Abstract
Spinal muscular atrophy (SMA) is a neuromuscular disease causing the most frequent genetic childhood lethality. Recently, nusinersen, an antisense oligonucleotide (ASO) that corrects SMN2 splicing and thereby increases full-length SMN protein, has been approved by the FDA and EMA for SMA therapy. However, the administration of nusinersen in severe and/or post-symptomatic SMA-affected individuals is insufficient to counteract the disease. Therefore, additional SMN-independent therapies are needed to support the function of motoneurons and neuromuscular junctions. We recently identified asymptomatic SMN1-deleted individuals who were protected against SMA by reduced expression of neurocalcin delta (NCALD). NCALD reduction is proven to be a protective modifier of SMA across species, including worm, zebrafish, and mice. Here, we identified Ncald-ASO3—out of 450 developed Ncald ASOs—as the most efficient and non-toxic ASO for the CNS, by applying a stepwise screening strategy in cortical neurons and adult and neonatal mice. In a randomized-blinded preclinical study, a single subcutaneous low-dose SMN-ASO and a single intracerebroventricular Ncald-ASO3 or control-ASO injection were presymptomatically administered in a severe SMA mouse model. NCALD reduction of >70% persisted for about 1 month. While low-dose SMN-ASO rescues multiorgan impairment, additional NCALD reduction significantly ameliorated SMA pathology including electrophysiological and histological properties of neuromuscular junctions and muscle at P21 and motoric deficits at 3 months. The present study shows the additional benefit of a combinatorial SMN-dependent and SMN-independent ASO-based therapy for SMA. This work illustrates how a modifying gene, identified in some asymptomatic individuals, helps to develop a therapy for all SMA-affected individuals.
Keywords: spinal muscular atrophy, SMA, neuromuscular disorder, motor neuron disorder, neuromuscular junction, SMN1, SMN2, NCALD, modifier gene, ASO therapy
Main Text
Spinal muscular atrophy (SMA) is an autosomal-recessive neurodegenerative disease characterized by the loss of α-motoneurons in the anterior horns of the spinal cord causing symmetrical muscle weakness and atrophy of limb and trunk muscles. SMA affects 1 in 6,000—10,000 live births and is the leading genetic cause of infant mortality.1, 2 In the European population 1:35 and worldwide 1:51 individuals are SMA carriers.3, 4 SMA is caused by deletions or loss-of-function mutations of survival of motor neuron 1 (SMN1 [MIM: 600354]) and a variable number of mainly non-functional SMN2 copies resulting in low levels of the survival motor neuron (SMN) protein.5, 6 While SMN1 produces only full-length (FL)-SMN1 transcripts and protein, SMN2 (MIM: 601627) mainly produces alternatively spliced transcripts (SMN2Δ7), which generate a truncated and unstable SMNΔ7 protein. Only a small amount of SMN2 transcripts (∼10%) are correctly spliced and are of full length, generating an SMN protein identical to the one produced from SMN1 copies.7, 8 There is an inverse correlation between the number of SMN2 copies and the severity of the disease. It can range from type I (SMA1 [MIM: 253300]), which represents the severe end of the spectrum and accounts for approximately 60% of 5q-SMA-affected individuals, to type IV, the mildest and adult form.1, 2, 4, 9 SMN is crucial for all cells, but abnormal low levels of SMN mainly affect spinal motoneurons innervating proximal muscles.10
Recently, nusinersen, the first antisense oligonucleotide (ASO)-based therapy for SMA-affected individuals, has been approved by the US Food & Drug Administration (FDA) and the European Medicines Agency (EMA).11 Nusinersen is an SMN-ASO that increases SMN levels by blocking an intronic splice silencer in SMN2, thereby facilitating the exon 7 inclusion and generation of FL-SMN2 transcripts.12 Clinical studies in all types of SMA-affected individuals treated with nusinersen showed significant amelioration in motoric abilities in about half of them.13, 14 However, the therapeutic increment of SMN through the ASO approach seems to be insufficient to fully counteract the SMA pathology.13, 14 Despite encouraging on-going studies, it might be that even presymptomatic therapy with nusinersen is unable to provide sufficient SMN protein support for motoneuron function and thus stop disease progression over the patient’s life-time in individuals with only one or two SMN2 copies. Moreover, results from different animal models have shown a critical “therapeutic time window” in SMA, as increasing SMN postsymptomatically either fails or shows only a modest amelioration of symptoms in mice.15, 16, 17, 18, 19, 20 Similarly, in SMN1-deleted individuals, the highest effects were observed when early or presymptomatic therapeutic intervention was applied.13, 14 Since only few countries started to include SMN1 deletion testing into the neonatal screening, in most instances SMA is detected only after the first clinical signs appear, which means that a large number of motoneurons are already affected; this fact drastically reduces the beneficial effect of any therapy. Therefore, the development of SMN-independent therapies can be beneficial (1) as an additional support of motoneurons and neuromuscular junction (NMJ) function under conditions when SMN elevation via SMN-dependent therapy is insufficient (e.g., ASO therapy in SMA-affected individuals with SMA1, who possess only one or two SMN2 copies) and (2) after the disease onset in all SMA types, to support the function of motoneurons and NMJs in an SMN-independent manner.
The strongest support of a potential beneficial impact of SMN-independent therapeutic approaches originates from our knowledge gained on SMA-protective genetic modifiers in humans.21, 22 In asymptomatic SMN1-deleted individuals, we found two SMA-protective modifiers able to counteract the SMN deficiency and prevent the disease phenotype. In 2008, we identified the first human SMA genetic modifier, Plastin 3 (PLS3 [MIM: 300131]), and provided conclusive evidence that its overexpression exerts protective effects in in vitro and in vivo SMA models.21, 23, 24, 25 Further studies using AAV9-PLS3 in SMA mice strengthened our findings.26 In 2017, we reported a second SMA-protective modifier gene, Neurocalcin delta (NCALD [MIM: 606722]).22 We found that reduced levels of NCALD, which is a neuronal Ca2+ sensor protein, acts protective in a four-generation discordant family with five asymptomatic and two SMA1-affected individuals. Multiple in vitro and in vivo experiments have shown that reducing NCALD levels significantly ameliorate SMA pathology across SMA species.22 Most importantly, heterozygous Ncald knockout in a severely affected SMA mouse model (SMA-Ncaldko/wt) injected with a low dose SMN-ASO (30 μg) at P1 to rescue multiorgan dysfunction ameliorates the neuromuscular pathology including motor axon development, both NMJ size and maturation, muscle size, proprioceptive input on motoneuron soma, as well as endocytic uptake of FM1-43 dye at the NMJ and motoric abilities.22 Moreover, heterozygous in contrast to homozygous Ncald knockout has no effect on brain development and adult neurogenesis, but enhances spinal motor neuron development.27 Collectively, these results demonstrate that genetically mediated NCALD reduction acts beneficially on SMA pathology.
Based on these encouraging results, we (1) developed and analyzed specific Ncald-ASOs to efficiently downregulate NCALD in mouse spinal cord and (2) used them in a randomized and blinded preclinical study in the severely affected SMA mouse model in a combination with low-dose SMN splice switching ASOs. This is a proof of concept for a combinatorial SMN-dependent and SMN-independent ASO-based therapy in SMA mice.
