Abstract
Objective:
To compare the treatment success and failure rates, as well as side effects and surgery rates between methotrexate protocols.
Data Sources:
PubMed, Embase and the Cochrane library searched up till July 2018.
Study eligibility criteria:
RCTs that compared women with ectopic pregnancies receiving the single dose, two dose or multi-dose methotrexate protocols.
Study appraisal and synthesis methods:
Odds of treatment success, treatment failure, side effects and surgery for tubal rupture as well as length of follow-up until treatment success compared using random and fixed effects meta-analysis. Sensitivity analyses compared treatment success in high hCG and large adnexal mass groups, as defined by individual studies. Cochrane’s collaboration tool used to assess risk of bias.
Results:
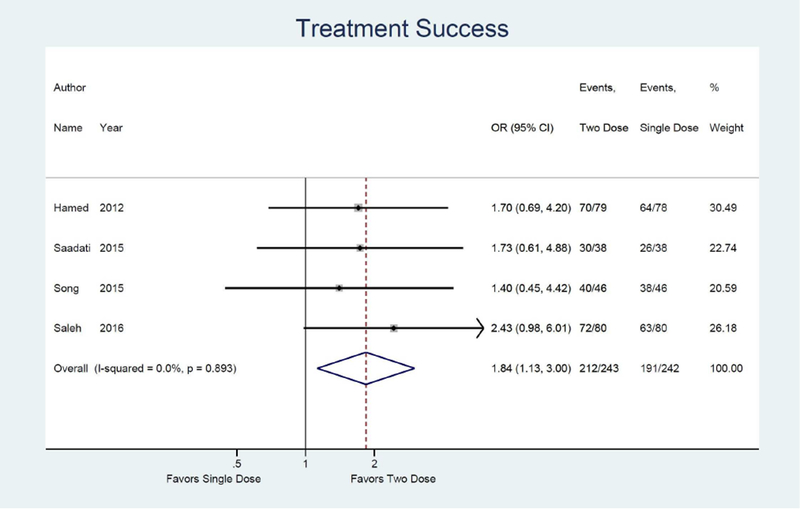
The two dose protocol was associated with higher treatment success compared to single dose protocol (OR: 1.84, 95% CI: 1.13, 3.00). The two dose protocol was more successful in women with high hCG (OR: 3.23, 95% CI: 1.53, 6.84) and in women with a large adnexal mass (OR: 2.93 95% CI: 1.23, 6.9). The odds of surgery for tubal rupture were lower in the two dose protocol (OR: 0.65, 95%CI: 0.26, 1.63), but not statistically significant. The length of follow up was 7.9 days shorter for the two dose protocol (95% CI: −12.2, −3.5). Odds of side effects were higher in the two dose protocol (OR: 1.53, 95% CI: 1.01, 2.30).
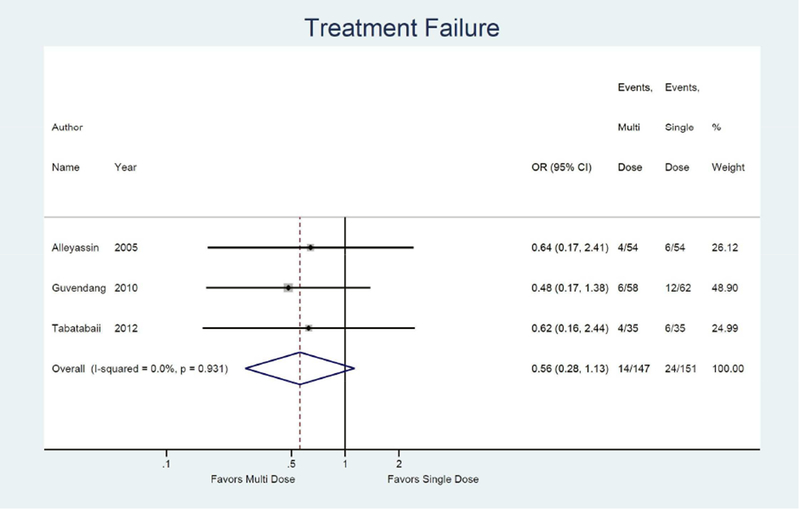
Compared to the single dose protocol the multi-dose protocol is associated with a nonsignificant reduction in treatment failure (OR: 0.56, 95% CI: 0.28, 1.13) and a higher chance of side effects (OR: 2.10, 95% CI: 1.24, 3.54). Odds of surgery for tubal rupture (OR: 1.62, 95% CI: 0.41, 6.49) and time to follow-up (−1.3, 95% CI: −5.4, 2.7) were similar.
Conclusion:
The two dose methotrexate protocol is superior to the single dose protocol for the treatment of ectopic pregnancy in terms of treatment success and time to success. Importantly, these findings hold true in patients thought to be at a lower likelihood of responding to medical management, such as those with higher hCGs and large adnexal mass.
Keywords: Doses, ectopic pregnancy, medical management, methotrexate, protocol, tubal pregnancy
Introduction
Ectopic pregnancies occur in approximately 2% of pregnancies, yet account for 9% of maternal mortality and are the leading cause of pregnancy-related death in the first trimester.1 Advancements in imaging technology and protocols to screen women at risk have led to earlier detection of ectopic pregnancies.2–4 As more women with ectopic pregnancies present clinically stable without concern for rupture, options for treatment have expanded beyond surgical management to medical management. The mainstay of medical management has been methotrexate, a folate antagonist that binds to dihydrofolate reductase leading to downstream inhibition of DNA synthesis and repair as well as in cell replication.3, 5 Multiple publications have demonstrated comparable efficacy of medical management to surgical management in the treatment of stable ectopic pregnancies.6–9 The most widely used methotrexate protocols include single dose, two dose and the multi-dose protocols.3 The multi-dose protocol can be beneficial for patients at higher risk of failing medical management and thus requiring additional doses.5, 10 However due to its frequency of administration, the multidose protocol requires the addition of folinic acid rescue, alternating with methotrexate doses, to decrease side effects. The single dose was introduced to reduce the number of visits, but often requires additional treatment and follow-up. The two dose protocol was introduced with the goal of achieving a balance between the benefits of increased treatment success from additional doses of methotrexate, while using the same convenient visit schedule as the single dose protocol.11
The success rates of medical management of ectopic pregnancies have varied with a range of 70–90% for the single dose,12–14 80–90%14–16 for the two dose and 89–96% for the multi dose protocols.12, 13, 17 Variation in rates may be influenced by the population being studied, criteria for administering the medication and definition of treatment success. Treatment failure rates, or chance of failure with a particular protocol, are also important to consider as they can be clinically useful when counseling patients and can be a driver in deciding which protocol to recommend.
