Abstract
The dual occurrence of Pseudomonas‐like and Wolbachia endobacteria has not been investigated in the Pederus beetles yet. We investigated pederin‐producing bacteria (PPB) infection in Paederus fuscipes specimens from the southern margins of the Caspian Sea by designed genus‐specific (OprF) and species‐specific (16S rRNA) primers. Wolbachia infection was studied through a nested‐PCR assay of Wolbachia surface protein (wsp) gene. Of the 125 analyzed beetles, 42 females (82.35%) and 15 males (20.27%) were positive to PPB infection; this is the first study reporting male P. fuscipes infection to PPB. Wolbachia infection was found in 45 female (88.23%) and 50 male (67.57%) analyzed beetles. Surprisingly, a number of 36 females (70.59%) and 13 males (17.57%) were found to be infected with both PPB and Wolbachia endosymbionts. In general, population infection rates to PPB and Wolbachia were determined to be 45.6% and 76%, respectively. The infection rates of female beetles to PPB and PPB‐Wolbachia were significantly higher than males. In Paederus species, only female beetles shelter PPB and the discovery of this bacterium in adult males may reflect their cannibalistic behavior on the contaminated stages. Phylogenetic analysis showed that the sequences of OprF gene were unique among Pseudomonas spp.; however, sequences of 16S rRNA gene were related to the PPB of Pederus species. The co‐occurrence and random distribution of these endobacteria may imply putative tripartite interactions among PPB, Wolbachia, and Paederus. In order to elucidate these possible tripartite interactions, further studies are required even at gender level.
Keywords: 16S rRNA, OprF, Paederus, pederin, Pseudomonas‐like, wsp
1. INTRODUCTION
Rove beetles of Staphylinidae are the largest family of beetles and are distributed in a wide range of habitats. They include more than 63,000 known species arranged into thousands of genera and 32 subfamilies worldwide (Grebennikov & Newton, 2009; Thayer, 2005). The genus Paederus Fabricius, 1775, which is classified into the tribe Paederini and subfamily Paederinae, currently comprises ~490 species (Nikbakhtzadeh, Naderi, & Safa, 2012; Vieira, Ribeiro‐Costa, & Caron, 2014).
Fourteen species and subspecies of the Paederus, including five subgenera occur in Iran. Among them, six species P. balachowskyi, P. balcanicus, P. duplex, P. fuscipes fuscipes, P. littoralis ilsae, and P. riparius are present in three Southern Caspian Provinces, Gilan, Mazandaran, and Golestan (Nikbakhtzadeh et al., 2012).
In natural ecosystems (predominantly moist environments), staphylinidaes are connected with various arthropods, higher plants, fungi, decomposing materials, mollusks, and vertebrates. Most of the rove beetles are predators of arthropods, and some of them are associated with social insects, while others are scavengers on decaying plant matter or live in nests of rodents (Frank & Thomas, 2016). Some species of rove beetles are important in terms of the biological control of insects of agricultural, medical, and veterinary importance (Echegaray & Cloyd, 2013).
Paederus species and its relatives are the agents of human dermatitis as well. They are active during daylight hours and can cause linear dermatitis on human skin and severe damage to human eyes (Nairobi eye). These beetles neither bite nor sting but release their hemolymph containing pederin, a potent vesicant toxin (C25H45NO9; MM: 503.63; LD50: 0.14 mg/kg rat i.p.), when they injured or crushed on human skin (Dettner, 2011; Iserson & Walton, 2012). This contact dermatitis is a distinctive stimulus form that can be distinguished by the rapid onset of erythematobullous lesions on the exposed areas (Mammino, 2011). If erythemas continue longer, other symptoms such as fever, edema, neuralgia, arthralgia, and vomiting may be observed as well (Rahman, 2006). It is supposed that pederin has antitumor and antiviral properties (Narquizian & Kocienski, 2000), presumably through the inhibition of DNA replication and protein synthesis (Dettner, 2011).
Pederin and its derivatives, namely, pseudoephedrine and pederone, are synthesized by uncultured Pseudomonas‐like endosymbionts located in the female accessory glands, restored in the hemolymph and transferred to the developmental stages through the contaminated eggs (Kellner, 1998, 2001; Kellner & Dettner, 1995). Studies based on 16S rRNA gene have shown that only female beetles contain the pederin‐producing bacteria (PPB; Kellner, 2002). These bacteria are distributed in the rove beetle populations, through the transovarial transmission (Kador, Horn, & Dettner, 2011).
Naturally, pederin is used by Paederus species as a defensive compound against insect and arachnid predators (Kellner & Dettner, 1996). The immature stages of P. fuscipes and P. riparius, which were pederin positive, were repulsive for spiders of the families Lycosidae and Salticidae but not for insect predators (Kellner & Dettner, 1996).
Wolbachia, obligate endosymbionts, are estimated to infect 40% of terrestrial arthropod species (Zug & Hammerstein, 2012) and many parasitic filarial nematodes (Taylor, Bandi, & Hoerauf, 2005). They manipulate reproduction properties of the hosts through the induction of cytoplasmic incompatibility, parthenogenesis, feminization, and male killing (Hughes, Pamilo, & Kathirithamby, 2004; Li et al., 2016; Li, Wang, Bourguet, & He, 2013; Vavre, Fleury, Lepetit, Fouillet, & Boulétreau, 1999; Werren, 1997; Yun, Peng, Liu, & Lei, 2011).
Wolbachia strains and their role in arthropod host fitness have been reviewed recently (Zug & Hammerstein, 2015). It has been indicated that a Wolbachia strain could protect alfalfa weevil, Hypera postica, against the parasitic wasp, Microctonus aethiopoides (Hsiao, 1996). Recently, it has been shown that Wolbachia can protect Culex pipiens mosquitoes against Plasmodium relictum‐induced mortality (Zele, Nicot, Duron, & Rivero, 2012). In addition, a new strain of Wolbachia has been reported in Cimex lectularius that appears to display an important role in bedbug fitness through provisioning of B vitamins (Nikoh et al., 2014). More recently, some strains of Wolbachia have been introduced as a weapon in the war against vector‐borne pathogens (Hughes & Rasgon, 2014; Kambris, Cook, Phuc, & Sinkins, 2009). Therefore, a variety of Wolbachia strains can have either mutualistic or parasitic outcomes in the insect/pathogens/parasitoids assemblages (van Nouhuys, Kohonen, & Duplouy, 2016), which should be studied in details when their properties are exploited.
