondly, despite these group differences, inspection of individual data within the PI group indicated that some individuals (~40%), which included both children and adolescents, did exhibit amygdala decreases to pictures of their adoptive parent. Finally, amygdala decreases to parent cues predicted change in anxiety symptoms across time. Decreased amygdala reactivity to parental stimuli at Time 1 was associated with a greater decrease in anxiety symptoms across time in youth from the PI group. Importantly, those individuals who exhibited amygdala decreases to parent did not differ in initial anxiety scores (Time 1) from those who did not; instead associations between amygdala response and anxiety symptoms revealed themselves across time, when examining change from baseline to follow-up. In other words, regardless of within group variation in initial anxiety levels, amygdala reactivity was predictive of intra-individual, long-term anxiety reductions (i.e., the anxiety slope). The fact that we saw Caregiving group differences in anxiety at Time 2, but not Time 1, likely reflects the fact that we are observing a phenomenon that emerges across development, as children are living with their parents. Despite our hypotheses that this effect would be specific to children, the association between amygdala reactivity to parent/stranger cues and anxiety symptom reductions was present for children as well as adolescents, suggesting that amygdala decreases to parent cues at any time in childhood or adolescence is protective against elevated anxiety for youth exposed to early caregiving adversity. That finding has important implications, suggesting that there is capacity for some youth (particularly in the context of high relationship security) to retain child-like plasticity in this circuit (i.e., amygdala decreases to parents), despite adversity exposure.
The group level findings are consistent with previous studies in both rodents and humans [16, 20, 21, 29, 31] suggesting that early parental deprivation changes amygdala development and may even do so through acceleration. In the current study, at the group level, amygdala responses to parental cues in children with a history of institutional care were more similar to adolescents’ responses. Such data is also, at least conceptually, consistent with ‘Life Course’ models, which postulate that certain early environments (i.e., instability or threat) favor accelerated development [45]. These outcomes support the idea that early exposure to adverse environments may recruit the activity of certain neurobiological systems (e.g., those involved in affective processes) at earlier ages, abbreviating developmental plasticity and shifting individuals towards a more adult-like, less plastic, state [30, 31]. Such abbreviated plasticity could result in a vulnerability to anxiety as neural circuits have less time to adapt to the environment across development [17].
The within-subject association between child-reported security in the attachment relationship and amygdala decreases to adoptive parent stimuli was a particularly important finding in the current data set. Several studies have demonstrated the importance of attachment security in the emergence of resilience following institutional care [32, 33, 46]. For example, in the Bucharest Early Intervention Project [32] establishment of a secure attachment mediated improvements of an experimental foster care intervention on internalizing disorders. The current findings provide a potential neurobiological mechanism for that effect. Specifically, we have shown that decreased amygdala to parental cues, which serves a protective function against long-term anxiety symptoms, occurs more frequently in the context of a secure relationship with the attachment figure. These data suggest that interventions targeting children’s feelings of security in the attachment relationship, which has been shown to protect against child internalizing disorders [47, 48], might enhance parental regulation of the amygdala. The fact that the association between security and amygdala reactivity to parent cues did not exist in the Comparison group (see Supplemental material) is not interpreted to mean that security is inconsequential in the Comparison children, but that individual differences in security might be particularly important following early caregiving adversity. Indeed, such findings are consistent with data demonstrating the critical importance of attachment security for resilience within populations that have experienced early institutional caregiving [32, 33, 49]. Interestingly, as there was no association between right amygdala reactivity and adoption timing variables, these data further emphasize the importance of the post-adoption environment (such as feelings of security with the parent), rather than the age at adoption, in amygdala reactivity. Indeed, as youth in this study were in the middle childhood – adolescent age, there has been ample time for the post adoption environment to exert its effects on amygdala development. In contrast, left amygdala signal amplification to parents was not associated with child-reported security in the attachment relationship [50].
Although the sample examined here overlaps with prior reports [13, 50], the novel longitudinal contributions and different analytic approaches used here innovate the work and merit reporting. Specifically, after the finding on left amygdala amplification to parents reported in Olsavsky was published, in Gee et al.,[13], we revisited this task with a renewed hypothesis that children should exhibit a different amygdala response from adolescents to parental stimuli [motivated by findings in rodent development; 15]. To address this question, we used age group as a variable of interest (children versus adolescents) and specifically probed the right amygdala (anatomically defined). This report showed that if children were considered separately from adolescents, the right amygdala exhibited a relative decrease in reactivity to parental stimuli. The current paper sought to apply this hypothesis-driven approach to the PI sample, and again specifically examined age groups (children versus adolescents) in the right (anatomically-defined) amygdala. Additionally, the current paper includes the more recently acquired longitudinal follow-up data, demonstrating a novel, and clinically important, association between amygdala responses to parents and long-term emotional functioning in humans.
