Abstract
A bacterial strain designated as P08T was isolated from laboratory tap water during a water quality assessment in University of Malaya, Malaysia. The strain was a Gram‐negative, rod‐shaped, nonmotile, and aerobic bacterium. Complete genome of P08T comprised of a 2,820,660 bp chromosome with a G + C content of 36.43%. Both 16S rRNA phylogeny and phylogenetic tree inferred from the core gene matrix demonstrated that P08T formed a hitherto unknown subline within the family Neisseriaceae. Ortho average nucleotide identity (OrthoANI) values and the percentage of conserved proteins (POCP) calculated from complete genome sequence indicated low relatedness between P08T and its phylogenetic neighbors. Respiratory quinone analysis revealed Q‐8 as the only detectable quinone. The predominant cellular fatty acids were identified as C14:0, iso‐C15:0, and summed feature 3 (C16:1 ω7c/C16:1 ω6c). The polar lipids consisted of uncharacterized aminolipid, phosphatidylglycerol, and phosphatidylethanolamine. All aspects of phenotypic and phylogenetic data suggested that strain P08T represents a novel genus within family Neisseriaceae, for which the name Aquella gen. nov. is proposed. The type species of the genus is Aquella oligotrophica sp. nov., and the type strain is P08T (=LMG 29629T =DSM 100970T).
Keywords: Aquella oligotrophica gen. nov. sp. nov., Neisseriaceae, OrthoANI, POCP, tap water
1. INTRODUCTION
The order Neisseriales, which constitutes a major branch of Betaproteobacteria, presently contains 36 genera spanning a wide range of morphologies, habitats, and growth requirements (Parte, 2013). The taxonomic classification in Neisseriales is primarily based on 16S rRNA sequence identity studies and phylogenetic analysis (Adeolu & Gupta, 2013), which the taxon delineation was mainly determined by distinct clade from the phylogenetic tree. The type family of Neisseriales is Neisseriaceae (Tønjum, 2015).
In 2013, Adeolu and Gupta proposed that a new family, Chromobacteriaceae, to be split from Neisseriaceae based on phylogenomic and molecular signatures evidence (Adeolu & Gupta, 2013). The amendment reclassified 19 genera from 32 genera of Neisseriaceae to Chromobacteriaceae. The emended Neisseriaceae contains Alysiella (Langeron, 1923), Bergeriella (Xie & Yokota, 2005), Conchiformibius (Xie & Yokota, 2005), Eikenella (Jackson & Goodman, 1972), Kingella (Henriksen & Bøvre, 1976), Neisseria (Trevisan, 1885), Simonsiella (Simons, 1922), Stenoxybacter (Wertz & Breznak, 2007), Uruburuella (Vela et al., 2005), and Vitreoscilla (Pringsheim, 1949).
Subsequently, 4 additional genera have been validly described and classified into family Neisseriaceae, namely Amantichitinum (Moß et al., 2013), Snodgrassella (Kwong & Moran, 2013), Rivicola (Sheu, Chen, Young, & Chen, 2014), Crenobacter (Dong et al., 2015), and Populibacter (Li, Xue, Sang, Lin, & Wang, 2017). At the time of writing, there are 17 validly described genera included in family Neisseriaceae. However, there are also 2 genera with uncertain taxonomic status that have been classified into Neisseriaceae; Adeolu and Gupta (2013) suggested that genus Morococcus (Long, Sly, Pham, & Davis, 1981) should be reclassified to Neisseria species and Prolinoborus (Pot, Willems, Gillis, & De Ley, 1992) is likely wrongly assigned to the order of Neisseriales, based on their phylogenetic analysis.
Other than 16S rRNA gene sequence similarities and phylogenetic analysis, the major distinguishing features among genera for Neisseriaceae are cell morphology, biochemical characteristics, such as oxidase and catalase tests, glucose fermentation, nitrite reduction, and mol% G + C content of the genomic DNA (Garrity et al., 2006). Most strains inhabit indigenously in mucosal membranes of humans and animals, although environmental species were recently included in this family with representatives isolated from anthill, hot spring sediment, and freshwater river (Dong et al., 2015; Garrity et al., 2006; Moß et al., 2013; Sheu et al., 2014). During an assessment on laboratory tap water quality, a bacterial strain designated as P08T was isolated on R2A agar. Here, we report the characterization of strain P08T that is the first representative of a newly proposed genus Aquella gen. nov. belonging to family Neisseriaceae.