Initially, insect's isolates of Wolbachia pipientis has been classified into two supergroups (A and B) and 12 groups based on the sequences of the major Wolbachia surface protein (wsp) gene (Zhou, Rousset, & O'Neill, 1998). Today, all invertebrate isolates of Wolbachia have been divided sequentially into 16 supergroups (A to Q) using the multilocus sequence typing (MLST) technique (Baldo et al., 2006; Glowska, Dragun‐Damian, Dabert, & Gerth, 2015).
Despite many advances in the study on Wolbachia infection in insects, our knowledge on the Wolbachia strain diversity/dispersion, or their effects on the beetle hosts is very limited. According to the findings of a review study (Kajtoch & Kotásková, 2018), Wolbachia infection was detected in 204 coleopteran species with average prevalence of 38.3%. The most intensively studied families have been herbivorous beetles of Curculionidae and Chrysomelidae. Coleoptera‐infecting Wolbachia strains belonged to three supergroups of A, B, and F with single, double, or multiple infections in the studied species. Wolbachia has had a lot of effects on its beetle hosts ranging from selective sweep with host mtDNA and cytoplasmic incompatibility to other changes related to the reproductive or developmental phenotypes (Kajtoch & Kotásková, 2018).
Survival and reproduction of many insects rely on the endosymbiotic bacteria (Eleftherianos, Atri, Accetta, & Castillo, 2013; Ratzka, Gross, & Feldhaar, 2012). Therefore, PPB as defensive (Oliver & Moran, 2009) and Wolbachia as reproductive (Kajtoch & Kotásková, 2018) symbionts may play an important role in evolution and adaptation of Paederus species. As a matter of fact, PPB seems to affect the capacity of the Paederus beetles to be causative agent of human linear dermatitis. It is also necessary to study the distribution of Wolbachia in the rove beetles to determine its function in host biology. Infection of Paederus species by PPB and Wolbachia has separately been investigated in a very few studies (Kador et al., 2011; Yun et al., 2011); however, dual occurring of these endobacteria has not been investigated yet. Hence, we studied co‐occurrence of PPB and Wolbachia in P. fuscipes. The achieved results can contribute to pave the way to address interesting open queries on the evolutionary consequences of the interactions between these inherited bacteria and their host biology with further experiments.
2. EXPERIMENTAL PROCEDURES
2.1. Study areas
The specimens were collected from nine locations of two provinces of southern coast of Caspian Sea in Iran, Gilan (Bijar Boneh [n = 6], Vashmeh Sara [n = 38], Kochesfehan [n = 10], Chini Jan [n = 8], Kalachai [n = 1], and Tajan Gukeh [n = 40]) and Mazandaran (Royan [n = 6], Shirud [n = 5], and Amol [n = 11]). Live adult beetles were gathered from humid areas, principally from rice fields, using hand collection method. The specimens were kept in 70% ethanol in 4°C refrigerator until experiments.
2.2. Morphological studies
The specimens were morphologically identified using available identification keys generated by Blackwelder (1957), Arnett and Thomas (2001), and Borror and DeLong's (Triplehorn & Johnson, 2005).
2.3. DNA extraction
Prior to molecular survey, to surface sterilize, the specimens were immersed twice in freshly prepared 70% ethanol for 2 min and rinsed vigorously with 0.9% normal saline. The whole bodies of adult beetles were homogenized in the DNA lysis buffer using sterile pestles. Genomic DNA of rove beetles was extracted using Collins DNA extraction method (Collins et al., 1987).
2.4. Detection of PPB infection
2.4.1. OprF primer designing and amplification
The major outer membrane protein of Pseudomonas, OprF, has been found only in Pseudomonas genus and considered as a diagnostic protein in Pseudomonas sensu stricto (Bodilis & Barray, 2006; Bouffartigues et al., 2011). A total of 44 sequences of OprF gene related to Pseudomonas isolates were extracted from the GenBank and aligned using Mega 5.0 software. The conserved regions among all Pseudomonas isolates were targeted to design genus‐specific primers. Two primers, OPRFF: 5'‐GTGGA(A/G)GTGGACGGGTACTGCTTCATG‐3' and OPRFR: 5'‐CAACGGTCACCAGGGCGAGTGGATG‐3', were designed based on the OprF‐specific sequences to amplify 327 bp of Pseudomonas spp. and PPB in the rove beetles. PCRs were done in a volume of 20 μl containing 5 pmol of each designed primer (Macrogen, Korea), 0.5 nmol dNTPs (Fermentas, USA), 1 U Taq DNA polymerase (CinnaGen, Iran), 2.5 μl buffer 10×, and 1–5 μl (~0.1 μg) of the extracted DNA from samples. The PCR thermal profile used with these primers was an initial denaturation at 95°C for 5 min, followed by 35 cycles of 94°C for 30 s, 66°C for 30 s, 72°C for 25 s, and a final extension step at 72°C for 10 min. All specimens were firstly screened with OprF gene, and then positive ones were examined via 16S rRNA gene.
2.4.2. 16S rRNA primer designing and amplification
Five available 16S rRNA sequences of PPB in rove beetles (P. fuscipes [AJ316016], P. riparius [AJ316018], P. melanurus [AJ316017], P. ruficollis [AJ316019], and P. sabaeus [AJ295331]) and five representative 16S rRNA sequences of other bacteria (Pseudomonas aeruginosa [AE004844], Escherichia fergusonii [NR_074902], Salmonella enteric [NR_119108], Klebsiella pneumoniae [NR_117686], and Proteus mirabilis [NR_114419]) were retrieved from the GenBank and subjected to PPB species‐specific primer designing. After alignment, 16S‐PPBF: 5'‐ACCGCATACGTCCTAAGGGAG‐3' and 16S‐PPBR: 5'‐CCTCCTTGCGGTTAGACCAG‐3’ primers were designed based on the 16S rRNA‐specific sequences of PPB in rove beetles to amplify a 1,265‐bp fragment of this gene. PCRs were the same as those performed for OprF primers. After an initial denaturation step of 5 min at 94°C, 35 cycles were carried out (denaturation for 30 s at 94°C, annealing for 30 s at 59°C, and elongation for 80 s at 72°C), followed by 10 min at 72°C.