A major strength of the current study was the use of a longitudinal design to assess how amygdala decreases to parental cues predicts future anxiety phenotypes. However, there were some important limitations. First, the use of parent images, rather than physical parental presence, limits the ecological validity of the findings that will have to be interrogated in future research. Nonetheless, cues, such as maternal odors in rodent studies [16], or pictures of social support figures in humans [51] are frequently used in buffering studies, and many seminal studies examining the effect of the parent on brain function do not attempt to induce fear to examine parent effects [52]. Further, familiarity of parents versus strangers was not controlled in this study. However, considering that adolescents have greater familiarity with parental stimuli than children, that PI youths had been with the family for many years (i.e., parents were familiar to all subjects), and that time with the adoptive family was not associated with amygdala decreases to parent cues, simple familiarity is unlikely to have influenced the current study outcomes. Also, in line with several studies examining developmental transitions in emotion circuitry [12, 13, 53], and because we did not expect to see linear changes in amygdala response with months of age, we chose to examine age as a categorical variable. In addition, though anxiety was assessed longitudinally in this study, fMRI data from this task was available at only one point, leaving developmental differences in amygdala reactivity in the task to be interpreted on the basis of cross-sectional data. As we had limited pre-adoption information for children in the PI group (including pre adoption mental health) we cannot address the influence of pre-adoption factors. Also, as the levels of anxiety were not clinically significant in the majority of participants, the findings reported here may not generalize to clinical populations, although they are compatible with the Research Domain Criteria objectives to assess psychological factors continuously. Finally, as parent reports of child anxiety can be influenced by parents own anxiety symptoms, it is possible that the change in child anxiety across time may instead have reflected change in parent anxiety across time. Though we did not have parent anxiety at Time 2, at Time 1 it was moderately correlated with child/adolescent anxiety in both groups (Comp r=.44, PI r=.33). However, while we saw change in youth anxiety across time, we have no reason to suspect that parents anxiety would change. Despite these limitations, finding that the quality of the post-adoption relationship was a good predictor of amygdala reactivity to parent cues, and that the amygdala response was associated with long-term mental health, emphasizes the value of post-adoption factors in promoting children’s emotional health
Supplementary Material
Acknowledgements
This research was supported by National Institute of Mental Health Grant R01MH091864 (to N. Tottenham), by the Dana Foundation (to N. Tottenham), by an NSF Conference Grant conference grant (BCS-1439258, co-I NT), by the National Health and Medical Research Council Early Career Fellowship 1091571 (to B. Callaghan), by the American Australian Association (to B. Callaghan), by the National Institute of Mental Health 1K99MH113821 (to B. Callaghan) and by the Brain Behavior Research Association, NARSAD grant (to B. Callaghan).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
None of the authors report any biomedical financial interests or potential conflicts of interest.
Contributor Information
Bridget Callaghan, Department of Psychology, Columbia University; Department of Psychiatry, The University of Melbourne.
Dylan G. Gee, Department of Psychology, Yale
Laurel Gabard-Durnam, Boston Children’s Hospital, Boston.
Eva H. Telzer, Department of Psychology, University of Illinois at Urbana Champagne.
Katherine L Humphreys, Department of Psychology, Stanford University, Stanford.
Bonnie Goff, Department of Psychology, University of California Los Angles.
Mor Shapiro, David Geffen School of Medicine, University of California Los Angeles, Los Angeles.
Jessica Flannery, Department of Psychology, University of Oregon, Eugene.
Daniel S. Lumian, Department of Psychology, University of Denver.
Dominic S. Fareri, Department of Psychology, Adelphi University.
Christina Caldera, Department of Psychology, University of California Los Angles.