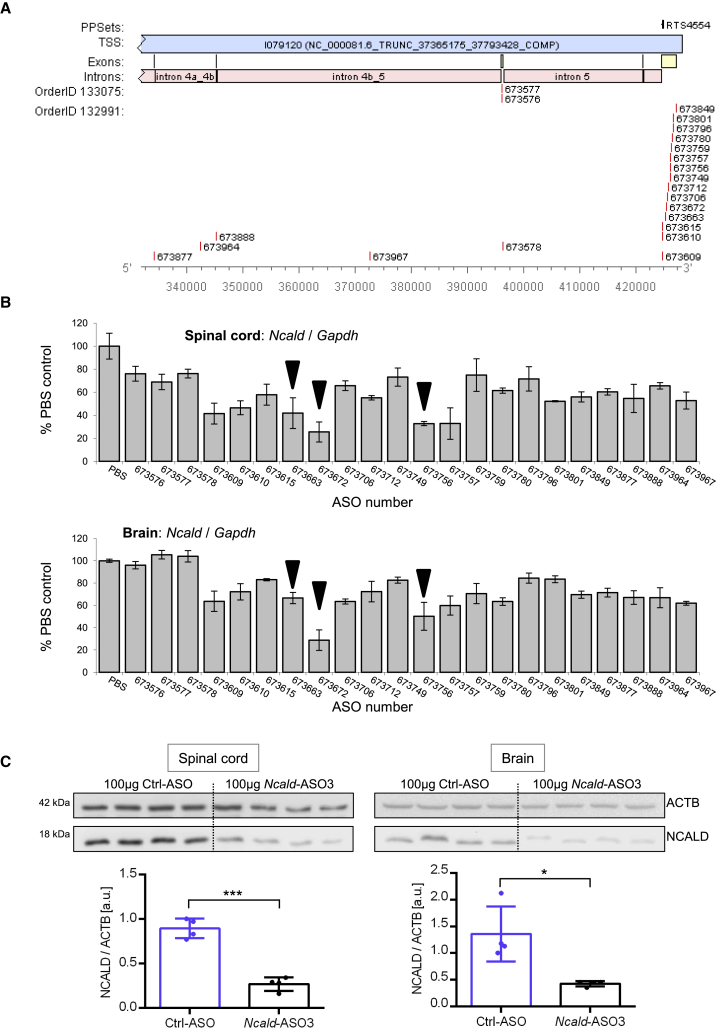
To study in vivo the effect of NCALD downregulation and evaluate its effect on SMA pathology, we first had to develop a non-toxic and efficient ASO. Therefore, we designed 450 different 2′-O-methoxyethyl (MOE)-gapmer Ncald ASOs on mixed backbone, which were first tested in cultured embryonic mouse cortical neurons for downregulation of Ncald mRNA using a quantitative RT-PCR assay. A subset of 22 hits from the cell culture screen, mainly targeting the 3′ UTR of Ncald RNA, were further tested in adult mice for tolerability and efficiency (Figure 1A). Adult mice were treated by intracerebroventricular (i.c.v.) bolus injection with 500 μg of each ASO. Two weeks later, Ncald expression was verified in spinal cord and brain lysates by qRT-PCR (Figure 1B). None of the ASOs overlapped with the primer probe set (PPS) which were flanking exon 5–6 junction; therefore, amplicon effect of oligonucleotides was not a concern (Figure S1A). Since the Taiwanese SMA mice (who carry two human SMN2 copies per allele and no functional murine Smn gene) on C57BL6/N background survive around 2 weeks23, 28 and the best clinical outcome is expected in a presymptomatic therapy, the three most efficient Ncald-ASOs (Ionis #673672, #673663, #673756; further referred as Ncald-ASO1, -ASO2, or -ASO3, respectively) or a control ASO (Ionis #676626, Ctrl-ASO) were tested in neonatal mice (Figures S1B and S1C). To evaluate the tolerability and efficiency and to determine the optimal dose in these young animals, i.c.v. injections with different doses, ranging from 30 to 60 μg of Ncald-ASO1, -ASO2, -ASO3, and Ctrl-ASO were carried out at postnatal day 2 (P2). At P10 we examined NCALD downregulation in spinal cord and brain lysates of mice injected with Ncald-ASOs in comparison to Ctrl-ASO-injected mice. We found that i.c.v. delivery of Ncald-ASO1 and Ncald-ASO2 showed acute toxicity, since 30% and 50% of the injected animals died (data not shown); Ctrl-ASO and Ncald-ASO3 were well tolerated by all animals. Since mice treated with 30 to 60 μg of Ncald-ASO3 exhibited only a moderate downregulation of NCALD (Figure S2), we increased the dose to 100 μg and obtained an 80% and 75% NCALD downregulation in the spinal cord and the brain, respectively (Figure 1C). Therefore, 100 μg of Ncald-ASO3 was applied for the whole study.
Figure 1.
Target Regions of Tested Ncald-ASOs and Knockdown Efficiency in Spinal Cord and Brain of Adult Wild-Type Mice
(A) Numbers of generated Ncald-ASOs and their respective target site in the mouse Ncald gene are shown. Used reference sequence is the Mus musculus strain C57BL/6J chromosome 15, GRCm38.p4 C57BL/6J (GenBank: NC_000081.6). Exons are labeled in yellow, introns in pink.
(B) The 22 most efficient Ncald-ASOs were applied in adult wild-type mice by intracerebroventricular (i.c.v.) bolus injection and knockdown efficiency was determined by qRT-PCR of Ncald (primer probe set flanks exon 5–6 junction) relative to Gapdh (control) expression in spinal cord and brain. Ncald-ASOs marked by black triangle were tested in neonatal mice.
(C) NCALD protein levels in the spinal cord and the brain of P10 animals (n = 4 animals per genotype) treated i.c.v. at P2 with 100 μg Ncald-ASO3 were more than 70% reduced compared to mice injected with 100 μg of Ctrl-ASO. Numbers on the left indicate respective band size in kDa. ACTB, loading control. Unpaired, two-tailed Student’s t test; ∗p < 0.05, ∗∗∗p < 0.001.
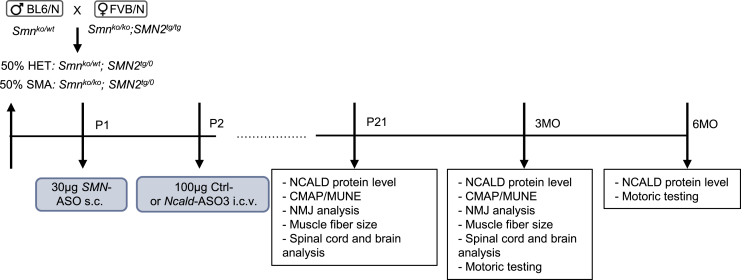
All our experiments were carried out in the Taiwanese severe SMA mouse model on a mixed50 background (50% FVB/N: 50% C57BL6/N), which is slightly more robust than congenic FVB/N or C57BL6/N mice; SMA mice on mixed50 background die at 16.5 days.22, 23, 24 By applying our previously developed breeding scheme (Figure 2),29 50% of mice develop SMA (Smnko/ko;SMN2tg/0) and 50% are healthy SMA carriers (Smnko/wt;SMN2tg/0, defined as heterozygous [HET] and used as controls). Since our previous studies showed that none of the genetic modifiers (PLS3, NCALD, or CHP1) are able to rescue the severe SMA phenotype,22, 23, 24, 30 we pharmacologically elevated the SMN levels—mainly in the non-central nervous system organs—by applying subcutaneously a single low dose of the SMN splice switching ASO (SMN-ASO, nusinersen). Therefore, all animals were subcutaneously injected with 30 μg of SMN-ASO at P1 (Figure 2A). We confirmed our previous results, showing that systemic injection of low-dose SMN-ASO has a major impact on survival, while the mice still show all SMA typical deficits (reduced compound muscle action potential [CMAP] and motor unit estimation [MUNE], impaired NMJ and muscle structure, reduced motoric abilities). Note also, we avoided a high dose of SMN-ASO since this provides a full rescue of SMA in mice,31 making the analysis of the effect of the Ncald ASO impossible. Thus, the low-dose nusinersen-treated SMA model resembles a mild SMA phenotype, similar to an SMA-affected individual carrying 3–4 SMN2 copies or the genotype found in our asymptomatic individuals or an SMA1-affected individual treated with nusinersen.32, 33
Figure 2.
Experimental Design and Time Points of Individual Analyses
A graph showing the breeding scheme to obtain severe SMA and HET mice on mixed50 background (upper left), ASO treatments (blue boxes), and individual analyses at the indicated time points P21 and 3 and 6 months (MO). s.c., subcutaneous; i.c.v., intracerebroventicular; CMAP, compound muscle action potential; MUNE, motor unit number estimation; NMJ, neuromuscular junction.
