Abstract
Background:
Children with severe asthma have frequent exacerbations despite guidelines-based treatment with high-dose corticosteroids. The importance of refractory lung inflammation and infectious species as factors contributing to poorly-controlled asthma in children are poorly understood.
Objective:
To identify prevalent granulocyte patterns and potential pathogens as targets for revised treatment, 126 children with severe asthma underwent clinically-indicated bronchoscopy.
Methods:
Diagnostic tests included BAL for cell count and differential, bacterial and viral studies, spirometry, and measurements of blood eosinophils, total IgE, and allergen-specific IgE. Outcomes were compared among 4 BAL granulocyte patterns.
Results:
Children with pauci-granulocytic BAL were most prevalent (52%), and compared to mixed granulocytic BAL, had less post-bronchodilator (BD) airflow limitation, less blood eosinophilia, and less detection of BAL enterovirus. Children with isolated neutrophilia BAL were differentiated by less blood eosinophilia than mixed granulocytic BAL, but greater prevalence of potential bacterial pathogens compared to pauci-granulocytic BAL. Children with isolated eosinophilia BAL had features similar to mixed granulocytic BAL. Children with mixed granulocytic BAL took more maintenance prednisone, and had greater blood eosinophilia and allergen sensitization compared to pauci-granulocytic BAL.
Conclusions:
In children with severe, therapy-resistant asthma, BAL granulocyte patterns and infectious species are associated with novel phenotypic features which can inform pathway-specific revisions in treatment. In 32% of children evaluated, BAL revealed corticosteroid-refractory eosinophilic infiltration amenable to anti-Th2 biological therapies, and in 12%, a treatable bacterial pathogen.
Keywords: severe asthma, bronchoalveolar lavage, asthma phenotypes
Introduction
Most children with asthma treated daily with low- to medium-dose inhaled corticosteroids attain symptom control with few exacerbations 1. In contrast, children with problematic asthma have frequent symptoms and exacerbations despite treatment with high-dose inhaled and systemic corticosteroids 2–5. A rigorous approach to the child with problematic asthma includes referral to a specialty center for a staged assessment to confirm the diagnosis, and address remediable factors including co-morbid diagnoses and mitigation of adverse environmental conditions 6–7. Controller therapies are then adjusted accordingly, and the child is followed by an asthma specialist before a diagnosis of “severe” asthma is made 8. Those children with frequent symptoms and exacerbations despite these steps have “poorly-controlled, therapy-resistant asthma.” However, the extent to which such children have therapy-resistant lung inflammation and detectable lower respiratory infectious species is not well understood. Although sputum, BAL, and blood granulocyte counts have been studied in children with therapy-resistant asthma 9–11, quantitative correlation among those compartments is unreliable, and limits their utility in guiding treatment. Nonetheless, studies of inflammatory markers in BAL and endobronchial biopises have been safely conducted in children with problematic asthma 12. These investigations demonstrate heterogenous patterns of lung fluid and bronchial wall inflammation, including eosinophilic and neutrophilic infiltration, type 2 innate and diverse helper Th1/Th2/Th17 responses, increased production of reactive chemical species, characteristic BAL cytokine clusters, and impaired macrophage phagocytic function 13–26.
These original reports provide insight into the pattern of lung inflammation in severe asthma of childhood. However, they are based on relatively small samples, with limited information as to whether prevalent inflammatory patterns correspond to specific clinical features. Furthermore, the role of lower respiratory pathogens in informing the lung inflammatory milieu and clinical features in severe asthma has received only limited study 16, despite the clear importance of infection in the pathobiology of asthma of childhood 27. Thus a broader understanding of prevalent lung inflammatory patterns and potential infectious species and how they might inform clinical features in children with poorly-controlled, therapy-resistant asthma is necessary. Published guidelines which contain algorithms to advance treatment in childhood asthma are based on studies focused primarily on children with relatively mild asthma 28–29. Henceforth these algorithms may not be appropriate for children with severe asthma with alternate patterns of inflammation. Therefore, we conducted a prospective study in a well-characterized sample of children referred from community providers with poorly-controlled, therapy-resistant asthma to identify prevalent BAL granulocyte patterns and infectious species and describe the clinical features associated with individual patterns.
Methods
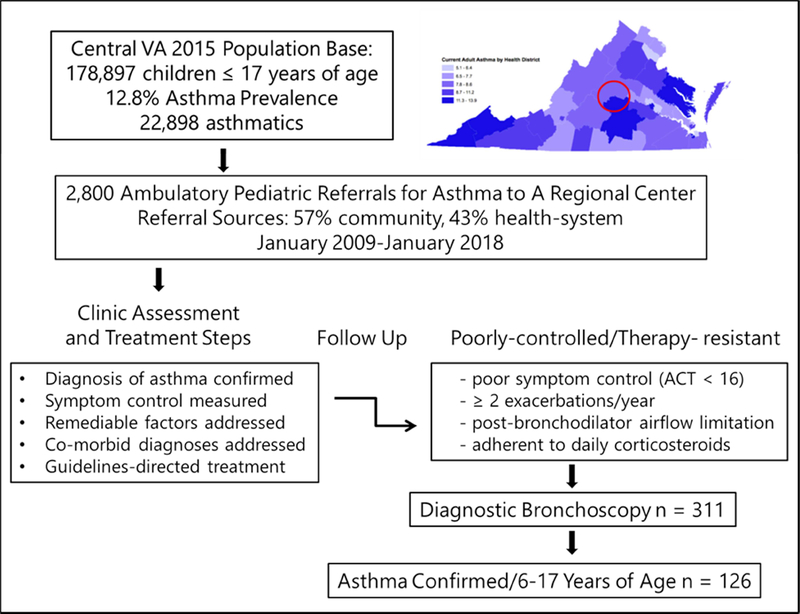
Children with poorly-controlled asthma were referred from a 35-county region in central Virginia to a university-based specialty clinic (Figure 1). These children had an initial assessment (see E-supplement, Methods) based on modification of an evidence-based approach published by the Brompton group 6–7 that included: a) confirmation of the diagnosis of asthma, b) correction of remediable factors, c) evaluation of adherence and co-morbid diagnoses, d) identification of allergen sensitization and appropriate avoidance measures, and e) measurements of lung function with bronchodilator response. After these steps, controller treatment was adjusted according to severity classification 8 and symptom control measures in accordance with standard guidelines 28–29. Combination high-dose inhaled corticosteroid /long-acting beta agonist therapy was prescribed based on symptom control status, but in some cases denied by the child’s payor of care. Children with severe, recurrent exacerbations and poor symptoms were treated with alternate day prednisone 0.5 mg/kg per dose. The children were then followed longitudinally in a specialty clinic by pediatric pulmonologists and allergists. Symptom control was assessed by measures recorded at the first clinic visit and repeated at follow up visits, with poor control defined by a composite index of symptom scores, exacerbations, adherence estimates 30, and lung function results (Figure 1 and E-Supplement).
Figure 1.
Consort plot and schematic of the geographic source and clinical assessment, treatment, characterization procedures, and indications for diagnostic bronchoscopy in the study sample.
