Abstract
Frailty is a geriatric condition characterized by increased vulnerability to physical impairments and limitations that may lead to disabilities and mortality. Although studies in the general population suggest that psychosocial factors affect frailty, less is known about whether similar associations exist among people living with HIV (PLWH). The purpose of this study was to examine psychosocial correlates of frailty among PLWH and HIV-uninfected adults. Our sample included 127 adults (51% PLWH) participating in the Multi-Dimensional Successful Aging among HIV-Infected Adults study at the University of California San Diego (average age 51 years, 80% male, 53% White). Frailty was assessed via the Fried Frailty Index. Psychosocial variables significant in bivariate models were included in principal component analysis to generate factor variables summarizing psychosocial correlates. Multivariate logistic regression models were fit to examine the independent effects of factor variables and their interaction terms with HIV status. In bivariate models, frailty was associated with multiple psychosocial variables, e.g. grit, optimism, personal mastery, social support, emotional support. Factor analysis revealed that psychosocial variables loaded on two factors – Positive Resources/Outlook and Support by Others. The multivariate model showed significant main effects of Support by Others and HIV status, and interactive effects HIV X Positive Resources/Outlook, such that Positive Resources/Outlook was negatively associated with frailty for PLWH but not for HIV-uninfected individuals. These analyses indicate that psychosocial factors may be associated with frailty among PLWH. Positive resources and outlook may play a role in frailty prevention. Future longitudinal studies are needed to establish causal links.
Keywords: Successful aging, AIDS, grit, optimism, social support
Introduction
Even though advances in antiretroviral therapy (ART) contribute to increasing longevity,1 aging people living with HIV (PLWH) experience greater morbidity and age-related complications than otherwise comparable HIV-uninfected adults.2,3 One of the geriatric syndromes that may be exacerbated by HIV infection is frailty, defined as a state of vulnerability that puts an individual at increased risk of adverse clinical outcomes when faced with internal or external stressors.4–7 A recent study among participants in the Multi-Center AIDS Cohort Study (MACS) found 12% frailty prevalence among HIV-infected men versus 9% among HIV-uninfected men (median age 53.8, IQR=47.6, 61.3).8 Similarly, frailty prevalence among women participating in the Women’s Interagency HIV Study (WIHS) was 17% among the HIV-infected and 10% among the HIV-uninfected (average age 39 years).5,9 Moreover, studies suggest that PLWH experience earlier onset and higher prevalence of frailty when compared to their HIV-uninfected counterparts.2,6,9–11 Research among PLWH shows that frailty is associated with increased likelihood of falls,12,13 hospitalizations,14 disability,15 and mortality.7,16 Given the increased frailty prevalence and its adverse effects among PLWH, it is important to understand factors associated with frailty in this population.
To date, little is known about psychosocial correlates of frailty among PLWH. The existing research has mostly focused on biomedical and sociodemographic predictors and correlates of frailty. Thus, the presence of frailty in the era of combination ART (cART) was found to be associated, though somewhat inconsistently, with greater age, female gender, lower education, non-Hispanic Black ethnicity, and low annual income by both cross-sectional and longitudinal studies.6,17,18 Other frailty predictors include HIV disease characteristics, such as low CD4 cell count or AIDS diagnosis,5,19,20 as well as the presence of inflammatory markers (e.g., c-reactive protein),2 and medical comorbidities, such as psychiatric disease, neurocognitive impairment, chronic kidney disease, hypertension, and diabetes mellitus.5,6 Additionally, research examined the effects of several lifestyle risk factors on frailty among PLWH, including protective effects of physical activity and low to moderate drinking, and detrimental effects of smoking and drug use.5,17,18
Even though many predictors and correlates of frailty have been identified, relatively little is known about psychosocial factors associated with frailty among PLWH. Research among those without HIV shows that frailty may be connected to multiple psychosocial factors, such as wellbeing, positive affect, perceived control, resilience, social support, and emotional support.21–26 For example, a recent cross-sectional study by Freitag and Schmidt24 examined multiple psychosocial correlates of frailty in a sample of community-dwelling older adults and found that higher frailty was associated with greater depressive symptomatology and lower resilience. Importantly, a number of longitudinal observational studies revealed that increases in frailty observed at study follow-up were associated with greater baseline levels of negative affect27 and depressive symptomatology25,28,29 as well as lower baseline levels of positive affect,22 personal mastery,30 and perceived social support.25
Therefore, the overall goal of this study is to assess the association between multiple psychosocial factors and the frailty phenotype as measured by the Fried Frailty Index (FFI) among PLWH. The FFI classifies individuals into three categories – robust, pre-frail, and frail – based on the criteria of weakness, slowness, physical exhaustion, low physical activity, and unintended weight loss.31 More specifically, the purpose of this research was among PLWH and HIV-uninfected adults: 1) to assess psychosocial correlates of frailty; 2) to explore whether psychosocial variables associated with frailty have an underlying structure such that they can be reduced to several summary factor variables, and 3) to evaluate whether the effects of psychosocial variables on frailty differ by HIV status. Based on research in the general population reviewed above, we hypothesize that frailty will be associated with: 1) higher levels of negative psychosocial factors, such as stress and depression, and 2) lower levels of positive psychosocial factors, such as grit and optimism. In the view of studies suggesting that aging PLWH are more likely to experience negative psychosocial factors as compared to HIV-uninfected counterparts,32 we also expect that psychosocial factors will interact with HIV status in such a way that their association with frailty will be stronger for PLWH.
