Abstract
Mercury (Hg) and cadmium (Cd) are the major toxic heavy metals and are known to induce neurotoxicity. Although many studies have shown that several heavy metals have neurotoxic effects, the cellular and molecular mechanisms thereof are still not clear. Oxidative stress is reported to be a common and important mechanism in cytotoxicity induced by heavy metals. However, the assays for identifying toxic mechanisms were not performed under the same experimental conditions, making it difficult to compare toxic properties of the heavy metals. In this study, we investigated the mechanisms underlying neurotoxicity induced by heavy metals and H2O2, focusing on cell death, cell proliferation, and oxidative stress under the same experimental condition. Our results showed that MeHg caused lactate dehydrogenase (LDH) release, caspase activation and cell-cycle alteration, and ROS generation in accordance with decreased cell viability. HgCl2 caused LDH release and cell-cycle alteration, but not caspase activation. CdCl2 had a remarkable effect on the cell cycle profiles without induction of LDH release, caspase activation, or ROS generation. Pretreatment with N-acetyl-l-cysteine (NAC) prevented the decrease in cell viability induced by MeHg and HgCl2, but not CdCl2. Our results demonstrate a clear difference in neurotoxic mechanisms induced by MeHg, HgCl2, CdCl2 or H2O2 in SH-SY5Y cells. Elucidating the characteristics and mechanisms of each heavy metal under the same experimental conditions will be helpful to understand the effect of heavy metals on health and to develop a more effective therapy for heavy metal poisoning.
Keywords: cell death, heavy metal, neurotoxicity, ROS generation, SH-SY5Y cell
Heavy metals are found ubiquitously, such as in the natural environment, workplace, food, and water supply [8], and heavy metal pollution has increased considerably worldwide because of high industrial activities. Humans and animals can be exposed to the heavy metals through inhalation of dust, consumption of contaminated drinking water and food, and direct ingestion of contaminated soil or industrial waste, thereby leading to potential health hazards [14].
Mercury (Hg) is one of the most widely used heavy metals. It exists in numerous chemical forms including elemental, inorganic, and organic Hg compounds. It has been reported that various forms of Hg can have diverse toxic effects on human populations [29]. Methylmercury (MeHg) is a potent environmental neurotoxic pollutant that is generated by bacterial methylation of inorganic Hg in an aquatic environment [21]. Although MeHg is one of the most important neurotoxic Hg toxins for humans, inorganic Hg compounds can accumulate in the brain and induce central nervous system damage in experimental animal models [30]. In addition, cadmium (Cd) is known as a major toxic heavy metal with Cd2+ as the most common form, which is produced by and used in various industrial processes. Contamination of agricultural soil with Cd causes the absorption of Cd compounds by plants, by which means humans and animals can be exposed to Cd. Accumulation of Cd in various organs causes severe damage to organs systems, including the nervous system [17, 32].
Although many studies have shown that several heavy metals induce neurotoxicity, the cellular and molecular mechanisms thereof are still not clearly understood. Oxidative stress is commonly reported as an important mechanism underlying the cytotoxicity induced by heavy metals including Hg and Cd [2, 27, 37]. However, the assays for identifying toxic mechanisms were not performed under the same experimental conditions, making it difficult to compare toxic properties of the heavy metals. In this study, we intended to characterize the neurotoxic effects and mechanisms of heavy metals under the same experimental conditions in SH-SY5Y human neuroblastoma cells, which are widely used as a model cell system for studying the neurotoxicity of chemical substances. We investigated the mechanisms involved in the toxicity of MeHg, HgCl2, and CdCl2 on cell death, cell proliferation, and oxidative stress. In addition, we also monitored the effect of hydrogen peroxide (H2O2)-induced oxidative stress and antioxidants on SH-SY5Y cells.
MATERIALS AND METHODS
Reagents
Dulbecco’s modified eagle’s medium nutrient mixture F-12 HAM (DMEM/F-12) was obtained from SIGMA (Tokyo, Japan). Fetal bovine serum (FBS) was purchased from Equitech-Bio, Inc. (Kerrville, TX, U.S.A.). Penicillin/streptomycin, Dulbecco’s phosphate-buffered saline (PBS) and menadione were purchased from Nacalai tesque (Kyoto, Japan). Camptothecin, nocodazole, 4% paraformaldehyde phosphate buffer solution (4% PFA), N-acetyl-l-cysteine (NAC), catalase, mercury (II) chloride (HgCl2), hydrogen peroxide (H2O2) were from Wako (Osaka, Japan), FxCycle PI/RNase staining solution and CellROX from Thermo Fisher Scientific (Carlsbad, CA, U.S.A.) and Accumax from Innovative Cell Technologies (San Diego, CA, U.S.A.). Methylmercury (II) chloride standard (MeHg) and cadmium chloride 2.5-hydrate (CdCl2) were obtained from Kanto Chemical (Tokyo, Japan). Cell counting Kit-8 was from Dojindo (Kumamoto, Japan). Cytotoxicity detection KitPLUS (LDH) and Cell proliferation ELISA, BrdU (colorimetric) were purchased from Roche (Basel, Switzerland). Amplite fluorimetric Caspase 3/7 assay kit was from AAT Bioquest (Sunnyvale, CA, U.S.A.).
Cell culture
SH-SY5Y cells were grown at 37°C in DMEM/F-12 containing 10% FBS with 100 units/ml penicillin and 100 µg/ml streptomycin in the presence of 5% CO2. In all of experiments, the cells were used at no more than 20 passages. Cells at 80–90% confluence in 100 mm dish were collected and adjusted to a density of 2.0 × 105 cells/ml, then 100 µl or 2 ml of the cell suspension was added to a well of 96-well plate or 35 mm dish, respectively, 2 days before the following experiments. Cells were serum-starved for 4 hr and then incubated with heavy metals, such as MeHg, HgCl2, and CdCl2, or H2O2 for 24 hr.
Cell viability assay
Cell viability assay was performed by using Cell counting Kit-8 (CCK-8) according to the manufacturer’s instructions. The absorbance of WST-8 formazan in SH-SY5Y cells grown on 96-well plates was measured at 450 nm using a microplate reader Infinite F200 (TECAN, Männedorf, Switzerland). Cells treated with vehicle were used as control and taken to have 100% viability. To analyze the effect of antioxidants, 2.5 mM NAC or 1,000 U/ml catalase were treated to SH-SY5Y cells at 4 hr before the treatment with the heavy metals or H2O2.