To better evaluate the requirement of NCALD from birth to adult age and beyond, we first determined endogenous NCALD level in spinal cord and brain of HET and SMA mice (injected with 30 μg SMN-ASO at P1) at P4, P21, 6, and 10 months. Our data show that NCALD is particularly abundant in spinal cord at very early developmental stages and gradually decreases when NMJs starts to develop and mature and muscles became more and more active. This is in line with our former finding that NCALD suppresses clathrin-mediated endocytosis, a process highly required for synaptic vesicle recycling at the NMJ.22 In contrast, NCALD level in the brain increases at P21 and stays high throughout the adulthood, although it slightly decreases at 10 months (Figure S3); this is in line with its important role in adult neurogenesis.27
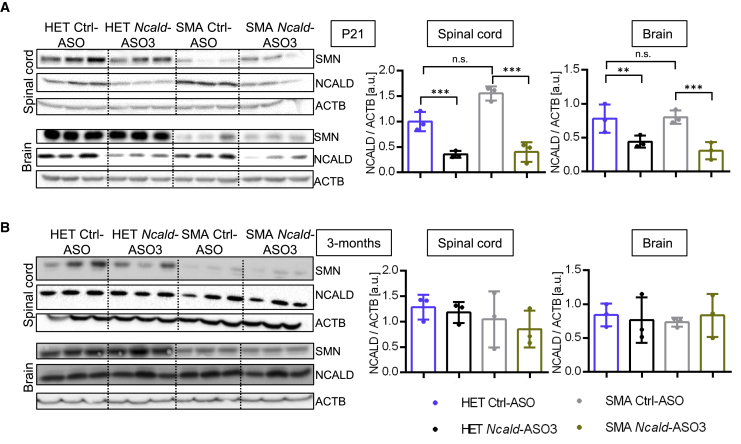
We next analyzed the efficiency of Ncald-ASO3 in spinal cord and brain. We obtained four different study groups originated from litters treated with Ncald-ASO3 (referred to as SMA Ncald-ASO3 and HET Ncald-ASO3 mice) and from litters injected with Ctrl-ASO (referred to as SMA Ctrl-ASO and HET Ctrl-ASO mice). Mice were sacrificed at P21 and 3 months to examine the efficiency of the Ncald-ASO3 in spinal cord and brain. At P21, the NCALD amount was significantly reduced in both SMA and HET mice treated with Ncald-ASO3 in comparison to Ctrl-injected mice (Figure 3A). However, no differences were observed at 3 months between Ncald- and Ctrl-ASO-treated mice (Figure 3B), suggesting that the Ncald-ASO3 effect persists for about 1 month and reaches Ctrl-ASO levels by 3 months. Importantly, the postnatal Ncald-ASO3 treatment has no effect on brain morphology, which is in line with our previous data from heterozygous Ncald knockout mice27 (Figure S4).
Figure 3.
Temporal Progression of NCALD Protein Levels in Mice Injected with SMN-ASO at P1 and Ctrl-ASO or Ncald-ASO3 at P2
Western blot analysis of NCALD and SMN levels in the spinal cord and the brain at (A) P21 and (B) 3 months after injection with respective ASOs (n = 3 per genotype and age). NCALD protein levels normalized to ACTB (loading control) are shown on the right side. NCALD levels are significantly decreased in both spinal cord and brain of HET and SMA animals co-injected with SMN-ASO and Ncald-ASO3 at P21 (A) but not at 3 months (B). Color legend for graphs is displayed at the bottom. For statistics, ordinary one-way ANOVA was applied with Tukey posthoc test for multiple comparisons; n.s., not significant, ∗∗p < 0.01, ∗∗∗p < 0.001.
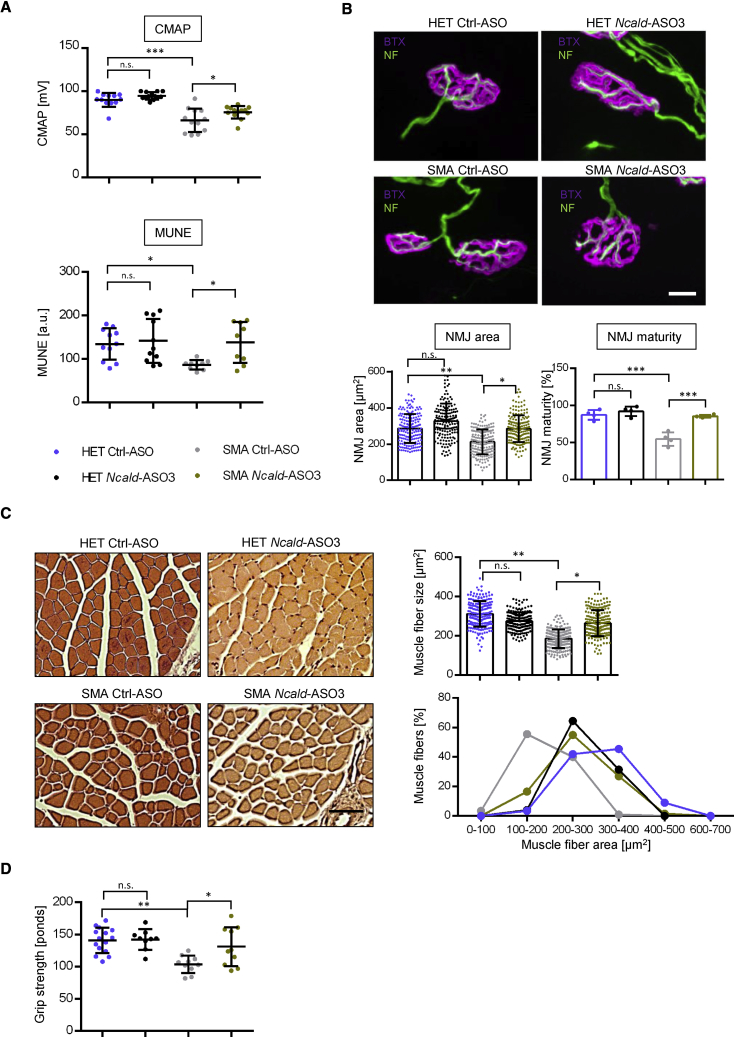
Since Ncald-ASO3 efficiently reduced NCALD levels in spinal cord at P21, we investigated the impact on main SMA-affected cell types, motoneurons and muscles, and studied neuromuscular circuitry and muscle strength by applying four independent approaches: (1) electrophysiological measurements, i.e., compound muscle action potential (CMAP) and motor unit estimation (MUNE), (2) immunostaining of histological sections to analyze neuromuscular junction (NMJ) size and maturity, (3) determination of muscle fiber size, and (4) examination of muscle force using the grip strength test.
First, we analyzed animals at P21, when NCALD was visibly reduced to >70%; moreover, this is a time point when NMJs are fully matured and functional in mice. Second, we analyzed the animals at 3 months. Although NCALD was no longer reduced upon a single injection, we speculated whether the effect on motoneuron development and function could be beneficial even at a later time point.
At P21, CMAP and MUNE, both known to be excellent predictors of muscle-nerve functionality at the NMJ, were performed. Both are well documented to be reduced in SMA mouse models and SMA individuals.19, 34, 35 CMAP represents the maximal response of a given muscle upon the stimulation of the efferent nerve, and MUNE gives information about the number of motor units that innervate a muscle or a group of muscles.36 The electrophysiological assays were performed in the gastrocnemius muscle. SMA Ctrl-ASO-treated mice exhibited highly decreased CMAP amplitude and MUNE in comparison with HET Ctrl-ASO-treated animals, which upon NCALD reduction was significantly ameliorated in SMA mice (Figure 4A).
Figure 4.