Children with severe, therapy-resistant, poorly-controlled asthma were offered a diagnostic bronchoscopy with BAL and assessment of blood inflammatory markers. Samples were shared between the clinical and research laboratories through protocols approved by the University of Virginia Institutional Review Board (UVA HSR # 17555, UVA HSR# 10905, and UVA HSR# 10634). Informed consent was provided by the parents or legal guardians, and children > 7 years provided assent. Details of the asthma definition criteria, assessment of remediable factors, adherence, co-morbid diagnoses, exclusion and inclusion criteria, bronchoscopy, BAL, bronchial brushings, and peripheral blood markers are provided in the E- Supplement.
Data Analysis.
The analysis was limited to children age 6 to 17 years with confirmed asthma, demonstrable adherence, with exacerbations and poor control (Figure E1). BAL granulocyte categories as the basis for comparison of phenotypic features were modified from published studies in adults with asthma 31–33. Cut points for BAL eosinophilia and neutrophilia (Figure 2) were derived from tables published in the ERS Task Force on BAL in Children 34 and earlier investigations in healthy children 35. The analytic approach was exploratory as described in the E-Supplement.
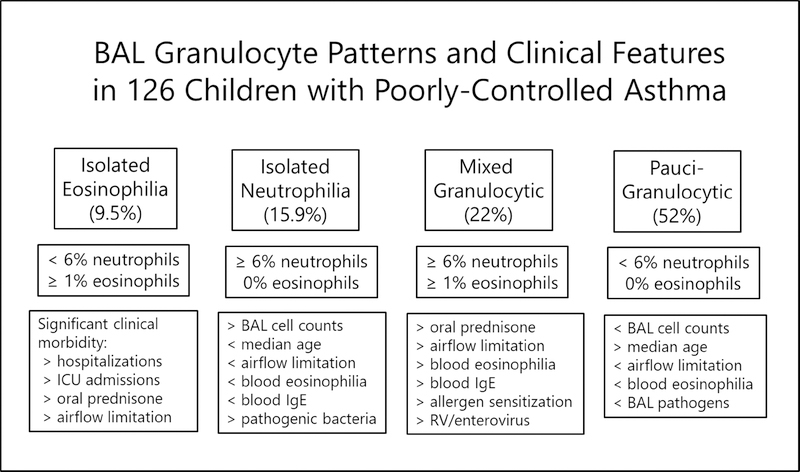
Figure 2.
Prevalent BAL granulocyte patterns and corresponding clinical features
Results
2,800 children with poorly-controlled asthma were referred to a regional asthma specialty clinic for assessment and treatment over a 9 year span (Figure 1). Among this sample, 311 children treated with high-dose inhaled and/or systemic corticosteroids with poor symptom control at follow up underwent diagnostic bronchoscopy. 126 of these children, 6 to 17 years of age, with confirmed, therapy-resistant severe asthma are the subject of this report. The features of children who had bronchoscopy but are not included in the analysis are provided in Figure E-1.
General Sample Features
The sample had significant asthma-related morbidity, over 2/3 had been admitted to the hospital in the past year despite treatment with three or more controller medications, with a median daily ICS dose of 800 μg per day fluticasone equivalents (Table E1). The proportion of children treated with daily ICS/LABA was approximately 2/3, lower than expected due to medication costs and non-coverage by the child’s payor of care. Co-morbid diagnoses were common, led by gastro-esophageal reflux disease (GERD), obesity, and type IB laryngeal clefts (Table E2).
Features Compared According to BAL Granulocyte Categories
Pauci-Granulocytic.
This was the most prevalent granulocyte category (52%). BAL total cell count was significantly lower in this category compared to the isolated neutrophilia category (Table I). Children with pauci-granulocytic BAL were significantly older than children with BAL isolated neutrophilia, and were less likely to be treated with maintenance prednisone compared to children with isolated eosinophilia or mixed granulocytic BAL (Table II). Children with pauci-granulocytic BAL had greater pre-bronchodilator (pre-BD) FEV1%, and greater pre-BD FVC% compared to children with isolated eosinophilia (Table III). The proportion of children with post-BD airflow limitation (based on an FEV1/FVC < 90%) was significantly less than it was in children with mixed granulocytic BAL. Blood eosinophil percentage and absolute blood eosinophil counts were significantly lower in pauci-granulocytic BAL compared to the mixed granulocytic category (Table IV). With regards to detection of potential pathogens in BAL, children in the pauci-granulocytic category had significantly lower prevalence of positive enterovirus/HRV transcripts compared to the mixed granulocytic category (Table V).
Table I.
Prevalent BAL Granulocyte Patterns and Constituents in 126 Children with Poorly-Controlled Asthma
| Isolated Eosinophilia | Isolated Neutrophilia | Mixed Granulocytic | Paucigranulocytic | |
|---|---|---|---|---|
| Sample n (%) * | 12 (9.5) | 20 (15.9) | 28 (22.2) | 66 (52.4) |
| Total Cell Count β (X 106 cells) | 1.78 (0.96–2.25) | 1.79 Ϯ (1.22–5.51) | 1.61 (0.57–2.92) | 1.08 (0.52–2.20) |
| Differential Cellular Constituents n (%) π | ||||
| Macrophages | 72 (59–82) | 61 (38–82) | 43 α (15–64) | 76 (64–90) |
| Neutrophils | 1 (0–2) | 12 (7–38) | 23 (11–56) | 1 (1–3) |
| Eosinophils | 4 (1–6) | 0 (0–0) | 3 (2–9) | 0 (0–0) |
| Lymphocytes | 3 (2–7 | 3 (1–6) | 3 (1–8) | 2 (1–5) |
| Ciliated epithelial cells | 16 (8–30) | 10 (4–14) | 11 (4–20) | 15 (5–28) |
| Aspiration Markerϴ | ||||
| Lipid-laden macrophage index | 0 (0–0) | 0 (0–2) | 0 (0–1) | 0 (0–1) |
row percentages
median (25th–75th percentile)
p = 0.02 vs pauci-granulocytic
expressed as median ± IQR % cells per smear
p < .05 vs pauci-granulocytic
nominal scale, range 0–4 in intensity.
Table II.
Demographic Features, Asthma Severity, and Treatment According to BAL Granulocyte Patterns
| Isolated Eosinophilia n=12 | Isolated Neutrophilia n=20 | Mixed Granulocytic n=28 | Pauci-Granulocytic n=66 | |
|---|---|---|---|---|
| Age (years) | 10 (8–13) | 8 Ϯ (6–12) | 9 (7–12) | 12 (8–15) |
| Male sex n (%) | 6 (50) | 12 (60) | 21 (75) | 34 (53) |
| Non-white n (%) | 9 (75) * | 4 (20) | 12 (43) | 29 (45) |
| Body Mass Index (kg/m2) | 19.8 (17.3–22.5) | 18.4 (15.6–25.8) | 20.6 (15.9–23.9) | 21.7 (17.2–27.2) |
| Asthma Severity Indicators | ||||
| Age at Symptom Onset (months) | 12 (6–12) | 12 (7–24) | 12 (12–36) | 12 (6–36) |
| Asthma duration (months) | 98 (78–144) | 73 (63–102) | 78 (57–126) | 107 (68–166) |
| ACT/cACT scores | 13 ± 3 | 16 ± 8 | 13 ± 7 | 16 ± 5 |
| Hospitalized in past year n (%) | 12 (100) • | 10 (50) | 21 (75) | 46 (70) |
| ICU admission n (%) | 6 (60) | 4 (21) | 8 (29) | 19 (30) |
| Treatment | ||||
| Number of daily controller meds. | 4 u (3–4) | 2 (2–3) | 2 (1–4) | 3 (1–3) |
| Daily ICS dose (μg fluticasone equiv.) | 800 (160–800) | 400 (227–800) | 800 (400–920) | 800 (320–800) |
| Prednisone Rx n (%) | 5 (42) | 2 (10) | 12 (43) Δ | 14 (22) |
| Omalizumab Rx n (%) | 2 (16) κ | 1 (5) | 3 (11) | 2 (3) |
| Mepolizumab Rx n (%) | 2 (16) | 0 | 2 (7) | 1 (2) |
| ICS/LABA Rx n (%) | 7 (58) | 13 (65) | 15 (54) | 41 (62) |
| Anti-leuk. n (%) | 10 (83)ϴ | 9 (45) | 11 (39) | 41 (62) |
Scaled results are median (25th–75th %ile)
p = 0.02 versus pauci-granulocytic
p =0.01
p= 0.04
p = 0.03 versus isolated neutrophilia
p = 0.01
p = 0.02
p = 0.004
Table III.
