Abstract
Objective:
Benefits of social support (SS) during cancer survivorship are complex. This study examines change in SS over time in cervical cancer (CXCA) survivors who have completed definitive treatment and how changing SS impacts quality of life (QOL) and T-helper type 2 (Th2) cytokines.
Methods:
We conducted a randomized trial in 204 CXCA survivors to test if psychosocial telephone counseling (PTC) could improve QOL compared to usual care (UC). Although PTC did not target SS, data were collected at baseline, 4 and 9 months post-enrollment using the MOS Social Support scale. Biospecimens were collected to investigate associations with patient-reported outcomes. Data were analyzed using multivariate linear models and stepwise regression.
Results:
Participants’ mean age was 43. PTC participants experienced increasing SS compared to UC at 4 months (PTC-UC=5.1; p=0.055) and 9 months (PTC-UC=6.0; p=0.046). Higher baseline SS and increasing SS were independently associated with improved QOL at 4 and 9 months after adjusting for patient characteristics (p<0.05). Differences between study arms were not statistically significant. Improvements in QOL at 4 months were observed with increases in emotional/informational and tangible SS. Increasing SS predicted significant longitudinal decreases in IL-4 and IL-13 at 4 months that were larger in the PTC arm (interactions p=0.041 and p=0.057 respectively).
Conclusion:
Improved SS was significantly associated with improved QOL independent of patient characteristics and study arm. Decreasing Th2 cytokines with increasing SS and QOL are consistent with a biobehavioral paradigm in which modulation of the chronic stress response is associated with shifts in immune stance.
Keywords: cancer survivorship, quality of life, social support
Introduction
Cervical cancer (CXCA) survivors have among the worst physical and mental health-related quality of life (QOL) reported in cancer survivor populations [1–4]. QOL disruptions persist after definitive therapy and long into survivorship [5–7]. This has negative implications for long-term survival in CXCA because of the well-established association between QOL and cancer survival [8]. One potential target for improving QOL is social support (SS), broadly defined as emotional, informational, and instrumental assistance provided by one’s social network [9, 10]. Longitudinal and cross-sectional studies have established strong positive associations for SS with physical and psychosocial aspects of long-term cancer survivorship [11–17]. In the broader population of cancer survivors, SS has been associated with decreased cancer-related stress and depressive symptoms [11, 12], positive psychosocial change [13], better QOL [14], and lower mortality [15–17]. In CXCA survivors, higher SS is significantly associated with better QOL 5-20 years after diagnosis [18, 19].
The longitudinal trajectory for SS in cancer survivors may be quite different from other QOL domains. In CXCA patients, QOL is generally below the level of healthy reference populations at diagnosis [6, 20] but increases significantly post-treatment [6, 20–23]. While some studies report QOL equal to general population norms 6 months post-diagnosis [20, 24], studies of advanced stage CXCA report physical and mental health components of QOL below the norm throughout survivorship [6, 25]. In contrast to the increasing trend for QOL, SS appears to decline over treatment and follow-up [6, 20, 23]. Ding et al [23] noted a significant decrease in social/family well-being (p=0.025) over 9 months in early stage CXCA while Kirchheiner et al [20] reported that social functioning in locally advanced CXCA recovered slightly at 6 months before decreasing over long-term follow-up. Dahiya et al [22] noted no significant change in social functioning in stage II-IV CXCA survivors 6 months post-treatment but longer follow-up was not available. Survivors treated with radiotherapy reported less opportunity to share worries with others compared to population-based controls, and decreasing interaction with others over time [6]. Thus, SS appears to decline over survivorship in CXCA. Sustained improvements in SS post-treatment may positively impact QOL and overall survival.
A growing body of literature has sought to elucidate the biologic mechanisms by which improvement in QOL affects health outcomes among cancer survivors [26]. Chronic stress may impact tumor growth and progression through activation of pathways in the sympathetic nervous system and hypothalamic-pituitary-adrenal-axis, influencing inflammatory responses by immune cells [27]. Potential effects of behavioral interventions include decreases in T-helper class 2 (Th2) inflammatory cytokines, reductions in helper:suppressor (Th1/Th2) ratios and higher levels of natural killer cell cytotoxicity (NKCC) [28]. CXCA survivors randomized to a healing touch intervention experienced greater decrease in depressive symptoms and less decline in NKCC compared to survivors randomized to UC [29]. Psychosocial intervention with breast cancer survivors reduced psychological distress and increased disease-free and overall survival [30, 31]. The intervention arm had significantly higher SS and greater immune activity (NKCC) compared to controls [31]. Higher NKCC and Th1/Th2 ratio, and decreases in the inflammatory cytokine IL-4 have also been observed with higher SS [32]. Less is known about how changes in SS are related to changes in such biomarkers.
We previously conducted a randomized controlled trial designed to test the hypothesis that a psychosocial telephone counseling intervention (PTC) improves QOL [33]. Eligible patients included CXCA survivors identified through the California Cancer Registries with stage I-IVA disease who had completed definitive treatment. Patients were randomized 1:1 to usual care (UC) or weekly PTC. The intervention was designed to help patients manage chronic stress, improve health and wellness, and manage sexual side effects of disease and treatment. Results showed non-significant improvements at 4 and 9-month follow-up in overall QOL (p=0.26 and p=0.13), and significant improvement in depression and gynecologic and cancer-specific concerns among the PTC participants relative to UC (p<0.05 for all). Improved QOL was significantly associated with decreases in Th2 and counter-regulatory cytokines, supporting a shift in immune stance and possible reduction in the chronic stress response regardless of study arm [26, 27, 34]. Although the PTC intervention was not designed to modify SS, we collected data on SS as a supplemental outcome at baseline and follow-up to better understand the role of SS on changing QOL and stress-related biomarkers. We report here on longitudinal changes in SS and associations with changes in QOL and stress-related biomarkers in CXCA survivors.
Methods
Participants
After approval by the Institutional Review Boards of the University of California, Irvine and California Cancer Registries, CXCA survivors were identified from the California Cancer Registries (Orange, Los Angeles, Imperial, and San Diego Counties). Eligibility criteria were 1) stage I to IVA CXCA (locally advanced but without disseminated metastasis), 2) completion of definitive treatment at least 2 months earlier, and 3) ability to speak and read English or Spanish. Exclusion criteria were 1) treatment with biologic response modifiers or prior immunotherapy within 4 weeks of study enrollment, 2) treatment with investigational drugs within 30 days, 3) required corticosteroids, and 4) immunosuppression. Participants were enrolled in the trial 9-30 months from diagnosis and randomly assigned to PTC or UC with stratification by ethnicity. Prior to enrolling, all participants provided informed consent.
