Abstract
The anesthetic effects of alfaxalone combined with medetomidine and butorphanol were investigated for ICR, BALB/c, and C57BL/6 mice. Mice were administered a combination of 0.5 or 0.75 mg/kg medetomidine and 5 mg/kg butorphanol with 30 or 40 mg/kg alfaxalone (0.5MBA30, 0.75MBA30 and 0.75MBA40, respectively). The drug combinations were administered subcutaneously and were compared with a widely used combination of 0.3 mg/kg medetomidine, 4 mg/kg midazolam, and 5 mg/kg butorphanol (MMB). All three MBA combinations achieved surgical anesthesia, although the recovery time was longer with 0.75MBA30 and 0.75MBA40 compared with 0.5MBA30. Furthermore, several mice exhibited a considerable jumping reaction immediately after injection with 0.75MBA30 and 0.75MBA40. Therefore, 0.5MBA30 may be suitable for inducing surgical anesthesia in the mouse strains tested. The anesthetic scores for 0.5MBA30 were improved compared with those of MMB in all three mouse strains; however, the body temperature drop in C57BL/6 mice was greater with 0.5MBA30. Our results show that the alfaxalone combination, 0.5MBA30, should allow surgical operations that are more stable in more strains of mice than MMB, although the combination may cause hypothermia, especially in C57BL/6 mice.
Keywords: alfaxalone, anesthesia, butorphanol, medetomidine, mice
The mixture of medetomidine, midazolam, and butorphanol (MMB) has been widely used in rodents as a narcotic-free anesthetic since ketamine was designated as a narcotic [7, 10,11,12]. However, various mixing ratios and administration routes have been reported for MMB [3, 13, 19]. In addition, none of these three drugs are anesthetics; and butorphanol is an analgesic, medetomidine is a sedative and an analgesic, and midazolam is a sedative.
Alfaxalone (3α-hydroxy-5α-pregnane-11, 20-dione) is an injectable neurosteroid anesthetic [27] that is widely used to induce anesthesia and provides satisfactory induction of anesthesia in dogs [1, 17, 25] and cats [18, 29]. In addition, alfaxalone anesthesia has been investigated in rats [14, 28], pigs [8], and horses [4, 5]. Alfaxalone has few or no cardiovascular effects when given at clinical doses, unlike propofol [9, 16, 20]. Several studies have reported that combining alfaxalone with sedatives and opioids improves the safety and quality of anesthetic induction in dogs [15, 23] and cats [21].
We previously examined alfaxalone as an alternative to MMB in female ICR mice [6]. In that study, administration of alfaxalone (100 mg/kg) alone gave an average anesthetic score of 1. Therefore, anesthesia using a mixture of medetomidine and butorphanol was examined in addition to that using alfaxalone. Anesthesia with 20, 40, 60, and 80 mg/kg of alphaxalone mixed with 0.3 mg/kg medetomidine and 5 mg/kg butorphanol was investigated. The mixture containing 80 mg/kg of alphaxalone caused death in some cases, and that containing 20 mg/kg alphaxalone did not reach surgical anesthetic depth. Mixtures containing 40 and 60 mg/kg alfaxalone appeared to be suitable, and sufficient anesthesia was accomplished in ICR mouse foster mothers during fertilized egg transplantation. However, when this combination was used in other strains of mice, the anesthesia was either ineffective or too deep. Therefore, a combination of anesthetics that is effective and safe for other strains of mice is required.
In the present study, we investigated three combinations of 0.5 or 0.75 mg/kg medetomidine and 5 mg/kg butorphanol with 30 or 40 mg/kg alfaxalone (0.5MBA30, 0.75MBA30, and 0.75MBA40) in male and female ICR, BALB/c, and C57BL/6 mice. We compared these combinations with MMB as an established anesthetic. We also monitored peripheral oxygen saturation (SpO2) and body temperature as vital signs to verify the safety of the anesthetics.
MATERIALS AND METHODS
This study was carried out in strict accordance with the Guidelines for Proper Conduct of Animal Experiments, Science Council of Japan (http://www.scj.go.jp/en/animal/index.html). All animal procedures and their care were approved by the Animal Care and Use Committee of Rakuno-Gakuen University in accordance with the Guide for the Care and Use of Laboratory Animals (approval number: VH16A8).
Mice
Specific pathogen-free ICR, BALB/c, and C57BL/6 mice, aged 6 weeks, were purchased from Japan SLC, Inc. (Hamamatsu, Japan). The mice were housed in autoclaved polycarbonate cages with autoclaved bedding under barrier-sustained conditions and controlled temperature (23 ± 2°C) and lighting (12 hr light/dark cycle). Mice were fed a commercial diet (CE-2, CLEA Japan, Inc., Tokyo, Japan) and received tap water ad libitum. Mice were allowed to acclimatize for at least 1 week before use at 7 weeks old.