Electrophysiological, Structural, and Motoric Analysis of Mice Injected with SMN-ASO at P1 and Ctrl-ASO or Ncald-ASO3 at P2
(A) Dot plots of CMAP and MUNE values at P21 in HET and SMA mice with mean ± SD. Animals used for CMAP: HET Ctrl-ASO n = 12; HET Ncald-ASO3 n = 13; SMA Ctrl-ASO n = 12; SMA Ncald-ASO3 n = 13. MUNE: HET Ctrl-ASO n = 11; HET Ncald-ASO3 n = 11; SMA Ctrl-ASO n = 9; SMA Ncald-ASO3 n = 9.
(B) Representative NMJ images in HET and SMA animals at P21 showing postsynaptic NMJ region stained with bungarotoxin (BTX, magenta) and presynaptic nerve with neurofilament (NF, green). Scale bar: 10 μm. Graphs below the images show single dot plot values of NMJ areas of all grouped animals with mean ± SD and quantification of maturity of NMJs with mean values of mice per group ± SD. Statistics was performed with mean values of animals per group. n = 4 animals per group, 30–55 NMJs per animal.
(C) Representative pictures and quantifications of hematoxylin and eosin-stained gastrocnemius (GC) muscle fibers from HET and SMA animals at P21. Scale bar: 50 μm. Graphs on the right show single dot plot values of GC areas of all grouped animals with mean ± SD (n = 4 animals per genotype, n = 50 fibers/mouse). For visualization, muscle fibers were grouped according to the area intervals of 100 μm2.
(D) Dot plot of grip strength in HET and SMA animals at 3 months. Single values of each animal are shown as mean ± SD. Number of animals used are: HET Ctrl-ASO n = 14, HET Ncald-ASO3 n = 9, SMA Ctrl-ASO n = 10, SMA Ncald-ASO3 n = 10.
Color legend for all graphs is displayed in (A). For all analyses, ordinary one-way ANOVA with Tukey posthoc test for multiple comparisons was applied. n.s., not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
To verify whether improved CMAP and MUNE are due to an increase in NMJ size and maturity, we next analyzed the NMJ architecture in the Transversus abdominis (TVA) muscle, which is a well-known vulnerable muscle in SMA.37 To discriminate NMJs individually, we stained the postsynaptic terminal with bungarotoxin, which reveals the distribution of the acetylcholine receptors (AChRs) and thus allows a determination of the size of the NMJ, and co-stained the presynaptic part with an antibody against neurofilament (Figure 4B). The area occupied by the AChRs in NMJs of SMA Ctrl-ASO-treated mice was reduced compared to HET Ctrl-ASO-treated mice. NCALD downregulation enhanced the amount of AChRs in SMA Ncald-ASO3-treated mice (Figure 4B). Moreover, NMJ maturity (defined as mature when ≥3 perforations and immature when <3 perforations are present19) was delayed in SMA Ctrl-ASO- compared to HET Ctrl-ASO-treated mice at P21, but rescued in SMA Ncald-ASO3-treated mice (Figure 4B).
Next, we assessed the effect of NCALD downregulation on muscle morphology by quantifying the diameter of gastrocnemius muscle fibers using transverse H&E-stained sections (Figure 4C). Muscle fibers were significantly smaller in SMA Ctrl-ASO mice compared to HET Ctrl-ASO-treated mice at P21. Upon NCALD downregulation, we observed that the mean size of the muscle fibers was rescued in SMA mice (Figure 4C). A detailed analysis of size-grouped fibers showed that the number of fibers with larger diameter, ranging from 200 to 400 μm, was significantly higher in SMA Ncald-ASO3 compared to SMA Ctrl-ASO mice (Figure 4C). These results suggest that NCALD downregulation in the nervous system of SMA mice ameliorates also muscle pathology.
Although the effect of a single injection of the Ncald-ASO3 at P2 was abolished at 3 months, we analyzed CMAP and MUNE at that age to ascertain whether the effect of early NCALD reduction that markedly restored motoneuron and muscle function to HET level could have a long-lasting effect and still improve the NMJ functionality and motor abilities. While SMA Ctrl-ASO-treated mice showed diminished CMAP amplitude and MUNE compared to HET Ctrl-ASO-treated mice, Ncald-ASO3 treatment had no positive effect on the electrophysiology biomarkers at 3 months (Figure S5A). While the area occupied by the AChRs in NMJs of SMA Ctrl-ASO-treated mice was reduced compared to HET Ctrl-ASO-treated mice, the early NCALD downregulation had no long-term effect on NMJ area (Figure S5B). Lastly, while the muscle fiber size was clearly decreased in SMA Ctrl-ASO-treated mice, it was not restored to HET Ctrl-ASO-treated level (Figure S5C). Strikingly, the number of fibers with larger diameter, ranging from 200 to 400 μm in SMA Ncald-ASO3-treated animals was similar to HET Ctrl-ASO-treated mice, suggesting a long-lasting effect of improved NMJs development and maturation under NCALD reduction on the muscle structure.
Lastly, to assess the motoric ability in these mice and to strengthen the electrophysiology and histological results, we performed a grip strength test with adult mice at 3 and 6 months.22 At both ages, SMA Ctrl-ASO-treated mice displayed reduced grip strength in comparison with HET Ctrl-ASO-treated mice. Interestingly, 3-month-old SMA Ncald-ASO3-treated animals performed significantly better in the grip strength test compared to SMA Ctrl-ASO mice (Figure 4D), but not at 6 months (Figure S5D). These data demonstrate a beneficial effect of neonatal NCALD downregulation on motor performance until 3 months in SMA mice, which might be driven by the early improvement in NMJ structure and innervation as well as by the increase in muscle fiber size.
In summary, we developed a Ncald-ASO that highly efficiently downregulates NCALD protein levels by about 70% for about 1 month and is non-toxic for CNS development and maturation. Previously, we and others have shown that endocytosis is strongly decreased in SMA. Through multiple in vitro and in vivo experiments, we have demonstrated that NCALD reduction restores impaired endocytosis in SMA.22 Thus, similar to the genetically induced NCALD reduction, a single i.c.v. injection of Ncald ASO3 reduces the NCALD amount especially during the most critical time of NMJ development and maturation and thus facilitates synaptic vesicles endocytosis and neurotransmission in SMA, which is severely affected.
Indeed, a single neonatal CNS injection of Ncald-ASO3 in a combinatorial therapy with a single low-dose SMN-ASO systemic injection improved all neuromuscular deficits in SMA mice including (1) electrophysiological properties (CMAP and MUNE) and thus NMJ neurotransmission, (2) NMJ area and maturity, and (3) muscle fiber size at P21. Furthermore, this beneficial effect of Ncald-ASO3 on the development and function of motoneurons and muscles has a long-lasting effect even 3 months later, when SMA Ncald-ASO3-treated mice show significantly increased motoric strength compared to SMA Ctrl-ASO-treated mice.
Since endogenous NCALD is gradually downregulated after P4 and reaches about 20%–25% at 10 months, it suggests that under physiological conditions, motoneurons downregulate NCALD levels postnatally to achieve the most efficient endocytic recycling of synaptic vesicles, required for proper NMJ function. Via Ncald ASO3 therapy, we achieve the facilitation of synaptic vesicle recycling earlier in the development, thus improving the neurotransmission and as a consequence the NMJ maturation and function in SMA.
