Abstract
Systemic amyloidosis is rarely reported among cetaceans, and a surveillance dedicated for its occurrence across a certain geographic location has not been reported. Between 2013 and 2018, comprehensive gross and histopathologic examinations were conducted on 54 animals comprising 11 species of stranded and bycaught cetaceans in Hokkaido, Japan. Systemic amyloidosis was diagnosed in 2 out of 3 Stejneger’s beaked whales (Mesoplodon stejnegeri), through Congo red staining and immunohistochemistry for amyloid A. The kidney and gastrointestinal tract had the largest amounts of amyloid deposits, representing a previously undescribed organ distribution in the species. The current study demonstrates the possibility of Stejneger’s beaked whales being prone to the development of systemic amyloidosis, and highlights the need for further investigations.
Keywords: epidemiology, pathology, prevalence, Stejneger’s beaked whale, systemic amyloidosis
Amyloidosis refers to a collection of debilitating proteopathies in which misfolded amyloid fibrils accumulate and deposit in extracellular spaces of organs and tissues [4]. There are about 10 types of amyloid fibril proteins recognized in animals to date, each having a distinct precursor protein [15]. Deposition can either be systemic or localized, and out of the 6 reported systemic types in animals, the predominant form is amyloid A (AA) amyloidosis [15]. With AA amyloidosis, an increase in the circulating acute phase protein serum amyloid A (SAA) level triggers the accumulation and subsequent deposition of AA in tissues, usually following chronic inflammation [4]. The disease has been diagnosed occasionally in a variety of species, both domestic and wild [5, 7, 9, 18]. In wild mammals, the island foxes (Urocyon littoralis) are known to show an unusually high prevalence of AA amyloidosis at the population/species level, signifying potential detrimental effects to the species’ survival [5].
Marine mammals are no exception to being affected by systemic amyloidosis. The condition has been reported sporadically in a total of 3 Stejneger’s beaked whales (Mesoplodon stejnegeri) [14, 16], while a more in-depth study has been conducted in bottlenose dolphins (Tursiops truncatus) [2] and California sea lions (Zalophus californianus) [1]. Out of these 3 species, stranding records have indicated that Stejneger’s beaked whales are the only regular residents around Hokkaido, Japan. Although the previous cases of amyloidosis in this species have been reported in detail especially on the histopathology of selected organs [14, 16], a thorough investigation on its tissue tropism has not been described. Furthermore, epidemiologic assessments of amyloidosis among various species of marine mammals occurring in a certain geographic location through comprehensive gross and histopathologic examinations have never been conducted. The objective of this study was to investigate the incidence of amyloidosis in cetaceans around Hokkaido, Japan, where prior information on cetacean health is scarce, and to further describe the pathology with its detailed organ distribution.
During 2013 to 2018, 366 cetacean strandings were reported along the coast of Hokkaido. Many of these animals were unsuitable for histopathologic examinations due to decomposition and hence not included in this study, but comprehensive gross and histopathologic investigations were attainable on 54 cetaceans comprising 11 species (Table 1), which include a fin whale (Balaenoptera physalus), 2 common minke whales (Balaenoptera acutorostrata), 1 pygmy sperm whale (Kogia breviceps), 6 Hubbs’ beaked whales (M. carlhubbsi), 3 Stejneger’s beaked whales, 3 killer whales (Orcinus orca), 3 Pacific white-sided dolphins (Lagenorhynchus obliquidens), 1 Risso’s dolphin (Grampus griseus), 6 striped dolphins (Stenella coeruleoalba), 12 Dall’s porpoises (Phocoenoides dalli) and 16 harbor porpoises (Phocoena phocoena). Most of the Dall’s and harbor porpoises were obtained from bycatch. The approximate age class was determined through a combination of body length, coloration, teeth eruption for beaked whales, size of thymus and histologic features of gonads, where the 4 age classes in this study were each defined as follows: calf (newborns and those with a similar body length), juvenile (larger than a calf but obviously smaller than a subadult with an immature body coloration), subadult (reaching or slightly falling below a full grown body length but reproductively immature) and adult (fully grown and reproductively mature) [6, 8]. All postmortem examinations were carried out systematically in the same manner including morphometry, necropsy and histopathology. For histopathology, tissue samples of the liver, spleen, kidney, heart, lung, thyroid gland, pancreas, adrenal gland, stomachs, intestine, urinary bladder, gonad, thymus (for immature individuals), various lymph nodes, laryngeal gland, brain, and others such as the skin and spinal cord when noted with gross abnormalities were fixed in 10–15% neutral buffered formalin and processed routinely. The above complete list of tissue collection was restricted to certain tissues in a small number of animals due to logistic constraints. Sections were stained with hematoxylin and eosin (HE), while an additional Congo red (CR) stain was performed for the definitive diagnosis of amyloidosis. All CR-stained sections were viewed under polarized light. Whenever deemed necessary, a selection of other special stains such as periodic acid Schiff (PAS), Gram, and Ziehl-Neelsen (ZN) stains were also conducted for tissues of some animals.