2.5. Detection of Wolbachia infection
Wolbachia infection was detected in rove beetles on the basis of Zhou et al.'s, (1998) introduced primers and through a nested‐PCR assay recruited by Karami et al., (2016). Originally, primers of 81F: 5'‐TGGTCCAATAAGTGATGAAGAAAC‐3' and 691R: 5'‐ AAAAATTAAACGCTACTCCA‐3' were applied to amplify a 632‐bp of partial sequence of the wsp gene. The PCR product of the first step was employed as a template for the second step. In this step, the primers of 691R and 183F: 5'‐AAGGAACCGAAGTTCATG‐3' were used to amplify a 501‐bp fragment. The PCR was performed in a total volume of 20 μl containing 5 μl (~0.5 μg) of genomic DNA for the first step, and 2.5 μl of PCR product for the second step of nested‐PCR, one‐time PCR buffer, 2.5 U Taq polymerase (CinnaGen, Iran), 1 μl of each primer (20 mM, Macrogen, Korea), 200 μM of each dATP, dTTP, dCTP, and dGTP (Fermentas, USA) and 1.5 mM of MgCl2 in an automated Thermocycler (Analytik Jena FlexCycler, Canada). The PCR conditions were set as an initial denaturation at 95°C for 5 min, followed by 35 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1 min, followed by a final extension at 72°C for 7 min.
2.6. Sequencing and phylogenetic analysis
All the PCR products from 16s rRNA, OprF, and wsp genes were analyzed by 1% agarose gel electrophoresis, followed by Green Viewer staining and visualization using a UV transilluminator. Amplicons of the representative specimens were extracted from the gel, and after purification was sequenced bidirectionally via the same amplification primers (Macrogen Company, Korea).
The raw sequences were initially edited by the Chromas 2.6.5 software through trimming of right and left cut‐off regions that may contain poor qualities. The consensus of confident sequences was analyzed using NCBI (nucleotide collection) database. Multiple alignments of the studied sequences were generated by the Clustal Omega package (Sievers et al., 2011). BLOSUM62 and Kimura‐2‐Parameter models were used to, respectively, score the pairs of aligned OprF/wsp amino acids and 16S rRNA nucleotides. Phylogenetic trees were constructed using the maximum likelihood method embedded in Mega 5 software (Tamura et al., 2011). Confidence of internal nodes was tested by Bootstrap test with 1,000 replications.
The phylogeny of various Pseudomonas spp., including PPB, was evaluated based on the OprF gene sequences. The relationships between 16S rRNA gene sequences of PPB in Paederus specimens and their close relative, Pseudomonas aeruginosa, was investigated through the phylogenetic tree construction.
2.7. Statistical analysis
SPSS 20 for windows (SPSS Inc., USA) was used for statistical analysis. Differences between the proportions of subjects positive for each one of the Wolbachia and BBP bacteria or their combination in females and males were assessed using Chi‐square (χ2) test. p values <0.05 were considered statistically significant.
3. RESULTS
3.1. Morphological study
In this research, a total of 125 adult rove beetles, including 74 males and 51 females, were studied. All the collected specimens were taxonomically identified as Paederus fuscipes Curtis, 1840 (Coleoptera: Staphilinidae) by using the morphological keys mentioned in the Experimental Procedures.
3.2. Detection of PPB and Wolbachia infection in P. fuscipes
Prior to practical procedures, the specificity of designed primers was tested in silico. Performing BLAST searches showed that OprF primers were able to find cultured and uncultured Pseudomonas spp., which is in accordance with the desired specificity we expect for our study to identify Pseudomonas and Pseudomonas‐like species, but not the other genera. Also, the 16S rRNA primers could amplify only PPB endosymbiont of P. fuscipes, and it did not even reproduce symbionts which were present in the GenBank other than P. fuscipes.
In practice, both Pseudomonas‐specific (OprF) and PPB‐specific (16S rRNA) primers resulted in amplicon sizes of 327 and 1,265 bp, respectively, as expected. Applying the nested‐PCR assay could easily detect the wsp, a single‐copy gene. The PCR products of the first and the second steps of nested‐PCR assay were roughly 650 and 500 bp, respectively.
3.3. PPB and Wolbachia infection rates in P. fuscipes
The designed OprF primers could amplify all Pseudomonas species, including Pseudomonas‐like PPB and P. aeruginosa (Figure 1). However, the species‐specific 16S rRNA primers that we designed could identify only Pseudomonas‐like PPB (Figure 2). In total, of the 125 (51 female and 74 male) analyzed beetles, 42 females (82.35%) and 15 males (20.27%) were positive to OprF primers and the same rates (82.35% and 20.27%) were also positive to the PPB‐specific 16S rRNA primers. PPB was detected not only in female beetles (as reported by Kellner, 2002) but also in male beetles. This is the first study reporting male P. fuscipes infection to PPB. Also, Wolbachia infection was found in 45 female (88.23%) and 50 male (67.57%) analyzed beetles. Surprisingly, a number of 36 females (70.59%) and 13 males (17.57%) were detected to be infected with both PPB and Wolbachia endosymbionts. Individual analysis of bacteria showed that six females (11.76%) and two males (2.7%) were PPB positive and nine females (17.65%) and 37 males (50%) were positive for wsp gene.
Figure 1.
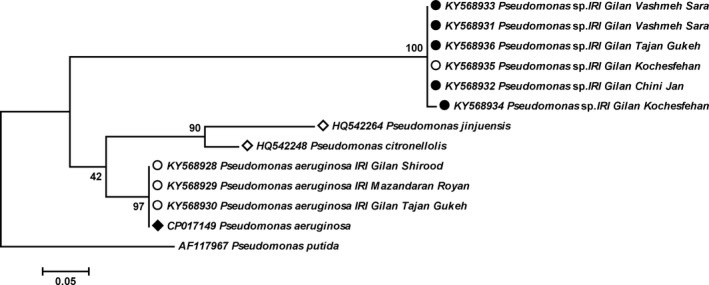
Maximum likelihood tree showing the phylogenetic relationships between the OprF gene sequences obtained in this study (solid/empty circles) and other isolates of Pseudomonas spp. Solid and empty circles: bacterial genome amplified from female and male Paederus fuscipes, respectively; solid diamond: clinical isolate of Pseudomonas spp.; empty diamonds: environmental isolate of Pseudomonas spp. Pseudomonas putida was designated as outgroup. The numbers at the branch points are bootstrap values based on 1,000 replicates
Figure 2.

Maximum likelihood tree showing the phylogenetic relationships among pederin‐producing bacteria (PPB) in Paederus spp. based on 16S rRNA gene sequences. Sequences obtained in this study are shown by solid circles. Pseudomonas aeruginosa was set as outgroup. The numbers at the branch points are bootstrap values based on 1,000 replicates
The Chi‐squared test showed no significant difference (p = 0.13) of Wolbachia infection among male and female beetles, either alone or in combination with Pseudomonas. The infection rates of females to PPB and PPB‐Wolbachia were significantly higher than males in both alone and combined analyses (χ2, p < 0.05). Combined analysis showed that Wolbachia infection rate in females was more than males; however, this difference was not significant (χ2, p = 0.015). Overall, our results pointed out that 45.6% and 76% of all the specimens were positive to PPB and Wolbachia endosymbionts, respectively. The infection results in both alone and combined analyses are depicted in Table 1.
