Abstract
Background and Aims
The presence and content of health warning labels (HWLs) on nicotine vaping products (NVPs), such as electronic cigarettes, varies by country and manufacturer. We compared proportions of people who report (i) noticing HWLs on NVPs and (ii) feeling concerned having noticed HWLs, by country and by smoking or vaping status. We also examined recall of HWL content and whether this varies by country.
Design
Cross-sectional survey
Setting
Australia (AU), Canada (CA), England (EN), and the United States (US). At the time of data collection, HWLs on NVPs were only mandatory in EN.
Participants
A total of 11561 respondents from the following samples in the 2016 International Tobacco Control 4-Country Project: (1) re-contacted smokers and quitters who had participated in the previous wave of the project; (2) newly recruited current smokers and recent quitters, and (3) newly recruited current vapers from CA, EN and US.
Measurements
Outcomes included: 1) having noticed HWLs on NVPs, 2) feeling concerned having noticed HWLs, and 3) recall of HWL message content.
Findings
Compared with respondents in EN, respondents in CA were more likely to report having noticed HWLs (OR=1.58, p=0.02) whereas respondents in AU (OR=0.76, p=1.00) and the US (OR=1.54, p=0.09) were not significantly more or less likely to report having noticed HWLs. Compared with concurrent smokers and vapers, daily smokers, non-daily smokers, and quitters were less likely to report having noticed HWLs, (ORs=0.21, 0.33 and 0.19 respectively, all p<0.001). There were no significant differences in reports of noticing HWLs when comparing concurrent smokers and vapers with daily (OR=1.62, p=0.91) or non-daily (OR=1.15, p=1.00) vapers. There were no significant differences by country in reporting that HWLs made them concerned about using NVPs. Daily vapers were less likely to report feeling concerned than concurrent users (OR=0.11, p=0.017). Among those who reported reading HWLs (n=688), there was little evidence of differences in recall of the HWL content.
Conclusions
Respondents in England, where health warning labels on nicotine vaping products are mandatory, were not significantly more likely to report having noticed such warnings than those in Australia, Canada and the US where warnings are not mandatory.
Keywords: Electronic cigarettes, consumer information, health warnings, tobacco health information, tobacco product labelling
Introduction
The use of nicotine vaping products (NVPs) such as electronic cigarettes is increasing worldwide. In England, where NVPs are available, ever use in smokers and recent ex-smokers increased from 2% to 20% between 2011 and 2017, with daily use increasing from 2% to 12% (1). Similar trends have also been seen in the US (2), where NVPs are similarly available, and in Canada (3, 4) and Australia (5), despite the sale of NVPs being prohibited in these countries. The increase in use of these products has been accompanied by much debate within the public health community regarding the potential impact of NVPs and strategies to regulate them. Recent reports from England (6–8) state that NVPs carry significantly lower risk than cigarettes or other tobacco combustibles and that they may be a useful tool to aid cessation. However, concerns about potential health harms have also been raised (9). Other studies have asserted that NVPs may have limited potential to facilitate smoking cessation and may even prolong smoking through dual use (10, 11), or may promote smoking uptake in younger populations (11, 12). Furthermore, the potential long-term health effects of NVPs are unknown (13). The lack of consensus on the absolute safety of NVP use means that a need to communicate their potential harms may be warranted. However, as NVP use can be considered as potentially harm-reducing for current smokers, but harm-elevating for those who do not use tobacco, the relative risks of NVPs and tobacco cigarettes may need to be communicated also.
Health warning labels (HWLs) on tobacco products have been used for many years to convey risks to users and non-users and have been shown to increase knowledge of health risks (14–17); increase quit attempts (18–21); discourage non-smokers from initiating smoking (22); and assist recent quitters to avoid relapse (23). To date there has been no consistent approach taken to HWLs on NVPs, at least partly due to these products not containing tobacco, meaning tobacco control policies do not apply in most cases. As a result, the NVP industry has largely been unregulated in the relatively short period in which use of these products has grown more prevalent.
At the time of data collection for the current study, of the four countries examined here, NVP HWLs were only required in England. European Union (EU) Classification, Labelling and Packaging (CLP) regulations require that all chemical substances placed on the market are appropriately classified, labelled and packaged according to the known hazards of those substances (24). This requires suppliers of NVPs and refill containers to label their products to convey the hazards of propellants, nicotine and other chemicals present. In addition, the EU Tobacco Products Directive (TPD) was enforced from May 2016 in the UK, with a new set of regulations for NVPs and refill containers (25). This regulation required these products to carry a large, prominent HWL consisting of the text “This product contains nicotine which is a highly addictive substance”. A transitional period was in place for one year from the implementation of the law on May 20 2016, following which the HWLs were required to be in place. Data for the current study was collected during this transitional period.
