Abstract
Atypical bovine spongiform encephalopathy (BSE), first identified in 2004, poses a threat due to the potential to spread the disease to cattle and other animals, including humans. Here, we estimated prion titers in various tissues of cattle infected with atypical BSE using a real-time quaking-induced conversion assay that detects amyloid seeding activity of a disease-specific prion protein, PrPSc, a major component of prions. PrPSc was detected both in and outside of nerve tissues, and some of the peripheral nerve tissues contained relatively high prion titers. Low titers of prions were also observed in masseter, jejunum, and adrenal glands. Quantitative data on prion infectivity in tissues of atypical BSE-affected cattle is useful to assess the risk of atypical BSE.
Keywords: atypical bovine spongiform encephalopathy, H-BSE, L-BSE, prion, RT-QuIC
Bovine spongiform encephalopathy (BSE) is a fatal neurodegenerative disease in cattle and is associated with the accumulation of a disease-specific isoform of prion protein, PrPSc, in the central nervous system (CNS). BSE emerged in the mid-1980s in the United Kingdom, spread globally, and is thought to be the result of a single prion strain (called classical BSE [C-BSE]) [5]. Two types of BSEs, whose PrPSc show different biochemical properties from that of C-BSE, have been identified since 2004 [3, 7]. These are called as atypical BSEs. Atypical BSE cases have been further classified into H- and L-BSE based on the higher and lower apparent molecular weight of the non-glycosylated PrPSc in immunoblot analysis, respectively [12]. More than 120 atypical BSE cases have been reported worldwide, including in Brazil, where no C-BSE had been reported before. Atypical BSE cases have been mainly identified in aged cattle over eight-year old, implying that atypical BSE is a sporadic disease in cattle, as is sporadic Creutzfeldt-Jakob disease (CJD) in humans [4].
Since C-BSE transmitted to human and caused variant CJD [6, 19], transmission of atypical BSE to humans has become a public health concern. L-BSE experimentally transmitted to non-human primates [8, 16] and transgenic mice expressing human prion protein [2, 13] with shorter incubation periods than C-BSE. These facts suggest zoonotic potential of L-BSE prions with a higher transmissibility to humans compared with C-BSE. On the other hand, concrete evidence on the zoonotic potential of H-BSE is still needed [2, 20].
The tissue distribution and the level of infectivity in atypical BSE-affected cattle is not fully understood. Immunohistochemistry and immunoblot analyses have revealed PrPSc accumulation in many peripheral nerve tissues, in adrenal glands, and in skeletal muscles of cattle infected with H- and L-BSE [11, 14, 15, 17]. Low levels of infectivity were also confirmed in peripheral nerve tissues, in adrenal glands, and in skeletal muscles obtained from clinical cases of cattle intracerebrally inoculated with L-BSE and/or field asymptomatic L-BSE cases by bioassay using transgenic mice expressing bovine prion protein (PrP) [11, 17]. Quantitative data on prion infectivity in various tissues of cattle infected with atypical BSE is needed to analyze the risk of atypical BSE for the spread to humans as well as to cattle and other animals through food and foodstuffs. Since atypical BSE exhibits different pathology than C-BSE, the distribution of prions should be carefully examined.
The gold standard for measuring prion infectivity is an end-point titration by bioassay. However, bioassays are not suitable to analyze large numbers of samples, as these methods are time-consuming and laborious. Real-time quaking-induced conversion assay (RT-QuIC) is an in vitro PrPSc amplification technique that can detect PrPSc as amyloid formation seeding activity. RT-QuIC can detect extremely low level of PrPSc with a high-throughput approach [1]. Henderson et al. recently reported a linear relationship between the lag-phase of RT-QuIC and prion titers in samples [10]. High sensitivity, a linear relationship, and the high throughput of RT-QuIC are major advantages for estimation of prion titers in tissues. Thus, we estimated prion infectivity in various tissues from atypical BSE-infected cattle by RT-QuIC.