Methods
Study sample
Data were collected as part of the NIMH-funded Multi-Dimensional Successful Aging among HIV Infected Adults Study at University of California, San Diego (UCSD), which is described in an earlier publication.33 Briefly, the study recruited community-dwelling PLWH and HIV-uninfected adults 35 to 65 years old. The exclusion criteria were: 1) history of psychotic disorder or a mood disorder with psychotic features; 2) the presence of a neurological condition not related to HIV infection and known to affect cognitive functioning, such as Alzheimer’s disease, stroke or traumatic brain injury; and 3) having a positive urine toxicology test for drugs of abuse during the baseline visit. During the screening, participants with unknown HIV status were tested with the HIV/HCV finger stick point of care test (Abbott Real-time HIV-1 test, Abbott Laboratories, Illinois, USA). Participants were compensated for participation. The UCSD Institutional Review Board approved the study and participants provided written informed consent to participate. The sample for the present cross-sectional analyses is based on biomedical and psychosocial data from the baseline visit and includes 65 PLWH and 62 HIV-uninfected participants who were administered frailty assessment.1
Primary outcome
Frailty was measured using the FFI criteria.31 Participants’ level of slowness and weakness was assessed, objectively, by gait speed (15 feet walk time) and grip strength tests. The three remaining criteria were evaluated by self-reports. Thus, unintentional weight loss was measured as a “Yes” to a question whether participant unintentionally lost more than 10 pounds in a previous year. Low physical activity was defined as expending less than 383 kilocalories per week for males and less than 270 kilocalories per week for females and measured by the International Physical Activity Questionnaire (IPAC).34 Exhaustion during the past week was evaluated as “Occasionally or a moderate amount of time” or “All of the time” answers to the two following questions from the CES-D scale:35 “I felt that everything I did was an effort” and “I could not ‘get going.’” Pre-frailty was defined as the presence of one or two of the FFI criteria, frailty – as three or more of these criteria. Since only 8.7% (N=11) participants in our sample were frail whereas 41.7% (N=53) were prefrail, for our primary analyses, we collapsed frail and prefrail into a “frail” category and others were categorized as “robust.” In secondary analyses, we also considered a categorical variable with robust, prefrail, and frail categories.
Psychosocial exposures
The psychosocial factors were assessed by standardized validated scales. Several Likert-type instruments evaluated positive psychological constructs. Resilience was measured by the Connor Davidson Resilience Scale – 10 Item (CD-RISC-10)36,37 and included items such as “I am not easily discouraged by failure” rated from 1 (not true at all) to 5 (true nearly all the time). To measure optimism, a six-item Lifetime Orientation Test-Revised (LOT-R)38,39 scale was used. The items on this scale ranged from 1 (strongly disagree) to 5 (strongly agree) and included statements like “Overall, I expect more good things to happen to me than bad.” Grit was assessed using the Grit Scale,40 which included 12 items (e.g., “I am diligent”), ranging from 1 (very much like me) to 5 (not at all like me). Personal mastery was measured by a 7-item Pearlin-Schooler Personal Mastery Scale (PMS),41 where responses to questions like “I have little control over the things that happen to me” ranged from 1 (strongly agree) to 4 (strongly disagree). To assess participants’ degree of religiousness, we used three-subscale sum from the Brief Multidimensional Measure of Religiousness/Spirituality42 (e.g., “To what extent do you consider yourself a spiritual person?”), with lower scores representing greater religiosity/spirituality. We assessed life satisfaction by a 5-item Satisfaction with Life Scale43 (e.g., “The conditions of my life are excellent”), which ranges from 1 (not at all true) to 7 (absolutely true). Additionally, we used the following two items to evaluate self-rated successful aging:44 “Using your own definition, where would you rate yourself in terms of “successful aging?” (from 1 “least successful” to 10 “most successful”) and “How old/young do you feel? (Please write a specific age).”
We also included several measures of interpersonal psychosocial factors. To measure social support, we used a four-item social interaction sub-scale of Duke Social Support Index (DSSI),45 which has items like “About how often did you go to meetings of clubs, religious meetings, or other groups that you belong to in the past week?” ranging from 0 (none) to 7 (seven or more times). Emotional Support Scale (ESS)46 was used to assess support by others in the three following domains: emotional support (e.g., “How often do your spouse, children, close friends and/or relatives make you feel loved and cared for?”), instrumental support (e.g., “How often do your spouse, children, close friends and/or relatives help with daily tasks like shopping, giving you a ride, or helping you with household tasks?”), and negative interactions with others (e.g., “How often are your spouse, children, close friends and/or relatives critical of what you do?”).
Lastly, we assessed participants’ emotional functioning by several well-known scales. Thus, depression was measured using the Center for Epidemiologic Studies Depression Scale (CESD),35 whereas participants’ level of stress was evaluated by the Perceived Stress Scale (PSS).47 For each of the individual scales described above, we computed a separate assessment summary score that we used in our analyses.
Covariates
As potential covariates, we considered multiple sociodemographic and biomedical variables identified by the literature as predictors of frailty and available in our dataset. Potentially confounding sociodemographic factors included continuous age, gender, race/ethnicity (Black, White, Hispanic, other), and years of education. We also considered a number of comorbidities coded as dichotomous variables – i.e., hepatitis C infection, diabetes mellitus, hypertension, hyperlipidemia, any cancer, ever smoking, chronic pulmonary disease, lifetime diagnosis of substance use disorder, and lifetime diagnosis of alcohol use disorder. Lastly, we considered the following HIV disease characteristics: current CD4 cell count, nadir CD4, undetectable plasma HIV viral load, AIDS diagnosis, and an estimated duration of HIV disease. Those variables that were at least marginally associated with frailty (p<=0.1) were included in our adjusted and multivariate models.
Statistical analyses
All statistical analyses were conducted using Stata 14 software. First, descriptive statistics were calculated to examine the sample distributions; based on the assumption of normal distribution, chi-square tests for dichotomous and t-tests for continuous variables were performed to determine the differences between the HIV serostatus groups. Second, bivariate logistic regression models were fit to estimate crude odds ratios and 95% confidence intervals for associations between psychosocial exposures and frailty. These models were adjusted as the next step by including biomedical and sociodemographic covariates as described above. Third, all psychosocial variables significantly associated with frailty in the adjusted models, were included in principal component analysis (PCA) with varimax orthogonal rotation to generate factor variables summarizing psychosocial effects. We used the Kaiser-Meyer-Olkin measure of sampling adequacy and Bartlett’s test of sphericity to check the appropriateness of PCA use. PCA automatically retains components with eigenvalues greater than 1. For those components retained in the analyses, factor scores were obtained using Stata predict command. The resulting summary factor variables represent the linear composites formed by standardizing psychosocial variables included in PCA, weighing them with factor score coefficients, and summing for each factor.48 Next, multivariate logistic regression models on frailty were fit to examine the independent effects of factor variables generated through PCA as well as their interaction terms with HIV status. The interactive effects were further explored through HIV-stratified analyses. Lastly, we repeated the multivariate models for a 3-level (robust, prefrail, and frail) categorical outcome variable, using multinomial logistic regression. Since these models showed patterns of results similar to those by logistic regression described above and the Wald test for combining outcome categories performed after the multinomial regression showed that frail and prefrail categories can be combined, we report results only for our primary dichotomous outcome variable, which combines prefrail and frail categories. All our analyses were based on non-missing data, so the number of observations in various models varied from 117 to 127.