In the US, the Food and Drug Administration assumed regulatory authority over NVPs in 2016. In addition to other regulations, these also required HWLs of the addictiveness of nicotine “WARNING: This product contains nicotine. Nicotine is an addictive chemical” to be displayed in the advertising and packaging of all NVP products. Full implementation was initially planned for August 2018, however this has now been delayed until 2022 (26). At the time of data collection, most manufacturers of NVPs in the US carried voluntary HWLs describing potential health and addiction impacts that vary widely in terms of content and length (27). In Canada and Australia there are no specific local regulations regarding labelling of NVPs due to the prohibition on the sale of these products. Despite this lack of regulation, NVPs are available and used in these countries (5, 28, 29). Further, as the inclusion of HWLs is determined not only by country, but also by manufacturer, the packaging of these products may also contain voluntary HWLs; an audit of retail outlets in Canada found that many NVPs sold contained warnings such as disclaimers that the product is not to be sold to persons under a certain age or that the content of e-liquid bottles could be harmful. See Figure 1 for examples of NVP packaging with CLP and TPD compliant HWLs visible in England at the time of data collection, and for manfacturers’ with voluntary HWLs on NVPs sold in Australia, Canada and the US.
Figure 1:
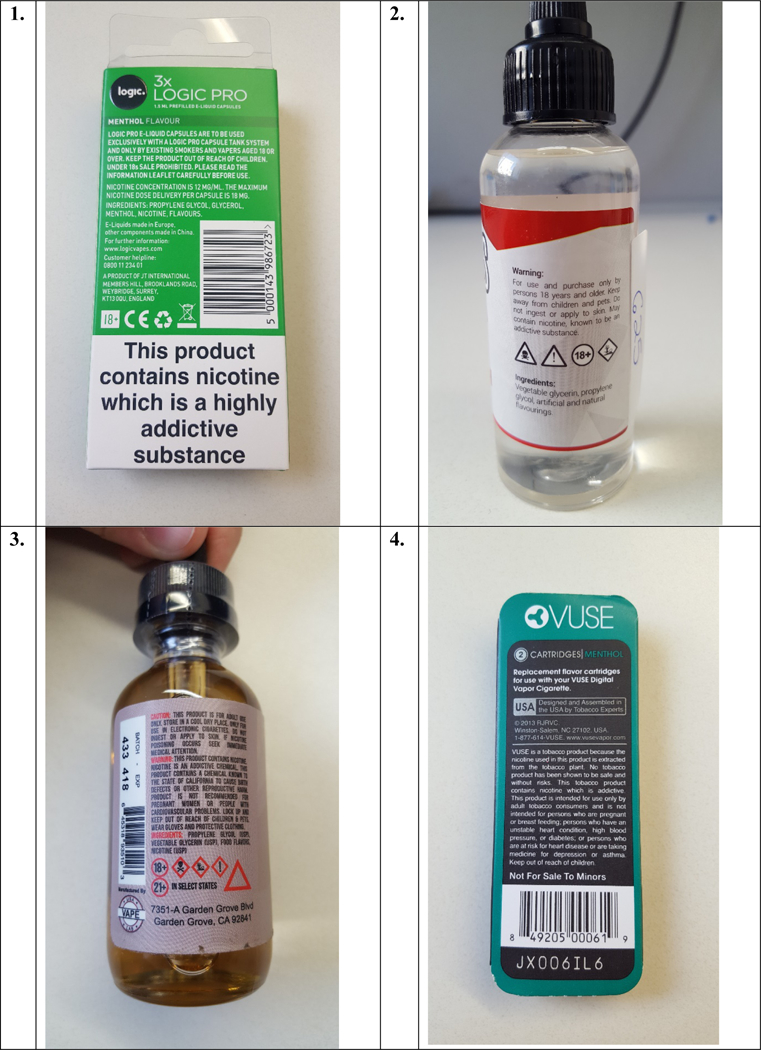
NVP packaging with CLP and TPD compliant warnings from the UK (1) and voluntary, manufacturer warning from Australia (2), Canada (3) and the US (4).
The primary aim of the current study is to examine country differences in the proportion of respondents who report noticing HWLs on NVPs and who report that noticing HWLs made them concerned about using NVPs, using data from the ITC Four Country Project (ITC4CV). The ITC4 has been collecting data since 2002 comparing the implementation of tobacco control activities, and more recently NVP regulations, in Australia, Canada, England and the US. As detailed above, these four countries have varying approaches to NVP regulation and labelling that could impact upon rates of noticing HWLs; specifically one might expect that respondents in England, where HWLs on NVPs are mandated, would be more likely to notice HWLs than those in countries where the presence of HWLs is determined by manufacturer. Further, it is also possible that differences in regulation and the social context could lead to differences in reactions to HWLs between countries. Reactions to warnings on tobacco products across countries can vary widely, even in cases where very similar HWLs are used such as in the EU (30). Smokers and ex-smokers in England report that ECs are more socially acceptable, and less harmful than conventional cigarettes compared to those in Australia (31, 32). HWLs on NVPs will be interpreted within these contexts meaning reactions may differ.
We will also examine differences in noticing HWLs and reactions to HWLs by smoking and vaping status, in particular between those who use NVPs (i.e. vapers), those who do not use NVPs and concurrent users (those who both vape and smoke at least monthly). There are two main reasons for examining this: first, previous studies have found differences between smokers, concurrent users and non-smokers in recall of HWLs (33) and reactions to HWLs (33, 34). Second, as mentioned above, any differences between groups could represent an unwanted effect of HWLs on NVPs. Whilst the aim of HWLs on traditional tobacco products is to stop individuals from smoking, either by discouraging uptake in non-users or encouraging cessation in users, the function of HWLs on NVPs is arguably more complex. HWLs on NVPs could have the desired impact of discouraging non-users from use, but may also discourage use in those groups for whom NVP use may be less harmful, i.e. current smokers and dual users.