Combinations of anesthetic drugs
Medetomidine (Dorbene Vet, Kyoritsu Seiyaku, Co., Tokyo, Japan), midazolam (Midazolam Sandoz, Sandoz Japan Co., Ltd., Tokyo, Japan), butorphanol (Vetorphale, Meiji Seika Pharma Co., Ltd., Tokyo, Japan), and alfaxalone (Alfaxan, Meiji Seika Pharma Co., Ltd.) were used in the combinations listed in Table 1. The drugs were diluted in normal saline (0.9% NaCl) to concentrations that could be administered in a total volume of 0.01 ml/g of body weight. MMB comprised 0.3 mg/kg medetomidine, 4 mg/kg midazolam, and 5 mg/kg butorphanol. Other groups of mice were administered 0.5 mg/kg medetomidine and 5 mg/kg butorphanol with 30 mg/kg alfaxalone (0.5MBA30), or 0.75 mg/kg medetomidine and 5 mg/kg butorphanol with 30 mg/kg (0.75MBA30) or 40 mg/kg (0.75MBA40) alfaxalone.
Table 1. Summary of the drugs and doses used in this study.
| Abbreviation | Product | Product concentration (mg/ml) |
Agent dose (mg/kg) |
Volume in 10 ml normal saline (ml) |
|---|---|---|---|---|
| MMB | Medetomidine | 1 | 0.3 | 0.3 |
| Midazolam | 5 | 4 | 0.8 | |
| Butorphanol | 5 | 5 | 1 | |
| 0.9% NaCl | - | - | 7.9 | |
| 0.5MBA30 | Medetomidine | 1 | 0.5 | 0.5 |
| Butorphanol | 5 | 5 | 1 | |
| Alfaxalone | 10 | 30 | 3 | |
| 0.9% NaCl | - | - | 5.5 | |
| 0.75MBA30 | Medetomidine | 1 | 0.75 | 0.75 |
| Butorphanol | 5 | 5 | 1 | |
| Alfaxalone | 10 | 30 | 3 | |
| 0.9% NaCl | - | - | 5.25 | |
| 0.75MBA40 | Medetomidine | 1 | 0.75 | 0.75 |
| Butorphanol | 5 | 5 | 1 | |
| Alfaxalone | 10 | 40 | 4 | |
| 0.9% NaCl | - | - | 4.25 | |
Experimental protocols
Twenty male and 20 female ICR, BALB/c, and C57BL/6 mice (total of 120 mice) were randomly allocated to 24 groups (5 mice per group), and each group received one anesthetic protocol. All drugs were administered subcutaneously. After drug injection, the mouse was kept on a heater plate (FHP450-S, Tokyo Glass Kikai, Co., Ltd., Tokyo, Japan) maintained at approximately 37°C. The reflex response to a stimulating noxious stimulus was tested every 5 min until these responses recovered after drug administration.
Assessment of anesthetic depth
The reflex response to a stimulus was assessed using a method reported by Kawai et al. with some modifications [7]. Five reflexes were evaluated: the righting reflex, the fore- and hindlimb pedal withdrawal reflexes, the tail pinch reflex, and the eyelid reflex. The righting reflex was assessed by placing the mouse on its back and observing the motion taken to correct its posture to determine the presence (score=0) or absence (score=1) of a reflex. The tail pinch reflex was assessed in six locations by pinching the proximal tail lightly with atraumatic forceps, and observing the presence (score=0) or absence (score=1) of a reflex. The pedal withdrawal reflex was assessed by lightly pinching the interdigital webbing of all four limbs using atraumatic forceps, and observing the presence (score=0) or absence (score=1) of a reflex in the fore- and hindlimbs. The eye reflex was assessed by blowing air onto the cornea using a Pasteur pipette with a 2 ml silicone nipple, and observing the presence (score=0) or absence (score=1) of a reflex. Each parameter was scored, and the anesthetic depth was expressed as the total score for each mouse. A score of ≥4 was defined as surgical anesthesia. The time to the loss of the righting reflex and the time to recovery of the righting reflex were also recorded. The immobilization time (i.e., the time during which the animal made no movements) was defined as the time from the loss of the righting reflex to the recovery of the righting reflex. An electric thermometer (ThemoScan, Braun, Kronberg, Germany) was used to measure ear temperature [22]. All tests were performed by investigators who were not blinded to the study treatments.
Measurement of SpO2 in 0.5MBA30 and MMB
A pulse oximeter (Hakubatec Lifescience Solutions, Tokyo, Japan) was used to measure SpO2. A total of 36 mice, comprising 12 ICR, BALB/c, and C57BL/6 mice (6 males and 6 females of each strain) were used, with 3 mice per group. Mice in each group were administered 0.5MBA30 or MMB subcutaneously. The sensor clip of the pulse oximeter was attached to the hind leg of the mouse and SpO2 was measured from anesthesia administration to recovery.
