Abstract
Background & Aims:
Statin use is associated with lower risk of developing hepatocellular carcinoma (HCC). However, it is unclear whether post-diagnosis statin use is associated with reduced risk of mortality in HCC patients.
Methods:
We used data from 15,422 patients with HCC in the VA Central Cancer Registry diagnosed between 2002 and 2016. We identified statin prescriptions that were filled before and after cancer diagnosis, and used time-dependent Cox regression models to calculate adjusted hazard ratios (HR) and 95% CIs for mortality risk. We used a time-varying exposure to avoid immortal-time bias, and a 3 month lag (following patients from 3 months after cancer diagnosis) to reduce reverse causation. A sensitivity analysis was conducted varying the lag duration between date of cancer diagnosis and start of follow-up.
Results:
Statin use after diagnosis was recorded in 14.9% of HCC patients. We found that postdiagnosis statin use was associated with a decreased risk of cancer specific (adjusted HR, 0.85; 95% CI, 0.77–0.93) and all-cause mortality (HR, 0.89; 95% CI, 0.83–0.95). The magnitudes of these inverse associations were consistent for HCC patients using both low- and high-dose statins, and the inverse associations remained across a range of lag periods (from 0 months to 12 months after HCC diagnosis). We found no evidence for effect modification by pre-diagnosis statin use, or by presentation or treatment-related factors, and no independent association with pre-diagnosis statin use.
Conclusion:
Post-diagnosis statin use was associated with reduced mortality in HCC patients.
Keywords: liver cancer, mortality, prognosis, medication use
INTRODUCTION
Hepatocellular carcinoma (HCC) is a rapidly increasing highly fatal cancer.1,2 For patients who present with early stage HCC, treatment modalities including transplant, resection and local ablation have been shown to improve overall survival.3 However, despite the existence of guidelines for screening and detection of incident HCC among high-risk patients, the majority of HCC patients, especially those with alcohol- or non-alcoholic fatty liver disease-related HCC,4 still present with advanced stage disease that is not amenable to curative treatment. Curative treatment modalities are only available to HCC patients with good functional status, and patients with decompensated cirrhosis are often not candidates.3 For patients with advanced disease the only available treatment until recently was sorafenib, but this drug may have low efficacy in advanced liver disease as well as increase risk for adverse effects.5 There is a need for additional treatment options. Statins (or 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors), commonly used because of their efficacy in preventing cardiovascular morbidity and mortality, have been shown to reduce the risk of developing as well as mortality from several types of cancer and may represent such a treatment.6–10
It is conceivable that there may be a biologically beneficial effect of statins on HCC prognosis. In various cancer cell lines, statins have been shown to increase apoptosis, inhibit proliferation and invasion, and decrease spread of tumors.11–13 Observational studies in humans have consistently shown that statin use is associated with lower risk of having HCC. 14–17 However, few studies have examined the association between post-diagnosis statin use and risk of mortality in HCC patients. A clinical trial of 91 patients with unresectable HCC published in 2001 showed a 9-month improvement in survival for advanced HCC patients treated with pravastatin compared to placebo.18 A prospective cohort study found that, compared with chemoembolization alone, combined therapy of chemoembolization with pravastatin was associated with improved median survival (20.9 months vs. 12.0 months) for patients with advanced HCC. However, these studies involved only advanced HCC cases.19 A recent study analyzing data from the NCI’s Surveillance, Epidemiology, and End Results (SEER)-Medicare data file found no association between post-diagnosis statin use and risk of mortality for patients (aged ≥65 years) with early stage HCC.20
We therefore examined the effects of statin use on cancer specific and all-cause mortality in a large cohort of HCC patients with comprehensive data on filled medications and potential confounders. To overcome immortal-time bias and selection bias, we used time-dependent exposure assignment methods and used varying exposure lag periods, respectively.
METHODS
Study Design and Population
We conducted a retrospective cohort study including all patients diagnosed with a first primary HCC between 2002 and 2016 in the VA Central Cancer Registry (VACCR). The VACCR was initiated in 1995 and is a national data repository for >750,000 Veterans with cancer.21,22 Cancer registrars at each VA Hospital manually abstract data, conforming to standards set by the North American Association of Central Cancer Registries, and data are then aggregated into the VACCR where cases are merged and quality assurance checks are conducted. The VACCR includes information on patient demographics, date of cancer diagnosis, primary site, histology, grade, tumor size, extension, staging, treatment, and cause of death. We identified patients with HCC using International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) primary site code C22 in combination with histology codes 81703–81753. This study was approved by the Institutional Review Board at Baylor College of Medicine.
Study Outcomes
HCC patients were followed up to the date of death or 12/31/2016. We identified cancer specific and all-cause mortality, where applicable, using the VA Vital Status file.23 Cancer specific deaths were those classified as “directly related” in the VACCR.
