Abstract
Mesophyll conductance (gm) is a critical variable for the use of stable carbon isotopes to infer photosynthetic water-use efficiency (WUE). Although gm is similar in magnitude to stomatal conductance (gs), it has been measured less often, especially under field conditions and at high temporal resolution. We mounted an isotopic CO2 analyser on a field photosynthetic gas exchange system to make continuous online measurements of gas exchange and photosynthetic 13C discrimination (Δ13C) on mature Pinus sylvestris trees. This allowed the calculation of gm, gs, net photosynthesis (Anet), and WUE. These measurements highlighted the asynchronous diurnal behaviour of gm and gs. While gs declined from around 10:00, Anet declined first after 12:00, and gm remained near its maximum until 16:00. We suggest that high gm played a role in supporting an extended Anet peak despite stomatal closure. Comparing three models to estimate WUE from ∆13C, we found that a simple model, assuming constant net fractionation during carboxylation (27‰), predicted WUE well, but only for about 75% of the day. A more comprehensive model, accounting explicitly for gm and the effects of daytime respiration and photorespiration, gave reliable estimates of WUE, even in the early morning hours when WUE was more variable. Considering constant, finite gm or gm/gs yielded similar WUE estimates on the diurnal scale, while assuming infinite gm led to overestimation of WUE. These results highlight the potential of high-resolution gm measurements to improve modelling of Anet and WUE and demonstrate that such gm data can be acquired, even under field conditions.
Electronic supplementary material
The online version of this article (10.1007/s11120-019-00645-6) contains supplementary material, which is available to authorized users.
Keywords: Carbon isotope ratio, Cavity ring-down absorption spectrometry, Online discrimination, Vapour pressure deficit, Photosynthesis
Introduction
Mesophyll conductance (gm) describes the ability of CO2 to diffuse across the interior of the leaf. In plants with C3 photosynthesis, gm is roughly similar in magnitude to stomatal conductance (gs), frequently accounting for about 40% of the decline in CO2 concentration from the ambient atmosphere to the chloroplasts (Cc) (Flexas et al. 2008; Warren 2008a). As a consequence, it has an important place in leaf-level photosynthesis models (von Caemmerer 2000; Dewar et al. 2017), but has been so infrequently quantified that it is seldom included in earth-system models (Rogers et al. 2017). It also has a critical role in the inference of water-use efficiency (WUE) from stable carbon isotope composition (δ13C) of plant tissues or, conversely, in the inference of δ13C from gas exchange (Rogers et al. 2017). This role is caused by the decrease in CO2 concentration at the enzyme rubisco, where δ13C is determined, relative to the substomatal cavities, where WUE is determined. Mesophyll conductance provides a means to calculate this difference. If gm could be accounted for, then δ13C could provide independent tests of the WUE predictions of leaf (von Caemmerer 2000; Wei et al. 2014), canopy (Keenan et al. 2013), and earth-system models (Rogers et al. 2017).
One reason for the relative paucity of gm data is that it is more difficult to estimate than gs. Stomatal conductance to CO2 is easily estimated from humidity, temperature and transpiration measurements, which might come from leaf-level gas exchange or sap-flux data. Given the relative ease of making such measurements, high temporal resolution gs data are available for many species and sites, and models of gs based on theory and empirical data have converged (Medlyn et al. 2011) and been incorporated into global models (Prentice et al. 2014; Rogers et al. 2017). In contrast, measuring gm requires simultaneous measurements of gas exchange and either chlorophyll fluorescence or photosynthetic discrimination against 13C (∆13C). Discrimination can be inferred from the δ13C signature of photosynthesis products, e.g. leaf soluble sugars, phloem contents, plant tissues (e.g.: Hu et al. 2010; Ubierna and Marshall 2011), or directly from leaf CO2 flux (e.g.: Evans et al. 1986; Warren et al. 2003; Bickford et al. 2009; Wingate et al. 2007, 2010; Maseyk et al. 2011; Campany et al. 2016). These methods are technically challenging, especially under field conditions, so that measurements are often made with low temporal resolution.
It has been difficult to model gm, because previous studies have found that gm and gs respond differently to changes in environmental conditions, suggesting that the two are not tightly coupled. Rapid responses of gm have been described to several environmental variables (for a review see Flexas et al. 2008; Warren 2008a; Flexas et al. 2012). These variables include light intensity or quality (Flexas et al. 2007; Tholen et al. 2008; Hassiotou et al. 2009; Loreto et al. 2009; Campany et al. 2016), intercellular CO2 concentration (Ci) (Flexas et al. 2007; Hassiotou et al. 2009; Vrábl et al. 2009; Bunce 2010; Douthe et al. 2011; Tazoe et al. 2011), and leaf temperature (Bernacchi et al. 2002; Yamori et al. 2006; Warren 2008b; Evans and von Caemmerer 2013). If gs responded to other variables, or at different rates, then the ratio gm/gs would change. For example, it has been shown that gm responds similarly, but more quickly, to variable Ci than gs (Flexas et al. 2007). In addition, the gm/gs ratio was found to be temperature dependent in a study exploring the thermal acclimation of gm in spinach (Yamori et al. 2006).
Vapour pressure deficit (VPD) is particularly interesting in this context, because gs responds so strongly to it (Marshall and Waring 1984; Oren et al. 1999; Medlyn et al. 2011). In contrast, the response of gm to VPD has not been extensively studied and the results so far are contradictory (Bongi and Loreto 1989; Warren 2008c, Loucos et al. 2017). Both temperature and VPD change dynamically under natural conditions, diurnally and seasonally, potentially influencing the gs to gm relationship. However, the magnitude and importance of this variability has yet to be explored.
Given constant gs, an increase in gm would increase water-use efficiency (WUE) (Flexas et al. 2010; Galmés et al. 2011), which is defined as the ratio of net carbon assimilation (Anet) to water loss through transpiration (E). This would happen because an increase in gm has no direct effect on transpiration, but it increases photosynthesis, resulting in an increase of the Anet/E ratio. Accounting for gm is especially important when estimating WUE from δ13C. For example, WUE is often inferred from historic tree-ring isotope data (Marshall and Monserud 1996; Seibt et al. 2008; Voelker et al. 2016). Such inferences require that some value for gm be assumed. This assumption is often embedded as a constant, empirical adjustment in the relationship between Ci/Ca and isotopic discrimination (Farquhar et al. 1982), or extrapolated based on its correlation with gs in models of WUE (Klein et al. 2015), although as noted above, the correlation with gs is not always strong.
In this manuscript, we present continuous, simultaneous measurements of shoot-scale gas exchange and 13C discrimination in a 100-year-old Pinus sylvestris stand in northern Sweden. We use these simultaneous data streams to obtain hourly gm estimates parallel to gs, Anet and E. We begin with a brief description of how the data were treated and evaluate the accuracy of our measurement system. We next explore the diurnal dynamics of gs and gm and their relationship to Anet. Finally, we compare estimates of WUE derived from gas exchange (WUEG) with estimates derived from photosynthetic discrimination (WUE∆). Three photosynthetic discrimination (∆13C) models were used to calculate WUE∆: a comprehensive model, a partial model and a simple model. Additionally, the comprehensive model was applied using three different assumptions for gm values. We compare the different models and calculations and discuss their impact on WUE∆ estimates.