Table 1. Details of the animals examined with their representative morphologic findings.
| Species | SNHa) ID | Sex | Length (cm) | Age class | Location | Representative morphologic findings |
|---|---|---|---|---|---|---|
| Fin whale (Balaenoptera physalus) | SNH16040 | Female | 498.6 | Calf | Setana | Fetal distress |
| Common minke whale (Balaenoptera acutorostrata) | SNH16022 | Male | 263.5 | Calf | Tomari | Pseudohermaphroditism with testicular hypoplasia |
| SNH16044 | Female | 460.0 | Subadult | Koshimizu | Peritonitis | |
| Pygmy sperm whale (Kogia breviceps) | SNH14045 | Male | 245.2 | Adult | Toyokoro | Myocardial degeneration |
| Hubbs’ beaked whale (Mesoplodon carlhubbsi) | SNH15011 | Male | 493.0 | Adult | Samani | Hepatic trematodiasis; Pulmonary nematodiasis |
| SNH16003 | Male | 436.4 | Juvenile | Hakodate | Myocardial degeneration | |
| SNH17030 | Female | 278.0 | Calf | Shinhidaka | Hepatic lipidosis; Adrenal lipidosis | |
| SNH17037 | Female | 248.3 | Calf | Shinhidaka | Esophageal ulcer; Sinus histiocytosis | |
| SNH18033 | Female | 548.0 | Adult | Samani | Multiple lymphadenitis; | |
| SNH18034 | Female | 510.0 | Adult | Kushiro | Hepatic trematodiasis; Pulmonary cestodiasis | |
| Stejneger’s beaked whale (Mesoplodon stejnegeri) | SNH17015 | Female | 495.5 | Adult | Hakodate | Systemic AA amyloidosis; Renal crassicaudiasis |
| SNH17034 | Male | 410.0 | Juvenile | Betsukai | Granulomatous myocarditis; Renal crassicaudiasis | |
| SNH18001 | Male | 448.0+b) | Adult | Rumoi | Systemic AA amyloidosis; Renal crassicaudiasis | |
| Killer whale (Orcinus orca) | SNH16006 | Male | 219.8 | Calf | Kushiro | Neonatal weakness |
| SNH16035 | Female | 227.3 | Calf | Rebun | Neonatal weakness | |
| SNH17011 | Male | 712.5 | Adult | Toyokoro | No remarkable changes | |
| Pacific white-sided dolphin (Lagenorhynchus obliquidens) | SNH17020 | Female | 203.4 | Adult | Hakodate | Pulmonary fibrosis; Pancreatitis |
| SNH17024 | Male | 102.9 | Calf | Hakodate | Pulmonary nematodiasis | |
| SNH17056 | Male | 228.3 | Adult | Esashi | Myocarditis | |
| Risso’s dolphin (Grampus griseus) | SNH16032 | Male | 288.7 | Adult | Shari | Meningoencephalitis |
| Striped dolphin (Stenella coeruleoalba) | SNH15032 | Male | 250.0 | Adult | Urahoro | Meningitis |
| SNH16002 | Female | 147.0 | Juvenile | Kushiro | Encephalitis; Lingual ulcer | |
| SNH16019 | Male | 244.0 | Adult | Oshamambe | Pulmonary aspiration | |
| SNH18025 | Male | 216.9 | Subadult | Tomakomai | Meningoencephalomyelitis | |
| SNH18038 | Male | 212.5 | Subadult | Samani | Meningitis; Multiple myocardial necrosis | |
| SNH18043 | Male | 224.3 | Adult | Noboribetsu | Meningitis; Epididymo-orchitis; Multiple hepatic abscess; Pulmonary nematodiasis | |
| Dall’s porpoise (Phocoenoides dalli) | SNH14017 | Male | 118.0 | Calf | Abashiri | Hepatic lipidosis |