2. MATERIALS AND METHODS
2.1. Isolation of the strain
Strain P08T was isolated from a laboratory tap water collected for water quality assessment in University of Malaya (3°07′20.9″N, 101°39′23.7″E) on 22 May 2015. After 3 days of incubation on R2A agar (BD Difco) at 37°C, bacteria isolation and purification were performed. The purified strain was routinely cultivated in R2A liquid medium, unless specified. Cells were preserved in 20% (v/v) glycerol at −80°C. The strain P08T has been deposited in the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany) and the Belgium Coordinated Collections of Microorganisms (BCCM/LMG, Gent, Belgium).
2.2. DNA extraction, genome sequencing, and functional gene annotation
The genomic DNA of strain P08T was extracted using the MasterPureTM DNA purification kit (Epicenter, WI, USA) following the manufacturer's protocol. Extracted genomic DNA was sheared and constructed into a template library according to the “Guidelines for Preparing 20 kb SMRTbell™ Templates.” Genome sequencing was performed in 1 SMRT cell using the PacBio RS II single‐molecule real‐time (SMRT) sequencing technology (Pacific Biosciences, CA, USA). The reads were de novo assembled using the hierarchical genome assembly process (HGAP) algorithm version 2 (Chin et al., 2013) into complete genome of P08T. The assembled genome was annotated using the NCBI Prokaryotic Genome Annotation Pipeline (PGAP) version 2.10 (Tatusova et al., 2016), Rapid Annotation using Subsystem Technology (RAST) version 3.0 (Aziz et al., 2008; Overbeek et al., 2014), and IMG ER pipeline (Markowitz et al., 2009). The genome project and the complete genome sequence were deposited in the Genomes OnLine Database (Liolios et al., 2010) and GenBank. A comparison on the genomes of P08T and available genomes of type strains in the family Neisseriaceae was performed.
2.3. Phylogenetic analyses
The 16S rRNA gene sequence was mined from the complete genome using RNAmmer 1.2 server (Lagesen et al., 2007). The sequence similarity to other validly described type strains was examined from pairwise sequence comparisons using EzBioCloud database (https://www.ezbiocloud.net) (Yoon et al., 2017). Phylogenetic analysis of 16S rRNA genes of strain P08T and type members of Neisseriaceae were performed using the software package MEGA version 6.0 (Tamura, Stecher, Peterson, Filipski, & Kumar, 2013), with neighbor‐joining (Saitou & Nei, 1987), maximum‐likelihood (Felsenstein, 1981), and maximum parsimony (Fitch, 1971) algorithms. In each case, bootstrap values were calculated based on 1,000 resamplings (Felsenstein, 1985). Kimura's two‐parameter model was used to calculate evolutionary distance matrices of the neighbor‐joining method and maximum‐likelihood method (Kimura, 1980). Vitreoscilla stercoraria was included in the analysis in place of the type species V. beggiatoides that has no sequence data available. For core genes (present in all genomes) analysis, the homologous clusters were determined using panX pan‐genome pipeline (Ding, Baumdicker, & Neher, 2018). The alignments were then built from these orthologous clusters before being concatenated and used for phylogenetic analysis in RAxML (Stamatakis, 2014). Generally, the phylogenetic tree was constructed by applying GAMMA for modeling rate heterogeneity, fast bootstrapping in conjunction with the autoMRE bootstopping criterion (Pattengale, Alipour, Bininda‐Emonds, Moret, & Stamatakis, 2010), and subsequent search for the best tree (Stamatakis, Hoover, & Rougemont, 2008). The core genes sequences of strain P08T were compared against members of the genera Alysiella, Amantichitinum, Bergeriella, Conchiformibius, Crenobacter, Eikenella, Kingella, Morococcus, Neisseria, Populibacter, Prolinoborus, Rivicola, Simonsiella, Snodgrassella, Stenoxybacter, and Vitreoscilla.
2.4. Average Nucleotide Identity (ANI), Average Amino acid Identity (AAI), and Percentage of conserved proteins (POCP) analysis
ANI between the complete genome of P08T and each reference genome was calculated using an online ANI calculation tool on the EzBioCloud web server with the OrthoANI algorithm (Lee, Kim, Park, & Chun, 2015). AAI between of complete genome of P08T and each reference genome was calculated using an online AAI calculation tool available at the web server http://enve-omics.ce.gatech.edu/(Rodriguez‐R & Konstantinidis, 2016). The available genome sequences of the family Neisseriaceae were retrieved from GenBank. The percentage of conserved proteins (POCP) (Qin et al., 2014) in each pair of genomes was calculated as [(C1+C2)/(T1+T2)]×100%, where C1 and C2 represent the numbers of conserved proteins in the two genomes being compared, respectively, and T1 and T2 represent the total numbers of predicted proteins in the two genomes being compared, respectively. Conserved proteins were defined as having a BLASTP match with an E‐value of less than 1e−5, sequence identity of more than 40% and an alignable region of the query protein sequence of more than 50%, as recommended by Qin et al. (2014).