Main Exposure
Pharmacy records were obtained from the pharmacy clinical national data extract of the VA Corporate Data Warehouse. Prescriptions for statins filled (i.e., dispensed) were identified for the period prior to (as far back as 10/01/1999) and after HCC diagnosis date. We included the following statins: simvastatin (Zocor), lovastatin (Mevacor and Altoprev), atorvastatin (Lipitor), fluvastatin (Lescol), pravastatin (Pravachol), and rosuvastatin (Crestor). We collected start and stop dates, daily dose, number of days’ supply, and number of pills for each filled prescription. The total duration of filled prescriptions was calculated by adding the duration of individual statin prescriptions irrespective of gaps between prescriptions. We calculated the cumulative dose of filled prescriptions by adding the dose of individual prescriptions (dose multiplied by the quantity of pills), and the defined daily dose (DDD) by dividing the total cumulative dose by the total days supplied. Statin users were categorized as low (<20 mg) or high (≥20 mg) dose users based on the mean daily dose for statin prescriptions collected 6 to 18 months prior to cancer diagnosis. Post-diagnosis cumulative dose-response or duration of use analyses were not examined a priori because the dose and duration of use categories would be expected to be a function of survival time.
Covariates
Data on cancer stage (1, 2, 3, 4, or missing), grade (I-II, III-IV, or missing) and treatment (radiotherapy, chemotherapy and surgery including liver transplant) were retrieved from the VACCR. Other study variables included age at cancer diagnosis, sex, race (categorized as White, Hispanic, Black, Other, and Missing), body mass index (BMI; kg/m2) within 1 year of cancer diagnosis date, smoking status prior to cancer diagnosis (never, ever), alcohol abuse prior to cancer diagnosis on the basis of outpatient or inpatient ICD codes (yes, no), positive tests for hepatitis B virus infection (i.e., HBV surface antigen) or hepatitis C virus infection (i.e., HCV RNA) prior to cancer diagnosis, and non-alcoholic fatty liver disease (NAFLD) and cirrhosis prior to cancer diagnosis on the basis of outpatient or inpatient ICD codes. Transarterial chemoembolization (TACE) and systemic chemotherapy were identified by ICD and CPT codes.24 We defined TACE by the presence of at least one ICD or CPT code for embolization with an ICD or CPT code indicative of chemotherapy within 30-days. Systemic chemotherapy was identified by the presence of an ICD or CPT code for chemotherapy in the absence of an embolization code within 30-days. Several indicators of liver disease dysfunction and severity, including the aspartate aminotransferase (AST) to Platelet Ratio Index (APRI), Model for End-Stage Liver Disease (MELD) score,25 and the presence of ascites, varices, and encephalopathy, were captured. APRI was calculated on the basis of AST and platelet values closest to HCC diagnosis date. MELD was calculated from laboratory test values from the year prior to HCC diagnosis date. Measures of overall performance, including Eastern Cooperative Oncology Group (ECOG) Performance Status and Deyo comorbidity index were derived on the basis of ICD codes. We also determined use of aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) as described above for statins.
Statistical Analysis
We compared baseline characteristics (i.e., at HCC diagnosis) between statin users and statin non-users using independent T-test for continuous variables and chi-square tests for categorical variables. Survival was estimated using the Kaplan-Meier method and compared using the logrank test.
We estimated hazard ratios (HRs) and 95% confidence intervals (95% CI) for the association between post-diagnosis statin use and risks of cancer specific mortality and all-cause mortality using time-varying covariate Cox proportional hazards regression models. This approach accounts for the varying initiation dates for statin use among the HCC patients and helps to overcome immortal-time bias.26,27 Furthermore, the resulting effect estimate is less prone to overestimation.28 In the primary analysis, we considered statin use from the date of HCC diagnosis as a time varying covariate with HCC patients classified as statin non-users until their first post-diagnosis prescription, from which point they were then reclassified as statin users until the end of follow-up.29 Patients who never used statins (“non-users”) were classified as non-users throughout follow-up (until death or 12/31/2016). We used a 3 month lag to help reduce reverse causation, whereby the accrual of person years at risk started from 3 months after HCC diagnosis date and we excluded HCC patients who died within the first 3 months after their HCC diagnosis date. Participants with missing or unknown data for covariables were included in the analyses using a missing category. We examined for a trend with increasing dose among statin users using the Cochran-Armitage test for trend.
We explored possible heterogeneity of the effect of post-diagnosis statin use and risks of cancer specific mortality and all-cause mortality through analyses stratified by pre-diagnosis statin use, MELD score, cirrhosis, HCV, alcohol abuse, NAFLD status, and stage. Potential interactions were assessed by fitting the interaction term between post-diagnosis statin use and the stratified variable into the model. Likelihood ratio tests of nested models with and without the interaction term were performed. In secondary analyses, we required that statin users have ≥1 month of filled prescriptions, and examined the effect of statin use on HCC mortality in a Cox model with a time-dependent covariate for statin use whereby patients were classified as non-users until after a one month period of prescription had lapsed. Finally, we conducted a sensitivity analysis whereby the duration of the lag was varied. Specifically, using the same time varying covariate analysis described above, but using no lag (following patients starting from cancer diagnosis and not excluding any deaths after cancer diagnosis), a 6 month lag and a 12 month lag.