Although several studies have attempted to compare one protocol to another they are limited by their retrospective nature, nonstandard protocols and heterogeneous definition of outcomes.18–21 Randomized controlled trials (RCTs) are considered the gold standard when evaluating such questions due to their systematic, reproducible approach with minimization of confounding through the process of randomization. While there have been other meta-analyses on this topic, several do not include more recent studies, thus providing an opportunity to update our understanding.2, 9, 12, 22 Others are limited by inclusion of retrospective studies and abstracts,12, 23 or lack of treatment failure rate reporting.22, 23 Our study sought to compare via a meta-analysis, quality RCTs evaluating the treatment success, side effect incidence and surgery rates between the methotrexate protocols.
Materials and Methods
Eligibility criteria
The systematic review and meta-analysis were performed by strictly following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The population being studied was women with an ectopic pregnancy diagnosed by transvaginal ultrasound. Interventions included the single dose, two dose or multi-dose methotrexate protocol (Table 1). Comparisons included single dose to two dose and single dose to multi-dose protocols. Associations of interest for binary outcomes are presented as odds ratios (OR) with 95% confidence intervals (CI). Only RCTs published as manuscripts with clear randomization protocols were included in this analysis.
Table 1:
Methotrexate protocols
| Single Dose | Two Dose | Multi-dose | |
|---|---|---|---|
| Day 1 | Administer MTX 50mg/m2 IM, obtain serum hCG | Administer MTX 50mg/m2 IM, obtain serum hCG | Administer MTX 1mg/kg IM, obtain serum hCG |
| Day 2 | -- | -- | -- |
| Day 3 | -- | -- | Administer 2nd dose MTX 1mg/kg IM, obtain serum hCG, if >15% drop, stop MTX and follow hCG levels weekly. If <15% drop, proceed with plan. |
| Day 4 | Obtain serum hCG | Administer 2nd dose MTX 50mg/m2 IM | Administer leucovorin 0.1 mg/kg IM |
| Day 5 | -- | -- | Obtain serum hCG. if >15% drop, stop MTX and follow hCG levels weekly. If <15% drop, proceed with plan. Administer 3rd dose MTX 1mg/kg IM |
| Day 6 | -- | -- | Administer leucovorin 0.1 mg/kg IM |
| Day 7 | Obtain serum hCG, if >15% drop, follow hCG levels weekly. If <15% drop, administer 2nd dose MTX 50mg/m2 IM | Obtain serum hCG, if >15% drop, follow hCG levels weekly. If <15% drop, administer 3rd dose MTX 50mg/m2 IM | Obtain serum hCG. if >15% drop, stop MTX and follow hCG levels weekly. If <15% drop, proceed with plan. Administer 4th dose MTX 1mg/kg IM |
| Day 8 | -- | -- | Administer leucovorin 0.1 mg/kg IM |
| Day 11 | -- | Obtain serum hCG, if >15% drop, follow hCG levels weekly. If <15% drop, administer 4th dose MTX 50mg/m2 IM | -- |
| Day 14 | Obtain serum hCG, if >15% drop, follow hCG levels weekly. If <15% drop, administer 3rd dose MTX 50mg/m2 IM | Obtain serum hCG, if >15% drop, follow hCG levels weekly. If <15% drop, consider surgery | Obtain serum hCG. if >15% drop, stop MTX and follow hCG levels weekly. If <15% drop, proceed with plan. Administer 5th dose MTX 1mg/kg IM |
| Day 21 | Obtain serum hCG, if >15% drop, follow hCG levels weekly. If <15% drop, consider surgery | -- | Obtain serum hCG, if >15% drop, follow hCG levels weekly. If <15% drop, consider surgery |
Information sources and search strategy
Studies were identified by searching PubMed, Embase and the Cochrane library in July 2018, with no starting date restrictions. Combinations of the following keywords were used to identify the studies: “methotrexate”, “ectopic pregnancy”, “tubal pregnancy”, “dose” and “protocol”. No filters were applied for language or location.
Study selection
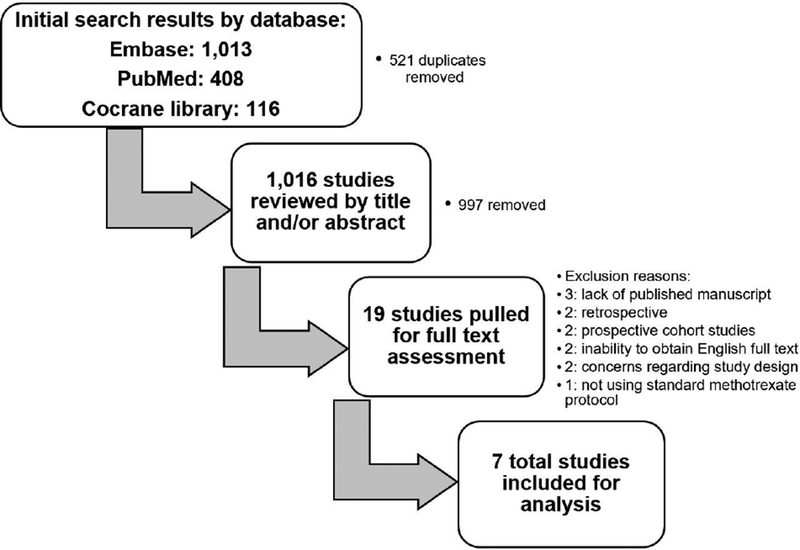
Two independent reviewers (SAG and LC) used the above stated eligibility criteria to screen all article titles and abstracts for inclusion. RCTs on human subjects with published manuscripts were considered eligible. A flow diagram of study selection is outlined in Figure 1.