Tissues of cattle intracerebrally inoculated with H- and L-BSE were provided from the National Institute of Animal Health (NIAH), National Agriculture and Food Research Organization (NARO), Japan. Tissues were collected from two cattle intracerebrally inoculated with Canadian H-BSE prions euthanized at 19 months post inoculation (mpi) (ID: 0728) and 18 mpi (ID: 9458) [15], or Japanese L-BSE prions [9] euthanized at 14 mpi (ID: 3383, clinical stage) and 9 mpi (ID: 4685, subclinical stage). Brain homogenates (BHs) (10% in phosphate-buffered saline [PBS]) from H- or L-BSE-affected cattle, which had been titrated by end-point titration using transgenic mice expressing bovine PrP, were also provided by NIAH/NARO as standard BHs for estimation of prion titers. The prion titers of standard BHs for H- and L-BSE were 107.4 and 106.9 50% lethal dose (LD50) /g tissue, respectively. The tissues, with the exception of skeletal muscles, were homogenized in sterile PBS at a concentration of 20% (w/v) using a Multi-Beads Shocker (Yasui Kikai, Osaka, Japan). Skeletal muscles were homogenized using a tissue grinder (Biomedical Polymers, Sterling, MA, U.S.A.).
We performed RT-QuIC as previously described with slight modifications [10, 18]. Reaction mixtures (95 µl) containing a final concentration of 10 mM phosphate buffer (pH 7.4), 500 mM NaCl, 10 µM thioflavin T (ThT), 1 mM ethylenediaminetetraacetic acid, 0.001% sodium dodecyl sulfate and 100 µg/ml recombinant cervid PrP (rCerPrP) residues 25–233 were added to wells of a black-walled 96-well plate with a clear bottom (Nunc, Roskilde, Denmark). We tested five different recombinant PrPs, including mouse, hamster, sheep, bovine and cervid. Among them, rCerPrP provided the most stable and reproducible results with satisfactory sensitivity (data not shown). Tissue homogenates (20%) were serially diluted (10-fold) with sterile PBS, and 5 µl of the diluted homogenates were added to wells. The plate was sealed and incubated in a Tecan Infinite F200 or M200 plate reader (TECAN, Männedorf, Switzerland) at 37°C with 360 cycles of incubation and detection. One cycle consisted of eight repetitions of 30 sec orbital shaking at 432 rpm and 30 sec of settling, and then measurement of fluorescence with excitation at 430 nm and emission at 485 nm. PBS was used as a negative control. The threshold was calculated as the average fluorescence intensity of negative control + 10 × standard deviation. Reactions were determined to be positive when ThT fluorescence intensity reached the threshold. The lag-phase (hr) was defined as the time of reaction that needed to cross the threshold. Amyloid formation rate (AFR) (1/hr) that is the inverse of lag-phase was used as an indicator of amyloid seeding activity: samples containing higher concentrations of prions show higher AFRs. AFRs were determined by two independent experiments with quadruplicate. Samples that were negative during the 60 hr measuring period were considered to be below the detection limit.
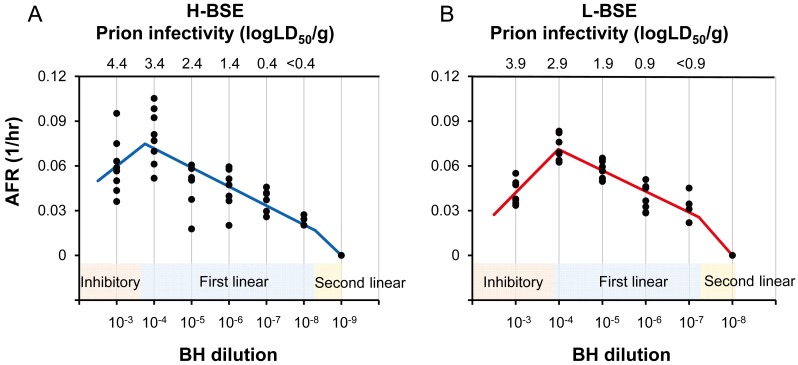
RT-QuIC was performed with 10-fold serial dilutions of standard BHs to establish the infectivity-AFR standard curves. Each standard curve was separated into three phases: inhibitory, first linear, and second linear phases (Fig. 1). Lower AFRs at the 10−3 dilution than at the 10−4 dilution indicate the inhibition of RT-QuIC in the presence of a high concentration of tissue homogenates and/or biological fluids [10, 18].