Pre- and Post-Bronchodilator Spirometry According to BAL Granulocyte Pattern
| Isolated Eosinophilia n=12 | Isolated Neutrophilia n=20 | Mixed Granulocytic n=28 | Pauci-Granulocytic n=66 | |
|---|---|---|---|---|
| Pre-BD FEV1 % * | 70 ± 21Ϯ | 98 ± 19 | 79 ± 24 α | 91 ± 18 |
| Post-BD FEV1 % | 82 ± 20 | 103 ± 19 | 89 ± 20 | 98 ± 24 |
| FEV1 BD % change Δ | 25 ± 14 | 7 ± 10 | 17 ± 23 | 12 ± 11 |
| Pre-BD FVC % | 81 ± 14 u | 102 ± 19 | 91 ± 22 | 101 ± 16 |
| Post-BD FVC % | 94 ± 11 | 105 ± 22 | 99 ± 18 | 106 ± 18 |
| FVC BD % change | 16 ± 12 κ | 4 ± 8 | 10 ± 11 μ | 3 ± 4 |
| Pre-BD FEV1/FVC % | 84 ± 13 | 95 ± 12 | 86 ± 14 | 89 ± 10 |
| Post-BD FEV1/FVC % | 87 ± 12 | 96 ± 9 | 90 ± 11 | 94 ± 8 |
| Pre-BD FEF25–75 % | 56 ± 43 | 93 ± 35 | 59 ± 34 π | 73 ± 30 |
| Post-BD FEF25–75 % | 66 ± 43 | 100 ± 30 | 73 ± 30 | 89 ± 31 |
| Pre-BD airflow limitation (%) | 62% | 31% | 56% | 50% |
| Post-BD airflow limitation (%) | 50% | 22% | 59% £ | 22% |
mean± SD
at the pre-bronchoscopy visit
p < 0.05 versus isolated neutrophilia and pauci-granulocytic
p = 0.03 versus isolated neutrophilia
p = 0.02 versus pauci-granulocytic
p < 0.05 versus isolated neutrophilia and pauci-granulocytic
p = 0.03 versus pauci-granulocytic
p = 0.02 versus isolated neutrophilia
p = 0.03.
Table IV.
Blood and Breath Markers of Inflammation According to BAL Granulocyte Pattern
| Isolated Eosinophilia n=12 | Isolated Neutrophilia n=20 | Mixed Granulocytic n=28 | Pauci-Granulocytic n=66 | |
|---|---|---|---|---|
| Blood eosinophil % β | 7 (0–10) | 2 (1–4) | 6 * (3–11) | 3 (1–8) |
| Absolute blood eosinophils (cells/ul) | 395 (0–662) | 170 (90–460) | 480 * (300–850) | 180 (90–495) |
| Peripheral blood eosinophilia n (%) π | 6 (50) | 5 (25) | 20 Ϯ (71) | 24 (37) |
| Absolute blood neutrophils (cells/ul) | 2830 (1760–3930) | 3680 (2945–7470) | 3370 (2175–5475) | 3145 (2110–4387) |
| Geometric mean total blood IgE (IU/ml) | 297 (97–1378) | 55 (16–342) | 322 α (170–1091) | 194 (56–700) |
| Number + allergenspecific IgE tests (of 16 tested) | 4 (0–12) | 1 (0–3) | 4 (0–11) | 3 (0–7) |
| Proportion with no allergens n (%) | 3 (25) | 7 (35) | 8 (29) | 20 (30) |
| Proportion ≥ 4 allergens n (%) | 6 (50) | 3 (19) | 15 γ (54) | 28 (42) |
| Serum C-reactive protein (mg/dl) | 0.42 (0.16–0.62) | 0.73 (0.19–1.61) | 0.39 (0.15—1.69) | 0.43 (0.20–1.86) |
| C-reactive protein ≥ 0.25 n (%) | 6 (50) | 14 (70) | 15 (54) | 41 (62) |
| Expired NO (ppb) | 51 (24–51) | 9 (5–8) | 27 (6–27) | 14 (6–14) |
Results are median and IQR
% eosinophils reported in blood count
p < 0.05 versus isolated neutrophilia and pauci-granulocytic
≥ 300 eosinophils per μl blood
p = 0.002
p = 0.05 versus isolated neutrophilia
p = 0.02.
Table V.
Potential Respiratory Pathogens in BAL According to Granulocyte Pattern
| Isolated Eosinophilia n (%)* | Isolated Neutrophilia n (%) | Mixed Granulocytic n (%) | Pauci-Granulocytic n (%) | |
|---|---|---|---|---|
| + Any Microbe n=39 (31%) ** | 1 (8) | 13 (65) Ϯ | 15 (54) | 10 (15) |
| + Any Respiratory Virus n=29 (24%) | 0 | 9 (45) | 13 (46) Ϯ | 7 (11) |
| + Any Bacteria n=18 (14%) | 1 (8) | 7 (35) β | 5 (18) | 5 (8) |
| + Virus and Bacteria n=8 (6%) | 0 | 3 (15) | 3 (11) | 2 (3) |
| Potential Viral Pathogens Identified n (%) | ||||
| Enterovirus/human rhinovirus | 0 | 4 (20) | 10 (36) u | 5 (8) |
| Human metapneumovirus | 0 | 2 (10) | 0 | 1 (2) |
| Influenza A | 0 | 1 (5) | 1 (4) | 0 |
| Influenza B | 0 | 0 | 1 (4) | 0 |
| RSV A | 0 | 1 (5) | 0 | 0 |
| RSV B | 0 | 0 | 1 (4) | 0 |
| Parainfluenza 1 | 0 | 1 (5) | 0 | 0 |
| Parainfluenza 2 | 0 | 0 | 0 | 1 (2) |
| Adenovirus | 0 | 0 | 0 | 0 |
| Potential Bacterial Pathogens Identified n (%) | ||||
| Streptococcus pneumoniae | 0 | 2 (10) | 3 (11) | 1 (2) |
| Moraxella catarrhalis | 1 (10) | 1 (5) | 0 | 2 (4) |
| Hemophilus influenzae | 0 | 3 (15) | 0 | 1 |
| Pseudomonas aeruginosa | 0 | 1 (5) | 0 | 0 |
column %
sample %
p < 0.001
n=121 cultures
p =0.02
p = 0.004.