Figure 1:
Flow diagram of study inclusion
Data extraction
Full text of potentially eligible studies was extracted and examined for the following data: year of study, number of subjects, location of subject recruitment, mean age of subjects, mean BMI of subjects, pretreatment hCG, pretreatment adnexal mass diameter, description of methotrexate protocols used, randomization and blinding processes, inclusion and exclusion criteria, definition of outcomes measured including treatment success, length of follow-up, side effects, surgery for tubal rupture and results for these outcomes. Several attempts were made to electronically contact authors of eligible studies that did not explicitly contain the above information. (See Figure 1)
Assessment of risk of bias
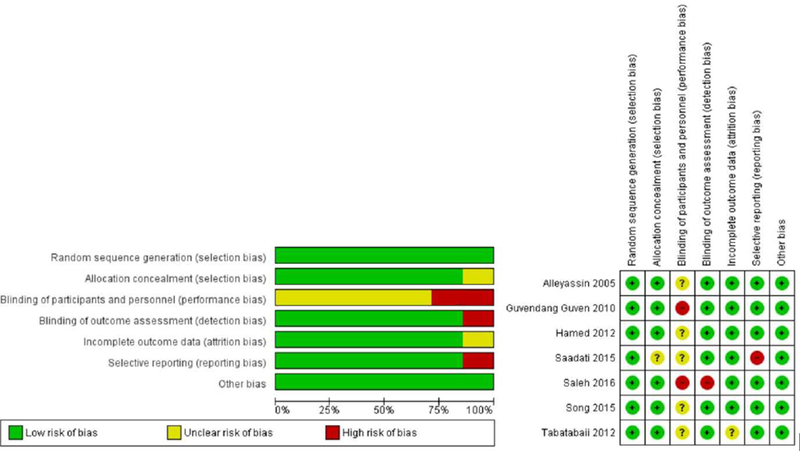
The Cochrane Collaboration’s tool was used to assess the risk of bias. Two authors (SAG and LC) independently reviewed included studies and assigned values of low, uncertain or high risk to the six domains outlined in the tool. (See Figure 2)
Figure 2:
Risk of bias assessment
Data synthesis for meta-analysis
The following primary outcome measure was analyzed for included studies: treatment success (as reported in individual studies). Treatment failure (defined as not achieving treatment success with the stated protocol), which is the weighted inverse of treatment success and provides an alternate tool to use when counseling patients, was also described. Secondary outcomes analyzed included side effects, surgery for tubal rupture and length of follow-up in days (as defined by individual studies). Side effects reported in individual studies included nausea, diarrhea, mucositis, abdominal pain and lab abnormalities. Fixed effects meta-analysis was used to report OR with 95% CI for the outcomes with low heterogeneity including treatment success and failure, surgery for tubal rupture and side effects whereas random effects meta-analysis was used to report mean days with 95% CI for the outcome of length of follow-up. (See Figures 3 and 4)
Figure 3A:
Forest plot: Two versus Single dose-Treatment success
Figure 4A:
Forest plot: Multi versus Single dose-Treatment failure
Sensitivity analyses were conducted to assess treatment success rates in high hCG groups (as reported in individual studies, with a range of greater than 3000–5500 mIU/mL for two dose versus single dose and greater than 800 IU/L for multi-dose versus single dose) and large adnexal mass groups (as reported in individual studies, with a range of greater than 2–3.5cm). The heterogeneity amongst studies was evaluated both via forest plots with 95% CI as well as through the I2 statistic, with p<0.05 considered as statistically significant. Publication bias was assessed via funnel plots of the log OR. (See Supplementary Figure 1) Analysis was conducted using STATA v14.2 (Stata-Corp).
Results
Study selection
The search strategy yielded 1013 results in Embase, 408 in PubMed and 116 in the Cochrane Library. The 521 duplicates were removed. The remaining articles’ titles and abstracts were screened along with the bibliography of recently published metaanalyses. Nineteen potentially eligible articles were identified. Three were excluded due to lack of a published manuscript24–26, two were excluded for being retrospective studies19, 20, two were excluded for being prospective cohort studies21, 27, two were excluded due to inability to obtain full text beyond the abstract in English despite attempts to contact authors28, 29, two were excluded for concerns regarding whether and how randomization was performed30, 31 and one was excluded for not using standard methotrexate protocols18. Therefore, seven final publications were found to meet inclusion criteria.
Study characteristics