Table 1.
Prevalence of PPB and Wolbachia infection in the Paederus fuscipes specimens collected from nine locations of two Northern provinces of Iran during 2016
| Endosymbiont beetle gender | Alone | Combined | |||
|---|---|---|---|---|---|
| PPB (%) | Wolbachia (%) | PPB‐Wolbachia (%) | PPB (%) | Wolbachia (%) | |
| Male | 2 (2.7) | 37 (50) | 13 (17.57) | 15 (20.27) | 50 (67.57) |
| Female | 6 (11.76) | 9 (17.65) | 36 (70.59) | 42 (82.35) | 45 (88.23) |
| Total | 8 (6.4) | 46 (36.8) | 49 (39.2) | 57 (45.6) | 95 (76) |
3.4. Sequence and phylogenetic analyses
Sequence analysis of OprF gene revealed the presence of two phylogenetically diverse groups in both male and female rove beetles; the first group of PPB sequences had 78% similarity to P. jinjuensis and P. citronellolis, and the second group of sequences was 100% identical to P. aeroginosa (Figure 1). Phylogenetic analysis showed that the sequences of OprF gene are unique among Pseudomonas spp.; however, the sequences of 16S rRNA gene were related to the PPB of Pederus species.
Comparative 16S rRNA gene sequence analysis showed that some specimens from Gilan (KY568938 & KY568939) and Mazandaran (KY568940) Provinces were 100% identical to each other. Nevertheless, there were minor differences between the samples from Gilan Province (KY568941 with 4 and KY568937 with 2 substitutions). In general, phylogenetic analysis of 16S rRNA gene from P. fuscipes specimens indicated that along with a sequence of the same species from Germany, the sequences of this study made a monophyletic clade were located as the sister clade of the sequences from P. ruficollis (France) and P. sabaeus (Cameroon; Figure 2).
The wsp gene sequence analysis displayed that all Wolbachia strains, obtained from the collected P. fuscipes in the study areas, were 100% identical to each other. In addition, the results of the BLAST search indicated that these strains were fully similar to the wsp sequence of Aedes albopictus [AF020059], Drosophila simulans [AF020069 and AF020074], Culex pipiens [AF020061], and Lasioderma serricorne [AB469359], the members of the Pip group of supergroup B.
3.5. Nucleotide sequence accession numbers
The nucleotide sequences determined in this study have been deposited into the GenBank database under the following accession numbers; OprF: KY568928–KY568936, 16s rRNA: KY568937–KY568941, and wsp gene: KY555600–KY555603. The representatives of each sequences group were applied to phylogenetic analysis (Figures 1 and 2).
4. DISCUSSION
We studied dual occurrence of PPB and Wolbachia endobacteria in P. fuscipes rove beetles. The overall population infection rates to PPB and Wolbachia endosymbionts were revealed to be 45.6% and 76%, respectively. The PPB infection has previously been reported only in adult females (Kellner, 2002); however, here, we report the infection not only in females but also in male specimens. Detection of PPB in male beetles does not necessarily mean the existence of pederin substance in the male beetles. The PPB infection in females was found to be four times that of males (Table 1). These results are rational because the female Paederus have to transmit PPB to offspring and protect them against both conspecific and other natural predators. Finding the PPB infection in adult males may be reflecting the cannibalistic behavior of the rove beetles, in part.
In this study, for the first time, PPB was detected at the genus and species levels, respectively, by OprF and 16S rRNA primers. The outcoming results from both genus and species levels were in agreement with the detection of PPB. Initially, the specimens were screened with OprF gene (copy numbers ≃ 200,000 per bacterial genome [Hancock, Siehnel, & Martin, 1990]), and then positive specimens were examined via 16S rRNA gene with copy numbers 1–15 per bacterial genome (Rainey, Ward‐Rainey, Janssen, & Hippe, 1996). The OprF is a protein that not only has widely been studied in vaccine researches (Rawling, Martin, & Hancock, 1995) but also considered as a diagnostic protein for Pseudomonas spp. (Bouffartigues et al., 2011). Our designed OprF primers could amplify both Pseudomonas‐like PPB and P. aeruginosa (Figure 1). The P. aeruginosa is extensively distributed in the environment and can be both opportunistic and pathogenic microbial agent of plants, animals, and humans (Balcht & Smith, 1994). It has frequently been isolated from medical and nonmedical insects (Bulla, Rhodes, & St. Julian, G., 1975; Maleki‐Ravasan et al., 2015; Mitscherlich & Marth, 1984). Pseudomonas strains found in insects have been shown to protect host against toxic compounds in some cases (e.g., Ceja‐Navarro et al., 2015); however, they display pathogenic characteristics in general (Vega & Kaya, 2012). The role of P. fuscipes originating Pseudomonas strains needs to be disclosed in future studies. Our designed species‐specific 16S rRNA primers could identify only Pseudomonas‐like PPB (Figure 2), an advantage that will be useful for the determination of PPB circulation pattern in the life cycle of Paederus beetles.
To raise the sensitivity and specificity of Wolbachia DNA amplification, we used a nested‐PCR assay (Karami et al., 2016). Generally, in many specimens, PCR products of the first step were positive; however, in a few cases, the density of Wolbachia indeed was so low (as indicated by Arthofer, Riegler, Avtzis, & Stauffer, 2009) that we have to perform the second step. The use of other techniques, including high‐quality polymerases, amplicon detection via DNA probes (Arthofer, Riegler, Schneider, et al., 2009) or high‐throughput sequencing methods (NGS), is recommended. The frequency of Wolbachia in 128 species of beetles belonging to seven families of Buprestidae, Hydraenidae, Dytiscidae, Hydrophilidae, Gyrinidae, Haliplidae, and Noteridae showed to be 31% (Sontowski, Bernhard, Bleidorn, Schlegel, & Gerth, 2015). Oliveira et al. (2015) used three markers (16S rRNA, wsp, and ftsZ) to screen a broad range of Brazilian insect species and found Wolbachia infection in 13% (n = 25) of the studied coleopterans (Oliveira et al., 2015). Infection of P. fuscipes by Wolbachia strains was originally reported by Yun et al., (2011). They did not track the prevalence of Wolbachia infection in the rove beetles but provided evidence for indirect horizontal transmission of Wolbachia between predators and preys (Yun et al., 2011). In the present study, Wolbachia (combined) infection rate in female and male specimens was 88.23% and 67.57%, respectively (χ2, p = 0.015). This difference is remarkable as the infection rates are in accordance with other studied insects including mosquitoes (Karami et al., 2016), and the fact is that no study has already been compared Wolbachia infection rates in the male and female beetles.