Results
Characteristics of participants
The clinical and demographic characteristics of our participants by HIV status are presented in Table 1. Mean age was 50.1 (SD=8.9, range: 35-65) for PLWH and 51.0 (SD=7.7, range: 38-65) for HIV-uninfected participants. A majority of the participants in our sample were men. While there were no group differences in age and sex, when compared to HIV-uninfected counterparts, PLWH had fewer years of education and a lower proportion of them were White. We also found significant group differences in frailty and comorbidities prevalence. In comparison to the HIV-uninfected individuals in our sample, significantly higher proportions of PLWH were frail or prefrail (67.7% v. 32.3%), had hypertension (44.6% v. 16.1%), malignancy (9.2% v. 0%), ever smoked (44.6% v. 12.9%), or were diagnosed with lifetime alcohol use disorder (50% v. 33.3%). Lastly, the estimated mean HIV disease duration in our sample was 15.3 years and HIV disease was relatively well-controlled, with median CD4 count of 637 cells/mL (IQR= 480-855), 68.3% of PLWH having undetectable plasma viral load, and 43.1% having no history of AIDS.
Table 1.
Comparison of Participant Characteristics by HIV Status.
| PLWH (N=65) | HIV-uninfected (N=62) | P-value | |
|---|---|---|---|
| N (%) or Mean (SD) | N (%) or Mean (SD) | ||
| DEMOGRAPHICS | |||
| Age | 50.1 (8.9) | 51.0 (7.7) | 0.55 |
| Gender (% male) | 49 (75.4%) | 52 (83.9%) | 0.24 |
| Race (% white) | 27 (41.5%) | 40 (64.5%) | 0.01 |
| Education, years | 13.8 (2.4) | 15.2 (2.1) | 0.001 |
| FRAILTY | |||
| Prefrail (FFI 1-2) | 34 (52.3%) | 19 (30.7%) | 0.01 |
| Frail (FFI 3-5) | 10 (15.4%) | 1 (1.6%) | 0.01 |
| Low physical activity | 23 (35.4%) | 5 (8.1%) | <0.001 |
| Slowness | 11 (16.9%) | 1 (1.6%) | 0.003 |
| Exhaustion | 29 (44.6%) | 6 (9.7%) | <0.001 |
| Weakness | 7 (10.8%) | 9 (14.5%) | 0.40 |
| Unintended weight loss | 14 (21.5%) | 3 (4.8%) | 0.01 |
| COMORBIDITIES | |||
| Ever smoking | 29 (44.6%) | 8 (12.9%) | <0.001 |
| Lifetime alcohol use disorder | 32 (50.0%) | 20 (33.3%) | 0.06 |
| Lifetime major depressive disorder | 36 (57.1%) | 13 (21.7%) | <0.001 |
| Hypertension | 29 (44.6%) | 10 (16.1%) | 0.001 |
| Diabetes | 8 (12.3%) | 4 (6.5%) | 0.26 |
| Hyperlipidemia | 29 (44.6%) | 11 (17.7%) | 0.001 |
| Malignancy | 6 (9.2%) | 0 (0%) | 0.03 |
| HIV DISEASE CHARACTERISTICS | |||
| Current CD4, median (IQR) | 637 (480; 855) | -- | -- |
| Nadir CD4, median (IQR) | 194.5 (40.5; 321.5) | -- | -- |
| Est. duration of HIV disease, years | 15.3 (8.3) | -- | -- |
| Undetectable plasma viral load | 43 (68.3%) | -- | -- |
FFI – Fried Frailty Index
Bivariate logistic regression models
Out of 14 psychosocial factors tested, 11 were significantly associated with frailty in the unadjusted bivariate models (Table 2). When adjusting for covariates (age, HIV status, ever smoking, hypertension, and hyperlipidemia), 9 out of 11 variables retained statistical significance. The adjusted analyses showed that positive psychosocial factors significantly reduced the odds of frailty in our combined sample. For example, an increase in one scale-point on participants’ optimism score, resulted in a 12% reduction in the odds of frailty (95% CI=0.80-0.96). The following factors reduced the risk of frailty: higher grit score (aOR=0.44, 95% CI=0.21-0.95), higher personal mastery score (aOR=0.87, 95% CI=0.78-0.96), higher successful aging score (aOR=0.7, 95% CI=0.55-0.88), higher emotional support score (aOR=0.4, 95% CI=0.18-0.72), and higher Duke social support index scores (aOR=0.60, 95% CI=0.44-0.82). In contrast, negative psychosocial factors such as higher depression score (aOR=1.13, 95% CI=1.06-1.22), higher perceived stress score (aOR=1.09, 95% CI=1.02-1.16), and a greater number of negative interactions with others (aOR=2.36, 95% CI=1.37-4.07) significantly increased the odds of frailty in our sample. All of these adjusted models were significant with p<0.01 and pseudo R2 ranging from 0.15 to 0.22.
Table 2.
