Abstract
Background:
While autoimmunity and hyperinflammation secondary to recombinase activating gene (RAG) deficiency have been associated with delayed diagnosis and even death, our current understanding is limited primarily to small case series.
Objective:
Understand the frequency, severity, and treatment responsiveness of autoimmunity and hyperinflammation in RAG deficiency.
Methods:
In reviewing the literature and our own database, we identified 85 patients with RAG deficiency, reported between 2001 and 2016, and compiled the largest case series to date of 63 patients with prominent autoimmune and/or hyperinflammatory pathology.
Results:
Diagnosis of RAG deficiency was delayed a median of 5 years from the first clinical signs of immune dysregulation. The majority of patients (55.6%) presented with more than one autoimmune or hyperinflammatory complication, with the most common etiologies being cytopenias (84.1%), granulomas (23.8%), and inflammatory skin disorders (19.0%). Infections, including live viral vaccinations, closely preceded the onset of autoimmunity in 28.6% of cases. Autoimmune cytopenias had early onset (median 1.9, 2.1, and 2.6 years for autoimmune hemolytic anemia (AIHA), immune thrombocytopenia (ITP) and autoimmune neutropenia (AN), respectively) and were refractory to intravenous immunoglobulin, steroids, and rituximab in the majority of cases (64.7%, 73.7%, and 71.4% for AIHA, ITP, and AN, respectively). Evans syndrome specifically was associated with lack of response to first-line therapy. Treatment-refractory autoimmunity/hyperinflammation prompted hematopoietic stem cell transplantation in 20 patients.
Conclusions:
Autoimmunity/hyperinflammation can be a presenting sign of RAG deficiency and should prompt further evaluation. Multi-lineage cytopenias are often refractory to immunosuppressive treatment and may require hematopoietic cell transplantation for definitive management.
Keywords: recombinase activating gene (RAG), severe combined immunodeficiency (SCID), immune dysregulation, autoimmune cytopenias, hematopoietic stem cell transplantation (HSCT)
Introduction
Recombinase activating genes (RAG1 and RAG2) initiate the V(D)J recombination process, supporting the development of a diverse repertoire of T and B lymphocytes (1). Mutations in RAG were first described in patients with severe combined immune deficiency with lack of T and B cells (T- B- SCID) (2). Subsequently, the clinical presentation of RAG deficiency was expanded to include Omenn syndrome, in which partial V(D)J recombination activity accounts for the generation of autologous oligoclonal T lymphocytes that infiltrate and damage end-organs (3, 4). More recently, hypomorphic RAG mutations have been associated with a broad clinical spectrum of atypical SCID (AS), including a phenotype with predominance of T cell receptor (TCR) γδ+ T cells (γδ AS) (5, 6), and a phenotype of combined immunodeficiency with granulomatous disease and/or autoimmunity (CID-G/AI), where frequent autoimmunity, granulomatous lesions, and occasionally Epstein-Barr virus (EBV)-driven lymphoproliferation are the predominant clinical features (6–10). Finally, RAG deficiency has been case reported to clinically mimic ‘idiopathic’ CD4+ T cell lymphopenia (ICL) (11), hyper-IgM syndrome (HIM) (12), common variable immunodeficiency (CVID) (13), and even refractory autoimmune entities such as chronic multifocal osteomyelitis and demyelinating neuropathy (9).
The development of autoimmunity in RAG deficiency has been linked to checkpoint breaks in both T and B cell tolerance, including abnormal thymic negative selection of autoreactive T cells (central T cell tolerance), impaired development and dysfunction of regulatory T cells (peripheral T cell tolerance), impaired B cell receptor editing in the bone marrow (central B cell tolerance), and elevated levels of B cell activating factor (BAFF) allowing survival of immature B cells with self-reactive specificity (peripheral B cell tolerance) (4, 14, 15). Environmental factors such as composition of the host intestinal microbiota may play an additional role in sustaining intestinal T cell infiltration and autoimmune/hyperinflammatory pathology (16).
To date, however, our understanding of the clinical spectrum of autoimmunity and hyperinflammatory pathology that can occur in RAG deficiency has been limited to small case series and a single review of the literature (17, 18). Moreover, there have been no larger studies addressing treatment outcomes for autoimmune and hyperinflammatory disease in the background of RAG deficiency. To address this deficit, we herein present the results of a literature search and review of our internal database and report on the largest case series of annotated and curated cases of RAG deficiency with prominent autoimmune and hyperinflammatory disease.
Methods
1. Literature search.
We reviewed all RAG deficient cases in PubMed published between September 2001 and 2016. We excluded reports that did not detail the presence or absence of autoimmune/hyperinflammatory complications. Data was extracted regarding RAG mutation, gender, clinical phenotype including autoimmune/hyperinflammatory complications, and age of hematopoietic stem cell transplant (HSCT), if utilized. We assigned clinical phenotypes according to criteria from the Primary Immune Deficiency Treatment Consortium (PIDTC) (19). The CID-G/AI phenotype was defined by a clinical history of recurrent infections and immune dysregulation (autoimmunity and/or granulomas) (6–10).
2. Patient database.
Based on the literature search above and our data repository of unpublished cases, we generated a highly annotated and curated patient database that included 63 cases. Information was collected as follows: gender, age (current as of November 2017, at clinical diagnosis of immunodeficiency and/or autoimmunity, at molecular diagnosis of RAG deficiency, and at death or HSCT where applicable), genotype (specific RAG1 or RAG2 mutations), immune phenotype (lymphocyte counts and function, immunoglobulin levels, and autoantibodies), autoimmune/hyperinflammatory complications (type, age at onset, preceding infections if available, length, and severity), and therapies trialed (including response and complications). Predicted V(D)J recombination activity was recorded as previously described (20, 21). The study was approved by the Institutional Review Board of the University of South Florida (protocol # Pro00025693).
3. Therapeutic Response Score.
Therapeutic response was scored for all annotated cases of autoimmune cytopenias and granulomas using the following criteria: ‘no’ = no clinical response to the intervention was seen or side effects were limiting; ‘partial’ = some clinical improvement to the intervention was seen but therapeutic escalation was ultimately required for stabilization; or ‘full’ = clinical improvement to the intervention was seen and no subsequent escalation has been required for stabilization to date. Across all centers, the term ‘treatment-refractory’ was applied only in cases where ‘no clinical response to intervention’ was specifically documented by the managing clinical care team.
4. Statistical analysis.
All data were assembled and analyzed using GraphPad Prism software. Groups were compared using a two-tailed Student’s t-test. Kaplan-Meier curves were compared using a log-rank (Mantel-Cox) test. Significance was defined as P < 0.05.
Results
1. RAG deficient cases based on literature search (n=85)
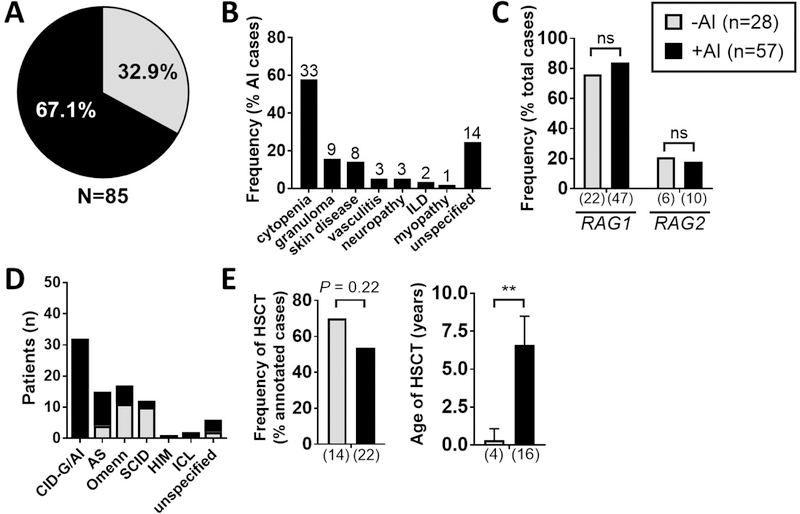
We performed a literature search of published cases of RAG deficiency between 2001 and 2016 and identified 134 cases, of which 85 met criteria for further analysis. In review of these 85 published cases, autoimmune and/or hyperinflammatory complications were identified in 57 patients (67.1% of total cases) (Figure 1A), and included autoimmune cytopenias (n=33, 57.9%), granulomas (n=9, 15.8%), skin disease (n=8, 14.0%), vasculitis (n=3, 5.3%), neuropathy (n=3, 5.3%), interstitial lung disease (ILD) (n=2, 3.5%), and myopathy (n=1, 1.8%) (Figure 1B).
Figure 1. Autoimmunity and hyperinflammation are frequent complications in published cases of RAG deficiency.
85 published cases of RAG deficiency were reviewed for the presence (+AI: n=57, shown in black) or absence (-AI: n=28, shown in grey) of autoimmune and/or hyperinflammatory complications with results shown as prevalence of: (A) +AI vs. -AI (frequency as % total cases, n=85), (B) individual autoimmune and hyperinflammatory complications (frequency as % AI subtype, n=57), (C) genotype (frequency as % total cases, n=85), (D) clinical phenotype (as absolute patient count), (D) occurrence of HSCT (frequency as % annotated total cases, n=36), (E) age of HSCT (median +/− 95% CI). Exact patient counts as shown with statistical difference indicated (ns = not significant; *P <0.05, **P <0.005, ***P <0.0001); interstitial lung disease (ILD).