LDH cytotoxicity assay
Lactate dehydrogenase (LDH) cytotoxicity assay was performed by using Cytotoxicity detection kit plus (LDH) according to the manufacturer’s instructions. In brief, SH-SY5Y cells were grown on 96-well plates and treated with heavy metals or H2O2 as described above. After 24 hr incubation, LDL cytotoxicity assay was performed, and LDL release was measured at absorbance at 490 nm using a microplate reader Infinite F200. Dissolved cells by treatment with lysis solution supplied with the kit were used as positive control and taken as 100% LDH release.
Caspase assay
SH-SY5Y cells grown on 96-well plates with black walls and clear bottoms were stimulated as described above. Caspase assay was performed by using Amplite fluorimetric caspase 3/7 assay kit according to the manufacturer’s instructions. In brief, stimulated cells were treated with the substrate for activated caspase 3/7 (Z-DEVD). Fluorescence at 450 nm was measured by 350 nm excitation using a microplate reader Infinite F200. Cells treated with 1 µM camptothecin, (an inducer of apoptosis through the inhibition of DNA topoisomerases) were used as positive control and taken as 100% caspase 3/7 activation.
Cell proliferation assay
Cell proliferation assay was performed in cells grown on 96-well plates by using Cell proliferation ELISA, BrdU (colorimetric) according to the manufacturer’s instructions. BrdU incorporation into newly synthesized DNA was detected by absorbance at 370 nm using a microplate reader. Cells treated with vehicle were used as control and taken as 100% proliferation.
Cell-cycle analysis using PI staining and flow cytometry
SH-SY5Y cells on 35 mm dishes were exposed to heavy metals or H2O2 as described above. Floating and attached cells harvested by using Accumax were collected by centrifuge at 2,000 rpm for 3 min. After, the cells were fixed using 4% PFA and stained by using FxCycle PI/RNase staining solution according to the manufacturer’s instructions. Cell-cycle analysis was performed using BD FACSCalibur (BD Biosciences, San Jose, CA, U.S.A.). The population of each cell-cycle phase (sub-G1, G1, S and G2/M) was determined based on the DNA amount. The sub-G1 phase population was used to determine apoptosis. Cells treated with 1 µM nocodazole (20 hr), an inhibitor for mitosis, were used as a positive control to confirm cell-cycle alteration.
Measurement of ROS generation
ROS detection assay was performed in cells on black 96-well plates by using CellROX according to the manufacturer’s instruction. Cells were serum-starved 4 hr using DMEM/F-12 without phenol red, and then treated with heavy metals or H2O2. Cells were incubated with CellROX reagent (final 5 µM) for 24 hr. Fluorescence at 520 nm was measured by 485 nm excitation using a microplate reader (Infinite F200). Cells treated with 20 µM menadione were used as positive control and taken as 100% ROS generation.
Statistical analysis
Data are expressed as mean ± SEM. Statistical differences between two means were evaluated by the Student’s t-test. Multiple comparisons were evaluated with one-way analysis of variance (ANOVA) followed by Tukey’s or Dunnett’s test. Differences were considered significant at P<0.05.
RESULTS
Effect of heavy metals and H2O2 on cell viability
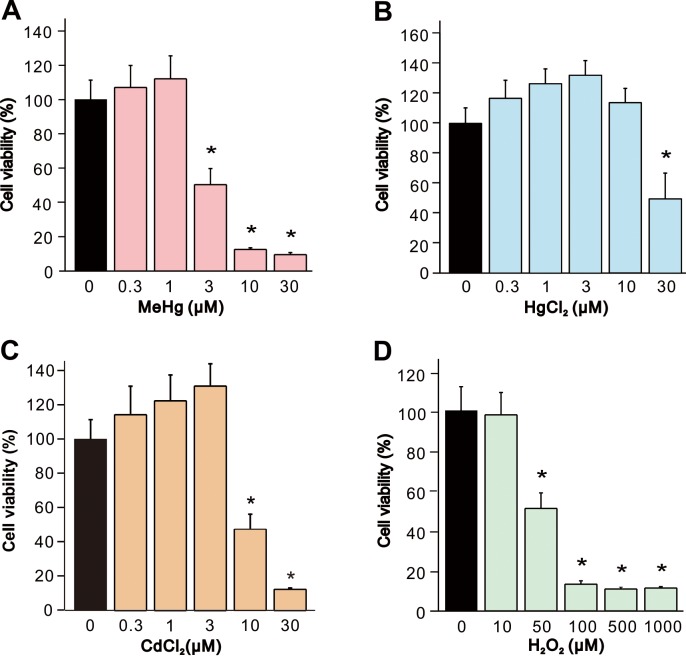
First, we examined the toxic effect of the neurotoxic reagents on SH-SY5Y cells by measurement of cell viability after 24 hr exposure with different concentrations of MeHg, HgCl2, CdCl2 (0.3–30 µM) or H2O2 (10–1,000 µM). Exposure of cells to MeHg, HgCl2, CdCl2, and H2O2 elicited decreases in the cell viability in a dose-dependent manner (Fig. 1). Significant decrease in comparison with vehicle-treated controls were observed at 3 µM MeHg, 30 µM in HgCl2, 10 µM in CdCl2, and 50 µM in H2O2, in which the neurotoxic reagent showed approximately 50% decrease in the cell viability. For the following experiments, we used maximum concentrations of the neurotoxic reagent in which decrease in cell viability were not statistically significant and minimum concentrations which showed significant decrease (1 and 3 µM in MeHg, 10 and 30 µM in HgCl2, 3 and 10 µM in CdCl2, and 10 and 50 µM in H2O2).
Fig. 1.
Decrease in cell viability of SH-SY5Y cells by MeHg, HgCl2, CdCl2, and H2O2. Cell viability of SH-SY5Y cells was evaluated 24 hr after exposure to (A) MeHg (0.3–30 µM, n=7), (B) HgCl2 (0.3–30 µM, n=7), (C) CdCl2 (0.3–30 µM, n=7) or (D) H2O2 (10–1,000 µM, n=7). Data are expressed as a percentage of vehicle-treated cells (control). Results are shown as mean ± SEM. *P<0.05 as compared with the control.