All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). Statistical significance was determined at α = 0.05, and all p-values for statistical significance were two-sided.
RESULTS
We included 15,422 HCC patients in the primary analysis; of whom 78.8% died during a total 28,680 person-years of follow-up. Among the entire HCC cohort, the median survival time was 17.24 months (interquartile range [IQR], 8.24–33.02 months). The distributions of baseline variables according to post-diagnosis and pre-diagnosis statin use are shown in Table 1 and Supplementary Table 1, respectively. Patients that used statins post-HCC diagnosis were older and had higher mean BMI but less severe liver disease at cancer diagnosis than patients that did not use statins after their cancer diagnosis. As expected, advanced tumor stage at diagnosis, ascites, hepatic encephalopathy, high APRI score and poor performance status were associated with higher risk of mortality. Conversely, receipt of surgery and TACE and higher BMI were associated with lower risk of mortality (Supplementary Table 2).
Table 1.
Characteristics of HCC patients according to post-diagnosis statin use
| Characteristics | Post-diagnosis statin non-user (N=13129) | Post-diagnosis statin user (N=2293) | p-value |
|---|---|---|---|
| Age, Mean (SD) | 62.7 (8.3) | 66.9 (8.4) | <0.001 |
| BMI, Mean (SD) | 27.0 (5.4) | 28.8 (5.8) | <0.001 |
| Male, N (%) | 13013 (99.1) | 2280 (99.4) | 0.26 |
| Ethnicity/race | 0.03 | ||
| White | 7691 (58.6) | 1352 (59.0) | |
| Hispanic | 1216 (9.3) | 242 (10.5) | |
| Black | 3203 (24.4) | 545 (23.8) | |
| Other | 276 (2.1) | 54 (2.3) | |
| Missing | 743 (5.6) | 100 (4.4) | |
| Pre-diagnosis statin use | <0.001 | ||
| No | 11891 (90.6) | 857 (37.4) | |
| Yes | 1238 (9.4) | 1436 (62.6) | |
| Alcohol abuse | <0.001 | ||
| No | 7434 (56.6) | 1444 (63.0) | |
| Yes | 5695 (43.4) | 849 (37.0) | |
| Smoking status | <0.001 | ||
| Never | 1351 (10.3) | 322 (14.0) | |
| Ever | 11257 (85.7) | 1965 (85.7) | |
| Missing | 521 (4.0) | 6 (0.3) | |
| NAFLD | <0.001 | ||
| No | 11770 (89.7) | 1983 (86.5) | |
| Yes | 1359 (10.3) | 310 (13.5) | |
| Hepatitis C infection | <0.001 | ||
| No | 4146 (31.6) | 1053 (45.9) | |
| Yes | 8983 (68.4) | 1240 (54.1) | |
| Hepatitis B infection | 0.05 | ||
| No | 11906 (90.7) | 2109 (92.0) | |
| Yes | 1223 (9.3) | 184 (8.0) | |
| Cirrhosis | <0.001 | ||
| No | 6662 (50.7) | 1310 (57.1) | |
| Yes | 6467 (49.3) | 983 (42.9) | |
| Grade | <0.001 | ||
| Grade I-II | 3082 (23.5) | 632 (27.6) | |
| Grade III-IV | 684 (5.2) | 125 (5.4) | |
| Missing | 9363 (71.3) | 1536 (67.0) | |
| TNM Stage | <0.001 | ||
| Stage 1 | 4092 (31.2) | 1059 (46.2) | |
| Stage 2 | 3301 (25.1) | 547 (23.9) | |
| Stage 3 | 2311 (17.6) | 301 (13.1) | |
| Stage 4 | 1486 (11.3) | 101 (4.4) | |
| Missing | 1939 (14.8) | 285 (12.4) | |
| Treatment | <0.001 | ||
| None | 4155 (31.7) | 439 (19.2) | |
| Surgery | 3091 (23.5) | 834 (36.4) | |