Measures
Surveys were conducted via mail with follow-up phone calls as needed. SS was measured using the Medical Outcomes Survey Social Support (MOS-SS) questionnaire, a 19-item multidimensional, self-administered survey of SS developed for patients with chronic conditions [35]. Responses are ranked on a Likert scale from 1 (none of the time) to 5 (all of the time), and indicate how often respondents perceive the availability of a particular source of support. Subdomains measured include 1) emotional/informational support, measuring perception of received empathy, displays of positive affect, encouragement of emotional expression, and perception of ready sources of advice and guidance; 2) positive social interaction, measuring perceived availability of others to engage in enjoyable activities; 3) affection, measuring perceived reception of love and affection; and 4) tangible support, measuring perceived receipt of material and behavioral assistance. Scores are calculated by taking the average of all items and transforming to a standardized score ranging from 0 to 100. This measure has been validated in both English and Spanish and is reliable and stable over time (Cronbach’s alpha ≥ 0.96 at each of 3 time points).
QOL was measured using the Functional Assessment of Cancer Therapy—Cervical (FACT-Cx). This instrument is a multidimensional, combined generic and disease-specific QOL questionnaire that includes the FACT-General (FACT-G) questionnaire (version 4) consisting of four subscales (Physical, Social, Emotional, and Functional Well-Being) plus the cervical cancer-specific Additional Concerns subscale. Subdomains are summed to produce the FACT-Cx score (Cronbach’s α = .92). Additional patient-reported measures included in the study but not in the present analysis can be found in Wenzel, et al. [33].
Biomarkers
Biospecimens were collected at the participant’s locale in accordance with verbal and written instructions. Standard phlebotomy was performed into EDTA Vacutainer® tubes. Blood was transported at ambient temperature from the collection site to the laboratory where it was processed, typically within 60-180 minutes. Plasma was collected by centrifugation, aliquoted and stored at −80 C until analyzed. All samples were tested in duplicate with Milliplex® MAP High Sensitivity Human Cytokine Magnetic Bead Kit, HSCYTMAG-60SK (EMD Millipore, Billerica, MA). Patient samples from multiple time points were run on a single plate. Data were collected with MAGPIX® xPONENT software (Luminex, Austin, TX) and analyzed with Milliplex® Analyst 5.1 software. Biomarkers analyzed for this work included Th2 cytokines IL-4, IL-5, and IL-13.
Statistical Methods
Comparisons of baseline characteristics between treatment arms were performed using t-tests and univariate analysis of variance for continuous variables. Chi-square analyses were used for categorical variables. Primary outcomes of interest are the MOS-SS standard score and the FACT-Cx measure of QOL. Changes over time in SS were compared between study arms using multivariate analysis of variance for repeated measures. Effect sizes were calculated as the difference between arms divided by the pooled baseline standard deviation (SD). Data were adjusted for baseline values. Trends over time were tested from baseline to 4 and 9 month follow-ups with significance level p<0.05.
The contribution of change in SS to change in QOL was assessed using stepwise general linear models and stepwise multivariable linear regression with a backward stepping procedure. Alphas to remove and enter were set at 0.015. Covariates included in the model were study arm, treatment, baseline QOL, baseline SS and SS change (baseline to 4 or 9 month follow-up). Cancer treatment was included as a covariate because we have previously shown a significant difference in change over time in QOL by treatment [34]. Interaction terms were investigated. Change in SS was included as either a continuous variable or as a categorical variable (ΔSS < 10, −10 < ΔSS < 10, and ΔSS > 10).
Associations between longitudinal changes in SS and cytokine levels were investigated across all subjects using stepwise multivariate linear models with change in cytokine level as the dependent variable. Independent variables included age, baseline cytokine level, study arm and change in SS from baseline to follow-up. Interactions were added to test if the effect of change in SS differed between PTC and UC. Specimens with variance from ambient transportation and overtly lipemic samples were excluded. Methods are described in detail elsewhere [33].
Results
Study Subjects
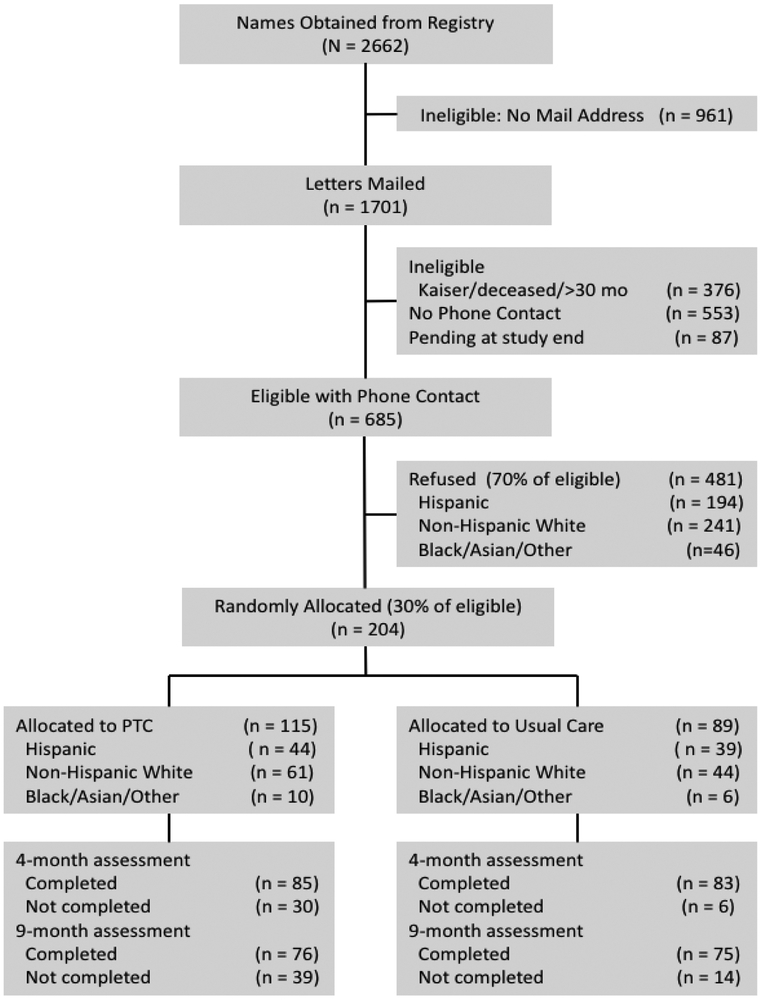
A total of 204 out of 685 eligible CXCA survivors with phone contact (30%) were enrolled in the study and randomized to either PTC (n=115) or UC (n=89). Subject recruitment and baseline characteristics are described in detail elsewhere [23]. Completion rates at 4 and 9-month follow-up were 74% and 66% in PTC and 93% and 84% in UC. Survivors were on average 45 years of age at study entry and 19.4 months from diagnosis (range: 9-30). There were no significant differences between subjects randomized to PTC and UC with respect to age, ethnicity, marital status, income, education, stage, treatment or prior comorbidities. Baseline SS and overall QOL (FACT-Cx) were 74.1 (SD=19.6) and 128.9 (SD=21.9) respectively in the PTC arm compared to 70.3 (SD=25.0) and 123.7 (SD=23.7) in the UC arm. These differences were not statistically significant (p=0.28 and p=0.14 respectively).
