Abstract
Sedentary lifestyles and obesity are known risk factors for breast cancer. Elevated estrogen levels correlate with obesity and, independently, with increased breast cancer risk. Lifestyle interventions that reduce obesity may mitigate this risk, potentially via estrogen pathways. In a 6-month lifestyle intervention, overweight/obese women with high breast cancer risk were randomized to control (n = 7) or intervention (n = 6) and analyzed for sex hormone levels. Serum and urine hormones were evaluated by UPLC-MS/MS, and sex hormone binding globulin (SHBG) by ELISA. Serum estrone (E1) and estradiol (E2) were reduced by 12.1% and 50.8%, respectively, at 9 months in the intervention group, which differed from controls (p = 0.043 and 0.020). This contrasted with a 73.3% increase in urine E1 at 6 months in the intervention group (p = 0.035). These results suggest that a lifestyle intervention led to a favorable estrogen profile in relation to breast cancer risk.
Keywords: PUBLIC HEALTH, BREAST NEOPLASMS, WEIGHT LOSS, MINDFULNESS, ESTROGENS
In 2018, an estimated 266,120 new cases of invasive breast cancer are expected to be diagnosed in women in the U.S. (Siegel, Miller, & Jemal, 2018). Risk factors for breast cancer include older age, overweight/obesity, and lack of exercise (Kamińska, Ciszewski, Łopacka-Szatan, Miotła, & Starosławska, 2015). Elevated concentrations of endogenous sex hormones in the context of obesity, in particular estradiol (E2) and estrone (E1), are associated with increased breast cancer risk in postmenopausal women (Key, Appleby, Barnes, & Reeves, 2002). Thus, this pilot study examined the impact of the Diet, Exercise, Emotional processing, and Mindfulness (DEEM) lifestyle intervention on sex hormones within biological pathways relevant to breast cancer development.
Sex Hormones and Breast Cancer Risk
Breast cancer poses a significant public health burden in the United States as the leading non-dermatologic cancer diagnosis among women (DeSantis et al., 2016). Among known risk factors for breast cancer, overweight/obesity and a sedentary lifestyle are thought to account for approximately 25% of cases (McTiernan, 2003). Breast cancer risk is lower in those who engage in regular physical activity (PA), practice healthy lifestyle behaviors (e.g., low alcohol consumption, nutritional diet, abstinence from smoking), and have a lower BMI (World Cancer Research Fund International, 2018; Thomson et al., 2014; Kamińska et al., 2015; Ellingjord-Dale et al., 2018). Studies have demonstrated that women at high risk for breast cancer who engage in moderate PA are at reduced risk of developing breast cancer (Tehard, Friedenreich, Oppert, & Clavel-Chapelon, 2006). While the relationship between static BMI and breast cancer risk is well-understood, studies on the impact of weight loss are more limited, as persistent weight loss is infrequently reported (Wolin & Colditz, 2008).
Of note, hormone-related factors are strongly related to sedentary lifestyles and obesity, which are associated with an elevated risk for developing breast cancer (Key et al., 2002). There is robust evidence linking higher levels of endogenous sex hormones with increased breast cancer risk in pre- and postmenopausal women. A pooled analysis of studies (N = 9) on endogenous sex hormone levels and breast cancer risk in postmenopausal women found a strong positive association of breast cancer risk with sex hormones (Key et al., 2002). Higher serum levels of estradiol (E2), estrone (E1), androstenedione (A-dione), dehydroepiandrosterone (DHEA), and testosterone (T), and lower levels of sex-hormone binding globulin (SHBG), have each been associated with breast cancer risk (Key et al., 2002; Woolcott et al., 2010), particularly for E2 and E1.
It is now understood that excess E2 acts via estrogen receptor (ER) to promote breast cancer tumorigenesis. In the setting of obesity, elevated estrogen production via aromatization of androgens in adipose tissue is thought to mediate the increased risk of breast cancer in women who are overweight/obese (Rose & Vona-Davis, 2014), which also explains the inverse relationship between estrogen levels and PA-induced weight loss (Schmitz et al., 2015). Yet, little is known about the relationship between weight loss and circulating hormones during the timeframe of adiposity loss. It is conceivable that during the period of adiposity loss, circulating estrogens increase due to adipose tissue serving as a source of aromatized estrogens, and thus estrogens are elevated temporarily. It is further unclear that, if this elevation occurs, whether it persists after the period of weight loss ends. Thus, a study that can evaluate the temporal trends in estrogen concentrations during weight loss and weight stabilization is needed.
Conceptual Framework
The theoretical conceptual framework (Figure 1) for the current study is based on Bandura’s Social Cognitive Theory (Bandura, 1986, 2011). Social Cognitive Theory is a learning theory based on the idea that people learn, absorb, process, and retain skills and knowledge during learning with behavioral changes by the reciprocal interactions of personal, behavioral, and environmental factors (Bandura, 1986, 2011). The central concepts include the ability to perform desired behaviors, expectations for the outcomes of such behaviors, self-efficacy, goal-setting, learning through observation, reinforcement, and social support (Bandura, 1986, 2011).
Figure 1. Theoretical framework for the Biobehavioral Study of the DEEM intervention based on Social Cognitive Theory (Bandura, 2011).
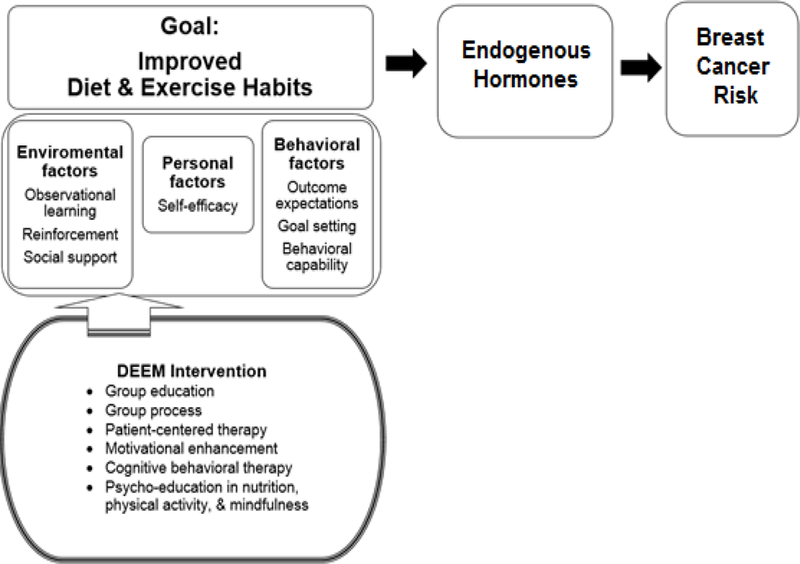
Note. DEEM = Diet, Exercise, Emotional, and Mindfulness.