While SMN-ASOs, small molecules, or SMN gene therapy show promising results in pre- or early-treated symptomatic SMA individuals, treatment in more advanced stages of the disease show only moderate or even no effect.1, 31, 33, 38, 39 For these SMA-affected individuals, amelioration or even stopping the disease progression is crucial and therefore requires further SMN-dependent and SMN-independent therapies.40, 41 Moreover, the capacity of SMN-ASOs or small molecules to restore endogenous SMN2 splicing is below 2-fold in spinal cord and brain, which is likely insufficient to counteract loss of SMN, especially in SMA1-affected individuals with only one or two SMN2 copies, even if treated presymtomatically.6, 33, 42, 43, 44 It has also been shown in SMA mice that SMN is mainly required before P17, corresponding to the period of NMJ development and maturation.18
Therefore, additional SMN-independent approaches allowing life-long maintenance of motoneurons and NMJ function are needed. The advantage of this system is that both genes (SMN and NCALD) will be targeted by the same system: an ASO approach. We accomplish NCALD downregulation (more than 70% of protein levels) in the targeted tissues by a specific Ncald-ASO administration in P2 mice via i.c.v. injection. However, the Ncald MOE gapmer ASOs used to downregulate the RNA in comparison to the SMN MOE ASOs (nusinersen) that restores SMN2 splicing were less metabolically stable. While the SMN-ASOs are very stable upon subcutaneous injection and have a positive effect in liver even after 6 months,31 the duration of the effect of the Ncald-ASO3 was highly efficient for about 1 month but disappeared after 3 months. This suggests that a monthly reinjection or further designs to optimize duration of action needs to be considered.
Our findings provide the proof of concept that NCALD-mediated ASO downregulation in CNS is possible and demonstrate that Ncald-ASO3 can ameliorate SMA pathology and motoric dysfunction upon a single presymptomatic injection in neonatal animals. Although NCALD protein and its repressor role is gradually downregulated in spinal cord from P4 to 10 months, it still could be that a repetitively monthly i.c.v. bolus injection of the Ncald-ASO3 in the first few months could further enhance the positive impact, resembling the effect of the genetically modified SMA-Ncaldko/wt mice.22 A future perspective of the present study is to design ASOs against human NCALD and analyze the effect in cultured motoneurons derived from human iPSC, which then might be used to treat SMA-affected individuals. Finally, this work illustrates how a modifying gene uncovered in some asymptomatic individuals contributes to development of a therapy for all SMA-affected individuals.
Declaration of interests
C.F.B., F.R., and K.K.L. are employees of IONIS Pharmaceuticals. B.W. holds US patent 9,988,626 B2 approved June 5, 2018, Neurocalcin Delta Inhibitors and Therapeutic and Non-Therapeutic Uses Thereof.
Acknowledgments
This study was support by DFG Wi945/17-1, RTG 1960, DFG Wi945/19-1, (B.W.), CMMC C16 (B.W.), DFG KO5091/2-1 (N.L.K.), SMA Europe (L.T.-B.), and Ottomar Päsel Stiftung (S.S.).
Published: June 20, 2019
Footnotes
Supplemental Data can be found online at https://doi.org/10.1016/j.ajhg.2019.05.008.
Supplemental Data
References
- 1.Finkel R.S., Mercuri E., Meyer O.H., Simonds A.K., Schroth M.K., Graham R.J., Kirschner J., Iannaccone S.T., Crawford T.O., Woods S., SMA Care group Diagnosis and management of spinal muscular atrophy: Part 2: Pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul. Disord. 2018;28:197–207. doi: 10.1016/j.nmd.2017.11.004. [DOI] [PubMed] [Google Scholar]; Finkel, R.S., Mercuri, E., Meyer, O.H., Simonds, A.K., Schroth, M.K., Graham, R.J., Kirschner, J., Iannaccone, S.T., Crawford, T.O., Woods, S., et al.; SMA Care group (2018). Diagnosis and management of spinal muscular atrophy: Part 2: Pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul. Disord. 28, 197-207. [DOI] [PubMed]
- 2.Mercuri E., Finkel R.S., Muntoni F., Wirth B., Montes J., Main M., Mazzone E.S., Vitale M., Snyder B., Quijano-Roy S., SMA Care Group Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul. Disord. 2018;28:103–115. doi: 10.1016/j.nmd.2017.11.005. [DOI] [PubMed] [Google Scholar]; Mercuri, E., Finkel, R.S., Muntoni, F., Wirth, B., Montes, J., Main, M., Mazzone, E.S., Vitale, M., Snyder, B., Quijano-Roy, S., et al.; SMA Care Group (2018). Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul. Disord. 28, 103-115. [DOI] [PubMed]
- 3.Sugarman E.A., Nagan N., Zhu H., Akmaev V.R., Zhou Z., Rohlfs E.M., Flynn K., Hendrickson B.C., Scholl T., Sirko-Osadsa D.A., Allitto B.A. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: clinical laboratory analysis of >72,400 specimens. Eur. J. Hum. Genet. 2012;20:27–32. doi: 10.1038/ejhg.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sugarman, E.A., Nagan, N., Zhu, H., Akmaev, V.R., Zhou, Z., Rohlfs, E.M., Flynn, K., Hendrickson, B.C., Scholl, T., Sirko-Osadsa, D.A., and Allitto, B.A. (2012). Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: clinical laboratory analysis of >72,400 specimens. Eur. J. Hum. Genet. 20, 27-32. [DOI] [PMC free article] [PubMed]
- 4.Feldkötter M., Schwarzer V., Wirth R., Wienker T.F., Wirth B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am. J. Hum. Genet. 2002;70:358–368. doi: 10.1086/338627. [DOI] [PMC free article] [PubMed] [Google Scholar]; Feldkotter, M., Schwarzer, V., Wirth, R., Wienker, T.F., and Wirth, B. (2002). Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am. J. Hum. Genet. 70, 358-368. [DOI] [PMC free article] [PubMed]
- 5.Lefebvre S., Bürglen L., Reboullet S., Clermont O., Burlet P., Viollet L., Benichou B., Cruaud C., Millasseau P., Zeviani M. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]; Lefebvre, S., Burglen, L., Reboullet, S., Clermont, O., Burlet, P., Viollet, L., Benichou, B., Cruaud, C., Millasseau, P., Zeviani, M., et al. (1995). Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80, 155-165. [DOI] [PubMed]
- 6.Wirth B. An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA) Hum. Mutat. 2000;15:228–237. doi: 10.1002/(SICI)1098-1004(200003)15:3<228::AID-HUMU3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]; Wirth, B. (2000). An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA). Hum. Mutat. 15, 228-237. [DOI] [PubMed]
- 7.Lorson C.L., Hahnen E., Androphy E.J., Wirth B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl. Acad. Sci. USA. 1999;96:6307–6311. doi: 10.1073/pnas.96.11.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lorson, C.L., Hahnen, E., Androphy, E.J., and Wirth, B. (1999). A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl. Acad. Sci. USA 96, 6307-6311. [DOI] [PMC free article] [PubMed]