| SNH14018 | Female | 118.5 | Calf | Abashiri | Hepatic lipidosis | |
| SNH14024 | Male | 108.5 | Calf | Saroma | Hepatic lipidosis | |
| SNH14026-1 | Male | 219.5 | Adult | Rausu | Hepatic trematodiasis; Pulmonary nematodiasis | |
| SNH14026-2 | Male | 207.7 | Adult | Rausu | Pancreatic trematodiasis; Pulmonary nematodiasis | |
| SNH14026-3 | Male | 210.5 | Adult | Rausu | Hepatic trematodiasis; Pulmonary nematodiasis | |
| SNH14030 | Female | 118.8 | Calf | Ishikari | Hepatic lipidosis | |
| SNH14031 | Female | 179.3 | Subadult | Ishikari | No remarkable changes | |
| SNH14034-1 | Male | 193.5 | Subadult | Rausu | Hepatic trematodiasis; Pulmonary nematodiasis | |
| SNH14034-2 | Male | 199.5 | Subadult | Rausu | Pancreatic trematodiasis; Pulmonary nematodiasis | |
| SNH14034-3 | Male | 198.6 | Subadult | Rausu | Hepatic trematodiasis; Pulmonary nematodiasis | |
| SNH18005 | Male | 211.0 | Adult | Matsumae | Bronchopneumonia; Pancreatic trematodiasis | |
| Harbor porpoise (Phocoena phocoena) | SNH13012 | Male | 131.0 | Subadult | Rausu | Hepatic trematodiasis; Pulmonary nematodiasis |
| SNH14011 | Female | 182.8 | Adult | Rausu | Hepatic trematodiasis; Pulmonary nematodiasis | |
| SNH16201 | Male | 135.0 | Subadult | Rausu | Hepatic trematodiasis; Pulmonary nematodiasis | |
| SNH16011-2 | Male | 127.2 | Subadult | Hakodate | Hepatic trematodiasis; Pulmonary nematodiasis; Lingual papillomatosis | |
| SNH16023 | Female | 186.5 | Adult | Urahoro | Aspiration pneumonia; Hepatic trematodiasis; Pulmonary nematodiasis | |
| SNH16031 | Male | 138.3 | Subadult | Erimo | Hepatic trematodiasis; Pulmonary nematodiasis | |
| SNH17002 | Male | 133.0 | Subadult | Hokuto | Hepatic trematodiasis; Pulmonary nematodiasis | |
| SNH17003 | Male | 139.5 | Subadult | Otaru | Hepatic trematodiasis; Pulmonary nematodiasis; Intrapancreatic accessory spleen | |
| SNH17004 | Male | 127.0 | Subadult | Otaru | Hepatic trematodiasis; Pulmonary nematodiasis | |
| SNH17005 | Female | 133.1 | Subadult | Tomakomai | Hepatic trematodiasis; Pulmonary nematodiasis | |
| SNH17007 | Female | 138.6 | Subadult | Tomakomai | Hepatic trematodiasis; Pulmonary nematodiasis | |
| SNH17046 | Male | 116.9 | Juvenile | Shiranuka | No remarkable changes | |
| SNH18009 | Male | 143.0 | Subadult | Otaru | Hepatic trematodiasis; Pulmonary nematodiasis | |
| SNH18011 | Female | 131.8 | Subadult | Hakodate | Hepatic trematodiasis; Pulmonary nematodiasis | |
| SNH18012 | Female | 152.2 | Adult | Hakodate | Hepatic trematodiasis; Pulmonary nematodiasis | |
| SNH18015 | Male | 134.4 | Subadult | Muroran | Hepatic trematodiasis; Pulmonary nematodiasis | |
a) SNH refers to ‘Stranding Network Hokkaido’, where the first 2 digits after the letters indicate the year of stranding and the following numbers indicate the chronological order of event. b) Individual had a broken maxilla, making accurate measurements unattainable.
