Abstract
A neutered male Golden Retriever was referred with a 2-week history of dry mouth. Multiple and bilateral enlargement of the lacrimal and salivary glands showing heterogeneous internal enhancement was identified on contrast-enhanced computed tomography (CT). Ultrasonographic examination detected multifocal hypoechoic areas within the swollen submandibular salivary glands, which were histopathologically diagnosed as lymphoplasmacytic sialoadenitis. As both imaging and histopathological findings were in accordance with those in human Sjögren’s syndrome, a provisional diagnosis of Sjögren’s-like syndrome was made. Immunosuppressive drugs promptly improved clinical signs concurrently with the abnormal sonographic findings, indicating the feasibility of ultrasonography in monitoring therapeutic outcomes. Herein, we discuss a proposed criteria set for diagnosis of Sjögren’s-like syndrome in veterinary medicine.
Keywords: computed tomography (CT), dog, exocrine glands, Sjögren’s syndrome, ultrasonography
Sjögren’s syndrome (SS) in humans is a chronic and systemic autoimmune disorder of the exocrine glands, mainly the lacrimal and salivary glands. It is characterized by focal lymphocytic inflammation of the affected organs, as well as clinical symptoms including dry eyes and dry mouth, resulting from the exocrine dysfunction [3, 4, 12]. In dogs and cats, a syndrome similar to SS in humans, a Sjögren’s-like syndrome, has been reported [14]. However, to the best of our knowledge, no detailed information on the corresponding diagnostic criteria, treatment, and prognosis has been available, probably owing to the low incidence of this syndrome in animals [1, 5, 6]. In this report, we describe a canine case of Sjögren’s-like syndrome and present a set of diagnostic criteria that are applicable to veterinary cases, wherein imaging diagnosis including both computed tomography (CT) and ultrasonography (US) plays a key role.
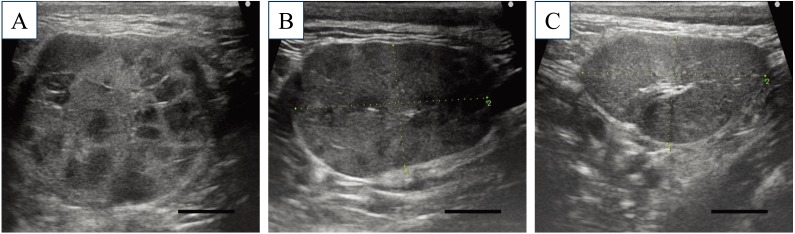
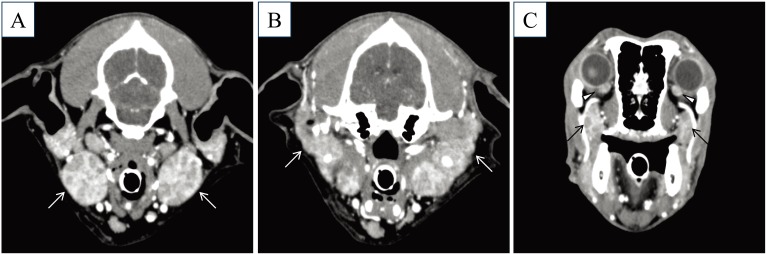
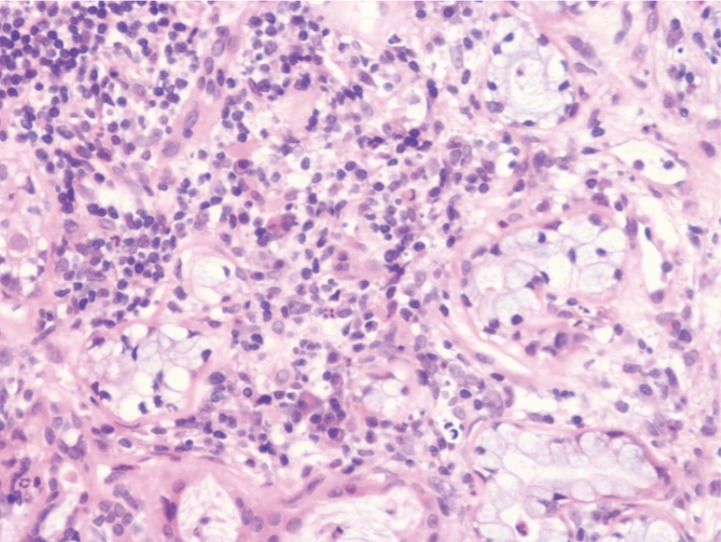
A neutered male Golden Retriever, weighing 26.4 kg, was referred to the Animal Medical Center at the Tokyo University of Agriculture and Technology for evaluation of dry mouth that had been present for the previous 2 weeks (Day 1). Since the dog was adopted from a shelter, his detailed disease and vaccination histories and his exact age were unclear. Physical examination revealed severe dryness of the oral mucosa, particularly on the surface of the tongue, resulting in loss of its glossy and moist appearance and its smoothness. Concurrently, swelling of the submandibular lymph nodes and the submandibular salivary glands was noticed. No ocular abnormalities