Changes over time in Social Support
Social Support increased significantly from baseline to 4-month follow-up in PTC (p<0.001) and non-significantly in UC (p=0.09) before declining at 9 months in both groups (Table 1, Figure 2A). Differences between PTC and UC were 5.1 at 4 months (effect size=0.28, p=0.055) and 6.0 at 9 months (effect size=0.30, p=0.046). Significantly more PTC participants experienced increasing SS compared to UC at 4 months (54% vs. 37%, p=0.016) and 9 months (47% vs. 33%, p=0.034).
Table 1:
Differences over time for MOS Social Support in PTC and UC
| Differences at 4 months | Differences at 9 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Absolute Differences (T2-T1)a |
Group difference in change T2-T1 |
Absolute Differences (T3-T1)a |
Group difference in change T3-T1 |
|||||||
| UC | PTC | PTC- UC |
Effect Sizeb |
p-value | UC | PTC | PTC- UC |
Effect Sizeb |
p-value | |
| Social Support-All | ||||||||||
| Total Score | 1.67 | 6.76 | 5.08 | 0.28 | 0.055 | −0.14 | 5.87 | 6.01 | 0.30 | 0.046 |
| Emotional/Informational | 3.40 | 8.02 | 4.61 | 0.26 | 0.142 | 2.18 | 7.39 | 5.21 | 0.26 | 0.110 |
| Tangible | −1.40 | 5.02 | 6.42 | 0.36 | 0.048 | −5.09 | 5.26 | 10.34 | 0.52 | 0.006 |
| Affection | 1.13 | 6.15 | 5.02 | 0.28 | 0.088 | −0.71 | 3.03 | 3.74 | 0.19 | 0.294 |
| Positive Interaction | 1.15 | 7.23 | 6.09 | 0.34 | 0.099 | −0.35 | 5.92 | 6.27 | 0.31 | 0.121 |
Differences adjusted for baseline value
Effect Size=Difference between arms/SD
Figure 2.
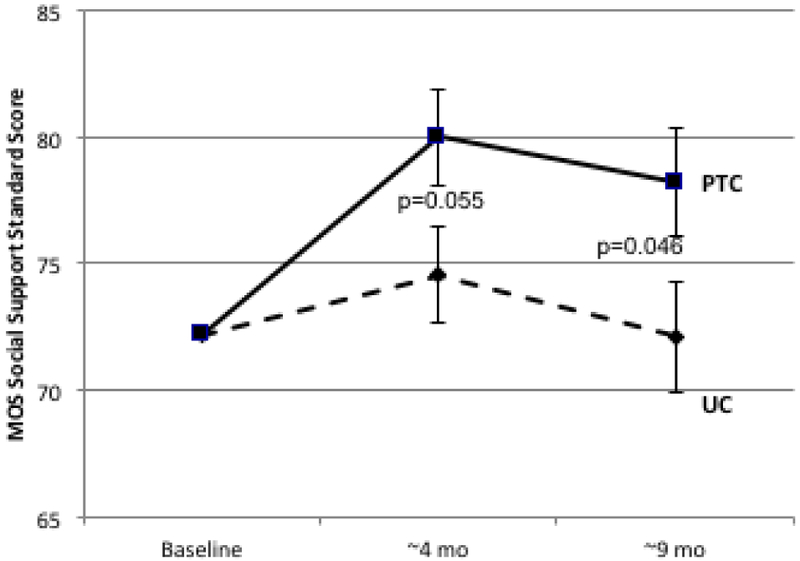
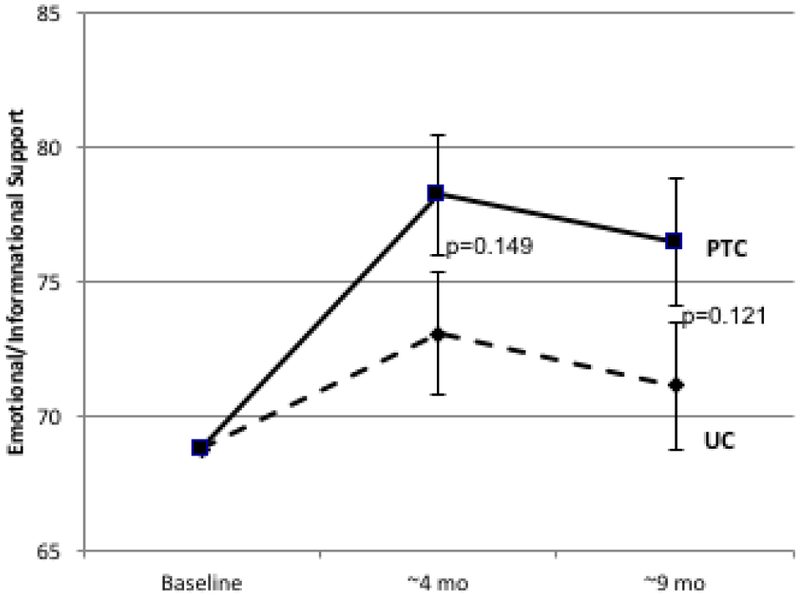
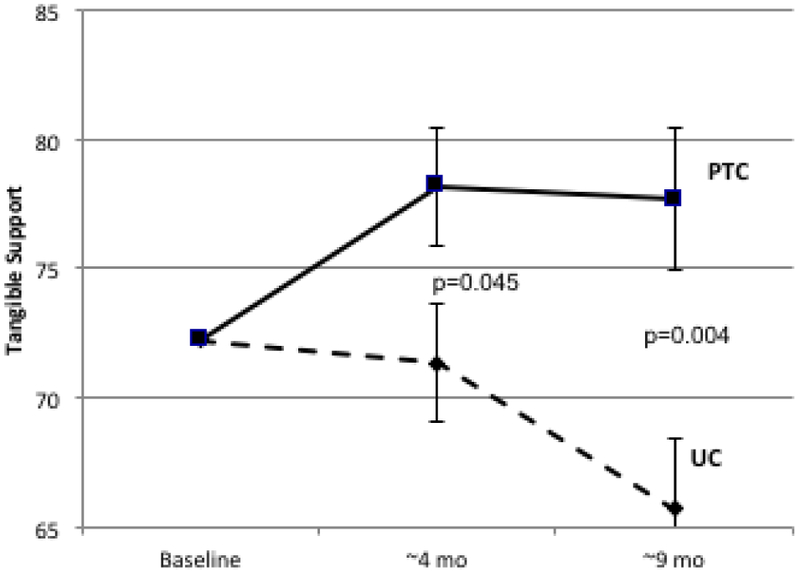
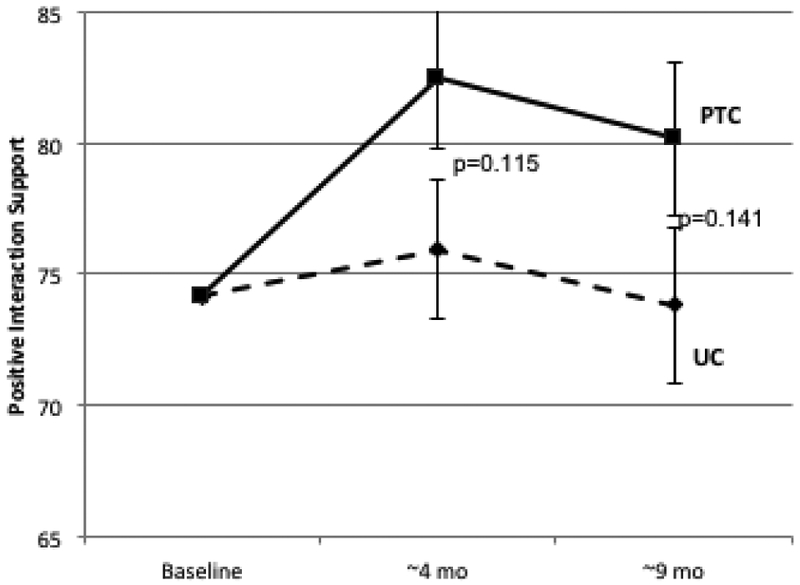
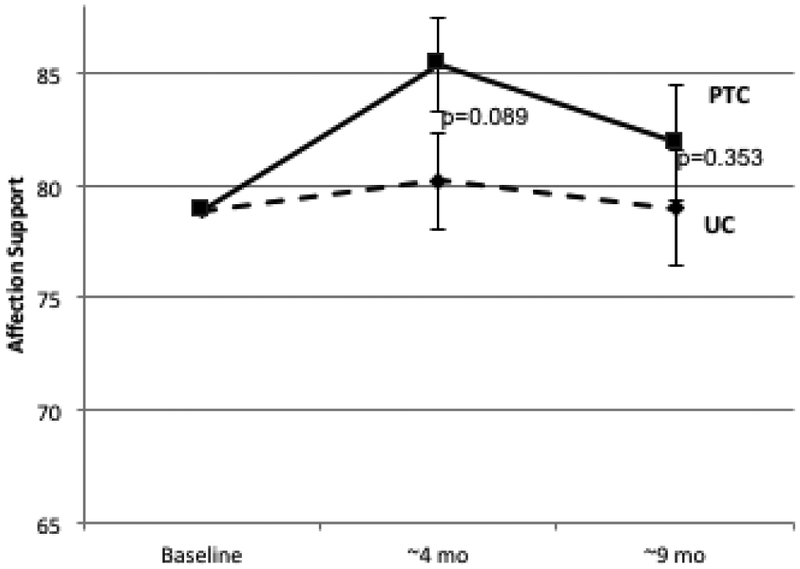