Study characteristics are presented in Table 2. All studies were RCTs. Study sizes ranged from 70 to 160 total patients. While all studies reported rates of treatment success, only half reported side effects or length of follow-up. Five reported on rates of surgery for tubal rupture: three in the single vs two dose group and two in the single vs multi-dose group. Although the studies by Saadati et. al14 and Guvendang et. al13 reported data on rates of surgery, indication for surgery as rupture or elective surgery was not specified therefore these studies were not included for this outcome.
Table 2:
Included study characteristics
| Author/Study location/Year | Ectopic pregnancy diagnostic criteria | Total patients per arm | Inclusion Criteria | Exclusion Criteria | Method of randomization | Definition of treatment success | Other outcomes studied | |
|---|---|---|---|---|---|---|---|---|
| Single versus Two dose | ||||||||
| Song South Korea 2015 | bHCG, TVUS, physical exam, Medical history | 46 | 46 | 1. bHCG <15,000 mIU/mL 2. GS <4cm 3. Hemodynamically stable |
1. Heterotopic pregnancy or persistent tubal
pregnancy 2. +FHR 3. Suspected tubal rupture 4. Lab tests contraindicating MTX use |
Randomly permuted blocks with allocation concealment (1:1 ratio) | bHCG <5 mIU/mL | 1. Side effects 2. Length of follow-up 3. Rate of operation 4. Need for repeat doses 3. Cost of care received 4. Days of work/school missed 5. Treatment satisfaction |
| Saadati Iran 2015 | bHCG,TVUS | 38 | 38 | 1. bHCG <15,000 mIU/mL 2. Hemodynamically stable |
1. Women with history of liver, kidney disease
or blood dyscrasia 2. Breastfeeding |
Block randomization with enclosed envelopes (1:1 ratio) | bHCG ≤ 200 mIU/mL | 1. Side effects 2. Length of follow-up 3. Rate of operation 4. Need for repeat doses |
| Hamed Saudi Arabia 2012 | bHCG, TVUS, progesterone and D&C when abortion suspected | 78 | 79 | 1. bHCG of <15,000 mIU/mL 2. GS ≤4cm 3. Hemodynamically stable 4. Absence of FHR |
1. Women suspected of having non- adnexal
ectopic pregnancy 2. Suspected tubal rupture 3. Free fluid extending beyond Douglas pouch on TVUS 4. Lab tests contraindicating MTX use |
Computer generated random numbers table with opaque envelopes | bHCG <15 mIU/mL within 6 weeks without surgery or repeat dose | 1. Side effects 2. Length of follow-up 3. Rate of operation 4. Need for repeat doses |
| Saleh Egypt 2016 | bHCG,TVUS | 80 | 80 | 1. bHCG ≤6000 mIU/mL 2. GS ≤4cm 3. Hemodynamically stable 4. Absence of FHR 5. <300mL hemoperitoneum on TVUS |
1. Hemodynamically unstable 2.Suspected tubal
rupture 2. Uncertain diagnosis 3. Falling bHCGs 4. Non-adnexal ectopic pregnancy 5. Lab tests contraindicating MTX use 6. Breastfeeding 7. Immunodeficiency or use of corticosteroids |
Computer generated randomization with sealed, opaque envelopes | bHCG <15 mIU/mL within 6 weeks without surgery or repeated dose | 1. Side-effects 2. Length of follow-up 3. Rate of operation 4. Need for repeat doses |
| Single versus Multi-dose | ||||||||
| Guvendang Guven Turkey 2010 | bHCG, TVUS, progesterone | 62 | 58 | 1. bHCG reaching plateau or increased by
≤50% in 48 hours 2. Adnexal mass ≤3.5cm 3. Hemodynamically stable |
1. Prior tubal surgery 2. Hemodynamically unstable 3. Hepatic or renal disease |
Computer assisted randomization with sealed envelopes | bHCG <5 mIU/mL | 1. Side-effects 2. Length of follow-up 3. Need for repeat doses |
| Alleyassin Iran 2005 | bHCG,TVUS | 54 | 54 | 1. bHCG <15,000 mIU/mL 2. Adnexal mass <3.5cm 3. Hemodynamically stable 4. Absence of FHR |
None listed. | Computer generated block randomization with sealed envelopes | bHCG <15mIU/mL within 6 weeks | 1. Side-effects 2. Length of follow-up 3. Rate of operation 4. Need for repeat doses |
| Tabatabaii iran 2012 | TVUS, Laparoscopic surgery | 35 | 35 | 1. bHCG <15,000 mIU/mL 2. Adnexal mass ≤4cm 3. Hemodynamically stable 4. Absence of FHR 5. Absence of bleeding in laparoscopic surgery or TVUS |
