Abstract
In an 8-year-old Labrador Retriever with progressive anorexia, constipation, and depression, CT revealed intussusception of the cecum into the ascending colon and a small cecal mass showing strong enhancement on arterial phase. The ileocecocolic junction was surgically resected and histologically diagnosed as cecocolic intussusception with carcinoid tumor. The carcinoid tumor worked as a lead point of intussusception in this case. Dual phasic CT is useful to assess the presence of gastrointestinal tumors as lead points in old dogs with intussusception.
Keywords: carcinoid tumor, computed tomography, dog, intussusception, lead point
An eight-year-old male Labrador Retriever was presented with progressive anorexia, constipation, diarrhea, and depression for 7 days. Intermittent discomfort during abdominal palpation was notable. On blood examination, serum lipase level (5,463 U/l; reference range, 200–1,800 U/l) and the C-reactive protein level (30 mg/l; reference range, 0–20 mg/l) were increased.
There was no remarkable finding on abdominal radiography. Abdominal ultrasonography revealed an intussusception and indistinctive wall layers of the intestinal segment proximal to the intussusception (Fig. 1). Invagination of the distal ileum into the proximal colon with focal necrosis or severe inflammation was suspected. Color Doppler application and scrutinization of the lesion including the extent and characteristics was impossible because of shadowing artifact of the intraluminal gas and the patient’s rapid breathing.
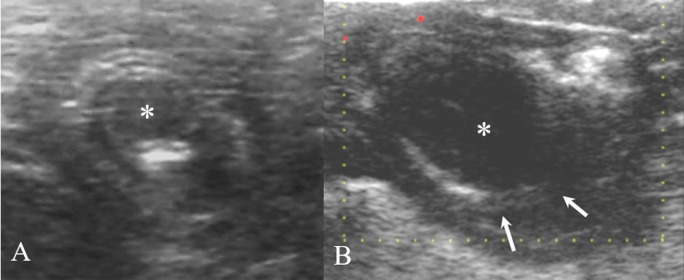
Fig. 1.
On transverse (A) and longitudinal (B) ultrasonography, the concentric ring sign and multi-layered pattern was identified around the distal part of the ileum and continuous with the proximal colon. Focal hypoechoic lesion (asterisk) was observed within the intussusceptum (arrows). Intussusception of the distal ileum into the proximal colon and edematous change of the vaginated ileum was suspected.
Computed tomography (CT) was performed using a 16-row multi-detector CT scanner (Siemens Emotion 16, Siemens, Forchheim, Germany) with the following settings: slice thickness=2 mm; pitch=0.8; rotation duration=600 msec; tube voltage=130 kV; and tube current=110 mA. Dynamic CT was performed to investigate the presence of a lead point and other lesion. A test bolus scan was conducted at the level of the intussusception, where the cranial mesenteric artery and cranial mesenteric vein were well-visualized together, after injection of 150 mgI/kg iohexol (300 mgI/ml; Omnipaque 300, GE Healthcare, Oslo, Norway) at a rate of 4.5 ml/sec. Based on the time to attenuation curves, arterial phase scan delay was set as the time to 15% of the peak enhancement of the cranial mesenteric artery and venous scan delay was determined as the time to maintain peak enhancement of the cranial mesenteric vein. Dual-phase CT was then performed after injection of 600 mgI/kg iohexol with the power injector (MEDRAD Vistron CT injection system, Medrad, PA, U.S.A.) at 4.5 ml/sec. The CT images were reconstructed using soft kernel, 1.5 mm of slice thickness, 1 mm increment and viewed using soft tissue window (window level=40 HU, window width=400 HU).
On CT images, a crescent-shaped tubular structure, cecum, was invaginated into the ascending colon and normal ileocolic junction was observed separately (Fig. 2). On arterial phase, the outer layer of the cecum was strongly enhanced (306 HU) compared with the inner layer (60–70 HU). A small mass (7.5 × 8 mm) with smooth and discrete margin was markedly enhanced (310 HU) within the inner layer of the cecum. On venous phase images, the attenuation of the cecal mass was decreased (120 HU) and the cecal mass became less distinguishable from the intestinal wall (50–60 HU). The mesenteric lymph nodes adjacent to the cecal mass were enlarged as 15 × 10 and 10 × 7 mm with oval shape. They were suspected to have reactive change or metastasis. Adipose tissue adjacent to the intestinal lesion looked normal without fat stranding or neovascularization. Pulmonary metastasis was not suspected. On exploratory laparotomy, the ileocolic junction looked dark red and the cecum was located within the dilated ascending colon. After ileocecocolic junction resection, end-to-end anastomosis was performed. The dog showed uneventful recovery from anesthesia. Constipation and diarrhea disappeared after 5 days.