Longitudinal Change in Patient-Reported Social Support. Psychosocial telephone counseling (PTC) (solid lines) and usual care (UC) (dotted lines) is represented at baseline (T1), 4 months (T2), and 9 months (T3). Error bars represent SEs. 2A: MOS Total Social Support Standard Scores; 2B: Emotional/Informational Social Support Subdomain; 2C: Tangible Social Support subdomain; 2D Positive Interaction Social Support Subdomain; 2E: Affection Social Support Subdomain.
Similar differences between PTC and UC were observed in longitudinal change for all subdomains of the MOS-SS scale (Table 1, Figure 2B-2E). Effect sizes ranged from 0.26 to 0.36 at 4 months and from 0.19 to 0.52 at 9 months. The largest differences between PTC and UC were for tangible support (effect size at 4 months=0.36, p=0.05; effect size at 9 months=0.52, p=0.006).
Changes in Social Support associated with Changes in QOL
Both baseline SS and change in SS (Table 2) were significantly associated with change in QOL at 4 and 9-month follow-up, however differences between study arms were not significant. In the PTC arm, a decrease in SS of 10 points at T2 (0.5 SD) was associated with a decrease in QOL of −4.3 while an increase in SS of 10 (0.5 SD) at T2 was associated with an increase in QOL of +12.1 (Figure 3A). In the UC arm, a decrease in SS of 10 points at T2 was associated with a decrease of −5.9 and an increase in SS of 10 at T2 was associated with an increase of +8.0. The difference between arms was not significant (p=0.46). Similar associations were observed in PTC and UC arms for changes in SS at 9 months (Figure 3B; p=0.47 for difference between arms). After adjustment for SS, the PTC-UC difference in QOL change was reduced from 2.4 to 1.4 at 4 months and from 3.3 to 1.5 at 9 months (Figure 3C). While change in QOL did not differ significantly between arms, adjustment for SS did reduce the magnitude of observed change in QOL by approximately 50%, suggesting that SS may partially mediate changes in QOL.