2.5. Morphological observations and physiological tests
Growth of strain P08T was tested on chocolate agar (Thermo Scientific), Luria‐Bertani agar (Merck), Pseudomonas agar (BD Difco), MacConkey agar (Merck), R2A agar (BD Difco), and trypticase soy agar (Merck), and the ability to grow in this media was recorded after incubation of 3 days at 37 °C. Oxidase and catalase activities were examined with solutions of oxidase reagent (bioMérieux) and 3% (v/v) hydrogen peroxide, respectively. The morphology of bacterial cells was observed using tabletop scanning electron microscopy (Hitachi TM3030, Germany). Cellular motility was tested by the hanging drop method (Beveridge, Lawrence, & Murray, 2007). Gram staining was performed using the standard Gram reaction and was confirmed by using the KOH lysis test method (Cerny, 1978). Growth temperature was investigated in R2A medium at 4, 12, 16, 20, 25, 30, 35, 37, 40, and 45 °C, up to 14 days of incubation. Growth at pH 4.0–11.0 (intervals of 1 unit) was determined in R2A medium after incubation for 14 days at 37 °C under speed 220 rpm. For pH adjustment of the basal medium, the following buffers were used as follows: 0.1 M citric acid/0.1 M sodium citrate (pH 4.0–5.0), 0.1 M KH2PO4/0.1 M NaOH (pH 6.0–8.0), 0.1 M NaHCO3/0.1 M Na2CO3 (pH 9.0–10.0), and 0.05 M Na2HPO4/0.1 M NaOH. Growth on medium added with sodium chloride (NaCl) was determined in R2A medium supplemented with 0, 0.5, 1%–6% (intervals of 1%) (w/v) NaCl after 14 days of incubation at 37 °C. Growth under anaerobic condition was determined by incubating strain P08T on R2A agar in the Oxoid AnaeroGen system.
P08T was tested on R2A agar supplemented with casein (2% skimmed milk, w/v), starch (0.2% soluble starch, w/v), cellulose (0.5% CM‐cellulose, w/v), urease, Tweens 20, 40, 60, and 80 for hydrolysis activities, respectively (Tindall, Sikorski, Smibert, & Krieg, 2007). Hydrolysis of DNA was determined using DNase test agar (BD Difco). The production of clear or opaque halo zones around colonies on agar plates was recorded as a positive result after incubation of 3 weeks, except for hydrolysis of starch, cellulose, urease, and DNA (observed on agar plates incubated for 2 weeks). The color and morphology of colonies were determined on R2A agar incubated for 2 days at 37 °C. Other physiological and biochemical tests were performed using API 20E, API 20NE, and API ZYM strips (bioMérieux) according to the manufacturer's instructions, and results were recorded after 2 days of incubation at 37 °C. Utilization of a variety of carbon sources was tested using GEN III MicroPlates (Biolog), along with other major biochemical and physiological properties. Cells grown for 2 days at 37 °C on R2A were suspended in sterilized inoculating fluid C (Biolog) and adjusted to a specific transmittance (60% T) using a turbidimeter according to the manufacturer's instruction. An aliquot (100 μl) of the cell suspension was transferred to each well, and the plate was immediately incubated for 3 days at 37 °C, before visual reading.
2.6. Antibiotic susceptibility testing
Sensitivity of strain P08T to antibiotics was tested by the disk diffusion method after spreading cell suspensions (0.5 McFarland) on R2A agar (BD Difco) plates. The disks (Oxoid) contained the following antibiotics: ampicillin (10 μg), ampicillin/sulbactam (20 μg), chloramphenicol (30 μg), gentamicin (10 μg), kanamycin (30 μg), nalidixic acid (30 μg), rifampicin (5 μg), penicillin G (10 μg), streptomycin (10 μg), sulfamethoxazole (23.75 μg) plus trimethoprim (1.25 μg), and tetracycline (30 μg). The effect of antibiotics on cell growth was assessed after incubation for 2 days at 37°C. The diameter of the antibiotic disks was 6 mm. The strain was considered susceptible when the diameter of the inhibition zone was>13 mm, intermediate at 10–12 mm, and resistant at <10 mm as described by Nokhal and Schlegel (1983).