None listed. | Computer generated block randomization with sealed envelopes | bHCG <15 mIU/mL within 6 weeks | 1. Side-effects 2. Length of follow-up 3. Rate of operation 4. Need for repeat doses 5. Outcomes of subsequent pregnancies |
Risk of bias of included studies
Results of bias assessment via the Cochrane’s collaboration tool are presented in Figure 2. While all studies discussed random sequence generation clearly, some were not clear as to how allocation concealment was performed. Although several studies were not explicit in reporting blinding to outcomes, reviewers felt this would be unlikely to significantly skew outcome measures which are mainly objective. The study by Saleh et. al32 however was scored as high degree of bias in this category due to description of differential counseling regarding elective surgery based upon group.32 Blinding of personnel was similarly not explicitly stated in multiple studies. While blinding of personnel may not have been as practically feasible due to requiring intramuscular injections, it is possible this could have affected patient reporting of side effects, which was one of the subjective outcomes measured. The study by Saleh et. al32 was scored high in this category as envelopes were opened in front of patients whereas the study by Guvendang et. al13 was also scored high as they discussed that the study team was not blinded. The study by Saadati et. al14 hospitalized patients during treatment and discharged them when hCG was <200 mIU/mL, therefore reporting of outcomes may have been biased away from the null due to a non-standard follow-up protocol as well as definition of treatment success. Publication bias was not noted to be significant when looking at studies that compared either the two dose to single dose protocol or ones that compared multi-dose to single dose protocol. (See Supplementary Figure 1)
Meta-analysis results
Single vs two dose protocols
Meta-analysis results are shown as forest plots in Figure 3. For the primary outcome of treatment success, four studies were identified comparing single dose to two dose protocol, with two dose protocol associated with 1.84 times the odds of achieving treatment success (95% CI: 1.13, 3.00) compared to single dose.14, 15, 32, 33 (Figure 3a) Odds of treatment failure were 0.54 times lower in the two dose protocol (95% CI: 0.33, 0.89). (Figure 3b) For the secondary outcome of side effects, four studies were identified with a combined odds of side effects that were 1.53 times higher in the two dose protocol compared to the single dose (95% CI: 1.01, 2.30).14, 15, 32, 33 (Figure 3e) Odds of surgery for tubal rupture were lower when comparing two dose to the single dose protocol (OR: 0.65; 95%CI: 0.26, 1.63), but the difference was not statistically significant. (Figure 3f) The length of follow up was 7.9 days shorter for the two dose protocol compared to single dose (95% CI: −12.2, −3.5). (Figure 3g)
Figure 3B:
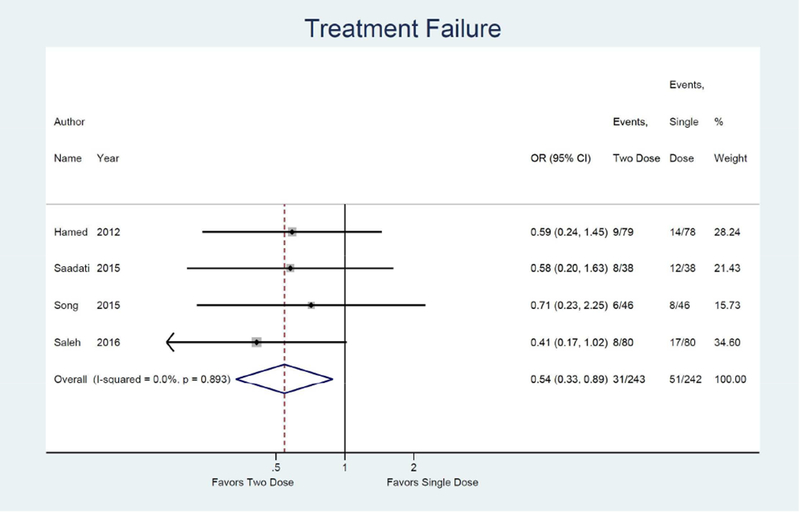
Forest plot: Two versus Single dose-Treatment failure
Figure 3E:
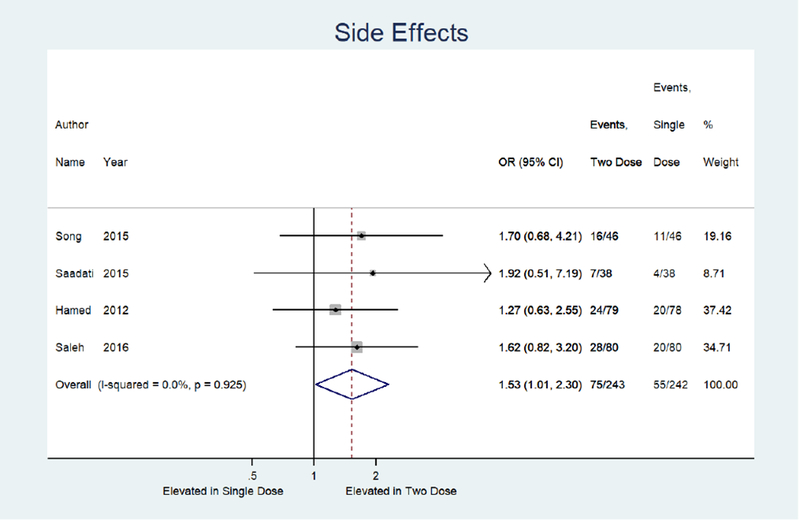
Forest plot: Two versus Single dose-Side effects
Figure 3F:
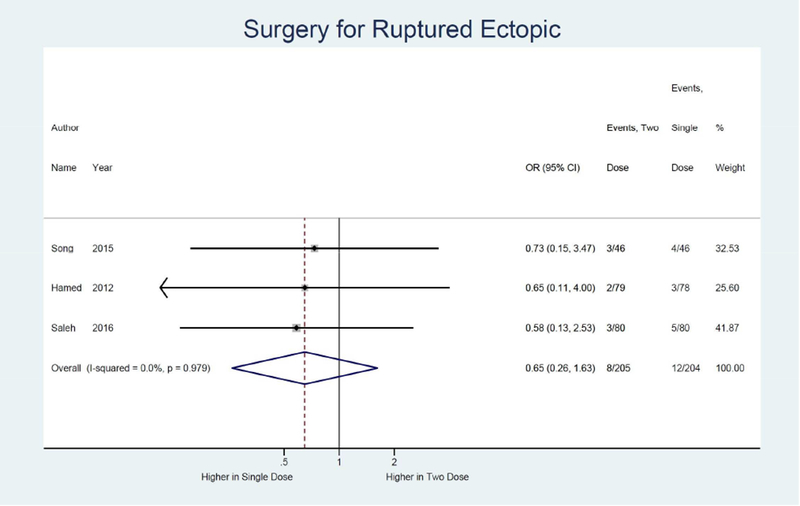
Forest plot: Two versus Single dose-Surgery for ruptured ectopic pregnancy
Figure 3G:
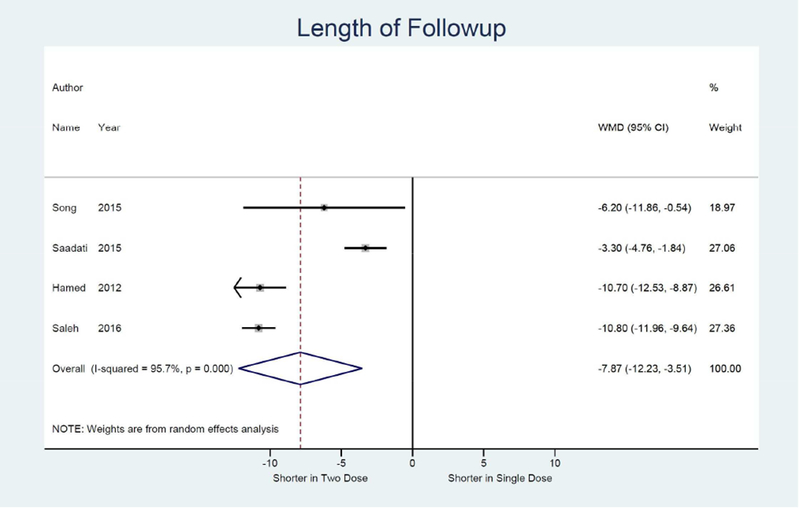
Forest plot: Two versus Single dose-Length of follow-up
Sensitivity analyses of 4 studies in high hCG groups revealed a 3.23 times higher odds of treatment success with the two dose protocol as compared to the single dose protocol (95% CI: 1.53, 6.84).14, 15, 32, 33 (Figure 3c) Evaluation of treatment success in large adnexal mass groups from 3 studies showed a 2.92 times higher odds of treatment success with use of the two dose protocol as compared to the single dose protocol (95% CI: 1.23, 6.93).14, 15, 32 (Figure 3d)
Figure 3C:
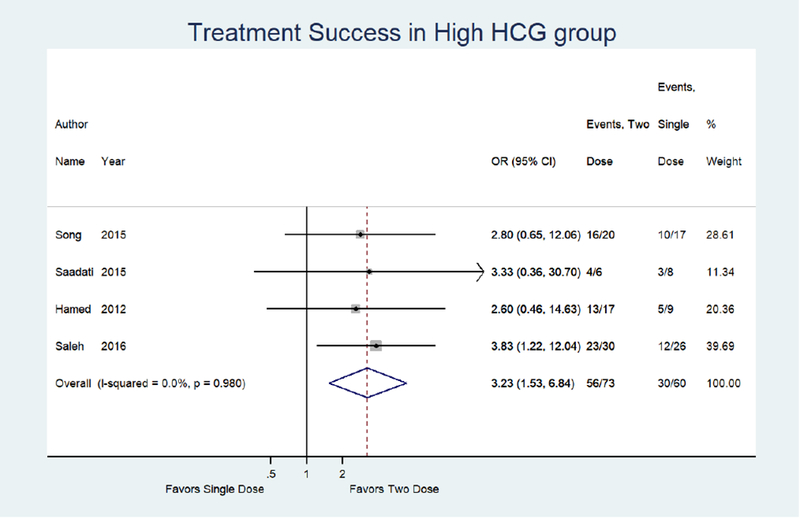
Forest plot: Two versus Single dose-Treatment success in high HCG group (defined by individual studies with a range of >3000–5500 mIUmL)