Fig. 1.
Infectivity-AFR standard curves. Standard curves were established by plotting AFRs to corresponding dilutions of the standard BHs of H- (A) and L-BSE (B). AFRs measured from two independent experiments (n=4 in each experiment) were plotted. Blue and red lines indicate standard curves for H- and L-BSE, respectively. Standard curves were separated into three phases: inhibitory, first linear, second linear phases. Y-axes show AFRs (1/hr), whereas x-axes show dilutions of BH. The standard curve of H-BSE for AFRs higher than 0.0167 (1/hr) was fitted as the first linear phase (LD50/g=e78.33x-2.71028), while that lower than 0.0167 (1/hr) was fitted as the 2nd linear phase (LD50/g=e41.76x-2.1). The standard curve of L-BSE for AFRs higher than 0.0257 (1/hr) was fitted as first linear phase (LD50/g=e71.37x-2.157), while that lower than 0.0257 (1/hr) was fitted as second linear phase (LD50/g=e30.17x-1.1).
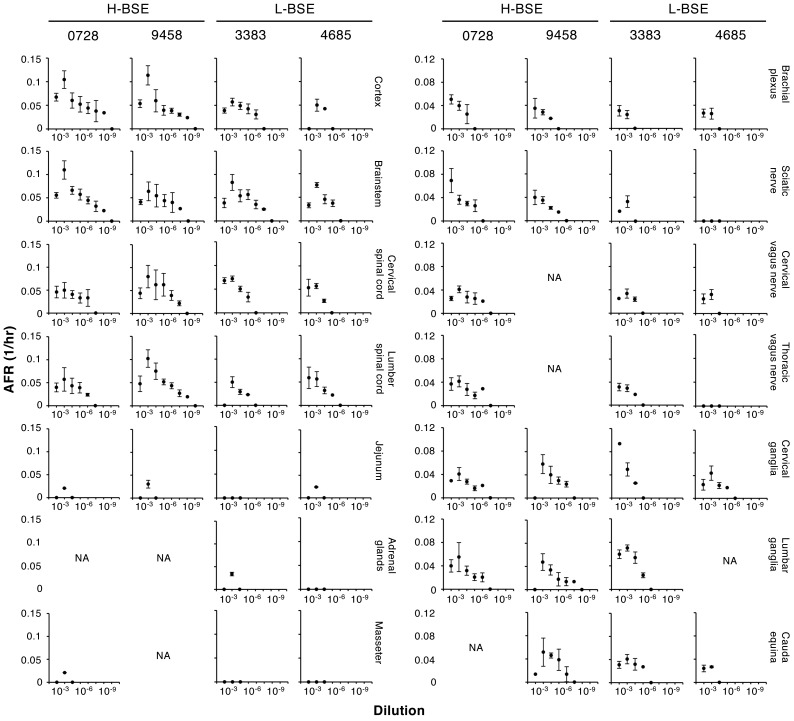
In the current study, CNS tissues (cortex, brainstem, and cervical and lumber spinal cord), peripheral nerves (brachial plexus, sciatic nerve, cervical and thoracic vagus nerve, cervical and lumber ganglia, and cauda equina), alimentary tracts (jejunum, ileum, caecum, and rectal), skeletal muscles (triceps, semitendinosus, quadriceps, longissimus, masseter, and diaphragm) and other tissues (heart, liver, tonsil, and adrenal glands) from the two cattle intracerebrally inoculated with H- or L-BSE were subjected to RT-QuIC. Seeding activities were detected in all CNS tissues and peripheral nerves tested (Fig. 2). The lower AFRs observed at the 10−3 dilution than at the 10−4 dilution indicate interference of the reaction in the presence of a high concentration of tissue homogenates. Thus, ARFs at 10−3 were not used for estimations. Prion titers in tissues were estimated from ARFs at a dilution of 10−4 and higher. If only one dilution was positive, an average of two independent experiments were calculated (Table 1). If several dilutions were positive, prion titers were estimated for each dilution, and averages and standard deviations are shown in Table 1.