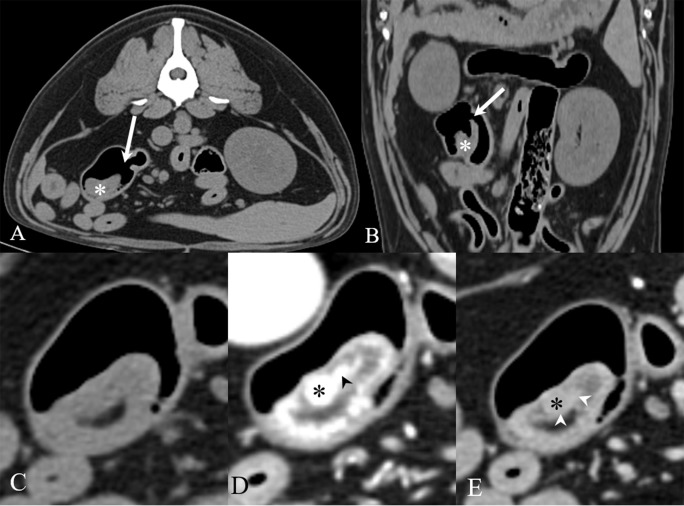
Fig. 2.
Transverse (A, C–E) and dorsally reformatted (B) soft tissue-window CT images of the dog. On pre-contrast images (A–C), normal ileocolic junction (white arrow) was observed, and the invaginated cecum (white asterisk) was observed in the proximal colon by intraluminal gas. On arterial phase (D), a small mass (black asterisk) with smooth and discrete margins was homogenously enhanced within the inner layer of the cecum. On venous phase (E), the mass became less enhanced. A tortuous linear line (arrowheads) ran medial to the mass and inner layer of the cecal wall.
In gross examination, the mucosa and serosa of the cecum were inverted completely such that the mucosa of the cecum and colon abutted each other (Fig. 3). On the perpendicularly cut surface of the cecum, the 7.5 × 8 mm sized, round and dark brown-colored tumor was located within the moderately thickened muscularis layer of the cecum at the mid portion. On microscopic examination and immunohistochemical examination, the tumor was diagnosed as carcinoid tumor. Dilated veins and lymphatic vessels were found within the mildly thickened mucosa and submucosa of the cecum, which was indicative of congestion and edema. There was no severe inflammation, ischemia, or necrosis of the intestinal wall. Hence, the lesion was diagnosed as lead point intussusception with a gastrointestinal carcinoid tumor.
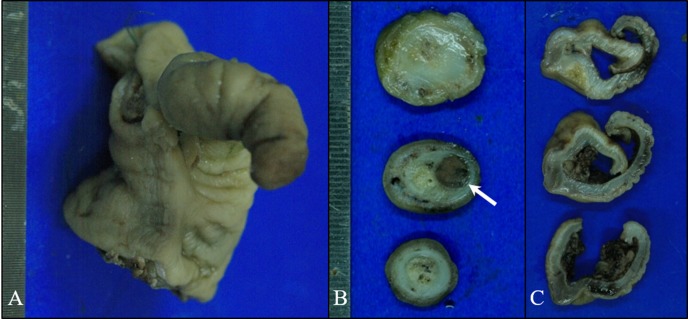
Fig. 3.
Gross findings. On the longitudinal cut surface of the ileocolic junction (A), whole cecum segment was protruded from the cecocolic orifice and located inside the lumen of the proximal colon. The mucosa and serosa of the cecum were completely inverted. On the perpendicularly cut surface of the cecum (B), the 7.5 × 8 mm sized, round and dark brown-colored tumor (arrow) was located within the moderately thickened muscularis layer of the cecum. On the cut surface of the colon (C), the mucus was edematous and mildly thickened.
Lead point intussusception is defined when any anatomical change of the intestine acts as a lead point to trigger the intussusception. In humans, lead point intussusception is mostly caused by neoplasia of the gastrointestinal tract, including malignant tumors such as adenocarcinoma, leiomyosarcoma, lymphoma, and metastatic tumors, and benign tumors such as lipoma, adenomatous polyp, Meckel’s diverticulum, leiomyoma, and gastrointestinal stromal tumors [13, 17, 21, 27, 32]. Due to the underlying causes, most intussusceptions, which occurs in adults, are lead point intussusception in humans [18, 27]. In veterinary medicine, to the best of our knowledge, only 15 dogs with lead point intussusception were reported. Most dogs with lead point intussusception were mid- to old-aged dogs, and are associated with gastrointestinal tumors including leiomyoma, leiomyosarcoma, lymphoma, mesenteric cyst, mast cell tumor, and schwannoma [1, 14, 15, 20, 25, 26, 33]. In only two young dogs with lead point intussusception, congenital mesenteric cysts functioned as the lead points [2, 14].