Figure 3D:
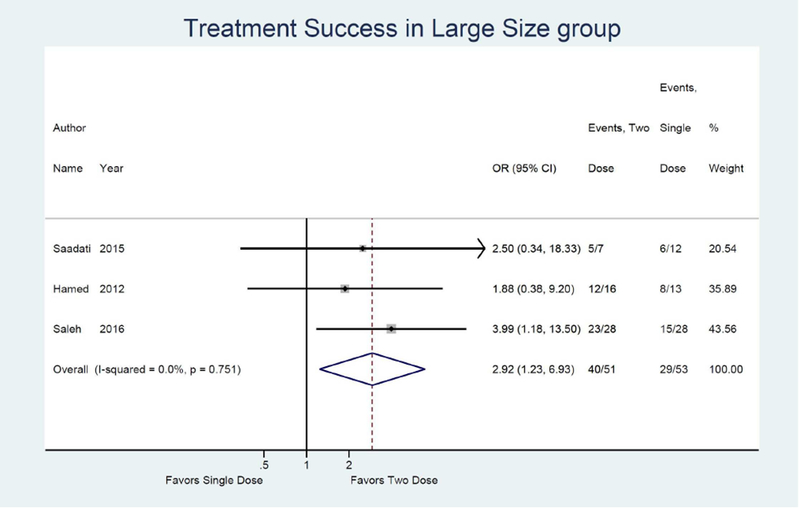
Forest plot: Two versus Single dose-Treatment success in large size group (defined by individual studies with a range of >2–3.5cm)
Single vs multidose protocols
We also identified three studies comparing treatment success rates for single dose versus multi-dose protocol. The multi-dose protocol is associated with a higher odds of treatment success, although non-significant, compared to the single dose (OR 1.79; 95% CI: 0.89, 3.62).13, 17, 22, 34 (not shown) Similarly, the multi-dose was associated with a non-significant 0.56 times lower odds of treatment failure (95% CI: 0.28, 1.13). (Figure 4a) The odds of side effects were significantly higher in the multi-dose protocol compared to the single dose (OR 2.10; 95% CI: 1.24, 3.54).13, 17, 34 (not shown) Odds of surgery for tubal rupture and length of follow-up were comparable between multi-dose and single dose protocol (OR: 1.62; 95% CI: 0.41, 6.49) (Figure 4d) and −1.3 days (95% CI: −5.4, 2.7). (Figure 4e)
Figure 4D:
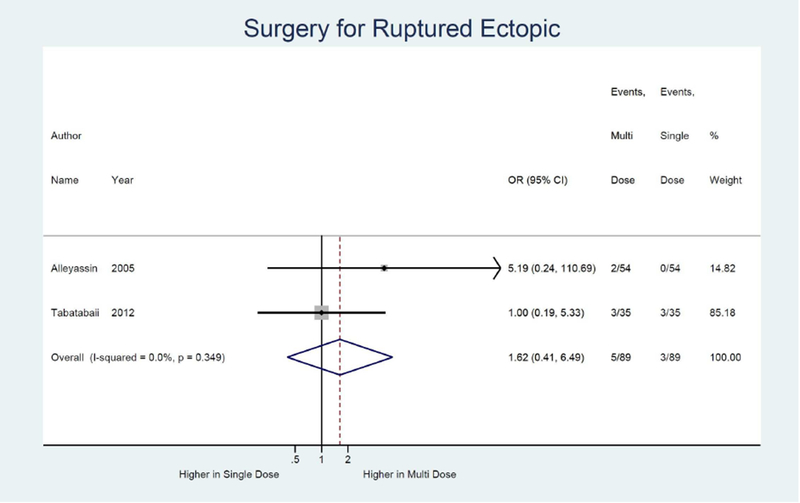
Forest plot: Multi versus Single dose-Surgery for ruptured ectopic pregnancy
Figure 4E:
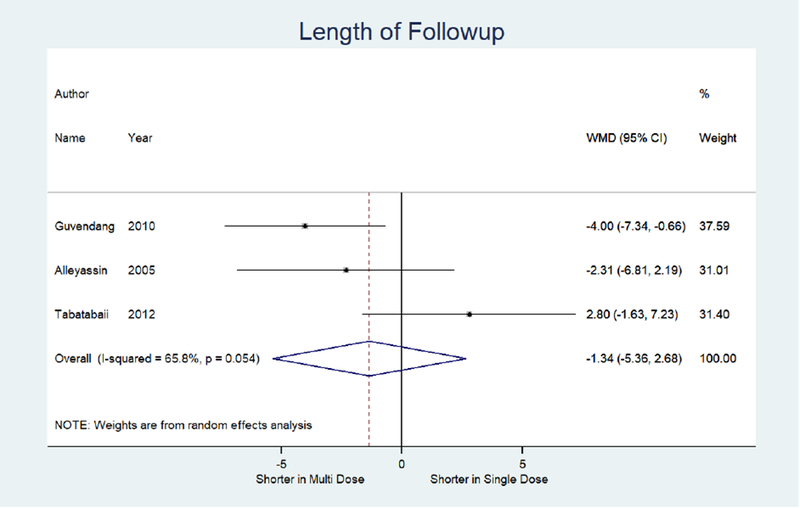
Forest plot: Multi versus Single dose-Length of follow-up
Only one study was identified assessing treatment success when initial hCG was high when comparing the multi-dose to single dose protocol and found a non-significant 2.00 times higher odds of treatment success (95% CI: 0.54, 7.44).13 (Figure 4b) Only one study was also identified assessing treatment success with larger adnexal mass when comparing the multi-dose to single dose protocol and found a non-significant 1.63 times higher odds of treatment success (95% CI: 0.38, 6.96).13 (Figure 4c)
Figure 4B:
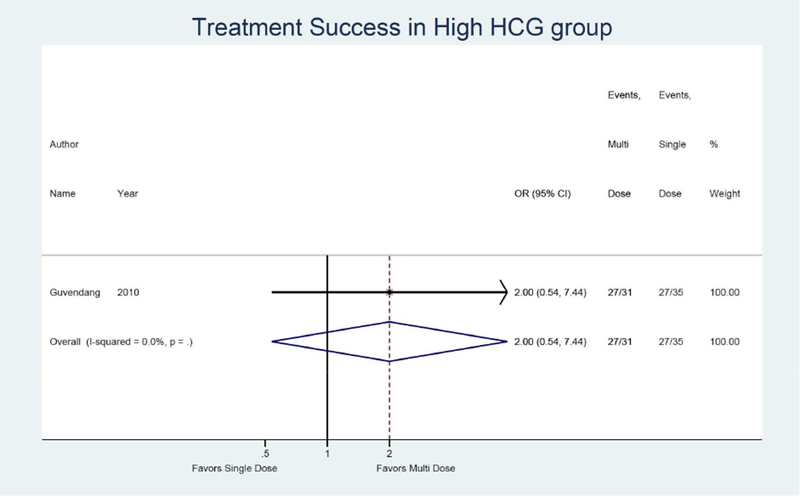
Forest plot: Multi versus Single dose-Treatment success in high HCG group (defined by study as >800 IU/L)
Figure 4C:
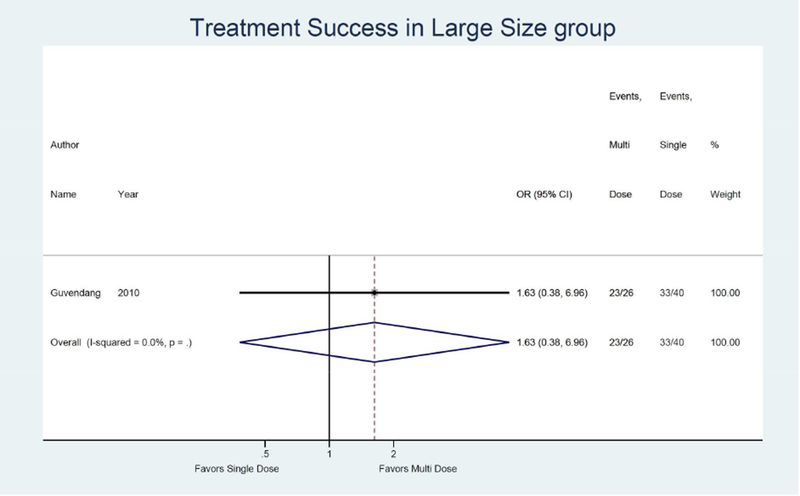
Forest plot: Multi versus Single dose-Treatment success in large size group (defined by study as >2 cm)
Comment
Overall, the two dose protocol was found to result in a significantly higher odds of treatment success and thus a significantly lower odds of treatment failure, when compared to the single dose protocol. These findings held true in patients with higher hCGs as well as those with large adnexal mass as defined by the individual studies. In addition, the length of follow-up for women receiving the two dose protocol was over a week shorter than for women receiving the single dose protocol. There was also a nonstatistically significant reduction in odds of surgery for tubal rupture with the use of the two dose protocol. The two dose protocol did have a higher rate of side effects, but it should be noted that most side effects described in included studies were mild and transient. No patients required hospitalization or long-term management, nor did side effects preclude continuation of treatment. Taken together, the two dose protocol is superior to the single dose protocol and should be considered first line therapy.
A meta-analysis has recently compared treatment success and side effects rates between multi-dose and single dose protocols.22 Given there have been no additional RCTs evaluating this comparison since this analysis was performed, we validated these findings and focused the analysis on odds of failure, odds of surgery for tubal rupture, length of follow-up and sensitivity analyses, which were not performed in the prior meta-analysis. Overall there is a non-statistically significant trend toward lower treatment failure and higher treatment success, with use of the multidose protocol. In addition, we found that the length of follow-up was only roughly 1 day shorter in the multi-dose protocol and odds of surgery for tubal rupture were not significantly different.