Statistical analysis
All data are presented as the mean ± standard error of the mean (SEM). Statistical analysis was performed using Kruskal-Wallis test with Steel-Dwass multiple pairwise post hoc comparisons for three or more groups, and the Mann-Whitney U test between two groups. Fisher’s exact test was used for the statistical analysis of the average difference. Statistical significance was defined as P<0.05. The free statistical software R (version 3.2.2) was used for all analyses (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Anesthetic scores
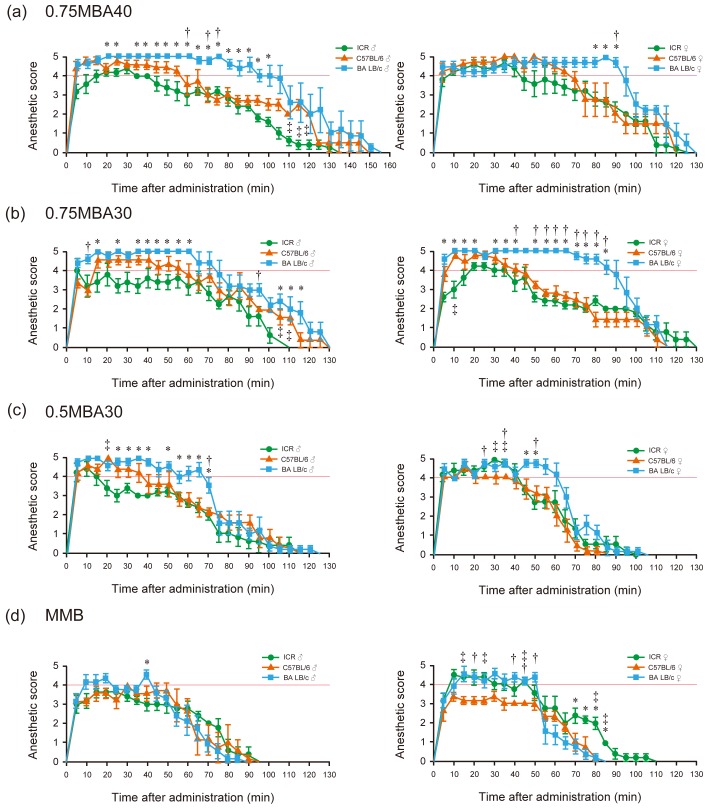
In our previous study, intraperitoneal administration of anesthesia did not reach the depth required for surgical anesthesia or significant variation was observed in the anesthetic score; thus, in this work, the anesthetics were administered subcutaneously [6]. For 0.75MBA40, surgical anesthesia (score ≥4) was reached with the male and female mice, but the anesthesia time of more than 2 hr was as long as that for injection anesthesia, and was particularly long for BALB/c mice (Fig. 1a). For 0.75MBA30, the recovery from anesthesia in all mouse strains was as slow as that for injection anesthesia, similar to 0.75MBA40. Recovery in male BALB/c mice was faster than for 0.75MBA40, although the BALB/c mice had significantly longer anesthesia times than the other strains of mice (Fig. 1b). For 0.5MBA30, surgical anesthesia was reached with all tested male and female mouse strains and the length of the anesthesia time in BALB/c was decreased (Fig. 1c). In this anesthesia group, the average of anesthesia score of the ICR male mouse was low compared with other strains and the surgical anesthesia time was about 14 min. In contrast, for MMB, which is widely used in anesthesia, the average score of male mice exceeded the surgical anesthesia score only in BALB/c mice, and the length of anesthesia was also shorter than that for MBA (Fig. 1d, left panel). Female ICR and BALB/c mice reached the surgical depth of anesthesia and were stable, but female C57BL/6 mice had an average anesthesia score of around 3, which was inappropriate for surgical procedures (Fig. 1d, right panel).
Fig. 1.
Anesthetic scores following subcutaneous administration of the anesthetic drug combinations. (a) 0.75MBA40 (0.75 mg/kg medetomidine, 5 mg/kg butorphanol, and 40 mg/kg alfaxalone). (b) 0.75MBA30 (0.75 mg/kg medetomidine, 5 mg/kg butorphanol, and 30 mg/kg alfaxalone). (c) 0.5MBA30 (0.5 mg/kg medetomidine, 5 mg/kg butorphanol, and 30 mg/kg alfaxalone). (d) MMB (0.3 mg/kg medetomidine, 4 mg/kg midazolam, and 5 mg/kg butorphanol). Green circles: ICR mice; orange triangles: C57BL/6 mice; blue squares: BALB/c mice. Left panels: males; right panels: females. Surgical anesthesia was defined as a total anesthetic score of ≥4 (red lines). Results are presented as the mean ± SEM of five mice. Statistical analysis was performed using the Kruskal-Wallis test with Steel-Dwass multiple pairwise post hoc comparisons. *Significant difference (P<0.05) in ICR vs. BALB/c mice, †Significant difference (P<0.05) in C57BL/6 vs. BALB/c mice. ‡Significant difference (P<0.05) in ICR vs. C57BL/6 mice.
Jumping reaction
Several mice jumped one to three times within 20 sec of MBA administration. This jumping reaction was not observed during anesthesia and awakening, and did not affect anesthesia. This reaction, which appears to be unique to alphaxalone, was observed in six mice (two ICR female mice, one C57BL/6 male mouse, one C57BL/6 female mouse, one BALB/c male mouse, and one BALB/c female mouse) for 0.75MBA40, and in three mice (one C57BL/6 male mouse, one C57BL/6 female mouse, and one BALB/c female mouse) for 0.75MBA30. In addition, the response was seen in only one mouse for 0.5MBA30 (one BALB/c male mouse) and not observed for MMB.