Isolated Neutrophilia.
16 % of the children fit the isolated neutrophilia BAL category. Compared to children with pauci-granulocytic BAL, children with isolated neutrophilia had significantly higher BAL total cell counts (Table I), and a lower proportion of non-white minorities and lower median age at bronchoscopy (Table II). Children with isolated neutrophilia had significantly greater pre-BD FEV1 % compared to isolated eosinophilia, and higher Pre-BD FEF25–75 % compared to mixed granulocytic BAL (Table III). With regards to inflammatory markers, children with isolated neutrophilia had significantly lower blood eosinophil %, lower absolute blood eosinophil counts, and lower blood total IgE values compared to children with mixed granulocytic BAL (Table IV). Detection of any microbe was most prevalent in children with isolated neutrophilia, and specifically detection of potentially pathogenic bacteria was more prevalent in children with isolated neutrophilia compared to children with pauci-granulocytic BAL (Table V).
Isolated Eosinophilia.
9.5% % of children had BAL Isolated eosinophilia. This BAL category was differentiated by the highest prevalence of non-white children (90%), the highest prevalence of hospitalization in the past year (100%), the highest prevalence of past ICU admissions (60%), and the highest prevalence of omalizumab (20%) and antileukotriene treatments among the four categories (p < .05, Fisher’s exact test, Table II). The pre-BD FEV1 % was lower in this category than in children with isolated neutrophilia and pauci-granulocytic BAL, and the pre-BD FVC % was lower than isolated neutrophilia (Table III). Isolated eosinophilia was not associated with significant differences in values of any inflammatory markers or prevalence of potential pathogens.
Mixed Granulocytic.
This was the 2nd most prevalent BAL category, 22% of the total. Children with mixed granulocytic BAL had signficantly lower median BAL macrophages (43%) compared to the pauci-granulocytic category (Table I), and significantly greater prevalence of treatment with maintenance prednisone (Table II). Mean pre-BD FEV1% was significantly lower in mixed granulocytic BAL compared to isolated neutrophilia (Table III). The prevalence of children with post-BD persistent airflow limitation was significantly higher in mixed granulocytic BAL. The mixed granulocytic category was also differentiated by a relatively higher absolute blood eosinophil count (480 cells/μl; p < 0.05 versus isolated neutrophilia and pauci-granulocytic groups; Table IV), and the highest prevalence of children with peripheral blood eosinophils > 300 cells/μl (p = 0.002). Furthermore children with mixed granulocytic BAL had the highest blood total IgE (322 IU/ml, p < 0.05 versus isolated neutrophilia and pauci-granulocytic groups), and significantly greater sensitization to four or more allergens (58%, p = 0.02). With regard to detection of BAL potential pathogens, children with mixed granulocytic BAL had the highest prevalence of enterovirus/HRV transcripts (Table V).
Correlations Between Inflammatory Markers in Blood and BAL
The % of eosinophils in the blood had a significant (p < 0.001) positive correlation with % of eosinophils in BAL, but the coefficient was low (r = 0.32). To test whether maintenance prednisone treatment might impact the correlation between systemic and lung eosinophils, we did a secondary analysis excluding children treated with oral prednisone. In this secondary analysis the correlation between blood and BAL eosinophil % remained low at 0.36.
ROC curve analysis to depict sensitivity and specificity of the absolute blood eosinophil count as a predictor of BAL eosinophilia was poor, with an area under the curve of 0.66 (Figure E-2). Overall, the sensitivity of an absolute blood eosinophil count > 300 cells/μl blood for BAL eosinophilia was fair, 71% (Table VI), with a positive predictive value of 50%. Likewise the specificity of an absolute blood eosinophil count for BAL eosinophilia was low, 65%, with a negative predictive value of 82%.
Table VI.
Diagnostic Performance of the Absolute Blood Eosinophil Count to Predict BAL Eosinophilia in Children with Poorly-Controlled, Treatment-Resistant Asthma
| True Negatives Absolute Blood Eosinophil Count < 300 cells/ul and BAL Eosinophils < 1% N = 54 (43.5% of total) |
False Negatives Absolute Blood Eosinophil Count < 300 cells/ul and BAL Eosinophils ≥ 1% N = 12 (9.7% of total) |
| True Positives Absolute Blood Eosinophil Count ≥ 300 cells/ul and BAL Eosinophils ≥ 1% N = 29 (23.4% of total) |
False Positives Absolute Blood Eosinophil Count ≥ 300 cells/ul and BAL Eosinophils < 1% N = 29 (23.4% of total) |
Performance Indicators of Absolute Blood Eosinophils > 300 cells/ul for BAL Eosinophilia Sensitivity 71% Specificity 65% Positive Predictive Value 50% Negative Predictive Value 82%
Other systemic markers of inflammation, including the total blood neutrophil count and serum C-reactive protein performed poorly as predictors of BAL granulocyte numbers. The sensitivity and specificity of the total blood neutrophil count to predict BAL neutrophilia depicted by ROC curve analysis was not significant with an area under the curve = 0.59. Likewise the serum C-reactive protein performed equally with a low ROC curve area of < 0.60 ROC for both BAL neutrophilia and eosinophilia.
Safety
Bronchoscopy with BAL and bronchial brushing was well tolerated and safe. Post-establishment of general anesthesia, the median time to do a complete examinaton and collect samples was 12 minutes. Minor adverse events included brief laryngospasm (6.5%), wheeze (4.9%), cough (3.3%%), and transient hypoxemia during BAL (1.6%). Two children (1.6%) were admitted electively post-bronchoscopy. No child had a major unexpected adverse event as a result of the bronchoscopy or shared sample procedure. The distribution of individual adverse events was no different according to age category, sex, race, asthma control status, granulocyte pattern, or the presence of pre-BD or post-BD airflow limitation. However obese children had a significantly higher (p = 0.02) prevalence of any adverse event (44%) compared to non-obese children (19%).
The lowest recorded SpO2 was < 90% in 2 children, 1.5% of the sample. The median SpO2 upon discharge from the post-anesthesia recovery unit was 98% (97.0–99.0) and no child was discharged with an SpO2 < 93% in room air. Permissive hypercarbia during emergence from anesthesia was common, 62% of the sample had peak end-tidal CO2 - values > 45 mm Hg and the highest recorded end-tidal CO2 was > 60 mm Hg in 14%. Adverse events were no more prevalant in children with permissive hypercarbia > 45 mm Hg compared to those with end-tidal CO2 < 45 mm Hg.