Ultrasonography is useful for detecting the intussusception, and assessing the degree of intestinal obstruction and swelling or loss of the layer of the intestinal wall [19, 24]. However, the accuracy of ultrasonography in localizing intestinal segments associated with intussusception is only 50% compared with that of surgical findings [14]. Moreover, ultrasonography has the low sensitivity (25%) for visualizing a lead point [12]. Among 8 human patients with lead point intussusception, only two lead points (lymphoma and intestinal duplication) were diagnosed using ultrasonography. Moreover, the gastrointestinal carcinoid tumor is usually small as lesser than 2 cm in human thus detection of the gastrointestinal carcinoid tumor is very low on ultrasonography [8, 10, 16]. In veterinary literature, the gastrointestinal carcinoid tumors in dogs were larger than human, from 1 to 15 cm, and have nonspecific ultrasonographic features including loss of layering, ulcer and thickening of the wall and decreased motility [24]. There was no description about the blood signal of the carcinoid tumor in the previous reports. In our case, ileocolic intussusception was misdiagnosed and the lead point was not detected on ultrasonography because intraluminal gas obscured the small carcinoid tumor.
CT study can determine the presence and types of underlying causes or lead points, with 58–100% sensitivity and 57–71% specificity [3, 4, 30]. CT allows to evaluate the location of intussusception, extent of intestinal segments involved, impairment of mesenteric vessels, and involvement of mesenteric fat or lymph nodes. CT is also utilized for tumor staging in patients with malignant lead points [6, 7, 31]. Only one study about serial changes in CT features of experimentally induced small intestinal intussusception has been reported in dogs [11]. The intussusception was noted as the target mass with a mixture of high and low attenuated band-like areas at the early stage, severely thickened bowel wall with less obvious layers at the mid-stage, and the amorphous mass with varied density and loss of wall layers at the late stage. In our case, the intussusception appeared as a target mass with a mixture of multiple band-like appearances and the cecal wall was moderately thickened with distinct layering. Based on the CT findings, this dog was considered as early stage of intussusception with concurrent intestinal edema. This finding was compatible with the pathological findings of edematous mucosa, submucosa, and muscularis layer of the cecum with mild inflammation without any evidence of ischemia, severe inflammation, or necrosis.
CT also detected gastrointestinal carcinoid tumor as a lead point in our case. The carcinoid tumor originated from well-differentiated neuroendocrine cells arising from the mucosa or submucosa of the intestinal wall [8, 10, 16, 23, 29]. Gastrointestinal carcinoids are rarely reported in dogs and the size of the tumor varies from 1–15 cm [9, 22, 28]. Although the cecal carcinoid tumor in this dog was small (8 mm), the tumor was distinguished from the surrounding cecal wall by markedly enhanced pattern on the arterial phase. This typical CT pattern of carcinoid tumors is characterized by high vascularization [5, 16]. Contrast enhancement patterns of most gastrointestinal tumors vary and overlap with each other; thus they are non-specific [34]. However, in our case, the dynamic CT study and interpretation of contrast-enhanced pattern was critical in the detection of a lead point with intussusception, even though the mass was very small.
In this report, dynamic CT was useful to detect a small-sized carcinoid tumor in an old dog based on strong enhanced pattern on arterial phase and diagnose this patient with lead point intussusception. In old dogs with intestinal intussusception, the presence of an intestinal tumor should be considered and dual-phase CT study may prove useful for assessing the presence of a lead point.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2018R1A2B6006775).