Fig. 2.
Detection of seeding activities in tissues of cattle intracerebrally inoculated with H- and L-BSE. Tissues from two cattle intracerebrally inoculated with H-BSE (IDs: 0728 and 9458) or L-BSE (IDs: 3383 and 4685) were subjected to RT-QuIC. Graphs show AFRs (mean ± standard deviations from a total of eight wells from the two independent experiments [n=4 in each experiment]). Tissues positive for H-BSE and/or L-BSE are indicated. Y-axes show AFRs (1/hr) whereas x-axes show the dilution of tissue homogenates. NA: tissues were not available.
Table 1. Estimated prion titersa).
| Tissue | H-BSE | L-BSE | |||
|---|---|---|---|---|---|
| 0728 | 9458 | 3383 | 4685 | ||
| CNS | |||||
| Cortex | 8.14 ± 0.95 | 7.76 ± 1.33 | 6.09 ± 0.73 | 4.98 ± 0.4 | |
| Brainstem | 8.12 ± 0.89 | 6.87 ± 0.51 | 6.79 ± 0.59 | 5.86 ± 0.37 | |
| Cervical spinal cord | 5.84 ± 0.74 | 7.47 ± 0.46 | 5.80 ± 0.13 | 4.81 ± 0.41 | |
| Lumbar spinal cord | 5.99 ± 0.32 | 7.94 ± 0.72 | 4.92 ± 0.46 | 4.79 ± 0.59 | |
| Peripheral nerves | |||||
| Brachial plexus | 4.96 ± 0.3 | 4.04 ± 0.03 | 3.61 | 3.68 | |
| Sciatic nerve | 5.30 ± 0.5 | 4.64 ± 0.17 | 4.22 | <3.40b) | |
| Cervical vagus nerve | 5.61 ± 0.57 | NAc) | 5.80 ± 0.13 | 4.19 | |
| Thoracic vagus nerve | 5.61 ± 1.01 | NA | 4.92 ± 0.46 | <3.40 | |
| Cervical ganglia | 5.38 ± 0.67 | 6.52 ± 0.38 | 5.02 ± 0.49 | 5.02 ± 0.44 | |
| Lumbar ganglia | 5.90 ± 0.67 | 5.55 ± 0.37 | 6.4 ± 0.67 | NA | |
| Cauda equina | NA | 6.46 ± 0.62 | 5.21 ± 0.53 | 3.74 | |
| Skeletal muscles | |||||
| Triceps | <2.60b) | NA | <3.40 | <3.40 | |
| Semitendinosus | <2.60 | NA | <3.40 | <3.40 | |
| Quadriceps | <2.60 | NA | <3.40 | <3.40 | |
| Longissimus | <2.60 | NA | <3.40 | <3.40 | |
| Masseter | 2.92 | NA | <3.40 | <3.40 | |
| Diaphragm | <2.60 | <2.60 | <3.40 | <3.40 | |
| Alimentary tracts | |||||
| Jejunum | 2.89 | 3.67 | <3.40 | 3.48 | |
| Ileum | <2.60 | <2.60 | <3.40 | <3.40 | |
| Caecum | <2.60 | <2.60 | <3.40 | <3.40 | |
| Rectal | <2.60 | <2.60 | <3.40 | <3.40 | |
| Other tissues | |||||
| Heart | <2.60 | <2.60 | <3.40 | <3.40 | |
| Liver | <2.60 | <2.60 | <3.40 | <3.40 | |
| Adrenal gland | NA | NA | 3.77 | <3.40 | |
| Tonsil | <2.60 | <2.60 | <3.40 | <3.40 | |
a) Estimated prion titers were expressed as Log (LD50) /g tissues. b) Prion titers indicated by <2.60 and <3.40 were below the detection limits for H- and L-BSE, respectively. c) Tissues were not available.