2.7. Fatty acid analyses
Fatty acid methyl esters are extracted from 40 mg cells scraped from Petri dishes by saponification, methylation, and extraction using minor modifications of the method of Kuykendall, Roy, O'neill, and Devine (1988) and Miller (1982). Fatty acid methyl esters were separated and analyzed by the Identification Service of the DSMZ, Braunschweig, Germany, using the Sherlock Microbial Identification System (MIDI Inc, Newark, USA, version 6.1 with database TSBA6) according to the standard protocol. For this purpose, strain P08T was grown for 2 days on R2A plates at 37 °C to get cultures of the same physiological age.
2.8. Polar lipid and respiratory quinones analyses
Polar lipid analyses and analyses of respiratory quinones were carried by the Identification Service of the DSMZ, Braunschweig, Germany. Cells were freeze‐dried before 100 mg cell material was used for extraction of respiratory lipoquinones using the two‐stage method described by Tindall (1990a) and Tindall (1990b). Respiratory quinones were then extracted using methanol:hexane, followed by phase separation into hexane (Tindall, 1990a, 1990b). Separation of respiratory lipoquinones into different classes was conducted by thin layer chromatography (TLC) on silica gel (Macherey‐Nagel Art. No. 805 023) using hexane:tertbutylmethylether [9:1 (v/v)] as the solvent. UV‐absorbing bands corresponding to different quinone classes were removed from the TLC plate and further analyzed on a LDC Analytical (Thermo Separation Products) HPLC fitted with a reverse phase column (Macherey–Nagel, 2 mm × 125 mm, 3 μm, RP18) using methanol:heptane [9:1 (v/v)] as the eluent. Respiratory lipoquinones were detected at 269 nm. Cells were freeze‐dried before 100 mg cell material was used for polar lipids extraction with a modified method using a chloroform:methanol:0.3% aqueous NaCl mixture 1:2:0.8 (v/v/v) (Bligh & Dyer, 1959). The mixture was stirred overnight, and cell debris was centrifuged for pellet. Polar lipids were obtained from the chloroform phase by adjusting the chloroform:methanol:0.3% aqueous NaCl mixture to a ratio of 1:1:0.9 (v/v/v). Recovered polar lipids were separated by a two‐dimensional silica gel TLC (Macherey‐Nagel Art. No. 818 135), first in chloroform:methanol:water (65:25:4, v/v/v) and the second in chloroform:methanol:acetic acid:water (80:12:15:4, v/v/v/v). Total lipid material was detected using molybdatophosphoric acid while specific functional groups detected using spray reagents specific for defined functional groups (Tindall et al., 2007).
3. RESULTS
3.1. Phylogenetic analyses
Complete 16S rRNA gene sequence of strain P08T is 1,532 bp. The 16S rRNA gene sequence similarity was highest to Neisseria animaloris LMG 23011T (90.1%), and lesser to all validly described type strains, for instance Neisseria iguana NVSL 85737T (89.4%), Neisseria flavescens ATCC 13120T (89.3%), Paludibacterium paludis KBP‐21T (89.3%), Uruburuella testudines 07_OD624T (89.3%), and Morococcus cerebrosus CIP 81.93T (89.3%) based on EzBioCloud similarity‐based search (Table S1). According to Ludwig et al. (1998), 16S rRNA gene sequence similarities of lesser than 95% between two bacteria are generally affiliated as a novel genus. Therefore, the low 16S rRNA gene sequence similarity to other validly described type strains highly supported that strain P08T belongs to a novel genus. Figure 1 shows the positions of the type species of genera within the family Neisseriaceae in a neighbor‐joining (NJ) phylogenetic tree based on 16S rRNA gene sequences. Strain P08T formed a branch clearly separated from the remaining genera from Neisseriaceae. As indicated in Figure 1, strain P08T appeared to be more closely related to Neisseriaceae, compared to the type species of genus Prolinoborus, which have a questionable taxonomic status in Neisseriaceae (Adeolu & Gupta, 2013). The NJ phylogenetic tree was congruent with the maximum‐likelihood (ML) (Figure S1) and maximum parsimony (MP) (Figure S2) phylogenetic trees, all constructed based on 16S rRNA gene sequences. For core genes analysis, we have identified 146 orthologous protein clusters (Table S2). The ML phylogenetic tree based on concatenated core gene (Figure 2) showed that all genera in the Neisseriaceae formed distinct phylogenetic lineages. Strain P08T was in a cluster distantly related to Snodgrassella and was clearly separated in the phylogenetic tree based on concatenated core gene from other genera of Neisseriaceae. This association was supported by a bootstrap value of 100%. Likewise, the concatenated core gene phylogenetic tree was congruent with the 16S rRNA gene phylogenetic analyses in which strain P08T represents a novel bacterial taxon in the family Neisseriaceae.