The present study uses data from Wave 1 of the ITC Four Country Smoking and Vaping 1 Survey (4CV1) to examine: (1) country and smoking or vaping status differences in the proportion of adult smokers, ex-smokers, and vapers from Australia, Canada, England and the US who notice HWLs on NVPs; (2) country and smoking or vaping status differences in whether HWLs made respondents concerned about using NVPs, and (3) what HWL messages were recalled and whether this varied by country.
Methods
Participants and design.
The study uses cross-sectional data from Wave 1 of the ITC Four Country Smoking and Vaping 1 Survey (4CV1). Methodological details for each country are available in Thompson et al. (35) and via the ITC website (http://www.itcproject.org/methods) (36). In brief, data for the survey was collected between July and November 2016. All surveys in AU, CA and EN were conducted online. A small number (n=46) of surveys conducted in the US were conducted by telephone, with the remainder online. The sample comprised the following cohorts: (1) re-contact smokers and quitters who participated in the previous wave of the ITC 4C Project, regardless of NVP use (retention rates ranged between 35.7% and 44.2%); (2) newly recruited current smokers and recent quitters (quit smoking in the past 24 months) from country-specific panels, regardless of NVP use, and (3) newly recruited current vapers (use NVPs at least weekly) from CA, EN and US. Sample sizes for smokers/recent quitters were 1504 in AU, 3006 in CA, 3773 in EN, and 2239 in the US. Sample sizes of additional at-least-weekly NVP users were 727 in CA, 551 in EN, and 494 in the US. The sample in each country was designed to be as representative as possible and used either probability-based sampling frames or non-probability quota samples. Respondents were recruited via random-digit-dialling (RDD) sampling frames, or web-based or address-based panels, or a combination of these frames. All participants were incentivised for participation, with incentives varying by country and sample source (37). Response rates by country for new recruits ranged from 15.2% to 49.6%, conditional on invitation by the survey firm; cooperation rates were above 90%. In total, responses from 11561 participants were included in analyses.
Ethical clearance
The survey protocols and all materials, including the survey questionnaires, were cleared for ethics by King’s College London, UK; Office of Research Ethics, University of Waterloo, Canada; and Human Research Ethics, Cancer Council Victoria, Australia. All participants provided consent to participate.
Measures
Primary outcomes
To assess whether respondents noticed HWLs on NVPs they were asked “Now thinking about e-cigarettes, in the last 30 days, have you noticed any health warnings on packaging for e-cigarettes, cartridges or e-liquid containers?” (answered yes/ no). Those reporting that they had noticed HWLs for NVPs were then asked a number of follow-up questions: “What effect have the health warnings had on your thoughts about using e-cigarettes?”, with responses: made me concerned about using them; had no effect; and reassured me about using them; and “What do you recall the health warning(s) saying?”. For this question respondents were given a number of options (each answered yes/ no): this product contains nicotine which is a highly addictive substance; e-cigarettes are not to be used by non-smokers; keep e-cigarettes out of reach of children; e-cigarettes are not a safe alternative to cigarettes; e-cigarettes are not to be sold to minors; and e-cigarettes contain cancer-causing chemicals. Participants could choose as many responses as they wished.
Smoking and vaping status
To ascertain smoking status, respondents were asked “how often, if at all, do you CURRENTLY smoke ordinary cigarettes (either factory-made or roll-your-own)?” NVP use was assessed by the question “have you ever used an e-cigarette or vaping device, even one time?” Those who answered yes were then asked “How often, if at all, do you CURRENTLY use e-cigarettes/vaping devices (i.e. vape)?”. Based on responses to these items, respondents were divided into six categories for analyses: smokers (daily and non-daily), vapers (daily and non-daily), concurrent users, and quitters.
Analysis
Because our survey samples in each country were designed to ensure that we recruited specific numbers of current vapers, quitters, and current smokers we developed sampling weights to recalibrate responses to questions based on population estimates from national surveys in each country. Logistic regression analyses were conducted to examine: (i) associations between noticing HWLs on NVPs, country and smoking or vaping status; (ii) associations between the impact of noticing HWLs on NVPs, country and smoking or vaping status and (iii) associations between what respondents recalled HWLs saying across countries. Analyses for (ii) were restricted to those who reported noticing HWLs. Analyses for (iii) were restricted to those who reported noticing HWLs and who reported having read any of the HWLs. All analyses controlled for age (18–24, 25–39, 40–54, 55 and up); sex; household income; education (both equated across the four countries and coded into low, moderate and high) and ethnicity (coded as white or non-white in CA, US & EN and English or non-English speaking in AU). Sampling weights were applied in each model and tests were adjusted by Bonferroni correction to control the familywise error rate (FWER) when performing multiple hypotheses tests within age group, country, and smoking and vaping status. Missing data in each logistic regression model was between 0.4% and 2.5%. Cases with missing data were removed from the model. The threshold for statistical significance was 0.05. Wald (“linear”) confidence limits were used. All statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results
Noticing HWLs on VDs by country and smoking or vaping status
Table 1 shows the proportion of participants who reported noticing HWLs on NVPs in each country and by smoking or vaping status. Overall 5.7% of respondents reported noticing HWLs on VDs. Logistic regression analyses indicated that the proportion of respondents noticing HWLs on packaging for NVPs differed significantly by country (p<.001). Compared to those in England, respondents in Canada (OR=1.58 (1.05, 2.38), p=0.019) had greater odds of noticing HWLs (Table 1). Those in Australia and the US were no more or no less likely to notice HWLs on NVPs than those in England.