Discussion
We found that the phenotypic features of children with severe, therapy-resistant asthma are informed by BAL granulocyte categories and detection of potential respiratory pathogens (Figure 2). The two categories with the highest degree of clinical morbidity were mixed granulocytic and isolated eosinophilia, and had in common increased BAL eosinophils despite relatively high prevalance of treatment with systemic prednisone. Although bronchoscopies were postponed in children with symptomatic colds and recent lower respiratory infections, respiratory viruses and/or pathogenic bacteria were detected in 31% of the sample. Our study is novel in so far as most studies of lung fluid or sputum granulocytes in adults and children with severe asthma do not include broad assessment of lower respiratory potential pathogens. Pathogenic bacteria were most prevalent in children with isolated neutrophilia, and rhinovirus/enterovirus transcripts were greatest in the mixed granulocytic category. Children with any respiratory pathogen detected had greater BAL cell counts and neutrophil percentages, were relatively younger, had fewer hospital admissions, shorter duration of asthma, and higher mid-expiratory flow rates (Table E-3). Detection of pathogenic bacteria could represent colonization or indolent survival in biofilms, and likewise viral detection can correspond to a carrier state, viral replication in the absence of organ involvement, or true infection. Whereas blood granulocyte numbers poorly correlated with their counterparts in BAL, we submit that a diagnostic bronchoscopy is helpful in the care of children with severe, treatment-refractory asthma so as to precisely guide treatment.
We found a considerable number of children adherent to treatment with high-dose corticosteroids had increased BAL and systemic eosinophils. Does this mean these children were corticosteroid-resistant 36? Glucorticoids decrease lung eosinophilic infiltration through inhibition of epithelial-derived chemotactic cytokines 37, direct induction of eosinophil apoptosis, and they further impede eosinophil production and survival through inhibition of IL-5 38. To test whether prednisone treatment might alter the study results, we analyzed the sample features after removing 33 children treated with maintenance prednisone. This did not change in a significant way the distribution of the granulocyte categories nor their salient phenotypic features (Table E-5). Corticosteroid-resistance may occur more frequently than realized in children with asthma. For example in a recent SARP investigation, stable adults and children with asthma given intramuscular triamcinolone had only minor improvements in clinical features and the adults had no significant decrease in sputum eosinophils 39. Mechanisms of corticosteroid-resistance in asthma are varied and range from impaired drug delivery to the lung per se, glucocorticoid receptor down-regulation, tobacco smoke exposure, and infection 40–43. Overall second-hand smoke exposure in the sample was not measured directly but is estimated at 46% 44.
This would be the first study to suggest chronic rhinovirus infection may be a factor in poorly-controlled asthma in so far as detection of rhinovirus RNA requires active intracellular replication. Enteroviradae transcripts were detected in 36% of children with mixed granulocytic BAL and may have contributed to corticosteroid resistance. Human rhinovirus infection opposes the anti-inflammatory effects of corticosteroids through promotion of eotaxins and mucosal type 2 inflammatory cytokine production including Il-4, IL-5, and IL-13 45, and disruption of lung epithelial barrier function 46. The current observations are similar to published observations following hematopoietic stem cell transplants where persistent human rhinovirus type C infection in the lower respiratory tract has been reported 47 and, in the setting of decreased T and NK cell expression, single strains may persist over months 48.
The present study supports a fundamental role of eosinophilic infiltration into the air spaces as an important feature in approximately one-third of children with severe asthma. We would point out that the numbers of children with isolated BAL eosinophilia were relatively few; BAL eosinophilia more often was accompanied by BAL neutrophilia in the sub-group with mixed granulocytic inflammation. In the past decade, an adult-onset “eosinophilic” asthma endotype has been described differentiated by nasal polyps, airflow limitation, and frequent exacerbations 49. Children with difficult asthma accompanied by corticosteroid-refractory airway mucosal eosinophilia were described by Payne and colleagues over a decade ago 50. Detection of airway eosinophilic activation best differentiates adults with asthma in complete remission versus those with clinical remission and current asthma 51. Accordingly, we found that children with BAL eosinophilia with or without neutrophilia had considerable asthma-related morbidity, despite treatment with maintenance prednisone, and therefore might be considered candidates for anti-eosinophil biological therapies even in the absence of peripheral blood eosinophila. However, although blood eosinophil counts are widely touted as useful markers for initiating biological therapies in asthma, we found blood eosinophils did not reliably predict lung fluid eosinophil counts.
Children with isolated BAL neutrophilia had unique features, with overall less morbidity compared to children with the other granulocyte patterns. They were younger, had more laryngeal clefts, greater detection of BAL bacteria, and relatively higher lung function (Figure 2). Our results are similar to those reported in a sample of children with severe asthma and airway mucosal neutrophilia by Andersson and colleagues 24. Various mechanisms have been proposed to account for lung neutrophilia in asthma including corticosteroid treatment 36 , adipocyte-mediated IL-6 inflammation 52, and respiratory viral 53–55 and bacterial 56- 58 infections. Corticosteroid treatment not only does not diminish neutrophilia, but by inhibiting apoptosis, increases the presence of neutrophils and augments neutrophilic inflammation. We did a secondary analysis limited to 20 children with isolated BAL neutrophilia to see if children without BAL potential pathogens had different features from those with pathogens detected. Children with BAL neutrophilia and no detected pathogens were older with longer duration of asthma and trended towards less allergen sensitization than children with BAL neutrophilia and potential pathogens present (Table E-4).
Over one-half the children we studied had pauci-granulocytic BAL with relatively fewer morbid clinical features. This result is different from findings in a previous study of children with severe asthma wherein pauci-granulocytic BAL was found in only 11% 59. Thirty-six % of adults with asthma in the SARP cohort reported by Hastie et al. had pauci-granulocytic sputum 32. In an earlier study based on sputum granulocyte categories that included both adults and children with stable asthma, the pauci-granulocytic category was most prevalent regardless of age 60. Adult asthmatics with pauci-granulocytic sputum had relatively lower IL-5 and IL-13 cytokine levels compared to asthmatics with raised sputum eosinophils 61. Comparable to our results in children with eosinophilic BAL, a large cohort of adults with asthma and eosinophilic sputum had significantly greater total serum IgE than adults with pauci-granulocytic and neutrophilic sputum 62. We speculate that children with pauci-granulocytic BAL may represent a sub-group that was originally “Th2 high” 63, but became corticosteroid-resistant, and thus the morbid features we observed in this category were likely driven by non-Th2 and/or non-eosinophilic inflammatory pathways. Thus, children with pauci-granulocytic inflammation already treated with high-dose corticosteroids may be candidates for nonsteroidal therapies and although confirmatory studies are indicated, might even be less responsive to anti-Th-2 biologics including mepolizumab, benralizumab, and dupilumab 64.
The results of our analysis are based on a community-referred sample and thus might be applied to clinical practice. The proportion of children treated with combination ICS/LABA was low in comparison to proportions reported in the European U-BIOPRED cohort (4), but higher than the proportions found in the U.S. SARP III pediatric cohort (5). Thus the study sample has better generalizability for a U.S. compared to a European pediatric severe asthma population. Hence, a sensitivity sub-analysis was done limited to 88 children who received ICS/LABA treatment (Table E-6). As shown the differentiating features among the granulocyte categories did not change in an important way in the sub-analysis. The granulocyte categories are cross-sectional “snap shots” of a heterogenous inflammatory process, and thus are subject to changes according to treatment, stress, and environmental exposures. In particular constituents of the large conducting airways, which admix with alveolar constituents in BAL, interface closely with the external environment. Thus we found and would expect that the granulocyte patterns are highly prone to variations imposed by environmental exposures including microbes, inhaled irritants, and allergens. BAL granulocyte patterns could have added value in informing adjustments to therapy. Anti-microbials, particularly the macrolides, may have a role in children with lower respiratory bacteria. Children with eosinophilic and mixed granulocytic patterns could be considered for biologicals with the added benefit that these might facilitate a reduction in corticosteroid dosing. Children with isolated neutrophilia in the absence of infection could be treated with novel anti-neutrophil therapies including anti-IL-1β, anti-IL-17, and anti-IL-6. Pauci-granulocytic severe asthma is perhaps the most challenging category, potentially treated with therapies targetting the bronchial epithelium such as macrolides and evolving non-Th-2 novel biologics. Finally, we found important differences in counts of blood and BAL eosinophils, thus assessment of BAL granulocytes via bronchoscopy we suggest could improve selection of biological therapies over utilization of blood alone.