Nim Tottenham, Department of Psychology, Columbia University
References
- 1.Repetti RL, Taylor SE, and Seeman TE, (2002): Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin 128: 330–366. [PubMed] [Google Scholar]
- 2.Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, et al. , (2010): Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Archives of General Psychiatry 67: 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, and Kessler RC, (2010): Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication II: associations with persistence of DSM-IV disorders. Archives of General Psychiatry 67: 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. , (2013): Early Developmental Emergence of Mature Human Amygdala-Prefrontal Phenotype following Maternal Deprivation: Evidence of Stress-Induced Acceleration. Proceedings of the National Academy of Sciences 110: 15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silvers JA, Lumian DS, Gabard-Durnam L, Gee DG, Goff B, Fareri DS, et al. , (2016): Previous Institutionalization Is Followed by Broader Amygdala–Hippocampal–PFC Network Connectivity during Aversive Learning in Human Development. Journal of Neuroscience 36: 6420–6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egliston K-A and Rapee RM, (2007): Inhibition of fear acquisition in toddlers following positive modelling by their mothers. Behaviour research and therapy 45: 1871–1882. [DOI] [PubMed] [Google Scholar]
- 7.Hibel LC, Granger DA, Blair C, and Finegood ED, (2014): Maternal-child adrenocortical attunement in early childhood: Continuity and change. Developmental psychobiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofer MA, (1994): Early relationships as regulators of infant physiology and behavior. Acta Paediatrica 83: 9–18. [DOI] [PubMed] [Google Scholar]
- 9.Hostinar CE, Sullivan RM, and Gunnar MR, (2014): Psychobiological mechanisms underlying the social buffering of the hypothalamic–pituitary–adrenocortical axis: A review of animal models and human studies across development. Psychological bulletin 140: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seltzer LJ, Prososki AR, Ziegler TE, and Pollak SD, (2012): Instant messages vs. speech: hormones and why we still need to hear each other. Evolution and Human Behavior 33: 42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorce JF, Emde RN, Campos JJ, and Klinnert MD, (1985): Maternal emotional signaling: Its effect on the visual cliff behavior of 1-year-olds. Developmental Psychology 21: 195. [Google Scholar]
- 12.Hostinar CE, Johnson AE, and Gunnar MR, (2015): Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Developmental science 18: 281–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gee DG, Gabard-Durnam LJ, Telzer EH, Humphreys KL, Goff B, Shapiro M, et al. , (2014): Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychological science: 0956797614550878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenberger NI, (2013): An Empirical Review of the Neural Underpinnings of Receiving and Giving Social Support: Implications for Health. Psychosomatic medicine 75: 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moriceau S and Sullivan RM, (2006): Maternal presence serves as a switch between learning fear and attraction in infancy. Nature neuroscience 9: 1004–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moriceau S, Shionoya K, Jakubs K, and Sullivan RM, (2009): Early-life stress disrupts attachment learning: the role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. The Journal of Neuroscience 29: 15745–15755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Callaghan BL and Tottenham N, (2015): The neuro-environmental loop of plasticity: a cross-species analysis of parental effects on emotion circuitry development following typical and adverse caregiving. Neuropsychopharmacology 41: 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hostinar CE, Johnson AE, and Gunnar MR, (2015): Early social deprivation and the social buffering of cortisol stress responses in late childhood: An experimental study. Developmental psychology 51: 1597–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winslow JT, Noble PL, Lyons CK, Sterk SM, and Insel TR, (2003): Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology 28: 910–918. [DOI] [PubMed] [Google Scholar]
- 20.Callaghan BL and Richardson R, (2012): The effect of adverse rearing environments on persistent memories in young rats: removing the brakes on infant fear memories. Translational Psychiatry 2: e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Callaghan BL and Richardson R, (2014): Early emergence of adult-like fear extinction in the rat following chronic corticosterone treatment of mother or pups. Behavioural Neuroscience 128. [DOI] [PubMed] [Google Scholar]
- 22.Cowan CSM, Callaghan B, and Richardson R, (2013): Acute early-life stress results in early emergence of adult-like fear retention and extinction relapse in infant rats. Behavioral Neuroscience 125: 703–711. [DOI] [PubMed] [Google Scholar]
- 23.Lupien SJ,S and Parent ea, (2011): Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proceedings of the National Academy of Sciences of the United States of America 108: 14324–14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tottenham N, (2012): Human amygdala development in the absence of species-expected caregiving. Developmental Psychobiology 54: 594–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, and Casey BJ, (2011): Elevated amygdala response to faces following early deprivation. Developmental Science 14: 190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, et al. , (2010): Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science 13: 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whittle S, Dennison M, Vijayakumar N, Simmons JG, Yücel M, Lubman DI, et al. , (2013): Childhood Maltreatment and Psychopathology Affect Brain Development During Adolescence. Journal of the American Academy of Child & Adolescent Psychiatry 52: 940–952.e1. [DOI] [PubMed] [Google Scholar]