Table 1.
Proportion of respondents noticing HWLs on NVPs and correlates of noticing HWLs on NVPs
| Noticed HWL on NVPs (yes/ otherwise) |
Correlates of noticing HWL on NVPs | ||
|---|---|---|---|
| n/N | % | OR (95% CI) | |
| Country | |||
| Australia | 63/1475 | 2.6 | 0.76 (0.40–1.44) |
| Canada | 330/3487 | 7.0 | 1.58 (1.05–2.38)* |
| United States | 397/2503 | 6.7 | 1.54 (0.96–2.47) |
| England | 260/4096 | 5.1 | 1.00 |
|
Age | |||
| 18–24 | 311/2268 | 8.5 | 1.86 (1.09–3.18)* |
| 25–39 | 425/2889 | 6.5 | 1.49 (0.94–2.37) |
| 40–54 | 183/3035 | 4.5 | 0.97 (0.60–1.58) |
| 55+ | 131/3369 | 4.4 | 1.00 |
|
Sex | |||
| Male | 676/5881 | 6.8 | 1.77 (1.40–2.25)** |
| Female | 374/5680 | 4.3 | 1.00 |
|
Income | |||
| Not stated | 35/687 | 4.3 | 0.71 (0.29–1.73) |
| High | 536/4886 | 5.4 | 0.75 (0.47–1.20) |
| Moderate | 274/3260 | 6.0 | 0.91 (0.56–1.49) |
| Low | 205/2728 | 6.2 | 1.00 |
|
Education | |||
| Not stated | 8/87 | 12.3 | 3.68 (0.37–36.58) |
| High | 457/3356 | 7.4 | 1.64 (1.10–2.44)** |
| Moderate | 349/4701 | 5.5 | 1.27 (0.88–1.84) |
| Low | 236/3417 | 4.6 | 1.00 |
|
Ethnicity | |||
| Non-whitea | 249/1637 | 9.9 | 1.75 (1.30–2.35)*** |
| Whiteb | 801/9924 | 5.1 | 1.00 |
|
Smoking/ vaping status | |||
| Daily smoker | 194/5578 | 3.6 | 0.21 (0.15–0.30)*** |
| Non-daily smoker | 102/1575 | 6.7 | 0.33 (0.21–0.51)*** |
| Daily vaper | 39/199 | 20.1 | 1.62 (0.76–3.45) |
| Non-daily vaper user | 8/66 | 15.7 | 1.15 (0.28–4.80) |
| Quitter | 22/785 | 3.4 | 0.19 (0.08–0.44)*** |
| Concurrent user | 685/3358 | 15.5 | 1.00 |
non-English in AU
English in AU
p<.05
p<.01
p<.001.
Percentages are based on weighted data; ‘Otherwise’ includes those who responded ‘no’ or ‘don’t know’ but excludes those who refused to respond or whose response was deemed NA.
Analyses also showed that smoking or vaping status was associated with noticing HWLs on NVPs (p<0.001). Compared to concurrent users, daily smokers (OR=0.21 (0.15–0.30), p<0.001), non-daily smokers (OR=0.33 (0.21–0.51), p<0.001), and quitters (OR=0.19 (0.08–0.44), p<0.001) had lower odds of noticing HWLs (see Table 1). When concurrent users were compared with daily or non-daily vapers users there were no significant differences.
Feeling concerned about using VDs amongst those who notice HWLs
Table 2 shows the proportion of participants who reported feeling concerned following noticing HWLs on NVPs in each country and by smoking or vaping status. Overall 31.2% of those who noticed HWLs on NVPs said that it made them concerned about using NVPs. Despite wide variation in the proportion of respondents reporting feeling concerned, there was no significant difference overall across country (AU=14.1%, CA=36.5%, EN=26.4%, US=33.7%, p=0.176). Analyses indicated that feeling concerned following noticing HWLs on NVPs varied by smoking or vaping status (p=0.003). Daily vapers had lower odds of reporting feeling concerned than concurrent users (OR=0.11 (0.01–0.80), p=0.017).
Table 2.