The current intervention was designed to improve health and reduce breast cancer risk by implementing dietary and exercise modifications for women at high risk for breast cancer. Social Cognitive Theory provided a theoretical framework to inform and guide the core content of the intervention, focusing on improvements in personal, behavioral, and environmental factors and incorporating multiple, comprehensive approaches for behavioral change throughout the intervention.
Purpose
The Diet, Exercise, Emotional processing, and Mindfulness (DEEM) pilot study, an intervention to improve physical activity and dietary habits, aimed to reduce adiposity and achieve sustained weight loss among women at high risk for breast cancer. The goal of this paper is to evaluate changes in sex steroid metabolites over the course of the intervention and at follow-up.
Methods
Study Design and Setting
We conducted a two-arm, randomized pilot study for a 6-month DEEM intervention with follow-up at 9 months (3 months post-intervention). The DEEM intervention has been shown to improve metabolic and inflammatory profiles of overweight/obese women at risk of breast cancer in our previous pilot study (Han et al., 2018). The study was approved by the Fred Hutchinson Cancer Research Center Human Subjects Institutional Review Board and reviewed annually. This study was registered with ClinicalTrials.gov (NCT01874184).
Participants
Participants were women at high risk for breast cancer who were recruited through the Seattle Cancer Care Alliance High-Risk Clinic, Cierra Sisters Breast support group, and other cancer support groups. The methods have been described in detail previously (Han et al., 2018). Women were eligible for inclusion if they (1) were aged 35–65 years; (2) were considered to be at high risk for breast cancer by meeting one of the following criteria: (a) Gail model risk of ≥ 1.7% over 5 years, (b) Claus model lifetime risk of > 20%, (c) International Breast Intervention Study (IBIS), known as Tyrer-Cuzick model, lifetime risk > 20%, (d) personal history of breast biopsy showing atypical ductal hyperplasia, atypical lobular hyperplasia, or lobular carcinoma in situ (LCIS), (e) ductal carcinoma in situ that has been previously treated, (f) deleterious mutation in BRCA1 or BRCA2 or another gene known to increase the risk of developing BC, or (g) risk assessment of ≥ 20% chance of carrying a BRCA1/2 gene mutation; (3) had a body mass index (BMI) of >25 and <45 for premenopausal women and a BMI of >28 and <40 for postmenopausal women; 4) were able to attend weekly group sessions and subsequent clinic visits; 5) could communicate in English; and 6) agreed to random assignment to study groups. Participants were excluded based on the following criteria: 1) regular moderate-to-vigorous activity lasting 90 or more minutes at the time of recruitment; 2) alcohol or drug abuse; 3) previous diagnosis of invasive cancer within the past 5 years (in situ BC or squamous cell carcinoma not included); 4) plans to leave the geographic area within six months; 5) contraindications for treadmill testing or entry into training program; or 6) pregnancy or wish to become pregnant.
A total of 6 women in the intervention arm and 7 in the control arm were used for analysis (Figure 2-a). Randomization was performed through a computer-generated table with stratification based on menopausal status. The staff collecting outcome data were blinded to randomization. After providing informed consent, participants attended a clinic visit for collection of baseline data, questionnaires, fasting blood samples, and urine samples. All assessment data were collected again at 6 months (post-intervention) and 9 months (follow-up) (Figure 2-a).
Figure 2.
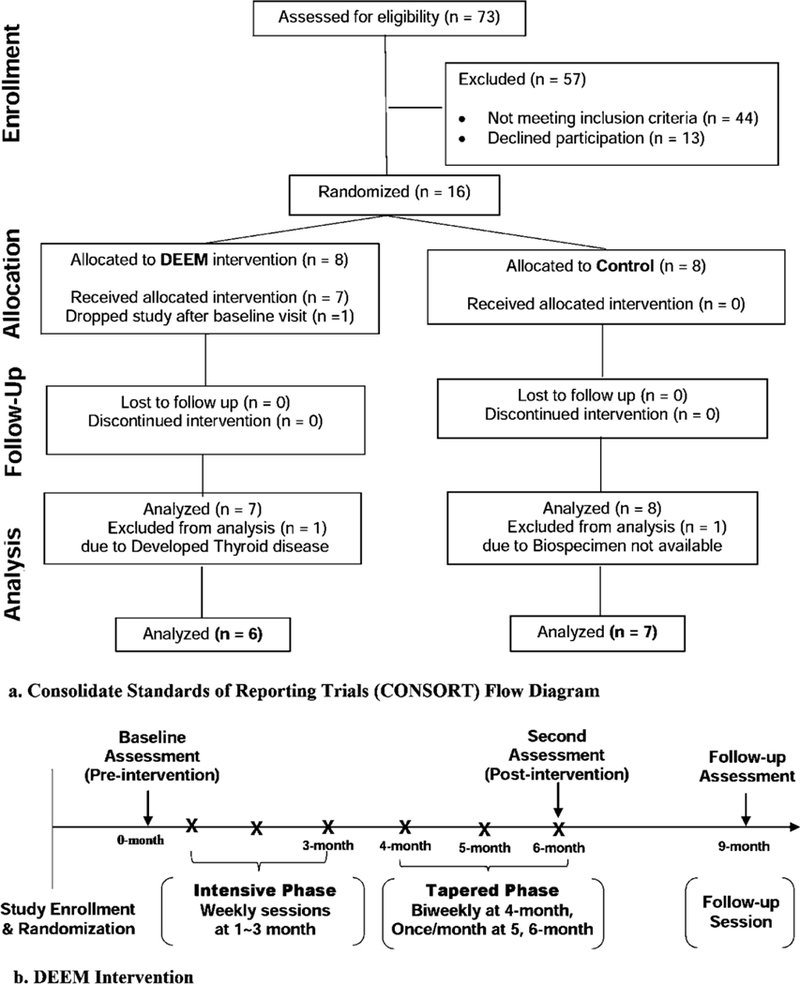
(a) Consolidate Standards of Reporting Trials (CONSORT) Flow Diagram and (b) DEEM intervention study design (Han et al., 2018).