Materials and methods
Description of the experimental site
The study was conducted in a ~ 100-year-old, naturally regenerated, even-aged stand of Pinus sylvestris (Scots pine) at the Rosinedalsheden experimental forest in northern Sweden (64°10′N, 19°45′E, 153 m above see level), during the growing season of 2017. The Rosinedalsheden experiment includes an intensive fertilisation treatment (Lim et al. 2015), but the current study was conducted entirely on the unfertilised area. The photosynthetic season typically extends from mid-April to mid-November, buds burst at the end of May, and stem diameter-growth ceases in late August (Tarvainen et al. 2018). The June to August mean temperature was 12.4 ± 0.8 °C (mean ± SD) and the mean monthly precipitation was 67.9 ± 8.6 mm (mean ± SD), based on the 15-year (2003–2017) data measured at the Vindeln-Sunnansjönäs meteorological station (Swedish Meteorological and Hydrological Institute, www.smhi.se) approximately 5 km from the experimental site. The site has weakly podzolised fine sandy soil with a thin (2–5 cm) organic layer (Hasselquist et al. 2012). The leaf area index was 2.7 and the average tree height was 18.6 ± 2.3 m (mean ± SD) in 2013 (Lim et al. 2015).
Experimental setup for continuous measurements of gas exchange
Shoot gas exchange (CO2 and H2O) was measured continuously on one 1-year-old upper canopy shoot on four trees. A 16-m tall scaffolding tower was used to reach the shoots and secure the equipment. The shoot-scale gas exchange was measured using a custom-built multichannel gas exchange system (GUS) (Wallin et al. 2001; Tarvainen et al. 2016), equipped with infrared gas analysers (IRGA, CIRAS-1, PP systems Hitchin Herts, U.K.) to measure CO2 and H2O partial pressure in the air from shoot cuvettes and reference channels. The 330 ml shoot cuvettes had a transparent acrylic plastic (Plexiglas) top for natural illumination. The cuvettes were temperature (T) controlled to track the ambient T and were equipped with a light sensor (PAR-1 M, PP systems, Hitchin, Herts, UK). The polyethylene tubing that connected the cuvettes to the IRGAs were insulated and heated with cables to avoid condensation. Nonetheless, morning condensation could occur in the cuvettes in connection with heavy rain events; we filtered those days out in the current analysis. The GUS cycled through the four shoot cuvettes and two non-cuvette lines once per hour, spending 7 min at each position, which were divided into 2 min of waiting time to allow instrument readings to stabilise and 5 min of measurement. We used the means from the 5-min measurement periods in the subsequent analyses, which yielded approximately one value/cuvette/hour throughout the 9 days. The non-cuvette lines were used for data quality assurance and for measurement of δ13C of ambient air (see details in next chapter). The IRGAs were calibrated with 400 µmol mol−1 CO2 gas at the beginning and at the end of the growing season. Additionally, every hour the IRGAs were zero calibrated and the system ran a cross-calibration protocol to match values in the sample and reference channels.
Continuous measurement of δ13C
The isotopic composition of the CO2 in the air entering and leaving the cuvettes was analysed with a cavity ring-down spectrophotometer (CRDS; G2131-i, Picarro Inc., California, USA). The CRDS was connected to the same central line as the GUS, in parallel to the sample IRGA. We tested the instrument at varying CO2 and H2O vapour concentrations and found that the δ13C values were dependent on both, with an asymptotic relationship of δ13C to CO2 concentration (Fig. S1) and a linear dependency to H2O vapour concentration (Fig. S2). The continuous δ13C readings were corrected to account for the CO2 and H2O concentration effects before the data were used in further analyses. The CRDS was factory-calibrated in 2017 and manually calibrated once per week, using two reference gases with known CO2 concentrations (411 µmol mol−1, SD = 5.1; 1606 µmol mol−1, SD = 13.1) and δ13C values (− 32.36‰, SD = 0.09; − 4.14‰, SD = 0.06). The reference gases were analysed at the SLU Stable Isotope Laboratory (Umeå, Sweden) with GB-IRMS (Gasbench II—Isotope Ratio Mass Spectrometer, Thermo Fisher Scientific, Bremen, Germany), which was calibrated against IAEA-co-9 and NBS 19 standards. We found the weekly calibrations to be sufficient, because the reference δ13C values were stable over the season (Fig. S3) and were offset from the reference gases by a constant 4.17‰ (SD = 0.1), after correction for CO2 concentration. The CRDS recorded δ13C values once per second during the 5-min calibration period, which were then combined into a mean for each calibration date and these means yielded standard deviations of 0.1‰ for δ13C.
Calculation of leaf gas exchange parameters and mesophyll conductance
In this paper, we present data collected on nine sunny days during the summer (28th of June—2nd of July and 6th of July—9th of July), with daily minimum and maximum temperatures of 6.2 ± 0.5 °C and 24.4 ± 0.6 °C, respectively, and daily maximum irradiation of 1964 ± 25 µmol m−2 s−1. Because of the high latitude and season, sunrise was typically around 02:15 and sunset was around 23:00. These days were chosen for high photosynthetic rates and lack of condensation in the cuvettes and tubing. We optimised the system setup to yield clear and consistent δ13C values with the CRDS, using 5-min integrations at approximately 1-second intervals. Because each of these measurements contributed to the mean δ13C value, it was appropriate to calculate the standard error of the mean from them. This yielded high precision, typically SE < 0.06‰.
Anet, E, gs, and Ci were calculated from the gas exchange data according to the model described by Farquhar et al. (1980). Boundary layer conductance has previously been found to be high (8.1 mol H2O m−2 s−1) (Uddling and Wallin 2012) in our gas exchange cuvettes, therefore, we assumed boundary layer resistance to be insignificant. Needles from the shoots enclosed in the cuvettes were collected at the end of the study campaign to determine the projected leaf area using a flat bed scanner (Epson 1600) equipped for dual scanning, and WinSEEDLE Pro 5.1a (Regent Instruments, Canada) analysis software.
Mesophyll conductance (gm) and Cc were estimated from the carbon isotope discrimination data collected by the CRDS. The gm was calculated from the comprehensive ∆13C model of Farquhar and Cernusak (2012) that includes ternary corrections. In particular, we used the formulation of Evans and von Caemmerer (2013) (see supplementary materials for details) that calculates gm as
| 1 |
where b, am and e are the fractionation factors during carboxylation (b = 29‰), dissolution and diffusion through water (am = 1.8‰) and respiration (e, see Eqn. S5), respectively. Rd is daytime respiration (Eqn. S1), and Cout1 is the CO2 concentration in the cuvette; ∆i, ∆o, ∆e and ∆f are, respectively, the discrimination when Ci = Cc (Eqn. S2), the observed discrimination during gas exchange (Eqn. S3 and S4), the discrimination associated with respiration (Eqn. S5 and S6) and with photorespiration (Eqn. S7). The term t is the ternary correction factor (Eqn. S8, Farquhar and Cernusak 2012). Note that Cout is lower than the atmospheric CO2 concentration (Cin), due to Anet within the cuvette. The CO2 concentration at the site of carboxylation (Cc) was calculated from gm through the following relationship:
| 2 |
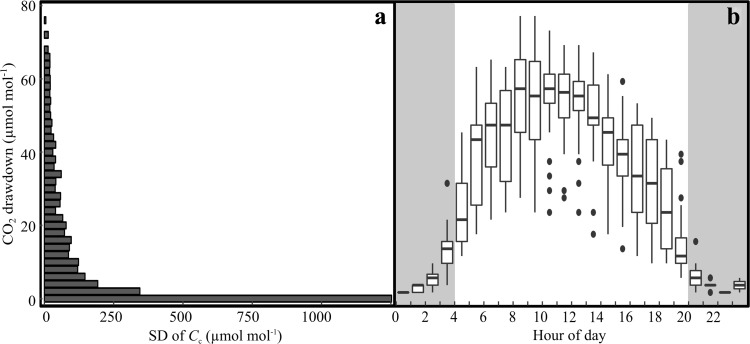
We evaluated how the magnitude of the net photosynthetic CO2 drawdown, calculated as Cin−Cout, affected our estimates of Cc. This drawdown, together with instrument precision, determines the error associated with ∆13C measurements, which ultimately determines the error in Cc and gm estimates (for a discussion see Ubierna et al. 2018). The concentration drop is evaluated with the parameter ζ = Cin/(Cin−Cout). Pons et al. (2009) showed that the error associated with gm estimates increased when ζ was large and the instrument precision was low. We likewise found that Cc became exponentially more variable as the CO2 drawdown in the cuvette decreased below 20 μmol CO2 mol−1 (Fig. 1a). Assuming an ambient CO2 concentration of 400 ppm, a drawdown of 20 μmol CO2 mol−1 corresponds to ζ = 20 (= 400/(400–380)). In this case, and with an instrument precision of 0.06‰ the error associated with ∆13C measurements was 1.7‰ (= . This low drawdown and large error mainly occurred during early mornings and evenings, so that it was not possible to acquire reasonable estimates before 04:00 and after 20:00 (Fig. 1b). For most of the day, the observed drawdown was substantially greater than 20 μmol CO2 mol−1 which resulted in ζ < 10 and associated errors in ∆o < 0.8‰ (Fig. 1b).