- 8.Lorson C.L., Strasswimmer J., Yao J.M., Baleja J.D., Hahnen E., Wirth B., Le T., Burghes A.H., Androphy E.J. SMN oligomerization defect correlates with spinal muscular atrophy severity. Nat. Genet. 1998;19:63–66. doi: 10.1038/ng0598-63. [DOI] [PubMed] [Google Scholar]; Lorson, C.L., Strasswimmer, J., Yao, J.M., Baleja, J.D., Hahnen, E., Wirth, B., Le, T., Burghes, A.H., and Androphy, E.J. (1998). SMN oligomerization defect correlates with spinal muscular atrophy severity. Nat. Genet. 19, 63-66. [DOI] [PubMed]
- 9.Wirth B., Brichta L., Schrank B., Lochmüller H., Blick S., Baasner A., Heller R. Mildly affected patients with spinal muscular atrophy are partially protected by an increased SMN2 copy number. Hum. Genet. 2006;119:422–428. doi: 10.1007/s00439-006-0156-7. [DOI] [PubMed] [Google Scholar]; Wirth, B., Brichta, L., Schrank, B., Lochmuller, H., Blick, S., Baasner, A., and Heller, R. (2006). Mildly affected patients with spinal muscular atrophy are partially protected by an increased SMN2 copy number. Hum. Genet. 119, 422-428. [DOI] [PubMed]
- 10.Tisdale S., Pellizzoni L. Disease mechanisms and therapeutic approaches in spinal muscular atrophy. J. Neurosci. 2015;35:8691–8700. doi: 10.1523/JNEUROSCI.0417-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tisdale, S., and Pellizzoni, L. (2015). Disease mechanisms and therapeutic approaches in spinal muscular atrophy. J. Neurosci. 35, 8691-8700. [DOI] [PMC free article] [PubMed]
- 11.Hoy S.M. Nusinersen: First Global Approval. Drugs. 2017;77:473–479. doi: 10.1007/s40265-017-0711-7. [DOI] [PubMed] [Google Scholar]; Hoy, S.M. (2017). Nusinersen: First Global Approval. Drugs 77, 473-479. [DOI] [PubMed]
- 12.Rigo F., Chun S.J., Norris D.A., Hung G., Lee S., Matson J., Fey R.A., Gaus H., Hua Y., Grundy J.S. Pharmacology of a central nervous system delivered 2′-O-methoxyethyl-modified survival of motor neuron splicing oligonucleotide in mice and nonhuman primates. J. Pharmacol. Exp. Ther. 2014;350:46–55. doi: 10.1124/jpet.113.212407. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rigo, F., Chun, S.J., Norris, D.A., Hung, G., Lee, S., Matson, J., Fey, R.A., Gaus, H., Hua, Y., Grundy, J.S., et al. (2014). Pharmacology of a central nervous system delivered 2′-O-methoxyethyl-modified survival of motor neuron splicing oligonucleotide in mice and nonhuman primates. J. Pharmacol. Exp. Ther. 350, 46-55. [DOI] [PMC free article] [PubMed]
- 13.Finkel R.S., Mercuri E., Darras B.T., Connolly A.M., Kuntz N.L., Kirschner J., Chiriboga C.A., Saito K., Servais L., Tizzano E., ENDEAR Study Group Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017;377:1723–1732. doi: 10.1056/NEJMoa1702752. [DOI] [PubMed] [Google Scholar]; Finkel, R.S., Mercuri, E., Darras, B.T., Connolly, A.M., Kuntz, N.L., Kirschner, J., Chiriboga, C.A., Saito, K., Servais, L., Tizzano, E., et al.; ENDEAR Study Group (2017). Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 377, 1723-1732. [DOI] [PubMed]
- 14.Mercuri E., Darras B.T., Chiriboga C.A., Day J.W., Campbell C., Connolly A.M., Iannaccone S.T., Kirschner J., Kuntz N.L., Saito K., CHERISH Study Group Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2018;378:625–635. doi: 10.1056/NEJMoa1710504. [DOI] [PubMed] [Google Scholar]; Mercuri, E., Darras, B.T., Chiriboga, C.A., Day, J.W., Campbell, C., Connolly, A.M., Iannaccone, S.T., Kirschner, J., Kuntz, N.L., Saito, K., et al.; CHERISH Study Group (2018). Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 378, 625-635. [DOI] [PubMed]
- 15.Murray L.M., Gillingwater T.H., Parson S.H. Using mouse cranial muscles to investigate neuromuscular pathology in vivo. Neuromuscul. Disord. 2010;20:740–743. doi: 10.1016/j.nmd.2010.06.013. [DOI] [PubMed] [Google Scholar]; Murray, L.M., Gillingwater, T.H., and Parson, S.H. (2010). Using mouse cranial muscles to investigate neuromuscular pathology in vivo. Neuromuscul. Disord. 20, 740-743. [DOI] [PubMed]
- 16.Lutz C.M., Kariya S., Patruni S., Osborne M.A., Liu D., Henderson C.E., Li D.K., Pellizzoni L., Rojas J., Valenzuela D.M. Postsymptomatic restoration of SMN rescues the disease phenotype in a mouse model of severe spinal muscular atrophy. J. Clin. Invest. 2011;121:3029–3041. doi: 10.1172/JCI57291. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lutz, C.M., Kariya, S., Patruni, S., Osborne, M.A., Liu, D., Henderson, C.E., Li, D.K., Pellizzoni, L., Rojas, J., Valenzuela, D.M., et al. (2011). Postsymptomatic restoration of SMN rescues the disease phenotype in a mouse model of severe spinal muscular atrophy. J. Clin. Invest. 121, 3029-3041. [DOI] [PMC free article] [PubMed]
- 17.Robbins K.L., Glascock J.J., Osman E.Y., Miller M.R., Lorson C.L. Defining the therapeutic window in a severe animal model of spinal muscular atrophy. Hum. Mol. Genet. 2014;23:4559–4568. doi: 10.1093/hmg/ddu169. [DOI] [PMC free article] [PubMed] [Google Scholar]; Robbins, K.L., Glascock, J.J., Osman, E.Y., Miller, M.R., and Lorson, C.L. (2014). Defining the therapeutic window in a severe animal model of spinal muscular atrophy. Hum. Mol. Genet. 23, 4559-4568. [DOI] [PMC free article] [PubMed]
- 18.Kariya S., Obis T., Garone C., Akay T., Sera F., Iwata S., Homma S., Monani U.R. Requirement of enhanced Survival Motoneuron protein imposed during neuromuscular junction maturation. J. Clin. Invest. 2014;124:785–800. doi: 10.1172/JCI72017. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kariya, S., Obis, T., Garone, C., Akay, T., Sera, F., Iwata, S., Homma, S., and Monani, U.R. (2014). Requirement of enhanced Survival Motoneuron protein imposed during neuromuscular junction maturation. J. Clin. Invest. 124, 785-800. [DOI] [PMC free article] [PubMed]
- 19.Bogdanik L.P., Osborne M.A., Davis C., Martin W.P., Austin A., Rigo F., Bennett C.F., Lutz C.M. Systemic, postsymptomatic antisense oligonucleotide rescues motor unit maturation delay in a new mouse model for type II/III spinal muscular atrophy. Proc. Natl. Acad. Sci. USA. 2015;112:E5863–E5872. doi: 10.1073/pnas.1509758112. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bogdanik, L.P., Osborne, M.A., Davis, C., Martin, W.P., Austin, A., Rigo, F., Bennett, C.F., and Lutz, C.M. (2015). Systemic, postsymptomatic antisense oligonucleotide rescues motor unit maturation delay in a new mouse model for type II/III spinal muscular atrophy. Proc. Natl. Acad. Sci. USA 112, E5863-E5872. [DOI] [PMC free article] [PubMed]
- 20.Zhou H., Meng J., Marrosu E., Janghra N., Morgan J., Muntoni F. Repeated low doses of morpholino antisense oligomer: an intermediate mouse model of spinal muscular atrophy to explore the window of therapeutic response. Hum. Mol. Genet. 2015;24:6265–6277. doi: 10.1093/hmg/ddv329. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhou, H., Meng, J., Marrosu, E., Janghra, N., Morgan, J., and Muntoni, F. (2015). Repeated low doses of morpholino antisense oligomer: an intermediate mouse model of spinal muscular atrophy to explore the window of therapeutic response. Hum. Mol. Genet. 24, 6265-6277. [DOI] [PMC free article] [PubMed]