Prion titers of all CNS tissues were estimated from 105.84 to 108.14 LD50/g for H-BSE and from 104.79 to 106.79 LD50/g for L-BSE, respectively. Prion titers of peripheral nerves were estimated to range from 104.04 to 106.52 LD50/g for H-BSE-infected cattle, and those of peripheral nerves for L-BSE-infected cattle were estimated from 103.61 to 106.4 LD50/g. Prion titers in the sciatic nerve and thoracic vagus nerve of one of the two cattle (ID: 4685) were below the detection limit. Dorsal root ganglia (cervical and lumber ganglia) contained the highest infectivity among peripheral nerves tested; estimated prion titers ranged from 105.38 to 106.52 and 105.02 to 106.4 LD50/g for H- and L-BSE, respectively (Table 1).
Among the other type of tissues than the nerve tissues, seeding activities were detected in jejunum and masseter for H-BSE and jejunum and adrenal glands for L-BSE. Infectivity in jejunum from the two H-BSE inoculated cattle was estimated from 102.89 to 103.67 LD50/g and from one of the two L-BSE inoculated cattle (ID: 4685) was estimated at 103.48 LD50/g. Seeding activity was also detected in the adrenal glands from one of the two L-BSE inoculated cattle (ID: 3383) and prion titer was estimated at 103.77 LD50/g. Seeding activity was detected in masseter from one of the two H-BSE inoculated cattle (ID: 0728) with a titer estimated at 102.92 LD50/g (Table 1). Estimated prion titers in jejunum were 1/100 to 1/100,000, those in adrenal glands was 1/1,000, and those in masseter was 1/100,000 lower than those in brainstems of the corresponding BSE type.
Iwamaru et al. reported that prion titers in the sciatic nerve, brachial plexus, vagus nerve, and adrenal glands of L-BSE-infected cattle were about 1/1,000 lower than that in the obex [11]. This is consistent with observed results presented herein. The estimated infectivities of sciatic nerves and adrenal glands of L-BSE-infected cattle (ID: 3383) were approximately 1/400 and 1/1,000 lower than that of the corresponding brainstem, respectively (Table 1). This demonstrates the advantage of RT-QuIC for the estimation of prion titers. However, dorsal root ganglia (cervical and lumbar ganglia) of L-BSE-infected cattle (ID: 3383) contained relatively high prion titers, roughly one-tenth lower prion titers compared to the brainstems (Table 1). A similar result was observed in the H-BSE-infected cattle (ID: 9458). Additionally, cervical and thoracic vagus nerves of H- (ID: 0728) and L-BSE-infected cattle (ID: 3383) contained prion titers comparable to the corresponding spinal cords (Table 1). Although PrPSc has been detected in peripheral nerves [15], quantitative data was insufficient for H-BSE. This is the first study that provided quantitative data on prion titers in peripheral nerves of H-BSE-infected cattle. Relatively high prion titers in peripheral nerves may provide rationale of the definition of specified risk materials.
In previous studies, a very low prion infectivity was detected in skeletal muscles (e.g., longissimus dorsi, gluteus, or intercostalis muscles) of cattle infected with L-BSE [11, 17]. Seeding activity in skeletal muscle of L-BSE infected cattle was below the detection level. The inhibition of RT-QuIC in the presence of a high concentration of tissue homogenates may be one of the reasons for the apparent discrepancy. The presence of PrPSc in skeletal muscles, such as triceps or gluteus medius, of cattle infected with both H- and L-BSE has also been reported [14]. However, infectivity in skeletal muscles of H-BSE-infected cattle has not been clarified yet. In the current study, seeding activity was detected only from the masseter among the skeletal muscles examined, suggesting that prion titers in skeletal muscles of H-BSE-infected cattle are extremely low even if they exist. Although a nerve tissue-concentrated distribution of prion infectivity in atypical BSE-affected cattle resembles to the tissue distribution of prions in C-BSE-affected cattle, the detection of seeding activity in jejunum of cattle intracranially inoculated with H- and L-BSE implies the efferent spread of atypical BSE prions through nerve systems. Therefore, further analysis of prion tissue distribution in atypical BSE-affected cattle will be required to assess the detailed pathogenesis of atypical BSE-affected cattle as well as the risk of atypical BSE infection to humans and other animals.