| TACE | 2097 (16.0) | 422 (18.4) | |
| Systemic chemotherapy | 596 (4.5) | 42 (1.8) | |
| Other | 3190 (24.3) | 556 (24.2) | |
| MELD score | <0.001 | ||
| <10 | 4164 (31.7) | 936 (40.8) | |
| ≥10 | 4385 (33.4) | 661 (28.8) | |
| Missing | 4580 (34.9) | 696 (30.4) | |
| APRI | <0.001 | ||
| <2 | 4164 (31.7) | 936 (40.8) | |
| ≥2 | 4385 (33.4) | 611 (28.8) | |
| Missing | 4580 (34.9) | 696 (30.4) | |
| Ascites | <0.001 | ||
| No | 9755 (74.3) | 1972 (86.0) | |
| Yes | 3374 (25.7) | 321 (14.0) | |
| Varices | <0.001 | ||
| No | 9911 (75.5) | 1886 (82.2) | |
| Yes | 3218 (24.5) | 407 (17.8) | |
| Encephalopathy | <0.001 | ||
| No | 11303 (86.1) | 2118 (92.4) | |
| Yes | 1826 (13.9) | 175 (7.6) | |
| Deyo comorbidity index | <0.001 | ||
| 0–1 | 5693 (43.4) | 700 (30.5) | |
| 2–3 | 3202 (24.4) | 714 (31.1) | |
| 4–5 | 2518 (19.2) | 483 (21.1) | |
| ≥6 | 1716 (13.1) | 396 (17.3) | |
| ECOG performance status | <0.001 | ||
| 0 | 2255 (17.2) | 514 (22.4) | |
| 1 | 1878 (14.3) | 312 (13.6) | |
| ≥2 | 654 (5.0) | 118 (5.2) | |
| Missing | 8345 (63.6) | 1349 (58.8) |
APRI, AST to Platelet Ratio Index; BMI, body mass index; HCC, Hepatocellular carcinoma; TACE, transarterial chemoembolization; SD, standard deviation. Patients with missing values were excluded from comparisons2
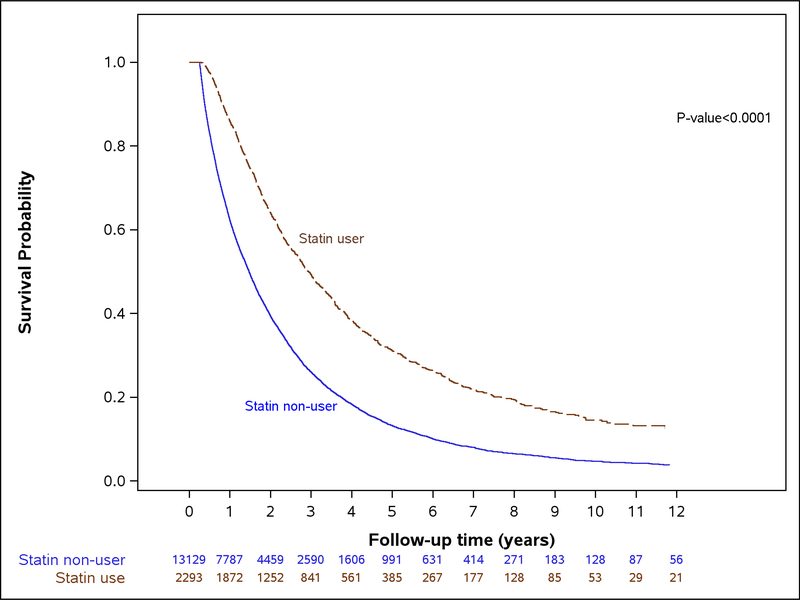
Post-diagnosis statin use was recorded in 2293 HCC patients (14.9%) in whom 5.6% of the entire cohort of HCC patients (N=857 of 15,422) used statins after HCC diagnosis but not prior to their cancer diagnosis. Among post-diagnosis statin users, the median time from start of follow-up (i.e., 3 months after HCC diagnosis date) to statin initiation was 2.37 months (IQR, 1.00–7.83 months). The median survival time differed between HCC patients who used statins after their cancer diagnosis (N=2293; 26.38 months, IQR, 14.82–47.41 months) and HCC patient who did not use statins post-diagnosis (N=13,129; 15.67 months, IQR, 7.58–30.68 months; p<0.0001) (Figure 1). For the primary analysis using a 3 month lag and time-varying multivariable models (Table 2), HCC patients who were post-diagnosis statin users had 15% lower risk of cancer specific mortality (adjusted HR, 0.85; 95% CI, 0.77–0.93) compared with non-users. Likewise, post-diagnosis statin use was associated with 11% lower risk of all-cause mortality (HR, 0.89; 95% CI, 0.83–0.95). However, we found no evidence for a dose-response relationship (Table 3; p for trend among statin users=0.85). The associations were essentially no different in a sensitivity analysis in patients with non-missing MELD and where MELD was included in the final model as a continuous term (cancer specific mortality, adjusted HR, 0.87; 95% CI, 0.81–0.94; all-cause mortality, adjusted HR, 0.81; 95% CI, 0.71–0.92).
Figure 1.
Kaplan-Meier curve comparing overall survival of patients with hepatocellular carcinoma according to post-diagnosis use of statins.
Table 2.