Immunohistochemistry using a mouse monoclonal antibody against AA (1:600, clone KM268; Kyowa Medex, Tokyo, Japan) was conducted on all collected tissues of Stejneger’s beaked whales in order to determine the nature of the deposited amyloid. The same immunohistochemistry was also applied in cases where amyloid deposition was suspected by the HE stain in other species. Briefly, antigen retrieval was performed by incubating the slides with pronase E (Kaken Pharmaceutical, Tokyo, Japan) for 60 min, while endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 10 min, both reactions performed at room temperature. The antigen was detected using a Histofine Simple Stain MAX-PO kit (Nichirei Biosciences, Tokyo, Japan) and labeling was visualized with 3′3-diaminobenzidine chromogen (Nichirei Biosciences). The sections were counterstained with Meyer’s hematoxylin. Tissue sections in which the primary antibody was replaced by normal mouse serum served as negative controls and a liver section of a Holstein cow (Bos taurus) with AA deposits was used as a positive control.
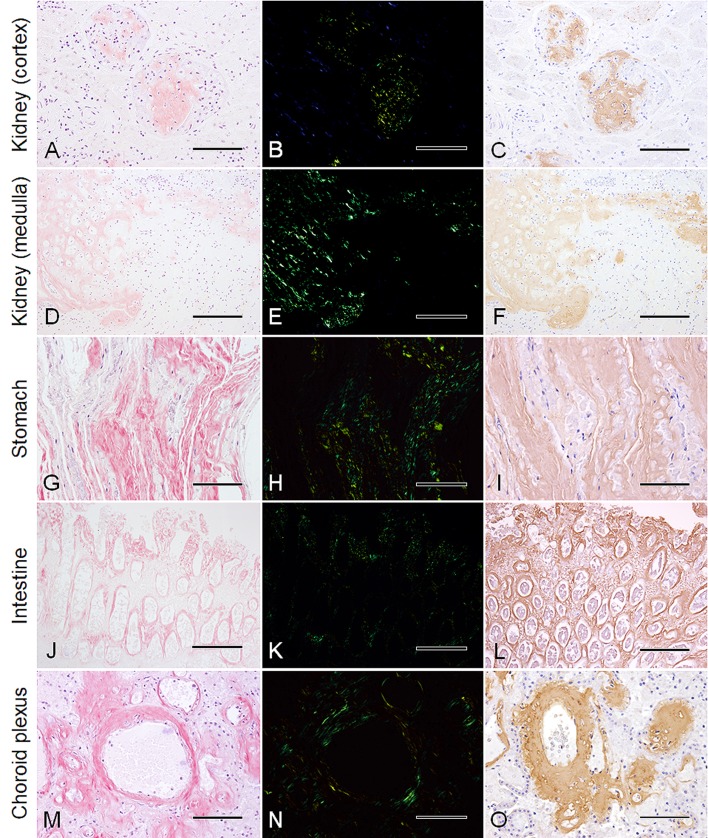
Out of the 54 animals across 11 species, amyloid deposits were found only in the adult male and female Stejneger’s beaked whales (SNH17015, SNH18001). In these 2 animals, although prominent gross abnormalities suggestive of amyloidosis were difficult to detect, slight hepatomegaly and splenomegaly were noted. The edges of the livers were slightly rounded, and the parenchyma appeared friable. With histopathology, deposits of amorphous pink material that stained positive on the CR stain and by birefringence were identified in multiple tissues. In particular, amyloid deposits were found in the vascular walls of various organs such as the liver (including the space of Disse) and choroid plexus, follicles of the spleen, glomeruli and medullary interstitium of the kidney, and lamina propria and muscularis of the stomachs and intestine (Fig. 1, Table 2). Additionally, in the adult female whale, amyloid was also detected in the pericellular spaces of the myocardium, endometrial mucosa of the uterus, and interstitium of the thyroid gland, adrenal gland and pancreas. The degree of amyloid deposition in each organ was assessed using a semi-quantitative scale [18], showing that the kidney and gastrointestinal tract had the largest amounts of deposits (Table 2). Through immunohistochemistry, all these deposits showed affinity to the anti-AA antibody. The 2 adult Stejneger’s beaked whales were diagnosed as systemic amyloidosis, while the juvenile male Stejneger’s beaked whale (SNH17034) did not have any discernible amyloid deposits in the examined tissue sections.
Fig. 1.