relating to dry eyes were detected in the physical examination. Initial clinical diagnostic evaluation, including complete blood count (CBC), serum biochemical analysis, radiography, US, and cytological examination, was performed. The CBC showed only microcytic (mean corpuscular volume [MCV], 60.0 fl; reference range 61.6–73.5 fl), normochromic, mild anemia (hematocrit [Hct], 35.4%; reference range 37.3–61.7%) and mild thrombocytosis (platelet count, 506 × 103/µl; reference range 148–484 × 103/µl). The serum biochemical profile exhibited no abnormalities, except a slightly elevated level of C-reactive protein (CRP) (1.6 mg/dl; reference range <1.0 mg/dl). The detailed blood test results are shown in Table 1. While thoracic and abdominal radiography revealed no significant abnormalities, US detected bilateral swelling of the submandibular salivary glands and the submandibular and retropharyngeal lymph nodes with no significant abnormalities in the abdomen. Within the swollen submandibular salivary glands, multifocal hypoechoic areas were noted (Fig. 1A). Cytological examination of aspirates from the lymph nodes suggested the presence of an inflammatory disease and ruled out lymphoma. In CT images obtained under general anesthesia, multiple and bilateral enlargement of the salivary glands, including the submandibular, parotid, and zygomatic glands, the lacrimal glands, and the nictitating membrane glands was observed. Swelling of the submandibular and retropharyngeal lymph nodes was also noted. After intravenous administration of the contrast medium, iodixanol, the parenchyma of these moisture-secreting glands was enhanced heterogeneously (Fig. 2). During general anesthesia, tissue sampling from the submandibular salivary glands was performed through Tru-cut biopsy for histopathological examination, which diagnosed lymphoplasmacytic sialoadenitis (Fig. 3). Schirmer tear test (STT), which assesses tear secretion, was chosen as a representative test to evaluate the exocrine function owing to the bilateral abnormal CT findings in the lacrimal glands. However, clear dysfunction of the glands could not be demonstrated using STT since the values obtained were around the lower limit of the normal range. Results of serum anti-nuclear antibody and rheumatoid factor tests (IDEXX Laboratories, Tokyo, Japan) on Day 1 were both negative. Because the specific findings with a solid consensus for human SS, multiple and bilateral involvement of the exocrine glands in the head and neck along with lymphocytic infiltration into the affected glands [7, 11, 13, 15], were also detected in this dog, a provisional diagnosis of Sjögren’s-like syndrome was made.
Table 1. Blood test results before treatment.