We next compared the RAG deficient patients without vs. with autoimmune and hyperinflammatory clinical manifestations. Gender and genotype were evenly distributed, and RAG1 mutations accounted for the majority of patients in both groups (Figure 1C). In review of the clinical phenotype, 32 patients with CID-G/AI accounted for the majority of the autoimmune/hyperinflammatory subset (32 out of 57 patients, 56.1%). Additionally, autoimmune and/or hyperinflammatory complications were prominent among patients with AS (11 out of 15 patients, 73.3%), but rare among patients with Omenn syndrome (6 out 17, 35.3%) and SCID (2 out of 12, 16.7%) (Figure 1D). Finally, no significant difference was observed in the proportion of patients who received HSCT among patients without vs. with immune dysregulation, but the latter group received HSCT at a significantly older age (median 0.3 vs. 6.6 years in patients without vs. with immune dysregulation, P = 0.0003) (Figure 1E). To gain more insights into the natural history of patients with RAG deficiency complicated by immune dysregulation, we created a curated longitudinal database and analyzed the data.
2. Annotated and curated patient database (n=63)
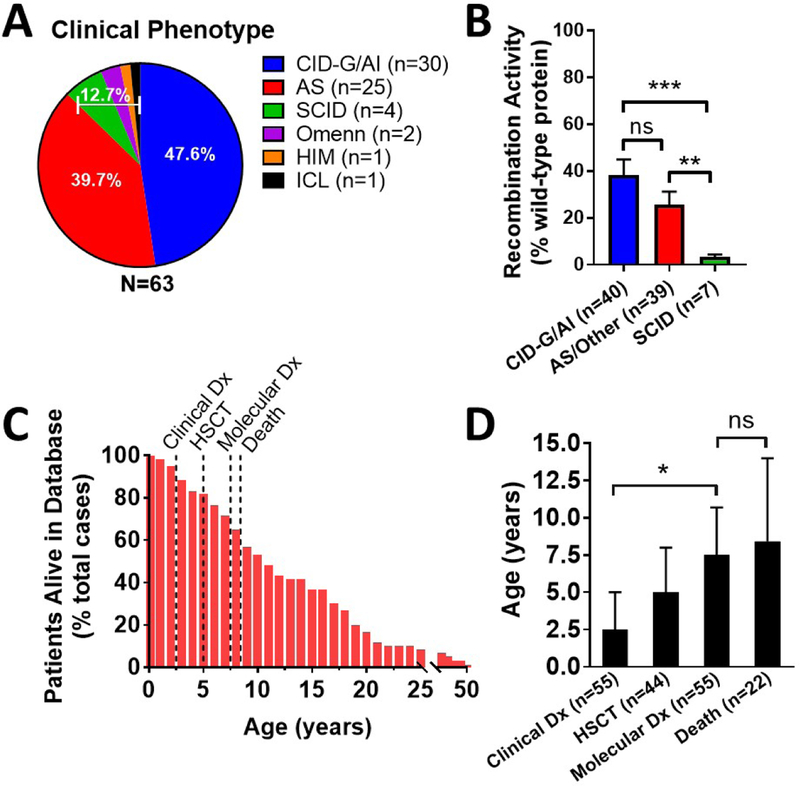
Based on the literature search and our own database of unpublished cases, we identified 63 total cases of RAG deficiency with prominent autoimmune and/or hyperinflammatory manifestations. The characteristics of this patient cohort are described in Table 1. There was a slight predominance of female patients (54.8% females; 45.2% males). The clinical phenotype was predominantly CID-G/AI (30 cases, 47.6%), followed by AS (25 cases, 39.7%), SCID (4 cases, 6.3%), Omenn syndrome (2 cases, 3.2%), and single cases of HIM and ICL (1.6% each) (Figure 2A). RAG1 and RAG2 mutations were present in 48 (76.2%) and 15 (23.8%) patients, respectively. Functional data of in vitro recombination activity were available for 63 of 96 RAG1 and 23 of 30 RAG2 alleles. Upon dividing patients into three groups (CID-G/AI; AS/Other; and SCID), the average recombination activity, expressed as percentage of wild-type protein, was 38.1, 25.5, and 3.4, respectively (Figure 2B). Thirty-nine patients (61.9%) were alive at the time of review at a median age of 10.6 years (Figure 2C). The median age at clinical diagnosis (immunodeficiency and/or autoimmunity) was 2.5 years. In comparison, the median age of genetic diagnosis of RAG deficiency was 7.5 years, with six cases identified post-mortem. In total, 45 patients (71.4%) had received HSCT at the time of review at a median of 5.0 years of age. Additionally, 8 patients (12.7%) were either being evaluated for HSCT or had passed away prior to anticipated HSCT at the time of review. There were no occurrences of solid organ transplantation. Twenty-four patients (38.1%) were deceased at the time of review at a median age of 8.4 years, which was statistically coincident with the age of genetic diagnosis (P = 0.70) (Figure 2D). Multi-organ failure and/or sepsis was the leading cause of death in ten cases (41.7%) (Figure E1A). Median patient survival was 14 vs. 21 years in untransplanted compared to hematopoietic cell transplanted patients, however, these Kaplan-Meier curves failed to reach statistical difference (Figure E1B; P = 0.42). Patient condition at the time of HSCT was unavailable in the majority of cases.
Table 1: Patient characteristics of curated RAG deficiency database (n=63).
Yes (+); No (−); not available (n.a.); acetylcholine receptor (AChR); anti-mitochondrial antibody (AMA); anti-nuclear antibody (ANA); anti-phospholipid antibody (APLA); atypical SCID (AS); autoimmune (ai); autoimmune hemolytic anemia (AIHA); autoimmune neutropenia (AN); combined immunodeficiency with granulomatous disease and/or autoimmunity (CID-G/AI); cytomegalovirus (CMV); diagnosis (dx); Diphtheria, Tetanus, Pertussis (DTap); double stranded DNA (dsDNA), evaluated (eval); female (F); glutamic acid decarboxylase (GAD); granulomatous and lymphointerstitial lung disease (GLILD); hematopoietic stem cell transplant (HSCT); hepatitis B virus (HBV); hyper-IgM syndrome (HIM); idiopathic CD4+ lymphopenia (ICL); immune thrombocytopenia (ITP); inflammatory bowel disease (IBD); interferon (IFN); interleukin (IL); male (M); measles-mumps-rubella (MMR); Miller Fisher syndrome (MFS); Omenn syndrome (Omenn); pneumococcal (PC); severe combined immune deficiency (SCID); upper respiratory tract infection (URI); varicella zoster virus (VZV).
| Case | Citation | Gender | Clinical Phenotype |
Gene | Mutation | Recombination Activity (% wild-type protein) |
Age, Current ( years) |
Age, Clinical Dx (years) |
Age, Molecular Dx (years) |
HSCT | Age, HSCT (years) |
AI indication for HSCT? |
Cytopenia | Granuloma | AI Other | AI preceded by infection? (etiology; timing) |
Auto-Antibody |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | (17, 27) | M | CID-G/AI | RAG1 | a. W522C; b. L541Cfs*30 | a. 41.6; b. 1.0 | 20 (deceased) | 10 | 17 | + | 19 | + | − | midline (oropharynx, skin) | myasthenia gravis | − | AChR, IFN-α/β/ω, IL-12p70, IL-22 |
| 2 | (17, 28) | F | AS | RAG1 | a.R474C; b. K983Nfs*9 | a. 125.4; b. 0.1 | 8 | n.a. | 2 | + | 2 & 4 | − | AN, ITP, AIHA | − | eczematous rash, nodular splenomegaly | + AN (vaccine-strain VZV; one month) | Coombs, neutrophil |
| 3 | (28) | F | AS | RAG1 | a. R474C; b. K983Nfs*9 | a. 125.4; b. 0.1 | 14 | n.a. | 7.9 | + | 8 | − | AIHA | − | nephrotic syndrome, splenomegaly | + AIHA (MMR/DTaP/HBV/PC vaccines; two weeks) | neutrophil, ANA, APLA, thyroid (TG/TPO) |
| 4 | (17) | F | CID-G/AI | RAG2 | a. G451A; b. M459L | a. 60; b. 30.8 | 7 | n.a. | n.a. | + | 1.5 | − | AN | − | − | − | Coombs, neutrophil, ANA, IFN-α/ω |
| 5 | (7, 17) | F | CID-G/AI | RAG1 | a. R778Q; b.R975W | a. 8.58; b. 0.1 | 9.5 (deceased) | 7.8 | 7.8 | + | 8.5 | + | − | skin, oropharynx, lung | − | n.a. | − |