Induction of necrosis and apoptosis by heavy metals and H2O2
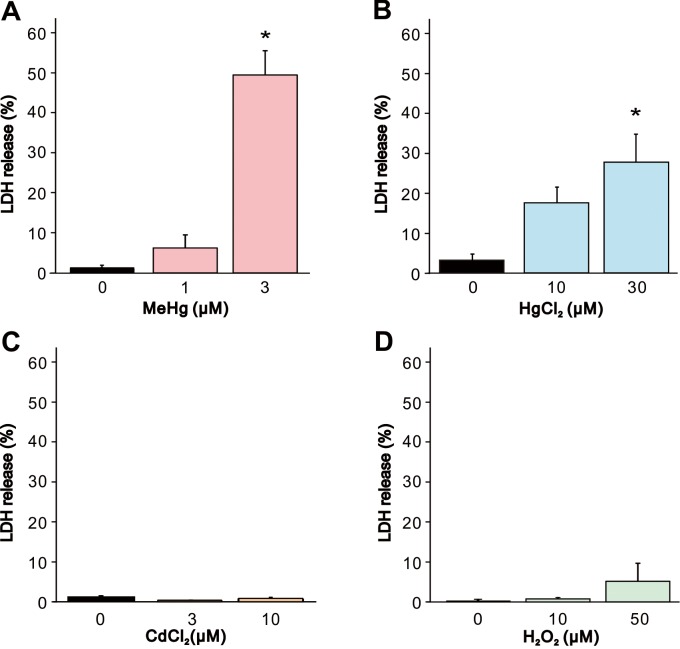
To explore the contribution of necrotic cell death on the effect of the heavy metals and H2O2, we first measured the LDH leakage, a general hallmark of cell membrane damage and necrotic cell death. MeHg or HgCl2 exposure (Fig. 2A and 2B) induced significant LDH leakage at 3 or 30 µM, respectively, compared to vehicle-treated controls in accordance with the result in cell viability assay. In contrast, CdCl2 or H2O2 exposure did not cause significant LDH leakage even at the highest concentration which elicited significant decrease in cell viability assay (Fig. 2C and 2D).
Fig. 2.
Increase in LDH release from SH-SY5Y cells by MeHg, HgCl2, CdCl2 and H2O2. LDH release from SH-SY5Y cells was evaluated 24 hr after exposure to (A) MeHg (1 and 3 µM, n=5), (B) HgCl2 (10 and 30 µM, n=5), (C) CdCl2 (3 and 10 µM, n=5) or (D) H2O2 (10 and 50 µM, n=5). Data are expressed as a percentage of vehicle-treated cells (control). Results are shown as mean ± SEM. *P<0.05 as compared with the control.
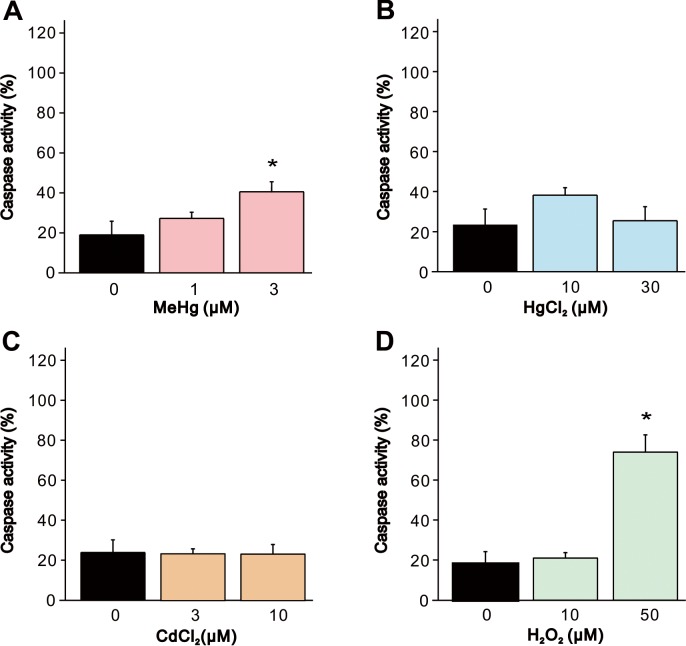
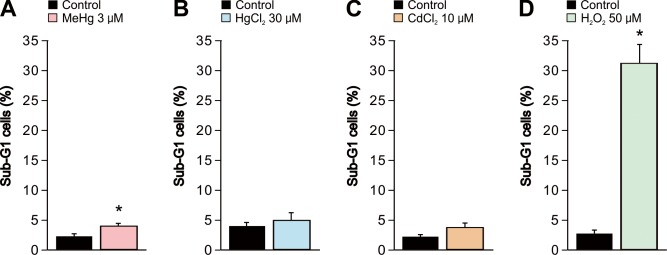
We quantified caspase-3 activity, which is a crucial biomarker of neuronal caspase-dependent apoptosis, to evaluate the effect of the heavy metals and H2O2 on apoptosis. Exposure of cells to 3 µM MeHg or 50 µM H2O2 significantly increased the caspase activity compared to the vehicle-treated control cells (Fig. 3A and 3D). However, in HgCl2 or CdCl2 exposure, significant increase in caspase activity were not observed (Fig. 3B and 3C). It was suggested that MeHg and H2O2 caused apoptosis through the caspase cascade. Furthermore, we also evaluated the effect of the heavy metals and H2O2 on apoptosis by sub-G1 analysis by flow cytometry (Fig. 4). Although a significant increase in sub-G1 population was observed in cells exposed to MeHg or H2O2 in accordance with the result in caspase activation, MeHg elicited only a small change in the sub-G1 population.
Fig. 3.
Increase in caspase activity in SH-SY5Y cells by MeHg, HgCl2, CdCl2 and H2O2. Caspase 3/7 activity in SH-SY5Y cells was evaluated 24 hr after exposure to (A) MeHg (1 and 3 µM, n=5), (B) HgCl2 (10 and 30 µM, n=5), (C) CdCl2 (3 and 10 µM, n=5) or (D) H2O2 (10 and 50 µM, n=5). Data are expressed as a percentage of 1 µM camptothecin-treated cells (positive control). Results are shown as mean ± SEM. *P<0.05 as compared with the vehicle-treated cells.
Fig. 4.