REFERENCES
- 1.Applewhite A. A., Cornell K. K., Selcer B. A.2002. Diagnosis and treatment of intussusceptions in dogs. Compendium 24: 110–127. [Google Scholar]
- 2.Applewhite A. A., Hawthorne J. C., Cornell K. K.2001. Complications of enteroplication for the prevention of intussusception recurrence in dogs: 35 cases (1989-1999). J. Am. Vet. Med. Assoc. 219: 1415–1418. doi: 10.2460/javma.2001.219.1415 [DOI] [PubMed] [Google Scholar]
- 3.Barussaud M., Regenet N., Briennon X., de Kerviler B., Pessaux P., Kohneh-Sharhi N., Lehur P. A., Hamy A., Leborgne J., le Neel J. C., Mirallie E.2006. Clinical spectrum and surgical approach of adult intussusceptions: a multicentric study. Int. J. Colorectal Dis. 21: 834–839. doi: 10.1007/s00384-005-0789-3 [DOI] [PubMed] [Google Scholar]
- 4.Beall D. P., Fortman B. J., Lawler B. C., Regan F.2002. Imaging bowel obstruction: a comparison between fast magnetic resonance imaging and helical computed tomography. Clin. Radiol. 57: 719–724. doi: 10.1053/crad.2001.0735 [DOI] [PubMed] [Google Scholar]
- 5.Bonekamp D., Raman S. P., Horton K. M., Fishman E. K.2015. Role of computed tomography angiography in detection and staging of small bowel carcinoid tumors. World J. Radiol. 7: 220–235. doi: 10.4329/wjr.v7.i9.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawes L. C., Hunt R., Wong J. K., Begg S.2004. Multiplanar reconstruction in adult intussusception: case report and literature review. Australas. Radiol. 48: 74–76. doi: 10.1111/j.1440-1673.2004.01249.x [DOI] [PubMed] [Google Scholar]
- 7.Eisen L. K., Cunningham J. D., Aufses A. H., Jr1999. Intussusception in adults: institutional review. J. Am. Coll. Surg. 188: 390–395. doi: 10.1016/S1072-7515(98)00331-7 [DOI] [PubMed] [Google Scholar]
- 8.Ganeshan D., Bhosale P., Yang T., Kundra V.2013. Imaging features of carcinoid tumors of the gastrointestinal tract. AJR Am. J. Roentgenol. 201: 773–786. doi: 10.2214/AJR.12.9758 [DOI] [PubMed] [Google Scholar]
- 9.Giles R. C., Jr, Hildebrandt P. K., Jr, Montgomery C. A., Jr1974. Carcinoid tumor in the small intestine of a dog. Vet. Pathol. 11: 340–349. doi: 10.1177/030098587401100406 [DOI] [PubMed] [Google Scholar]
- 10.Horton K. M., Kamel I., Hofmann L., Fishman E. K.2004. Carcinoid tumors of the small bowel: a multitechnique imaging approach. AJR Am. J. Roentgenol. 182: 559–567. doi: 10.2214/ajr.182.3.1820559 [DOI] [PubMed] [Google Scholar]
- 11.Iko B. O., Teal J. S., Siram S. M., Chinwuba C. E., Roux V. J., Scott V. F.1984. Computed tomography of adult colonic intussusception: clinical and experimental studies. AJR Am. J. Roentgenol. 143: 769–772. doi: 10.2214/ajr.143.4.769 [DOI] [PubMed] [Google Scholar]
- 12.Kenney I. J.1990. Ultrasound in intussusception: a false cystic lead point. Pediatr. Radiol. 20: 348–348. doi: 10.1007/BF02013173 [DOI] [PubMed] [Google Scholar]
- 13.Kim Y. H., Blake M. A., Harisinghani M. G., Archer-Arroyo K., Hahn P. F., Pitman M. B., Mueller P. R.2006. Adult intestinal intussusception: CT appearances and identification of a causative lead point. Radiographics 26: 733–744. doi: 10.1148/rg.263055100 [DOI] [PubMed] [Google Scholar]
- 14.Lamb C. R., Mantis P.1998. Ultrasonographic features of intestinal intussusception in 10 dogs. J. Small Anim. Pract. 39: 437–441. doi: 10.1111/j.1748-5827.1998.tb03752.x [DOI] [PubMed] [Google Scholar]
- 15.Levien A. S., Baines S. J.2011. Histological examination of the intestine from dogs and cats with intussusception. J. Small Anim. Pract. 52: 599–606. doi: 10.1111/j.1748-5827.2011.01128.x [DOI] [PubMed] [Google Scholar]
- 16.Levy A. D., Sobin L. H.2007. From the archives of the AFIP: Gastrointestinal carcinoids: imaging features with clinicopathologic comparison. Radiographics 27: 237–257. doi: 10.1148/rg.271065169 [DOI] [PubMed] [Google Scholar]
- 17.Lorigan J. G., DuBrow R. A.1990. The computed tomographic appearances and clinical significance of intussusception in adults with malignant neoplasms. Br. J. Radiol. 63: 257–262. doi: 10.1259/0007-1285-63-748-257 [DOI] [PubMed] [Google Scholar]