Figure 1.

16s rRNA gene phylogeny of strain P08T and other members of Neisseriaceae. Neighbor‐joining phylogenetic tree based on 16S rRNA gene sequences showing the positions of strain P08T (MF371416) and the most closely related members of the family Neisseriaceae. Bootstrap percentage values (1,000 replications) greater than or equal to 50% are shown at nodes. Bradyrhizobium japonicum ATCC 10324T (U69638) is used as an out‐group. Bar, 0.02 substitutions per nucleotide position
Figure 2.

Phylogeny of the Neisseriaceae. ML tree reconstructed from concatenated core gene sequences (146 genes, 133,677 characters). Only bootstrap values of 50% or greater are shown. Asterisks adjacent to nodes indicated 100% bootstrap support. Bar, 0.05 substitutions per nucleotide position
3.2. Genomic features and functional gene annotation
A total of 1,985,842,173 reads with a mean read length of 11,876 bp were generated from whole genome sequencing. The reads were de novo assembled using the hierarchical genome assembly process (HGAP) algorithm version 2 (Chin et al., 2013) to generate the complete genome of P08T, which consists of 1 polished contig with an average genome coverage of 472.61‐fold. The genome of P08T is 2,820,660 bp in length, consists of a circular chromosome, with G + C contents of 36.43 mol%. The assembled and annotated genome of P08T described in this paper has been deposited in GenBank (accession number: CP024847.1) and JGI portal (GOLD ID: Gp0293937; IMG Taxon ID: 2,770,939,448). Out of a total of 2,625 genes in the genome, 2,564 protein‐coding gene, 12 rRNAs, and 46 tRNAs were predicted from the chromosome by PGAP analysis (Table 1 and Figure 3). Complete genome sequencing analysis revealed that the protein‐coding gene constituted 97.68% of the total genes in the genome of P08T but only 76.11% are predicted with functions. Notably, several genes that confer resistance to beta‐lactam antibiotics were also identified in the genome and were annotated as class A beta‐lactamase [NCBI locus tag =CUN60_09190] and class D beta‐lactamase [CUN60_03820]. Furthermore, there were 1621 genes assigned to different functional categories based on the clusters of orthologous genes (COG) designation (Tatusov, Galperin, Natale, & Koonin, 2000), 756 genes were connected to KEGG pathways, and 619 genes connected to MetaCyc pathways (Table 1). Majority of the genes were categorized into classes responsible for central metabolism of the P08T including translation, ribosomal structure and biogenesis and amino acid transport and metabolism. There are also a number of genes with unknown functions. Figure S3 represents the gene's distribution into different clusters of orthologous groups (COGs) functional categories.
Table 1.
Genome statistics of Aquella oligotrophica gen. nov. sp. nov. (strain P08T)
| Attribute | Value | % of total |
|---|---|---|
| Genome size (bp) | 2,820,660 | 100.00 |
| DNA coding region (bp) | 2,571,724 | 91.17 |
| DNA G + C content (bp) | 1,027,475 | 36.43 |
| Total genes | 2,625 | 100.00 |
| Protein‐coding gene | 2,564 | 97.68 |
| RNA genes | 61 | 2.32 |
| rRNA genes | 12 | 0.46 |
| tRNA genes | 46 | 1.75 |
| Genes with function prediction | 1998 | 76.11 |
| Genes assigned to COGs | 1621 | 61.75 |
| Genes assigned to KEGG pathways | 756 | 28.80 |
| Genes assigned to MetaCyc pathways | 619 | 23.58 |
Figure 3.