DEEM Intervention
Details of the DEEM intervention are described elsewhere (Han et al., 2018). Briefly, the intervention design was guided by Social Cognitive Theory and the Stages-of-Change Model, utilizing motivational interviewing, patient-centered therapy, and cognitive-behavioral skills training. The intervention consisted of sixteen 2-hour group sessions that incorporated modules on nutrition and exercise education, mindfulness meditation, experiential exercise activities, and open-format emergent processing that reinforced interpersonal connection as well as skills for coping with unhelpful thoughts, emotional discomfort, and initiation of new adaptive behavior (Figure 2-b). The group sessions were led by a Licensed Mental Health Counselor and experts in nutrition, PA, and mindfulness, who facilitated emotional processing in each session. At the end of the intervention, control women were offered 3 supervised exercise sessions at the Fred Hutch Prevention Center exercise gym.
Measures
Baseline data.
Participants completed a 44-item online questionnaire including demographics, clinical characteristics, and general health history including lifestyle habits.
Body composition.
Dual-Energy X-Ray Absorptiometry (DEXA; GE Lunar) was used to assess body composition including body weight and adiposity at baseline, 6 months, and 9 months follow-up (Han et al., 2018).
Sex hormone biomarkers.
Blood and urine samples were collected at baseline, 6 months, and 9 months after an overnight fast of 12 hours. Blood samples were processed within 3 hours of collection and stored at –80°C. Blood sex hormones included serum E1, serum E2, plasma A-dione, plasma DHEA, plasma T, and plasma sex hormone binding globulin (SHBG). Due to the low concentration of detectable levels of plasma E1 and E2, only serum E1 and serum E2 were included for the current study. Urine sex hormones included E1, E2, A-dione, DHEA, and T.
SHBG and urine creatinine were detected by enzyme-linked immunosorbent assay (ELISA; RayBiotech, Norcross, GA) using a VersaMax analyzer (Molecular Devices, Sunnyvale, CA). Intra-assay and inter-assay coefficients of variation (CV) for creatinine were 12.0–16.3% and 6.2%, respectively, and the intra-assay CV for SHBG ranged from 3.5–5.7%. Urine metabolites were normalized to urinary creatinine for analysis.
Blood and urine concentrations of E1, E1, A-dione, DHEA, and T were determined by ultraperformance liquid chromatography (UPLC)/tandem mass spectrometry (MS/MS). Reference and internal standards were purchased from Steraloids (Newport, RI) and Sigma-Aldrich (St. Louis, MO) and used to optimize run conditions before analysis. An internal standard mixture containing d2–17β-estradiol, C13-estrone, d3-androstenedione, d5-DHEA, and d3-testosterone was added to all samples. Calibration curve and quality control standards were prepared in mock urine. Samples were subjected to liquid-liquid extraction with diethyl ether and injected on an Accucore column (2.1 × 100 mm, 2.6 μm) (Thermo Fisher Scientific, Grand Island, NY) at a flow rate of 0.5 ml/min for a total run time of 18 minutes, using 0.1% formic acid in water and methanol:acetonitrile (1:1) as mobile phases. Analytes were detected by UPLC-MS/MS using electrospray ionization (ESI) in positive ion mode on a Xevo TQ-S triple quadrupole mass spectrometer (Waters, Milford, MA). Intra-batch CV ranged from 2.6–12.2% for urine samples and 1.0–5.8% for blood samples. Data were processed using MassLynx 4.1 and QuanLynx software. Lab personnel were blinded to study group for all analyses.
Statistical Analysis
The primary analysis examined the effect of the DEEM intervention on blood and urine sex hormone profiles (from baseline to 6 months, and from baseline to 9 months). Fisher’s exact test was used for categorical variables in group comparisons of demographic and clinical characteristics. For group comparisons (interventions vs. controls) with a small sample size, a Kruskal-Wallis test was used to test the significance of group differences in continuous variables of demographic and clinical data and sex hormone profiles. We controlled for the baseline values of sex hormones for the comparisons of sex hormone variables between the intervention and control groups. We first compared changes in sex hormones from baseline to 6 months and from baseline to 9 months between the two groups. Then we compared the differences of post-intervention values at each time point between the two groups.
For post-hoc analysis, correlations between the changes in sex hormones and body composition from baseline to 6 months and from baseline to 9 months were examined using a Spearman rank correlation. The statistically significant threshold was set at 0.05 based on the rank-transformed data. To maintain statistical power with a small sample size, we did not adjust for other possible covariates such as age, BMI, menopausal status, or family history of breast cancer. Analyses were performed using SPSS version 19.0 for Windows.
Results
Demographic and Clinical Characteristics
As seen in the CONSORT flow diagram (Figure 2-a), 16 participants were randomized, and outcome data were available on 13 participants. Demographic and clinical characteristics are presented in Table 1. There were no statistically significant differences in age (64.5 ± 5.2 y.o. vs. 55.4 ± 5.2 y.o., p = 0.762) or body composition (body weight: 89.7 ± 10.0 kg vs. 84.0 ± 7.2 kg, p = 0.211; BMI: 34.9 ± 4.4 kg/m2 vs. 31.2 ± 4.0 kg/m2, p = 0.412; % adiposity: 48.0 ± 5.0 vs. 45.6 ± 4.4, p = 0.847) between the intervention and control groups, respectively.
Table 1.