Fig. 1.
a Standard deviation of the CO2 concentration in the chloroplast (Cc) in relation to CO2 drawdown in the cuvette, defined as the difference between the atmospheric concentration (Cin), and the concentration inside the cuvette (Cout). The SD of Cc was estimated for every 2 µmol mol−1 change in the CO2 drawdown. The figure is based on non-filtered data. b The diurnal time course of the CO2 drawdown. The whiskers of the box-plots extend to 1.5 times the interquartile range. The grey area marks the time of day excluded from the analysis. The figure is based on non-filtered data
Comparison of models to estimate water-use efficiency from ∆13C
Instantaneous WUE can be derived from ∆13C (WUE∆) or from gas exchange (WUEG) measurements as (Seibt et al. 2008; Hu et al. 2010; Wang et al. 2014; Klein et al. 2015; Guerrieri et al. 2016):
| 3 |
where Ci is solved from a theoretical ∆13C model.
We considered three models for ∆13C, which resulted in three estimations of WUE∆. First we estimated Ci from the simple model by Farquhar et al. (1982), as
| 4 |
where is taken as 27‰, a standard value for C3 plants, that was derived empirically from relationships between δ13C of leaf bulk material and Ci/Ca values (Farquhar et al. 1982; Cernusak et al.2013; Ubierna and Farquhar 2014). This model does not account specifically for the dependency of ∆13C on gm, Rd or photorespiration (Rp); instead it includes these effects empirically within , which is often sufficient in practice (Cernusak et al. 2013; Bloomfield et al. 2019). Second, we estimated Ci from a model proposed by Seibt et al. (2008), subsequently referred to as the partial model, as
| 5 |
where Γ* is the CO2 compensation point, derived from an Arrhenius function (Bernacchi et al. 2001; Medlyn et al. 2002 Eq. 12). This model accounts explicitly for the effect of gm and Rp and assumes negligible effect of Rd. Finally, from the comprehensive model of Farquhar and Cernusak (2012) Ci can be solved as (see supplemental materials in Cernusak et al. 2018)
| 6 |
where equations I, II and III are given in the supplementary materials (Eqn S9–S11). This model accounts explicitly for gm, Rp and Rd, and it includes a correction for the ternary effect.
To avoid circularity, we divided our data set into two parts. We used 4 days’ data to estimate a mean value of gm (0.29 mol CO2 m−2 s−1 bar−1). Subsequently, this mean gm was used on the remaining 5 days’ data to estimate Ci from ∆13C (Eqs. 4, 5 and 6) and WUE (Eq. 3). Note that the 4-day mean gm was slightly different from the mean for all 9 days together, which was 0.31 mol CO2 m−2 s−1 bar−1. For the comprehensive model, besides using a constant mean gm, we also performed the calculations using either a constant gm/gs (2.9) or infinite gm and compared these estimates as well to WUEG.
Data analysis
A statistical filter was applied to the dataset to discard outliers in Ci−Cc and gm. Any data point outside the range of mean ± 3 SD was considered an outlier and removed. This filter removed 4.5% of the data. Despite the filtering, some few negative conductances remained. Although they are not theoretically possible, we retained them in the analysis because they represent the tails of the statistical distributions and they influenced the means. The sole exception was when we analysed the dependency of net photosynthetic rates on the conductances. In this one analysis, the negative values were deleted. The four cuvettes were treated as biological replicates, from which the mean hourly values were used for further analysis. Regression analysis was used to evaluate diurnal patterns. This included linear, polynomial, and nonlinear regression, as deemed appropriate. Correlations were treated as significant for p ≤ 0.05. All variability is given as standard error, unless stated otherwise. All statistical analyses were performed using the base package of R (version 3.3.2).
Results
Diurnal trends of gs and gm
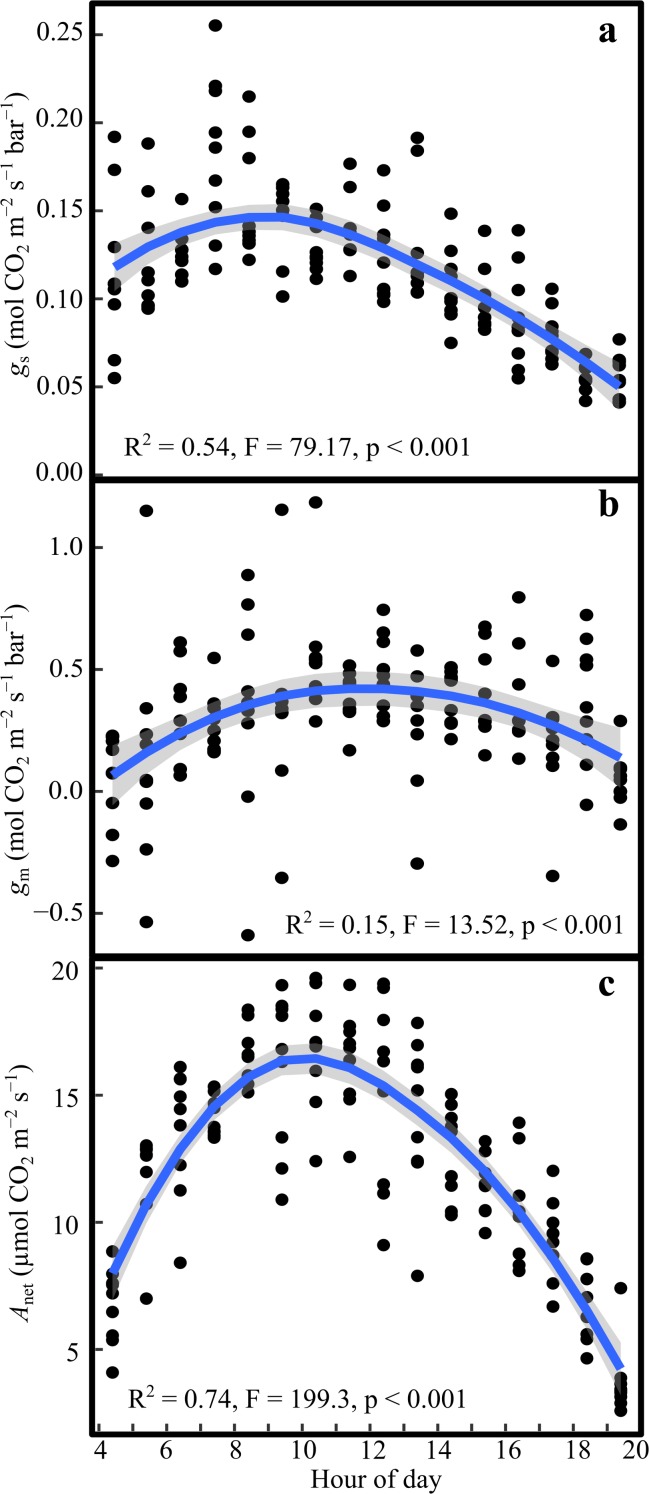
We evaluated the diurnal trends in stomatal and mesophyll conductance, and in their ratio. Mean gs was 0.115 (SE = 0.002) mol CO2 m−2 s−1 bar−1. As expected, gs showed a significant diurnal pattern (F = 79.17, p < 0.001), with peak values between 09:00 and 10:00, and decreased thereafter (Fig. 2a). We found a mean gm value of 0.31 (SE = 0.02) mol CO2 m−2 s−1 bar−1. Furthermore gm also had a significant diurnal pattern (F = 13.52, p < 0.001; Fig. 2b) with relatively stable mean values between 08:00 and 16:00 and lower values in the early morning and towards the evening. The mean for the unitless ratio gm/gs was 2.67 (SE = 0.3); with a weak, but significant diurnal pattern (F = 3.9, p = 0.02).