Proportion of respondents reporting feeling concerned following noticing HWLs on NVPs and correlates of feeling concerned
| Felt concerned about using NVPs following noticing HWL (yes/ otherwise) |
Correlates of feeling concerned about using NVPs following noticing HWL |
||
|---|---|---|---|
| n/N | % | OR (95% CI) | |
| Country | |||
| Australia | 10/63 | 14.1 | 0.35 (0.08–1.63) |
| Canada | 118/328 | 36.5 | 1.18 (0.55–2.57) |
| United States | 188/396 | 33.7 | 1.13 (0.45–2.85) |
| England | 97/259 | 26.4 | 1.00 |
|
Age | |||
| 18–24 | 114/310 | 34.4 | 0.92 (0.36–2.34) |
| 25–39 | 201/424 | 31.9 | 0.78 (0.32–1.91) |
| 40–54 | 60/182 | 28.5 | 0.66 (0.25–1.73) |
| 55+ | 38/130 | 28.5 | 1.00 |
|
Sex | |||
| Male | 272/675 | 29.9 | 0.88 (0.57–1.36) |
| Female | 141/371 | 33.8 | 1.00 |
|
Income | |||
| Not stated | 6/34 | 12.3 | 0.36 (0.07–1.91) |
| High | 250/534 | 35.8 | 1.27 (0.56–2.90) |
| Moderate | 86/274 | 26.4 | 0.82 (0.34–1.97) |
| Low | 71/204 | 32.9 | 1.00 |
|
Education | |||
| Not stated | 3/8 | 11.1 | 0.20 (0.01–3.70) |
| High | 218/455 | 34.1 | 1.21 (0.54–2.74) |
| Moderate | 113/349 | 31.0 | 0.95 (0.46–1.99) |
| Low | 79/234 | 30.2 | 1.00 |
|
Ethnicity | |||
| Non-whitea | 97/247 | 44.9 | 1.88 (1.09–3.24)* |
| Whiteb | 316/799 | 27.3 | 1.00 |
|
Smoking/ vaping status | |||
| Daily smoker | 58/193 | 31.1 | 0.711 (0.36–1.39) |
| Non-daily smoker | 31/101 | 27.3 | 0.47 (0.21–1.10) |
| Daily vaper | 3/39 | 6.0 | 0.11 (0.01–0.80)* |
| Non-daily vaper | 3/8 | 22.4 | 0.42 (0.02–9.73) |
| Quitter | 9/22 | 38.1 | 0.99 (0.25–3.98) |
| Concurrent user | 309/683 | 41.3 | 1.00 |
non-English in AU
English in AU
p<.05
p<.01
p<.001
percentages are based on weighted data; analyses only include those who reported noticing HWLs on NVPs; ‘Otherwise’ includes those who responded ‘no’ or ‘don’t know’ but excludes those who refused to respond or whose response was deemed NA.
Recall of messages on HWLs
Across all respondents, the most recalled HWLs (n=688) were ‘This product contains nicotine which is a highly addictive substance’ (78.7%), ‘Keep e-cigarettes out of reach of children’ (72.1%), and ‘E-cigarettes are not to be sold to minors’ (63.7%) (see Table 3). There were some differences by country. Respondents in US had greater odds of recalling having seen the HWL ‘E-cigarettes are not a safe alternative to cigarettes’ than those in EN (OR=3.38 (1.10–10.35), p=0.025). For the HWL ‘E-cigarettes contain cancer causing chemicals’, respondents in EN had lower odds of recalling this message than respondents in AU (OR=4.39 (1.13, 17.04), p=0.024), CA (OR=5.03 (1.90–13.35), p<0.001) and US (OR=3.52 (1.16–10.69), p=0.017).
Table 3.
Recall of HWL content by country and smoking/ vaping status and correlates of recalling HWL content
| This product contains nicotine which is a highly addictive substance |
E-cigarettes are not to be used by non- smokers |
Keep e- cigarettes out of reach of children |
E-cigarettes are not a safe alternative to cigarettes |
E-cigarettes are not to be sold to minors |
E-cigarettes contain cancer causing chemicals |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | OR | % | OR | % | OR | % | OR | % | OR | % | OR | |
| Country | ||||||||||||
| Australia | 63.2 | 0.30 | 31.9 | 1.07 | 72.9 | 2.13 | 32.7 | 1.48 | 67.9 | 2.08 | 52.4 | 4.39* |
| Canada | 78.1 | 0.56 | 29.0 | 0.83 | 72.8 | 2.03 | 38.9 | 1.61 | 63.3 | 1.22 | 51.9 | 5.03*** |
| United States | 77.5 | 0.64 | 33.7 | 0.98 | 77.6 | 1.99 | 54.8 | 3.38* | 65.2 | 1.44 | 47.3 | 3.52* |
| England | 82.2 | 1.0 | 32.5 | 1.00 | 65.0 | 1.00 | 23.3 | 1.00 | 61.6 | 1.00 | 23.0 | 1.00 |
| Total | 78.7 | 31.6 | 72.1 | 39.1 | 63.7 | 42.3 | ||||||
Percentages are based on weighted data
p<.05
p<.01
p<.001
analyses only include those who reported reading HWLs on NVPs; analyses also controlled for age, sex, income, education and ethnicity; quitters were excluded from these analyses as their inclusion prevented models from reaching acceptable levels of fit. For ‘keep e-cigarettes out of reach of children’ all vapers were combined in the analysis to achieve acceptable model fit.
Discussion
The main finding from this study was that respondents in England, the only country which had requirements for HWLs on NVPs, were not more likely to report noticing HWLs than respondents in countries where the presence of HWLs was determined by manufacturer. Despite differences in regulations and societal attitudes towards NVPs, there was also no evidence that reactions to HWLs differed between countries. There were some differences in reactions to HWLs by smoking and vaping status, however, with daily vapers being less likely to report feeling concerned about using NVPs than concurrent users.