Increase in Sub-G1 apoptotic SH-SY5Y cells by MeHg, HgCl2, CdCl2 and H2O2. Sub-G1 population of SH-SY5Y cells was evaluated using flow cytometry 24 hr after exposure to (A) MeHg (3 µM, n=5), (B) HgCl2 (30 µM, n=5), (C) CdCl2 (10 µM, n=5) or (D) H2O2 (50 µM, n=5). Data are expressed as a percentage of the total number of cells. Results are shown as mean ± SEM. *P<0.05 as compared with the vehicle-treated cells.
Inhibition of cell growth by heavy metals and H2O2
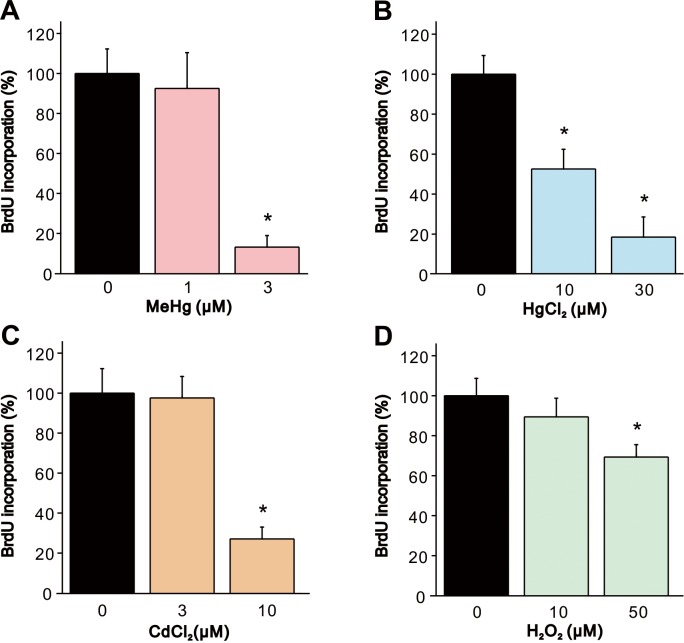
To determine whether the heavy metals and H2O2 affect cell proliferation, we measured incorporation of BrdU which is an analog of thymidine that is incorporated in DNA during replication. In exposure of cells to MeHg or CdCl2, BrdU incorporation was significantly decreased compared to vehicle-treated controls at the concentrations which elicited significant decrease in cell viability (Fig. 5A and 5C). BrdU incorporation was significantly decreased in a dose-dependent manner by exposure to 10 and 30 µM HgCl2 (Fig. 5B). Exposure of cells to 50 µM H2O2 induced significant decrease in BrdU incorporation, although the extent of decrease in BrdU incorporation was less than those observed in the heavy metals (Fig. 5D).
Fig. 5.
Decrease in BrdU incorporation in SH-SY5Y cells by MeHg, HgCl2, CdCl2 and H2O2. BrdU incorporation in SH-SY5Y cells was evaluated 24 hr after exposure to (A) MeHg (1 and 3 µM, n=5), (B) HgCl2 (10 and 30 µM, n=5), (C) CdCl2 (3 and 10 µM, n=5) or (D) H2O2 (10 and 50 µM, n=5). Data are expressed as a percentage of vehicle-treated cells (control). Results are shown as mean ± SEM. *P<0.05 as compared with the control.
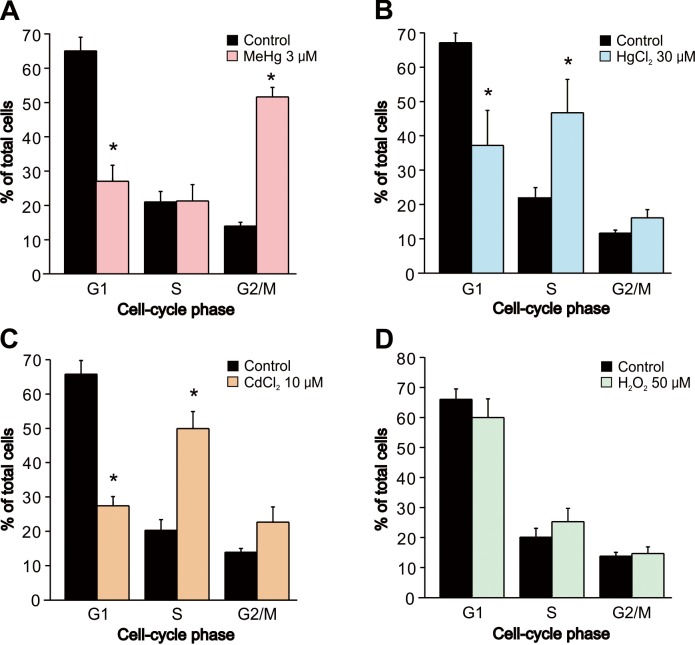
Since the heavy metals and H2O2 inhibited cell proliferation, we confirmed whether the inhibition was related to cell-cycle alteration by using flow cytometry. The percentages of subpopulations of cells in cell cycle phases G1, S, and G2/M are shown in Fig. 5. Exposure of cells to 3 µM MeHg induced a dramatic decrease in the population of G1 cells and a corresponding increase in the population of G2 cells, indicating a G2 cell-cycle arrest (Fig. 6A). On the other hand, exposure to 30 µM HgCl2 or 10 µM CdCl2 induced a similar change in cell cycle distribution in which the proportion of cells in the G1 phase was lower, and that of cells in the S phase was higher, than in vehicle-treated controls (Fig. 6B and 6C). In contrast to the heavy metals, exposure to 50 µM H2O2 exposure did not induce cell-cycle alteration (Fig. 6D).
Fig. 6.
Alteration of cell cycle in SH-SY5Y cells by MeHg, HgCl2, CdCl2 and H2O2. Alteration of cell cycle in SH-SY5Y cells was evaluated using flow cytometry 24 hr after exposure to (A) MeHg (3 µM, n=5), (B) HgCl2 (30 µM, n=5), (C) CdCl2 (10 µM, n=5) or (D) H2O2 (50 µM, n=5). Data are expressed as a percentage of total cells in G1, S and G2/M population. Results are shown as mean ± SEM. *P<0.05 as compared with the vehicle-treated cells.