Fig. 2.
Diurnal variation in a stomatal conductance (gs), b mesophyll conductance (gm), and c net photosynthesis (Anet). The points represent the cuvette means (n = 4) for each hour and day. The blue line is the second order polynomial fit to the data and the shaded grey area is the standard error of the fit
The relationship of Anet to gs and gm
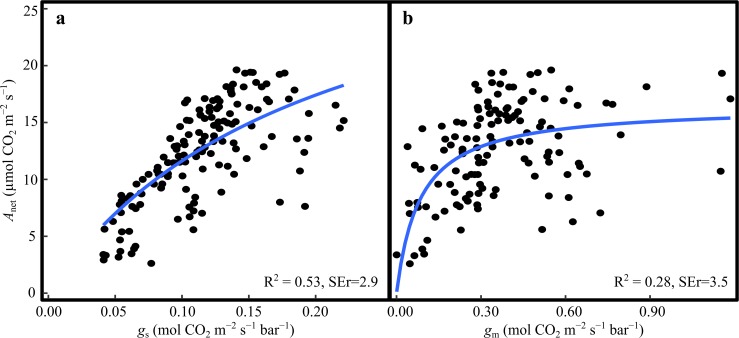
Anet followed a typical diurnal pattern, with highest rates between 08:00 and 12:00, with a mean of 16.2 (SE = 0.26) µmol CO2 m−2 s−1, and gradually declining rates in the afternoon (Fig. 2c). We found a significant asymptotic relationship between Anet and gs (p < 0.001, R2 = 0.53, Fig. 3a). Similarly there was a significant asymptotic relationship between Anet and gm (p < 0.001, R2 = 0.28, Fig. 3b).
Fig. 3.
Relationship between net photosynthesis (Anet) and a stomatal conductance (gs), and b mesophyll conductance (gm). The points represent the cuvette means (n = 4) for each hour and day. The blue line represents the asymptotic fit to the data
Relationship of gs and gm to VPD
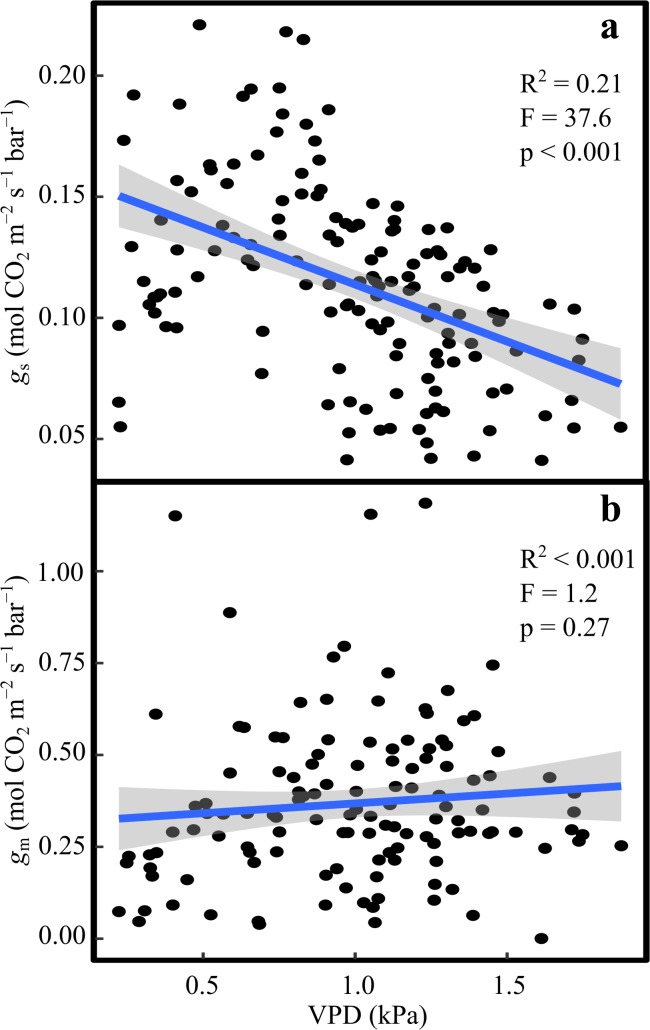
The average hourly VPD varied from 0.26 kPa to 1.82 kPa during the day, with sharp increase during the mornings until about 12:00 (Fig S4). We found a significant linear relationship between gs and VPD (F = 37.6, p < 0.001, R2 = 0.26) (Fig. 4a) but no relationship between gm and VPD (F = 1.12, p = 0.3, R2 = 0.001) (Fig. 4b).
Fig. 4.
Response of a stomatal (gs), and b mesophyll (gm) conductance to vapour pressure deficit (VPD). The points represent the cuvette means (n = 4) for each hour and day. The blue line represents the regression fit to the data and the shaded grey area is the standard error of the fit
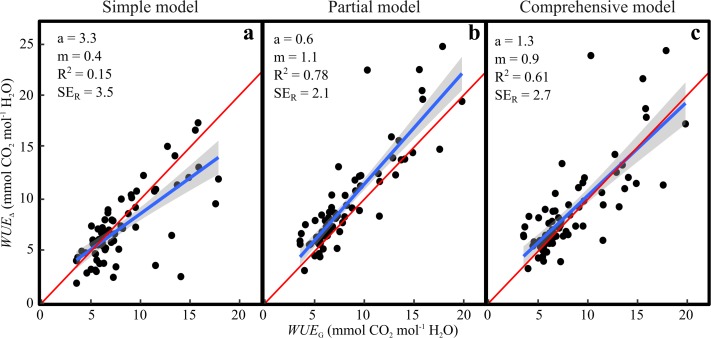
Contrasting estimates of WUE from ∆13C
We compared the performance of the three models to estimate WUE from ∆13C (WUE∆) against direct measurements of WUE as Anet/E by the gas exchange system (WUEG). Theoretically WUE∆ and WUEG should be identical with an ideal fit, where slope m = 1 and intercept a = 0. Using the simple model resulted in a poor fit to our data (Fig. 5a). Further analysis revealed that this model could not predict WUEG in the early hours (04:00–08:00), but fit the data well between 08:00 and 20:00 (a = − 0.4, m = 1.0, R2 = 0.69, Fig S5). The partial model consistently overestimated WUEG by ca. 15% with no diurnal pattern (Fig. 5b). The comprehensive model matched the data well on average (Fig. 5c), but had a slight tendency to overestimate WUEG in the low range and underestimate it in the high range. Furthermore, it introduced more variability into the estimates compared to the partial model, with a residual standard error (SEr) of 2.7 mmol CO2 mol H2O−1 (R2 = 0.61) compared to 2.1 mmol CO2 mol H2O−1 (R2 = 0.78). In the comprehensive model, representing gm as a constant ratio to gs overestimated WUEG by ca. 9% compared to observations (a = 0.7, m = 1.05, Fig. S6a). Assuming infinite gm resulted in a poor fit to observed data (a = 0.7, m = 1.5, Fig. S6b) and an overestimation of WUEG by 49%.