Supplementary Material
Highlights Box.
1. What is already known about this topic?
Severe asthma, despite treatment with high-dose corticosteroids, has varied phenotypic features, but the characteristic patterns of lung granulocytic inflammation and infectious species are not well studied in a community-based setting.
2. What does the article add to our knowledge?
In children with severe asthma, BAL granulocyte categories and detection of lower respiratory microbes correspond to phenotypic differences in morbid outcomes, airflow limitation, eosinophilia, and degree of allergen sensitization.
3. How does the study impact currrent management guidelines?
BAL is a safe and effective means to identify corticosteroid-refractory lung eosinophilia and potential bacterial pathogens amenable to revised treatment in children with severe asthma.
Acknowledgements
The authors wish to acknowledge the children and family who volunteered to be in this study and share clinical samples with the research laboratory. We likewise wish to acknowledge the steadfast efforts of the research coordinators, Kristin Wavell, Denise Thompson-Batt, and Theresa Altherr, and laboratory technicians, Kimberly De Ronde and Martha Spanno. The efforts of the anesthesiology attending staff and residents were appreciated, as well as the clinical laboratory managers and staff, and attending pathologists. Administrative staff included Lyn Melton and Wendy Cline, and grants administrators included Angela Rogers and Michelle Haynes.
Funding sources: NIH/NHLBI Severe Asthma Research Program U10HL109250-07 (WGT), NIAAID U01A123337 (LB).
Conflict of interest disclosures: W. Gerald Teague received honoraria payments as a speaker for Genentech/Novartis; Monica G. Lawrence reports no conflicts; Debbie-Ann Shirley reports no conflicts; Andrea S. Garrod reports no conflicts; Steven V. Early reports no conflicts; Jackie Payne reports no conflicts; Julia Wisniewski reports no conflicts; Peter W. Heymann has received funding to support research devoted to rhinovirus and asthma from the NIH (U01-AI100799) and from Novartis Pharmaceuticals; James Daniero reports no conflicts; Deborah Froh reports no conflicts; John W. Steinke reports royalty payments from the LUVA cell line; Thomas Braciale reports no conflicts; Michael Ellwood reports no conflicts; Drew Harris reports no conflicts; and Larry Borish reports no relevant conflicts.
Abbreviations
- BAL
Bronchoalveolar lavage
- BD
bronchodilator
- EoE
Eosinophilic esophagitis
- FEV1
Forced expired volume in one second
- FVC
Forced vital capacity
- FEF
Forced expiratory flow
- LABA
Long acting beta agonists
- ROC
Reciever operating characteristic
- RSV
Respiratory syncytial virus
- SARP
Severe asthma research program
- SpO2
Peripheral capillary oxygen saturation
- Th1
T helper one
- Th2
T helper two
- Th17
T helper seventeen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The Childhood Asthma Management Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000; 343: 105401063. [DOI] [PubMed] [Google Scholar]
- 2.Fitzpatrick AM, Gaston B, Erzurum S, Teague WG. Features of severe asthma in school age children: Atopy and increased exhaled NO. J Allergy Clin Immunol 2006; 118: 1218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, Wenzel SE, Castro M, Becharier L, Gaston BM, Bleecker ER, Moore WC. Heterogeneity of severe asthma in childhood: Confirmation by cluster analysis of children in the NIH/NHLBI Severe Asthma Research Network. J Allergy Clin Immunol, 2011; 127: 382–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleming L, Murray C, Bansal AT, Hashimoto S, Bisgaard H, Bush A, Frey U, Hedlin G, Singer F, van Aalderen W, Vissing NH, Zolkpili Z, Selby A, Fowler S, Shaw D, Chung KF, Sousa AR, Wagers S, Corfield J, Pandis I, Rowe A, Formaggio E, Sterk PJ, Roberts G, on behalf of the U-BIOPRED Study Group. The burden of severe asthma in childhood and adolescence: Results from the paediatric U-BIOPRED cohorts. Eur Respir J 2015; 46:1322–1333. [DOI] [PubMed] [Google Scholar]
- 5.Teague WG, Phillips BR, Fahy JV, Wenzel SE, Fitzpatrick AM, Moore WC, Hastie AT, Bleecker ER, Meyers DA, Peters SP, Castro M, Coverstone AM, Bacharier LB, Ly NP, Peters MC, Denlinger LC, Ramratnam S, Sorkness RL, Gaston BM, Erzurum SC, Comhair SAA, Ross EM, Zein J, DeBoer MD, Irani AM, Israel E, Levy B, Cardet JC, Phipatanakul W, Gaffin JM, Holguin F, Fajt ML, Aujla SH. Mauger DT, Jarjour NN. Baseline features of the Severe Asthma Research Program (SARPIII) Cohort: Differences with age. J Allergy Clin Immunol in Practice, 2017. August 30pii:S2213–2198(17)30526–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bush A, Saglani S. Management of severe asthma in children. Lancet 2010; 376: 814–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharples J, Gupta A, Fleming L, Bossley CF, Bracken-King M, Hall P, Hayward A, Puckey M, BALour-Lynn IM, Rosenthal M, Bush A, Saglani S. Long-term effectiveness of a staged assessment for paediatric problematic severe asthma. Eur Resp J 2012; 40: 264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk P, Adcock IM, Bateman E, Bel E, Bleecker E, Boulet LP, Brightling C, Chanez P, Dahlen SE, Djukanovic R, Frey U, Gaga M, Gibson P, Hamid Q, Jarjour N, Mauad T, Sorkness R, Teague WG. International ERS/ATS Consensus Definition, Mechanisms, Evaluation and Treatment of Severe Asthma. Eur Respir J 2014; 43 (2): 343–73. [DOI] [PubMed] [Google Scholar]
- 9.Fleming L, Tsartsali L, Wilson N, Regamey N, Bush A. Longitudinal relationship between sputum eosinophils and exhaled nitric oxide in children with asthma. Am J Respir Crit Care Med 2013; 188: 400–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lex C, Ferreira F, Zacharasiewicz A, Nicholson AG, Haslam PL, Wilson NM, Hansel TT, Payne DN, Bush A. Airway eosinophilia in children with severe asthma: predictive values of noninvasive tests. Am J Respir Crit Care Med 2006; 174: 1286–91. [DOI] [PubMed] [Google Scholar]
- 11.Ullmann N, Bossley CJ, Fleming L, Silvestri M, Bush A, Saglani S. Blood eosinophil counts rarely reflect airway eosinophilia in children with severe asthma. Allergy 2013; 68:402–6. [DOI] [PubMed] [Google Scholar]
- 12.Payne D, McKenzie SA, Stacey S, Misra D, Haxby E, Bush A. Safety and ethics of bronchoscopy and endobronchial biopsy in difficult asthma. Arch Dis Child 2001; 84: 423–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson AC, Wong FWM. Bronchial hyperresponsiveness in asthmatic children: Correlation with macrophages and eosinophils in bronchoalveolar lavage fluid. Chest 1989; 96:988–91. [DOI] [PubMed] [Google Scholar]
- 14.Stevenson EC, Turner G, Heaney LG, Schock BC, Taylor R, Gallagher T, Ennis M, Shields MD. Bronchoalveolar lavage findings suggest two different forms of childhood asthma. Clin Exp Allergy 1997; 27:1027–35. [DOI] [PubMed] [Google Scholar]