Herein, the phylogeny of P. fuscipes‐infecting Wolbachia was not investigated; nonetheless, they were previously classified in the supergroup B, based on the 16S rRNA and wsp markers (Yun et al., 2011). MLST data are needed to determine their exact position among 16 supergroups.
Surprisingly, the coinfection rates of both PPB and Wolbachia were 70.59% in females and 17.57% in males. The frequency of both bacteria in females was four times that of males (χ2, p < 0.0001). This co‐occurrence may imply putative interactions among these endosymbionts.
Our results highlighted the coexistence of PPB (as defensive) and Wolbachia (as reproductive) secondary endosymbionts not only in females but also in males of P. fuscipes. These bacteria will potentially interact with the host beetle and with each other as well. As defined in defensive symbiosis, the symbionts protect their host against hostile agents, including pathogens, parasites, parasitoids, or predators by the production of diverse metabolites, antimicrobial compounds, or toxins (Flórez, Biedermann, Engl, & Kaltenpoth, 2015). Defensive compounds such as pederin, piericidin, streptochlorin, and diaphorin have been characterized from bacterial symbionts of diverse insects (Beemelmanns, Gio, Rischer, & Poulsen, 2016). Although pederin can protect Paederus species from predation by natural enemies (Kellner & Dettner, 1995, 1996), its protective role against parasitoid wasps or entomopathogenic nematodes has not been inspected (Oliver & Moran, 2009). Also, the effects of Wolbachia infection on the life history of Paederus spp. are unclear. The reproductive phenotypes caused by Wolbachia in the P. fuscipes will need to be determined in the future surveys.
Given the transovarial transmission of Wolbachia as well as its relation to the reproductive phenotypes, the attention of researchers on Wolbachia infections should be drawn to the reproductive tissues. Dobson et al. (1999) have conversely demonstrated that Wolbachia infections not only are distributed in germ line but also are present throughout insect somatic tissues. They have also reported that the distribution of Wolbachia in somatic tissues is varied between different Wolbachia/host associations (Dobson et al., 1999). Distribution of Wolbachia in the somatic and reproductive tissues of Paederus species needs to be determined in future.
The interaction between the PPB and Wolbachia has not been studied in any case. However, the asymmetrical interaction of Wolbachia and Spiroplasma endosymbionts had been indicated in the Drosophila melanogaster by Goto, Anbutsu, and Fukatsu (2006) who showed that Wolbachia could not affect the population of Spiroplasma, while Spiroplasma could negatively restrict the population of Wolbachia. Remarkably, they could not detect Wolbachia from the fly hemolymph, the principal location of Spiroplasma (Goto et al., 2006). Insect hemolymph is an operational area for innate immune responses where the phenol oxidase cascade factors, antimicrobial peptides, phagocytosis, and encapsulation of exotic agents are produced by hemocytes (Lavine & Strand, 2002; Naitza & Ligoxygakis, 2004; Theopold, Li, Fabbri, Scherfer, & Schmidt, 2002). In Paederus beetles, the addition of pederin toxin to the hostile environment of the hemolymph may render the condition more difficult for dwelling microorganisms, requiring further investigation.
Our results reported more frequency of both bacteria in females than that of males (χ2, p < 0.0001). This observation may indicate tripartite interactions among Paederus, Wolbachia, and PPB. Recently, it has been proposed that the nature of the interaction between the insect host and Wolbachia bacterium is parasitic or mutualistic, and the induction/inhibition of reactive oxygen species would be an essential player in the new and native hosts (Zug & Hammerstein, 2015). The nature of Paederus–Wolbachia interaction is not known and requires being determined in upcoming studies. Moreover, it has previously been reported that antimicrobial peptides keep the insect's endosymbionts under governor (Login et al., 2011). It is unclear whether the PPB regulates the population of Wolbachia via pederin or not. Hence, co‐occurrence of Wolbachia and PPB in rove beetles may infer that Wolbachia is adapted to cope with adverse conditions triggered by PPB. Numerous Wolbachia strains have already been found in beetle's eggs containing antimicrobially active components (Pankewitz, Zollmer, Hilker, & Graser, 2007). Thus, it seems that these kinds of adaptations are common features among the Wolbachia strains. As a conclusion, on the side of symbiosis, PPB and Wolbachia may interact with each other and Paederus beetles, while on the side of insect host, Paederus beetles exploit these defensive and reproductive symbionts to warrant their fitness in the environment. Details and nature of these interactions (even at gender level) call for further investigation and testing.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS CONTRIBUTION
NDD, NMR, and AAR conceived and designed the experiments. NMR and MJ collected the samples. NA performed the molecular experiments. NMR wrote the paper. NMR and AAR went through bioinformatics analyses. NMR and NDD analyzed and interpreted total data. NMR, NDD,SZ, and AAR involved in critical revision of manuscript. NDD and SZ financially supported the research. All authors read, discussed the results, and contributed to the final version of manuscript.
ETHICS STATEMENT
None required.
ACKNOWLEDGEMENTS
The authors would like to give sincere acknowledgment to the MVRG staffs (especially J. Sani) in the national insectary of Iran. We also appreciate Dr A. Abouei and Dr. A. Karami for their kind assistance in statistical analysis.
Maleki‐Ravasan N, Akhavan N, Raz A, Jafari M, Zakeri S, Dinparast Djadid N. Co‐occurrence of pederin‐producing and Wolbachia endobacteria in Paederus fuscipes Curtis, 1840 (Coleoptera: Staphilinidae) and its evolutionary consequences. MicrobiologyOpen. 2019;8:e777 10.1002/mbo3.777
DATA ACCESSIBILITY
All data are included in the main manuscript. Sequences were also have been deposited at the NCBI GenBank under accession number of KY568928–KY568936, KY568937–KY568941, and KY555600–KY555603.