Fig. 5.
Water-use efficiency calculated from ∆13C data (WUE∆) using a the simple model b the partial model, and c comprehensive model presented here. The results are compared to water-use efficiency calculated from continuous gas exchange data (WUEG). In model b and c we assume a constant vale for gm (0.29 mol CO2 m−2 s−1 bar−1). The points represent the cuvette means (n = 4) for each hour and day. The blue line is the linear fit to the data, the shaded grey area is the standard error of the fit, and m is the slope of the fit. The red line represents the theoretical 1:1 fit for comparison
Discussion
Here, we report the first gm estimates for mature Pinus sylvestris, one of the most widespread coniferous species of the northern hemisphere. Our mean value (0.31, SE = 0.02 mol m−2 s−1 bar−1) is somewhat higher than previously reported for other Pinus species (Flexas et al. 2008), but it is within the range of values reported for various conifers (Table 1) (De Lucia et al. 2003; Flexas et al. 2008; Bown et al. 2009; Bickford et al. 2010; Han 2011; Maseyk et al. 2011; Ubierna and Marshall 2011; Veromann-Jürgenson et al. 2017). Some of this variation may be due to differences in the methods used (Flexas et al. 2008). In particular, the “variable J method”, which uses simultaneous measurements of gas exchange and chlorophyll fluorescence to infer gm, generally yields lower gm values than do isotopic techniques. If we exclude the “variable J” estimates from the list in Table 1, then our estimate of gm matches the other values for conifers quite well. Furthermore, our estimates of δ13C of Anet were in the range reported previously (e.g. Wingate et al. 2010) (Fig. S7), and are the first based on measurements using the CRDS (cavity ring-down spectroscopy) technology. The good agreement encourages us to suggest that this method, which is less expensive than most alternatives, produces reliable δ13C measurements and is suitable for field applications. The CRDS was steady under the variable conditions of field-measurements, exemplified by the fact that we did not see any drift in the δ13C values of the reference gases during several weeks of continuous measurements (Fig. S3). Nevertheless, it was crucial to correct the data for the CRDS’ sensitivity to changing CO2 and H2O vapour concentrations. The sensitivity of isotope measurements to CO2 concentration is a known phenomenon and is commonly corrected for in other laser technologies, like in lead alloy tunable diode lasers (Ubierna et al. 2018). Furthermore, drying the gas before isotope analysis will avoid having to correct for H2O concentrations dependency, which is likely to improve measurement accuracy.
Table 1.
Mesophyll conductance values reported for various coniferous species
| Measurement method | Species | gm mol m−2 s−1 bar−1** | References |
|---|---|---|---|
| Instantaneous ∆13C and gas exchange (CRDS) | Pinus sylvestris | 0.33 ± 0.03 | Current paper |
| Instantaneous ∆13C and gas exchange | Pinus halepensis | 0.2–0.4 | Maseyk et al. (2011) |
| Instantaneous ∆13C and gas exchange (TDL) | Juniperus monosperma | 0.04–4.0 | Bickford et al. (2009) |
| Instantaneous ∆13C and gas exchange | Pseudotsuga menziesii | 0.14–0.20 | Warren et al. (2003) |
| Phloem δ13C | Pseudotsuga menziesii | 0.22 ± 0.11 | Ubierna and Marshall (2011) |
| Phloem δ13C | Thuja plicata | 0.09 ± 0.04 | Ubierna and Marshall (2011) |
| Phloem δ13C | Larix occidentalis | 0.41 ± 0.21 | Ubierna and Marshall (2011) |
| Variable J method | Pinus radiata | 0.153 ± 0.002 | De Lucia et al. (2003) |
| Variable J method | Pinus sylvestris | 0.124 ± 0.009 | Veromann-Jürgensson et al. (2017) |
| Variable J method | Pinus radiata | 0.16–0.2 | Bown et al. (2009) |
| Variable J method | Pinus densiflora | 0.107–0.250 | Han (2011) |
| Various methods | Pinus spp.* | 0.04–0.17 | Flexas et al. (2008) |
*Pinus lambertiana, Pinus monticola, Pinus pinaster, Pinus radiata
**If published values were presented as mol m−2 s−1, then we assumed barometric pressure of 1 bar and converted the units to mol m−2 s−1 bar−1
We observed an asynchronous reduction in gm and gs over the day (Fig. 2a, b). This may happen because gs is under strong control by ambient VPD (Fig. 4a), whereas we found no correlation between gm and VPD (Fig. 4b). The response of gm to VPD has only been investigated in few studies, and with contrasting results. While Bongi and Loreto (1989) and Loucos et al. (2017) found a significant negative correlation between gm and VPD, a study looking at the effect of air humidity and soil moisture (Warren 2008c) on gs and gm found a strong correlation of both conductances to soil moisture, while VPD only affected gs and not gm. All studies involved different species, and higher VPD ranges (1–3 kPa and 1–2 kPa, respectively) compared to our study (0.23–1.82 kPa). These discrepancies highlight the need for further investigation including a wider range of VPD conditions for Pinus sylvestris.
We fitted an asymptotic relationship of Anet to gs and gm. The asymptotic response agrees with theoretical expectations of CO2 saturation at high conductances. In a diurnal context, Anet was maintained at high rates until mid-day, despite declining gs from mid-morning (Fig. 2). We suggest that high gm helped to maintain Anet during the late morning, enabling high Cc and compensating for the decline of gs. This diurnal asynchrony between gs and gm is qualitatively similar to the observations by Theroux-Rancourt et al. (2014) on hybrid poplar cuttings exposed to soil drying over 12 days. They suggested based on daily measurements of gs and gm that a delayed gm response reduced the decline in photosynthesis and enhanced WUE during the beginning of the drought treatment. Our finding suggests, that even within a diurnal context, the asynchronous response of gm and gs to environmental conditions has significant influence on Anet and presumably WUE.
We compared three models to estimate WUE from δ13C. We found that the simple model can estimate WUE well for most of the day. This model uses , an empirical value that accounts for the drop of concentration between Ci and Cc, the different fractionations occurring during photosynthetic discrimination, as well as possible post-photosynthetic discrimination. Many studies have shown that works well as an approximation (e.g. Farquhar et al. 1982; Seibt et al. 2008; Bloomfield et al. 2019). However, it performed poorly during the early morning, when WUE (Fig. S8), and especially δ13C of photosynthesis (Fig. S7) were more variable. This meant that it produced unreliable WUE estimates for 25% of the photosynthetically active period of the day. Our analysis clearly shows that a more complete model that accounts explicitly for the effects of photorespiration, or both photorespiration and daytime respiration, performs better under such variable conditions and provides more accurate estimates of WUE. The relatively high variability in the estimates highlight the need to further refine some of the model assumptions.
We have shown that it is critical to account for gm in the estimation of WUE from ∆13C. This point has been made before (Seibt et al. 2008; Klein et al. 2015), but is still often neglected. Our data is yet an other example of WUE being overestimated if gm is assumed to be infinite, and we show that assuming constant gm/gs or constant gm both yield better estimates than infinite gm. Estimating WUE from gm/gs had the further advantage of accounting for some of the diurnal change in gm, resulting in a slope closer to 1 than when gm was assumed constant. Nevertheless, this approach does not take into account the diurnality of gm/gs itself, and neglects the fact that gm is much less strongly correlated with VPD than gs.