Association between post-diagnosis statin use and cancer specific and all-cause mortality for HCC patients
| Post-diagnosis statin use | Number of Patients | Number of Deaths | Person-Years | Mortality rate (95% CI) (per 100 person-years) | Unadjusted HR (95% CI) | Adjusted HR (95% CI)* |
|---|---|---|---|---|---|---|
| Cancer specific mortality | ||||||
| Statin non-user | 13129 | 5460 | 22418.09 | 24.36 (23.72–25.01) | 1.00 (Ref) | 1.00 (Ref) |
| Statin user | 2293 | 648 | 6262.37 | 10.35 (9.58–11.17) | 0.79 (0.72–0.85) | 0.85 (0.77–0.93) |
| All-cause mortality | ||||||
| Statin nonuser | 13129 | 10686 | 22418.09 | 47.67 (46.77–48.60) | 1.00 (Ref) | 1.00 (Ref) |
| Statin user | 2293 | 1463 | 6262.37 | 23.36 (22.19–24.59) | 0.84 (0.80–0.89) | 0.89 (0.83–0.95) |
CI, confidence interval; HCC, Hepatocellular carcinoma; HR, hazard ratio.
Adjusted for age, sex, race, BMI, alcohol abuse, smoking status, HCV infection, HBV infection, cirrhosis, NAFLD, stage, MELD score, APRI, ascites, hepatic encephalopathy, varices, Deyo comorbidity score, treatment (surgery, TACE, systemic chemotherapy, other), ECOG performance status, pre-diagnosis statin use, and post-diagnosis aspirin/NSAID use (time-varying).
Table 3.
Dose-response associations between post-diagnosis statin use and cancer specific and all-cause mortality for HCC patients
| Post-diagnosis statin use | Number of Patients | Number of Deaths | Person-Years | Mortality rate (95% CI) (per 100 person-years) | Unadjusted HR (95% CI) | Adjusted HR (95% CI)* |
|---|---|---|---|---|---|---|
| Cancer specific mortality | ||||||
| Statin non-user | 13129 | 5460 | 22418.09 | 24.36 (23.72–25.01) | 1.00 (Ref) | 1.00 (Ref) |
| Low-dose statin use | 1419 | 399 | 4069.91 | 9.08 (8.89–10.81) | 0.46 (0.41–0.50) | 0.47 (0.42,0.52) |
| High-dose statin use | 874 | 249 | 2192.46 | 11.35 (10.04–12.86) | 0.50 (0.44–0.57) | 0.48 (0.42,0.56) |
| All-cause mortality | ||||||
| Statin non-user | 13129 | 10686 | 22418.09 | 47.67 (46.77–48.60) | 1.00 (Ref) | 1.00 (Ref) |
| Low-dose statin use | 1419 | 924 | 4069.91 | 22.70 (21.29–24.22) | 0.52 (0.48–0.55) | 0.50 (0.47,0.54) |
| High-dose statin use | 874 | 539 | 2192.46 | 24.58 (22.59–26.75) | 0.54 (0.50–0.59) | 0.51 (0.46,0.56) |
CI, confidence interval HCC, Hepatocellular carcinoma; HR, hazard ratio.
Adjusted for age, sex, race, BMI, alcohol abuse, smoking status, HCV infection, HBV infection, cirrhosis, NAFLD, stage, MELD score, APRI, ascites, hepatic encephalopathy, varices, Deyo comorbidity score, treatment (surgery, TACE, systemic chemotherapy, other), ECOG performance status, pre-diagnosis statin use, and post-diagnosis aspirin/NSAID use (time-varying).High-dose was defined as mean daily dose ≥20 mg, low-dose as <20mg.
We found some evidence for statistical interaction of pre-diagnosis statin use on the association between post-diagnosis statin use and risk of cancer specific mortality (p-interaction=0.06). The magnitude of the inverse association between post-diagnosis use of statins and risk of cancer specific mortality was greater for HCC patients with a history of pre-diagnosis statin use (HR, 0.69; 95% CI, 0.60–0.79) than for those HCC patients without a history of pre-diagnosis statin use (HR, 0.87; 95% CI, 0.75–1.01). Similar results were seen for associations with all-cause mortality (pre-diagnosis statin users, HR, 0.74; 95% CI, 0.67–0.82; pre-diagnosis statin nonusers, HR, 0.93; 95% CI, 0.84–1.03); p-interaction=0.259). The inverse association between post-diagnosis statin use and the risk of mortality was no different in HCC patients with (cancer specific mortality, HR, 0.80; 95% CI, 0.68–0.94) and without cirrhosis (cancer specific mortality, HR, 0.79; 95% CI, 0.69–0.90). Further sub-group analyses did not reveal any differences in the association between post-diagnosis statin use and mortality by cancer stage, treatment, or by HCC etiology or MELD score (Supplementary Table 3). We found no association between prediagnosis statin use and either cancer specific mortality or all-cause mortality.