Table 2:
Stepwise Linear Regression Model Predicting Change in Quality of Life (FACT-Cx)
| Dependent Variable | Independent Variable* | Coefficient | SE | Standard Coefficient |
t | p- value |
|---|---|---|---|---|---|---|
| Change in FACT-Cx at 4 months (T2-T1) | ||||||
| A. Model with SS Total | Constant | 30.17 | 5.39 | 0.00 | 5.59 | <0.001 |
| N=165 | FACT-Cx (baseline) | −0.33 | 0.05 | −0.52 | 6.29 | <0.001 |
| Multiple r=0.557 | Social Support (baseline) | 0.19 | 0.06 | 0.29 | 3.06 | 0.003 |
| Δ Social Support (T2-T1) | 0.35 | 0.06 | 0.44 | 5.72 | <0.001 | |
| Study Arm (PTC) | 1.40 | 1.91 | 0.05 | 0.74 | 0.462 | |
| B. Model with SS Subdomains | Constant | 29.96 | 5.45 | 0.00 | 5.50 | <0.001 |
| N=164 | FACT-Cx (baseline) | −0.33 | 0.05 | −0.53 | 6.23 | <0.001 |
| Multiple r=0.561 | Social Support (baseline) | 0.19 | 0.06 | 0.30 | 3.17 | 0.002 |
| Δ Tangible Support | 0.11 | 0.05 | 0.16 | 2.07 | 0.040 | |
| ΔEmotional/informational Support | 0.22 | 0.05 | 0.36 | 4.23 | <0.001 | |
| Study Arm (PTC) | 1.42 | 1.91 | 0.05 | 0.74 | 0.459 | |
| Change in FACT-Cx at 9 months (T3-T1) | ||||||
| C. Model with SS Total | Constant | 36.11 | 6.29 | 0.00 | 5.74 | <0.001 |
| N=150 | FACT-Cx (baseline) | −0.33 | 0.06 | −0.49 | 5.67 | <0.001 |
| Multiple r=0.581 | Chemo-radiation (0 vs 1) | −4.23 | 2.10 | −0.14 | 2.01 | 0.046 |
| Social Support (baseline) | 0.15 | 0.06 | 0.23 | 2.47 | 0.015 | |
| Δ Social Support (T3-T1) | 0.34 | 0.06 | 0.44 | 5.82 | <0.001 | |
| Study Arm (PTC) | 1.52 | 2.09 | 0.05 | 0.73 | 0.469 | |
| D. Model with SS Subdomains | Constant | 34.40 | 6.28 | 0.00 | 5.48 | <0.001 |
| N=150 | FACT-Cx (baseline) | −0.32 | 0.06 | −0.47 | 5.53 | <0.001 |
| Multiple r=0.596 | Chemo-radiation (0 vs 1) | −4.25 | 2.09 | −0.14 | 2.04 | 0.043 |
| Social Support (baseline) | 0.15 | 0.06 | 0.22 | 2.45 | 0.016 | |
| ΔEmotional/informational Support | 0.24 | 0.05 | 0.36 | 4.45 | <0.001 | |
| Δ Positive Interaction Support | 0.87 | 0.04 | 0.17 | 2.16 | 0.032 | |
| Study Arm (PTC) | 1.68 | 2.06 | 0.06 | 0.81 | 0.418 | |
Independent variables included in stepwise model: study arm, treatment, baseline FACT-Cx, baseline social support, Δ social support)
Figure 3.
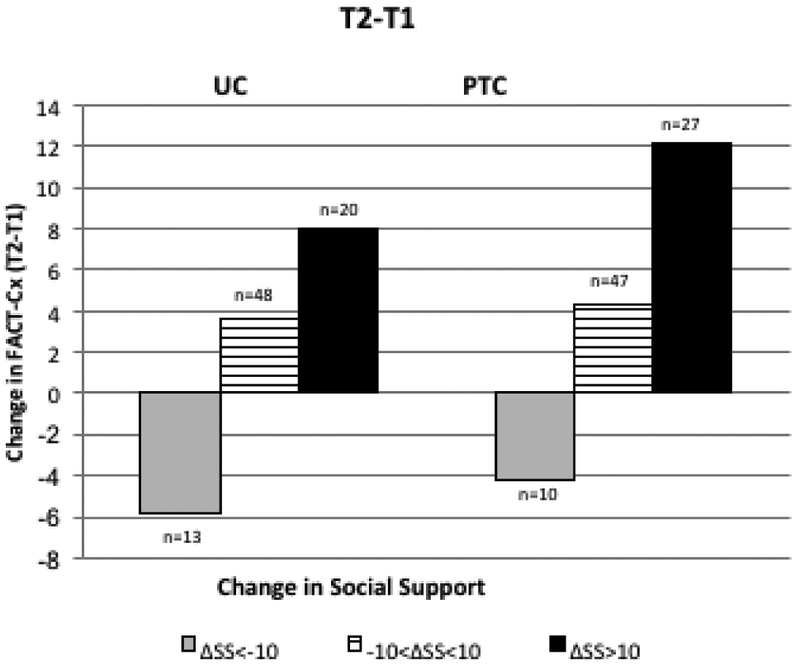
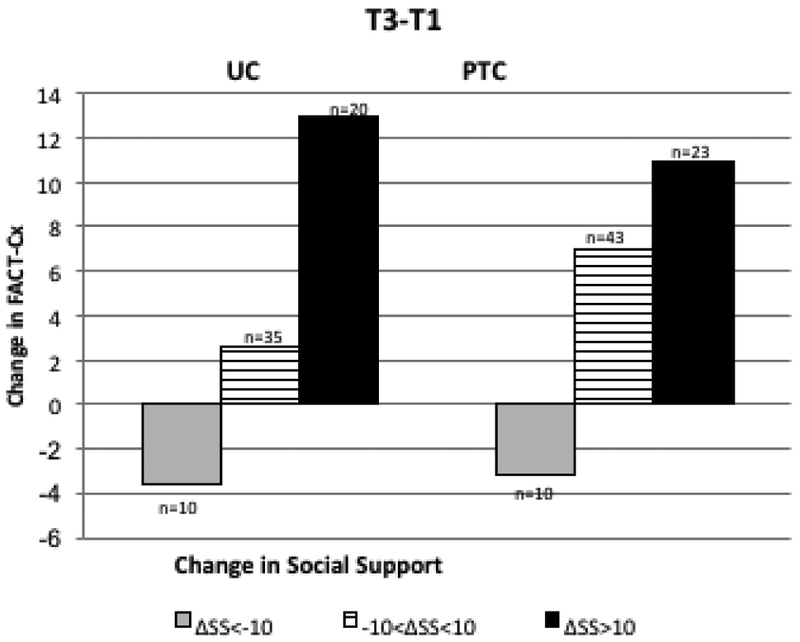
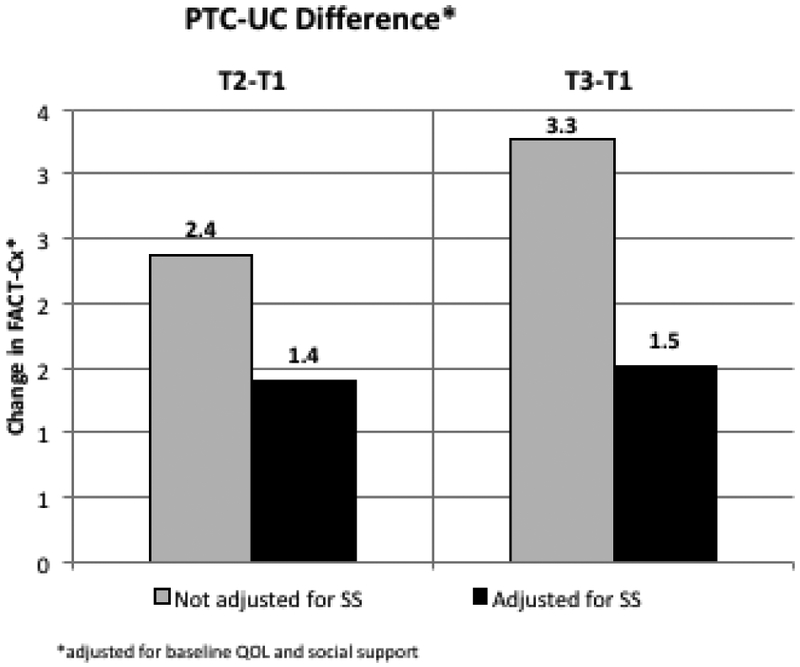
Longitudinal Change in QOL (Fact-Cx) with Change in SS. Changes in SS for PTC and UC are categorized as ΔSS <−10 (dark gray), −10< ΔSS <10 (pattern) and ΔSS>10 (black). 3A: Change in QOL from T1 to T2: trend in QOL change across ΔSS categories is significant (p<0.001) after adjusting for baseline values; 3B: Change in QOL from T1 to T3, trend in QOL change across ΔSS categories is significant (p<0.001) after adjusting for baseline values. 3C: PTC-UC difference in QOL change. Adjustment for SS change reduces the difference in QOL change between PTC and UC from 2.4 to 1.4 (T2-T1) and from 3.3 to 1.5 (T3-T1).