- 21.Oprea G.E., Kröber S., McWhorter M.L., Rossoll W., Müller S., Krawczak M., Bassell G.J., Beattie C.E., Wirth B. Plastin 3 is a protective modifier of autosomal recessive spinal muscular atrophy. Science. 2008;320:524–527. doi: 10.1126/science.1155085. [DOI] [PMC free article] [PubMed] [Google Scholar]; Oprea, G.E., Krober, S., McWhorter, M.L., Rossoll, W., Muller, S., Krawczak, M., Bassell, G.J., Beattie, C.E., and Wirth, B. (2008). Plastin 3 is a protective modifier of autosomal recessive spinal muscular atrophy. Science 320, 524-527. [DOI] [PMC free article] [PubMed]
- 22.Riessland M., Kaczmarek A., Schneider S., Swoboda K.J., Löhr H., Bradler C., Grysko V., Dimitriadi M., Hosseinibarkooie S., Torres-Benito L. Neurocalcin Delta Suppression Protects against Spinal Muscular Atrophy in Humans and across Species by Restoring Impaired Endocytosis. Am. J. Hum. Genet. 2017;100:297–315. doi: 10.1016/j.ajhg.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; Riessland, M., Kaczmarek, A., Schneider, S., Swoboda, K.J., Lohr, H., Bradler, C., Grysko, V., Dimitriadi, M., Hosseinibarkooie, S., Torres-Benito, L., et al. (2017). Neurocalcin Delta Suppression Protects against Spinal Muscular Atrophy in Humans and across Species by Restoring Impaired Endocytosis. Am. J. Hum. Genet. 100, 297-315. [DOI] [PMC free article] [PubMed]
- 23.Ackermann B., Kröber S., Torres-Benito L., Borgmann A., Peters M., Hosseini Barkooie S.M., Tejero R., Jakubik M., Schreml J., Milbradt J. Plastin 3 ameliorates spinal muscular atrophy via delayed axon pruning and improves neuromuscular junction functionality. Hum. Mol. Genet. 2013;22:1328–1347. doi: 10.1093/hmg/dds540. [DOI] [PubMed] [Google Scholar]; Ackermann, B., Krober, S., Torres-Benito, L., Borgmann, A., Peters, M., Hosseini Barkooie, S.M., Tejero, R., Jakubik, M., Schreml, J., Milbradt, J., et al. (2013). Plastin 3 ameliorates spinal muscular atrophy via delayed axon pruning and improves neuromuscular junction functionality. Hum. Mol. Genet. 22, 1328-1347. [DOI] [PubMed]
- 24.Hosseinibarkooie S., Peters M., Torres-Benito L., Rastetter R.H., Hupperich K., Hoffmann A., Mendoza-Ferreira N., Kaczmarek A., Janzen E., Milbradt J. The Power of Human Protective Modifiers: PLS3 and CORO1C Unravel Impaired Endocytosis in Spinal Muscular Atrophy and Rescue SMA Phenotype. Am. J. Hum. Genet. 2016;99:647–665. doi: 10.1016/j.ajhg.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hosseinibarkooie, S., Peters, M., Torres-Benito, L., Rastetter, R.H., Hupperich, K., Hoffmann, A., Mendoza-Ferreira, N., Kaczmarek, A., Janzen, E., Milbradt, J., et al. (2016). The Power of Human Protective Modifiers: PLS3 and CORO1C Unravel Impaired Endocytosis in Spinal Muscular Atrophy and Rescue SMA Phenotype. Am. J. Hum. Genet. 99, 647-665. [DOI] [PMC free article] [PubMed]
- 25.Heesen L., Peitz M., Torres-Benito L., Hölker I., Hupperich K., Dobrindt K., Jungverdorben J., Ritzenhofen S., Weykopf B., Eckert D. Plastin 3 is upregulated in iPSC-derived motoneurons from asymptomatic SMN1-deleted individuals. Cell. Mol. Life Sci. 2016;73:2089–2104. doi: 10.1007/s00018-015-2084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; Heesen, L., Peitz, M., Torres-Benito, L., Holker, I., Hupperich, K., Dobrindt, K., Jungverdorben, J., Ritzenhofen, S., Weykopf, B., Eckert, D., et al. (2016). Plastin 3 is upregulated in iPSC-derived motoneurons from asymptomatic SMN1-deleted individuals. Cell. Mol. Life Sci. 73, 2089-2104. [DOI] [PMC free article] [PubMed]
- 26.Kaifer K.A., Villalón E., Osman E.Y., Glascock J.J., Arnold L.L., Cornelison D.D.W., Lorson C.L. Plastin-3 extends survival and reduces severity in mouse models of spinal muscular atrophy. JCI Insight. 2017;2:e89970. doi: 10.1172/jci.insight.89970. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kaifer, K.A., Villalon, E., Osman, E.Y., Glascock, J.J., Arnold, L.L., Cornelison, D.D.W., and Lorson, C.L. (2017). Plastin-3 extends survival and reduces severity in mouse models of spinal muscular atrophy. JCI Insight 2, e89970. [DOI] [PMC free article] [PubMed]
- 27.Upadhyay A., Hosseinibarkooie S., Schneider S., Kaczmarek A., Torres-Benito L., Mendoza-Ferreira N., Overhoff M., Rombo R., Grysko V., Kye M.J. Neurocalcin Delta Knockout Impairs Adult Neurogenesis Whereas Half Reduction Is Not Pathological. Front. Mol. Neurosci. 2019;12:19. doi: 10.3389/fnmol.2019.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]; Upadhyay, A., Hosseinibarkooie, S., Schneider, S., Kaczmarek, A., Torres-Benito, L., Mendoza-Ferreira, N., Overhoff, M., Rombo, R., Grysko, V., Kye, M.J., et al. (2019). Neurocalcin Delta Knockout Impairs Adult Neurogenesis Whereas Half Reduction Is Not Pathological. Front. Mol. Neurosci. 12, 19. [DOI] [PMC free article] [PubMed]
- 28.Hosseinibarkooie S., Schneider S., Wirth B. Advances in understanding the role of disease-associated proteins in spinal muscular atrophy. Expert Rev. Proteomics. 2017;14:581–592. doi: 10.1080/14789450.2017.1345631. [DOI] [PubMed] [Google Scholar]; Hosseinibarkooie, S., Schneider, S., and Wirth, B. (2017). Advances in understanding the role of disease-associated proteins in spinal muscular atrophy. Expert Rev. Proteomics 14, 581-592. [DOI] [PubMed]
- 29.Riessland M., Ackermann B., Förster A., Jakubik M., Hauke J., Garbes L., Fritzsche I., Mende Y., Blumcke I., Hahnen E., Wirth B. SAHA ameliorates the SMA phenotype in two mouse models for spinal muscular atrophy. Hum. Mol. Genet. 2010;19:1492–1506. doi: 10.1093/hmg/ddq023. [DOI] [PubMed] [Google Scholar]; Riessland, M., Ackermann, B., Forster, A., Jakubik, M., Hauke, J., Garbes, L., Fritzsche, I., Mende, Y., Blumcke, I., Hahnen, E., and Wirth, B. (2010). SAHA ameliorates the SMA phenotype in two mouse models for spinal muscular atrophy. Hum. Mol. Genet. 19, 1492-1506. [DOI] [PubMed]
- 30.Janzen E., Mendoza-Ferreira N., Hosseinibarkooie S., Schneider S., Hupperich K., Tschanz T., Grysko V., Riessland M., Hammerschmidt M., Rigo F. CHP1 reduction ameliorates spinal muscular atrophy pathology by restoring calcineurin activity and endocytosis. Brain. 2018;141:2343–2361. doi: 10.1093/brain/awy167. [DOI] [PMC free article] [PubMed] [Google Scholar]; Janzen, E., Mendoza-Ferreira, N., Hosseinibarkooie, S., Schneider, S., Hupperich, K., Tschanz, T., Grysko, V., Riessland, M., Hammerschmidt, M., Rigo, F., et al. (2018). CHP1 reduction ameliorates spinal muscular atrophy pathology by restoring calcineurin activity and endocytosis. Brain 141, 2343-2361. [DOI] [PMC free article] [PubMed]
- 31.Hua Y., Sahashi K., Rigo F., Hung G., Horev G., Bennett C.F., Krainer A.R. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature. 2011;478:123–126. doi: 10.1038/nature10485. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hua, Y., Sahashi, K., Rigo, F., Hung, G., Horev, G., Bennett, C.F., and Krainer, A.R. (2011). Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature 478, 123-126. [DOI] [PMC free article] [PubMed]