Involvement of ROS in the toxicity by heavy metals and H2O2
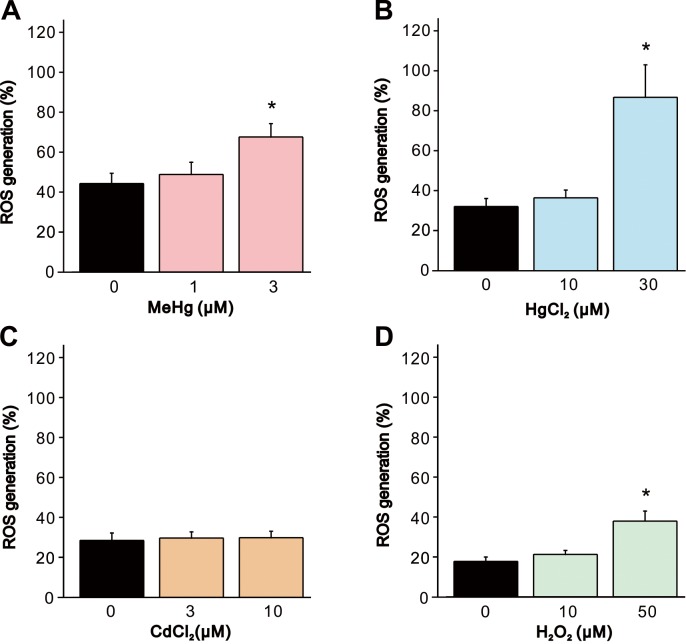
Since it has been suggested that ROS plays an important role in the toxicity induced by heavy metals including MeHg, HgCl2 and CdCl2, we determined the effect of heavy metals and H2O2 on ROS generation by using a fluorogenic indicator for ROS. Exposure of cells to MeHg, HgCl2 and H2O2 significantly increased ROS generation compared to the vehicle-treated controls (Fig. 7A, 7B and 7D), whereas changes in ROS generation caused by CdCl2 exposure were not observed (Fig. 7C).
Fig. 7.
Increase in ROS generation in SH-SY5Y cells by MeHg, HgCl2, CdCl2 and H2O2. ROS generation in SH-SY5Y cells was evaluated 24 hr after exposure to (A) MeHg (1 and 3 µM, n=6), (B) HgCl2 (10 and 30 µM, n=6), (C) CdCl2 (3 and 10 µM, n=6) or (D) H2O2 (10 and 50 µM, n=6). Data are expressed as a percentage of 20 µM menadione-treated cells (positive control). Results are shown as mean ± SEM. *P<0.05 as compared with the vehicle-treated cells.
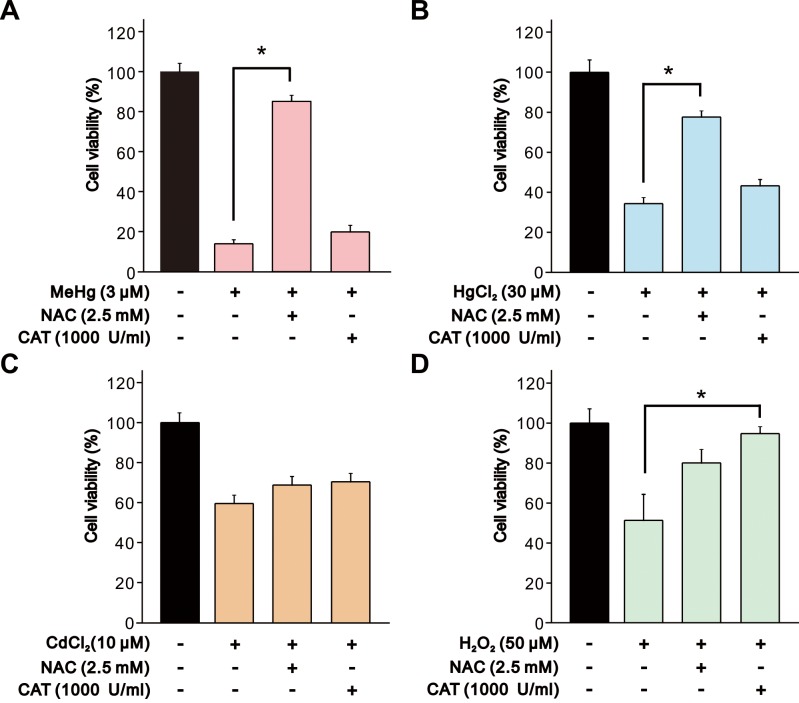
Finally, we confirmed the relationship between toxicity of heavy metals and oxidative stress by measurement of cell viability with or without pretreatments of antioxidants, NAC and catalase. Cells were pretreated with 2.5 mM NAC or 1,000 U/ml catalase for 4 hr before the addition of the heavy metals and H2O2. The decrease in cell viability induced by MeHg and HgCl2 exposure were significantly attenuated by pretreatment with NAC (Fig. 8A and 8B) in accordance with the results in ROS generation. On the other hand, H2O2-induced decrease in cell viability was attenuated by pretreatment with catalase but not with NAC (Fig. 8D). In contrast, both NAC and catalase did not cause a significant change in the effect of CdCl2 on cell viability (Fig. 8C).
Fig. 8.
Effect of NAC and catalase on decrease in cell viability of SH-SY5Y cells induced by MeHg, HgCl2, CdCl2 and H2O2. Cell viability of SH-SY5Y cells was evaluated 24 hr after exposure to (A) MeHg (3 µM, n=5), (B) HgCl2 (30 µM, n=5), (C) CdCl2 (10 µM, n=5) or (D) H2O2 (50 µM, n=5). NAC (2.5 mM) or catalase (1,000 U/ml) was added 4 hr before the addition of heavy metals and H2O2. Data are expressed as a percentage of vehicle-treated cells (control). Results are shown as mean ± SEM. *P<0.05 as compared with the control.
DISCUSSION
First, we determined the toxic effect and concentration for each neurotoxic reagent on SH-SY5Y cells by assessing cell viability. MeHg, HgCl2, CdCl2, and H2O2 decreased cell viability in a dose-dependent manner at concentrations between 0.3–30 µM. The rank-order of toxicity of heavy metals on cell viability was MeHg >CdCl2 >HgCl2 in SH-SY5Y cells under our experimental conditions.