Psychosocial Correlates of Frailty: Bivariate Logistic Regression Models.
| Crude, OR | 95% CI | Adjusted, aOR1 | 95% CI | |
|---|---|---|---|---|
| Resilience | 0.93 | (0.87; 0.98) | 0.95 | (0.89; 1.01) |
| Grit | 0.27 | (0.13; 0.55) | 0.44 | (0.21; 0.95) |
| Optimism | 0.86 | (0.78; 0.94) | 0.88 | (0.80; 0.96) |
| Personal mastery | 0.84 | (0.76; 0.93) | 0.87 | (0.78; 0.96) |
| Religiosity | 1 | (0.99; 1.02) | -- | -- |
| Life satisfaction | 0.93 | (0.89; 0.97) | 0.95 | (0.90; 1.00) |
| Successful aging | 0.65 | (0.52; 0.82) | 0.70 | (0.55; 0.88) |
| Subjective age | 1.01 | (0.99; 1.05) | -- | -- |
| Depression score | 1.15 | (1.07; 1.24) | 1.13 | (1.06; 1.22) |
| Perceived stress | 1.12 | (1.06; 1.18) | 1.09 | (1.02; 1.16) |
| Negative interactions | 2.08 | (1.29; 3.34) | 2.36 | (1.37; 4.07) |
| Instrumental support | 0.99 | (0.71; 1.37) | -- | -- |
| Emotional support | 0.38 | (0.20; 0.73) | 0.40 | (0.18; 0.72) |
| Duke social support index | 0.65 | (0.52; 0.83) | 0.60 | (0.44; 0.82) |
Models adjusted for ever smoking, age, HIV status, hypertension, and hyperlipidemia
Principal component analysis
The nine psychosocial variables significantly associated with frailty in the adjusted models were further considered for inclusion in PCA. First, the nine variables showed high intercorrelation (see Appendix 1) and internal consistency (Cronbach alpha=0.78). The Kaiser-Meyer-Olkin measure of sampling adequacy for the nine variables was 0.86, well above the cut-off of 0.5 suggested in the literature,49 and Bartlett’s test of sphericity was significant (chi2 (36)=433.86, p<0.001). We thus concluded that the use of the PCA was appropriate.
The PCA with orthogonal (varimax) rotation yielded two factors with eigenvalues>1, which together explained 59.6% of variance (Table 3). Factor 1, which explained 43.1% of variance, was labeled “Positive Resources/Outlook” since it had high positive loadings on grit, optimism, personal mastery, and successful aging; and high negative loadings on depression, perceived stress, and negative interactions. Two remaining psychosocial variables, emotional support and Duke social support index, had high loadings on Factor 2, which was labelled “Support by Others” and explained 16.5% of variance. The PCA also showed adequate communality among the included psychosocial variables. Only one variable (negative interactions with others) showed low communality of 0.2, and the rest of them had a communality of 0.5 or higher. Since the loading of negative interactions on Factor 1 was higher than the recommended cut-off of 0.4,50 it was retained in the analysis. Thus, composite scores for Factor 1 (Positive Resources/Outlook) and Factor 2 (Support by Others) were created based on the nine psychosocial variables as the last step.
Table 3.
Principal Component Analysis with Varimax Rotation.
| Factor 1 (Positive Resources/Outlook) | Factor 2 (Support by Others) | Communality | |
|---|---|---|---|
| Rotated factor loadings | |||
| Grit | 0.78 | −0.04 | 0.61 |
| Optimism | 0.80 | 0.21 | 0.69 |
| Personal mastery | 0.78 | 0.26 | 0.67 |
| Successful aging | 0.69 | 0.28 | 0.56 |
| Depression score | −0.70 | −0.09 | 0.50 |
| Perceived stress | −0.90 | −0.17 | 0.83 |
| Negative interactions | −0.45 | 0.02 | 0.20 |
| Emotional support | 0.13 | 0.83 | 0.71 |
| Duke social support | 0.20 | 0.75 | 0.60 |
| Eigenvalue | 3.88 | 1.49 | |
| % of total variance | 43.09% | 16.54% | |
| Total variance | 59.63% | ||
Multivariate logistic regression models
Both Factor 1 (Positive Resources/Outlook) and Factor 2 (Support by Others) were included in multivariate logistic regression models presented in Table 4. Model 1 also included the interaction terms of Factor 1 and Factor 2 with HIV status. This model showed significant main effects of Factor 2 (aOR=0.3, 95% CI=0.12-0.77) and HIV status (aOR=3.4, 95% CI=1.23-9.37), as well as interactive effects between HIV status and Factor 1 (aOR=0.23, 95% CI=0.06-0.87). The model was significant with p=0.001 and pseudo R2=0.30. The post-regression diagnostics were conducted, including tests for multicollinearity, model fit, and specification error. No problems were identified.
Table 4.
Psychosocial Correlates of Frailty: Multivariate Logistic Regression Models.
| Model 1 – Interactions | Model 2 – HIV-uninfected | Model 3 – PLWH | Model 4 – PLWH | |||||
|---|---|---|---|---|---|---|---|---|
| aOR | 95% CI | aOR | 95% CI | aOR | 95% CI | aOR | 95% CI | |
| Factor 1 (Positive Resources/Outlook) | 0.97 | (0.41; 2.32) | 1.06 | (0.44; 2.52) | 0.23 | (0.10; 0.56) | 0.25 | (0.10; 0.64) |
| Factor 2 (Support by Others) | 0.30 | (0.12; 0.77) | 0.31 | (0.12; 0.81) | 0.53 | (0.27; 1.01) | 0.55 | (0.24; 1.24) |
| HIV status (infected) | 3.40 | (1.23; 9.37) | -- | -- | -- | -- | -- | -- |
| HIV-infected × Factor 1 | 0.23 | (0.06; 0.87) | -- | -- | -- | -- | -- | -- |
| HIV-infected × Factor 2 | 1.73 | (0.58; 5.22) | -- | -- | -- | -- | -- | |
| Participant’s age | 0.36 | (0.10; 1.31) | 0.98 | (0.89; 1.07) | 0.95 | (0.87; 1.04) | 0.95 | (0.87; 1.05) |
| Hypertension | 2.34 | (0.77; 7.08) | 4.0 | (0.9; 16.17) | 1.47 | (0.44; 4.95) | 1.34 | (0.39; 4.66) |
| Hyperlipidemia | 0.74 | (0.24; 2.32) | -- | -- | -- | -- | -- | -- |
| Ever smoking | 1.21 | (0.10; 1.31) | -- | -- | -- | -- | -- | -- |
| Nadir CD4 | -- | -- | -- | -- | -- | -- | 1.0 | (1.00; 1.01) |
| Model N | 117 | 53 | 64 | 63 | ||||
| Model Chi2 and P | 29.9 | 0.001 | 8.7 | 0.07 | 15.3 | 0.004 | 16.6 | 0.01 |
| Model pseudo R2 | 0.30 | 0.15 | 0.28 | 0.29 | ||||
The interactive effects of HIV-status X Factor 1 are further illustrated by Figure 1, which shows that Positive Resources/Outlook was more strongly associated with reduced odds of frailty for the PLWH than for the HIV-uninfected participants. These synergistic effects were further explored through HIV-stratified analyses (Models 2-4, Table 4). Models 2 and 3 showed that Factor 1 was associated with reduced odds of frailty among PLWH (aOR=0.23, 95% CI=0.1-0.56) but not among the HIV-uninfected participants (aOR=1.06, 95% CI=0.44-2.52). These models also suggested that Factor 2 (Support by Others) was negatively associated with frailty irrespective of HIV status: it was significant among the HIV-uninfected participants (aOR=0.31, 95% CI=0.12-0.81) and approached significance among PLWH (aOR=0.53, 95% CI=0.27-1.01). Lastly, Model 4 repeated the analyses in Model 3 with inclusion of an HIV-disease characteristic (nadir CD4). Factor 1 (Positive Resources/Outlook) retained its significance in this last model.