Changes in body temperature
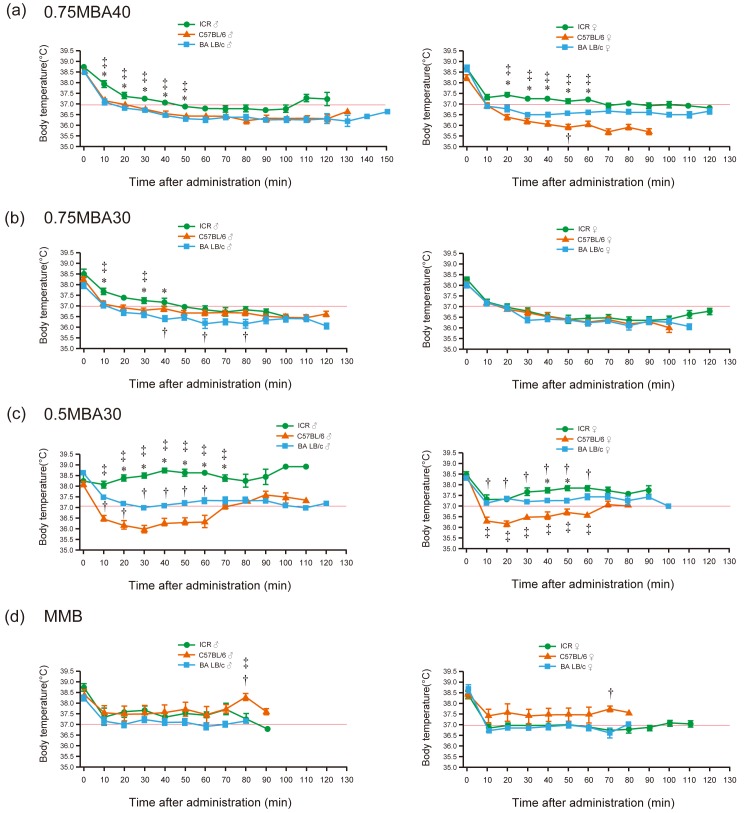
Prior to anesthesia, the body temperatures in the three strains of mice remained nearly constant in the range of 38 to 39°C (Fig. 2). For 0.75MBA40, all tested mice maintained a low body temperature in the range of 36–37.5°C from 20 min after administration to recovery and similar results were obtained for 0.75MBA30, in which the alphaxalone concentration was reduced to 30 mg/kg (Fig. 2a, 2b). For 0.5MBA30, body temperatures did not drop below 37°C throughout the anesthesia period in all male and female ICR and BALB/c mice (Fig. 2c). In C57BL/6 mice, the average body temperature was lower than 37°C after 10 min of anesthesia and did not exceed 37°C until 70 min after anesthesia (Fig. 2c). In contrast, the body temperatures of the mice in the MMB group were stable and did not fall significantly below 37°C during the observation period (Fig. 2d).
Fig. 2.
Body temperature following subcutaneous administration of the anesthetic drug combinations. (a) 0.75MBA40 (0.75 mg/kg medetomidine, 5 mg/kg butorphanol, and 40 mg/kg alfaxalone). (b) 0.75MBA30 (0.75 mg/kg medetomidine, 5 mg/kg butorphanol, and 30 mg/kg alfaxalone). (c) 0.5MBA30 (0.5 mg/kg medetomidine, 5 mg/kg butorphanol, and 30 mg/kg alfaxalone). (d) MMB (0.3 mg/kg medetomidine, 4 mg/kg midazolam, and 5 mg/kg butorphanol). Green circles: ICR mice; orange triangles: C57BL/6 mice; blue squares: BALB/c mice. Left panels: males; right panels: females. Body temperature of 37°C is shown by the red lines. Results are presented as the mean ± SEM of five mice. Statistical analysis was performed using the Kruskal-Wallis test with Steel-Dwass multiple pairwise post hoc comparisons. *Significant difference (P<0.05) in ICR vs. BALB/c mice, †Significant difference (P<0.05) in C57BL/6 vs. BALB/c mice. ‡Significant difference (P<0.05) in ICR vs. C57BL/6 mice.
Surgical anesthesia and immobilization times
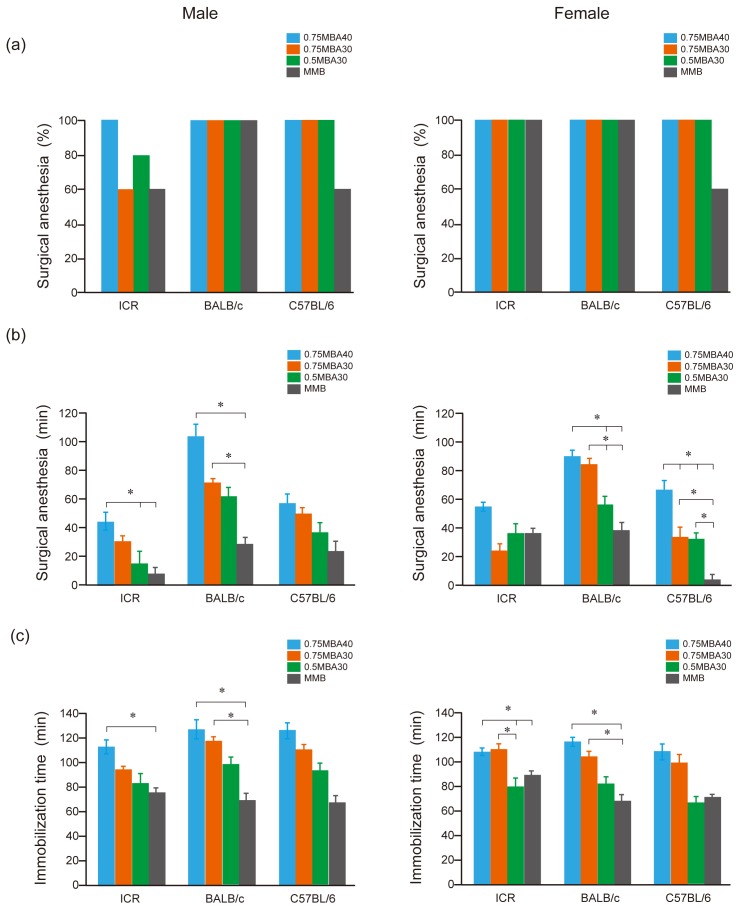
In the MMB group, two male ICR mice, and two male and two female C57BL/6 mice did not reach surgical anesthetic depth. Two male ICR mice in the 0.75MBA30 group and one male ICR mouse in the 0.5MBA30 group did not reach surgical anesthetic depth; however, the other mice treated with MBA did reach surgical anesthetic depth (Fig. 3a).