We observed stronger inverse associations with cancer specific mortality (HR, 0.47; 95% CI, 0.43–0.52) and all-cause mortality (HR, 0.51; 95% CI, 0.47–0.54) when we required that users had ≥1 month of filled statin prescriptions (11.2% of HCC patients were classified as postdiagnosis statin users). As expected, the magnitudes of the inverse associations with cancer specific mortality (HR, 0.56; 95% CI, 0.51–0.62) and all-cause mortality (HR, 0.60; 95% CI, 0.560.64) were significantly stronger in naïve analyses using a Cox model without a time-varying covariate for statin use. Finally, the inverse associations between post-diagnosis statin use and cancer specific mortality and all-cause mortality were consistent across a range of lag periods (from 0 months to 12 months after HCC diagnosis; Table 4).
Table 4.
Mortality HRs for post-diagnosis statin use using varying lag times between date of cancer diagnosis and start of follow-up
| Post-diagnosis statin use | Number of Patients | Number of Deaths | Person-Years | Mortality rate (95% CI) (per 100 person-years) | Unadjusted HR (95% CI) | Adjusted HR (95% CI)* |
|---|---|---|---|---|---|---|
| Cancer specific mortality | ||||||
| No lag | 2884 | 1025 | 7282.59 | 14.07 (13.24–14.96) | 0.88 (0.83–0.94) | 0.97 (0.88–1.07) |
| 3-month lag | 2293 | 648 | 6262.37 | 10.35 (9.58–11.17) | 0.79 (0.72–0.85) | 0.85 (0.77–0.93) |
| 6-month lag | 1899 | 503 | 5463.74 | 9.21 (8.44–10.05) | 0.79 (0.72–0.87) | 0.82 (0.73–0.91) |
| 12-month lag | 1482 | 345 | 4310.63 | 8.00 (7.20–8.89) | 0.80 (0.72–0.90) | 0.83 (0.73–0.95) |
| All-cause mortality | ||||||
| No lag | 2284 | 2005 | 7282.59 | 27.53 (26.35–28.76) | 0.87 (0.83–0.91) | 0.91 (0.85–0.97) |
| 3-month lag | 2293 | 1463 | 6262.37 | 23.36 (22.19–24.59) | 0.84 (0.80–0.89) | 0.89 (0.83–0.95) |
| 6-month lag | 1899 | 1130 | 5463.74 | 20.68 (19.51–21.92) | 0.80 (0.75–0.85) | 0.81 (0.75–0.87) |
| 12-month lag | 1482 | 824 | 4310.63 | 19.12 (17.85–20.47) | 0.81 (0.75–0.87) | 0.81 (0.74–0.89) |
CI, confidence interval; HR, hazard ratio.
Adjusted for age, sex, race, BMI, alcohol abuse, smoking status, HCV infection, HBV infection, cirrhosis, NAFLD, stage, MELD score, APRI, ascites, hepatic encephalopathy, varices, Deyo comorbidity score, treatment (surgery, TACE, systemic chemotherapy, other), ECOG performance status, pre-diagnosis statin use, and post-diagnosis aspirin/NSAID use (time-varying).
DISCUSSION
Our findings suggest that statin use after cancer diagnosis is associated with lower mortality risk for patients with HCC. In the 3-month lagged analyses, we found that HCC patients who used statins after their cancer diagnosis date had 15% lower risk of cancer specific and 11% lower risk of all-cause mortality than HCC patients who did not use statins. The inverse association remained unchanged in a series of sensitivity analyses, including increasing the lag period to address the potential issue of selection bias. The possible protective effect inferred from the inverse association between statin use and cancer specific as well as overall mortality seems to be mostly related to post-HCC diagnosis statin use. While we found 11–15% lower risk of mortality for post-diagnosis statin users, there was no independent association between pre-diagnosis statin use and mortality risk in HCC patients.
Although a number of studies have shown a strong inverse relationship between use of statins and the risk of developing HCC, 14–17 there are limited data regarding the influence of postdiagnosis statin use on the risk of mortality in HCC patients. Consistent with our results, Joen et al. found an inverse relationship between post-diagnosis statin use and mortality when incorporating statin use in the model as a non-time-dependent variable.20 However, this disregard for time of statin initiation is known to introduce unintended bias and overestimation of survival advantage. Furthermore, reverse causality may occur if cancer patients are not prescribed medications because they are obviously close to death. These issues can be address by using a time-varying analytic approach and introducing a lag period, respectively. After incorporating in the model a time-dependent statin variable with a 2-month lag, Jeon et al. found no association between post-diagnosis statin use and mortality in HCC patients.20 Conversely, although the magnitude of the inverse association was attenuated, we observed a strong inverse relationship with mortality using a time-varying model and with a range of lag periods. It is not entirely clear why our results would differ from those of the only previous observational study; however, our results are consistent with that of the only randomized clinical trial to date.18 That study showed that patients with advanced HCC using pravastatin had 9month improvement in survival compared with patients in the placebo arm, and that tumor growth was slower in HCC patients treated with pravastatin.