The current study presents the first estimate of gm for mature Pinus sylvestris trees, one of the most wide-ranging tree species in the world. Those estimates were derived with a CRDS/gas exchange system, which presents opportunities for simplifying the measurement of online ∆13C discrimination. The measurements were made continuously and in the field over several sunny days in the summer. The high temporal resolution of our data allowed us to evaluate diurnal trends in conductance in relation to Anet, and test different models to estimate WUE form ∆13C. Our analysis revealed that the simple model to account for 13C discrimination worked well, but only under stable conditions, and that the comprehensive model has the potential to account for variable conditions and provide reliable estimates of Ci and WUE. We highlight the need for further work under a broader range of environmental conditions, and including seasonal phenology. Our gm estimate provides a means of improving inferences of WUE from ∆13C and our continuous measurements provide a path forward to improve the modelling of gm in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Knut & Alice Wallenberg Foundation (#2015.0047), and the Swedish strategic research area BECC (Biodiversity and Ecosystem Services in a Changing Climate). The authors want to acknowledge the SLU Stable Isotope Laboratory (SSIL) (www.slu.se/en/departments/forest-ecology-management/ssil) for the isotopic analysis of the calibration gases.
Abbreviations
- Anet
Net photosynthesis rate (µmol CO2 m−2 s−1)
- ∆e
Discrimination associated with respiration (‰)
- ∆f
Discrimination associated with photorespiration (‰)
- ∆i
Discrimination when Ci = Cc (‰)
- ∆o
Observed photosynthetic discrimination (‰)
- am
12C/13C fractionation during dissolution and diffusion through water 1.8 (‰)
- as
12C/13C fractionation during diffusion through air 4.4 (‰)
- b
12C/13C fractionation during carboxylation 29 (‰)
12C/13C net fractionation during carboxylation 27 (‰)
- Cc
CO2 concentration in the chloroplast (µmol mol−1)
- Ci
CO2 concentration in the intercellular space (µmol mol−1)
- Cin
CO2 concentration in the air entering the cuvette (µmol mol−1)
- Cout
CO2 concentration in the cuvette (µmol mol−1)
- E
Transpiration rate (mmol H2O m−2 s−1)
- e
Fractionation during respiration (‰)
- f
Fractionation during photorespiration 16.2 (‰)
- gm
Mesophyll conductance (mol CO2 m−2 s−1 bar−1)
- gs
Stomatal conductance (mol CO2 m−2 s−1 bar1)
- Rd
Day time respiration rate (µmol CO2 m−2 s−1)
- Rp
Photorespiration rate (µmol CO2 m−2 s−1)
- t
Ternary correction factor (–)
- VPD
Vapour pressure deficit (kPa)
- WUE
Water-use efficiency (mmol CO2 mol−1 H2O)
- WUEG
WUE from gas exchange (mmol CO2 mol−1 H2O)
- WUE∆
WUE calculated from ∆13C (mmol CO2 mol−1 H2O)
- Γ*
CO2 compensation point (µmol mol−1)
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to declare.
Footnotes
In calculations based on on-line discrimination, we consider the CO2 concentration and the isotopic composition inside the cuvette (noted with the subscript ‘out’) as ‘ambient’ because they represent the immediate environment of the shoot or leaf. If the models presented here (Eqs. 1, 3, 4, 5) are to be applied elsewhere, Cout can be replaced with Ca and 13Cout can be replaced with 13Ca.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bernacchi C, Singsaas E, Pimentel C, Portis AR, Jr, Long SP. Improved temperature response functions for models of Rubisco limited photosynthesis. Plant, Cell Environ. 2001;24(2):253–259. [Google Scholar]
- Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol. 2002;130(4):1992–1998. doi: 10.1104/pp.008250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford C, McDowell N, Erhardt E, Hanson D. High-frequency field measurements of diurnal carbon isotope discrimination and internal conductance in a semi-arid species, Juniperus monosperma. Plant, Cell Environ. 2009;32(7):796–810. doi: 10.1111/j.1365-3040.2009.01959.x. [DOI] [PubMed] [Google Scholar]
- Bickford CP, Hanson DT, McDowell NG. Influence of diurnal variation in mesophyll conductance on modelled 13C discrimination: results from a field study. J Exp Bot. 2010;61(12):3223–3233. doi: 10.1093/jxb/erq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield KJ, Prentice IC, Cernusak LA, Eamus D, Medlyn BE, Rumman R, Wright IJ, Boer MM, Cale P, Cleverly J, Egerton JJ, Ellsworth DS, Evans BJ, Hayes LS, Hutchinson MF, Liddell MJ, Macfarlane C, Meyer WS, Togashi HF, Wardlaw T, Zhu L, Atkin OK. The validity of optimal leaf traits modelled on environmental conditions. New Phytol. 2019;221:1409–1423. doi: 10.1111/nph.15495. [DOI] [PubMed] [Google Scholar]
- Bongi G, Loreto F. Gas-exchange properties of salt-stressed olive (Olea europea L.) leaves. Plant Physiol. 1989;90(4):1408–1416. doi: 10.1104/pp.90.4.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bown HE, Watt MS, Mason EG, Clinton PW, Whitehead D. The influence of nitrogen and phosphorus supply and genotype on mesophyll conductance limitations to photosynthesis in Pinus radiata. Tree Physiol. 2009;29(9):1143–1151. doi: 10.1093/treephys/tpp051. [DOI] [PubMed] [Google Scholar]
- Bunce JA. Variable responses of mesophyll conductance to substomatal carbon dioxide concentration in common bean and soybean. Photosynthetica. 2010;48(4):507–512. [Google Scholar]
- Campany CE, Tjoelker MG, von Caemmerer S, Duursma RA. Coupled response of stomatal and mesophyll conductance to light enhances photosynthesis of shade leaves under sunflecks. Plant, Cell Environ. 2016;39(12):2762–2773. doi: 10.1111/pce.12841. [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Ubierna N, Winter K, Holtum JAM, Marshall JD, Farquhar GD. Environmental and physiological determinants of carbon isotope discrimination in terrestrial plants. New Phytol. 2013;200:950–965. doi: 10.1111/nph.12423. [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Ubierna N, Jenkins MW, Garrity SR, Rahn T, Powers HH, Hanson DT, Sevanto S, Wong SC, McDowell NG, Farquhar GD. Unsaturation of vapour pressure inside leaves of two conifer species. Sci Rep. 2018;8(1):7667. doi: 10.1038/s41598-018-25838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucia EH, Whitehead D, Clearwater MJ. The relative limitation of photosynthesis by mesophyll conductance in co-occurring species in a temperate rainforest dominated by the conifer Dacrydium cupressinum. Funct Plant Biol. 2003;30(12):1197–1204. doi: 10.1071/FP03141. [DOI] [PubMed] [Google Scholar]
- Dewar R, Mauranen A, Mäkelä A, Hölttä T, Medlyn B, Vesala T. New insights into the covariation of stomatal, mesophyll and hydraulic conductances from optimization models incorporating nonstomatal limitations to photosynthesis. New Phytol. 2017;217:571–585. doi: 10.1111/nph.14848. [DOI] [PubMed] [Google Scholar]