When all subdomains (emotional/informational, tangible, affection, and positive interaction) were included separately as independent variables in stepwise regression models, emotional/informational (p<0.001) and tangible SS (p=0.04) were significant independent predictors of improvement in QOL at 4 months (multiple r=0.56) whereas emotional/informational (p<0.001) and positive interaction (p=0.047) subdomains were independently associated with improvement in QOL at 9 months (multiple r=0.60).
Associations between Change in Social Support and Th2 Cytokines
Increasing SS from baseline to 4 months was significantly associated with decreasing IL-4 (p=0.010) and decreasing IL-13 (p=0.005) after adjusting for age and baseline cytokine level in multivariate linear models (Table 3). Furthermore, the decreases in IL-4 and IL-13 with increasing SS were greater in the PTC arm than for UC (interaction effects: p=0.041 and p=0.057 for IL-4 and IL-13 respectively). Change in IL-5 at 4 months was not associated with changes in SS, nor were changes in cytokine levels at 9 months associated with changing SS (p=0.59, p=0.57 and p=0.08 for change in IL-4, IL-5 and IL-13 respectively).
Table 3:
Change in Th2/Counter-regulatory cytokines with change in Social Support
| Dependent Variable | Independent Variable | Coefficient | SE | Standard Coefficient |
F | p- value |
|---|---|---|---|---|---|---|
| Change in IL-4 (T2-T1) | Constant | 18.96 | ||||
| (N=103) | Baseline IL-4 | −0.21 | 0.04 | −0.47 | 29.99 | <0.001 |
| Multiple r=0.537 | Age | −0.32 | 0.21 | −0.13 | 2.40 | 0.124 |
| Δ Social Support (T2- T1) | −0.26 | 0.10 | −0.23 | 6.99 | 0.010 | |
| Study Arm × ΔSS | 0.19 | 0.09 | 0.18 | 4.28 | 0.041 | |
| Change in IL-13 (T2-T1) | Constant | 1.11 | ||||
| (N=102) | Baseline IL-13 | −0.22 | 0.04 | −0.51 | 37.58 | <0.001 |
| Multiple r=0.576 | Δ Social Support (T2-T1) | −0.08 | 0.03 | −0.24 | 8.40 | 0.005 |
| Study Arm × ΔSS | 0.05 | 0.03 | 0.16 | 3.71 | 0.057 |
Discussion
The present study describes changes in social support over time in a randomized controlled trial of PTC to improve QOL in CXCA survivors. Although changing SS was not a focus of the PTC intervention, SS data were collected as a supplemental study aim. Our aims in this analysis were to describe longitudinal changes in SS following an intervention designed to improve overall QOL and to investigate associations with changes in QOL and stress-related biomarkers. Longitudinal trends showed an increase in SS post-intervention and a decline in SS at long-term follow-up in both arms. There was a significant difference between study arms at the 9-month follow-up (p=0.046) and a nearly significant difference at 4 months (p=0.055). The effect size of 0.28-0.30 was small to moderate and the study was under-powered to detect significance (power=54% in post-hoc analysis). A decline in overall SS at extended follow-up has been consistently observed in other studies [20, 23]. Perhaps the most parsimonious explanation is that as health improves over time, there is less perceived need for SS. Alternatively, as noted by Moyer et al [36], there may be a discrepancy between the perceptions of patients and their supporters, with supporters believing they are continuing to provide needed support while providing less support over time. Thus, the drop off in SS may not necessarily reflect less need for support from the patient’s view, as suggested by associations between decreased SS and increased distress and depression at follow-up reported in the literature [11, 12, 36, 37].
It is noteworthy that the PTC intervention achieved greater improvement relative to UC in all subdomains of SS, though statistical significance was only reached for tangible support. Tangible support includes the perceived availability of help with aspects of daily life such as transportation, meals and housework. Effect sizes (0.36 at 4 months and 0.52 at 9 months) reflect a clinically meaningful difference in tangible support between PTC and UC participants. While tangible support increased in PTC following the intervention and remained stable at long term follow-up, a continuous decrease was observed in UC, consistent with other studies [20, 23, 36, 37]. Even without a PTC module focusing specifically on SS, counselors’ use of problem- and emotion-focused coping strategies may have contributed to improved SS and, more specifically, to sustained improvement in tangible support. In the future, sustained efforts via targeted sessions devoted to SS plus continued contact between the counselor and survivor through text messaging and/or booster sessions over long-term might help sustain an increase in all SS domains.
Greater SS at a single time point has been shown to have a positive effect on QOL in both cross-sectional and longitudinal studies [14, 19, 23, 36, 37]. A notable limitation of prior studies, however, is the absence of data on the impact of changes in SS during survivorship on QOL change over time. In our study, both baseline SS and change in SS were independently associated with QOL change. A decrease in SS of 10 points (approximately 0.5 SD) is associated with decreases in QOL of 4.3 and 5.9 points for PTC and UC subjects respectively, while an increase in SS of 10 points is associated with increases in QOL of 12.1 and 8.0 points for PTC and UC, independent of baseline SS. Improvements in QOL with increasing SS were clinically meaningful (effect size ≥0.5 SD) [38, 39], persisted over time, and were reported by both PTC and UC participants.
Although significantly more patients in the PTC arm reported improved SS compared to UC, there was no significant difference between study arms in the effect of improved SS on QOL change. There are several possible explanations for this finding. First, improvement in SS related to PTC may be modest because the intervention was not designed to improve SS, rather focusing on improving QOL through decreasing stress, managing relationships, and improving health behaviors. It is conceivable that contact with study personnel in both arms might have contributed some support to all cancer survivors, thus diluting the difference between study arms. It is also reasonable to hypothesize that among PTC participants, counseling on stress management, enhanced communication, and improvement in health and wellness may have included some discussion about seeking and obtaining SS as a meaningful dialogue to bolster PTC success.