Fig. 3.
Comparison of surgical anesthesia and immobilization times after administration of 0.75MBA40, 0.75MBA30, 0.5MBA30, and MMB. (a) Percentage of mice (n=5) that reached surgical anesthesia. A score of ≥4 was defined as surgical anesthesia. Statistical analysis was performed using Fisher’s exact test. (b) Time to surgical anesthesia (i.e., score ≥4). Results are presented as the mean ± SEM of five mice. Statistical analysis was performed using the Kruskal-Wallis test with Steel-Dwass multiple pairwise post hoc comparisons. *P<0.05. (c) Immobilization time. The immobilization time was defined as the time from the loss of the righting reflex to the recovery of the righting reflex. Results are presented as the mean ± SEM of five mice. Statistical analysis was performed using the Kruskal-Wallis test with Steel-Dwass multiple pairwise post hoc comparisons. *P<0.05. Blue bars: 0.75MBA40; orange bars: 0.75MBA30; green bars: 0.5MBA30: gray bars: MMB. Left panels: males; right panels: females.
Fisher’s exact test detected no statistically significant difference between MMB and the combination containing alphaxalone. The surgical anesthesia time for MBA, which contained alphaxalone, was significantly longer than that for MMB, especially in female C57BL/6 mice (Fig. 3b). In male ICR mice, the surgical anesthesia time for 0.5MBA30 was significantly shorter than that for 0.75MBA40. In contrast, for 0.75MBA40, the surgical anesthesia time for male and female BALB/c mice was >80 min, which was too long, and it was considered unsuitable as an injectable anesthetic. Even for 0.75MBA30, the surgical anesthesia time in female BALB/c mice was 80 min or more. For 0.5MBA30, the surgical anesthesia time was about 60 min in male and female BALB/c mice, which was better than the short surgical anesthesia time for MMB in female C57BL/6 mice.
The immobilization time was defined as the time from the loss of the righting reflex to the recovery of the righting reflex. For 0.75MBA40 and 0.75MBA30, immobilization times were more than 100 min in male and female BALB/c mice and more than 100 min in female ICR mice (Fig. 3c). In contrast, for 0.5MBA30, the average immobilization time was less than 100 min in all tested mice, and was similar to that of MMB in C57BL/6 female mice, whose surgical anesthesia time was very short for MMB (Fig. 3b, 3c, right panel).
Changes in SpO2 for 0.5MBA30 and MMB
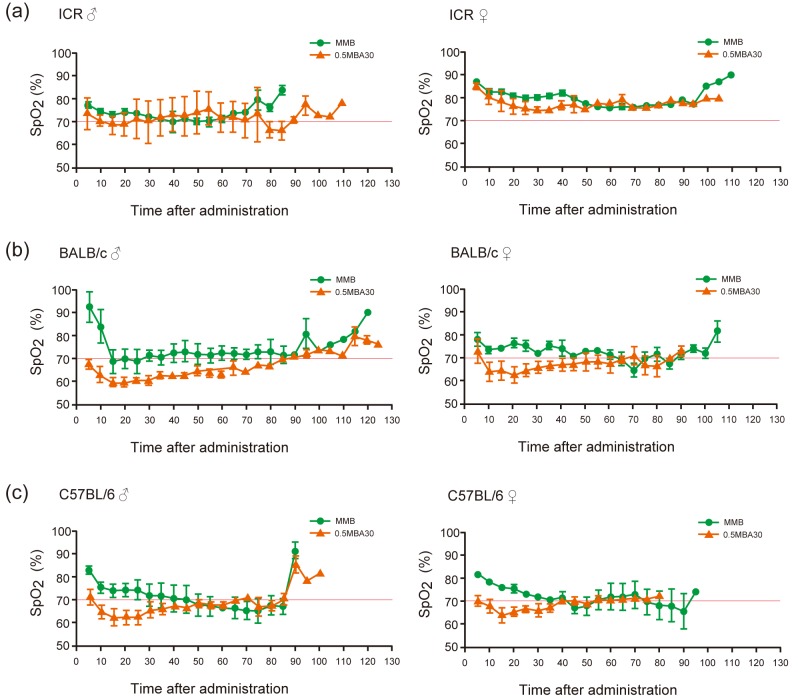
The changes in SpO2 over time in the 0.5MBA30 and MMB groups are shown in Fig. 4. SpO2 was measured every 5 min, starting 5 min after drug administration. SpO2 rarely fell below an average of 70% in the MMB group, and tended to be lower throughout the experiment in the 0.5MBA30 group. In ICR mice, SpO2 values remained around 70–80% for both MMB and 0.5MBA30 (Fig. 4a). In BALB/c mice in the 0.5MBA30 group, SpO2 values were lower than those in the MMB group for up to 80 min after administration in males and up to 60 min in females (Fig. 4b). In C57BL/6 mice in the 0.5MBA30 group, SpO2 values tended to be lower compared with the MMB group until about 40 min after administration of the anesthetic (Fig. 4c).