- Douthe C, Dreyer E, Epron D, Warren CR. Mesophyll conductance to CO2, assessed from online TDL-AS records of 13CO2 discrimination, displays small but significant short-term responses to CO2 and irradiance in Eucalyptus seedlings. J Exp Bot. 2011;62(15):5335–5346. doi: 10.1093/jxb/err141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR, von Caemmerer S. Temperature response of carbon isotope discrimination and mesophyll conductance in tobacco. Plant, Cell Environ. 2013;36(4):745–756. doi: 10.1111/j.1365-3040.2012.02591.x. [DOI] [PubMed] [Google Scholar]
- Evans J, Sharkey T, Berry J, Farquhar G. Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 Diffusion in Leaves of higher plants. Funct Plant Biol. 1986;13(2):281–292. [Google Scholar]
- Farquhar GD, Cernusak LA. Ternary effects on the gas exchange of isotopologues of carbon dioxide. Plant, Cell Environ. 2012;35(7):1221–1231. doi: 10.1111/j.1365-3040.2012.02484.x. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149(1):78–90. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Ball MC, von Caemmerer S, Roksandic Z. Effect of salinity and humidity on δ13C value of halophytes-evidence for diffusional isotope fractionation determined by the ratio of intercellular/atmospheric partial pressure of CO2 under different environmental conditions. Oecologia. 1982;52:121–124. doi: 10.1007/BF00349020. [DOI] [PubMed] [Google Scholar]
- Flexas J, Diaz-Espejo A, Galmés J, Kaldenhoff R, Medrano H, Ribas-Carbo M. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant, Cell Environ. 2007;30(10):1284–1298. doi: 10.1111/j.1365-3040.2007.01700.x. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbo M, Diaz-Espejo A, Galmés J, Medrano H. Mesophyll conductance to CO2: current knowledge and future prospects. Plant, Cell Environ. 2008;31(5):602–621. doi: 10.1111/j.1365-3040.2007.01757.x. [DOI] [PubMed] [Google Scholar]
- Flexas J, Galmés J, Gallé A, Gulias J, Pou A, Ribas-Carbo M, Tomas M, Medrano H. Improving water use efficiency in grapevines: potential physiological targets for biotechnological improvement. Aust J Grape Wine Res. 2010;16(s1):106–121. [Google Scholar]
- Flexas J, Barbour MM, Brendel O, Cabrera HM, Carriquí M, Díaz-Espejo A, Douthe C, Dreyer E, Ferrio JP, Gago J, Gallé A, Galmés J, Kodama N, Medrano H, Niinemets Ü, Peguero-Pina JJ, Pou A, Ribas-Carbó M, Tomás M, Tosens T, Warren CR. Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Sci. 2012;193:70–84. doi: 10.1016/j.plantsci.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Galmés J, Conesa MA, Ochogavia JM, Perdomo JA, Francis DM, Ribas-Carbo M, Savé R, Flexas J, Medrano H, Cifre J. Physiological and morphological adaptations in relation to water use efficiency in Mediterranean accessions of Solanum lycopersicum. Plant, Cell Environ. 2011;34(2):245–260. doi: 10.1111/j.1365-3040.2010.02239.x. [DOI] [PubMed] [Google Scholar]
- Guerrieri R, Lepine L, Asbjornsen H, Xiao J, Ollinger SV. Evapotranspiration and water use efficiency in relation to climate and canopy nitrogen in U.S. forests. J Geophys Res. 2016;121(10):2610–2629. [Google Scholar]
- Han Q. Height-related decreases in mesophyll conductance, leaf photosynthesis and compensating adjustments associated with leaf nitrogen concentrations in Pinus densiflora. Tree Physiol. 2011;31(9):976–984. doi: 10.1093/treephys/tpr016. [DOI] [PubMed] [Google Scholar]
- Hasselquist NJ, Metcalfe DB, Högberg P. Contrasting effects of low and high nitrogen additions on soil CO2 flux components and ectomycorrhizal fungal sporocarp production in a boreal forest. Glob Change Biol. 2012;18(12):3596–3605. [Google Scholar]
- Hassiotou F, Ludwig M, Renton M, Veneklaas EJ, Evans JR. Influence of leaf dry mass per area, CO2, and irradiance on mesophyll conductance in sclerophylls. J Exp Bot. 2009;60(8):2303–2314. doi: 10.1093/jxb/erp021. [DOI] [PubMed] [Google Scholar]
- Hu J, Moore DJP, Riveros-Iregui DA, Burns SP, Monson RK. Modeling whole-tree carbon assimilation rate using observed transpiration rates and needle sugar carbon isotope ratios. New Phytol. 2010;185(4):1000–1015. doi: 10.1111/j.1469-8137.2009.03154.x. [DOI] [PubMed] [Google Scholar]
- Keenan TF, Hollinger DY, Bohrer G, Dragoni D, Munger JW, Schmid HP, Richardson AD. Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature. 2013;499:324. doi: 10.1038/nature12291. [DOI] [PubMed] [Google Scholar]
- Klein T, Rotenberg E, Tatarinov F, Yakir D. Association between sap flow-derived and eddy covariance-derived measurements of forest canopy CO2 uptake. New Phytol. 2015;209(1):436–446. doi: 10.1111/nph.13597. [DOI] [PubMed] [Google Scholar]
- Lim H, Oren R, Palmroth S, Tor-ngern P, Mörling T, Näsholm T, Lundmark T, Helmisaari HS, Leppälammi-Kujansuu J, Linder S. Inter-annual variability of precipitation constrains the production response of boreal Pinus sylvestris to nitrogen fertilization. For Ecol Manag. 2015;348:31–45. [Google Scholar]
- Loreto F, Tsonev T, Centritto M. The impact of blue light on leaf mesophyll conductance. J Exp Bot. 2009;60(8):2283–2290. doi: 10.1093/jxb/erp112. [DOI] [PubMed] [Google Scholar]
- Loucos K, Simonin K, Barbour M. Leaf hydraulic conductance and mesophyll conductance are not closely related within a single species. Plant, Cell Environ. 2017;40(2):203–215. doi: 10.1111/pce.12865. [DOI] [PubMed] [Google Scholar]
- Marshall JD, Monserud RA. Homeostatic gas-exchange parameters inferred from 13C/12C in tree rings of conifers. Oecologia. 1996;105(1):13–21. doi: 10.1007/BF00328786. [DOI] [PubMed] [Google Scholar]
- Marshall JD, Waring RH. Conifers and broadleaf species: stomatal sensitivity differs in western Oregon. Can J For Res. 1984;14:905–908. [Google Scholar]
- Maseyk K, Hemming D, Angert A, Leavitt SW, Yakir D. Increase in water-use efficiency and underlying processes in pine forests across a precipitation gradient in the dry Mediterranean region over the past 30 years. Oecologia. 2011;167(2):573–585. doi: 10.1007/s00442-011-2010-4. [DOI] [PubMed] [Google Scholar]
- Medlyn B, Dreyer E, Ellsworth D, Forstreuter M, Harley P, Kirschbaum M, Roux X, Montpied P, Strassemeyer J, Walcroft A, Wang K, Loustau D. Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant, Cell Environ. 2002;25(9):1167–1179. [Google Scholar]
- Medlyn B, Duursma R, Eamus D, Ellsworth D, Prentice I, Barton C, Crous K, Angelis P, Fremman M, Wingate L. Reconciling the optimal and empirical approaches to modelling stomatal conductance. Glob Change Biol. 2011;17(6):2134–2144. [Google Scholar]
- Oren R, Sperry JS, Katul GG, Pataki DE, Ewers BE, Phillips N, Schäfer KV. Survey and synthesis of intra-and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant, Cell Environ. 1999;22:1515–1526. [Google Scholar]
- Pons TL, Flexas J, von Caemmerer S, Evans JR, Genty B, Ribas-Carbo M, Brugnoli E. Estimating mesophyll conductance to CO2: methodology, potential errors, and recommendations. J Exp Bot. 2009;60(8):2217–2234. doi: 10.1093/jxb/erp081. [DOI] [PubMed] [Google Scholar]
- Prentice IC, Dong N, Gleason SM, Maire V, Wright IJ. Balancing the costs of carbon gain and water transport: testing a new theoretical framework for plant functional ecology. Ecol Lett. 2014;17(1):82–91. doi: 10.1111/ele.12211. [DOI] [PubMed] [Google Scholar]