Necrosis and apoptosis are two major forms of cell death observed physiologically and pathophysiologically. To explore the effects of neurotoxic reagents on cell death, we first measured LDH leakage, a general hallmark of cell membrane damage and necrotic cell death. In cells exposed to MeHg or HgCl2, significant LDH leakage was detected at the concentrations that caused significant decrease in cell viability, indicating that induction of necrosis was one of the major causes of the decrease in cell viability. In contrast, no changes in LDH leakage were detected following exposure to CdCl2 or H2O2, although concurrent decreases in cell viability and LDH leakage in SH-SY5Y cells exposed to CdCl2 or H2O2 had been reported previously [16, 25, 33]. On the other hand, we detected an increase in caspase activity following MeHg or H2O2 exposure, suggesting that MeHg or H2O2 caused apoptosis through the caspase cascade as reported previously in SH-SY5Y cells [10, 13, 15, 31]. Although it has also been reported that HgCl2 or CdCl2 promotes caspase-dependent apoptosis in SH-SY5Y cells [19, 31, 34], we did not detect increases in caspase activity. Furthermore, we also detected increase in sub-G1 population by MeHg or H2O2, but not by HgCl2 or CdCl2, suggesting that apoptotic cell death is caused by toxicity of MeHg or H2O2. However, the MeHg-induced increase in caspase activity and the sub-G1 population was noticeably lower than that induced by H2O2. These results may indicate that apoptosis does not play a major role in the induction of cell death by MeHg exposure. It has been reported that lower (~1 µm) concentration of Cd induced LDL release, caspase-3 activation, and apoptosis in primary rat cortical neurons [22]. The type of cell, such as the origin species, and primary culture or cell line, probably explain the discrepancy.
The BrdU incorporation assay clearly indicated that MeHg, HgCl2, CdCl2, and H2O2 inhibited cell proliferation in SH-SY5Y cells. Heavy metal exposure remarkably decreased BrdU incorporation in accordance with cell viability data, suggesting that not only cell death but also inhibition of cell proliferation may be a major cause of decrease in cell viability by MeHg, HgCl2, CdCl2. Therefore, we investigated whether the inhibition of cell proliferation was related to the toxic effects of cell-cycle regulation by using flow cytometry. Cell-cycle analysis revealed that MeHg, HgCl2 and CdCl2, but not H2O2, changed cell-cycle profiles of SH-SY5Y cells, confirming the disturbance of the cell cycle. MeHg promoted distinct changes in cell-cycle in which the percentage of cell in G1 phase was decreased and that in G2/M phase was increased. However, HgCl2 or CdCl2 exposure promoted distinct cell-cycle changes in which the percentage of cells in the G1 phase was decreased and that of cells in the S phase was increased. These results suggested that the cell-cycle dysregulation was, at least in part, involved in the inhibition of cell proliferation by MeHg, HgCl2, or CdCl2. There have been conflicting reports about the effects of heavy metals on the cell cycle. For example, Cd exposure results in not only G1 phase cell cycle arrest with increasing p21 levels [6], but also G2/M phase arrest in the absence of p21 induction in Cd-exposed human fibroblasts [3]. However, previous reports in rat glioma c6 cell line, rat pheochromocytoma PC12 cell line, and HeLa cell line [7, 23] are in concordance with our findings that exposure to MeHg results in G2 phase cell cycle arrest. In addition, arrest of S-phase by MeHg has also been reported in SH-SY5Y cells [20]. Besides, stimulation of cell proliferation through oxidative stress (activation of NOX) by SH-reactive metals, including Hg and Cd, has been reported in human promyelocytic leukemia PLB-985 cell line and human keratinocyte HaCat cells line [24]. Furthermore, MeHg and CdCl2 caused cell cycle arrest via changes in the expression of wide variety of genes and proteins related to cell cycle regulation [36], such as p21, cyclin E, and CDK2 [1, 4, 12, 26, 35]. Although effects on the cell cycle appear to depend on cellular condition, differences in the molecular mechanisms involved in cell cycle arrest by heavy metals in SH-SY5Y cells should be clarified in further studies.
Finally, we measured ROS generation caused by exposure of cells to heavy metals and confirmed that ROS is related to toxicity by using antioxidant reagents. We showed that exposure of cells to MeHg, HgCl2, and H2O2 induced significant ROS generation. Pretreatment of antioxidant NAC or catalase significantly suppressed decreases in cell viability induced by MeHg, HgCl2, or H2O2. These results indicated that ROS generation was involved in the mechanism underlying toxicity of MeHg and HgCl2. It has been known that excessive ROS levels cause considerable cellular damage, leading to cell death, including via apoptosis [28]. Although HgCl2 induced a remarkable increase in ROS generation, caspase activity and the population of sub-G1 cells were not altered, suggesting that ROS may not play an important role in induction of apoptosis in cells exposed to HgCl2. However, we did not observe a significant increase in ROS generation by exposure to CdCl2 under our experimental conditions. Although it has been reported that ROS generation plays an important role in neuronal apoptosis induced by CdCl2 in SH-SY5Y cells [18, 34, 37], relatively higher concentrations of CdCl2 (10 µM~) were used in their experiments. The present study showed that a relatively lower concentration of CdCl2 (~10 µM) did not cause a significant increase in ROS generation and apoptosis induction. Furthermore, pretreatment of antioxidants did not change the decrease in cell viability caused by exposure to CdCl2, suggesting that ROS did not play a major role in the toxicity of CdCl2 under our experimental conditions. It has been indicated that neuronal characteristics of SH-SY5Y cells were changed with increasing passage numbers [11], which accompany the change in sensitivity to pneumolysin toxicity [9]. Therefore, although induction of ROS generation by lower concentration of Cd (2.5 µM) was reported in SH-SY5Y cells [5], different culture conditions (passage number, composition of medium, presence of serum during exposure etc.) are predicted to result in different effects of heavy metals.
In this study, we examined the mechanisms underlying the neurotoxicity of heavy metals and H2O2, focusing on cell death, cell proliferation, and oxidative stress, under the same experimental conditions. We showed that MeHg caused LDH release, caspase activation, cell-cycle alteration, and ROS generation in accordance with a decrease in cell viability. HgCl2 caused LDH release and cell cycle alteration, but not caspase activation. CdCl2 had a remarkable effect on cell-cycle without induction of LDH release, caspase activation and ROS generation. Pretreatment of NAC prevented the decrease in cell viability induced by MeHg and HgCl2, but not CdCl2. Our results demonstrate a clear difference in neurotoxic mechanisms induced by MeHg, HgCl2, CdCl2 or H2O2 in SH-SY5Y cells. It is assumed that the disparity of the results is related to the origin species of cells, primary culture or cell line, model of cells, culture conditions, differentiated or undifferentiated status, reagent concentrations, exposure time, which lead to conflicting conclusions regarding the mechanisms of heavy metal toxicity. Elucidating the characteristics and mechanisms of each heavy metal under same experimental conditions will be helpful to understand the effect of heavy metals on health and to develop more effective therapies for heavy metal poisoning.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (C) (No. 26450407) from the Japan Society for the Promotion of Science (JSPS).