Acknowledgments
This work was supported by a Grant-in-Aid for Science Research (A) (JSPS KAKENHI Grant Number JP 15H02475) and a grant from the Program for Leading Graduate Schools (F01) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. This work was also supported by grants for TSE research (H29-Shokuhin-Ippan-004) from the Ministry of Health, Labour and Welfare of Japan. We thank Zensho Co., Ltd, for the BSL3 facility.
REFERENCES
- 1.Atarashi R., Sano K., Satoh K., Nishida N.2011. Real-time quaking-induced conversion: a highly sensitive assay for prion detection. Prion 5: 150–153. doi: 10.4161/pri.5.3.16893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Béringue V., Herzog L., Reine F., Le Dur A., Casalone C., Vilotte J. L., Laude H.2008. Transmission of atypical bovine prions to mice transgenic for human prion protein. Emerg. Infect. Dis. 14: 1898–1901. doi: 10.3201/eid1412.080941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biacabe A. G., Laplanche J. L., Ryder S., Baron T.2004. Distinct molecular phenotypes in bovine prion diseases. EMBO Rep. 5: 110–115. doi: 10.1038/sj.embor.7400054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biacabe A. G., Morignat E., Vulin J., Calavas D., Baron T. G.2008. Atypical bovine spongiform encephalopathies, France, 2001–2007. Emerg. Infect. Dis. 14: 298–300. doi: 10.3201/eid1402.071141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruce M., Chree A., McConnell I., Foster J., Pearson G., Fraser H.1994. Transmission of bovine spongiform encephalopathy and scrapie to mice: strain variation and the species barrier. Philos. Trans. R. Soc. Lond. B Biol. Sci. 343: 405–411. doi: 10.1098/rstb.1994.0036 [DOI] [PubMed] [Google Scholar]
- 6.Bruce M. E., Will R. G., Ironside J. W., McConnell I., Drummond D., Suttie A., McCardle L., Chree A., Hope J., Birkett C., Cousens S., Fraser H., Bostock C. J.1997. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature 389: 498–501. doi: 10.1038/39057 [DOI] [PubMed] [Google Scholar]
- 7.Casalone C., Zanusso G., Acutis P., Ferrari S., Capucci L., Tagliavini F., Monaco S., Caramelli M.2004. Identification of a second bovine amyloidotic spongiform encephalopathy: molecular similarities with sporadic Creutzfeldt-Jakob disease. Proc. Natl. Acad. Sci. U.S.A. 101: 3065–3070. doi: 10.1073/pnas.0305777101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comoy E. E., Casalone C., Lescoutra-Etchegaray N., Zanusso G., Freire S., Marcé D., Auvré F., Ruchoux M. M., Ferrari S., Monaco S., Salès N., Caramelli M., Leboulch P., Brown P., Lasmézas C. I., Deslys J. P.2008. Atypical BSE (BASE) transmitted from asymptomatic aging cattle to a primate. PLoS One 3: e3017. doi: 10.1371/journal.pone.0003017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagiwara K., Yamakawa Y., Sato Y., Nakamura Y., Tobiume M., Shinagawa M., Sata T.2007. Accumulation of mono-glycosylated form-rich, plaque-forming PrPSc in the second atypical bovine spongiform encephalopathy case in Japan. Jpn. J. Infect. Dis. 60: 305–308. [PubMed] [Google Scholar]