| 6 | (7, 17) | F | CID-G/AI | RAG1 | a. R314W; b. R507W/R737H | a. 24.3; b. 0.09 | 18 | 3 | 3 | + | 6 | n.a. | − | skin | EBV-driven B cell lymphoma | n.a. | − |
| 7 | (7, 17) | F | CID-G/AI | RAG2 | a. T77N; b. G451A | a. 0.73; b. 0.75 | 22 | 9.9 | 10.7 | + | 14 | n.a. | ITP, AN | spleen, lung, bone | − | n.a. | Coombs |
| 8 | (17) | F | AS | RAG2 | a./b. G35A | a./b. 22.1 | 10.83 (deceased) | 1.33 | 9 | -(eval) | AIHA, AN | − | psoriasis, splenomegaly | − | Coombs | ||
| 9 | (17) | F | CID-G/AI | RAG1 | a./b. C176F | n.a. | 16 | 3.5 | 11 | + | 12.5 | n.a. | − | skin | − | − | − |
| 10 | (17) | M | CID-G/AI | RAG1 | a. I102Sfe*15; b. P118Lfs*21 | n.a. | 11 | 7 | 7.5 | + | 8 | − | AIHA | skin | − | − | Coombs |
| 11 | (17, 29) | F | CID-G/AI | RAG1 | a. K86fs*33; b. H612R | a. 2.7; b. 121.6 | 20 | 3 | 15 | + | 18 | + | ITP, AIHA | lung | GLILD, duodenitis, vitiligo | − | Coombs, thyroid (TPO) |
| 12 | (17) | F | CID-G/AI | RAG2 | a./b. T215I | a./b. 48.4 | 7.33 (deceased) | 6.5 | 6.5 | + | 7.25 | n.a. | AN | − | − | n.a. | neutrophil |
| 13 | (30) | M | CID-G/AI | RAG1 | a./b. S480G | n.a. | 10.25 (deceased) | 6 | 8 | + | 9.5 | n.a. | AIHA, AN | − | − | − | Coombs, neutrophil |
| 14 | (30) | M | ICL | RAG1 | a./b. S480G | n.a. | 19 (lost to follow-up) | 15 | 15 | − | − | − | vitiligo | n.a. | − | ||
| 15 | (30) | M | CID-G/AI | RAG1 | a./b. H612R | a./b. 121.6 | 18 (lost to follow-up) | 7 | 9 | + | 7.5 | n.a. | AN, AIHA, ITP | skin | − | − | Coombs |
| 16 | (9) | M | CID-G/AI | RAG1 | a./b. R699W | a./b. 19.3 | 11 (deceased) | 9 | 11 (post-mortem) | − | AIHA | skin, lung, liver, bone, pancreas, testes | vitiligo | − | Coombs, ANA, dsDNA | ||
| 17 | (13) | M | CID-G/AI | RAG1 | a./b. C358Y | a./b. 48.8 | 14 (deceased) | 10 | 10 | -(died) | AN | liver | MALT lymphoma, splenomegaly | + AN (Leishmania; three months) | neutrophil (ANCA) | ||
| 18 | (17) | M | AS | RAG2 | a./b. E407* | a./b. 2.9 | 25 | 0.1 | 0.1 | + | 19 | + | AIHA | − | partial alopecia, IBD | − | − |
| 19 | (17, 31) | M | AS | RAG1 | a. R699W; b.M435V | a. 19.3; b. 23.6 | 17 | n.a. | n.a. | + | 6.5 | n.a. | AIHA, ITP, AN | − | vitiligo, psoriasis, Guillain-Barré syndrome | − | Coombs, neutrophil, platelet |
| 20 | (17) | F | AS | RAG1 | a./b. R108* | a./b. 1.8 | 5.5 | 0.25 | 0.33 | + | 0.5 | − | AN | − | − | − | Coombs |
| 21 | (17) | M | AS | RAG1 | a./b. K86Vfs*33 | a./b. 2.7 | 6 | 0.91 | 1.08 | + | 1.5 | − | AIHA | − | Miller Fisher syndrome | + MFS (CMV reactivation; 96 hours) | Coombs |
| 22 | (17) | F | AS | RAG1 | a. H612R; b. A857V | a. 121.6; b. 121.6 | 6 | 1.91 | 2.5 | + | 5 | n.a. | AIHA | − | thyroiditis | − | neutrophil (ANCA), B2GPI, microsomal |
| 23 | − | M | AS | RAG1 | a. W522C; b. M435V/M1006 V | a. 41.6; b. 23.6/105.6 | 4.8 (deceased) | 2 | n.a. | + | 3 | + | AIHA, ITP | − | − | + AIHA (VZV vaccine; 10 months) | Coombs, platelet |
| 24 | − | F | CID-G/AI | RAG1 | a. R474C; b. R975W | a. 125.4; b. 0.1 | 21 (deceased) | 9 | n.a. | + | 20 | + | AIHA, ITP | skin | − | − | Coombs, platelet |
| 25 | − | M | CID-G/AI | RAG1 | a. W522C; b. H994R | a. 41.6; b. n.a. | 6 (deceased) | 2.5 | 3 | + | 5 | + | AIHA | − | vasculitis | + vasculitis (vaccine-strain VZV; coincident) | − |
| 26 | (12, 17) | F | Omenn | RAG2 | a./b.M459L | a./b. 30.8 | n.a. (deceased) | 1.33 | 2.17 | + | 1.58 | n.a. | AIHA | − | − | n.a. | Coombs, C3 |
| 27 | (12) | M | HIM | RAG2 | a./b. M459L | a./b. 30.8 | n.a. (deceased) | 2 | 5.17 | − | AIHA | − | − | n.a. | APLA | ||
| 28 | (32) | F | CID-G/AI | RAG1 | a./b. R764C | n.a. | 20.5 | 8 | 11 | -(eval) | AIHA, ITP | skin, bone | − | − | − | ||
| 29 | − | M | AS | RAG1 | a. R396C; b. M435V | a. 0.6; b. 23.6 | 2.67 (deceased) | 1.42 | 1.5 | + | 1.75 & 2.5 | + | AIHA | − | vasculitis | − | Coombs, IFN-α |
| 30 | (33) | F | AS | RAG2 | a. P180H; b. R73H | a. 31.3; b.11.0 | 1.25 (deceased) | 1.08 | 1.25 (post-mortem) | -(died) | AIHA, ITP | − | − | + AIHA (VZV/MMR vaccines; three weeks) | Coombs | ||
| 31 | (34) | F | CID-G/AI | RAG1 | a. M1V; b. R737H | a. n.a.; b. 0.2 | 48 (deceased) | 20 | 46 | − | − | − | vasculitis | n.a. | ANA, dsDNA, APLA, RF, thyroid (TG/TPO/TSHR) | ||
| 32 | (8) | F | CID-G/AI | RAG1 | a. R841Q; b. F974L | a. 0; b. 56.5 | 2 (deceased) | 1 | 2 (post-mortem) | -(died) | AIHA, ITP, AN | − | vasculitis, myopathy, central demyelinating neuropathy | − | Coombs, platelet | ||
| 33 | − | M | AS | RAG1 | a./b. R841W | a./b. 10 | 1.75 (deceased) | 0.5 | 0.75 | + | 0.83 | + | AIHA | − | − | + AIHA (acute viral URI; coincident) | Coombs |
| 34 | − | M | SCID | RAG1 | a. N766l; b.K86VfsX33 | a. n.a.; b 2.7 | 19.17 | n.a. | 13 | + | 0.42 & 0.67 | n.a. | AIHA, AN | − | thyroiditis, hepatitis, urticaria | − | Coombs |
| 35 | (17) | F | CID-G/AI | RAG2 | a./b. F62L | a./b. 19.6 | 31 | 5 | 27 | − | ITP | lung | − | − | − | ||
| 36 | − | M | AS | RAG2 | a. G35A; b. E437K | a. 22.1; b. 0.9 | 7 (deceased) | 0.37 | 0.46 | -(died) | AIHA | − | − | + AIHA (CMV; coincident) | Coombs | ||
| 37 | − | F | AS | RAG1 | a./b. C335R | n.a. | 16(deceased) | 5 | 15 | + | 16 | − | ITP | − | T cell cutaneous lymphoma, uveitis | + ITP (VZV; coincident) | − |
| 38 | − | F | AS | RAG1 | a. K86VfsX33; b. R108X | a. 2.7; b. 1.8 | 16 | 14 | 14 | + | 15 | + | AN | − | − | − | − |
| 39 | − | F | CID-G/AI | RAG1 | a./b. H612R | a./b. 121.6 | 18 | 13.6 | 15.6 | + | 17 | n.a. | AIHA, AN | − | alopecia areata, thyroiditis | − | Coombs, IFN-α, thyroid (TPO/TG) |
| 40 | (35) | M | CID-G/AI | RAG1 | a./b. R507G | a./b. 19.2 | 8 | 2.5 | 5 | + | 5.25 | + | AIHA, AN | − | hepato-splenomegaly | + AIHA (CMV; coincident) | Coombs, neutrophil |
| 41 | − | F | CID-G/AI | RAG1 | a. A472V; b. H612R | a. n.a.; b. 121.6 | 8 | 2 | 2 | + | 4.33 | n.a. | − | − | aseptic encephalitis | − | AChR, GAD, CV2/CRMP5 |
| 42 | − | F | SCID | RAG1 | a./b. K86VfsX33 | a./b. 2.7 | 10.42 | 0.08 | 1.33 | + | 1.25 | − | AIHA | − | − | − | − |