REFERENCES
- 1.Bose R., Onishchenko N., Edoff K., Janson Lang A. M., Ceccatelli S.2012. Inherited effects of low-dose exposure to methylmercury in neural stem cells. Toxicol. Sci. 130: 383–390. doi: 10.1093/toxsci/kfs257 [DOI] [PubMed] [Google Scholar]
- 2.Caballero B., Olguin N., Campos F., Farina M., Ballester F., Lopez-Espinosa M. J., Llop S., Rodríguez-Farré E., Suñol C.2017. Methylmercury-induced developmental toxicity is associated with oxidative stress and cofilin phosphorylation. Cellular and human studies. Neurotoxicology 59: 197–209. doi: 10.1016/j.neuro.2016.05.018 [DOI] [PubMed] [Google Scholar]
- 3.Cao F., Zhou T., Simpson D., Zhou Y., Boyer J., Chen B., Jin T., Cordeiro-Stone M., Kaufmann W.2007. p53-Dependent but ATM-independent inhibition of DNA synthesis and G2 arrest in cadmium-treated human fibroblasts. Toxicol. Appl. Pharmacol. 218: 174–185. doi: 10.1016/j.taap.2006.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee S., Kundu S., Sengupta S., Bhattacharyya A.2009. Divergence to apoptosis from ROS induced cell cycle arrest: effect of cadmium. Mutat. Res. 663: 22–31. doi: 10.1016/j.mrfmmm.2008.12.011 [DOI] [PubMed] [Google Scholar]
- 5.Chen L., Xu B., Liu L., Luo Y., Zhou H., Chen W., Shen T., Han X., Kontos C. D., Huang S.2011. Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free Radic. Biol. Med. 50: 624–632. doi: 10.1016/j.freeradbiomed.2010.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi Y. J., Yin H. Q., Suh H. R., Lee Y. J., Park S. R., Lee B. H.2011. Involvement of E2F1 transcriptional activity in cadmium-induced cell-cycle arrest at G1 in human lung fibroblasts. Environ. Mol. Mutagen. 52: 145–152. doi: 10.1002/em.20593 [DOI] [PubMed] [Google Scholar]
- 7.Crespo-Lopez M. E., Costa-Malaquias A., Oliveira E. H., Miranda M. S., Arrifano G. P., Souza-Monteiro J. R., Sagica F. E., Fontes-Junior E. A., Maia C. S., Macchi B. M., do Nascimento J. L.2016. Is low non-lethal concentration of methylmercury really safe? A report on genotoxicity with delayed cell proliferation. PLoS One 11: e0162822. doi: 10.1371/journal.pone.0162822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorne J. L., Kass G. E., Bordajandi L. R., Amzal B., Bertelsen U., Castoldi A. F., Heppner C., Eskola M., Fabiansson S., Ferrari P., Scaravelli E., Dogliotti E., Fuerst P., Boobis A. R., Verger P.2011. Human risk assessment of heavy metals: principles and applications. Met. Ions Life Sci. 8: 27–60. [PubMed] [Google Scholar]
- 9.Ebert S., Dietz G. P., Mitchell T. J., Michel U., Bähr M., Nau R.2005. Limited protection of TAT-Bcl-X(L) against pneumolysin-induced neuronal cell death. Neurosci. Lett. 384: 349–353. doi: 10.1016/j.neulet.2005.05.027 [DOI] [PubMed] [Google Scholar]
- 10.Fernández-Moriano C., Divakar P. K., Crespo A., Gómez-Serranillos M. P.2017. In vitro neuroprotective potential of lichen metabolite fumarprotocetraric acid via intracellular redox modulation. Toxicol. Appl. Pharmacol. 316: 83–94. doi: 10.1016/j.taap.2016.12.020 [DOI] [PubMed] [Google Scholar]
- 11.Forsby A.2011. Neurite degeneration in human neuronal SH-SY5Y Cells as an indicator of axonopathy. pp. 255–268. In: Cell Culture Techniques. Neuromethods, Vol. 56 (Aschner, M., Sunol, C. and Bal-Price, A. eds.), Humana Press, New York. [Google Scholar]
- 12.Fujimura M., Usuki F.2015. Low concentrations of methylmercury inhibit neural progenitor cell proliferation associated with up-regulation of glycogen synthase kinase 3β and subsequent degradation of cyclin E in rats. Toxicol. Appl. Pharmacol. 288: 19–25. doi: 10.1016/j.taap.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 13.Guida N., Laudati G., Anzilotti S., Sirabella R., Cuomo O., Brancaccio P., Santopaolo M., Galgani M., Montuori P., Di Renzo G., Canzoniero L. M., Formisano L.2016. Methylmercury upregulates RE-1 silencing transcription factor (REST) in SH-SY5Y cells and mouse cerebellum. Neurotoxicology 52: 89–97. doi: 10.1016/j.neuro.2015.11.007 [DOI] [PubMed] [Google Scholar]
- 14.Hejna M., Gottardo D., Baldi A., Dell’Orto V., Cheli F., Zaninelli M., Rossi L.2018. Review: Nutritional ecology of heavy metals. Animal 12: 2156–2170. doi: 10.1017/S175173111700355X [DOI] [PubMed] [Google Scholar]
- 15.Hu X. L., Niu Y. X., Zhang Q., Tian X., Gao L. Y., Guo L. P., Meng W. H., Zhao Q. C.2015. Neuroprotective effects of Kukoamine B against hydrogen peroxide-induced apoptosis and potential mechanisms in SH-SY5Y cells. Environ. Toxicol. Pharmacol. 40: 230–240. doi: 10.1016/j.etap.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 16.Hu X. L., Gao L. Y., Niu Y. X., Tian X., Wang J., Meng W. H., Zhang Q., Cui C., Han L., Zhao Q. C.2015. Neuroprotection by Kukoamine A against oxidative stress may involve N-methyl-D-aspartate receptors. Biochim. Biophys. Acta 1850: 287–298. doi: 10.1016/j.bbagen.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 17.Karri V., Schuhmacher M., Kumar V.2016. Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ. Toxicol. Pharmacol. 48: 203–213. doi: 10.1016/j.etap.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 18.Kim S., Cheon H. S., Kim S. Y., Juhnn Y. S., Kim Y. Y.2013. Cadmium induces neuronal cell death through reactive oxygen species activated by GADD153. BMC Cell Biol. 14: 4. doi: 10.1186/1471-2121-14-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S. D., Moon C. K., Eun S. Y., Ryu P. D., Jo S. A.2005. Identification of ASK1, MKK4, JNK, c-Jun, and caspase-3 as a signaling cascade involved in cadmium-induced neuronal cell apoptosis. Biochem. Biophys. Res. Commun. 328: 326–334. doi: 10.1016/j.bbrc.2004.11.173 [DOI] [PubMed] [Google Scholar]