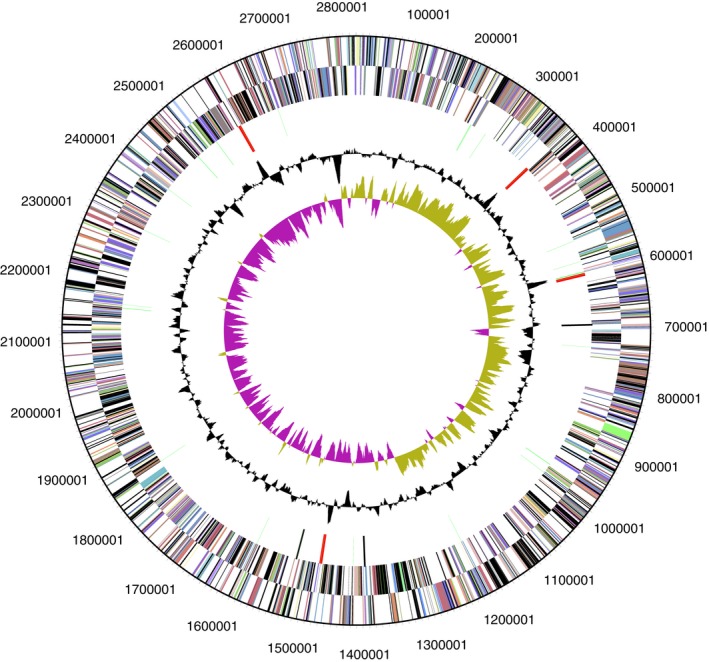
Circular map of the chromosome and plasmid of A. oligotrophica P08T. From the outside to the centre: genes on forward strand (colored by COG categories), genes on reverse strand (colored by COG categories), RNA genes (tRNAs green, rRNAs red, other RNAs black), GC content, and GC skew
3.3. Genome comparisons with type strains of type species in the family Neisseriaceae
Genome of P08T was compared to genome of other members in the family Neisseriaceae. With the exception to Crenobacter luteus, Vitreoscilla beggiatoides, and Uruburuella suis, publicly available genomes of all type strains of type species in the family were included. Genome of C. luteus strain CN10 was selected as the type strain of the type species C. luteus has no available genome information while the genome of V. stercoraria DSM 513T was incorporated into analysis as the type species V. beggiatoides has no available culture. Uruburuella is the only genus currently without genomic data. Most of the genomes currently available were draft genomes, and complete genomes obtained were only limited to strains P08T, Simonsiella muelleri ATCC 29453T, and Snodgrassella alvi wkB2T (Table S3). While an accurate comparison on the size would require the complete genomes of the other type strains, it is noteworthy that the complete genome size of P08T is 2.82 Mb, which is among the largest and only smaller than the genome size of Amantichitinum ursilacus IGB‐41T, Crenobacter luteus CN10, Prolinoborus fasciculus CIP103579T, and Rivicola pingtungensis DSM 29661T (4.93, 2.85, 3.45, and 3.71 Mb, respectively). Intriguingly, strain P08T has the lowest G + C content among these genomes. The range of G + C contents ranged widely from the lowest 36.43% in strain P08T to the highest 68.30% in genome of Crenobacter luteus CN10 (Table S3).
3.4. Average Nucleotide Identity (ANI), Average Amino acid Identity (AAI), and Percentage of Conserved Proteins (POCP) analysis
Genome‐to‐genome similarity between strain P08T and close taxa was performed using OrthoANI algorithm (Lee et al., 2015). OrthoANI values between strain P08T and phylogenetically related genera in Neisseriaceae ranged from 63.0% to 65.5% (Table 2), which were significantly below the proposed boundary of 95%–96% for defining a novel species (Richter & Rosselló‐Móra, 2009). Despite ANI is not suitable for genus delimitation (Qin et al., 2014), this analysis briefly suggested that strain P08T is not the species or the subspecies of aforementioned taxa. Similar observation was obtained from AAI analysis, and strain P08T has AAI values ranged from 40.62% to 46.71% with all reference genomes, which is much lower than 95%–96% for proposing a novel species (Table S4) (Konstantinidis & Tiedje, 2005). POCP has been applied to support the designation of P08T as a novel genus, using a suggested threshold value of 50% (Qin et al., 2014). POCP using the cutoff threshold for genus circumscription highly supported that strain P08T represented a novel genus in family Neisseriaceae, as strain P08T had an extremely low POCP compared to other representative members of family Neisseriaceae. The intergenera POCP analyses between P08T and strains from 13 genera of family Neisseriaceae had values ranged from 24% to 31%, which clearly indicated that strain P08T does not belong to any validly described genera from Neisseriaceae (Table 2).
Table 2.