- Rogers A, Medlyn BE, Dukes JS, Bonan G, von Caemmerer S, Dietze MC, Kattge J, Leakey ADB, Mercado LM, Niinemets Ü, Prentice CI, Serbin SP, Sitch S, Way DA, Zaehle S. A roadmap for improving the representation of photosynthesis in Earth system models. New Phytol. 2017;213(1):22–42. doi: 10.1111/nph.14283. [DOI] [PubMed] [Google Scholar]
- Seibt U, Rajabi A, Griffiths H, Berry JA. Carbon isotopes and water use efficiency: sense and sensitivity. Oecologia. 2008;155(3):441–454. doi: 10.1007/s00442-007-0932-7. [DOI] [PubMed] [Google Scholar]
- Tarvainen L, Lutz M, Räntfors M, Näsholm T, Wallin G. Increased needle nitrogen contents did not improve shoot photosynthetic performance of mature nitrogen-poor Scots pine trees. Front Plant Sci. 2016;7:1051. doi: 10.3389/fpls.2016.01051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarvainen L, Wallin G, Lim H, Linder S, Oren R, Ottosson Löfvenius M, Räntfors M, Tor-ngern P, Marshall JD. Photosynthetic re-fixation varies along stems and reduces CO2 efflux in mature boreal Pinus sylvestris trees. Tree Physiol. 2018;38(4):558–569. doi: 10.1093/treephys/tpx130. [DOI] [PubMed] [Google Scholar]
- Tazoe Y, von Caemmerer S, Estavillo GM, Evans JR. Using tunable diode laser spectroscopy to measure carbon isotope discrimination and mesophyll conductance to CO2 diffusion dynamically at different CO2 concentrations. Plant, Cell Environ. 2011;34(4):580–591. doi: 10.1111/j.1365-3040.2010.02264.x. [DOI] [PubMed] [Google Scholar]
- Théroux-Rancourt G, Éthier G, Pepin S. Threshold response of mesophyll CO2 conductance to leaf hydraulics in highly transpiring hybrid poplar clones exposed to soil drying. J Exp Bot. 2014;65(2):741–753. doi: 10.1093/jxb/ert436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholen D, Boom C, Noguchi K, Ueda S, Katase T, Terashima I. The chloroplast avoidance response decreases internal conductance to CO2 diffusion in Arabidopsis thaliana leaves. Plant, Cell Environ. 2008;31(11):1688–1700. doi: 10.1111/j.1365-3040.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- Ubierna N, Farquhar GD. Advances in measurements and models of photosynthetic carbon isotope discrimination in C3 plants. Plant, Cell Environ. 2014;37:1494–1498. doi: 10.1111/pce.12346. [DOI] [PubMed] [Google Scholar]
- Ubierna N, Marshall JD. Estimation of canopy average mesophyll conductance using δ13C of phloem contents. Plant, Cell Environ. 2011;34(9):1521–1535. doi: 10.1111/j.1365-3040.2011.02350.x. [DOI] [PubMed] [Google Scholar]
- Ubierna N, Holloway-Phillips MM, Farquhar GD. Using stable carbon isotopes to study C3 and C4 photosynthesis: MODELS and calculations. In: Covshoff S, editor. Photosynthesis. Methods in molecular biology. New York: Humana Press; 2018. [DOI] [PubMed] [Google Scholar]
- Uddling J, Wallin G. Interacting effects of elevated CO2 and weather variability on photosynthesis of mature boreal Norway spruce agree with biochemical model predictions. Tree Physiol. 2012;32(12):1509–1521. doi: 10.1093/treephys/tps086. [DOI] [PubMed] [Google Scholar]
- Veromann-Jürgenson LL, Tosens T, Laanisto L, Niinemets Ü. Extremely thick cell walls and low mesophyll conductance: welcome to the world of ancient living! J Exp Bot. 2017;68(7):1639–1653. doi: 10.1093/jxb/erx045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker S, Brooks R, Meinzer F, Anderson R, Bader M, Battipaglia G, Becklin K, Beerling D, Bert D, Betancourt J, Dawson TE, Domec J-C, Guyette R, Koerner C, Leavitt SW, Linder S, Marshall JD, Mildner M, Ogée J, Panyushkina I, Plumpton H, Pregitzer K, Saurer M, Smith A, Siegwolf R, Stambaugh M, Talhelm A, Tardif J, Van de Water P, Ward J, Wingate L. A dynamic leaf gas-exchange strategy is conserved in woody plants under changing ambient CO2: evidence from carbon isotope discrimination in paleo and CO2 enrichment studies. Glob Change Biol. 2016;22:889–902. doi: 10.1111/gcb.13102. [DOI] [PubMed] [Google Scholar]
- Von Caemmerer S. Biochemical models of leaf photosynthesis. Techniques in plant science. Collingwood: CSIRO Publishing; 2000. [Google Scholar]
- Vrábl D, Vašková M, Hronková M, Flexas J, Šantrůček J. Mesophyll conductance to CO2 transport estimated by two independent methods: effect of variable CO2 concentration and abscisic acid. J Exp Bot. 2009;60(8):2315–2323. doi: 10.1093/jxb/erp115. [DOI] [PubMed] [Google Scholar]
- Wallin G, Linder S, Lindroth A, Räntfors M, Flemberg S, Grelle A. Carbon dioxide exchange in Norway spruce at the shoot, tree and ecosystem scale. Tree Physiol. 2001;21(12–13):969–976. doi: 10.1093/treephys/21.12-13.969. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhao P, Zou LL, McCarthy HR, Zeng XP, Ni GY, Rao XQ. CO2 uptake of a mature Acacia mangium plantation estimated from sap flow measurements and stable carbon isotope discrimination. Biogeosciences. 2014;11(5):1393–1411. [Google Scholar]
- Warren CR. Stand aside stomata, another actor deserves centre stage: the forgotten role of the internal conductance to CO2 transfer. J Exp Bot. 2008;59(7):1475–1487. doi: 10.1093/jxb/erm245. [DOI] [PubMed] [Google Scholar]
- Warren CR. Does growth temperature affect the temperature responses of photosynthesis and internal conductance to CO2? A test with Eucalyptus regnans. Tree Physiol. 2008;28(1):11–19. doi: 10.1093/treephys/28.1.11. [DOI] [PubMed] [Google Scholar]
- Warren CR. Soil water deficits decrease the internal conductance to CO2 transfer but atmospheric water deficits do not. J Exp Bot. 2008;59(2):327–334. doi: 10.1093/jxb/erm314. [DOI] [PubMed] [Google Scholar]
- Warren CR, Ethier GJ, Livingston NJ, Grant NJ, Turpin DH, Harrison DL, Black TA. Transfer conductance in second growth Douglas-fir (Pseudotsuga menziesii (Mirb.)Franco) canopies. Plant, Cell Environ. 2003;26:1215–1227. [Google Scholar]
- Wei L, Marshall JD, Link TE, Kavanagh KL, Du E, Pangle RE, Gag PJ, Ubierna N. A new δ13C submodel for 3-PG. Plant, Cell Environ. 2014;37:82–100. doi: 10.1111/pce.12133. [DOI] [PubMed] [Google Scholar]
- Wingate L, Seibt U, Moncrieff J, Jarvis P, Lloyd J. Variations in 13C discrimination during CO2 exchange by Picea sitchensis branches in the field. Plant, Cell Environ. 2007;30(5):600–616. doi: 10.1111/j.1365-3040.2007.01647.x. [DOI] [PubMed] [Google Scholar]
- Wingate L, Ogée J, Burlett R, Bosc A, Devaux M, Grace J, Loustau D, Gessler A. Photosynthetic carbon isotope discrimination and its relationship to the carbon isotope signals of stem, soil and ecosystem respiration. New Phytol. 2010;188(2):576–589. doi: 10.1111/j.1469-8137.2010.03384.x. [DOI] [PubMed] [Google Scholar]
- Yamori W, Noguchi K, Hanba YT, Terashima I. Effects of internal conductance on the temperature dependence of the photosynthetic rate in spinach leaves from contrasting growth temperatures. Plant Cell Physiol. 2006;47(8):1069–1080. doi: 10.1093/pcp/pcj077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.