- 20.Kim Y. J., Kim Y. S., Kim M. S., Ryu J. C.2007. The inhibitory mechanism of methylmercury on differentiation of human neuroblastoma cells. Toxicology 234: 1–9. doi: 10.1016/j.tox.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 21.Lin C.C., Yee N., Barkay T.2012. Microbial transformations in the mercury cycle, pp. 155–191. In: Environmental Chemistry and Toxicology of Mercury (Liu, G., Cai, Y. O. and Driscoll, N. eds.), John Wiley & Sons, Hoboken. [Google Scholar]
- 22.López E., Figueroa S., Oset-Gasque M. J., González M. P.2003. Apoptosis and necrosis: two distinct events induced by cadmium in cortical neurons in culture. Br. J. Pharmacol. 138: 901–911. doi: 10.1038/sj.bjp.0705111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miura K., Koide N., Himeno S., Nakagawa I., Imura N.1999. The involvement of microtubular disruption in methylmercury-induced apoptosis in neuronal and nonneuronal cell lines. Toxicol. Appl. Pharmacol. 160: 279–288. doi: 10.1006/taap.1999.8781 [DOI] [PubMed] [Google Scholar]
- 24.Mohammadi-Bardbori A., Rannug A.2014. Arsenic, cadmium, mercury and nickel stimulate cell growth via NADPH oxidase activation. Chem. Biol. Interact. 224: 183–188. doi: 10.1016/j.cbi.2014.10.034 [DOI] [PubMed] [Google Scholar]
- 25.Nirmaladevi D., Venkataramana M., Chandranayaka S., Ramesha A., Jameel N. M., Srinivas C.2014. Neuroprotective effects of bikaverin on H2O2-induced oxidative stress mediated neuronal damage in SH-SY5Y cell line. Cell. Mol. Neurobiol. 34: 973–985. doi: 10.1007/s10571-014-0073-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ou Y. C., Thompson S. A., Ponce R. A., Schroeder J., Kavanagh T. J., Faustman E. M.1999. Induction of the cell cycle regulatory gene p21 (Waf1, Cip1) following methylmercury exposure in vitro and in vivo. Toxicol. Appl. Pharmacol. 157: 203–212. doi: 10.1006/taap.1999.8685 [DOI] [PubMed] [Google Scholar]
- 27.Park E. J., Park K.2007. Induction of reactive oxygen species and apoptosis in BEAS-2B cells by mercuric chloride. Toxicol. In Vitro 21: 789–794. doi: 10.1016/j.tiv.2007.01.019 [DOI] [PubMed] [Google Scholar]
- 28.Redza-Dutordoir M., Averill-Bates D. A.2016. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 1863: 2977–2992. doi: 10.1016/j.bbamcr.2016.09.012 [DOI] [PubMed] [Google Scholar]
- 29.Syversen T., Kaur P.2012. The toxicology of mercury and its compounds. J. Trace Elem. Med. Biol. 26: 215–226. doi: 10.1016/j.jtemb.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 30.Teixeira F. B., Fernandes R. M., Farias-Junior P. M., Costa N. M., Fernandes L. M., Santana L. N., Silva-Junior A. F., Silva M. C., Maia C. S., Lima R. R.2014. Evaluation of the effects of chronic intoxication with inorganic mercury on memory and motor control in rats. Int. J. Environ. Res. Public Health 11: 9171–9185. doi: 10.3390/ijerph110909171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toimela T., Tähti H.2004. Mitochondrial viability and apoptosis induced by aluminum, mercuric mercury and methylmercury in cell lines of neural origin. Arch. Toxicol. 78: 565–574. doi: 10.1007/s00204-004-0575-y [DOI] [PubMed] [Google Scholar]
- 32.Wang B., Du Y.2013. Cadmium and its neurotoxic effects. Oxid. Med. Cell. Longev. 2013: 898034.doi: 10.1155/2013/898034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang B., Xiao J. L., Ling Y. H., Meng X. J., Wu B., Yang X. Y., Zou F.2014. BNIP3 upregulation by ERK and JNK mediates cadmium-induced necrosis in neuronal cells. Toxicol. Sci. 140: 393–402. doi: 10.1093/toxsci/kfu091 [DOI] [PubMed] [Google Scholar]
- 34.Xu B., Chen S., Luo Y., Chen Z., Liu L., Zhou H., Chen W., Shen T., Han X., Chen L., Huang S.2011. Calcium signaling is involved in cadmium-induced neuronal apoptosis via induction of reactive oxygen species and activation of MAPK/mTOR network. PLoS One 6: e19052. doi: 10.1371/journal.pone.0019052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu M., Yan C., Tian Y., Yuan X., Shen X.2010. Effects of low level of methylmercury on proliferation of cortical progenitor cells. Brain Res. 1359: 272–280. doi: 10.1016/j.brainres.2010.08.069 [DOI] [PubMed] [Google Scholar]
- 36.Yu X., Robinson J. F., Sidhu J. S., Hong S., Faustman E. M.2010. A system-based comparison of gene expression reveals alterations in oxidative stress, disruption of ubiquitin-proteasome system and altered cell cycle regulation after exposure to cadmium and methylmercury in mouse embryonic fibroblast. Toxicol. Sci. 114: 356–377. doi: 10.1093/toxsci/kfq003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang R., Zhang N., Zhang H., Liu C., Dong X., Wang X., Zhu Y., Xu C., Liu L., Yang S., Huang S., Chen L.2017. Celastrol prevents cadmium-induced neuronal cell death by blocking reactive oxygen species-mediated mammalian target of rapamycin pathway. Br. J. Pharmacol. 174: 82–100. doi: 10.1111/bph.13655 [DOI] [PMC free article] [PubMed] [Google Scholar]