Baseline comparisons of participant characteristics in women at high risk of breast cancer (N =13).
| Baseline Characteristics | Intervention | Control | pa | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Race/Ethnicity | Non-Hispanic White | 4 | 66.7 | 4 | 57.1 | .835 |
| Non-Hispanic Black | 2 | 33.3 | 2 | 28.6 | ||
| American Indian/Alaskan Native |
0 | 0.0 | 1 | 14.3 | ||
| Education | ≥ College | 6 | 100.0 | 7 | 100.0 | .985 |
| Smoking | Never | 6 | 100.0 | 7 | 100.0 | .956 |
| Healthy dietary habit | Never | 3 | 50.0 | 2 | 28.6 | .789 |
| Ever | 3 | 40.0 | 5 | 71.4 | ||
| Frequency of physical activity | Never | 2 | 33.3 | 2 | 28.6 | .763 |
| Ever | 4 | 66.7 | 5 | 71.4 | ||
| Ductal carcinoma in situ | Yes | 1 | 14.3 | 1 | 16.7 | .923 |
| Gail model risk score | ≥ 1.7 % over 5 years | 5 | 83.3 | 5 | 71.4 | .786 |
Note. SD = standard deviation.
Testing for baseline differences between intervention (n = 6) and control (n = 7).
The majority of participants identified as non-Hispanic white (66.7% vs. 57.1%), non-smokers (100%), and college graduates (100%). One participant each in each the control and intervention groups were pre-menopausal, with samples collected at mid-luteal phase of the menstrual cycle, and the remaining 11 participants were post-menopausal. None of the participants were receiving medication containing hormones or blocking hormones (e.g., aromatase inhibitors) at the time of study. There were no significant differences in demographic features, clinical characteristics, or diet and exercise behaviors between the intervention and control groups.
Blood Sex Hormones
In Table 2, the blood sex hormones (E2, E1, A-dione, DHEA, T, and SHBG levels) were compared at each time point (baseline, 6 months, and 9 months) between the intervention and control groups. Baseline blood sex hormone levels did not differ between the intervention and control groups. In the intervention group, serum E1 decreased by 12% at 9 months, but no change (0%) was observed in the control group (p = 0.043). Serum E2 also decreased 51% in the intervention group at 9 months, while it increased 8% in the control group (p = 0.020). When we compared the post-intervention concentrations at 9 months, serum E1 and E2 concentrations were significantly lower in the intervention group compared to the control group (p = 0.048, and 0.039, respectively) (Table 4). Changes in blood sex hormones (from baseline to 6 months and from baseline to 9 months) other than E1 and E2 did not differ between the two groups over time.
Table 2.
Blood sex hormones in intervention and control groups (N = 13).
| Blood Sex | Baseline (M ± SD) | 6 months (M ± SD, % changea) | 9 months (M ± SD, % changea) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hormones | DEEM | Control | pb,d | DEEM | Control | pcd | DEEM | Control | pc,d |
| Serum E1, pg/ml | 21.5 ± 6.6 | 40.4 ± 14.2 | .176 | 19.2 ± 1.9 (−10.7%) | 40.7 ± 14.8 (0.7%) | .142 | 18.9 ± 1.6 (−12.1%) | 40.4 ± 24.5 (0.0%) | .043 |
| Serum E2, pg/ml | 6.3 ± 3.3 | 24.2 ± 17.2 | .339 | 12.9 ± 12.8 (104.8%) | 38.7 ± 31.2 (59.9%) | .143 | 3.1 ± 4.7 (−50.8%) | 26.2 ± 51.3(8.2%) | .020 |
| A-dione, ng/ml | 0.5 ± 0.1 | 0.5 ± 0.2 | .751 | 0.79 ± 0.3 (35.5%) | 0.59 ± 0.1 (23.6%) | .657 | 0.4 ± 0.1 (−6.8%) | 0.658 ± 0.1 (0.3%) | .352 |
| DHEA, ng/ml | 2.6 ± 0.4 | 3.5 ± 1.8 | .264 | 3.1 ± 1.3 (20.3%) | 3.11 ± 0.7 (−11.1%) | .456 | 2.2± 0.2 (−14.0%) | 3.0 ± 0.7 (−0.1%) | .517 |
| T, ng/ml | 0.2 ± 0.1 | 0.2 ± 0.1 | .553 | 0.3 ± 0.1 (20.8%) | 0.2 ± 0.1 (0.0%) | .954 | 0.2 ± 0.1 (−1.7%) | 0.2 ± 0.1 (0.3%) | .440 |
| SHBG, nM | 173.1 ± 13.3 | 162.3 ± 9.7 | .864 | 164.4 ± 13.9 (−5.7%) | 174.7 ± 9.9 (0.1%) | .172 | 160.0 ± 10.6 (0.8%) | 136.6 ± 7.2 (−0.2%) | .785 |
Note. M = mean; SD = standard deviation.
A-dione = androstenedione; DHEA = dehydroepiandrosterone; E1 = estrone; E2 = estradiol; SHBG = sex-hormone binding globulin;
T = testosterone.
% changes from mean at baseline to each time point (at 6 months and at 9 months).
Testing the differences of baseline between DEEM intervention and control groups.
Testing the differences of changes in blood sex hormones from baseline to each time point (at 6 months and at 9 months) between DEEM and control groups, controlling for baseline sex hormones.
p < 0.05, p-values were determined with rank-transformation using a Kruskal-Wallis test.
Table 4.
Differences in blood sex hormones at each time point between intervention and control groups (N = 13).
| Blood Sex | Baseline (M ± SD) | 6 months (M ± SD) | 9 months (M ± SD) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hormones | DEEM | Control | pa,c | DEEM | Control | pb,c | DEEM | Control | pb,c |
| Serum E1, pg/ml | 21.5 ± 6.6 | 40.4±14.2 | .439 | 19.2 ± 1.9 | 40.7 ± 14.8 | .439 | 18.9 ± 1.6 | 40.4 ± 24.5 | .048 |
| Serum E2, pg/ml | 6.3 ± 3.3 | 24.2±17.2 | .137 | 12.9 ± 12.8 | 38.7 ± 31.2 | .143 | 3.1 ± 4.7 | 26.2 ± 51.3 | .039 |
| A-dione, ng/ml | 0.51 ± 0.1 | 0.5 ± 0.2 | .751 | 0.6 ± 0.3 | 0.5 ± 0.1 | .775 | 0.4 ± 0.1 | 0.5 ± 0.1 | .475 |
| DHEA, ng/ml | 2.6 ± 0.4 | 3.5 ± 1.8 | .253 | 3.1 ± 1.3 | 3.1 ± 0.7 | .668 | 2.1 ± 0.2 | 3.0 ± 0.7 | .063 |
| T, ng/ml | 0.2 ± 0.1 | 0.2 ± 0.1 | .775 | 0.2 ± 0.1 | 0.2 ± 0.1 | .520 | 0.2 ± 0.1 | 0.2 ± 0.1 | .999 |
| SHBG, nM | 173.1 ± 13.3 | 162.3 ± 9.7 | .775 | 164.4 ± 13.9 | 174.7 ± 9.9 | .568 | 160.0 ± 10.6 | 136.6 ± 7.2 | .886 |
Note. M = mean; SD = standard deviation.