Our current findings are biologically plausible since statins inhibit not only cholesterol synthesis but also reduce other important downstream products, including membrane integrity maintenance, cell signaling, protein synthesis, and cell-cycle progression.11–13 Not only can statins have a direct impact on cancer cells through inhibition of the mevalonate pathway within the cancer cells, but the reduction of circulating cholesterol levels through hepatic pathways is indeed considered important. Future studies that specifically examine these basic mechanisms are undoubtedly warranted.
This study has several limitations. First, the study could have missed statin prescriptions dispensed at non-VA pharmacies. However, the likelihood of this happening is low, as previous studies have shown that veterans who use the VA health care system tend to disproportionately or exclusively use VA pharmacy services.30 While drug adherence (e.g., where a patient did not adhere to statin therapy) may be misclassified, any misclassification would likely attenuate an association with statin use and result in underestimation of the ‘true’ effect. We used filled prescriptions, which is a more accurate measure of adherence as compared to overall prescriptions). Second, only 5.6% of HCC patients in our study used statins after cancer diagnosis without a history of statin use in the period 6 to 18 months prior to their diagnosis. Therefore, we may have been underpowered to detect an association between exclusive postdiagnosis statin use and mortality risk. Nonetheless, we still observed an inverse association with post-diagnosis statin use in HCC patients without a history of statin use prior to their cancer diagnosis. Third, this study included only veterans with HCC and the results may not be generalizable to the general population. Fourth, we relied on data recorded in the VACCR to determine cancer-specific death. However, we know from our prior validation study of HCC patients in the VA that almost all deaths observed in these patients are cancer-specific.4 In the case of highly fatal cancers like HCC, the associations with all-cause mortality are therefore a valid proxy for cancer-specific mortality. Fifth, overall, approximately half of HCC patients in our cohort were classified as being without cirrhosis. While this fraction is higher than expected, it is consistent with prior studies among HCC patients in the VA administrative databases.24 While we likely misclassified some patients as without cirrhosis when they actually had cirrhosis, it is unclear how this misclassification would have influenced our study results. Lastly, there were differences in baseline characteristics between statin users and statin non-users that could have confounded our results (e.g., post-diagnosis statin users were older and had earlier stage tumors). However, when adjusting for these confounders, a clear association between statin use and lower risk of mortality remained. However, residual confounding by poorly measured or unmeasured factors such clinical, laboratory or patient provider decisions could still have influenced our results.
This study has multiple strengths. We identified all possible newly diagnosed HCC cases using an expanded search strategy of automated data, which resulted in the largest study to date with over 15,000 HCC patients with long average follow-up time. Prospective prescription records within the pharmacy clinical national data extract of the VA CDW helped to avoid recall bias compared with self-reported medication use, and measurement error of drug exposures was likely to be minimal given the accuracy of prescription records in the VA. Another strength of our study was the completeness of our cancer staging data; which enabled investigation of stage as a confounder of the association with statin use. We found that the risk estimates for statin use were similar in models with and without adjustment for staging suggesting that confounding by reverse causation mediated by cancer stage is unlikely to explain the observed association. We also adjusted for several other important confounders, including tumor grade and treatmentrelated factors, etiology (e.g., HCV infection), lifestyle factors, and other medication use (e.g., NSAID and aspirin use). Our analytic strategy using a time-varying exposure and introducing a lag period ensured that our findings were less likely to be affected by immortal-time bias and reverse causality. Furthermore, because statins may not be prescribed to patients with more advanced disease, we examined the association among various patients subgroups through stratified analyses. We found a consistent association between post-diagnosis statin use and 10–15% lower risk of HCC mortality.
In summary, among 15,422 HCC patients, when applying a methodological approach that minimizes the likelihood of selection bias and immortal-time bias, we found a strong and statistically significant inverse association between post-diagnosis statin use and risk of mortality.
Supplementary Material
What You Need to Know
Background:
Observational studies have consistently shown that use of statins is associated with lower risk of developing hepatocellular carcinoma (HCC). Whether statin use also improves survival in HCC patients is unclear.
Findings:
In this large retrospective cohort study among over 15,000 patients, use of statins after cancer diagnosis was associated with 10–15% lower risk of mortality in patients with HCC.
Implications for patient care:
Together with data from prior studies of HCC risk, our findings from an observational study indicate that use of statins not only reduce the risk of developing HCC in the first place, but also improve outcomes after cancer diagnosis.
Acknowledgments
Grant support: This work was supported in part by National Institutes of Health grant P30 DK056338 (Study Design and Clinical Research Core), which supports the Texas Medical Center Digestive Diseases Center. HES is supported by NIDDK K24–04-107. This research was supported in part with resources at the VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (#CIN 13–413), at the Michael E. DeBakey VA Medical Center, Houston, TX. The opinions expressed reflect those of the authors and not necessarily those of the Department of Veterans Affairs, the US government or Baylor College of Medicine.