Fig. 4.
Comparison of SpO2 over time after administration of MMB and 0.5MBA30. (a) ICR, (b) BALB/c, and (c) C57BL/6 mice. Left panels: males; right panels: females. Green circles: MMB; orange triangles: 0.5MBA30. 70% SpO2 is shown by the red lines. Results are presented as the mean ± SEM of three mice. Statistical analysis was performed using the Mann-Whitney U test. No statistically significant difference was detected between MMB and 0.5MBA30.
DISCUSSION
In a previous study, we reported that a combination of 0.3 mg/kg medetomidine, 5 mg/kg butorphanol, and 60 mg/kg alfaxalone (0.3MBA60) is suitable for anesthesia in female ICR mice [6]. Female ICR mice were chosen because they are commonly used as foster mothers in the embryo transfer technique. However, 0.3MBA60 produced either ineffective or overly deep anesthesia in other mouse strains. Therefore, the concentrations were changed to find a combination of medetomidine, butorphanol, and alfaxalone that can be used effectively and safely in other mouse strains. Combinations of anesthetics that included alfaxalone caused death at high concentrations of alfaxalone (>60 mg/kg) in preliminary experiments; therefore, the alphaxalone concentration was gradually decreased from 60 mg/kg to 40, 30, 20, and 10 mg/kg. We used a maximum concentration of medetomidine of 0.75 mg/kg, which is 2.5 times the usual dose, and decreased the concentration gradually to determine the minimum dose with an anesthetic effect. For a medetomidine concentration of 0.75 mg/kg and an alphaxalone concentration of 30 mg/kg (0.75MBA30), deep anesthesia was achieved in BALB/c mice; therefore, a medetomidine concentration of 0.5 mg/kg (0.5MBA30) was also examined.
In this study, we compared the combinations of 0.5MBA30, 0.75MBA30, and 0.75MBA40, which were promising in preliminary experiments in three mouse strains. However, 0.75MBA30 and 0.75MBA40 produced long, deep anesthesia, a large decrease in body temperature, and an SpO2 concentration below 60% (data not shown). In a previous study, alfaxalone caused a spectrum of activities, including popcorn-like jumping movements after injection, intense scratching of the face, hyperresponsiveness to noise or touch, and marked limb jerking during recovery in C57BL/6 mice [24]. A combination of alfaxalone and xylazine was also tested, in which the alfaxalone concentration was 80 mg/kg administered intraperitoneally, more than twice the dose used in the present study. We also observed jumping in six of the 30 mice for 0.75MBA40 and three of the 30 mice for 0.75MBA30 after administration, although this activity was observed in only one mouse out of 30 after administering 0.5MBA30. No other marked clinical side effects were observed in the three combinations.
MMB is the most common anesthetic for experimental mice, but the combination of concentrations differs between laboratories [3, 13]. Ochiai et al. [19] reported that MMB affects blood biochemistry in male C57BL/6 mice. They subcutaneously administered a low-dose combination (0.45 mg/kg medetomidine, 6 mg/kg midazolam, 7.5 mg/kg butorphanol), which was the minimum dose that enabled surgery, and a high-dose combination (0.9 mg/kg medetomidine, 12 mg/kg midazolam, 15 mg/kg butorphanol), which was the maximum non-lethal dose. Their experiments showed that 1.5 times the conventional dose (0.3 mg/kg medetomidine, 4 mg/kg midazolam, 5 mg/kg butorphanol) was required for surgical treatment in C57BL/6 mice. In our experiments, only 60% of C57BL/6 mice showed surgical anesthesia at the conventional dose. Furthermore, the drugs in the MMB combination are sedatives and analgesics, and not anesthetics.
Based on the anesthesia score and anesthesia time, 0.5MBA30 appeared to be a versatile combination that could be used in any mouse strain compared with MMB. Especially in female C57BL/6 mice, the surgical anesthesia time for MMB was about 5 min, whereas that of 0.5MBA30 was about 30 min, and the recovery time was similar. Male rats and mice require a much higher dose of alfaxalone to reach and maintain surgical anesthesia than female animals [2, 24]. The surgical anesthesia of time for 0.5MBA30 displayed sex-dependent effects in ICR mice. However, even for MMB, which did not contain alphaxalone, sex-dependent effects were observed in ICR and C57BL/6 mice, so it is unlikely that alphaxalone alone is the cause; thus, it is necessary to examine the sex-dependent effects of anesthetics in mice.