| Analyte | Value | Reference range |
|---|---|---|
| White blood cell count (×103/µl) | 6.84 | (5.05–16.76) |
| Neutrophils (×103/µl) | 5.07 | (2.95–11.64) |
| Lymphocytes (×103/µl) | 0.93 | (1.05–5.10) |
| Monocytes (×103/µl) | 0.59 | (0.16–1.12) |
| Eosinophils (×103/µl) | 0.19 | (0.06–1.23) |
| Basophils (×103/µl) | 0.06 | (0.0–0.10) |
| Red blood cell count (×106/µl) | 5.9 | (5.65–8.87) |
| Hemoglobin (g/dl) | 13.3 | (13.1–20.5) |
| Hematocrit (%) | 35.4 | (37.3–61.7) |
| Mean corpuscular volume (fl) | 60.0 | (61.6–73.5) |
| Mean corpuscular hemoglobin (pg) | 22.5 | (21.2–25.9) |
| Mean corpuscular hemoglobin concentration (g/dl) | 37.6 | (32.0–37.9) |
| Platelet count (×103/µl) | 506.0 | (148–484) |
| Reticulocyte count (×103/µl) | 50.7 | (10.0–110.0) |
| Total protein (g/dl) | 7.0 | (5.2–8.2) |
| Albumin (g/dl) | 3.2 | (2.3–4.0) |
| Blood urea nitrogen (mg/dl) | 9.0 | (7–27) |
| Creatinine (mg/dl) | 0.7 | (0.5–1.8) |
| Phosphorus (mg/dl) | 4.4 | (2.5–6.8) |
| Calcium (mg/dl) | 9.0 | (7.9–12.0) |
| Alanine aminotransferase (units/l) | 20.0 | (10–125) |
| Alkaline phosphatase (units/l) | 45.0 | (23–212) |
| Total bilirubin (mg/dl) | 0.3 | (0.0–0.9) |
| Glucose (mg/dl) | 98.0 | (74–143) |
| Sodium (mmol/l) | 150.0 | (144–160) |
| Potassium (mmol/l) | 4.8 | (3.5–5.8) |
| Chloride (mmol/l) | 116.0 | (109–122) |
| C-reactive protein (mg/dl) | 1.6 | (<1.0) |
| Total T4 (µg/dl) | 2.1 | (1.0–4.0) |
Fig. 1.
Ultrasonography of the submandibular salivary glands. At initial presentation (Day 1), the submandibular salivary glands were severely enlarged and multifocal hypoechogenic areas were identified within them (A). After 1 week of treatment with PSL (Day 8), the enlarged glands had decreased in size and the echogenic abnormalities within the glands were smaller (B). On Day 29, the size and echogenicity of the affected glands had returned to normal (C). Bar=1.0 cm.
Fig. 2.
CT images of the salivary glands and lacrimal glands after intravenous administration of contrast medium (iodixanol). The submandibular glands (A, arrows), parotid glands (B, arrows), zygomatic glands (C, arrows), and nictitating membrane glands (C, arrowheads) were bilaterally enlarged with heterogeneous enhancement by contrast medium.
Fig. 3.
Histopathological section of the submandibular salivary glands. Tissue samples obtained from the submandibular salivary glands by Tru-cut biopsy on Day 1 were subjected to hematoxylin-eosin staining according to a conventional method. The section showed mild to moderate lymphoplasmacytic infiltration with a small number of neutrophils in the stroma. Pyknotic or degenerated epithelial cells were occasionally seen. There was no evidence of neoplastic proliferation or infectious agents.
For treatment, prednisolone (PSL) was administered starting on Day 1 at a dose of 1 mg/kg, every (q) 12 hr. On Day 8, a reduction in the size of the submandibular salivary glands and lymph nodes was noted on cervical US (Fig. 1B). Concurrently, serum CRP levels returned to the normal range (0.05 mg/dl). Since gastrointestinal symptoms including diarrhea and hematochezia, presumably as adverse effects of PSL, were observed, administration of the immunosuppressive agent azathioprine (AZP) at an induction dose of 2 mg/kg, q 24 hr, was started on Day 12, and PSL was discontinued on Day 43 after a suitable tapering period. With the AZP induction period for 14 days, the dosage was tapered to a maintenance level of 0.5 mg/kg, q 24 hr by Day 51. During the treatment, the multifocal hypoechoic areas within the submandibular glands, originally observed on Day 1, gradually decreased in size and completely disappeared on Day 29 (Fig. 1C). Until Day 134, AZP, at the maintenance dose, successfully maintained the disease-free interval with no clinical recurrence based on salivary gland US, serum CRP levels, and clinical symptoms. Follow-up CT during the disease-free period confirmed that the abnormal findings observed on Day 1 completely disappeared. Mild anemia and thrombocytosis, which were found on Day 1, gradually improved as the immunosuppressive treatment progressed.
In humans, there are several sets of criteria for the diagnosis of SS proposed by the Japanese Ministry of Health (JPN), the American-European Consensus Group (AECG), the American College of Rheumatology (ACR), and the European League Against Rheumatism (EULAR) [7, 13, 15]. These sets of criteria are dependent on 1) clinical evaluation for secretory functions of the lacrimal or salivary glands as evidence of exocrine glands disorder and 2) histopathological evaluation of the glands and serological evaluation for autoantibodies including human SS-specific autoantibodies as evidence of autoimmune disease. However, it is impossible to apply any of these criteria sets to veterinary cases since the criteria require clinical evaluations that are not currently available in animals.