- 15.Payne DN, Wilson NM, James A, Hablas H, Agrafoti C, Bush A. Evidence for different subgroups of difficult asthma in children. Thorax 2001; 56: 345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Just J, Fournier L, Momas I, Zambetti C, Sahraoui F, Grimfeld A. Clinical significance of bronchoalveolar eosinophils in childhood asthma. J Allergy Clin Immunol 2002; 110: 42–4. [DOI] [PubMed] [Google Scholar]
- 17.De Blic J Tillie-Leblond I, Tonnel AB, Jaubert F, Scheinmann P, Gosset P. Difficult asthma in children: An analysis of airway inflammation. J Allergy Clin Immunol 2004; 113: 94–100. [DOI] [PubMed] [Google Scholar]
- 18.Fitzpatrick AM, Holguin F, Teague WG, Brown LA. Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J Allergy Clin Immunol 2008; 121:1372–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzpatrick AM, Teague WG, Yeh MY, Brown LS. Airway glutathione homeostasis is altered in children with severe asthma: Evidence for oxidant stress. J Allergy Clin Immunol 2009; 123: 146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzpatrick AM, Brown LA, Holguin F, Teague WG. Nitric oxide oxidation products are increased in the epithelial lining fluid of children with severe asthma. J Allergy Clin Immunol, 2009; 124: 990–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzpatrick AM, Higgins M, Holguin F, Brown LAS, Teague WG. The molecular phenotype of severe asthma in children. J Allergy Clin Immunol 2010, 125 (4): 851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bossley CJ, Fleming L, Gupta A, Regamey N, Frith J, Oates T, Tsartsali L, Lloyd CM, Bush A, Saglani S. Pediatric severe asthma is characterized by eosinophilia and remodeling without Th2 cytokines. J Allergy Clin Immunol 2012; 129: 974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta A, Dimeloe S, Richards DF, Chambers ES, Black C, Urry Z, Ryanna K, Xystrakis E, Bush A, Saglani S, Hawrylowicz CM. Defective IL-10 expression and in vitro steroidinduced IL-17A in paediatric severe therapy-resistant asthma. Thorax 2014; 69: 508–15. [DOI] [PubMed] [Google Scholar]
- 24.Andersson CK, Adams A, Nagakumar P, Bossley C, Gupta A, De Vries D, Adnan A, Bush A, Saglani S, Lloyd CM. Intraepithelial neutrophils in pediatric severe asthma are associated with better lung function. J Allergy Clin Immunol 2017; 139: 1819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wisniewski JA, Muehling LM, Eccles JD, Agrawal R, Capaldo BJ, Shirley DA, Patrie JT, Workman L, Lawrence MG, Teague WG, Woodfolk JA. Th1 cells in a mixed cytokine milieu define the lower airways of children with severe asthma, regardless of allergic status. J Allergy Clin Immunol 2017; pii: S0091–6749(17)31463-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagakumar P, Denney L, Fleming L, Bush A, Lloyd CM, Saglani S. Type 2 innate lymphoid cells in induced sputum from children with severe asthma. J Allergy Clin Immunol 2016; 137: 624–26. [DOI] [PubMed] [Google Scholar]
- 27.Beigelman A, Weinstock GM, Bacharier LB. The relationships between environmental bacterial exposure, airway bacterial colonization, and asthma. Curr Opin Allergy Clin Immunol. 2014; 14:137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention 2017. Available from: www.ginasthma.org.
- 29.National Heart, Lung, and Blood Institute National Asthma Education and Prevention Program. Expert Panel 3: Guidelines for the Diagnosis and Management of Asthma. Full Report 2007. U.S. Department of Health and Human Services; Available at www.nhlbi.nih.gov/guidelines/asthma/asthmagdln.pdf [Google Scholar]
- 30.Fitzpatrick AM, Kir T, Naeher LP, Fuhrman SC, Hahn K, Teague WG. Prescription refill frequencies for tablet and inhaled controller medications in children with asthma. J Pediatr Nursing 2008; 24: 81–89. [DOI] [PubMed] [Google Scholar]
- 31.Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology 2006; 11:54–61. [DOI] [PubMed] [Google Scholar]
- 32.Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, Bleecker ER; National Heart, Lung, and Blood Institute Severe Asthma Research Program. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol 2010; 125: 1028–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, Wenzel SE, Peters SP, Meyers DA, Bleecker ER. National Health Lung and Blood Institute’s Severe Asthma Research Program. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol 2014; 133: 1557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Blic J, Midulla F, Barbato A, Clement A, Dab I, Eber E, et al. for the ERS Task Force on Bronchoalveolar Lavage in Children. Eur Respir J 2000; 15:217–231. [DOI] [PubMed] [Google Scholar]
- 35.Heaney G, Stevenson EC, Turner G, et al. Investigating paediatric airways by non-bronchoscopic lavage: normal cellular data. Clin Exp Allergy 1996; 26: 799–806. [PubMed] [Google Scholar]
- 36.McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM et al. A large subgroup of mild-to-moderate asthmatics is persistently noneosinophilic. Am J Respir Crit Care Med 2012; 185: 612–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lilly CM, Nakamura H, Kesselman H, Nagler-Anderson C, Asano K, Garcia Zepeda EA, Rothenberg ME, Drazen JM, Luster AD. Expression of eotaxin by human lung epithelial cells – induction by cytokines and inhibition by glucocoticoids. J Clin Invest 1997; 99: 1767–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallen N, Kita H, Weller D, Gleich GJ. Glucocorticoids inhibit cytokine-mediated eosinophil survival. J Immunol 1991; 147:3490–3495. [PubMed] [Google Scholar]
- 39.Phipatanakul W, Mauger DT, Sorkness RL, Gaffin JM, Holguin F, Woodruff PG, Ly NP, Bacharier LB, Bhakta NR, Moore WC, Bleecker ER, Hastie AT, Meyers DA, Castro M, Fahy J, Fitzpatrick A, Gaston BM, Jarjour NN, Levy BD, Peters SP, Teague WG, Fajt M, Wenzel SE, Erzurum SC, Israel E and the Severe Asthma Research Program. Effects of age and disease severity on systemic corticosteroid responses in asthma. Am J Respir Crit Care Med 2017; 195: 1439–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito K, Chung KF, Adcock IM. Update on glucorticoid action and resistance. J Allergy Clin Immunol 2006; 117: 522–543. [DOI] [PubMed] [Google Scholar]
- 41.Carmichael J, Paterson IC, Diaz P, Crompton OK, Kay AB, Grant IW. Corticosteroid resistance in chronic asthma. Br Med J 1981; 282: 1419–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dente FL, Bacci E, Bartoli ML, Cianchetti S, Costa F, Di Franco A, Malagrino L, Vagaggini B, Paggiaro P. Effect of oral prednisone on sputum eosinophils and cytokines in patients with severe refractory asthma. Ann Allergy Asthma Immunol 2010; 464–70. [DOI] [PubMed] [Google Scholar]
- 43.Wark PA, McDonald VM, Gibson PG. Adjusting prednisone using blood eosinophils reduces exacerbations and improves asthma control in difficult patients with asthma. Respirology 2015; 20: 1282–4. [DOI] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention. Smoking and tobacco use in Virginia. www.cdc.gov/tobacco/data_statistics/state_highlights_2012/states/virginia. Accessed 2/22/2018.