OrthoANI and POCP values for pairs of genomes between strain P08T and other phylogenetically related genera in the family Neisseriaceae
| Bacteria | OrthoANI (%) | POCP (%) |
|---|---|---|
| Alysiella crassa DSM 2578T | 64.0 | 23 |
| Amantichitinum ursilacus IGB−41T | 63.4 | 23 |
| Bergeriella denitrificans NBRC 102155T | 63.7 | 27 |
| Conchiformibius steedae DSM 2580T | 64.4 | 26 |
| Crenobacter luteus CN10 | 63.0 | 29 |
| Eikenella corrodens ATCC 23834T | 63.9 | 26 |
| Kingella kingae ATCC 23330T | 64.5 | 27 |
| Morococcus cerebrosus CIP 81.93T | 64.4 | 27 |
| Neisseria gonorrhoeae DSM 9188T | 64.1 | 26 |
| Populibacter corticis 15–3−5T | 64.6 | 25 |
| Prolinoborus fasciculus CIP 103579T | 63.6 | 19 |
| Rivicola pingtungensis DSM 29661T | 63.1 | 26 |
| Simonsiella muelleri ATCC 29453T | 64.7 | 25 |
| Snodgrassella alvi wkB2T | 65.5 | 26 |
| Stenoxybacter acetivorans DSM 19021T | 64.6 | 25 |
| Vitreoscilla stercoraria DSM 513T | 64.5 | 26 |
3.5. Fatty acid, polar lipid, and respiratory quinones composition
The fatty acid composition of strain P08T is listed in Table 3. The major fatty acids (>5%) were C14:0 (15.9%), iso‐C15:0 (36.2%), C16:0 (9.9%), summed feature 3 (C16:1 ω7c/C16:1 ω6c) (16.4%), and summed feature 8 (C18:1 ω7c) (6.8%). Strain P08T exhibited a polar lipid profile consisting of uncharacterized aminolipid, phosphatidylglycerol, and phosphatidylethanolamine (Figure 4). Strain P08T had Q‐8 as the major respiratory quinone, which is the same as its closest phylogenetic relatives (Dong et al., 2015; Moß et al., 2013; Sheu et al., 2014; Srinivas et al., 2013; Su et al., 2013).
Table 3.
Cellular fatty acid compositions of Aquella oligotrophica gen. nov. sp. nov. strain P08T
| Fatty acid | Composition |
|---|---|
| Straight‐chain | |
| C12:0 | – |
| C14:0 | 15.9 |
| C16:0 | 9.9 |
| Branched‐chain | |
| iso‐C15:0 | 36.2 |
| C17:0 cyclo | – |
| Unsaturated | |
| C14:1 ω5c | 4.7 |
| C15:1 ω6c | 1.7 |
| C16:1 ω7c | – |
| C18:1 ω7c | – |
| Hydroxy | |
| C12:0 3‐OH | – |
| C16:0 3‐OH | 1.4 |
| Summed featuresa | |
| 3 | 16.4 |
| 8 | 6.8 |
| 9 | 1.9 |
Strain P08T was grown on R2A agar at 37 °C for 2 days. Values are in percentages of the total fatty acids; fatty acids that make up <1% of the total are not listed or are indicated by a dash.
Summed features represent groups of two or three fatty acids that cannot be separated by GLC with the MIDI system. Summed feature 3 comprises C16:1 ω7c and/or C16:1 ω6c; summed feature 8 comprises C18:1 ω7c and/or C18:1 ω6c.
Figure 4.

Thin layer chromatography image of polar lipid analysis of P08T; AL, aminolipid; PG, phosphatidylglycerol; PE, phosphatidylethanolamine
4. DISCUSSION
The 16S rRNA gene sequence similarity of strain P08T with other validly described strains is lower than the proposed cutoff (95%) value suggested by Ludwig et al. (1998) for a novel bacterial genus. EzBioCloud similarity‐based search in Table S1 shows that strain P08T has highest 16S rRNA gene sequence similarity with most of the type strains of species belonging to the genus Neisseria. This suggested that even though strain P08T does not belong to any validly described genera, it is highly related to family Neisseriaceae. The divergent branching pattern between strain P08T and the cluster of genera of the family Neisseriaceae was highly reproducible with solid bootstrap recovery using the neighbor‐joining algorithm (Figure 1). This was further confirmed in phylogenetic tree constructed using concatenated core genes (Figure 2). In the phylogenetic tree based on concatenated core genes, 146 orthologous protein clusters were concatenated and subjected to ML analysis. Strain P08T is distantly related to other members of family Neisseriaceae. Interestingly, even though Adeolu and Gupta (2013) proposed that Moroccocus should be reclassified into genus Neisseria, phylogenetic tree based on concatenated core genes showed that Moroccocus is rather closely related to Bergeriella. However, these 3 taxa clustered into the same clade and showed that they are more related to each other than to other members from family Neisseriaceae.
We reported in this study the complete genome sequence of P08T that is important for pairwise comparison with closely related species in terms of ANI and AAI analyses. The level of genome‐to‐genome similarity between strain P08T and the closest phylogenetic neighbors was significantly below the interspecies level of sequence similarity (Table 2). The genus delimitation as determined by POCP between strain P08T and Neisseria gonorrhoeae DSM 9188T, Amantichitinum ursilacus IGB‐41T, and Crenobacter luteus CN10 was of values 26%, 23%, and 29%, respectively (Table 2). This is far below the proposed threshold for genus delimitation of 50% (Qin et al., 2014), which indicates that strain P08T does not belong to any of these mentioned genera. In fact, the relatedness among all these genome sequences is extremely low, which also suggests that strain P08T represents a novel genus.