Recently, in human medicine, diagnostic imaging for assessment of exocrine gland involvement has received increasing attention as an objective examination for SS diagnosis [2, 8, 10, 11] and has been recommended for inclusion in criteria sets [2, 8]. In fact, the human CT demonstrates multiple and bilateral swelling with the heterogeneous parenchyma of the exocrine glands, which is a key finding for the diagnosis of human SS [10, 11]. Notably, our CT findings obtained from this canine case were compatible with the key findings in human SS patients. Therefore, clinical evaluation of the exocrine glands by CT should be included in the diagnostic criteria set in veterinary medicine.
During the long-term follow-up examination of this dog, salivary gland US could track the characteristic abnormality, i.e. multiple hypoechoic areas within the parenchyma, which has been reported as a specific finding in human SS [2, 9]. Interestingly, this US finding completely disappeared concurrently with clinical improvement after the treatment with immunosuppressive drugs and reappeared at an early stage of recurrence with no clinical symptoms relating to impaired secretory function. This suggests the feasibility of salivary gland US not only in detecting subclinical cases but also in evaluating therapeutic outcomes and predicting prognosis.
The histopathological finding of focal lymphocytic infiltration into the salivary glands, which is significant for diagnosis of SS in humans [7, 13, 15], was also noted in this case. Since SS-specific autoantibodies are not available in veterinary cases, the histopathological evaluation of exocrine glands providing supporting evidence of an autoimmune disease should be required for diagnosis of Sjögren’s-like syndrome.
In conclusion, based on this rare but informative case, we here propose the following two evaluations together as a veterinary criteria set for diagnosis of Sjögren’s-like syndrome: 1) diagnostic imaging evaluation as evidence of multiple exocrine gland involvement and 2) histopathological evaluation as evidence of autoimmune disease. Thus, salivary gland US should be performed in veterinary cases with symptoms of either ocular or oral dryness. Thereafter, in cases wherein the characteristic finding is noted on US, further evaluation of the multiple exocrine glands should be conducted using CT and subsequent histopathological confirmation.
Acknowledgments
This work was supported by the Training Program for Asian Veterinarians (TP-FAVII) by the Japan Veterinary Medical Association (JVMA). The authors are grateful to Dr. Koji Nishifuji, Laboratory of Veterinary Internal Medicine, for his support with the biopsy and to Dr. Ikki Mitsui, No Boundaries Animal Pathology, LLC, for his histopathological evaluation. The authors also would like to thank Ms. Namiko Ikeda, Animal Medical Center (Dr. Itoh laboratory), and Ms. Natsuki Utsumi, Laboratory of Veterinary Clinical Oncology, for their help in taking care of the dog as faculty students.
REFERENCES
- 1.Cannon M. S., Paglia D., Zwingenberger A. L., Boroffka S. A., Hollingsworth S. R., Wisner E. R.2011. Clinical and diagnostic imaging findings in dogs with zygomatic sialadenitis: 11 cases (1990–2009). J. Am. Vet. Med. Assoc. 239: 1211–1218. doi: 10.2460/javma.239.9.1211 [DOI] [PubMed] [Google Scholar]
- 2.Cornec D., Jousse-Joulin S., Pers J. O., Marhadour T., Cochener B., Boisramé-Gastrin S., Nowak E., Youinou P., Saraux A., Devauchelle-Pensec V.2013. Contribution of salivary gland ultrasonography to the diagnosis of Sjögren’s syndrome: toward new diagnostic criteria? Arthritis Rheum. 65: 216–225. doi: 10.1002/art.37698 [DOI] [PubMed] [Google Scholar]
- 3.Dumusc A., Rao V., Bowman J. S.2018. Sjogren’s syndrome. Medicine 46: 126–130. [Google Scholar]