More respondents in Canada (7.0%) reported having noticed HWLs than in England (5.1%); this is in spite of there being no specific requirements for HWLs on NVPs in Canada at the time that data was collected, whereas in England EU CLP regulations were in place, meaning that all NVP packaging would have carried mandatory warnings, and EU TPD HWLs were being introduced. There are a number of potential reasons for this finding. It is possible that EU CLP warnings, which, for example, convey the toxicity of mixtures containing nicotine using a pictogram, were not recognised by respondents as health warnings. With regards to the warnings required by EU TPD regulations, it is possible that respondents in England may have had limited opportunities to be exposed to these warnings as they were being phased in gradually during the data collection period. In keeping with this, there was no evidence that those in England were more likely to recall having seen the mandated nicotine addiction warning than those in the other three countries. Finally, despite these regulatory differences, it is possible that voluntary, manufacturer-written HWLs were more likely to be present and were more prominent on NVP packaging distributed in Canada than the mandated HWLs required in England. Certainly the lack of regulatory requirements for HWLs has not precluded a broad variety of HWLs appearing on NVP products in the US (27). It is also worth noting that although differences between countries were significant, overall rates of noticing HWLs on NVPs were low across all countries and the proportion reporting that they noticed HWLs on NVPs did not vary widely.
Concern about using NVPs prompted by HWLs was not found to vary by country, a finding that contrasts with that of a previous study (30). This was unexpected, as other studies have found differences in attitudes towards NVPs that could have mediated the effects of HWLs on concerns about using NVPs (31, 32). It may be that the underlying factors that translate into cross-country differences in reactions to NVPs on traditional tobacco products do not translate into differences in reactions to HWLs on NVPs; the current findings are broadly in line with a recent study using ITC data which found that reactions to NVP advertising did not differ between the different regulatory environments of England and Australia (38). The absence of cross-country differences in reactions to HWLs on NVPs compared to traditional tobacco products warrants further investigation.
Vapers were more likely to report noticing HWLs than non-vapers. This is most likely due to greater levels of exposure to packaging in those using NVPs, although vapers may also be primed to notice and recall HWLs on NVP packaging compared to non-vapers (33). We also found differences in reactions to NVP between different smoking and NVP user groups. Daily vapers had significantly lower odds of feeling concerned than concurrent users. One potential explanation for this effect is that sole NVP use leads to subjectively experienced improvements in health and wellbeing, as has been reported elsewhere (11), which may lead these users to discount HWLs compared to those still smoking. This finding is in contrast, however, to experimental studies examining the impact on risk perceptions of HWLs on print advertisements for NVPs, which found no differences between vapers and concurrent users (39, 40). Current vapers are one group for whom being discouraged from using NVPs by HWLs would be advantageous, provided they are not at risk for relapse to smoking. However, for other groups, such as concurrent users, switching to sole NVP use could reduce exposure to harm, yet in this study concurrent users were more likely to report concern than daily vapers. This is an important finding, if HWLs are to be introduced more widely in those countries where not currently mandated, messages will need to be carefully calibrated to ensure that smokers who are considering switching completely to NVPs are not discouraged from doing so.
This study had several limitations. Detail on the specific HWLs viewed by respondents was limited meaning few inferences regarding links between HWL type and reactions to HWLs could be drawn. For example, we had no information on, size and format of HWLs and size of HWL text, which have been found in previous studies to influence responses to HWLs (34, 39, 41). However, there were few country differences in what messages on HWLs respondents recalled seeing suggesting all were similarly exposed to a broad range of warnings. In any case, isolating the effects of specific HWLs on NVPs in real-life settings may prove difficult as most NVP packaging, even where specific HWLs are mandated, contains multiple warnings reflecting the complexity of NVPs as nicotine- products, chemical products and electrical goods. Another limitation is the use of cross-sectional data which means we cannot conclude, for example, that noticing HWLs led to feeling concerned about using NVPs, or whether smoking or vaping status caused respondents’ reactions to HWLs. Finally, cell sizes for some comparisons were small, which may have limited power to detect differences.
Further research
The current study did not include never smokers or those aged under 18 and these are two important groups for whom it would be worth gathering data on rates of noticing HWLs on NVPs and their reactions. Future research should also be conducted to understand differences in reactions to HWLs between different smoker and NVP user groups: this would necessitate research on the impact of specific HWLs, in particular the impact of HWLs on the addictiveness of nicotine because these warnings are likely to be included in any future NVPs HWL policy. Future research should ensure that health warnings do not exacerbate common misperceptions of nicotine (6, 42), which may undermine accurate perceptions of relative risk between smoking and vaping, and discourage the use of e-cigarettes for quitting smoking.
Acknowledgements
This study was supported by grants from the National Cancer Institute of the US (R01 CA100362, P01 CA138389, P01CA200512), the Canadian Institutes of Health Research (MOP 115016, FDN 148477), and by the National Health and Medical Research Council of Australia (APP1106451). Professor Fong was supported by a Senior Investigator Award from the Ontario Institute for Cancer Research. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit for publication.