REFERENCES
- Arnett, R. H. Jr , & Thomas, M. C. (Eds.) (2001). American beetles, Volume 1. Archostemata, Myxophaga, Adephaga, Polyphaga: Staphyliniformia. Boca Raton, FL: CRC Press. [Google Scholar]
- Arthofer, W. , Riegler, M. , Avtzis, D. N. , & Stauffer, C. (2009). Evidence for low‐titre infections in insect symbiosis: Wolbachia in the bark beetle Pityogenes chalcographus (Coleoptera, Scolytinae). Environmental Microbiology, 11, 1923–1933. [DOI] [PubMed] [Google Scholar]
- Arthofer, W. , Riegler, M. , Schneider, D. , Krammer, M. , Miller, W. J. , & Stauffer, C. (2009). Hidden Wolbachia diversity in field populations of the European cherry fruit fly, Rhagoletis cerasi (Diptera, Tephritidae). Molecular Ecology, 18, 3816–3830. [DOI] [PubMed] [Google Scholar]
- Balcht, A. , & Smith, R. (1994). Pseudomonas aeruginosa: Infections and treatment. Informa Health Care, 2, 83–84. [Google Scholar]
- Baldo, L. , Dunning Hotopp, J. C. , Jolley, K. A. , Bordenstein, S. R. , Biber, S. A. , Choudhury, R. R. , … Werren, J. H. (2006). Multilocus sequence typing system for the endosymbiont Wolbachia pipientis . Applied and Environment Microbiology, 72, 7098–7110. 10.1128/AEM.00731-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemelmanns, C. , Gio, H. , Rischer, M. , & Poulsen, M. (2016). Natural products from microbes associated with insects. Beilstein Journal of Organic Chemistry, 12, 314–327. 10.3762/bjoc.12.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwelder, R. E. (1957). Checklist of the coleopterous insects of Mexico, Central America, the West Indies and South America. Part 6. Bulletin of the United States National Museum, 185, 927–1492. [Google Scholar]
- Bodilis, J. , & Barray, S. (2006). Molecular evolution of the outer‐membrane protein gene (oprF) of Pseudomonas . Microbiology, 152, 1075–1088. [DOI] [PubMed] [Google Scholar]
- Bouffartigues, E. , Gicquel, G. , Bazire, A. , Fito‐Boncompte, L. , Taupin, L. , & Maillot, O. (2011). The major outer membrane protein Oprf is required for Rhamnolipid production in Pseudomonas aeruginosa . Journal of Bacteriology and Parasitology, 2, 118 10.4172/2155-9597.1000118 [DOI] [Google Scholar]
- Bulla, L. A. , Rhodes, R. A. , & St. Julian, G. (1975). Bacteria as insect pathogens. Annual Review of Microbiology, 29, 163–190. 10.1146/annurev.mi.29.100175.001115 [DOI] [PubMed] [Google Scholar]
- Ceja‐Navarro, J. A. , Vega, F. E. , Karaoz, U. , Zhao, H. , Jenkins, S. , Lim, H. C. , … Brodie, E. L. (2015). Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nature Communications, 6, 7618 10.1038/ncomms8618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, F. H. , Mendez, M. A. , Rasmussen, M. O. , Mehaffey, P. C. , Besansky, N. J. , & Finnerty, V. (1987). A ribosomal RNA gene probe differentiates member species of the Anopheles gambiae complex. American Journal of Tropical Medicine and Hygiene, 37, 37–41. 10.4269/ajtmh.1987.37.37 [DOI] [PubMed] [Google Scholar]
- de Oliveira, C. D. , Goncalves, D. S. , Baton, L. A. , Shimabukuro, P. H. , Carvalho, F. D. , & Moreira, L. A. (2015). Broader prevalence of Wolbachia in insects including potential human disease vectors. Bulletin of Entomological Research, 105, 305–315. 10.1017/S0007485315000085 [DOI] [PubMed] [Google Scholar]
- Dettner, K. (2011). Potential pharmaceuticals from insects and their co‐occurring microorganisms In Vilcinskas A. (Ed.), Insect biotechnology, series: Biologically‐inspired systems (Vol. 2, pp. 95–119). Dordrecht, The Netherlands: Springer. [Google Scholar]
- Dobson, S. L. , Bourtzis, K. , Braig, H. R. , Jones, B. F. , Zhou, W. , Rousset, F. , & O’Neill, S. L. (1999). Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochemistry and Molecular Biology, 29, 153–160. 10.1016/S0965-1748(98)00119-2 [DOI] [PubMed] [Google Scholar]
- Echegaray, E. R. , & Cloyd, R. A. (2013). Life history characteristics of the rove beetle, Dalotia coriaria (Coleoptera: Staphylinidae) under laboratory conditions. Journal of the Kansas Entomological Society, 86, 145–154. [Google Scholar]
- Eleftherianos, I. , Atri, J. , Accetta, J. , & Castillo, J. C. (2013). Endosymbiotic bacteria in insects: Guardians of the immune system? Frontiers in Physiology, 4, 46 10.3389/fphys.2013.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flórez, L. V. , Biedermann, P. H. W. , Engl, T. , & Kaltenpoth, M. (2015). Defensive symbioses of animals with prokaryotic and eukaryotic microorganisms. Natural Products Reports, 32, 904–936. 10.1039/C5NP00010F [DOI] [PubMed] [Google Scholar]
- Frank, J. H. , & Thomas, M. C. (2016). Rove beetles of the world, Staphylinidae (Insecta: Coleoptera: Staphylinidae). EENY, 114, 1–8. [Google Scholar]
- Glowska, E. , Dragun‐Damian, A. , Dabert, M. , & Gerth, M. (2015). New Wolbachia super groups detected in quill mites (Acari: Syringophilidae). Infection, Genetics and Evolution, 30, 140–146. 10.1016/j.meegid.2014.12.019 [DOI] [PubMed] [Google Scholar]
- Goto, S. , Anbutsu, H. , & Fukatsu, T. (2006). Asymmetrical interactions between Wolbachia and Spiroplasma endosymbionts coexisting in the same insect host. Applied and Environment Microbiology, 72, 4805–4810. 10.1128/AEM.00416-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebennikov, V. V. , & Newton, A. F. (2009). Good‐bye Scydmaenidae, or why the ant‐like stone beetles should become megadiverse Staphylinidae sensu latissimo (Coleoptera). European Journal of Entomology, 106, 275–301. 10.14411/eje.2009.035 [DOI] [Google Scholar]
- Hancock, R. E. W. , Siehnel, R. , & Martin, N. (1990). Outer membrane proteins of Pseudomonas . Molecular Microbiology, 4, 1069–1075. 10.1111/j.1365-2958.1990.tb00680.x [DOI] [PubMed] [Google Scholar]