- 4.Fox R. I.2005. Sjögren’s syndrome. Lancet 366: 321–331. doi: 10.1016/S0140-6736(05)66990-5 [DOI] [PubMed] [Google Scholar]
- 5.McGill S., Lester N., McLachlan A., Mansfield C.2009. Concurrent sialocoele and necrotising sialadenitis in a dog. J. Small Anim. Pract. 50: 151–156. doi: 10.1111/j.1748-5827.2009.00706.x [DOI] [PubMed] [Google Scholar]
- 6.Pérez-Écija A., Estepa J. C., Mendoza F. J.2012. Granulomatous giant cell submandibular sialadenitis in a dog. Can. Vet. J. 53: 1211–1213. [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen A., Ice J. A., Li H., Grundahl K., Kelly J. A., Radfar L., Stone D. U., Hefner K. S., Anaya J. M., Rohrer M., Gopalakrishnan R., Houston G. D., Lewis D. M., Chodosh J., Harley J. B., Hughes P., Maier-Moore J. S., Montgomery C. G., Rhodus N. L., Farris A. D., Segal B. M., Jonsson R., Lessard C. J., Scofield R. H., Sivils K. L.2014. Comparison of the American-European Consensus Group Sjogren’s syndrome classification criteria to newly proposed American College of Rheumatology criteria in a large, carefully characterised sicca cohort. Ann. Rheum. Dis. 73: 31–38. doi: 10.1136/annrheumdis-2013-203845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salaffi F., Carotti M., Iagnocco A., Luccioli F., Ramonda R., Sabatini E., De Nicola M., Maggi M., Priori R., Valesini G., Gerli R., Punzi L., Giuseppetti G. M., Salvolini U., Grassi W.2008. Ultrasonography of salivary glands in primary Sjögren’s syndrome: a comparison with contrast sialography and scintigraphy. Rheumatology (Oxford) 47: 1244–1249. doi: 10.1093/rheumatology/ken222 [DOI] [PubMed] [Google Scholar]
- 9.Shimizu M., Okamura K., Yoshiura K., Ohyama Y., Nakamura S., Kinukawa N.2006. Sonographic diagnostic criteria for screening Sjögren’s syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 102: 85–93. doi: 10.1016/j.tripleo.2005.09.012 [DOI] [PubMed] [Google Scholar]
- 10.Sun Z., Zhang Z., Fu K., Zhao Y., Liu D., Ma X.2012. Diagnostic accuracy of parotid CT for identifying Sjögren’s syndrome. Eur. J. Radiol. 81: 2702–2709. doi: 10.1016/j.ejrad.2011.12.034 [DOI] [PubMed] [Google Scholar]
- 11.Tonami H., Matoba M., Yokota H., Higashi K., Yamamoto I., Sugai S.2002. CT and MR findings of bilateral lacrimal gland enlargement in Sjögren syndrome. Clin. Imaging 26: 392–396. doi: 10.1016/S0899-7071(02)00455-2 [DOI] [PubMed] [Google Scholar]
- 12.Tong L., Koh V., Thong B. Y.2017. Review of autoantigens in Sjögren’s syndrome: an update. J. Inflamm. Res. 10: 97–105. doi: 10.2147/JIR.S137024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuboi H., Hagiwara S., Asashima H., Takahashi H., Hirota T., Noma H., Umehara H., Kawakami A., Nakamura H., Sano H., Tsubota K., Ogawa Y., Takamura E., Saito I., Inoue H., Nakamura S., Moriyama M., Takeuchi T., Tanaka Y., Hirata S., Mimori T., Matsumoto I., Sumida T.2017. Comparison of performance of the 2016 ACR-EULAR classification criteria for primary Sjögren’s syndrome with other sets of criteria in Japanese patients. Ann. Rheum. Dis. 76: 1980–1985. doi: 10.1136/annrheumdis-2016-210758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uzal A. F., Plattner L. B., Hostetter M. J.2016. Alimentary system. p. 29. In: Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, Vol. 2, 6th ed. (Maxie, M. G. ed.), Elsevier, Amsterdam. [Google Scholar]
- 15.Vitali C., Bombardieri S., Jonsson R., Moutsopoulos H. M., Alexander E. L., Carsons S. E., Daniels T. E., Fox P. C., Fox R. I., Kassan S. S., Pillemer S. R., Talal N., Weisman M. H., European Study Group on Classification Criteria for Sjögren’s Syndrome.2002. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 61: 554–558. doi: 10.1136/ard.61.6.554 [DOI] [PMC free article] [PubMed] [Google Scholar]