- 18.Marinis A., Yiallourou A., Samanides L., Dafnios N., Anastasopoulos G., Vassiliou I., Theodosopoulos T.2009. Intussusception of the bowel in adults: a review. World J. Gastroenterol. 15: 407–411. doi: 10.3748/wjg.15.407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattoon J. S., Nyland T. G.2014. Gastrointestinal tract. pp. 482–484. In: Small Animal Diagnostic Ultrasound, 3rd ed., Elsevier Health Sciences, St. Louis. [Google Scholar]
- 20.Myers N. C., Penninck D. G.1994. Ultrasonographic diagnosis of gastrointestinal smooth muscle tumors in the dog. Vet. Radiol. Ultrasound 35: 391–397. doi: 10.1111/j.1740-8261.1994.tb02059.x [DOI] [Google Scholar]
- 21.Onkendi E. O., Grotz T. E., Murray J. A., Donohue J. H.2011. Adult intussusception in the last 25 years of modern imaging: is surgery still indicated? J. Gastrointest. Surg. 15: 1699–1705. doi: 10.1007/s11605-011-1609-4 [DOI] [PubMed] [Google Scholar]
- 22.Patnaik A. K., Hurvitz A. I., Johnson G. F.1980. Canine intestinal adenocarcinoma and carcinoid. Vet. Pathol. 17: 149–163. doi: 10.1177/030098588001700204 [DOI] [PubMed] [Google Scholar]
- 23.Pelage J. P., Soyer P., Boudiaf M., Brocheriou-Spelle I., Dufresne A. C., Coumbaras J., Rymer R.1999. Carcinoid tumors of the abdomen: CT features. Abdom. Imaging 24: 240–245. doi: 10.1007/s002619900488 [DOI] [PubMed] [Google Scholar]
- 24.Penninck D. G., Moore A. S., Gliatto J.1998. Ultrasonography of canine gastric epithelial neoplasia. Vet. Radiol. Ultrasound 39: 342–348. doi: 10.1111/j.1740-8261.1998.tb01618.x [DOI] [PubMed] [Google Scholar]
- 25.Runyon C. L., Merkley D. F., Hagemoser W. A.1984. Intussusception associated with a paracolonic enterocyst in a dog. J. Am. Vet. Med. Assoc. 185: 443. [PubMed] [Google Scholar]
- 26.Schwandt C. S.2008. Low-grade or benign intestinal tumours contribute to intussusception: a report on one feline and two canine cases. J. Small Anim. Pract. 49: 651–654. doi: 10.1111/j.1748-5827.2008.00607.x [DOI] [PubMed] [Google Scholar]
- 27.Somma F., Faggian A., Serra N., Gatta G., Iacobellis F., Berritto D., Reginelli A., Di Mizio V., Cappabianca S., Di Mizio R., Grassi R.2015. Bowel intussusceptions in adults: the role of imaging. Radiol. Med. (Torino) 120: 105–117. doi: 10.1007/s11547-014-0454-4 [DOI] [PubMed] [Google Scholar]
- 28.Sykes G. P., Cooper B. J.1982. Canine intestinal carcinoids. Vet. Pathol. 19: 120–131. doi: 10.1177/030098588201900203 [DOI] [PubMed] [Google Scholar]
- 29.Sugimoto E., Lörelius L. E., Eriksson B., Öberg K.1995. Midgut carcinoid tumours. CT appearance. Acta Radiol. 36: 367–371. doi: 10.1177/028418519503600407 [DOI] [PubMed] [Google Scholar]
- 30.Sundaram B., Miller C. N., Cohan R. H., Schipper M. J., Francis I. R.2009. Can CT features be used to diagnose surgical adult bowel intussusceptions? AJR Am. J. Roentgenol. 193: 471–478. doi: 10.2214/AJR.08.1801 [DOI] [PubMed] [Google Scholar]
- 31.Valentini V., Buquicchio G. L., Galluzzo M., Ianniello S., Di Grezia G., Ambrosio R., Trinci M., Miele V.2016. Intussusception in Adults: The Role of MDCT in the Identification of the Site and Cause of Obstruction. Gastroenterol. Res. Pract. 2016: 5623718. doi: 10.1155/2016/5623718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang N., Cui X. Y., Liu Y., Long J., Xu Y. H., Guo R. X., Guo K. J.2009. Adult intussusception: a retrospective review of 41 cases. World J. Gastroenterol. 15: 3303–3308. doi: 10.3748/wjg.15.3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson G. P., Burt J. K.1974. Intussusception in the dog and cat: a review of 45 cases. J. Am. Vet. Med. Assoc. 164: 515–518. [PubMed] [Google Scholar]
- 34.Zubaidi A., Al-Saif F., Silverman R.2006. Adult intussusception: a retrospective review. Dis. Colon Rectum 49: 1546–1551. doi: 10.1007/s10350-006-0664-5 [DOI] [PubMed] [Google Scholar]