Footnotes
Declaration of interests
KMC has received payment as a consultant to Pfizer, Inc., for service on an external advisory panel to assess ways to improve smoking cessation delivery in health care settings. KMC also has served as paid expert witness in litigation filed against the tobacco industry. DH, GTF and JFT have served on behalf of governments in response to legal challenges from the tobacco industry. All other authors have no conflicts of interest to declare.
References
- 1.West R, Beard E, Brown J. Trends in electronic cigarette use in England 2017. [Available from: http://www.smokinginengland.info/downloadfile/?type=latest-stats&src=11.
- 2.McMillen RC, Gottlieb MA, Shaefer RMW, Winickoff JP, Klein JD. Trends in electronic cigarette use among US adults: use is increasing in both smokers and nonsmokers. Nicotine & Tobacco Research. 2014;17(10):1195–202. [DOI] [PubMed] [Google Scholar]
- 3.Adkison SE, O’Connor RJ, Bansal-Travers M, Hyland A, Borland R, Yong HH, et al. Electronic nicotine delivery systems: international tobacco control four-country survey. American journal of preventive medicine. 2013;44(3):207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reid JL, Rynard VL, Czoli CD, Hammond D. Who is using e-cigarettes in Canada? Nationally representative data on the prevalence of e-cigarette use among Canadians. Preventive Medicine. 2015;81(Supplement C):180–3. [DOI] [PubMed] [Google Scholar]
- 5.Yong HH, Borland R, Balmford J, McNeill A, Hitchman S, Driezen P, et al. Trends in E-Cigarette Awareness, Trial, and Use Under the Different Regulatory Environments of Australia and the United Kingdom. Nicotine & Tobacco Research. 2015;17(10):1203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNeill A, Brose L, Calder R, Bauld L, Robson D. Evidence review of e-cigarettes and heated tobacco products 2018. A report commissioned by Public Health England. London; 2018. [Google Scholar]
- 7.McNeill A, Brose L, Calder R, Hitchman S, Hajek P, McRobbie H. E-cigarettes: an evidence update A report commissioned by Public Health England. 2015. PHE publications gateway; 2016. [Google Scholar]
- 8.Tobacco Advisory Group of the Royal College of Physicians, editor Nicotine without smoke—tobacco harm reduction. Royal College of Physicians; 2016.
- 9.National Academies of Sciences Engineering & Medicine. Public health consequences of e-cigarettes. 2018.
- 10.Grana RA, Popova L, Ling PM. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Internal Medicine. 2014;174(5):812–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pepper JK, Brewer NT. Electronic nicotine delivery system (electronic cigarette) awareness, use, reactions and beliefs: a systematic review. Tobacco control. 2014;23(5):375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azagba S, Baskerville NB, Foley K. Susceptibility to cigarette smoking among middle and high school e-cigarette users in Canada. Preventive Medicine. 2017;103:14–9. [DOI] [PubMed] [Google Scholar]
- 13.Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho YO, Thrasher J, Swayampakala K, Lipkus I, Hammond D, Cummings KM, et al. Does adding information on toxic constituents to cigarette pack warnings increase smokers’ perceptions about the health risks of smoking? A longitudinal study in Australia, Canada, Mexico, and the United States. . Health Education and Behavior. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond D, Fong GT, McNeill A, Borland R, Cummings KM. Effectiveness of cigarette warning labels in informing smokers about the risks of smoking: findings from the International Tobacco Control (ITC) Four Country Survey. Tobacco control. 2006;15:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swayampakala K, Thrasher JF, Hammond D, Yong H-H, Bansal-Travers M, Krugman D, et al. Pictorial health warning label content and smokers’ understanding of smoking-related risks—a cross-country comparison. Health education research. 2014;30(1):35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thrasher JF, Pérez-Hernández R, Arillo-Santillán E, Barrientos-Gutiérrez I. Hacia el consumo informado de tabaco en México: efecto de las advertencias con pictogramas en población fumadora. salud pública de méxico. 2012;54:242–53. [PMC free article] [PubMed] [Google Scholar]
- 18.Borland R, Yong HH, Wilson N, Fong GT, Hammond D, Cummings KM, et al. How reactions to cigarette packet health warnings influence quitting: findings from the ITC Four-Country survey. Addiction. 2009;104(4):669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho YJ, Thrasher JF, Yong H-H, Szklo AS, O’Connor RJ, Bansal-Travers M, et al. Path analysis of warning label effects on negative emotions and quit attempts: A longitudinal study of smokers in Australia, Canada, Mexico, and the US. Social Science & Medicine. 2018;197:226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thrasher JF, Abad-Vivero EN, Huang L, O’Connor RJ, Hammond D, Bansal-Travers M, et al. Interpersonal communication about pictorial health warnings on cigarette packages: Policy-related influences and relationships with smoking cessation attempts. Social Science & Medicine. 2016;164:141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thrasher JF, Swayampakala K, Cummings KM, Hammond D, Anshari D, Krugman DM, et al. Cigarette package inserts can promote efficacy beliefs and sustained smoking cessation attempts: a longitudinal assessment of an innovative policy in Canada. Preventive medicine. 2016;88:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moodie C, MacKintosh AM, Hammond D. Adolescents’ response to text-only tobacco health warnings: results from the 2008 UK Youth Tobacco Policy Survey. European journal of public health. 2009;20(4):463–9. [DOI] [PubMed] [Google Scholar]
- 23.Partos TR, Borland R, Yong HH, Thrasher J, Hammond D. Cigarette packet warning labels can prevent relapse: findings from the International Tobacco Control 4-Country policy evaluation cohort study. Tobacco control. 2013;22(E1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(HSE) HaSE. GUIDANCE ON E-CIGARETTES AND THE CLP REGULATION – from the Health and Safety Executive (HSE) 2016. [Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/656493/CLP_Regulation_application_to_e-cigarettes_and_e-liquids_-_Final_Version_-_9_December_2016.pdf.