0.5MBA30 produced lower body temperatures and SpO2 values than MMB. Body temperature and SpO2 are important indicators for assessing the depth and side effects of anesthesia [26]. We used an infrared clinical thermometer to measure the ear temperature and our results were similar to those of previous reports [22]. For MMB, the body temperature rarely fell below 37°C throughout the anesthesia period, whereas a sharp drop in body temperature was observed for 0.5MBA30 in C57BL/6 mice. Thus, warming is essential when using this anesthetic. Under MMB anesthesia, SpO2 rarely fell below 70% in any mouse strain, similar to previous reports [11]. For ICR mice, 0.5MBA30 showed similar SpO2 values to MMB, but the concentrations tended to be lower in BALB/c and C57BL/6 mice. In particular, BALB/c mice took longer to recover to the same SpO2 as with MMB.
In summary, the combination 0.5MBA30 of alfaxalone, medetomidine, and butorphanol may allow surgical anesthesia that is more stable in more strains of mice than is possible with MMB. However, the combination requires precautions against hypothermia, especially in C57BL/6 mice.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Acknowledgments
This work was supported in part by the Japan Leukemia Research Fund.
REFERENCES
- 1.Ferré P. J., Pasloske K., Whittem T., Ranasinghe M. G., Li Q., Lefebvre H. P.2006. Plasma pharmacokinetics of alfaxalone in dogs after an intravenous bolus of Alfaxan-CD RTU. Vet. Anaesth. Analg. 33: 229–236. doi: 10.1111/j.1467-2995.2005.00264.x [DOI] [PubMed] [Google Scholar]
- 2.Fink G., Sarkar D. K., Dow R. C., Dick H., Borthwick N., Malnick S., Twine M.1982. Sex difference in response to alphaxalone anaesthesia may be oestrogen dependent. Nature 298: 270–272. doi: 10.1038/298270a0 [DOI] [PubMed] [Google Scholar]
- 3.Fujita T., Yamashita D., Katsunuma S., Hasegawa S., Tanimoto H., Nibu K.2012. Increased inner ear susceptibility to noise injury in mice with streptozotocin-induced diabetes. Diabetes 61: 2980–2986. doi: 10.2337/db11-1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodwin W., Keates H., Pasloske K., Pearson M., Sauer B., Ranasinghe M. G.2012. Plasma pharmacokinetics and pharmacodynamics of alfaxalone in neonatal foals after an intravenous bolus of alfaxalone following premedication with butorphanol tartrate. Vet. Anaesth. Analg. 39: 503–510. doi: 10.1111/j.1467-2995.2012.00734.x [DOI] [PubMed] [Google Scholar]
- 5.Goodwin W. A., Keates H. L., Pasloske K., Pearson M., Sauer B., Ranasinghe M. G.2011. The pharmacokinetics and pharmacodynamics of the injectable anaesthetic alfaxalone in the horse. Vet. Anaesth. Analg. 38: 431–438. doi: 10.1111/j.1467-2995.2011.00634.x [DOI] [PubMed] [Google Scholar]
- 6.Higuchi S., Yamada R., Hashimoto A., Miyoshi K., Yamashita K., Ohsugi T.2016. Evaluation of a combination of alfaxalone with medetomidine and butorphanol for inducing surgical anesthesia in laboratory mice. Jpn. J. Vet. Res. 64: 131–139. [PubMed] [Google Scholar]
- 7.Kawai S., Takagi Y., Kaneko S., Kurosawa T.2011. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim. 60: 481–487. doi: 10.1538/expanim.60.481 [DOI] [PubMed] [Google Scholar]
- 8.Keates H.2003. Induction of anaesthesia in pigs using a new alphaxalone formulation. Vet. Rec. 153: 627–628. doi: 10.1136/vr.153.20.627 [DOI] [PubMed] [Google Scholar]
- 9.Keates H., Whittem T.2012. Effect of intravenous dose escalation with alfaxalone and propofol on occurrence of apnoea in the dog. Res. Vet. Sci. 93: 904–906. doi: 10.1016/j.rvsc.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 10.Kirihara Y., Takechi M., Kurosaki K., Kobayashi Y., Kurosawa T.2013. Anesthetic effects of a mixture of medetomidine, midazolam and butorphanol in two strains of mice. Exp. Anim. 62: 173–180. doi: 10.1538/expanim.62.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirihara Y., Takechi M., Kurosaki K., Kobayashi Y., Saito Y., Takeuchi T.2015. Anesthetic effects of a three-drugs mixture--comparison of administrative routes and antagonistic effects of atipamezole in mice. Exp. Anim. 64: 39–47. doi: 10.1538/expanim.14-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirihara Y., Takechi M., Kurosaki K., Kobayashi Y., Saito Y., Takeuchi T.2016. Effects of an anesthetic mixture of medetomidine, midazolam, and butorphanol in rats-strain difference and antagonism by atipamezole. Exp. Anim. 65: 27–36. doi: 10.1538/expanim.15-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuroda N., Inoue K., Ikeda T., Hara Y., Wake K., Sato T.2014. Apoptotic response through a high mobility box 1 protein-dependent mechanism in LPS/GalN-induced mouse liver failure and glycyrrhizin-mediated inhibition. PLoS One 9: e92884. doi: 10.1371/journal.pone.0092884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau C., Ranasinghe M. G., Shiels I., Keates H., Pasloske K., Bellingham M. C.2013. Plasma pharmacokinetics of alfaxalone after a single intraperitoneal or intravenous injection of Alfaxan(®) in rats. J. Vet. Pharmacol. Ther. 36: 516–520. doi: 10.1111/jvp.12055 [DOI] [PubMed] [Google Scholar]