- 10.Henderson D. M., Davenport K. A., Haley N. J., Denkers N. D., Mathiason C. K., Hoover E. A.2015. Quantitative assessment of prion infectivity in tissues and body fluids by real-time quaking-induced conversion. J. Gen. Virol. 96: 210–219. doi: 10.1099/vir.0.069906-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwamaru Y., Imamura M., Matsuura Y., Masujin K., Shimizu Y., Shu Y., Kurachi M., Kasai K., Murayama Y., Fukuda S., Onoe S., Hagiwara K., Yamakawa Y., Sata T., Mohri S., Okada H., Yokoyama T.2010. Accumulation of L-type bovine prions in peripheral nerve tissues. Emerg. Infect. Dis. 16: 1151–1154. doi: 10.3201/eid1607.091882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs J. G., Langeveld J. P., Biacabe A. G., Acutis P. L., Polak M. P., Gavier-Widen D., Buschmann A., Caramelli M., Casalone C., Mazza M., Groschup M., Erkens J. H., Davidse A., van Zijderveld F. G., Baron T.2007. Molecular discrimination of atypical bovine spongiform encephalopathy strains from a geographical region spanning a wide area in Europe. J. Clin. Microbiol. 45: 1821–1829. doi: 10.1128/JCM.00160-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong Q., Zheng M., Casalone C., Qing L., Huang S., Chakraborty B., Wang P., Chen F., Cali I., Corona C., Martucci F., Iulini B., Acutis P., Wang L., Liang J., Wang M., Li X., Monaco S., Zanusso G., Zou W. Q., Caramelli M., Gambetti P.2008. Evaluation of the human transmission risk of an atypical bovine spongiform encephalopathy prion strain. J. Virol. 82: 3697–3701. doi: 10.1128/JVI.02561-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konold T., Bone G. E., Clifford D., Chaplin M. J., Cawthraw S., Stack M. J., Simmons M. M.2012. Experimental H-type and L-type bovine spongiform encephalopathy in cattle: observation of two clinical syndromes and diagnostic challenges. BMC Vet. Res. 8: 22. doi: 10.1186/1746-6148-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okada H., Iwamaru Y., Imamura M., Masujin K., Matsuura Y., Shimizu Y., Kasai K., Mohri S., Yokoyama T., Czub S.2011. Experimental H-type bovine spongiform encephalopathy characterized by plaques and glial- and stellate-type prion protein deposits. Vet. Res. (Faisalabad) 42: 79. doi: 10.1186/1297-9716-42-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ono F., Tase N., Kurosawa A., Hiyaoka A., Ohyama A., Tezuka Y., Wada N., Sato Y., Tobiume M., Hagiwara K., Yamakawa Y., Terao K., Sata T.2011. Atypical L-type bovine spongiform encephalopathy (L-BSE) transmission to cynomolgus macaques, a non-human primate. Jpn. J. Infect. Dis. 64: 81–84. [PubMed] [Google Scholar]
- 17.Suardi S., Vimercati C., Casalone C., Gelmetti D., Corona C., Iulini B., Mazza M., Lombardi G., Moda F., Ruggerone M., Campagnani I., Piccoli E., Catania M., Groschup M. H., Balkema-Buschmann A., Caramelli M., Monaco S., Zanusso G., Tagliavini F.2012. Infectivity in skeletal muscle of cattle with atypical bovine spongiform encephalopathy. PLoS One 7: e31449. doi: 10.1371/journal.pone.0031449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilham J. M., Orrú C. D., Bessen R. A., Atarashi R., Sano K., Race B., Meade-White K. D., Taubner L. M., Timmes A., Caughey B.2010. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog. 6: e1001217. doi: 10.1371/journal.ppat.1001217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Will R. G., Ironside J. W., Zeidler M., Cousens S. N., Estibeiro K., Alperovitch A., Poser S., Pocchiari M., Hofman A., Smith P. G.1996. A new variant of Creutzfeldt-Jakob disease in the U.K. Lancet 347: 921–925. doi: 10.1016/S0140-6736(96)91412-9 [DOI] [PubMed] [Google Scholar]
- 20.Wilson R., Dobie K., Hunter N., Casalone C., Baron T., Barron R. M.2013. Presence of subclinical infection in gene-targeted human prion protein transgenic mice exposed to atypical bovine spongiform encephalopathy. J. Gen. Virol. 94: 2819–2827. doi: 10.1099/vir.0.052738-0 [DOI] [PubMed] [Google Scholar]