A number of studies have suggested that SS may serve as a mediator or moderator for psychosocial outcomes [40]. In a study of end stage renal disease, SS was a partial mediator for the effect of depressive symptoms on QOL [41], and SS partially mediated the relationship between optimism and depression in women at high risk for breast cancer [42]. SS was not found to moderate the effect of a nutritional intervention on psychological functioning in women with breast cancer [43]. In our study, it would be reasonable to ask whether SS might mediate the effect of PTC on change in QOL. Although the PTC-UC difference in QOL change is reduced by approximately 50% after adjusting for change in SS (Figure 2C), the overall effect of PTC on change in QOL did not reach statistical significance [33]. Even though PTC influences change in SS (p=0.055 at 4 months), and change in SS clearly impacts change in QOL (p<0.001), the requirements for a mediation model [44, 45] are not met because PTC did not have a significant effect on QOL change, perhaps due to lack of power [33]. Furthermore, SS does not appear to moderate the effect of PTC on change in QOL in our study since both study arms experience similar effects (as evidenced by the non-significant interaction for SS change by study arm). With post-treatment CXCA survivors in our study, SS appears to influence QOL independent of the PTC intervention. Evaluation of the role of SS as mediator and/or moderator in future studies where the intervention is specifically designed to improve SS would be of interest.
The beneficial effect of SS on QOL and general health has been hypothesized to reflect a buffering of the effects of stress on the neuroendocrine and immune systems [26–29, 34]. Consistent with this hypothesis, Miyazaki et al [32] also showed significantly higher NK activity, lower IL-4 and a shift in the Th1/Th2 balance associated with high SS. Our results showing that SS is associated with a decrease in the Th2 cytokines IL-4 (p=0.010) and IL-13 (p=0.005) at 4-month follow-up independent of baseline cytokine level, most prominently in the PTC arm as supported by the observed interaction effect, further support the integration of SS into the biobehavioral paradigm for cancer survivorship.
Limitations of this study include the sample size and limited power to detect significant differences over time. Subjects who dropped from the study had significantly lower QOL and non- significantly lower SS at baseline, particularly in the PTC arm. These subjects presumably had the most to gain from the intervention, thus their absence from the analysis may have reduced effect sizes and limited our ability to detect differences between study arms. Nevertheless, both study arms improved in SS over time. Contact with study personnel at multiple time points for completion of questionnaires and blood draws may have provided some support for cancer survivors in the UC arm, again reducing the differences between arms.
In summary, we found that increases in SS are strongly associated with improved QOL over time and that this is true across all dimensions of QOL (physical, social, emotional, functional, and additional concerns) after adjusting for differences in baseline level of SS, regardless of study arm. Although the intervention in the present study was not designed to improve SS, the strong and durable relationship between SS and QOL provides reason to believe that an intervention targeted to improve SS throughout survivorship would potentially yield even greater improvements in SS, QOL and potentially additional biobehavioral outcomes in this population.
Figure 1.
CONSORT diagram of ascertainment and recruitment. PTC: Pyschosocial Telephone Counseling
Acknowledgments:
The authors acknowledge the support and contributions of Paige Green, PhD MPH.
Financial Support: This work was supported by the National Cancer Institute of the National Institutes of Health under award numbers RO1 CA118136-01, P30 CA062203 and P20 CA174188. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: All authors have declared no conflict of interest. All primary data is under full control of LW and may be reviewed by the journal if requested.
References
- 1.Weaver KE, Forsythe LP, Reeve BB, Alfano CM, Rodriguez JL, Sabatino SA, Hawkins NA, and Rowland JH Mental and Physical Health–Related Quality of Life among U.S. Cancer Survivors: Population Estimates from the 2010 National Health Interview Survey, Cancer Epidemiology Biomarkers & Prevention 21, 2108–2117 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashing-Giwa KT, Tejero JS, Kim J, Padilla GV, Kagawa-Singer M, Tucker MB, and Lim J.-w. Cervical cancer survivorship in a population based sample, Gynecologic Oncology 112, 358–364 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Pfaendler KS, Wenzel L, Mechanic MB, and Penner KR Cervical cancer survivorship: long-term quality of life and social support, Clinical Therapeutics 37, 39–48 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wenzel L, DeAlba I, Habbal R, Kluhsman BC, Fairclough D, Krebs LU, Anton-Culver H, Berkowitz R, and Aziz N Quality of life in long-term cervical cancer survivors, Gynecologic oncology 97, 310–317 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Maguire R, Kotronoulas G, Simpson M, and Paterson C A systematic review of the supportive care needs of women living with and beyond cervical cancer, Gynecologic Oncology 136, 478–490 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Klee M, Thranov I, and Machin D Life after Radiotherapy: The Psychological and Social Effects Experienced by Women Treated for Advanced Stages of Cervical Cancer, Gynecologic Oncology 76, 5–13 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Le Borgne G, Mercier M, Woronoff A-S, Guizard A-V, Abeilard E, Caravati-Jouvenceaux A, Klein D, Velten M, and Joly F Quality of life in long-term cervical cancer survivors: A population-based study, Gynecologic oncology 129, 222–228 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Montazeri A Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008., Health Qual Life Outcomes 7, 102 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thoits PA Mechanisms Linking Social Ties and Support to Physical and Mental Health, Journal of Health and Social Behavior 52, 145–161 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Helgeson VS Social support and quality of life, Quality of life research 12, 25–31 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Carpenter KM, Fowler JM, Maxwell GL, and Andersen BL Direct and buffering effects of social support among gynecologic cancer survivors, Annals of Behavioral Medicine 39, 79–90 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fong AJ, Scarapicchia TMF, McDonough MH, Wrosch C, and Sabiston CM Changes in social support predict emotional well-being in breast cancer survivors, Psycho-Oncology 26, 664–671 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Schroevers MJ, Helgeson VS, Sanderman R, and Ranchor AV Type of social support matters for prediction of posttraumatic growth among cancer survivors, Psycho-Oncology 19, 46–53 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Kroenke CH, Kwan ML, Neugut AI, Ergas IJ, Wright JD, Caan BJ, Hershman D, and Kushi LH Social networks, social support mechanisms, and quality of life after breast cancer diagnosis, Breast Cancer Research and Treatment 139, 515–527 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou AF, Stewart SL, Wild RC, and Bloom JR Social support and survival in young women with breast carcinoma, Psycho-Oncology 21, 125–133 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, and