- 15.Lee J., Suh S., Choi R., Hyun C.2016. Cardiorespiratory and anesthetic effects produced by the combination of butorphanol, medetomidine and alfaxalone administered intramuscularly in Beagle dogs. J. Vet. Med. Sci. 77: 1677–1680. doi: 10.1292/jvms.15-0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muir W., Lerche P., Wiese A., Nelson L., Pasloske K., Whittem T.2008. Cardiorespiratory and anesthetic effects of clinical and supraclinical doses of alfaxalone in dogs. Vet. Anaesth. Analg. 35: 451–462. doi: 10.1111/j.1467-2995.2008.00406.x [DOI] [PubMed] [Google Scholar]
- 17.O’Hagan B., Pasloske K., McKinnon C., Perkins N., Whittem T.2012. Clinical evaluation of alfaxalone as an anaesthetic induction agent in dogs less than 12 weeks of age. Aust. Vet. J. 90: 346–350. doi: 10.1111/j.1751-0813.2012.00974.x [DOI] [PubMed] [Google Scholar]
- 18.O’Hagan B. J., Pasloske K., McKinnon C., Perkins N. R., Whittem T.2012. Clinical evaluation of alfaxalone as an anaesthetic induction agent in cats less than 12 weeks of age. Aust. Vet. J. 90: 395–401. doi: 10.1111/j.1751-0813.2012.00983.x [DOI] [PubMed] [Google Scholar]
- 19.Ochiai Y., Iwano H., Sakamoto T., Hirabayashi M., Kaneko E., Watanabe T., Yamashita K., Yokota H.2016. Blood biochemical changes in mice after administration of a mixture of three anesthetic agents. J. Vet. Med. Sci. 78: 951–956. doi: 10.1292/jvms.15-0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Psatha E., Alibhai H. I., Jimenez-Lozano A., Armitage-Chan E., Brodbelt D. C.2011. Clinical efficacy and cardiorespiratory effects of alfaxalone, or diazepam/fentanyl for induction of anaesthesia in dogs that are a poor anaesthetic risk. Vet. Anaesth. Analg. 38: 24–36. doi: 10.1111/j.1467-2995.2010.00577.x [DOI] [PubMed] [Google Scholar]
- 21.Ramoo S., Bradbury L. A., Anderson G. A., Abraham L. A.2013. Sedation of hyperthyroid cats with subcutaneous administration of a combination of alfaxalone and butorphanol. Aust. Vet. J. 91: 131–136. doi: 10.1111/avj.12034 [DOI] [PubMed] [Google Scholar]
- 22.Saegusa Y., Tabata H.2003. Usefulness of infrared thermometry in determining body temperature in mice. J. Vet. Med. Sci. 65: 1365–1367. doi: 10.1292/jvms.65.1365 [DOI] [PubMed] [Google Scholar]
- 23.Seo J. I., Han S. H., Choi R., Han J., Lee L., Hyun C.2015. Cardiopulmonary and anesthetic effects of the combination of butorphanol, midazolam and alfaxalone in Beagle dogs. Vet. Anaesth. Analg. 42: 304–308. doi: 10.1111/vaa.12223 [DOI] [PubMed] [Google Scholar]
- 24.Siriarchavatana P., Ayers J. D., Kendall L. V.2016. Anesthetic activity of alfaxalone compared with ketamine in mice. J. Am. Assoc. Lab. Anim. Sci. 55: 426–430. [PMC free article] [PubMed] [Google Scholar]
- 25.Tamura J., Ishizuka T., Fukui S., Oyama N., Kawase K., Miyoshi K., Sano T., Pasloske K., Yamashita K.2015. The pharmacological effects of the anesthetic alfaxalone after intramuscular administration to dogs. J. Vet. Med. Sci. 77: 289–296. doi: 10.1292/jvms.14-0368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsukamoto A., Serizawa K., Sato R., Yamazaki J., Inomata T.2015. Vital signs monitoring during injectable and inhalant anesthesia in mice. Exp. Anim. 64: 57–64. doi: 10.1538/expanim.14-0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Visser S. A., Smulders C. J., Gladdines W. W., Irth H., van der Graaf P. H., Danhof M.2000. High-performance liquid chromatography of the neuroactive steroids alphaxalone and pregnanolone in plasma using dansyl hydrazine as fluorescent label: application to a pharmacokinetic-pharmacodynamic study in rats. J. Chromatogr. B Biomed. Sci. Appl. 745: 357–363. doi: 10.1016/S0378-4347(00)00296-6 [DOI] [PubMed] [Google Scholar]
- 28.White K. L., Paine S., Harris J.2017. A clinical evaluation of the pharmacokinetics and pharmacodynamics of intravenous alfaxalone in cyclodextrin in male and female rats following a loading dose and constant rate infusion. Vet. Anaesth. Analg. 44: 865–875. doi: 10.1016/j.vaa.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 29.Zaki S., Ticehurst K., Miyaki Y.2009. Clinical evaluation of Alfaxan-CD(R) as an intravenous anaesthetic in young cats. Aust. Vet. J. 87: 82–87. doi: 10.1111/j.1751-0813.2009.00390.x [DOI] [PubMed] [Google Scholar]