A-dione = androstenedione; DHEA = dehydroepiandrosterone; E1 = estrone; E2 = estradiol; SHBG = sex-hormone binding globulin;
T = testosterone.
Testing the differences of baseline between DEEM intervention and control groups.
Testing the differences in blood sex hormones at each time point (6 months and 9 months) between DEEM and control groups, controlling for baseline outcome variables.
p < 0.05, p-values were determined with rank-transformation using a Kruskal-Wallis test.
Urine Sex Hormones
In Table 3, the urine sex hormones (E2, E1, A-dione, DHEA, and T levels) were compared between intervention and control groups at baseline, 6 months, and 9 months. Baseline urine sex hormone levels did not differ between the intervention and control groups. Urinary E1 increased 73% in the intervention group at 6 months, which differed significantly from a 55% decrease in the control group (p = 0.035). Urinary E2 levels also increased in the intervention group (20%) and decreased in controls (–10%) at 6 months but did not reach statistical significance (p = 0.085) (Table 3). When we compared the post-intervention value of urinary E1 at 6 months, urinary E1 levels were higher in the intervention group compared to the control group at 6 months (p = 0.043) (Table 5). No significant differences in changes in other urine sex hormones were observed between the two groups at 6 or 9 months (Table 3).
Table 3.
Urine sex hormones in intervention and control groups (N = 13).
| Urine Sex | Baseline (M ± SD) | 6 months (M ± SD, % changea) | 9 months (M ± SD, % changea) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hormones | DEEM | Control | pb,d | DEEM | Control | pc,d | DEEM | Control | pc,d | |
| Urine E1, ng/dl | 1.1 ± 0.1 | 2.9 ± 0.2 | .212 | 2.6 ± 0.3 (73.3%) | 1.3 ± 0.2 (−55.2%) | .035 | 1.5 ± 1.1 (36.4%) | 2.5 ± 2.0 (−13.8%) | .253 | |
| Urine E2, ng/dl | 1.6 ± 0.1 | 1.1 ± 0.8 | .365 | 1.9 ± 0.1 (20.3%) | 1.0 ± 0.1 (−9.9%) | .085 | 1.6 ± 1.6 (6.4%) | 1.3 ± 1.6 (24.8%) | .140 | |
| A-dione, ng/dl | 47.1 ± 4.3 | 62.9 ± 6.0 | .622 | 48.3 ± 4.8 (2.5%) | 54.2 ± 3.4 (−13.8%) | .808 | 65.1 ± 6.0 (38.3%) | 54.2 ± 3.4 (−13.8%) | .685 | |
| DHEA, ng/dl | 323.6 ± 33.7 | 97.2 ± 3.3 | .215 | 42.4 ± 3.5 (−87%) | 60.4 ± 3.2 (−38.0%) | .462 | 190.1 ± 2.5 (−137.6%) | 60.4 ± 3.2 (−38.0%) | .626 | |
| T, ng/dl | 2.0 ± 0.2 | 3.6 ± 0.3 | .305 | 2.20 ± 0.2 (10.0%) | 2.9 ± 0.3 (−19.0%) | .935 | 3.1 ± 0.3 (31%) | 2.0 ± 0.2 (−19.4%) | .935 | |
Note. M = mean; SD = standard deviation.
A-dione = androstenedione; DHEA = dehydroepiandrosterone; E1 = estrone; E2 = estradiol; T = testosterone.
% changes from mean at baseline to each time point (at 6 months and at 9 months).
Testing the differences of baseline between DEEM intervention and control groups.
Testing the differences of changes in urine sex hormones from baseline to each time point (at 6 months and at 9 months) between DEEM and control groups, controlling for baseline sex hormones.
p < 0.05, p-values were determined with rank-transformation using a Kruskal-Wallis test.
Table 5.
Differences in urine sex hormones at each time point between intervention and control groups (N = 13).
| Urine Sex | Baseline (M ± SD) | 6 months (M ± SD) | 9 months (M ± SD) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hormones | DEEM | Control | pa,c | DEEM | Control | pb,c | DEEM | Control | pb,c |
| Urine E1, ng/dl | 1.1 ± 0.1 | 2.9 ± 0.2 | .062 | 2.3 ± 0.3 | 1.3 ± 0.2 | .043 | 1.5 ± 1.1 | 2.5 ± 2.0 | .617 |
| Urine E2, ng/dl | 1.6 ± 0.1 | 1.0 ± 0.8 | .061 | 1.8 ± 0.1 | 0.9 ± 0.1 | .099 | 1.6 ± 1.6 | 1.3 ± 1.6 | .317 |
| A-dione, ng/dl | 47.1 ± 4.3 | 62.8 ± 6.0 | .465 | 48.3 ± 4.8 | 54.1 ± 3.4 | .568 | 65.1 ± 6.0 | 54.1 ± 3.4 | .668 |
| DHEA, ng/dl | 323.6 ± 33.7 | 97.1 ± 3.3 | .817 | 42.4 ± 3.5 | 60.3 ± 3.2 | .174 | 190.0 ± 2.5 | 60.3 ± 3.2 | .153 |
| T, ng/dl | 1.9 ± 0.2 | 3.5 ± 0.3 | .167 | 2.2 ± 0.2 | 2.9 ± 0.3 | .352 | 3.0 ± 0.3 | 2.8 ± 0.2 | .886 |
Note. M = mean; SD = standard deviation.
A-dione = androstenedione; DHEA = dehydroepiandrosterone; E1 = estrone; E2 = estradiol; T = testosterone.
Testing the differences of baseline between DEEM intervention and control groups.