Kawachi I Social Networks, Social Support, and Survival After Breast Cancer Diagnosis, Journal of Clinical Oncology 24, 1105–1111 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Kim M-K, Sim JA, Yun YH, Bae D-S, Nam JH, Park CT, Cho C-H, Lee J-M, and Park SY Health-related quality of life and sociodemographic characteristics as prognostic indicators of long-term survival in disease-free cervical cancer survivors, International Journal of Gynecological Cancer 26, 743–749 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Khalil J, Bellefqih S, Sahli N, Afif M, Elkacemi H, Elmajjaoui S, Kebdani T, and Benjaafar N Impact of cervical cancer on quality of life: beyond the short term (Results from a single institution), Gynecologic Oncology Research and Practice 2, 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C-C, Chen M-L, Chang T-C, Chou H-H, and Chen M-Y Social support buffers the effect of self-esteem on quality of life of early-stage cervical cancer survivors in Taiwan, European Journal of Oncology Nursing 19, 486–494 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Kirchheiner K, Pötter R, Tanderup K, Lindegaard JC, Haie-Meder C, Petrič P, Mahantshetty U, Jürgenliemk-Schulz IM, Rai B, and Cooper R Health-related quality of life in locally advanced cervical cancer patients after definitive chemoradiation therapy including image guided adaptive brachytherapy: an analysis from the EMBRACE study, International Journal of Radiation Oncology Biology Physics 94, 1088–1098 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Ferrandina G, Mantegna G, Petrillo M, Fuoco G, Venditti L, Terzano S, Moruzzi C, Lorusso D, Marcellusi A, and Scambia G Quality of life and emotional distress in early stage and locally advanced cervical cancer patients: A prospective, longitudinal study, Gynecologic Oncology 124, 389–394 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Dahiya N, Acharya AS, Bachani D, Sharma D, Gupta S, Haresh K, and Rath G Quality of Life of Patients with Advanced Cervical Cancer before and after Chemoradiotherapy., Asian Pac J Cancer Prev 17, 3095–3099 (2016). [PubMed] [Google Scholar]
- 23.Ding Y, Hu Y, and Hallberg IR Health-related quality of life and associated factors in Chinese women with cervical cancer: a 9-month follow-up., Cancer Nurs 36, E18–26 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Korfage IJ, Essink-Bot M-L, Mols F, van de Poll-Franse L, Kruitwagen R, and van Ballegooijen M Health-Related Quality of Life in Cervical Cancer Survivors: A Population-Based Survey, International Journal of Radiation Oncology Biology Physics 73, 1501–1509 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Frumovitz M, Sun CC, Schover LR, Munsell MF, Jhingran A, Wharton JT, Eifel P, Bevers TB, Levenback CF, and Gershenson DM Quality of life and sexual functioning in cervical cancer survivors, Journal of Clinical Oncology 23, 7428–7436 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Costanzo ES, Sood AK, and Lutgendorf SK Biobehavioral Influences on Cancer Progression, Immunology and Allergy Clinics of North America 31, 109–132 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powell ND, Tarr AJ, and Sheridan JF Psychosocial stress and inflammation in cancer, Brain, Behavior, and Immunity 30, S41–S47 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Lutgendorf SK, and Andersen BL Biobehavioral approaches to cancer progression and survival: Mechanisms and interventions., American Psychologist 70, 186 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutgendorf SK, Mullen-Houser E, Russell D, DeGeest K, Jacobson G, Hart L, Bender D, Anderson B, Buekers TE, Goodheart MJ, Antoni MH, Sood AK, and Lubaroff DM Preservation of immune function in cervical cancer patients during chemoradiation using a novel integrative approach, Brain, Behavior, and Immunity 24, 1231–1240 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersen BL, Yang H-C, Farrar WB, Golden-Kreutz DM, Emery CF, Thornton LM, Young DC, and Carson WE Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial., Cancer 113, 3450–3458 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersen BL, Thornton LM, Shapiro CL, Farrar WB, Mundy BL, Yang H-C, and Carson WE Biobehavioral, immune, and health benefits following recurrence for psychological intervention participants, Clinical Cancer Research 16, 3270–3278 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyazaki T, Ishikawa T, Nakata A, Sakurai T, Miki A, Fujita O, Kobayashi F, Haratani T, Iimori H, and Sakami S Association between perceived social support and Th1 dominance, Biological psychology 70, 30–37 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Wenzel L, Osann K, Hsieh S, Tucker JA, Monk BJ, and Nelson EL Psychosocial Telephone Counseling for Survivors of Cervical Cancer: Results of a Randomized Biobehavioral Trial, Journal of Clinical Oncology 33, 1171–1179 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson EL, Wenzel LB, Osann K, Dogan-Ates A, Chantana N, Reina-Patton A, Laust AK, Nishimoto KP, Chicz-DeMet A, du Pont N, and Monk BJ Stress, Immunity, and Cervical Cancer: Biobehavioral Outcomes of a Randomized Clinical Trail, Clinical Cancer Research 14, 2111–2118 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherbourne CD, and Stewart AL The MOS social support survey, Social Science & Medicine 32, 705–714 (1991). [DOI] [PubMed] [Google Scholar]
- 36.Moyer A, and Salovey P Predictors of Social Support and Psychological Distress in Women with Breast Cancer, Journal of Health Psychology 4, 177–191 (1999). [DOI] [PubMed] [Google Scholar]
- 37.Thompson T, Rodebaugh TL, Pérez M, Schootman M, and Jeffe DB Perceived Social Support Change in Patients with Early-stage Breast Cancer and Controls, Health Psychology 32, 886 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yost KJ, and Eton DT Combining distribution-and anchor-based approaches to determine minimally important differences: the FACIT experience, Evaluation & the health professions 28, 172–191 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Rothrock NE, Hays RD, Spritzer K, Yount SE, Riley W, and Cella D Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS), Journal of Clinical Epidemiology 63, 1195–1204 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bloom JR Improving the health and well-being of cancer survivors: past as prologue, Psycho-Oncology 17, 525–532 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Khalil AA, and Abed MA Perceived social support is a partial mediator of the relationship between depressive symptoms and quality of life in patients receiving hemodialysis, Archives of psychiatric nursing 28, 114–118 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Garner MJ, McGregor BA, Murphy KM, Koenig AL, Dolan ED, and Albano D Optimism and depression: a new look at social support as a mediator among women at risk for breast cancer, Psycho-Oncology 24, 1708–1713 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheier MF, Helgeson VS, Schulz R, Colvin S, Berga SL, Knapp J, and Gerszten K Moderators of Interventions Designed to Enhance Physical and Psychological Functioning Among Younger Women With Early-Stage Breast Cancer, Journal of Clinical Oncology 25, 5710–5714 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Baron RM, and Kenny DA The moderator--mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations., Journal of personality and social psychology 51, 1173 (1986). [DOI] [PubMed] [Google Scholar]
- 45.Fairchild AJ, and MacKinnon DP A general model for testing mediation and moderation effects., Prevention Science 10, 87–99 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]