Histologic features of systemic amyloidosis in Stejneger’s beaked whales (Mesoplodon stejnegeri). Columns: left, Congo red; middle, Congo red under crossed polars; right, immunohistochemistry for amyloid A with Mayer’s hematoxylin counterstain. (A)–(C) Cortex of kidney (serial sections). Segmental amyloid deposits expand the glomeruli (SNH18001). Bar=100 µm. (D)–(F) Medulla of kidney (serial sections). Marked amyloid deposits in the tubular basement membrane and interstitium at the renal papilla (SNH17015). Bar=200 µm. (G)−(I) Main stomach. Prominent amyloid deposits in the interstitium of the lamina propria (SNH18001). Bar=100 µm. (J)–(L) Intestine. Striking amyloid deposits with a periglandular pattern and at the tip of the lamina propria (SNH17015). Bar=200 µm. (M)–(O) Choroid plexus. Marked amyloid deposits expanding the vascular walls while nodular deposits intersperse within the interstitium (SNH17015). Bar=100 µm.
Table 2. Severity of amyloid deposition in various tissues of 3 Stejneger’s beaked whales (Mesoplodon stejnegeri) .
| Liver | Spleen | Kidney (cortex) | Kidney (medulla) | Heart | Lung | Thyroid gland | Pancreas | Adrenal gland | Stomachs | Intestine | Urinary bladder | Mammary gland | Uterus | Gonad | Brain | Choroid plexus | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNH17015 (adult female) | ++ | ++ | ++ | ++ | + | + | ++ | ++ | + | +++ | +++ | − | − | + | − | − | ++ |
| SNH17034 (juvenile male) | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ||
| SNH18001 (adult male) | + | ++ | +++ | +++ | N. E. | N. E. | N. E. | N. E. | N. E. | +++ | +++ | − | N. E. | − | ++ |
−=no amyloid detected, +=mild, ++=moderate, +++=severe (see Terio et al. [18] for details of the grading criteria, a) N. E.: not examined.
Other pathologic changes shared among the 3 Stejneger’s beaked whales were moderate renal crassicaudiasis characterized by granulomatous inflammation and fibrosis. Furthermore, the 2 adults showed moderate polyglucosan body deposition in the cerebellum and intracytoplasmic lipofuscin in the neurons of the brainstem, while the juvenile had multiple granulomas in the heart. These granulomas did not reveal any associated pathogens with HE, PAS, Gram and ZN stains.
The current patho-epidemiologic study is a first of its kind to assess the occurrence of amyloidosis in cetaceans over a particular geographic area during a set time period. It is worthy to note that the individuals included in this study were not based on the kind of species or other biological factors, and that the majority of fresh to moderately decomposed individuals reported within the study period were covered. Amyloidosis was diagnosed only in adult Stejneger’s beaked whales, while none of the others, including the juvenile and the closely related Hubbs’ beaked whales, exhibited this condition. Amyloid deposits were detected in multiple tissues of adult Stejneger’s beaked whales, whilst the kidney and gastrointestinal tract were affected the most. It is noteworthy that neither of the kidney, stomachs nor intestines demonstrated obvious gross changes to suspect amyloidosis such as the classic renal enlargement [9], emphasizing the need of histopathologic examinations to diagnose this disease in cetaceans. The kidney is a commonly affected organ in humans and many other reported animals with systemic amyloidosis [9, 11], and hence extensive involvement of the kidney was to be expected. Meanwhile, prominent deposits in the stomachs and intestine were previously undescribed findings in the systemic amyloidosis of this species. Since heavy amyloid burdens in the kidney and gastrointestinal tract can potentially cause renal failure [11] or malabsorption through the alimentary system [19], respectively, future investigations to correlate these histopathologic findings with clinical presentations will be ideal.
Systemic amyloidosis in marine mammals is a disease still needing much research attention. The type of deposited amyloid has only been immunohistochemically confirmed as AA in California sea lions [1] and proposed as AA in Stejenger’s beaked whales and bottlenose dolphins by pretreatment of CR stains with potassium permanganate and sulfuric acid [2, 16]. Our immunohistochemistry results for AA follow these previous propositions, and also, the rather similar systemic deposition patterns compared to well studied species with AA amyloidosis such as dogs (Canis lupus) and cattle [9], suggest that systemic amyloidosis in Stejnger’s beaked whales is likely to be of the AA type.