- 32.Helmken C., Hofmann Y., Schoenen F., Oprea G., Raschke H., Rudnik-Schöneborn S., Zerres K., Wirth B. Evidence for a modifying pathway in SMA discordant families: reduced SMN level decreases the amount of its interacting partners and Htra2-beta1. Hum. Genet. 2003;114:11–21. doi: 10.1007/s00439-003-1025-2. [DOI] [PubMed] [Google Scholar]; Helmken, C., Hofmann, Y., Schoenen, F., Oprea, G., Raschke, H., Rudnik-Schoneborn, S., Zerres, K., and Wirth, B. (2003). Evidence for a modifying pathway in SMA discordant families: reduced SMN level decreases the amount of its interacting partners and Htra2-beta1. Hum. Genet. 114, 11-21. [DOI] [PubMed]
- 33.Finkel R.S., Chiriboga C.A., Vajsar J., Day J.W., Montes J., De Vivo D.C., Yamashita M., Rigo F., Hung G., Schneider E. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet. 2016;388:3017–3026. doi: 10.1016/S0140-6736(16)31408-8. [DOI] [PubMed] [Google Scholar]; Finkel, R.S., Chiriboga, C.A., Vajsar, J., Day, J.W., Montes, J., De Vivo, D.C., Yamashita, M., Rigo, F., Hung, G., Schneider, E., et al. (2016). Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet 388, 3017-3026. [DOI] [PubMed]
- 34.Arnold W.D., Porensky P.N., McGovern V.L., Iyer C.C., Duque S., Li X., Meyer K., Schmelzer L., Kaspar B.K., Kolb S.J. Electrophysiological Biomarkers in Spinal Muscular Atrophy: Preclinical Proof of Concept. Ann. Clin. Transl. Neurol. 2014;1:34–44. doi: 10.1002/acn3.23. [DOI] [PMC free article] [PubMed] [Google Scholar]; Arnold, W.D., Porensky, P.N., McGovern, V.L., Iyer, C.C., Duque, S., Li, X., Meyer, K., Schmelzer, L., Kaspar, B.K., Kolb, S.J., et al. (2014). Electrophysiological Biomarkers in Spinal Muscular Atrophy: Preclinical Proof of Concept. Ann. Clin. Transl. Neurol. 1, 34-44. [DOI] [PMC free article] [PubMed]
- 35.Swoboda K.J., Prior T.W., Scott C.B., McNaught T.P., Wride M.C., Reyna S.P., Bromberg M.B. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann. Neurol. 2005;57:704–712. doi: 10.1002/ana.20473. [DOI] [PMC free article] [PubMed] [Google Scholar]; Swoboda, K.J., Prior, T.W., Scott, C.B., McNaught, T.P., Wride, M.C., Reyna, S.P., and Bromberg, M.B. (2005). Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann. Neurol. 57, 704-712. [DOI] [PMC free article] [PubMed]
- 36.Arnold W.D., Sheth K.A., Wier C.G., Kissel J.T., Burghes A.H., Kolb S.J. Electrophysiological Motor Unit Number Estimation (MUNE) Measuring Compound Muscle Action Potential (CMAP) in Mouse Hindlimb Muscles. J. Vis. Exp. 2015;(103) doi: 10.3791/52899. [DOI] [PMC free article] [PubMed] [Google Scholar]; Arnold, W.D., Sheth, K.A., Wier, C.G., Kissel, J.T., Burghes, A.H., and Kolb, S.J. (2015). Electrophysiological Motor Unit Number Estimation (MUNE) Measuring Compound Muscle Action Potential (CMAP) in Mouse Hindlimb Muscles. J. Vis. Exp. (103). [DOI] [PMC free article] [PubMed]
- 37.Murray L.M., Lee S., Bäumer D., Parson S.H., Talbot K., Gillingwater T.H. Pre-symptomatic development of lower motor neuron connectivity in a mouse model of severe spinal muscular atrophy. Hum. Mol. Genet. 2010;19:420–433. doi: 10.1093/hmg/ddp506. [DOI] [PubMed] [Google Scholar]; Murray, L.M., Lee, S., Baumer, D., Parson, S.H., Talbot, K., and Gillingwater, T.H. (2010). Pre-symptomatic development of lower motor neuron connectivity in a mouse model of severe spinal muscular atrophy. Hum. Mol. Genet. 19, 420-433. [DOI] [PubMed]
- 38.Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W., Lowes L., Alfano L., Berry K., Church K. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]; Mendell, J.R., Al-Zaidy, S., Shell, R., Arnold, W.D., Rodino-Klapac, L.R., Prior, T.W., Lowes, L., Alfano, L., Berry, K., Church, K., et al. (2017). Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 377, 1713-1722. [DOI] [PubMed]
- 39.Naryshkin N.A., Weetall M., Dakka A., Narasimhan J., Zhao X., Feng Z., Ling K.K., Karp G.M., Qi H., Woll M.G. Motor neuron disease. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science. 2014;345:688–693. doi: 10.1126/science.1250127. [DOI] [PubMed] [Google Scholar]; Naryshkin, N.A., Weetall, M., Dakka, A., Narasimhan, J., Zhao, X., Feng, Z., Ling, K.K., Karp, G.M., Qi, H., Woll, M.G., et al. (2014). Motor neuron disease. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science 345, 688-693. [DOI] [PubMed]
- 40.Wirth B., Garbes L., Riessland M. How genetic modifiers influence the phenotype of spinal muscular atrophy and suggest future therapeutic approaches. Curr. Opin. Genet. Dev. 2013;23:330–338. doi: 10.1016/j.gde.2013.03.003. [DOI] [PubMed] [Google Scholar]; Wirth, B., Garbes, L., and Riessland, M. (2013). How genetic modifiers influence the phenotype of spinal muscular atrophy and suggest future therapeutic approaches. Curr. Opin. Genet. Dev. 23, 330-338. [DOI] [PubMed]
- 41.Talbot K., Tizzano E.F. The clinical landscape for SMA in a new therapeutic era. Gene Ther. 2017;24:529–533. doi: 10.1038/gt.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]; Talbot, K., and Tizzano, E.F. (2017). The clinical landscape for SMA in a new therapeutic era. Gene Ther. 24, 529-533. [DOI] [PMC free article] [PubMed]
- 42.Poirier A., Weetall M., Heinig K., Bucheli F., Schoenlein K., Alsenz J., Bassett S., Ullah M., Senn C., Ratni H. Risdiplam distributes and increases SMN protein in both the central nervous system and peripheral organs. Pharmacol. Res. Perspect. 2018;6:e00447. doi: 10.1002/prp2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]; Poirier, A., Weetall, M., Heinig, K., Bucheli, F., Schoenlein, K., Alsenz, J., Bassett, S., Ullah, M., Senn, C., Ratni, H., et al. (2018). Risdiplam distributes and increases SMN protein in both the central nervous system and peripheral organs. Pharmacol. Res. Perspect. 6, e00447. [DOI] [PMC free article] [PubMed]
- 43.Kletzl H., Marquet A., Günther A., Tang W., Heuberger J., Groeneveld G.J., Birkhoff W., Mercuri E., Lochmüller H., Wood C. The oral splicing modifier RG7800 increases full length survival of motor neuron 2 mRNA and survival of motor neuron protein: Results from trials in healthy adults and patients with spinal muscular atrophy. Neuromuscul. Disord. 2019;29:21–29. doi: 10.1016/j.nmd.2018.10.001. [DOI] [PubMed] [Google Scholar]; Kletzl, H., Marquet, A., Gunther, A., Tang, W., Heuberger, J., Groeneveld, G.J., Birkhoff, W., Mercuri, E., Lochmuller, H., Wood, C., et al. (2019). The oral splicing modifier RG7800 increases full length survival of motor neuron 2 mRNA and survival of motor neuron protein: Results from trials in healthy adults and patients with spinal muscular atrophy. Neuromuscul. Disord. 29, 21-29. [DOI] [PubMed]
- 44.Stevens G., Yawitch T., Rodda J., Verhaart S., Krause A. Different molecular basis for spinal muscular atrophy in South African black patients. Am. J. Med. Genet. 1999;86:420–426. [PubMed] [Google Scholar]; Stevens, G., Yawitch, T., Rodda, J., Verhaart, S., and Krause, A. (1999). Different molecular basis for spinal muscular atrophy in South African black patients. Am. J. Med. Genet. 86, 420-426. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.