Figure 1.
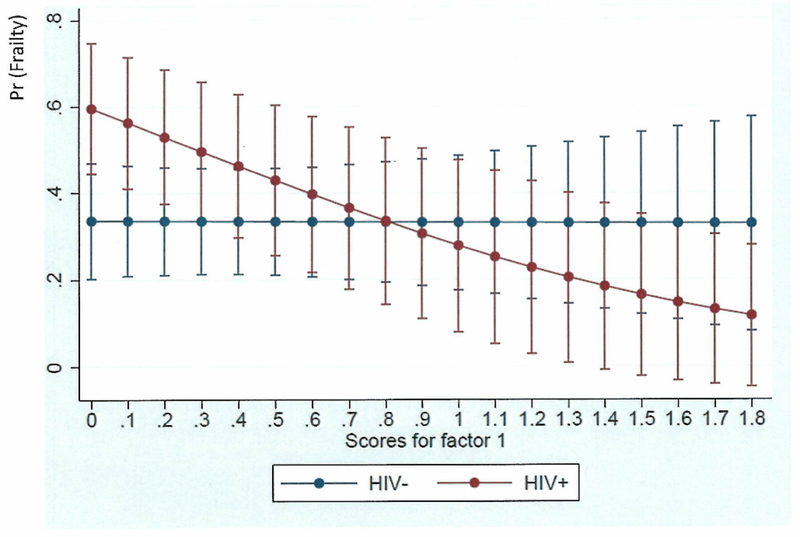
Adjusted predictions of frailty according to Positive Resources/Outlook (Factor 1) scores in PLWH versus HIV-uninfected adults: significant interaction HIV X Positive Resources/Outlook (aOR=0.23; 95% CI=0.06-0.87), such that higher Positive Resources/Outlook scores were significantly associated with lower likelihood of frailty for PLWH but not for HIV-uninfected individuals.
Discussion
Our study represents one of the first efforts to examine psychosocial correlates of frailty among PLWH. The existing research identified multiple biomedical and sociodemographic factors associated with frailty among PLWH but little is known about psychosocial correlates of frailty among this population.2,6,9–11 Our findings indicate that positive psychosocial factors reduce the risk of frailty and negative psychosocial factors increase the risk of frailty among PLWH and HIV-uninfected adults. Additionally, our results revealed that the associations of psychosocial factors with frailty may differ by HIV status. Thus, empirically-derived psychosocial factors related to Positive Resources/Outlook reduced the likelihood of frailty for PLWH but not for HIV-uninfected adults, whereas Support by Others was inversely associated with frailty irrespective of HIV status. Below, we discuss these findings in greater detail.
Multiple psychosocial factors were significantly associated with frailty in our bivariate analyses using a combined sample. Similar to research in the general population, 21–26 we found that higher levels of positive psychosocial factors, such as grit, optimism, personal mastery, or social support, lowered the odds of frailty. In contrast, higher levels of negative psychosocial factors, such as perceived stress, depression, or negative interactions, increased the odds of frailty. Contrary to the existing research,24 we found that resilience and life satisfaction reduced the likelihood of frailty in unadjusted but not adjusted analyses, which may be due to our somewhat limited sample size. We also found no statistically significant effects of religiosity on frailty. Based on the existing literature,51 however, we hypothesize that religiosity may have an indirect effect on frailty via increasing individuals’ wellbeing, which shall be explored by future research.
Through exploratory factor analysis (PCA), we also found that the numerous psychosocial variables that were associated with frailty in bivariate analyses could be reduced to two composite scores – Factor 1 (Positive Resources/Outlook) and Factor 2 (Support by Others). Factor 2 received its name since it had high positive loadings on social support and emotional support variables. We further conceptualized Factor 1 as positive outlook and resources since it had high positive loadings on grit, optimism, personal mastery, and successful aging, and high negative loadings on depression, stress, and negative interactions with others. It has been noted in the literature that such positive characteristics as grit, optimism, a sense of mastery or personal control, and few conflictual relationships represent psychosocial resources unequally distributed among social classes.52 Research suggests that lower socio-economic status (SES) is associated with higher likelihood of conflictual relationships and lower scores on grit, optimism, and personal mastery.52–54 Conversely, availability of psychosocial resources is closely related to a more positive outlook, wellbeing, and better mental health outcomes, such as decreased stress and depression.52 Given the above considerations, Factor 1 was termed as Positive Resources & Outlook. Future research is needed to further refine this concept and to understand the additional elements that may influence our identified factor structure. Moreover, this is one of the first studies that simultaneously considered multiple psychosocial correlates of frailty and examined factor variables summarizing psychosocial effects, and used this approach specifically among PLWH. Future studies will examine whether psychosocial factors associated with frailty have a similar underlying structure in other samples and populations.