AA amyloidosis usually occurs as a result of chronic inflammatory disease through the persistent elevation of SAA or due to a genetic factor [4, 15]. Assuming that the amyloid deposits in Stejneger’s beaked whales are formed from SAA-derived protein, the only common causative factor of chronic inflammation was renal crassicaudiasis. However, all 3 Stejneger’s beaked whales, including the individual without amyloidosis were affected with the same parasite, and inflammatory lesions including those owing to parasitic infections were a common finding among the examined cetaceans (34 out of 40 animals excluding Stejneger’s beaked whales and calves of other species; Table 1). On the other hand, genetic predisposition may be a contributing factor in the development of amyloidosis in Stejneger’s beaked whales, as genetic pleomorphism seems to be rather limited in this species [10]. This condition is comparable to that of the island foxes, where its high prevalence of AA amyloidosis is suggested to be due to a lack in heterozygosity, owing to their isolated insular habitat [5, 13]. Nonetheless, confirmation of the amyloid precursor protein in Stejneger’s beaked whales at this point seems premature since recent studies have suggested that immunohistochemistry alone is not enough and additional proteomic and/or genetic analyses are required, especially for little-studied wildlife species [5, 7].
The Stejneger’s beaked whale is a species with even the most basic biological information yet to be revealed [17]. Virtually nothing is known on their status of abundance, but like other beaked whale species, they are probably prone to anthropogenic threats such as loud acoustic exposure and ingestion of plastic, making them already vulnerable to the ever changing marine environment [3, 12]. Although it is unlikely that stranded animals represent disease prevalence in an entire population, the result of this study implies that systemic amyloidosis may be a substantial factor contributing to morbidity and mortality in adult Stejneger’s beaked whales. Our sample size of Stejneger’s beaked whales is limited, but the presence of earlier sporadic reports of amyloidosis in adult individuals is supportive of these conclusions [14, 16]. Stejneger’s beaked whales may possibly be more prone to the development of systemic amyloidosis among various cetaceans, and therefore, further pathologic research focusing specifically on the species is necessary to elucidate the pathogenesis of amyloidosis and its true prevalence in a population.
Acknowledgments
This study could not have been conducted without the tremendous support from Stranding Network Hokkaido, especially the following people: Dr. Ayaka Matsuda, Dr. Mika Kuroda, Ms. Natsuki Matsui and Ms. Saki Maeda. This work was partially supported by the Sasakawa Scientific Research Grant from The Japan Science Society (28-736 and 2018-7012) and the Leading Academia in Marine and Environmental Research (LaMer) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (29-26).
REFERENCES
- 1.Colegrove K. M., Gulland F. M. D., Harr K., Naydan D. K., Lowenstine L. J.2009. Pathological features of amyloidosis in stranded California sea lions (Zalophus californianus). J. Comp. Pathol. 140: 105–112. doi: 10.1016/j.jcpa.2008.08.003 [DOI] [PubMed] [Google Scholar]
- 2.Cowan D. F.1995. Amyloidosis in the bottlenose dolphin, Tursiops truncatus. Vet. Pathol. 32: 311–314. doi: 10.1177/030098589503200314 [DOI] [PubMed] [Google Scholar]
- 3.Cox T. M., Ragen T. J., Read A. J., Vos E., Baird R. W., Balcomb K., Barlow J., Caldwell J., Cranford T., Crum L., D’Amico A., D’Spain G., Fernández A., Finneran J., Gentry R., Gerth W., Gulland F., Hildebrand J., Houser D., Hullar T., Jepson P. D., Ketten D., Macleod C. D., Miller P., Moore S., Mountain D. C., Palka D., Ponganis P., Rommel S., Rowles T., Taylor B., Tyack P., Wartzok D., Gisiner R., Mead J., Benner L.2006. Understanding the impacts of anthropogenic sound on beaked whales. J. Cetacean Res. Manag. 7: 177–187. [Google Scholar]
- 4.Gaffney P. M.2017. Amyloid A amyloidosis: the influence of genetics and seeding on pathogenesis and the utility of mass spectrometry. Vet. Pathol. 54: 5–8. doi: 10.1177/0300985816677150 [DOI] [PubMed] [Google Scholar]
- 5.Gaffney P. M., Imai D. M., Clifford D. L., Ghassemian M., Sasik R., Chang A. N., O’Brien T. D., Coppinger J., Trejo M., Masliah E., Munson L., Sigurdson C.2014. Proteomic analysis of highly prevalent amyloid A amyloidosis endemic to endangered island foxes. PLoS One 9: e113765. doi: 10.1371/journal.pone.0113765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geraci J. R., Lounsbury V. J.2005. Marine Mammals Ashore: A Field Guide for Strandings, 2nd ed., National Aquarium in Baltimore, Baltimore. [Google Scholar]
- 7.Jansson D. S., Bröjer C., Neimanis A., Mörner T., Murphy C. L., Otman F., Westermark P.2018. Post mortem findings and their relation to AA amyloidosis in free-ranging Herring gulls (Larus argentatus). PLoS One 13: e0193265. doi: 10.1371/journal.pone.0193265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jefferson T. A., Webber M. A., Pitman R. L.2008. Marine Mammals of the World: A Comprehensive Guide to Their Identification, Academic Press, Elsevier, London.