The quality of a meta-analysis is directly dependent on the quality of the studies included in the analysis. Previous meta analyses have been limited by including retrospective or observational studies12. Moreover, additional randomized trials have been conducted since publication of recent analyses. For example, the meta-analysis by Yang et. al22 did not include the study by Saleh et. al32 performed in 2016 and therefore did not find statistically significant differences when comparing the two dose to single dose protocol. The meta-analysis by Yuk et al.23 included poor quality data including a meeting abstract without a full published manuscript26 as well as RCTs which did not specify how randomization was performed nor how many patients were in each arm30 and which described their randomization process as “patients were alternatively selected”31 (and yet had different numbers of patients in each arm). Attempts were made to electronically contact both authors regarding these study details prior to exclusion from our meta-analysis.
This meta-analysis has several strengths, most importantly its rigid inclusion criteria. Only RCTs with clearly stated methodology were included, limiting the chances that results are biased by flaws in study design or execution. In addition to treatment success reporting, odds of failure were also calculated, which can provide an additional useful tool and way to conceptualize data when counseling patients.
The meta-analysis was limited by the relatively few RCTs done on this topic, particularly for the single versus multi-dose protocols. In this category, three total quality RCTs were identified assessing the main outcomes of treatment success. It is possible that the reason our data cannot confirm a higher success rate (and lower failure rate) for the multi-dose protocol is lack of power or inherent bias in the few studies comparing multi-dose to single dose therapy. Only one of these studies contained data for subgroup sensitivity analyses. Based upon the odds of treatment success found with this meta-analysis, we calculated that an additional study with 70 patients per arm would be needed for the odds of treatment success to be significantly higher in the multi-dose group as compared to the single dose (assuming the same differences were to be found in one additional study). Additionally, the meta-analysis is limited in the ability to evaluate effectiveness in multiple subgroups. For example, it is possible that there are clinical situations when a single dose of methotrexate is sufficient for the treatment of an ectopic pregnancy, such as women with a low hCG value. However, the limited data from included randomized clinical trials does not allow such a conclusion from this meta-analysis and could be the focus of future study.
Based on pharmacokinetics of methotrexate, it is logical that a second dose would improve success rates compared to a single dose because a second dose will affect a greater percentage of trophoblast cells in the S phase (DNA synthesis). It is not clear why an even greater number of doses, as part of the multi dose protocol, does not result in greater efficacy. This may be related to low power as stated earlier or it is also possible that the use of alternating doses of leucovorin, in an attempt to decrease side effects, may also limit efficacy of the treatment. Multi-dose treatment is not currently considered first line therapy and may be best reserved for women with advanced ectopic pregnancy or those in unusual locations such as cervical, intestinal, or ovarian ectopic pregnancy.35, 36
In conclusion, the two dose protocol is significantly superior to the single dose protocol in terms of odds of treatment success and treatment failure. These findings hold true in patients thought to be at a lower likelihood of responding to medical management, such as those with higher hCGs and large adnexal mass. While the multi-dose protocol showed similar trends when compared to the single dose protocol, none of these parameters reached statistical significance. Therefore, we would recommend the two dose protocol as the first line protocol in patients being medically managed for ectopic pregnancies.
Supplementary Material
AJOG at a Glance:
A: This study was conducted to compare the odds of treatment success, side effects, surgery for ruptured ectopic pregnancy and length of follow-up of commonly used methotrexate protocols for the treatment of ectopic pregnancy.
B: Two dose protocol was superior to the single dose protocol in treatment success, including in women at higher risk of failure such as those with high hCGs and large adnexal mass.
C: Updated meta-analysis of two dose versus single dose protocol and additional analyses of multi-dose versus single dose protocol using only quality RCTs.
Acknowledgments
Funding:
Dr. Kurt Barnhart is supported by R01 HD 036455. Snigdha Alur-Gupta and Laura Cooney are supported by the NIH T32 Training Grant: HD007440. These sources did not play any role in study design; in the analysis or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest:
The authors report no conflict of interest.
Paper presentation information:
Poster presentation at the 73rd Scientific Congress and Expo, American Society for Reproductive Medicine, San Antonio, Texas, October 28-November 1, 2017
Condensation:
The two dose methotrexate protocol is superior to the single in the treatment of ectopic pregnancy, including those with high hCGs or large adnexal masses.
References
- 1.Marion LL, Meeks GR. Ectopic pregnancy: History, incidence, epidemiology, and risk factors. Clin Obstet Gynecol 2012;55:376–86. [DOI] [PubMed] [Google Scholar]
- 2.Hajenius PJ, Mol F, Mol BW, Bossuyt PM, Ankum WM, van der Veen F. Interventions for tubal ectopic pregnancy. Cochrane Database Syst Rev 2007:Cd000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ACOG Practice Bulletin No. 193: Tubal Ectopic Pregnancy. Obstet Gynecol 2018;131:e91–e103. [DOI] [PubMed] [Google Scholar]
- 4.Barnhart KT. Clinical practice. Ectopic pregnancy. N Engl J Med 2009;361:379–87. [DOI] [PubMed] [Google Scholar]
- 5.Barnhart K, Coutifaris C, Esposito M. The pharmacology of methotrexate. Expert Opin Pharmacother 2001;2:409–17. [DOI] [PubMed] [Google Scholar]
- 6.Hajenius PJ, Engelsbel S, Mol BW, et al. Randomised trial of systemic methotrexate versus laparoscopic salpingostomy in tubal pregnancy. Lancet 1997;350:774–9. [DOI] [PubMed] [Google Scholar]
- 7.Kazandi M, Turan V. Ectopic pregnancy; risk factors and comparison of intervention success rates in tubal ectopic pregnancy. Clin Exp Obstet Gynecol 2011;38:67–70. [PubMed] [Google Scholar]