The multivariate analyses further revealed that Support by Others was negatively associated with frailty irrespective of HIV status, while Positive Resources & Outlook reduced the odds of frailty for PLWH but not for HIV-uninfected individuals. The significant findings for Support by Others across HIV status groups are not surprising given the existing research linking social and/or emotional support to improved health outcomes among PLWH46 and to the decreased frailty among the general population.22,25 The differential effects of Positive Resources & Outlook are best understood within the context of research showing that PLWH and HIV-uninfected adults may experience different sets of psychosocial exposures. In particular, PLWH as a group are known to face greater levels of adversity than HIV-uninfected counterparts.32 Perhaps, given this amplified adversity, even small increases in positive outlook and resources may have stronger associations with decreased frailty for PLWH as compared to HIV-uninfected individuals.
Lastly, it is important to note that our findings should be understood within the context of social class or SES, which in the US maintains intersectionality with gender and race,55 and can underlie multiple issues in HIV. Research suggests that, among PLWH in the cART era as well as HIV-uninfected adults, higher frailty may be associated with lower SES (e.g. fewer years of education, lower income) and related disadvantaged social statuses (e.g., non-Hispanic Black ethnicity, and female gender).6,17,18 The possible mechanisms for these sociodemographic differences in frailty can be decreased access to care, housing, and transportation, as well as the increased levels of food insecurity and stress associated with low SES. Moreover, as noted above, the psychosocial resources allowing to cope with stress and adversity may also be unequally distributed among the social classes. Our sample had relatively high SES, as measured by years of education (M=14.4, SD=2.4) and prevalence of male gender and White race/ethnicity. We hypothesize that among PLWH with lower SES, frailty will be not only more prevalent than in our sample but it may be associated with different psychosocial effects – whereas positive psychosocial resources may be less prevalent in lower SES samples, the increase in optimism, grit, and personal mastery scores may have stronger effects on frailty reduction.
Limitations
Our analyses had several important limitations. First, with mean age of 51 (35 to 65 range), our sample was relatively young. Examining frailty in this age range is not uncommon for research among PLWH.5 In fact, due to the earlier onset of frailty among PLWH, multiple studies of frailty among this population had samples with a mean and/or median age well below 50 years old.5,11,20,56 This younger mean age, however, may be the root of our relatively low frailty (FFI 3-5) prevalence of 8.7% (N=11) in our combined sample. Given the low frailty prevalence, and in order to have sufficient numbers for our analyses, we chose to combine frail and prefrail categories for our primary analyses. Additional limitations were a cross-sectional nature of our data, somewhat small sample size and missing data for some variables; although our overall level of missingness was low. Future studies may consider using larger longitudinal samples to examine the effects of psychosocial variables on frailty across the life-course.
A further limitation of this study is that our findings may not generalize to other populations of PLWH. Our sample had high proportions of men (80%) and Whites (53%) residing in the greater San Diego area of California and may not be applicable to other demographic groups, such as women, racial/ethnic minorities, or rural populations. In particular, it is not clear whether and how our results would be applicable to HIV-infected women, since they are demographically different from HIV-infected men and are disproportionately African American. 57 Future studies will be necessary to examine psychosocial correlates of frailty among specific sub-populations of PLWH.
Conclusions
Despite these limitations, this study represents an essential first step towards our understanding of psychosocial factors related to frailty among PLWH. Importantly, our findings indicate that psychosocial factors related to positive outlook and psychosocial resources are associated with reduced likelihood of frailty among PLWH. Given the cross-sectional nature of our research, we cannot yet make inferences about the directionality of relationship between the psychosocial factors and frailty among this population. Nevertheless, the existing longitudinal research among those without HIV25,28,29 shows that higher levels of negative psychosocial factors and lower levels of positive psychosocial factors during the baseline increased the odds of frailty incidence during the follow-up. We therefore hypothesize that impaired psychosocial functioning may precede development of frailty among PLWH and thus may play an important role in frailty prevention. We also acknowledge that there may also be a reciprocal relationship between psychosocial factors and frailty, such that psychosocial factors may affect the likelihood of frailty development but, once emergent, frailty may result in the development of negative psychosocial factors, such as stress, depression, and loneliness.
Our findings also have important clinical and research implications. Similar to previous research, this study shows that frailty and prefrailty are common and more prevalent among PLWH than HIV-uninfected adults.6 Our novel results additionally suggest that negative psychosocial factors, such as stress and depression, are associated with greater likelihood of frailty, whereas positive psychosocial factors were tied to lower likelihood of frailty in PLWH. Therefore, from a clinical perspective, screening for frailty, stress, and depression are advisable among the aging PLWH. There are numerous therapies and interventions available to clinicians, which have been shown to reduce stress and depression and enhance wellbeing among PLWH: e.g., cognitive behavioral therapy,58 transcendental meditation,59 mindfulness-based therapies,60 as well as the antidepressants use.61 From a research perspective, psychological factors should be further examined and, perhaps, interventions that enhance wellbeing and prevent frailty among PLWH should be designed. Future research is needed to uncover the biological mechanisms underlying the association of psychosocial factors with frailty.
Acknowledgements
The San Diego HIV Neurobehavioral Research Program group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Reena Deutsch, Ph.D., Anya Umlauf, M.S.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Funding
Supported by the Sustained Training in HIV and Aging (STAHR) training grant (R25 MH108389) and R01 MH099987.
Appendix 1.
Correlation Matrix for Psychosocial Factors Included in PCA.
| Grit | Optimism | Personal mastery | Successful aging | Depression score | Perceived stress | Negative interactions | Emotional Support | Duke social support | |
|---|---|---|---|---|---|---|---|---|---|
| Grit | 1.00 | ||||||||
| Optimism | 0.60 | 1.00 | |||||||
| Personal mastery | 0.47 | 0.65 | 1.00 | ||||||
| Successful aging | 0.47 | 0.53 | 0.55 | 1.00 | |||||
| Depression score | −0.49 | −0.41 | −0.46 | −0.39 | 1.00 | ||||
| Perceived stress | −0.59 | −0.70 | −0.76 | −0.64 | 0.58 | 1.00 | |||
| Negative interactions | −0.21 | −0.25 | −0.24 | −0.17 | 0.22 | 0.36 | 1.00 | ||
| Emotional support | 0.15 | 0.33 | 0.33 | 0.29 | −0.09 | −0.24 | −0.16 | 1.00 | |
| Duke social support | 0.19 | 0.28 | 0.26 | 0.28 | −0.29 | −0.31 | −0.09 | 0.36 | 1.00 |
Footnotes
The frailty assessment was introduced after the study began and was administered to all participants enrolled after the introduction of this measure. We observed no statistically significant demographic differences among the participants who received the frailty assessment and those who did not, except for in race/ethnicity: the percentage of nonwhite participants was higher among those who received the frailty assessment than among those who did not (47.2% v. 27.7%, p=0.003).