Footnotes
Potential competing interests: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer 2016;122:1312–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White DL, Thrift AP, Kanwal F, et al. Incidence of Hepatocellular Carcinoma in All 50 United States, From 2000 Through 2012. Gastroenterology 2017;152:812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301–1314. [DOI] [PubMed] [Google Scholar]
- 4.Mittal S, Sada YH, El-Serag HB, et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol. 2015;13:594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Federico A, Orditura M, Cotticelli G, et al. Safety and efficacy of sorafenib in patients with advanced hepatocellular carcinoma and Child-Pugh A or B cirrhosis. Oncol Lett 2015;9:1628–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardwell CR, Hicks BM, Hughes C, et al. Statin use after colorectal cancer diagnosis and survival: a population-based cohort study. J Clin Oncol 2014;32:3177–83. [DOI] [PubMed] [Google Scholar]
- 7.Voorneveld PW, Reimers MS, Bastiaannet E, et al. Statin Use After Diagnosis of Colon Cancer and Patient Survival. Gastroenterology 2017;153:470–479. [DOI] [PubMed] [Google Scholar]
- 8.Ahern TP, Pedersen L, Tarp M, et al. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J Natl Cancer Inst 2011;103:1461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexandre L, Clark AB, Bhutta HY, et al. Association Between Statin Use After Diagnosis of Esophageal Cancer and Survival: A Population-Based Cohort Study. Gastroenterology 2016;150:854–865. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen T, Khan A, Liu Y, et al. The Association Between Statin Use After Diagnosis and Mortality Risk in Patients With Esophageal Cancer: A Retrospective Cohort Study of United States Veterans. Am J Gastroenterol 2018. [DOI] [PubMed] [Google Scholar]
- 11.Dimitroulakos J, Marhin WH, Tokunaga J, et al. Microarray and biochemical analysis of lovastatin-induced apoptosis of squamous cell carcinomas. Neoplasia 2002;4:337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park HJ, Kong D, Iruela-Arispe L, et al. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors interfere with angiogenesis by inhibiting the geranylgeranylation of RhoA. Circ Res 2002;91:143–150. [DOI] [PubMed] [Google Scholar]
- 13.Kusama T, Mukai M, Iwasaki T, et al. 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors reduce human pancreatic cancer cell invasion and metastasis. Gastroenterology 2002;122:308–317. [DOI] [PubMed] [Google Scholar]
- 14.Singh S, Singh PP, Singh AG, et al. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology 2013;144:323–32. [DOI] [PubMed] [Google Scholar]
- 15.Pradelli D, Soranna D, Scotti L, et al. Statins and primary liver cancer: a meta-analysis of observational studies. Eur J Cancer Prev 2013;22:229–34. [DOI] [PubMed] [Google Scholar]
- 16.Simon TG, Bonilla H, Yan P, et al. Atorvastatin and fluvastatin are associated with dosedependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: Results from ERCHIVES. Hepatology 2016;64:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGlynn KA, Hagberg K, Chen J, et al. Statin use and risk of primary liver cancer in the Clinical Practice Research Datalink. J Natl Cancer Inst 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawata S, Yamasaki E, Nagase T, et al. Effect of pravastatin on survival in patients with advanced hepatocellular carcinoma. A randomized controlled trial. Br J Cancer 2001;84:886–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graf H, Jungst C, Straub G, et al. Chemoembolization combined with pravastatin improves survival in patients with hepatocellular carcinoma. Digestion 2008;78:34–8. [DOI] [PubMed] [Google Scholar]
- 20.Jeon CY, Goodman MT, Cook-Wiens G, et al. Statin Use and Survival with Early-Stage Hepatocellular Carcinoma. Cancer Epidemiol Biomarkers Prev 2016;25:686–92. [DOI] [PubMed] [Google Scholar]
- 21.Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol 2010;28:3176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med 2012;177:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohn MW, Arnold N, Maynard C, et al. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr 2006;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davila JA, Duan Z, McGlynn KA, El-Serag HB. Utilization and outcomes of palliative therapy for hepatocellular carcinoma: a population-based study in the United States. J Clin Gastroenterol 2012; 46:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33:464–70. [DOI] [PubMed] [Google Scholar]
- 26.Levesque LE, Hanley JA, Kezouh A, et al. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 2010;340:b5087. [DOI] [PubMed] [Google Scholar]
- 27.Targownik LE, Suissa S. Understanding and Avoiding Immortal-Time Bias in Gastrointestinal Observational Research. Am J Gastroenterol 2015;110:1647–50. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Z, Rahme E, Abrahamowicz M, et al. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: a comparison of methods. Am J Epidemiol 2005;162:1016–23. [DOI] [PubMed] [Google Scholar]
- 29.Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health 1999;20:145–57. [DOI] [PubMed] [Google Scholar]
- 30.Morgan RO, Petersen LA, Hasche JC, et al. VHA pharmacy use in veterans with Medicare drug coverage. Am J Manag Care 2009;15:e1–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.