Testing the differences in urine sex hormones at each time point (6 months and 9 months) between DEEM and control groups, controlling for baseline outcome variables.
p < 0.05, p-values were determined with rank-transformation using a Kruskal-Wallis test.
Post-Hoc Analysis
Body weight, adiposity, and WC decreased over time in the intervention group as we previously reported (Han et al., 2018). The current study shows patterns of reduced serum E1 and/or E2 and increased urine E1 and/or E2 over time in the intervention group (Figure 3). This result indicates that serum E1/E2 and urine E1/E2 changed over time in opposite directions, suggesting a possible relationship between changes in serum E1/E2 levels, urine E1/E2 levels, and body composition (Figure 3). Therefore, we conducted a post-hoc analysis examining associations between changes in body composition and changes in blood E1and E2 levels (Table 6) and urine E1 and E2 levels (Figure 4) in all participants.
Figure 3. Changes in body composition and serum and urine estrogens in the intervention and control groups.
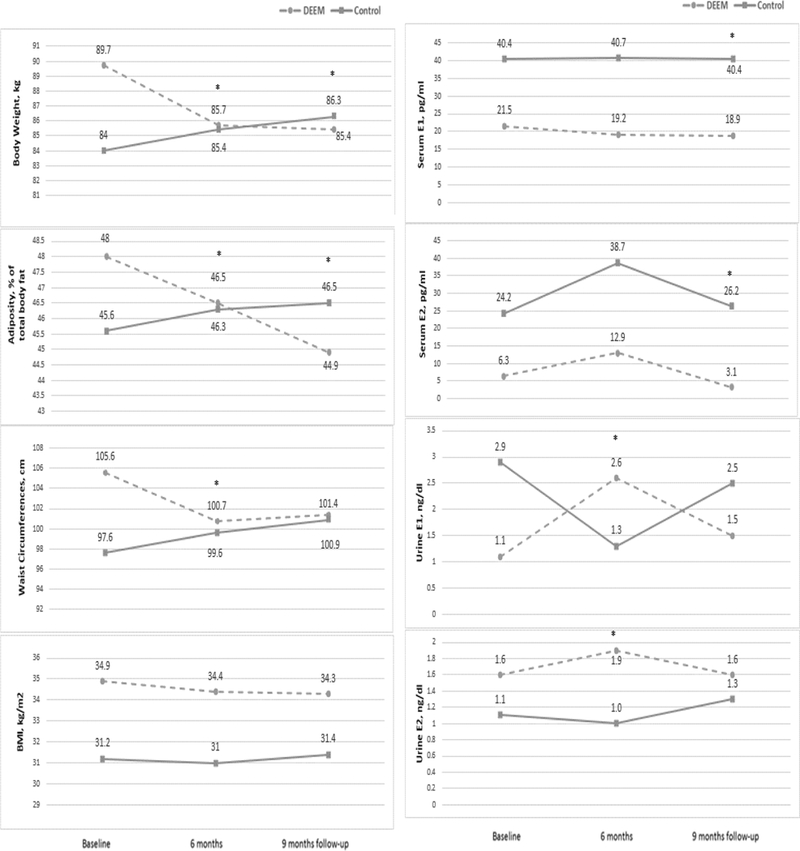
E1 = estrone; E2 = estradiol; * indicates statistically significant differences in changes of each outcome variable between the intervention and control groups (p < 0.05).
Table 6.
Correlations between changes in body composition (body weight/adiposity) and changes in blood estrone (E1) and estradiol (E2) (N = 13).
| Hormone changes from baseline to 6 months | Hormone changes from baseline to 9 months | |||
|---|---|---|---|---|
| Serum estrone (E1) | Serum estradiol (E2) | Serum estrone(E1) | Serum estradiol (E2) | |
| Body composition changes from baseline to 6 months | ||||
| Body Weight | .685** | .471 | .261 | .568 |
| Adiposity | .458 | .486* | .201 | .415 |
| Body Mass Index (BMI) | -.225 | -.327 | .064 | -.490 |
| Waist Circumference (WC) | .125 | -.029 | .584* | .284 |
| Body composition changes from baseline to 9 months | ||||
| Body Weight | NA | NA | .322 | .167 |
| Adiposity | NA | NA | .418 | .274 |
| Body Mass Index (BMI) | NA | NA | .125 | .089 |
| Waist Circumference (WC) | NA | NA | .591* | .420 |
Note. NA = not applicable. Data were presented with Spearman’s rank correlation coefficient (i.e., Spearman’s rho). For this post-hoc analysis, we combined control and intervention groups (N = 13). p-values were determined using rank-transformation using a Spearman correlation test with adjustments for baseline values of body composition and serum E1 and E2.
p < 0.05,
p < 0.01
Figure 4. Relationship between the change in estrone excretion and adiposity loss at 6 months.
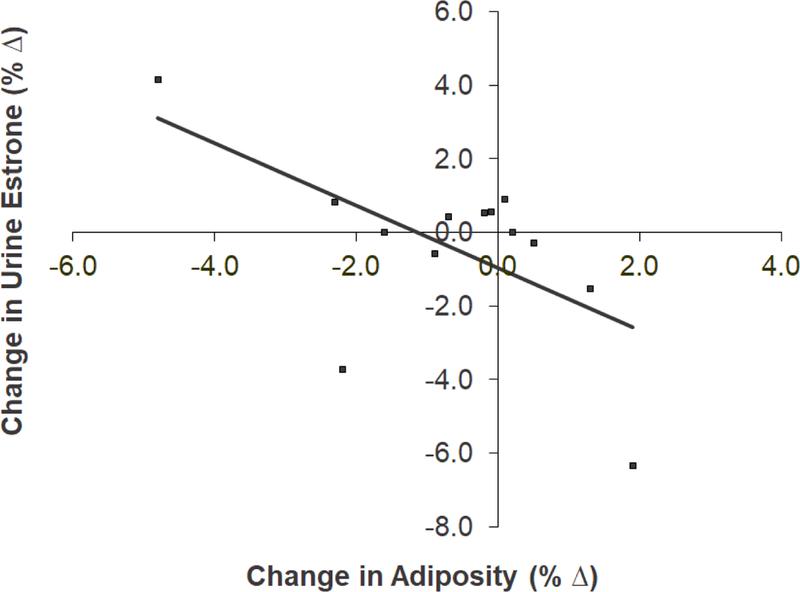
A statistically significant negative correlation (r = −0.592, N = 13, p = 0.032) was found between the change in urinary estrone excretion and change in adiposity at 6 months.