- 9.Johnson K. H., Westermark P., Sletten K., O’Brien T. D.1996. Amyloid proteins and amyloidosis in domestic animals. Amyloid 3: 270–289. doi: 10.3109/13506129609014375 [DOI] [Google Scholar]
- 10.Kakuda T., Yamada T. K.2001. Genetic variability of Stejneger’s beaked whale (Mesoplodon stejnegeri) stranded on the shore of Sea of Japan based on mitochondrial DNA sequences. Mamm. Sci. Supplementary Issue 3: 93–96 (in Japanese with English abstract).
- 11.Lachmann H. J., Goodman H. J. B., Gilbertson J. A., Gallimore J. R., Sabin C. A., Gillmore J. D., Hawkins P. N.2007. Natural history and outcome in systemic AA amyloidosis. N. Engl. J. Med. 356: 2361–2371. doi: 10.1056/NEJMoa070265 [DOI] [PubMed] [Google Scholar]
- 12.Lusher A. L., Hernandez-Milian G., O’Brien J., Berrow S., O’Connor I., Officer R.2015. Microplastic and macroplastic ingestion by a deep diving, oceanic cetacean: the True’s beaked whale Mesoplodon mirus. Environ. Pollut. 199: 185–191. doi: 10.1016/j.envpol.2015.01.023 [DOI] [PubMed] [Google Scholar]
- 13.Robinson J. A., Ortega-Del Vecchyo D., Fan Z., Kim B. Y., vonHoldt B. M., Marsden C. D., Lohmueller K. E., Wayne R. K.2016. Genomic flatlining in the endangered island fox. Curr. Biol. 26: 1183–1189. doi: 10.1016/j.cub.2016.02.062 [DOI] [PubMed] [Google Scholar]
- 14.Shindo J., Yamato A.1995. Pathology of Stenjengeris beaked whale Mesoplodon stejnegeri stranded at Johetsu-city, Niigata-prefecture. Nihonkai Cetol. 5: 27–29(in Japanese). [Google Scholar]
- 15.Sipe J. D., Benson M. D., Buxbaum J. N., Ikeda S. I., Merlini G., Saraiva M. J. M., Westermark P.2016. Amyloid fibril proteins and amyloidosis: chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid 23: 209–213. doi: 10.1080/13506129.2016.1257986 [DOI] [PubMed] [Google Scholar]
- 16.Tajima Y., Shimada A., Yamada T. K., Cowan D. F.2007. Amyloidosis in two Stejneger’s beaked whales (Mesoplodon stejnegeri) stranded at the Sea of Japan. J. Zoo Wildl. Med. 38: 108–113. doi: 10.1638/05-108.1 [DOI] [PubMed] [Google Scholar]
- 17.Taylor B. L., Baird R., Barlow J., Dawson S. M., Ford J., Mead J. G., Notarbartolo di Sciara G., Wade P., Pitman R. L.2008. Mesoplodon stejnegeri. The IUCN Red List of Threatened Species 2008: e.T13252A3431272. 10.2305/IUCN.UK.2008.RLTS.T13252A3431272.en [accessed on September 12, 2018]. [DOI]
- 18.Terio K. A., O’Brien T., Lamberski N., Famula T. R., Munson L.2008. Amyloidosis in black-footed cats (Felis nigripes). Vet. Pathol. 45: 393–400. doi: 10.1354/vp.45-3-393 [DOI] [PubMed] [Google Scholar]
- 19.Uzal F. A., Plattner B. L., Hostetter J. M.2016. Alimentary system. pp. 1–257. In: Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, Vol. 2, 6th ed. (Maxie, M. G. ed), Elsevier, St. Louis. [Google Scholar]