| 43 | − | M | CID-G/AI | RAG1 | a./b. K86VfsX33 | a./b. 2.7 | 7.67 | 3 | 4 | + | 4 | + | AIHA, AN, ITP | − | − | − | Coombs |
| 44 | − | M | SCID | RAG1 | a./b. K86VfsX33 | a./b. 2.7 | 8.67 | 0.5 | 0.58 | + | 0.75 | + | ITP | − | − | − | − |
| 45 | − | M | Omenn | RAG2 | n.a. | n.a. | 16.58 | 0.08 | 0.17 | + | 0.75 | − | − | − | dermatitis, hepatitis, & severe diarrhea | − | − |
| 46 | (36) | F | SCID | RAG1 | a. K992E; b. A444V | a. 9.1; b.1.4 | 2.5 | 2.17 | n.a. | + | 2.5 | + | ITP | − | polyclonal gammopathy, isolated ALP elevation | + ITP (VZV; two months) | ANA, IFN-α/ω, IL-12 |
| 47 | (31) | n.a. | CID-G/AI | RAG1 | a. R396C; b. R975Q | a. 0.6; b. 57.9 | 5 (deceased) | n.a. | n.a. | − | ITP | skin | − | − | − | ||
| 48 | − | M | CID-G/AI | RAG2 | a. N173S; b. E437K | a. n.a.; b. 0.9 | 36 | 31 | 36 | + | n.a. | n.a. | − | − | myopathy | − | − |
| 49 | − | F | AS | RAG2 | a./b. G35A | a./b. 22.1 | 2.67 (deceased) | 1.33 | 2.67 (post-mortem) | + | 2.67 | + | ITP, AN, AIHA | − | − | + AIC relapses (viral infections; õne week) | Coombs |
| 50 | − | M | CID-G/AI | RAG1 | a./b. N855S | n.a. | 11 (deceased) | 5 | 11 (post-mortem) | -(died) | AIHA | − | enteropathy | + enteropathy & AIHA (Salmonella; 2 & 2.5 months) | Coombs, enterocyte/ goblet cell | ||
| 51 | (34) | F | CID-G/AI | RAG2 | a. S381*; b. G95R | a. n.a.; b. 0 | 48 | 35 | 46 | − | AIHA | − | − | − | Coombs | ||
| 52 | − | F | CID-G/AI | RAG1 | a. R314W; b. R396C | a. 24.3; b. 0.6 | n.a. | 5 | 9 | + | 9 | − | − | − | thyroiditis, vitiligo, diabetes, nail dystrophy | − | GAD, ICA, thyroid (TG) |
| 53 | − | F | AS | RAG1 | a./b. R474H | n.a. | 12 | 10 | 11 | n.a. | AIHA, ITP | − | amyloidosis | + AIHA relapses (severe URIs; õne week) | Coombs | ||
| 54 | − | M | AS | RAG1 | a. R561H; b. R778Q | a. 2.0; b. 8.6 | 17 | 11 | 17 | -(eval) | AIHA | − | − | + AIHA (VZV; coincident) | − | ||
| 55 | − | M | AS | RAG1 | a. N855S; b. K992E | a. n.a.; b. 9.1 | 8.58 | 2.5 | 2.75 | + | 3 | − | AIHA | − | hepatitis | − | − |
| 56 | − | F | AS | RAG1 | a. R112H; b. K86Vfs33* | a. n.a.; b. 2.7 | 5 | n.a. | n.a. | n.a. | AN | − | − | − | − | ||
| 57 | − | F | AS | RAG1 | a. R142*;b. T477S | a. 9.0; b. n.a. | 3.33 (deceased) | 2.5 | 2.83 | + | 3.25 | + | AIHA, ITP, AN | − | − | − | Coombs, platelet |
| 58 | − | M | CID-G/AI | RAG1 | a./b. G816R | n.a. | 9.5 | 1.5 | 7.5 | − | AIHA | − | sclerosing cholangitis | + AIHA (CMV; two weeks) | − | ||
| 59 | − | F | AS | RAG1 | a. R112L; b. H735Q | n.a. | 3.74 | 0.92 | n.a. | + | 1.33 | + | ITP, AIHA | − | − | + ITP (VZV; several weeks) | Coombs, thyroid (TPO) |
| 60 | − | F | AS | RAG2 | a. G35A; b. A456D | a. 22.1; b. n.a. | 3.44 | 0.25 | 1.67 | + | 0.5 | + | ITP | − | vasculitis | − | − |
| 61 | − | F | AS | RAG1 | a. T708A; b. E669K | n.a. | 7.25 | 2.25 | 2.5 | + | 2.67 | − | AN | − | − | − | neutrophil |
| 62 | (37) | M | CID-G/AI | RAG1 | a. H375D; b. Y562C | n.a. | 18.67 | 9 | 15 | + | 15.83 | + | ITP | skin, liver, spleen | − | − | − |
| 63 | − | M | AS | RAG1 | n.a. | n.a. | 14.5 (deceased) | n.a. | 14.5 (post-mortem) | + | 14 | + | AN, ITP, AIHA | − | vitiligo, IBD | − | − |
Figure 2. Demographics of curated RAG deficiency database (n=63).
(A) Clinical diagnosis (frequency as % total cases). (B) Recombination activity from all available RAG1 (n=61) and RAG2 (n=23) alleles (average +/− SEM as % wild-type protein and in color by clinical phenotype). (C) Patients alive in database (% by age with clinical milestones annotated). (D) Age of clinical milestones (median +/− 95% CI). Exact patient counts (A,C,D) and allele counts (B) as shown with statistical difference indicated (ns = not significant; *P <0.05, **P <0.005, ***P <0.0001); diagnosis (dx); hematopoietic stem cell transplant (HSCT).
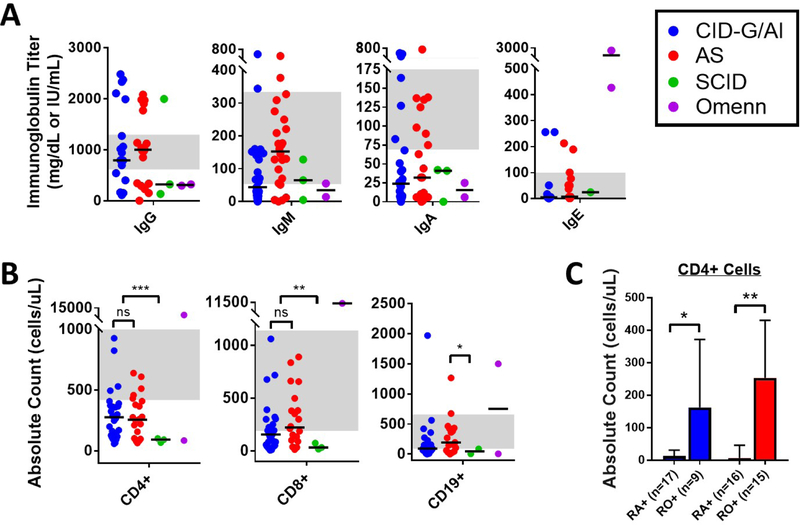
Next, we reviewed the immunological phenotype. Immunoglobulin serum levels were highly variable with a median native IgG of 890 mg/dL (25–75% IQR 296–1770 mg/dL), IgA of 25.5 mg/dL (25–75% IQR 6–73.3 mg/dL), IgM of 87.3 mg/dL (25–75% IQR 28.8–162.8 mg/dL), and IgE of 5 IU/mL (25–75% IQR 3.3–51.3 IU/mL) (Figure 3A). Interestingly, 26.3% of patients with CID-G/AI and AS manifested hypergammaglobulinemia. Increased serum IgE levels were present in the two patients with Omenn syndrome (IgE 427 and 2,448 IU/mL, respectively). T and B lymphocyte counts were decreased overall in the curated patient database (median CD3+ 599 cells/μL, median CD19+ 102.5 cells/μL), whereas NK cells were in the normal range (median 279.5 cells/μL) (Table E1). As expected by clinical phenotype, loss of T and B lymphocytes was most pronounced for patients with SCID vs. CID-G/AI and AS (Figure 3B). Within the CD4+ T cell compartment, CD45RA+/RO+ subtyping was available for 26 and 31 patients with CID-G/AI and AS, respectively, and demonstrated a predominance of memory (CD45RO+) CD4+ T cells in circulation for both groups (Figure 3C). Expansion of TCRγδ+ T cells was documented in three patients with AS and in the single patient with HIM (data not shown). Data on T cell proliferation to phytohemagglutinin (PHA) were available in 33 cases and were low to severely low in the majority (26 patients) (Table E1).
Figure 3. Immunophenotype of curated RAG deficiency database (n=63).
(A) Immunoglobulin titers (shown in color by clinical phenotype with symbols representing individual patients and bars representing clinical subset medians). (B) Lymphocyte counts (shown in color by clinical phenotype with symbols representing individual patients and bars representing clinical subset medians). (C) CD4+ T cell subsets, CD45RA+ ‘RA+’ and CD45RO+ ‘RO+’ (median +/− 95% CI, shown in color by clinical phenotype). Grey background indicates normal adult reference ranges from the Massachusetts General Hospital. Exact patient counts as shown with statistical difference indicated (ns = not significant; *P <0.05, **P <0.005, ***P <0.0001).