In Table 6, significant positive correlations were observed between serum E1 and body weight changes (rho = 0.685, p < 0.01) and between E2 and adiposity changes (rho = 0.486, p < 0.05) from baseline to 6 months. There were also significant correlations between serum E1 changes (from baseline to 9 months) and changes in waist circumference (WC) (rho = 0.584, p < 0.05 from baseline to 6 months, and rho = 0.591, p < 0.05 from baseline to 9 months). By merging intervention and control groups (N = 13) in analyzing the relationships between urine estrogen and body composition changes, an inverse relationship was found between changes in adiposity and urinary E1 excretion at 6 months (r = –0.592, N = 13, p = 0.032) (Figure 4). None of the relationships were significant between other body composition variables (i.e., body weight, BMI, and WC) and urine E1 or E2 levels (data not shown).
Discussion
Taken together, these results show that a reduction in adiposity was accompanied by a reduction in serum estrogen levels. Over the course of this study, reductions in serum E1 and E2 levels correlated with weight and adiposity loss, which is consistent with evidence in the literature (Ennour-Idrissi, Maunsell, & Diorio, 2015). Prior studies have shown the success of 6- to 12-month diet and exercise interventions in promoting weight loss in women in tandem with reductions in blood E1 and E2 levels (Stolzenberg-Solomon et al., 2012; Campbell et al., 2012). As lower estrogen levels reduce the risk of developing breast cancer, this intervention holds promise as a low-risk protocol that could promote healthful biometric and hormonal changes in women at heightened risk for breast cancer.
Importantly, statistically significant drops in serum E1 and E2 levels were not detected during the time of active adiposity loss at the 6-month follow-up, but rather were detected during a period of weight stability following the intervention at the 9-month follow-up. Due to the transient nature of hormones and the time required for endocrine modulation as a result of weight loss, it may be difficult to detect changes in estrogen concentrations in the blood in the short-term. However, after a period of weight stability, estrogen levels are more likely to have stabilized, and measurements may more accurately reflect the new hormonal profile in the long-term. This is supported by the timing of hormonal changes captured in this study. Thus, to promote stable, lasting reductions in serum estrogens, a long-term intervention followed by a period of weight stability is more likely to be successful than is a short-term intervention.
Although the results did not demonstrate a direct relationship between blood and urine estrogen levels, there is the interesting possibility that at the time of active adiposity loss, estrogen stored in adipose tissue is liberated via lipolysis into circulation and metabolized for urinary excretion, and that this might be responsible for a transient elevation in urine E1 and E2 levels. In fact, our findings indicate that urine E1 levels were inversely correlated with adiposity loss among all groups at the time of active adiposity loss. As a result, it seems reasonable to speculate that urinary excretion of estrogen may have been accelerated during the time of adipose shrinkage, resulting in estrogens collecting in the urine even while serum levels were lowered or maintained. While serum estrogens tend to correlate modestly with urine estrogen levels, urinary excretion patterns are known to vary based on stages of the menstrual cycle, routes of excretion, and estrogen metabolism pathways (Maskarinec, Beckford, Morimoto, Franke, & Stanczyk, 2015), the last of which is altered by physical activity (Dallal et al., 2016).
The strengths of the DEEM intervention are its design as a two-arm randomized controlled trial, collection of multiple biospecimen types at multiple timepoints, as well as the use of reliable biomarker assays. The study is limited, however, by a small sample size and thus is underpowered to detect subtler hormonal changes. Due to the pilot nature of this study, it remains possible that the randomization of this small sample and the absence of adjustment for confounders did not adequately control for the presence of confounding. Future work should focus on the timing of hormonal changes concurrent with adiposity and weight loss, with long-term follow-up on biomarker outcomes in a larger sample. One of the aims of the DEEM intervention was to create sustainable dietary and exercise changes with the intention of supporting long-term commitment to these lifestyle changes and, ultimately, reduced cancer risk. Thus, this enabled our research study to examine weight maintenance during the post-intervention period.
The significance of this study is the prospective assessment of both serum and urinary hormone concentrations during the time of weight and adiposity loss. The DEEM intervention relied upon a conceptual framework rooted in Social Cognitive Theory with the direct aim of addressing the personal, behavioral, and environmental factors required for sustainable behavioral change and, consequently, adiposity loss and endogenous hormonal changes. The success of this approach is evidenced by the improvements in body composition—namely, weight and adiposity loss—and the associated reductions in blood estrogen levels, which could infer a lowering of breast cancer risk. Interestingly, reductions in blood estrogen were accompanied by elevated urine estrogen concentrations, suggesting that enhanced urine excretion of estrogens may be a natural consequence of fat loss during adipose turnover.
By focusing on behavioral modification, the DEEM intervention demonstrates the promise of promoting healthy lifestyle behaviors that can maintain body fat loss (e.g., body weight, adiposity, WC) and may reduce estrogen exposure over the long-term. Diet- and exercise-centered lifestyle interventions not only are less costly and invasive, but also potentially more effective in reducing cancer mortality than are other treatments by protecting against carcinogenesis, via reductions in blood estrogens, before it occurs.
Acknowledgements
Funding: This work was supported by the National Institute of Nursing Research R00 NR012232. Claire Han is supported by the National Health Institute (NIH) National Cancer Institute (NCI) T32 training grant T32 CA092408–27. This study was registered as a clinical trial (ClinicalTrials.gov #NCT01874184).
Footnotes
Declaration of Conflicting Interests
The Authors declare that there is no conflict of interest.
Contributor Information
Sophia A. Stone, MD/PhD Fellow, Medical Scientist Training Program, University of Washington, Seattle, WA, sophia-stone@outlook.com
Claire J. Han, Postdoctoral Fellow, BCPT cancer fellowship (Biobehavioral Cancer Prevention and Control Training Program) University of Washington, Depts of Public Health and Health Service, Fred Hutchinson Cancer Research Center, Seattle. WA, jyh0908@uw.edu.