References
- 1.Vance DE, McGuinness T, Musgrove K, Orel NA, Fazeli PL. Successful aging and the epidemiology of HIV. Clin Interv Aging. 2011;6:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willig AL, Overton ET, Saag MS. The Silent Epidemic - Frailty and Aging with HIV. Total Patient Care HIV HCV. 2016;1(1):6–17. [PMC free article] [PubMed] [Google Scholar]
- 3.Stoff DM, Goodkin K, Jeste D, Marquine M. Redefining Aging in HIV Infection Using Phenotypes. Curr HIV/AIDS Rep. 2017;14(5):184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greene M, Justice AC, Covinsky KE. Assessment of geriatric syndromes and physical function in people living with HIV. Virulence 2017;8(5):586–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gustafson DR, Shi Q, Thurn M, et al. Frailty and Constellations of Factors in Aging HIV-infected and Uninfected Women--The Women’s Interagency HIV Study. J Frailty Aging 2016;5(1):43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levett TJ, Cresswell FV, Malik MA, Fisher M, Wright J. Systematic Review of Prevalence and Predictors of Frailty in Individuals with Human Immunodeficiency Virus. J Am Geriatr Soc 2016;64(5):1006–1014. [DOI] [PubMed] [Google Scholar]
- 7.Piggott DA, Erlandson KM, Yarasheski KE. Frailty in HIV: Epidemiology, Biology, Measurement, Interventions, and Research Needs. Curr HIV/AIDS Rep 2016;13(6):340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Althoff KN, Jacobson LP, Cranston RD, et al. Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci. 2014;69(2):189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thurn M, Gustafson DR. Faces of Frailty in Aging with HIV Infection. Curr HIV/AIDS Rep. 2017;14(1):31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kooij KW, Wit FW, Schouten J, et al. HIV infection is independently associated with frailty in middle-aged HIV type 1-infected individuals compared with similar but uninfected controls. AIDS. 2016;30(2):241–250. [DOI] [PubMed] [Google Scholar]
- 11.Desquilbet L, Jacobson LP, Fried LP, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci. 2007;62(11):1279–1286. [DOI] [PubMed] [Google Scholar]
- 12.Erlandson KM, Allshouse AA, Jankowski CM, et al. Risk factors for falls in HIV-infected persons. J Acquir Immune Defic Syndr. 2012;61(4):484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tassiopoulos K, Abdo M, Wu K, et al. Frailty is strongly associated with increased risk of recurrent falls among older HIV-infected adults. AIDS. 2017;31(16):2287–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piggott DA, Muzaale AD, Varadhan R, et al. Frailty and Cause-Specific Hospitalization Among Persons Aging With HIV Infection and Injection Drug Use. J Gerontol A Biol Sci Med Sci. 2017;72(3):389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guaraldi G, Malagoli A, Theou O, et al. Correlates of frailty phenotype and frailty index and their associations with clinical outcomes. HIV Med 2017;18(10):764–771. [DOI] [PubMed] [Google Scholar]
- 16.Gustafson DR, Shi Q, Holman S, et al. Predicting death over 8 years in a prospective cohort of HIV-infected women: the Women’s Interagency HIV Study. BMJ Open. 2017;7(6):e013993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brothers TD, Kirkland S, Theou O, et al. Predictors of transitions in frailty severity and mortality among people aging with HIV. PLoS One 2017;12(10):e0185352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erlandson KM, Wu K, Koletar SL, et al. Association Between Frailty and Components of the Frailty Phenotype With Modifiable Risk Factors and Antiretroviral Therapy. J Infect Dis. 2017;215(6):933–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Branas F, Jimenez Z, Sanchez-Conde M, et al. Frailty and physical function in older HIV-infected adults. Age Ageing 2017;46(3):522–526. [DOI] [PubMed] [Google Scholar]
- 20.Terzian AS, Holman S, Nathwani N, et al. Factors associated with preclinical disability and frailty among HIV-infected and HIV-uninfected women in the era of cART. J Womens Health (Larchmt). 2009;18(12):1965–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrew MK, Fisk JD, Rockwood K. Psychological well-being in relation to frailty: a frailty identity crisis? Int Psychogeriatr. 2012;24(8):1347–1353. [DOI] [PubMed] [Google Scholar]
- 22.Aranda MP, Ray LA, Snih SA, Ottenbacher KJ, Markides KS. The protective effect of neighborhood composition on increasing frailty among older Mexican Americans: a barrio advantage? Journal of aging and health. 2011;23(7):1189–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng Z, Lugtenberg M, Franse C, et al. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: A systematic review of longitudinal studies. PLoS One. 2017;12(6):e0178383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freitag S, Schmidt S. Psychosocial Correlates of Frailty in Older Adults. Geriatrics 2016;1(26):doi: 10.3390/geriatrics1040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lurie I, Myers V, Goldbourt U, Gerber Y. Perceived social support following myocardial infarction and long-term development of frailty. European journal of preventive cardiology. 2015;22(10):1346–1353. [DOI] [PubMed] [Google Scholar]
- 26.Mooney CJ, Elliot AJ, Douthit KZ, Marquis A, Seplaki CL. Perceived Control Mediates Effects of Socioeconomic Status and Chronic Stress on Physical Frailty: Findings From the Health and Retirement Study. J Gerontol B Psychol Sci Soc Sci 2016;[Epub ahead of print]:doi: 10.1093/geronb/gbw1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ottenbacher KJ, Graham JE, Al Snih S, et al. Mexican Americans and frailty: findings from the Hispanic established populations epidemiologic studies of the elderly. Am J Public Health. 2009;99(4):673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lakey SL, LaCroix AZ, Gray SL, et al. Antidepressant use, depressive symptoms, and incident frailty in women aged 65 and older from the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2012;60(5):854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monin J, Doyle M, Levy B, Schulz R, Fried T, Kershaw T. Spousal Associations Between Frailty and Depressive Symptoms: Longitudinal Findings from the Cardiovascular Health Study. J Am Geriatr Soc 2016;64(4):824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoogendijk EO, van Hout HP, Heymans MW, et al. Explaining the association between educational level and frailty in older adults: results from a 13-year longitudinal study in the Netherlands. Ann Epidemiol 2014;24(7):538–544.e532. [DOI] [PubMed] [Google Scholar]
- 31.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 32.Rueda S, Law S, Rourke SB. Psychosocial, mental health, and behavioral issues of aging with HIV. Curr Opin HIV AIDS. 2014;9(4):325–331. [DOI] [PubMed] [Google Scholar]
- 33.Blinded citation.