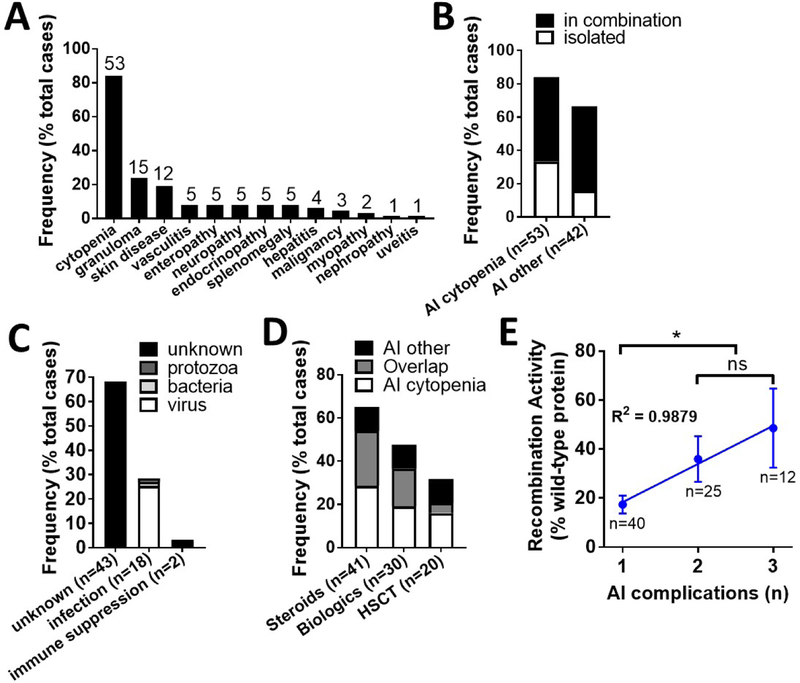
The most frequent autoimmune and/or hyperinflammatory complications were autoimmune cytopenias (n=53, 84.1%), granulomas (n=15, 23.8%), and skin manifestations including vitiligo, psoriasis, and alopecia (n=12, 19.0%) (Figure 4A). 55.6% of patients had more than one autoimmune or hyperinflammatory complication, specifically 60.4% of cytopenia cases presented with an additional autoimmune/hyperinflammatory manifestation (Figure 4B). Infections closely preceded the onset of autoimmunity in 28.6% of cases (Figure 4C). Viruses were the most frequent etiology in 16 cases and included both live vaccinations and natural infections (Table 1). Infections due to Leishmania and Salmonella preceded development of autoimmunity in a single patient each. The burden of treating autoimmune and hyperinflammatory complications was substantial as measured by use of steroids, biological agents, and HSCT (Figure 4D). Treatment-refractory autoimmunity and/or hyperinflammation were an indication to HSCT in 20 cases (44.4% of total HSCT) and included: autoimmune cytopenias (n=12), inflammatory bowel disease (IBD)/enteropathy (n=4), granulomas (n=3), vasculitis (n=3), and progressive pulmonary disease (n=1). Finally, type of immune dysregulation (cytopenia, granuloma, or ‘other’) did not correlate with the average predicted level of patient recombination activity (29.9, 36.5, and 34.8% of wild-type protein, respectively) (Figure E2), perhaps due to the high co-occurrence of these conditions (Figure 4B). However, cumulative number of autoimmune/hyperinflammatory complications per patient did correlate both positively and linearly with the average predicted level of patient recombination activity (17.3, 36.0, and 49.6% of wild-type protein for patients with one, two, or three autoimmune/hyperinflammatory complications, respectively) (Figure 4E).
Figure 4. Autoimmune and hyperinflammatory outcomes of curated RAG deficiency database (n=63).
(A) Prevalence of individual autoimmune and hyperinflammatory complications (frequency as % total cases). (B) Occurrence of autoimmune and hyperinflammatory complications in isolation or combination (frequency as % total cases). (C) Clinician-annotated triggers for autoimmune and hyperinflammatory disease development (frequency as % total cases). (D) Burden of treatment for autoimmune and hyperinflammatory complications (frequency as % total cases). (E) Correlation between number of autoimmune complications (cumulative per patient) and recombination activity (average +/− SEM as % wild-type protein), linear regression of mean Y values with R2 shown. Exact patient counts (A-D) and allele counts (E) as shown with statistical difference indicated (ns = not significant; *P <0.05, **P <0.005, ***P <0.0001); autoimmune (AI); hematopoietic stem cell transplant (HSCT).
3. Autoimmune Cytopenias: Occurrence, Outcomes, and Treatment
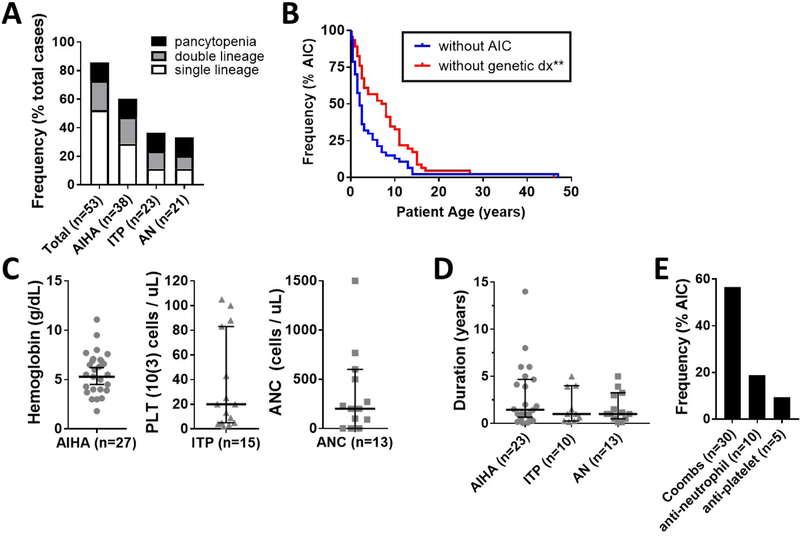
Autoimmune hemolytic anemia (AIHA) was the most frequent autoimmune complication identified in the curated patient database (n=38, 60.3%), followed by immune thrombocytopenia (ITP) (n=23, 36.5%), and autoimmune neutropenia (AN) (n=21, 33.3%). Evans syndrome was observed in 13 cases (20.6%), and pancytopenia was observed in 8 cases (12.7%) (Figure 5A). The median age at onset was 1.9 years for AIHA, 2.1 years for ITP, and 2.6 years for AN, which coincided with the clinical diagnosis of immunodeficiency/autoimmunity, but statistically preceded the molecular diagnosis of RAG deficiency by a median of 5.5 years (Figure 5B). Moreover, the cytopenias were often severe. The median cell nadir during disease flare was hemoglobin of 5.5 g/dL for AIHA, platelet count of 20,000 cells/μL for ITP, and absolute neutrophil count (ANC) of 200 cells/μL for AN (Figure 5C). Additionally, median duration of relapsing/remitting cytopenia disease course in total was 1.5 years for AIHA and 1 year for ITP and AN (Figure 5D). Finally, the majority of patients with cytopenias had positive auto-antibodies to at least one cell lineage, including Coombs (n=30, 55.6%), anti-granulocyte (n=10, 18.5%), and anti-platelet antibodies (n=5, 9.3%) (Figure 5E). All cytopenias occurred in the pre-transplant period apart from one patient who developed AIHA at 23 months of age (5 months post-HSCT) and another patient who underwent two consecutive HSCT and developed AIHA at 26 months (18 months post-final HSCT) and AN at 13 years.
Figure 5. Autoimmune cytopenias are a frequent and early-onset complication in patients with RAG deficiency.
(A) Prevalence of single- and multi-lineage cytopenias (frequency as % total cases). (B) Kaplan-Meier curves of RAG deficient patients with autoimmune cytopenias (n=53), showing difference in timing of cytopenia onset (blue line) and genetic diagnosis of RAG deficiency (red line). Severity of autoimmune cytopenias by (C) cell nadir and (D) duration (symbols representing individual patients, median +/− 95% CI shown). (E) Prevalence of positive autoimmune cytopenia autoantibodies (frequency as % total cases). Exact patient counts as shown with statistical difference indicated (**P <0.005); absolute neutrophil count (ANC); autoimmune cytopenia (AIC); diagnosis (dx); platelet (PLT).
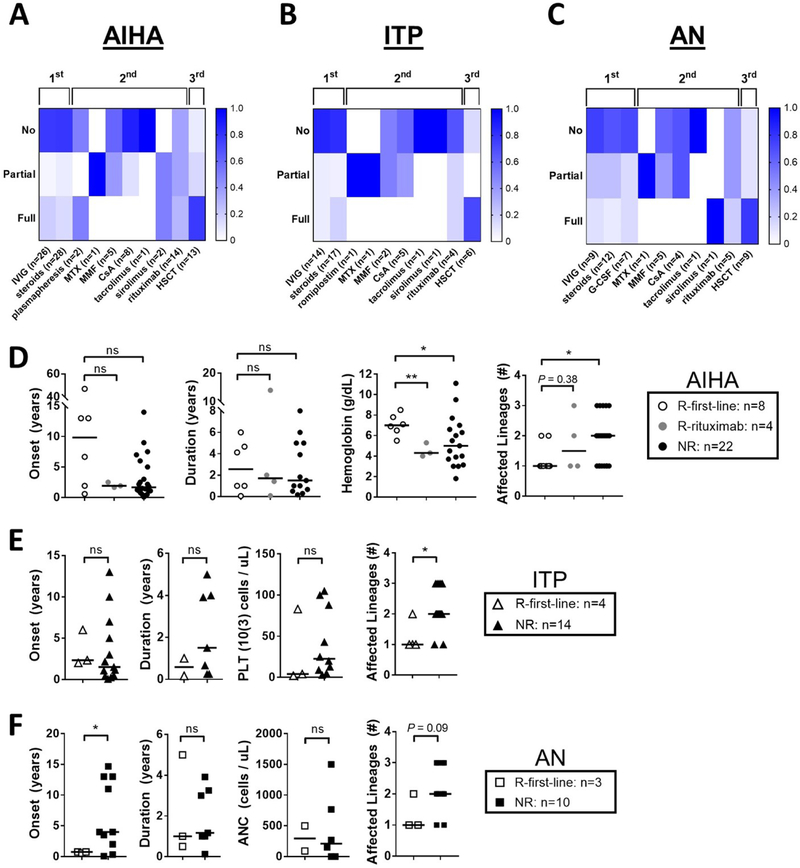
Treatment outcomes as available were reviewed in detail for cases of AIHA (n=34), ITP (n=19), and AN (n=14) (Figure 6A–C). Intravenous immunoglobulin (IVIG), steroids, and granulocyte-colony stimulating factor (G-CSF) in the context of AN specifically, were frequently used as first-line agents. However, definitive control to first-line therapy was achieved in only a subset of patients (23.5% in AIHA, 21.1% in ITP, and 21.4% in AN). The majority of patients progressed to second-line therapy, which most frequently included B cell depletion using rituximab (AIHA: n=14 (41%), ITP: n=4 (21%), AN: n=5 (35%)). Even this approach often failed to control the disease. Specifically, complete remission after use of rituximab was observed in only 28.9%, 16.7%, and 20.0% of patients with AIHA, ITP, and AN, respectively. Sirolimus was utilized only in two patients, leading to full remission of AIHA and AN in one of them. At the time of review, 64.7% of AIHA cases, 73.7% of ITP cases, and 71.4% of AN cases had no or only partial disease control to all first- and second-line therapeutics trialed. Among patients who received HSCT because of treatment-refractory autoimmune cytopenias, complete remission was observed in 76.9% of AIHA, 71.4% of ITP, and 77.8% of AN cases, respectively.