Taurence Senn, Research Scientist, Dept. of Medicinal Chemistry, University of Washington, Seattle, WA, tarheel@uw.edu.
Larissa A. Korde, Associate Professor, Medical Oncology, Univ. of Washington School of Medicine, Seattle, WA, lkorde@uw.edu.
Kristen Allott, Consultant, Dynamic Paths, Inc., Tacoma, WA, allott@dynamicpaths.com.
Scott Reding, Executive Director, Integrative Counseling Services, Seattle, WA, sreding@icswa.com.
Dale Whittington, Mass Spectrometry Lab Manager, Dept. of Medicinal Chemistry, University of Washington, Seattle, WA, dalewhit@uw.edu.
References
- Bandura A (1986). Social foundations of thought and action: A social cognitive theory. Rockville MD: Prentice-Hall. [Google Scholar]
- Bandura A (2011). Social cognitive theory In van Lange PAM, Kruglanski AW, & Higgins ET (Eds.). Handbook of social psychological theories. (pp. 349–373). London, UK: Sage. [Google Scholar]
- Campbell KL, Foster-Schubert KE, Alfano CM, Wang CC, Wang CY, Duggan CR, … McTiernan A (2012). Reduced-calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: Randomized controlled trial. Journal of Clinical Oncology, 30(19), 2314–2326. doi:10.1200/JCO.2011.37.9792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallal CM, Brinton LA., Matthews CE, Pfeiffer RM, Hartman TJ, Lissowska J,…Gierach GL (2016). Association of active and sedentary behaviors with postmenopausal estrogen metabolism. Medicine & Science in Sports & Exercise, 48(3), 439–448. doi:10.1249/MSS.0000000000000790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis CE, Fedewa SA, Sauer AG, Kramer JL, Smith RA, & Jemal A (2016). Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA: A Cancer Journal for Clinicians, 66(1), 31–42. doi:10.3322/caac.21320 [DOI] [PubMed] [Google Scholar]
- Ellingjord-Dale M, Vos L, Hjerkind KV, Hjartåker A, Russnes HG, Tretli S, … Ursin G (2018). Number of risky lifestyle behaviors and breast cancer risk. JNCI Cancer Spectrum, 2(3), pky030. doi:10.1093/jncics/pky030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennour-Idrissi K, Maunsell E, & Diorio C (2015). Effect of physical activity on sex hormones in women: A systematic review and meta-analysis of randomized controlled trials. Breast Cancer Research, 17(1), 139. doi:10.1186/s13058–015-0647–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CJ, Korde LA, Reding S, Allott K, Van Doren M, Schwarz Y, … Reding KW. (2018). Investigation of a lifestyle intervention in women at high risk of breast cancer. Western Journal of Nursing Research, 40, 976–996. doi:10.1177/0193945917697227 [DOI] [PubMed] [Google Scholar]
- Kamińska M, Ciszewski T, Łopacka-Szatan K, Miotła P, & Starosławska E (2015). Breast cancer risk factors. Menopause Review, 14(3), 196–202. doi:10.5114/pm.2015.54346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key T, Appleby P, Barnes I, & Reeves G (2002). Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studies. Journal of the National Cancer Institute, 94(8), 606–616. doi:10.1093/jnci/94.8.606 [DOI] [PubMed] [Google Scholar]
- Maskarinec G, Beckford F., Morimoto Y., Franke AA, & Stanczyk FZ. (2015). Association of estrogen measurements in serum and urine of premenopausal women. Biomarkers in Medicine, 9(5), 417–424. doi:10.2217/bmm.15.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTiernan A (2003). Behavioral risk factors in breast cancer: Can risk be modified? The Oncologist, 8(4), 326–334. doi:10.1634/theoncologist.8–4-326 [DOI] [PubMed] [Google Scholar]
- Rose DP, & Vona-Davis L (2014). Biochemical and molecular mechanisms for the association between obesity, chronic inflammation, and breast cancer. Biofactors. 40(1), 1–12. doi:10.1002/biof.1109 [DOI] [PubMed] [Google Scholar]
- Schmitz KH, Williams NI, Kontos D, Domchek S, Morales KH, Hwang WT, … Good J. (2015). Dose-response effects of aerobic exercise on estrogen among women at high risk for breast cancer: A randomized controlled trial. Breast Cancer Research and Treatment, 154(2), 309–318. doi:10.1007/s10549–015-3604-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, & Jemal A (2018). Cancer statistics, 2018. CA: A Cancer Journal for Clinicians, 68(1), 7–30. doi:10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- Stolzenberg-Solomon RZ, Falk RT, Stanczyk F, Hoover RN, Appel LJ, Ard JD, … Katki H. (2012). Sex hormone changes during weight loss and maintenance in overweight and obese postmenopausal African-American and non-African-American women. Breast Cancer Research, 14(5), R141. doi:10.1186/bcr3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehard B, Friedenreich CM, Oppert JM, & Clavel-Chapelon F (2006). Effect of physical activity on women at increased risk of breast cancer: Results from the E3N cohort study. Cancer Epidemiology, Biomarkers and Prevention, 15(1), 57–64. doi:10.1158/1055–9965.EPI-05–0603 [DOI] [PubMed] [Google Scholar]
- Thomson CA, McCullough ML, Wertheim BC, Chlebowski RT., Martinez ME., Stefanick ML., … Neuhouser ML. (2014). Nutrition and physical activity cancer prevention guidelines, cancer risk, and mortality in the Women’s Health Initiative. Cancer Prevention Research, 7(1), 42–53. doi:10.1158/1940–6207.CAPR-13–0258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin KY, & Colditz GA (2008). Can weight loss prevent cancer? British Journal of Cancer 99(7), 995–999. doi:10.1038/sj.bjc.6604623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolcott CG, Shvetsov YB, Stanczyk FZ., Wilkens LR., Henderson BE., Le Marchand L., … Goodman MT. (2010). Plasma sex hormone concentrations and the risk of breast cancer in postmenopausal women: The Multiethnic Cohort Study. Endocrine-Related Cancer, 17(1), 125–134. doi:10.1677/ERC-09–0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Cancer Research Fund International. (2018). Cancer preventability estimates for diet, nutrition, body fatness, and physical activity. Retrieved from http://www.wcrf.org/cancer-preventability-estimates