- 25.UK Government. The Tobacco and Related Products Regulations 2016. [Available from: http://www.legislation.gov.uk/uksi/2016/507/regulation/56/made.
- 26.Food & Drug Administration. Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products. Final rule. Federal Register. 2016;81(90):28973. [PubMed] [Google Scholar]
- 27.Durbin R, Waxman H, Harkin T, Rockefeller J, Blumenthal R, Markey E, editors. Gateway to addiction? A survey of popular electronic cigarette manufacturers and targeted marketing to youth. US Congress; 2014. [Google Scholar]
- 28.Gravely S, Hitchman S, McNeil A, Cummings K, Borland R, Yong H, et al. , editors. Use of electronic cigarettes across 13 ITC countries with different regulatory environments. Tobacco induced diseases; 2018: EUROPEAN PUBLISHING SCIENCE & TECHNOLGY PARK CRETE,(STEP-C), N PLASTIRA 100, VASSILIKA VOUTWN, HERAKLION, CRETE 00000, GREECE. [Google Scholar]
- 29.Hammond D, White CM, Czoli CD, Martin CL, Magennis P, Shiplo S. Retail availability and marketing of electronic cigarettes in Canada. Canadian Journal of Public Health/Revue Canadienne de Santé Publique. 2015;106(6):e408–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hitchman SC, Mons U, Nagelhout GE, Guignard R, Mcneill A, Willemsen MC, et al. Effectiveness of the European Union text-only cigarette health warnings: findings from four countries. The European Journal of Public Health. 2011;22(5):693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee C, Yong H-H, Borland R, McNeill A, Hitchman SC. Acceptance and patterns of personal vaporizer use in Australia and the United Kingdom: Results from the International Tobacco Control survey. Drug Alcohol Depen. 2018;185:142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yong HH, Borland R, Balmford J, Hitchman SC, Cummings KM, Driezen P, et al. Prevalence and Correlates of the Belief That Electronic Cigarettes are a Lot Less Harmful Than Conventional Cigarettes Under the Different Regulatory Environments of Australia and the United Kingdom. Nicotine & Tobacco Research. 2017;19(2):258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee YO, Shafer PR, Eggers ME, Kim AE, Parvanta SA, Nonnemaker JM. Effect of a voluntary e-cigarette warning label on risk perceptions. Tobacco Regulatory Science. 2016;2(1):82–93. [Google Scholar]
- 34.Katz SJ, Lindgren B, Hatsukami D. E-cigarettes Warning Labels and Modified Risk Statements: Tests of Messages to Reduce Recreational Use. Tobacco Regulatory Science. 2017;3(4):445–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson ME, Fong GT, Boudreau C, Driezen P, Li G, Gravely S, et al. Methods of the ITC Four Country Smoking and Vaping Survey, Wave 1 (2016). Addiction. 2018;XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Project ITCPE. International Tobacco Control Policy Evaluation Project Methods 2013. [Available from: http://www.webcitation.org/711htw4OJ.
- 37.Project. I. ITC Four Country Smoking and Vaping Survey Wave 1 (2017) Technical Report. . University of Waterloo, Waterloo, Ontario, Canada; Medical University of South Carolina, Charleston, South Carolina, United States; Cancer Council Victoria, Melbourne, Australia; King’s College; London, London, United Kingdom; 2017. [Google Scholar]
- 38.Wadsworth E, McNeill A, Li L, Hammond D, Thrasher J, Yong H-H, et al. Reported exposure to E-cigarette advertising and promotion in different regulatory environments: Findings from the International Tobacco Control Four Country (ITC-4C) Survey. Preventive medicine. 2018;112:130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berry C, Burton S, Howlett E. The Impact of E-Cigarette Addiction Warnings and Health-Related Claims on Consumers’ Risk Beliefs and Use Intentions. J Public Policy Mark. 2017;36(1):54–69. [Google Scholar]
- 40.Berry C, Burton S, Howlett E. Are Cigarette Smokers’, E-Cigarette Users’, and Dual Users’ Health-Risk Beliefs and Responses to Advertising Influenced by Addiction Warnings and Product Type? Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2017;19(10):1185–91. [DOI] [PubMed] [Google Scholar]
- 41.Popova L, Ling PM. Nonsmokers’ responses to new warning labels on smokeless tobacco and electronic cigarettes: an experimental study. BMC public health. 2014;14:997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borland R, Cooper J, McNeill A, O’Connor R, Cummings KM. Trends in beliefs about the harmfulness and use of stop-smoking medications and smokeless tobacco products among cigarettes smokers: Findings from the ITC four-country survey. Harm Reduct J. 2011;8. [DOI] [PMC free article] [PubMed] [Google Scholar]