Figure 6. Autoimmune cytopenias in RAG deficiency are refractory to first- and second-line therapy.
Autoimmune cytopenia treatment response, scored by individual treatment modality for each incidence of (A) AIHA, (B) ITP, (C) and AN (% response per trialed therapeutic shown by color gradation as indicated; therapeutic grouping by first-line (IVIG, steroids, and/or G-CSF), second-line (all biologics), and third-line (HSCT) agents as shown; number of annotated therapeutic trials shown). Clinical response at first-line therapy (R-first-line) vs. at rituximab therapy (R-rituximab) vs. non-responders to all first- and second-line therapies trialed to date (NR) is compared for (D) AIHA, (E) ITP, and (F) AN according to cytopenia onset, cytopenia duration, cell line nadir, and number of cell lineages involved (symbols representing individual patients, median shown, exact patient counts shown); absolute neutrophil count (ANC); cyclosporine A (CsA); granulocyte colony-stimulating factor (G-CSF); hematopoietic stem cell transplant (HSCT); intravenous immunoglobulin (IVIG); methotrexate (MTX); mycophenolate mofetil (MMF); platelet (PLT).
To further investigate clinical features that correlate with response to treatment for cytopenias, we analyzed patients that had definitive control at first-line therapy (R-first-line) vs. patients that had definitive control following rituximab (R-rituximab) vs. patients with incomplete response (‘no’ or ‘partial’) to all first- and/or second-line therapies trialed to date (NR). For AIHA, in comparison to R-first-line, we observed lower hemoglobin nadirs in the NR (median 4.3 vs. 7.0 g/dL, P = 0.035) and the R-rituximab (median 5.0 g/dL vs. 7.0 g/dL, P = 0.0047). In addition, we observed more frequent occurrence of multi-lineage cytopenias in the NR (median 2 vs. 1 cell lineage affected, P = 0.015). There was also a trend towards earlier age at onset of cytopenias in the NR and R-rituximab that did not meet statistical significance (Figure 6D). For ITP and AN, we had only a single patient who met criteria for R-rituximab, precluding further subset analysis. However, a similar observation of multi-lineage cytopenias in the NR vs. R-first-line was seen for ITP (median 2 vs. 1 cell lineage affected, P = 0.018), with a trend towards significance for AN (median 2 vs. 1 cell lineage affected, P = 0.097) (Figure 6E & F). Finally, for AN we observed a later age at onset in the NR vs. R-first-line (0.75 vs. 4 years, P = 0.0099) (Figure 6F). Together these data suggest that several factors correlate with lack of response to first-line therapy in autoimmune cytopenias, in particular: 1) Evans syndrome (≥ 2 affected lineages); 2) low hemoglobin nadir (≤ 5.0 g/dL) in patients with AIHA; and, 3) delayed age at onset (≥ 4 years) in patients developing AN.
4. Other Autoimmune and Hyperinflammatory Complications: Occurrence, Outcomes, and Treatment
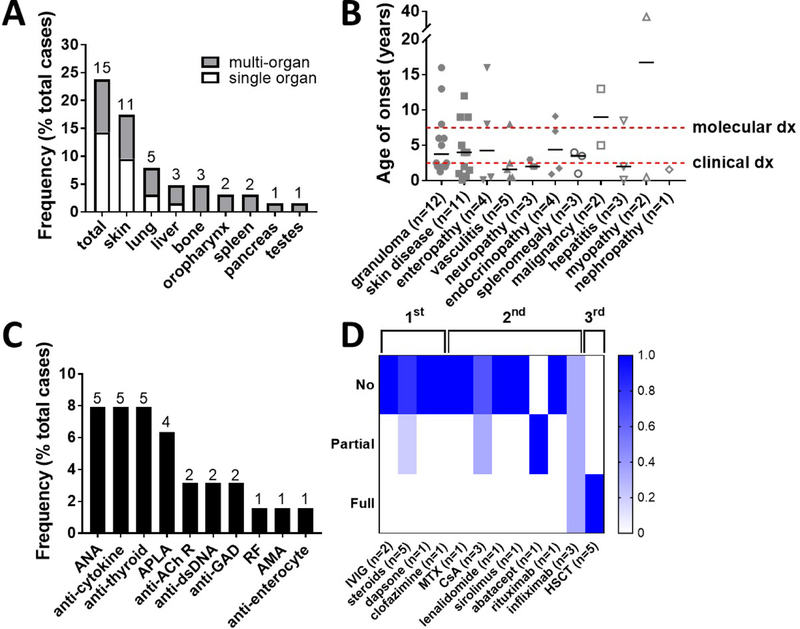
In total, 42 patients (66.7%) presented with other autoimmune or hyperinflammatory complications either alternatively (15.9%) or additionally (50.7%) to cytopenias. Granulomas were the most common, occurring in 15 patients (23.8%). Most granulomas were confined to a single organ (60.0%) with a subset of patients who developed multi-organ disease (40.0%). Single organ granulomas were predominantly limited to the skin (n=6) with the exception of two patients with lung granulomas and one patient with liver granulomas. However, a variety of organs may be affected by granulomas, including skin (n=10), lungs (n=5), liver (n=3), bone (n=3), oropharynx (n=2), spleen (n=2), pancreas (n=1), and testes (n=1) (Figure 7A). Inflammatory skin disorders were also prominent in the curated patient database, occurring in 12 patients (19.0%), and included combinations of vitiligo (n=6), psoriasis (n=2), alopecia (n=2), eczema/dermatitis (n=2), urticaria (n=1), and non-infectious nail dystrophy (n=1). Vasculitis occurred in 5 patients (7.9%), and when further annotated, was complicated by digital necrosis (n=2), stroke and Henoch-Schönlein purpura (n=1), and skin manifestations only (n=1). Enteropathy occurred in 5 patients (7.9%) and was annotated as IBD (n=2), autoimmune enteropathy (n=1), duodenitis (n=1), and severe non-infectious diarrhea (n=1). Autoimmune neuropathy occurred in 5 patients (7.9%) and was recorded as Guillain-Barré syndrome, Miller Fisher syndrome, myasthenia gravis, central demyelinating neuropathy, and aseptic encephalitis in one patient each. Endocrinopathies occurred in 5 patients (7.9%) and included autoimmune thyroiditis (n=4) and type I diabetes mellitus (n=1). Hepatitis occurred in 4 patients (6.3%) and included autoimmune hepatitis (n=3) and sclerosing cholangitis (n=1). Malignancy occurred in 3 patients (4.8%) and was exclusively lymphoma (one cutaneous T cell lymphoma, one mucosa-associated lymphoid tissue (MALT) lymphoma, and one EBV-driven B cell lymphoma of the tonsil). Finally, there were rare cases of inflammatory myopathy (n=2), minimal change nephropathy (n=1), and uveitis (n=1).
Figure 7. A spectrum of other autoimmune and hyperinflammatory diseases occur in RAG deficiency.
(A) Prevalence of single- and multi-organ granulomas listed by anatomic location (frequency as % total cases). (B) Age of onset for the other autoimmune and hyperinflammatory complications (dots representing individual patients, median +/− 95% CI shown, clinical milestones annotated). (C) Prevalence of positive autoantibodies (frequency as % total cases). (D) Granuloma treatment response, scored by individual treatment modality for each incidence of granulomatous disease (% response per trialed therapeutic shown by color gradation as indicated; therapeutic grouping by first-line (IVIG, steroids, and/or anti-infectives), second-line (all biologics), and third-line (HSCT) agents as shown; number of annotated therapeutic trials shown); acetylcholine receptor (AChR); anti-mitochondrial antibody (AMA); anti-nuclear antibody (ANA); anti-phospholipid antibody (APLA); cyclosporin A (CsA); double stranded DNA (dsDNA); glutamic acid decarboxylase (GAD); hematopoietic stem cell transplant (HSCT); intravenous immunoglobulin (IVIG); methotrexate (MTX); mycophenolate mofetil (MMF); rheumatoid factor (RF).
Despite wide patient-to-patient variability, the median age of onset of vasculitis (1.6 years), nephropathy (1.6 years), thyroiditis (1.75 years), hepatitis (2.0 years), and neuropathy (2.0 years) indicated that these were among the earliest immune dysregulatory complications (Figure 7B). In contrast to the autoimmune cytopenias, however, none of these complications statistically preceded the timing of genetic diagnosis, suggesting lower yield benefit in terms of facilitating the diagnosis of RAG deficiency. Autoantibody production was prominent, with anti-nuclear (ANA), anti-cytokine, and anti-thyroid antibodies being most common (Figure 7C).