- Hsiao, T. (1996). Studies of interactions between alfalfa weevil strains, Wolbachia endosymbionts and parasitoids In Symondson W. O. C., & Liddell J. E. (Eds.), The ecology of agricultural pests (pp. 51–72). London, UK: Chapman & Hall. [Google Scholar]
- Hughes, D. P. , Pamilo, P. , & Kathirithamby, J. (2004). Horizontal transmission of Wolbachia by strepsipteran endoparasites? A response to Noda et al., 2001. Molecular Ecology, 13, 507–509. [DOI] [PubMed] [Google Scholar]
- Hughes, G. L. , & Rasgon, J. L. (2014). Transinfection: A method to investigate Wolbachia‐host interactions and control arthropod‐borne disease. Insect Molecular Biology, 23, 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iserson, K. V. , & Walton, E. K. (2012). Nairobi fly (Paederus) dermatitis in South Sudan: A case report. Wilderness & Environmental Medicine, 23, 251–254. 10.1016/j.wem.2012.03.005 [DOI] [PubMed] [Google Scholar]
- Kador, M. , Horn, M. A. , & Dettner, K. (2011). Novel oligonucleotide probes for in situ detection of pederin‐producing endosymbionts of Paederus riparius rove beetles (Coleoptera: Staphylinidae). FEMS Microbiology Letters, 319, 73–81. 10.1111/j.1574-6968.2011.02270.x [DOI] [PubMed] [Google Scholar]
- Kajtoch, L. , & Kotásková, N. (2018). Current state of knowledge on Wolbachia infection among Coleoptera: A systematic review. PeerJ, 6, e4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambris, Z. , Cook, P. E. , Phuc, H. K. , & Sinkins, S. P. (2009). Immune activation by life‐shortening Wolbachia and reduced filarial competence in mosquitoes. Science, 326, 134–136. 10.1126/science.1177531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karami, M. , Moosa‐Kazemi, S. H. , Oshaghi, M. A. , Vatandoost, H. , Sedaghat, M. M. , Rajabnia, R. , … Ferdosi‐Shahandashti, E. (2016). Wolbachia Endobacteria in natural populations of Culex pipiens of Iran and its phylogenetic congruence. Journal of Arthropod‐Borne Diseases, 10, 347–363. [PMC free article] [PubMed] [Google Scholar]
- Kellner, R. L. L. (1998). When do Paederus riparius rove beetles (Coleoptera: Staphylinidae) biosynthesize their unique hemolymph toxin pederin? Zeitschrift Fuer Naturforschung, C: Journal of Biosciences, 53, 1081–1086. 10.1515/znc-1998-11-1222 [DOI] [Google Scholar]
- Kellner, R. L. L. (2001). Horizontal transmission of biosynthetic capabilities for pederin in Paederus melanurus (Coleoptera: Staphylinidae). Chemoecology, 11, 127–130. 10.1007/PL00001842 [DOI] [Google Scholar]
- Kellner, R. L. L. (2002). Molecular identification of an endosymbiotic bacterium associated with pederin biosynthesis in Paederus sabaeus (Coleoptera: Staphylinidae). Insect Biochemistry and Molecular Biology, 32, 389–395. 10.1016/S0965-1748(01)00115-1 [DOI] [PubMed] [Google Scholar]
- Kellner, R. L. L. , & Dettner, K. (1995). Allocation of pederin during lifetime of Paederus rove beetles (Coleoptera: Staphylinidae): Evidence for polymorphism of hemolymph toxin. Journal of Chemical Ecology, 21, 1719–1733. 10.1007/BF02033672 [DOI] [PubMed] [Google Scholar]
- Kellner, R. L. L. , & Dettner, K. (1996). Differential efficacy of toxic pederin in deterring potential arthropod predators of Paederus (Coleoptera: Staphylinidae) offspring. Oecologia, 107, 293–300. 10.1007/BF00328445 [DOI] [PubMed] [Google Scholar]
- Lavine, M. D. , & Strand, M. R. (2002). Insect hemocytes and their role in immunity. Insect Biochemistry and Molecular Biology, 32, 1295–1309. 10.1016/S0965-1748(02)00092-9 [DOI] [PubMed] [Google Scholar]
- Li, J. , Wang, Z. , Bourguet, D. , & He, K. (2013). Wolbachia infection in populations of Ostrinia furnacalis: Diversity, prevalence, phylogeny and evidence for horizontal transmission. Journal of Integrative Agriculture, 12, 283–295. 10.1016/S2095-3119(13)60227-0 [DOI] [Google Scholar]
- Li, S. J. , Ahmed, M. Z. , Lv, N. , Shi, P. Q. , Wang, X. M. , Huang, J. L. , & Qiu, B. L. (2016). Plant–mediated horizontal transmission of Wolbachia between whiteflies. ISME Journal, 11, 1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Login, F. H. , Balmand, S. , Vallier, A. , Vincent‐Monegat, C. , Vigneron, A. , Weiss‐Gayet, M. , … Heddi, A. (2011). Antimicrobial peptides keep insect endosymbionts under control. Science, 334, 362–365. 10.1126/science.1209728 [DOI] [PubMed] [Google Scholar]
- Maleki‐Ravasan, N. , Oshaghi, M. A. , Afshar, D. , Arandian, M. H. , Hajikhani, S. , Akhavan, A. A. , … Durvasula, R. (2015). Aerobic bacterial flora of biotic and abiotic compartments of a hyperendemic Zoonotic Cutaneous Leishmaniasis (ZCL) focus. Parasit Vectors, 8, 63 10.1186/s13071-014-0517-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammino, J. J. (2011). Paederus dermatitis. An outbreak on a medical mission boat in the Amazon. The Journal of Clinical and Aesthetic Dermatology, 4, 44–46. [PMC free article] [PubMed] [Google Scholar]
- Mitscherlich, E. , & Marth, E. H. (1984). Microbial survival in the environment. New York, NY: Springer‐Verlag. [Google Scholar]
- Naitza, S. , & Ligoxygakis, P. (2004). Antimicrobial defences in Drosophila: The story so far. Molecular Immunology, 40, 887–896. 10.1016/j.molimm.2003.10.008 [DOI] [PubMed] [Google Scholar]
- Narquizian, R. , & Kocienski, P. J. (2000). The pederin family of antitumor agents: Structures, synthesis and biological activity In Mulzer R., & Bohlmann R. (Eds.), The role of natural products in drug discovery (Vol. 32, pp. 25–56). Heidelberg, Germany: Springer‐Verlag. [DOI] [PubMed] [Google Scholar]
- Nikbakhtzadeh, M. R. , Naderi, M. , & Safa, P. (2012). Faunal diversity of Paederus fabricius 1775 (Coleoptera: Staphylinidae) in Iran. Insecta Mundi, 267, 1–9. [Google Scholar]