- 34.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. [DOI] [PubMed] [Google Scholar]
- 35.Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 36.Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety 2003;18(2):76–82. [DOI] [PubMed] [Google Scholar]
- 37.Cambell-Sills L, Stein MB. Psychometric analysis and refinement of the Connor-Davidson Resilience Scale (CD-RISC): validation of a 10-item measure of resilience. J Trauma Stress. 2007;20(6):1019–1028. [DOI] [PubMed] [Google Scholar]
- 38.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A re-evaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67:1063–1078. [DOI] [PubMed] [Google Scholar]
- 39.Schou I, Ekeberg O, Ruland CM, Sandvik L, Kåresen R. Pessimism as a predictor of emotional morbidity one year following breast cancer surgery. Psycho-Oncology. 2004;13(5):309–320. [DOI] [PubMed] [Google Scholar]
- 40.Duckworth AL, Peterson C, Matthews MD, Kelly DR. Grit: Perseverance and passion for long-term goals. Journal of Personality and Social Psychology. 2007;9:1087–1101. [DOI] [PubMed] [Google Scholar]
- 41.Pearlin LI, Schooler C. The structure of coping. J Health Soc Behav. 1978;19:2–21. [PubMed] [Google Scholar]
- 42.Fetzer Insitute; National Institute on Aging Working Group. Multidimensional Measurement of Religiousness, Spirituality for Use in Health Research. A Report of a National Working Group. Supported by the Fetzer Institute in Collaboration with the National Institute on Aging. Kalamazoo, MI: Fetzer Institute; 2003. (1999). [Google Scholar]
- 43.Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction with Life Scale. Journal of Personality Assessment. 1985;49:71–75. [DOI] [PubMed] [Google Scholar]
- 44.Montross LP, Depp C, Daly J, et al. Correlates of Self-Rated Successful Aging Among Community-Dwelling Older Adults. Am J Geriatr Psychiatry 2006;14(1):43–51. [DOI] [PubMed] [Google Scholar]
- 45.Koening HG, Westlund RE, George LK, Hughes DC, Blazer DG, Hybels C. Abbreviating the Duke Social Support Index for use in chronically ill elderly individuals. Psychosomatics 1993;34(1):61–69. [DOI] [PubMed] [Google Scholar]
- 46.Seeman TE, Lusignolo TM, Albert M, Berkman L. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychol. 2001;20(4):243–255. [DOI] [PubMed] [Google Scholar]
- 47.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 48.Hamilton LC. Statistics with Stata. Belmont, CA: Brooks/Cole, Cengage Learning; 2009. [Google Scholar]
- 49.Kaiser H An index of factorial simplicity. Psychometrika 1974;39:31–36. [Google Scholar]
- 50.Stevens JP. Applied multivariate statistics for the social sciences (2nd edition). Hillsdael, NJ: Erlbaum; 1992. [Google Scholar]
- 51.Kirby SE, Coleman PG, Daley D. Spirituality and well-being in frail and nonfrail older adults. J Gerontol B Psychol Sci Soc Sci. 2004;59(3):P123–129. [DOI] [PubMed] [Google Scholar]
- 52.Taylor SE, Seeman TE. Psychosocial resources and the SES-health relationship. Ann N Y Acad Sci. 1999;896:210–225. [DOI] [PubMed] [Google Scholar]
- 53.Gallo LC, de Los Monteros KE, Shivpuri S. Socioeconomic Status and Health: What is the role of Reserve Capacity? Curr Dir Psychol Sci October 2009;18(5):269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: do negative emotions play a role? Psychol Bull January 2003;129(1):10–51. [DOI] [PubMed] [Google Scholar]
- 55.Collins PH. Black Feminist Thought : Knowledge , Consciousness, and the Politics of Empowerment. New York: Routledge; 1991. [Google Scholar]
- 56.Piggott DA, Muzaale AD, Mehta SH, et al. Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PLoS One. 2013;8(1):e54910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Centers for Disease Control and Prevention. HIV Among Women. https://www.cdc.gov/hiv/group/gender/women/index.html Published (last updated) March 9, 2018. Accessed March 28, 2018.
- 58.Safren SA, Bedoya CA, O’Cleirigh C, et al. Cognitive behavioural therapy for adherence and depression in patients with HIV: a three-arm randomised controlled trial. Lancet HIV. 2016;3(11):e529–e538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chhatre S, Metzger DS, Frank I, et al. Effects of behavioral stress reduction Transcendental Meditation intervention in persons with HIV. AIDS Care. 2013;25(10):1291–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gonzalez-Garcia M, Ferrer MJ, Borras X, et al. Effectiveness of Mindfulness-Based Cognitive Therapy on the Quality of Life, Emotional Status, and CD4 Cell Count of Patients Aging with HIV Infection. AIDS Behav. 2014;18(4):676–685. [DOI] [PubMed] [Google Scholar]
- 61.Eshun-Wilson I, Siegfried N, Akena DH, Stein DJ, Obuku EA, Joska JA. Antidepressants for depression in adults with HIV infection. Cochrane Database Syst Rev 2018;1:Cd008525. [DOI] [PMC free article] [PubMed] [Google Scholar]