Treatment outcomes were well annotated in 10 of the 15 patients who developed granulomas (Figure 7D). Spontaneous granuloma resolution was seen in two patients with skin manifestations only, while the remainder of patients (80.0%) did not respond to first-line IVIG and/or steroids. Of the second-line agent trialed, only infliximab resulted in full response in one patient with multi-organ disease as well as partial response (temporizing for years) in one patient with isolated lung granulomas. Ultimately, HSCT was required for definitive management in 5 cases (50.0%) without granuloma recurrence to date.
Among the five patients with vasculitis, topical and systemic steroids were sufficient to induce remission in one case of late-onset (8 years) disease limited to skin manifestations. In contrast first- and second-line treatment with steroids, IVIG, cyclophosphamide, alemtuzumab, and/or rituximab failed to achieve a sustained response in the remaining four cases of early-onset (median 1.0 years) and severe disease (complicated by digital necrosis, stroke, and Henoch-Schönlein purpura). Ultimately, three of these patients were stabilized with HSCT while the final patient passed away prior to anticipated HSCT.
Three of the five cases of enteropathy had well annotated treatment outcomes. There was limited response to first- and/or second-line therapy with steroids, non-steroidal anti-inflammatories, cyclosporine, and sirolimus in all three cases. Adalimumab (Humira) was temporizing for a year in one case of duodenitis, however, all three cases ultimately required progression to transplant for definitive management.
Discussion
Herein, we present the largest assembled case series of RAG deficiency with prominent autoimmune and/or hyperinflammatory complications. The compilation of this patient database allowed for the first systematic analysis of autoimmune and hyperinflammatory complications secondary to RAG deficiency in terms of frequency, outcome, and response to therapeutic intervention. We observed a high prevalence of autoimmune and hyperinflammatory complications in published cases of RAG deficiency (67.1%). However, we do acknowledge a potential publication bias towards unusual clinical presentations of RAG deficiency that may skew towards an overrepresentation of autoimmune and/or hyperinflammatory comorbidities in the literature (22).
In our curated patient database, we observed a median 5-year delay between the clinical recognition of immune dysregulation (immunodeficiency and/or autoimmunity) and the final diagnosis of RAG deficiency. This diagnostic delay likely reflects lack of recognition that hypomorphic RAG mutations are often associated with severe manifestations of immune dysregulation and with normal to elevated IgG serum levels, in contrast to what has been observed in patients with T- B- SCID due to null RAG mutations (2). However, because of the retrospective nature of this study, it included many patients whose clinical manifestations of immune dysregulation occurred before the clinical phenotype of CID-G/AI was reported in 2008 (7). A prospective collection of clinical, immunological, and molecular data will help to assess whether improved awareness of the phenotypic spectrum of the disease may lead to more prompt recognition of cases with hypomorphic mutations and more prevalent autoimmune and hyperinflammatory manifestations. Experience with newborn screening for SCID and related disorders has highlighted that RAG mutations are more often associated with AS and Omenn syndrome than with T- B- SCID (23). Whether newborn screening is also capable of identifying patients who will manifest a CID-G/AI phenotype remains to be studied. Alternative screening approaches such as analysis of TCRα bias using the PROMIDISα biomarker may additionally prove clinically beneficial (24). Finally, as we demonstrated reduced T cell counts and diminished proportion of peripheral naïve CD4+ cells across multiple RAG deficient clinical phenotypes, including CID-G/AI specifically, detailed CD4+ immunophenotyping may be of particular utility in suspecting RAG deficiency in those patients manifesting primarily with features of immune dysregulation.
Infections frequently preceded the onset of autoimmunity/hyperinflammation in the patient dataset by a temporal association of days to months, with a majority of naturally acquired viral infections and live viral vaccinations. These data highlight the clinical importance of diagnosing RAG deficiency prior to administering live viral vaccines. However, how viral infections may precipitate immune dysregulation in patients with RAG deficiency remains unclear.
Cytopenias were the most frequent autoimmune/hyperinflammatory manifestation in our series and presented early in life (median onset 1.9 years for AIHA, 2.1 years for ITP, and 2.6 years for AN). A lack of response to first-line therapy (predominantly IVIG and steroids) and second-line therapy (predominantly rituximab) was observed in the majority of cases. In particular, complete remission after use of rituximab was achieved in only 28.9% of AIHA cases, 16.7% of ITP cases, and 20.0% of AN cases. These data are in contrast to the benefit of rituximab that has been reported in the literature previously in CVID patients with autoimmune cytopenias (85% initial complete patient response rate for AIHA and/or ITP) (25), and more closely resemble the intermittent rituximab responsiveness for autoimmune cytopenias reported previously in patients with combined T cell dysfunction syndromes, including autoimmune lymphoproliferative syndrome (ALPS) (Table E2). However, we acknowledge the limitation of our retrospective, international, multicenter study, which relied on physician annotation to score therapeutic response as compared to the more objective measure of cell counts used in CVID previously (25). In our case series, multi-lineage cytopenias, a low nadir of hemoglobin (≤ 5.0 g/dL) during AIHA episodes, and later age of onset (≥ 4 years) for AN were associated with lack of response to first-line treatment of autoimmune cytopenias. Sirolimus has been shown to be beneficial in the management of refractory cytopenias in patients with ALPS and CVID (26); however, it was used in only two patients in the present case series, and additional experience must be collected to document its efficacy in RAG deficiency. Definitive therapy with HSCT was successful in the majority of RAG deficient patients with severe autoimmune cytopenias in this series. Thus, while RAG deficiency is a small contributor to the overall incidence of autoimmune cytopenias in the general population, these data suggest that consideration of RAG deficiency in the differential diagnosis of treatment-refractory multi-lineage disease specifically may have potential therapeutic benefit, specifically early consideration of HSCT for definitive management.
Granulomas were the second most prevalent autoimmune/hyperinflammatory complication identified (23.8%) in this series. Single organ disease was more frequent and often limited to the skin. TNF inhibitors were used in three patients in this series and led to full remission in one patient with multi-organ disease and partial and transient response in another patient with lung granulomas. Additional clinical experience must be collected to evaluate the efficacy of this treatment. On the other hand, 50% of the patients with treatment-refractory granulomas ultimately required HSCT for definitive management in this series.
Finally, vasculitis occurred early in the course of RAG deficiency (median 1.6 years), was often complicated by significant end-organ involvement, and in most cases was not responsive to first- or second-line therapy but required HSCT for definitive management in this series. Similarly, the majority of patients with severe gastrointestinal manifestations required HSCT for definitive management in this series. One patient experienced initial benefit from adalimumab.
Overall, our data demonstrate that immune dysregulation is a common feature of RAG deficiency and is often refractory to conventional medical management. Characterization of factors associated with lack of response to first- and second-line treatment may help to identify patients in which HSCT should be considered early in the course of the disease, before development of severe organ damage.
Supplementary Material
3. Highlights.
1) What is already known about this topic? Knowledge of autoimmunity in RAG deficiency has been limited to small case series; herein we introduce the largest international database of RAG deficient cases with prominent autoimmune and hyperinflammatory disease facilitating detailed outcomes and treatment analysis.
2) What does this article add to our knowledge? RAG diagnosis is delayed in the setting of autoimmunity or hyperinflammation (median 5 years); autoimmune cytopenias are prevalent (84.1%), have early-onset (median 1.9–2.6 years), and lack of first-line treatment response correlates strongly with multi-lineage disease.
3) How does this study impact current management guidelines? RAG deficiency can present with autoimmunity/hyperinflammation; low naïve (CD45RA+) T cells counts are a useful diagnostic tool; multi-lineage cytopenias are refractory to immunosuppressive treatment in most cases and should prompt expedited hematopoietic cell transplantation evaluation.
7. Acknowledgements
This work was supported by the National Institutes of Health (T32-HL116275 to J.R.F.; 5K08AI103035 and sub-R01AI100887–05 to J.E.W.; R01AR059703 to P.J.F.; 5K12HD043245 to S.E.H.), the Clinical Immunology Society and the AAAAI Foundation (to S.E.H.), Robert A. Good Endowment, University of South Florida (to J.E.W.), and by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (to L.D.N.).
5. Abbreviations
- ai
autoimmune
- AIC
autoimmune cytopenia
- AIHA
autoimmune hemolytic anemia
- AN
autoimmune neutropenia
- CID-G/AI
combined immunodeficiency with granulomatous disease and/or autoimmunity
- CVID
common variable immunodeficiency
- ICL
idiopathic CD4+ T cell lymphopenia
- ITP
immune thrombocytopenia
- IBD
inflammatory bowel disease
- HSCT
hematopoietic stem cell transplantation
- HIM
hyper-IgM syndrome
- RAG
recombinase activating gene
- SCID
severe combined immunodeficiency
- AS
atypical SCID
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures
The authors have no conflicts of interest to disclose regarding the content of this paper.