- 45.Hansel TT, Tunstall T, Trujillo-Torralbo MD, Shamji B, Del-Rosario A, Dhariwal KJ, Kirk PDW, Stumpf MPH, Koopman J, Telcian A, Aniscenko J, Gogsadze L, Bakhsikuabu E, Stanciu L, Bartlett N, Edwards M, Walton R, Mallia P, Hunt TM, Hunt DG, Westwick J, Edwards M, Kon OM, Jackson DJ, Johnston SL. A comprehensive evaluation of nasal and bronchial cytokines and chemokines following experimental rhinovirus infection in allergic asthma: Increased interferons (IFN-gamma and IFN-lambda) and type 2 inflammation (IL-5 and IL-13). EBioMedicine 2017; 19:128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Looi K, Buckley AG, Rigby PJ, Garratt LW, Iosfidis T, Zosky GR, Larcombe AN, Lannigan FJ, Ling KM, Martinovich KM, Kicic-Starcevich E, Shaw NC, Sutanto EN, Knight DA, Kicic A, Stick SM. Effects of human rhinovirus on epithelial barrier integrity and function in children with asthma. Clin Expir Allergy 2018; doi 10.1111/cea13097. [DOI] [PubMed] [Google Scholar]
- 47.Pathak AK, Adams RH, Shah NC, Gustin KE. Persistant human rhinovirus type C infection of the lower respiratory tract in a pediatric cord blood transplant recipient. Bone Marrow Transplant 2013; 48: 747–8. [DOI] [PubMed] [Google Scholar]
- 48.Piralla A, Zecca M, Comoli P, Girello A, Maccario R, Baldanti F. Persistent rhinovirus infection in pediatric hematopoietic stem call tranplant recipients with impaired cellular immunity. J Clin Virol 2015; 67: 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Groot JC, Storm H, Amelink M, de Nijs SB, Eichhorn E, Reitsma BH, Bel EH, Ten Brinke A. Clinical profile of patients with adult-onset eosinophilic asthma. ERJ Open Res 2016. May 26; 2(2). pii: 00100–2015. eCollection 2016 Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Payne DN, Adcock IM, Wilson NM, Oates T, Scallan M, Bush A. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisilone. Am J Respir Crit Care Med 2001; 164: 1376–81. [DOI] [PubMed] [Google Scholar]
- 51.Broekema M, Timens W, Vonk JM, Volbeda F, Lodewijk ME, Hylkema N, ten Hacken NHT, Postma DS. Persistent remodeling and less airway wall eosinophil activation in complete remission of asthma. Am J Respir Crit Care Med 2011; 183: 310–316. [DOI] [PubMed] [Google Scholar]
- 52.Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, Phillips BR, Mauger DT, Comhair SA, Erzurum SC, Johansson MW, Jarjour NN, Coverstone AM, Castro M, Holguin F, Wenzel SE, Woodruff PG, Bleecker ER, Fahy J for the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of of two cohorts. Lancet Respir Med 2016; 4: 574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jarjour NN, Gern JE, Kelly EA, Swenson CA, Dick CR, Busse WW. The effect of an experimental rhinovirus-16 infection on bronchial lavage neutrophils. J Allergy Clin Immunol 2000; 105: 1169–77. [DOI] [PubMed] [Google Scholar]
- 54.Bourgeois ML, Goncalves M, Le Clainche L, Benoist MR, Fournet JC, Scheinmann P, de Blic J. Bronchoalveolar cells in children < 3 years old with severe recurrent wheezing. Chest 2002; 122:791–97. [DOI] [PubMed] [Google Scholar]
- 55.Malmstrom K, Lehto M, Majuri ML, Paavonen T, Sarna S, Pelkonen AS, Malmberg LP, Lindahl H, Kajosaari M, Saglani S, Alenius H, Makela MJ. Bronchoalveolar lavage in infants with recurrent lower respiratory symptoms. Clin Transl Allergy 2014; 4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Green BJ, Wiriyachaiporn S, Grainge C, Rogers GB, Kehagia V, Lau L, Carrol MP, Bruce KD, Howarth PH. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment-resistant severe asthma. PloS One 2014; 9: e100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simpson JL, Daly J, Baines KJ, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, Hugenholtz P, Willner D, Gibson PG. Airway dysbiosis: Haemophilus influenzae and tropheryma in poorly controlled asthma. Eur Respir J 2016; 47: 792–800. [DOI] [PubMed] [Google Scholar]
- 58.Essilfie A-T, Simpson JL, Horyat JC, Preston JA, Dunkley ML, Foster PS, Gibson PG, Hansbro PM. Haemophilus influenzea infections drives IL-17-mediated neutrophilic allergic airways disease. PloS Pathogens 2011; 7 (10): e1002244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Brien CE, Tsirilakis K, Santiago MT, Goldman DL, Vincenio AG. Heterogeneity of lower airway inflammation in children with severe-persistent asthma. Pediatr Pulmonol 2015; 50:1200–4. [DOI] [PubMed] [Google Scholar]
- 60.Wang F, He XY, Baines KJ, Gunawardhana LP, Simpson JL, Gibson PG. Different inflammatory phenotypes in adults and children with acute asthma. Eur Respir J 2011. 38: 567–74. [DOI] [PubMed] [Google Scholar]
- 61.Manise M, Holtappels G, Van Crombruggen K, Schliech f, Bachert C, Louis R. Sputum IgE and cytokines in ashtma: relationship with sputum cellular profile. PloS One 2013; 8: e58388. doi 10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manise M, Barkayoko B, Schleich F, Corhay JL, Louis R. IgE mediated sensitization to aeroallergens in an asthmatic cohort: relationship with inflammatory phenotypes and disease severity. Int J Clin Pract 2016; 70: 596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stokes JR, Casale TB. Characterization of asthma endotypes: implications for therapy. Ann Allergy Asthma Immunol 2016: 117:121–5. [DOI] [PubMed] [Google Scholar]
- 64.Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, Pirozzi G, Sutherland ER, Evans RR, Joish VN, Eckert L, Graham NM, Stahl N, Yancopoulos GD, Louis-Tisserand M, Teper A. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inaled corticosteroids plus a long-acting β2 agonist: A randomized double-blind placebo-controlled phase 2b dose-ranging trial. Lancet 2016; 388:31–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.