- 28.Whittle S, Lichter R, Dennison M, Vijayakumar N, Schwartz O, Byrne ML, et al. , (2014): Structural Brain Development and Depression Onset During Adolescence: A Prospective Longitudinal Study. American Journal of Psychiatry 171: 564–571. [DOI] [PubMed] [Google Scholar]
- 29.Ono M, Kikusui T, Sasaki N, Ichikawa M, Mori Y, and Murakami-Murofushi K, (2008): Early weaning induces anxiety and precocious myelination in the anterior part of the basolateral amygdala of male Balb/c mice. Neuroscience 156: 1103–1110. [DOI] [PubMed] [Google Scholar]
- 30.Bath K, Manzano-Nieves G, and Goodwill H, (2016): Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Hormones and behavior 82: 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Callaghan BL and Richardson R, (2011): Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behavioral Neuroscience 125: 20–28. [DOI] [PubMed] [Google Scholar]
- 32.McLaughlin KA, Zeanah CH, Fox NA, and Nelson CA, (2012): Attachment security as a mechanism linking foster care placement to improved mental health outcomes in previously institutionalized children. Journal of Child Psychology and Psychiatry 53: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smyke AT, Zeanah CH, Fox NA, Nelson CA, and Guthrie D, (2010): Placement in foster care enhances quality of attachment among young institutionalized children. Child development 81: 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jovanovic T, Nylocks KM, Gamwell KL, Smith A, Davis TA, Norrholm SD, et al. , (2014): Development of fear acquisition and extinction in children: Effects of age and anxiety. Neurobiology of learning and memory 113: 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Callaghan B, Meyer H, Opendak M, Van Teighem M, Harmon C, Li A, et al. , (In Press): Using a developmental ecology framework to align fear neurobiology across species. Annual Reviews in Clinical Psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Achenbach TM, (1991): Integrative guide for the 1991 CBCL/4–18, YSR, and TRF profiles. Department of Psychiatry, University of Vermont. [Google Scholar]
- 37.Chorpita BF, Yim L, Moffitt C, Umemoto LA, and Francis SE, (2000): Assessment of symptoms of DSM-IV anxiety and depression in children: a revised child anxiety and depression Scale. Behavior Research and Therapy 38: 835–855. [DOI] [PubMed] [Google Scholar]
- 38.Ebesutani C, Tottenham N, and Chorpita B, (2015): The Revised Child Anxiety and Depression Scale - Parent Version: Extended Applicability and Validity for Use with Younger Youth and Children with Histories of Early-Life Caregiver Neglect. Journal of Psychopathology and Behavioral Assessment 37: 705–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kerns KA, Klepac L, and Cole A, (1996): Peer relationships and preadolescents’ perceptions of security in the child-mother relationship. Developmental psychology 32: 457. [Google Scholar]
- 40.Boldt LJ, Kochanska G, Yoon JE, and Koenig Nordling J, (2014): Children’s attachment to both parents from toddler age to middle childhood: links to adaptive and maladaptive outcomes. Attachment & human development 16: 211–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brumariu LE, Madigan S, Giuseppone KR, Movahed Abtahi M, and Kerns KA, (2018): The Security Scale as a measure of attachment: meta-analytic evidence of validity. Attachment & Human Development 20: 600–625. [DOI] [PubMed] [Google Scholar]
- 42.Talairach J and Tournoux P, (1988): Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: An approach to cerebral imaging. New York, NY: Thieme. [Google Scholar]
- 43.Cox RW, (1996): AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical research 29: 162–173. [DOI] [PubMed] [Google Scholar]
- 44.JASPTeam, (2018): JASP. Version 0.9 [Computer Software].
- 45.Ellis BJ and Essex MJ, (2007): Family Environments, Adrenarche, and Sexual Maturation: A Longitudinal Test of a Life History Model. Child Development 78: 1799–1817. [DOI] [PubMed] [Google Scholar]
- 46.Callaghan BL and Tottenham N, (2016): The Stress Acceleration Hypothesis: effects of early-life adversity on emotion circuits and behavior. Current Opinion in Behavioral Sciences 7: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bernard K, Hostinar CE, and Dozier M, (2015): Intervention effects on diurnal cortisol rhythms of Child Protective Services–referred infants in early childhood: Preschool follow-up results of a randomized clinical trial. Journal of the American Medical Association-Pediatrics 169: 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lind T, Bernard K, Ross E, and Dozier M, (2014): Intervention effects on negative affect of CPS-referred children: Results of a randomized clinical trial. Child abuse & neglect 38: 1459–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chisholm K, Carter MC, Ames EW, and Morison SJ, (1995): Attachment security and indiscriminately friendly behavior in children adopted from Romanian orphanages.Development and psychopathology 7: 283–294. [Google Scholar]
- 50.Olsavsky AK, Telzer EH, Shapiro M, Humphreys KL, Flannery J, Goff B, et al. , (2013): Indiscriminate amygdala response to mothers and strangers after early maternal deprivation. Biol Psychiatry 74: 853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eisenberger NI, Master SL, Inagaki TK, Taylor SE, Shirinyan D, Lieberman MD, et al. , (2011): Attachment figures activate a safety signal-related neural region and reduce pain experience. Proceedings of the National Academy of Sciences 108: 11721–11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Courtiol E, Wilson DA, Shah R, Sullivan RS, and Teixeira CM, (2018): Maternal Regulation of Pups’ Cortical Activity: role of Serotonergic Signaling. E Neuro 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, et al. , (2013): A Developmental Shift from Positive to Negative Connectivity in Human Amygdala–Prefrontal Circuitry. The Journal of Neuroscience 33: 4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.