8. References
- 1.Oettinger MA, Schatz DG, Gorka C, Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science 1990;248(4962):1517–23. [DOI] [PubMed] [Google Scholar]
- 2.Schwarz K, Gauss GH, Ludwig L, Pannicke U, Li Z, Lindner D, et al. RAG mutations in human B cell-negative SCID. Science 1996;274(5284):97–9. [DOI] [PubMed] [Google Scholar]
- 3.Villa A, Santagata S, Bozzi F, Giliani S, Frattini A, Imberti L, et al. Partial V(D)J recombination activity leads to Omenn syndrome. Cell 1998;93(5):885–96. [DOI] [PubMed] [Google Scholar]
- 4.Walter JE, Rucci F, Patrizi L, Recher M, Regenass S, Paganini T, et al. Expansion of immunoglobulin-secreting cells and defects in B cell tolerance in Rag-dependent immunodeficiency. J Exp Med 2010;207(7):1541–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehl S, Schwarz K, Enders A, Duffner U, Pannicke U, Kuhr J, et al. A variant of SCID with specific immune responses and predominance of gamma delta T cells. J Clin Invest 2005;115(11):3140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Villartay JP, Lim A, Al-Mousa H, Dupont S, Dechanet-Merville J, Coumau-Gatbois E, et al. A novel immunodeficiency associated with hypomorphic RAG1 mutations and CMV infection. J Clin Invest 2005;115(11):3291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuetz C, Huck K, Gudowius S, Megahed M, Feyen O, Hubner B, et al. An immunodeficiency disease with RAG mutations and granulomas. The New England journal of medicine 2008;358(19):2030–8. [DOI] [PubMed] [Google Scholar]
- 8.Henderson LA, Frugoni F, Hopkins G, de Boer H, Pai SY, Lee YN, et al. Expanding the spectrum of recombination-activating gene 1 deficiency: a family with early-onset autoimmunity. The Journal of allergy and clinical immunology 2013;132(4):969–71 e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiff A, Bassuk AG, Church JA, Campbell E, Bing X, Ferguson PJ. Exome sequencing reveals RAG1 mutations in a child with autoimmunity and sterile chronic multifocal osteomyelitis evolving into disseminated granulomatous disease. J Clin Immunol 2013;33(8):1289–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharapova SO, Migas A, Guryanova I, Aleshkevich S, Kletski S, Durandy A, et al. Late-onset combined immune deficiency associated to skin granuloma due to heterozygous compound mutations in RAG1 gene in a 14 years old male. Hum Immunol 2013;74(1):18–22. [DOI] [PubMed] [Google Scholar]
- 11.Kuijpers TW, Ijspeert H, van Leeuwen EM, Jansen MH, Hazenberg MD, Weijer KC, et al. Idiopathic CD4+ T lymphopenia without autoimmunity or granulomatous disease in the slipstream of RAG mutations. Blood 2011;117(22):5892–6. [DOI] [PubMed] [Google Scholar]
- 12.Chou J, Hanna-Wakim R, Tirosh I, Kane J, Fraulino D, Lee YN, et al. A novel homozygous mutation in recombination activating gene 2 in 2 relatives with different clinical phenotypes: Omenn syndrome and hyper-IgM syndrome. The Journal of allergy and clinical immunology 2012;130(6):1414–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abolhassani H, Wang N, Aghamohammadi A, Rezaei N, Lee YN, Frugoni F, et al. A hypomorphic recombination-activating gene 1 (RAG1) mutation resulting in a phenotype resembling common variable immunodeficiency. The Journal of allergy and clinical immunology 2014;134(6):1375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villa A, Marrella V, Rucci F, Notarangelo LD. Genetically determined lymphopenia and autoimmune manifestations. Curr Opin Immunol 2008;20(3):318–24. [DOI] [PubMed] [Google Scholar]
- 15.Kreuzaler M, Rauch M, Salzer U, Birmelin J, Rizzi M, Grimbacher B, et al. Soluble BAFF levels inversely correlate with peripheral B cell numbers and the expression of BAFF receptors. J Immunol 2012;188(1):497–503. [DOI] [PubMed] [Google Scholar]
- 16.Rigoni R, Fontana E, Guglielmetti S, Fosso B, D’Erchia AM, Maina V, et al. Intestinal microbiota sustains inflammation and autoimmunity induced by hypomorphic RAG defects. J Exp Med 2016;213(3):355–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter JE, Rosen LB, Csomos K, Rosenberg JM, Mathew D, Keszei M, et al. Broad-spectrum antibodies against self-antigens and cytokines in RAG deficiency. J Clin Invest 2015;125(11):4135–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delmonte OM, Schuetz C, Notarangelo LD. RAG Deficiency: Two Genes, Many Diseases. J Clin Immunol 2018. [DOI] [PMC free article] [PubMed]
- 19.Shearer WT, Dunn E, Notarangelo LD, Dvorak CC, Puck JM, Logan BR, et al. Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: the Primary Immune Deficiency Treatment Consortium experience. J Allergy Clin Immunol 2014;133(4):1092–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YN, Frugoni F, Dobbs K, Walter JE, Giliani S, Gennery AR, et al. A systematic analysis of recombination activity and genotype-phenotype correlation in human recombination-activating gene 1 deficiency. J Allergy Clin Immunol 2014;133(4):1099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tirosh I, Yamazaki Y, Frugoni F, Ververs FA, Allenspach EJ, Zhang Y, et al. Recombination activity of human recombination-activating gene 2 (RAG2) mutations and correlation with clinical phenotype. The Journal of allergy and clinical immunology 2018. [DOI] [PMC free article] [PubMed]
- 22.Modell V, Knaus M, Modell F, Roifman C, Orange J, Notarangelo LD. Global overview of primary immunodeficiencies: a report from Jeffrey Modell Centers worldwide focused on diagnosis, treatment, and discovery. Immunol Res 2014;60(1):132–44. [DOI] [PubMed] [Google Scholar]
- 23.Kwan A, Abraham RS, Currier R, Brower A, Andruszewski K, Abbott JK, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA 2014;312(7):729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berland A, Rosain J, Kaltenbach S, Allain V, Mahlaoui N, Melki I, et al. PROMIDISalpha: A T-cell receptor alpha signature associated with immunodeficiencies caused by V(D)J recombination defects. The Journal of allergy and clinical immunology 2018. [DOI] [PubMed]
- 25.Gobert D, Bussel JB, Cunningham-Rundles C, Galicier L, Dechartres A, Berezne A, et al. Efficacy and safety of rituximab in common variable immunodeficiency-associated immune cytopenias: a retrospective multicentre study on 33 patients. Br J Haematol 2011;155(4):498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bride KL, Vincent T, Smith-Whitley K, Lambert MP, Bleesing JJ, Seif AE, et al. Sirolimus is effective in relapsed/refractory autoimmune cytopenias: results of a prospective multi-institutional trial. Blood 2016;127(1):17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Ravin SS, Cowen EW, Zarember KA, Whiting-Theobald NL, Kuhns DB, Sandler NG, et al. Hypomorphic Rag mutations can cause destructive midline granulomatous disease. Blood 2010;116(8):1263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen K, Wu W, Mathew D, Zhang Y, Browne SK, Rosen LB, et al. Autoimmunity due to RAG deficiency and estimated disease incidence in RAG½ mutations. The Journal of allergy and clinical immunology 2014;133(3):880–2 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchbinder D, Baker R, Lee YN, Ravell J, Zhang Y, McElwee J, et al. Identification of patients with RAG mutations previously diagnosed with common variable immunodeficiency disorders. J Clin Immunol 2015;35(2):119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuetz C, Pannicke U, Jacobsen EM, Burggraf S, Albert MH, Honig M, et al. Lesson from hypomorphic recombination-activating gene (RAG) mutations: Why asymptomatic siblings should also be tested. The Journal of allergy and clinical immunology 2014;133(4):1211–5. [DOI] [PubMed] [Google Scholar]
- 31.Avila EM, Uzel G, Hsu A, Milner JD, Turner ML, Pittaluga S, et al. Highly variable clinical phenotypes of hypomorphic RAG1 mutations. Pediatrics 2010;126(5):e1248–52. [DOI] [PubMed] [Google Scholar]
- 32.Patiroglu T, Akar HH, Van Der Burg M. Three faces of recombination activating gene 1 (RAG1) mutations. Acta Microbiol Immunol Hung 2015;62(4):393–401. [DOI] [PubMed] [Google Scholar]
- 33.Dutmer CM, Asturias EJ, Smith C, Dishop MK, Schmid DS, Bellini WJ, et al. Late Onset Hypomorphic RAG2 Deficiency Presentation with Fatal Vaccine-Strain VZV Infection. J Clin Immunol 2015;35(8):754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geier CB, Piller A, Linder A, Sauerwein KM, Eibl MM, Wolf HM. Leaky RAG Deficiency in Adult Patients with Impaired Antibody Production against Bacterial Polysaccharide Antigens. PLoS One 2015;10(7):e0133220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westermann-Clark E, Grossi A, Fioredda F, Giardino S, Cappelli E, Terranova P, et al. RAG deficiency with ALPS features successfully treated with TCRalphabeta/CD19 cell depleted haploidentical stem cell transplant. Clinical immunology (Orlando, Fla) 2018;187:102–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goda V, Malik A, Kalmar T, Maroti Z, Patel B, Ujhazi B, et al. Partial RAG deficiency in a patient with varicella infection, autoimmune cytopenia, and anticytokine antibodies. The journal of allergy and clinical immunology In practice 2018. [DOI] [PMC free article] [PubMed]
- 37.Neven B, Perot P, Bruneau J, Pasquet M, Ramirez M, Diana JS, et al. Cutaneous and Visceral Chronic Granulomatous Disease Triggered by a Rubella Virus Vaccine Strain in Children With Primary Immunodeficiencies. Clin Infect Dis 2017;64(1):83–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.