- 8.Krag Moeller LB, Moeller C, Thomsen SG, et al. Success and spontaneous pregnancy rates following systemic methotrexate versus laparoscopic surgery for tubal pregnancies: a randomized trial. Acta Obstet Gynecol Scand 2009;88:1331–7. [DOI] [PubMed] [Google Scholar]
- 9.Mol F, Mol BW, Ankum WM, van der Veen F, Hajenius PJ. Current evidence on surgery, systemic methotrexate and expectant management in the treatment of tubal ectopic pregnancy: a systematic review and meta-analysis. Hum Reprod Update 2008;14:309–19. [DOI] [PubMed] [Google Scholar]
- 10.Lipscomb GH, Mccord ML, Stovall TG, Huff G, Portera SG, Ling FW. Predictors of success of methotrexate treatment in women with tubal ectopic pregnancies. N Engl J Med 1999;341:1974–8. [DOI] [PubMed] [Google Scholar]
- 11.Barnhart K, Hummel AC, Sammel MD, Menon S, Jain J, Chakhtoura N. Use of “2-dose” regimen of methotrexate to treat ectopic pregnancy. Fertil Steril 2007;87:250–6. [DOI] [PubMed] [Google Scholar]
- 12.Barnhart KT, Gosman G, Ashby R, Sammel M. The medical management of ectopic pregnancy: a meta-analysis comparing “single dose” and “multidose” regimens. Obstet Gynecol 2003;101:778–84. [DOI] [PubMed] [Google Scholar]
- 13.Guvendag Guven ES, Dilbaz S, Dilbaz B, Aykan Yildirim B, Akdag D, Haberal A. Comparison of single and multiple dose methotrexate therapy for unruptured tubal ectopic pregnancy: a prospective randomized study. Acta Obstet Gynecol Scand 2010;89:889–95. [DOI] [PubMed] [Google Scholar]
- 14.Saadati N, Najafian M, Masihi S, Safiary S, Abedi P. Comparison of Two Different Protocols of Methotrexate Therapy in Medical Management of Ectopic Pregnancy. Iranian Red Crescent medical journal 2015;17:e20147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamed HO, Ahmed SR, Alghasham AA. Comparison of double- and single-dose methotrexate protocols for treatment of ectopic pregnancy. Int J Gynaecol Obstet 2012;116:67–71. [DOI] [PubMed] [Google Scholar]
- 16.Gungorduk K, Asicioglu O, Yildirim G, Gungorduk OC, Besimoglu B, Ark C. Comparison of single-dose and two-dose methotrexate protocols for the treatment of unruptured ectopic pregnancy. J Obstet Gynaecol 2011;31:330–4. [DOI] [PubMed] [Google Scholar]
- 17.Tabatabaii Bafghi A, Zaretezerjani F, Sekhavat L, Dehghani Firouzabadi R, Ramazankhani Z. Fertility outcome after treatment of unruptured ectopic pregnancy with two different methotrexate protocols. International journal of fertility & sterility 2012;6:189–94. [PMC free article] [PubMed] [Google Scholar]
- 18.Chen YX, Mao YY, Xie X. Efficacy and side effects of methotrexate in the treatment of ectopic pregnancy. Zhonghua fu chan ke za zhi 2003;38:749–51. [PubMed] [Google Scholar]
- 19.Lipscomb GH, Givens VM, Meyer NL, Bran D. Comparison of multidose and single-dose methotrexate protocols for the treatment of ectopic pregnancy. Am J Obstet Gynecol 2005;192:1844–7; discussion 47–8. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Jung YM, Lee DY, Jee BC. Pretreatment serum human chorionic gonadotropin cutoff value for medical treatment success with single-dose and multi-dose regimen of methotrexate in tubal ectopic pregnancy. Obstetrics & gynecology science 2017;60:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mergenthal MC, Senapati S, Zee J, et al. Medical management of ectopic pregnancy with single-dose and 2-dose methotrexate protocols: human chorionic gonadotropin trends and patient outcomes. Am J Obstet Gynecol 2016;215:590.e1–90.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang C, Cai J, Geng Y, Gao Y. Multiple-dose and double-dose versus single dose administration of methotrexate for the treatment of ectopic pregnancy: a systematic review and meta-analysis. Reprod Biomed Online 2017;34:383–91. [DOI] [PubMed] [Google Scholar]
- 23.Yuk JS, Lee JH, Park WI, Ahn HS, Kim HJ. Systematic review and meta-analysis of single-dose and non-single-dose methotrexate protocols in the treatment of ectopic pregnancy. Int J Gynaecol Obstet 2018;141:295–303. [DOI] [PubMed] [Google Scholar]
- 24.Cardamakis E, Kourounis G, Tzeferakos A, Korantzis A, Ginopoulos P. Three Different Regimens of Methotrexate for the Treatment of Ectopic Pregnancy. XV FIGO world congress of obstetrics & gynecology 1997:92. [Google Scholar]
- 25.Nct. Comparison of Single and Multiple-dose Methotrexate Therapy for Ectopic Pregnancy. Https://clinicaltrialsgov/show/nct01662167 2012. [Google Scholar]
- 26.Klauser CK, May WL, Johnson VK, Cowan BD, Hines RS. Methotrexate for Ectopic Pregnancy: A Randomized “Single Dose” Compared With “Multidose” Trial. Obstet Gynecol 2005;105:64S. [Google Scholar]
- 27.Olofsson JI, Poromaa IS, Ottander U, Kjellberg L, Damber MG. Clinical and pregnancy outcome following ectopic pregnancy; a prospective study comparing expectancy, surgery and systemic methotrexate treatment. Acta Obstet Gynecol Scand 2001;80:744–9. [DOI] [PubMed] [Google Scholar]
- 28.Golmohammadlou S, Hajishafiha M, Hasankhani Z. Comparison of single and two dose of methotrexate in treatment of ectopic pregnancy. Iranian journal of reproductive medicine 2012;10:74. [Google Scholar]
- 29.Fakheri T, Nankali A, Shahlazadeh H, Ataee M, Shahri MN. Comparison of single-dose and multiple-dose systemic methotrexate regimen for ectopic pregnancy. Journal of Isfahan Medical School 2014;32:1054–60. [Google Scholar]
- 30.Zargar M, Razi T, Barati M. Comparision of single and multidose of methotrexate in medical treatment of ectopic pregnancy. Pakistan journal of medical sciences 2008;24:586–89. [Google Scholar]
- 31.Amirian M, Rajaee M, Moayedi U, Mohammadi F, Hosseini S. Comparison of single and multiple-dose methotrexate therapy for ectopic pregnancy: A clinical trail. Life Science Journal 2013;10:564–67. [Google Scholar]
- 32.Saleh H Double Versus Single Dose Methotrexate Regimens in Management of Undisturbed Ectopic Pregnancy. Critical Care Obstetrics and Gynecology 2016;6. [Google Scholar]
- 33.Song T, Kim MK, Kim ML, Jung YW, Yun BS, Seong SJ. Single-dose versus twodose administration of methotrexate for the treatment of ectopic pregnancy: a randomized controlled trial. Hum Reprod 2016;31:332–8. [DOI] [PubMed] [Google Scholar]
- 34.Alleyassin A, Khademi A, Aghahosseini M, Safdarian L, Badenoosh B, Hamed EA. Comparison of success rates in the medical management of ectopic pregnancy with single-dose and multiple-dose administration of methotrexate: a prospective, randomized clinical trial. Fertil Steril 2006;85:1661–6. [DOI] [PubMed] [Google Scholar]
- 35.Ramkrishna J, Kan GR, Reidy KL, Ang WC, Palma-Dias R. Comparison of management regimens following ultrasound diagnosis of nontubal ectopic pregnancies: a retrospective cohort study. BJOG 2018;125:567–75. [DOI] [PubMed] [Google Scholar]
- 36.Hunt SP, Talmor A, Vollenhoven B. Management of non-tubal ectopic pregnancies at a large tertiary hospital. Reprod Biomed Online 2016;33:79–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