- Nikoh, N. , Hosokawa, T. , Moriyama, M. , Oshima, K. , Hattori, M. , & Fukatsu, T. (2014). Evolutionary origin of insect‐Wolbachia nutritional mutualism. Proceedings of the National Academy of Sciences of the United States of America, 111, 10257–10262. 10.1073/pnas.1409284111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, K. M. , & Moran, N. A. (2009). Defensive symbionts in aphids and other insects In White J. F., & Torres M. S. (Eds.), Defensive mutualism in microbial symbiosis (pp. 129–147). London, UK: Taylor & Francis. [Google Scholar]
- Pankewitz, F. , Zollmer, A. , Hilker, M. , & Graser, Y. (2007). Presence of Wolbachia in insect eggs containing antimicrobially active anthraquinones. Microbial Ecology, 54, 713–721. 10.1007/s00248-007-9230-5 [DOI] [PubMed] [Google Scholar]
- Rahman, S. (2006). Paederus dermatitis In Sierra Leone. Dermatology Online Journal, 12, 9. [PubMed] [Google Scholar]
- Rainey, F. A. , Ward‐Rainey, N. L. , Janssen, P. H. , & Hippe, H. (1996). Clostridium paradoxum DSM 7308 (T) contains multiple 16S rRNA genes with heterogeneous intervening sequences. Microbiology, 142, 2087–2095. 10.1099/13500872-142-8-2087 [DOI] [PubMed] [Google Scholar]
- Ratzka, C. , Gross, R. , & Feldhaar, H. (2012). Endosymbiont tolerance and control within insect hosts. Insects, 3, 553–572. 10.3390/insects3020553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawling, E. , Martin, N. , & Hancock, R. (1995). Epitope mapping of the Pseudomonas aeruginosa major outer membrane porin protein OprF. Infection and Immunity, 63, 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers, F. , Wilm, A. , Dineen, D. G. , Gibson, T. J. , Karplus, K. , Li, W. , … Higgins, D. G. (2011). Fast, scalable generation of high‐quality protein multiple sequence alignments using Clustal Omega. Molecular Systems Biology, 7, 539 10.1038/msb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontowski, R. , Bernhard, D. , Bleidorn, C. , Schlegel, M. , & Gerth, M. (2015). Wolbachia distribution in selected beetle taxa characterized by PCR screens and MLST data. Ecology and Evolution, 5, 4345–4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, K. , Peterson, D. , Peterson, N. , Stecher, G. , Nei, M. , & Kumar, S. (2011). MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28, 2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, M. J. , Bandi, C. , & Hoerauf, A. (2005). Wolbachia bacterial endosymbionts of filarial nematodes. Advances in Parasitology, 60, 245–284. [DOI] [PubMed] [Google Scholar]
- Thayer, M. K. (2005). Staphylinidae Latreille, 1802 In Beutel R. G., & Leschen R. A. B. (Eds.), Handbuch der Zoologie/Handbook of Zoology, Vol. IV (Arthropoda: Insecta), Part 38 Coleoptera, Beetles. Volume 1: Morphology and systematic (Archostemata, Adephaga, Myxophaga, Polyphaga partim) (pp. 296–344). Berlin, Germany: Walter de Gruyter. [Google Scholar]
- Theopold, U. , Li, D. , Fabbri, M. , Scherfer, C. , & Schmidt, O. (2002). The coagulation of insect hemolymph. Cellular and Molecular Life Sciences, 59, 363–372. 10.1007/s00018-002-8428-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplehorn, C. A. , & Johnson, N. F. (2005). Borror and DeLong’s introduction to the study of insects (7th ed.). Blemont, CA: Thomson Brooks/Cole. [Google Scholar]
- van Nouhuys, S. , Kohonen, M. , & Duplouy, A. (2016). Wolbachia increases the susceptibility of a parasitoid wasp to hyperparasitism. Journal of Experimental Biology, 219, 2984–2990. [DOI] [PubMed] [Google Scholar]
- Vavre, F. , Fleury, F. , Lepetit, D. , Fouillet, P. , & Boulétreau, M. (1999). Phylogenetic evidence for horizontal transmission of Wolbachia in host‐parasitoid associations. Molecular Biology and Evolution, 16, 1711–1723. 10.1093/oxfordjournals.molbev.a026084 [DOI] [PubMed] [Google Scholar]
- Vega, F. E. , & Kaya, H. K. (2012). Insect pathology (2nd ed.). San Diego, CA: Academic Press. [Google Scholar]
- Vieira, J. S. , Ribeiro‐Costa, C. S. , & Caron, E. (2014). Rove beetles of medical importance in Brazil (Coleoptera, Staphylinidae, Paederinae). Revista Brasileira De Entomologia, 58, 244–260. 10.1590/S0085-56262014000300005 [DOI] [Google Scholar]
- Werren, J. H. (1997). Biology of Wolbachia . Annual Review of Entomology, 42, 587–609. [DOI] [PubMed] [Google Scholar]
- Yun, Y. , Peng, Y. , Liu, F. X. , & Lei, C. (2011). Wolbachia screening in spiders and assessment of horizontal transmission between predator and prey. Neotropical Entomology, 40, 164–169. [PubMed] [Google Scholar]
- Zele, F. , Nicot, A. , Duron, O. , & Rivero, A. (2012). Infection with Wolbachia protects mosquitoes against Plasmodium‐induced mortality in a natural system. Journal of Evolutionary Biology, 25, 1243–1252. 10.1111/j.1420-9101.2012.02519.x [DOI] [PubMed] [Google Scholar]
- Zhou, W. , Rousset, F. , & O'Neill, S. (1998). Phylogeny and PCR‐based classification of Wolbachia strains using wsp gene sequences. Proceedings of the Royal Society of London. Series B: Biological Sciences, 265, 509–515. 10.1098/rspb.1998.0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zug, R. , & Hammerstein, P. (2012). Still a host of hosts for Wolbachia: Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE, 7, e38544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zug, R. , & Hammerstein, P. (2015). Wolbachia and the insect immune system: What reactive oxygen species can tell us about the mechanisms of Wolbachia–host interactions. Frontiers in Microbiology, 6, 1201 10.3389/fmicb.2015.01201 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in the main manuscript. Sequences were also have been deposited at the NCBI GenBank under accession number of KY568928–KY568936, KY568937–KY568941, and KY555600–KY555603.