Several beta‐lactamase gene were identified in the genome of P08T, and the drug‐resistant phenotype of P08T toward antibiotics was furthered confirmed by antibiotic susceptibility test. Consistent with the complete genome analysis, P08T was resistant to beta‐lactam antibiotics including ampicillin and penicillin G tested in this study. The class A and D beta‐lactamases identified are known to confer broad‐spectrum resistance toward beta‐lactam antibiotics (Bush & Jacoby, 2010).
The predominant fatty acids of strain P08T consisted of C14:0, iso‐C15:0, and summed feature 3 (C16:1 ω7c/C16:1 ω6c) (Table 3). Polar lipid analyses using two‐dimensional TLC (Figure 4) revealed differences between the polar lipid composition of cell membrane of strains P08T and phylogenetically related genera. Strain P08T harbored uncharacterized aminolipid, phosphatidylglycerol, and phosphatidylethanolamine. No unidentified phospholipid was found in P08T.
Physiological and biochemical characteristics such as absence of catalase and oxidase, rod‐shaped, absence of motility, inability to reduce nitrate to nitrite, low DNA G + C content, and cellular fatty acid profile clearly distinguished strain P08T from other members of the family Neisseriaceae (Table 3 & 4). The ability to grow in up to 43°C is also useful in differentiating strain P08T from the genera Amantichitinum and Rivicola (Table 4). Based on these morphological, physiological, chemotaxonomic, phylogenetic, and phylogenomic properties, strain P08T is considered to represent a novel species within a new genus, for which the name Aquella oligotrophica gen. nov., sp. nov. is proposed.
Table 4.
Characteristics that differentiate Aquella oligotrophica P08T from other phylogenetically related genera in the family Neisseriaceae
| Characteristics | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Isolation source | Tap water | Thumb wound | Ant hill soil | Hot spring sediment | Freshwater river |
| Colony pigmentation | Milky white | Opaque, shiny | Milky white to beige | Yellowish | Cream |
| Cell size (μm) | 0.7–1.4 × 0.3–0.5 | NA | 0.7–0.8 × 1.5–3.0 | ND | 0.3–0.6 × 1.4–3.2 |
| Cell shape | Rod‐shaped | Circular | Rod‐shaped | Short‐rod‐shaped | Rod‐shaped |
| Motility | ‒ | ‒ | + | + | ‒ |
| Oxygen requirement | Strictly aerobic | Facultative anaerobic | Facultative anaerobic | Strictly aerobic | Facultative anaerobic |
| Temperature range for growth (°C) (optimal) | 13‒43 (35‒40) | 37 (18‒22) | 10‒35 (20‒25) | 10‒55 (40‒50) | 10‒37 (30‒35) |
| pH range for growth (optimal) | 5.0‒8.0 (5.0‒7.0) | ND | 6.0‒9.0 (7.0) | 6.0‒10.0 (8.0‒9.0) | 6.0‒8.0 (6.0‒7.0) |
| Salinity range for growth (w/v,%) | 0‒1 (0‒1) | ND | 0 | 0‒3 (0‒1) | 0‒1 (0) |
| Nitrate reduction | ‒ | + | + | + | + |
| DNA G + C content (mol%) | 36.4 | 49.3 | 61.5 | 67.3 | 64.1 |
| Production of | |||||
| Catalase | ‒ | + | + | + | + |
| Oxidase | ‒ | + | + | + | + |
| Hydrolysis of starch | ‒ | ‒ | + | ‒ | ‒ |
| Fermentation of | |||||
| Arabinose | + | ‒ | + | ‒ | + |
| D‐mannitol | + | ‒ | + | ‒ | + |
| Maltose | + | ‒ | + | + | + |
| Polar lipids | Uncharacterized aminolipid, phosphatidylglycerol, phosphatidylethanolamine | ND | Phosphoaminolipids, aminolipids, glycoaminolipids | Diphosphatidylglycerol, phosphatidylethanolamine, phosphatidylmethylethanolamine, phospholipids of unknown structure containing aminoglycophospholipid, three unidentified polar lipids. | Phosphatidylethanolamine,phosphatidylglycerol, diphosphatidylglycerol, an uncharacterized aminolipid, three uncharacterized phospholipids |
| Major quinones | Q−8 | ND | Q−8 | Q−8 | Q−8 |
Taxa: 1, Aquella oligotrophica gen. nov. sp. nov. (strain P08T); 2, Neisseria (Vandamme, Holmes, Bercovier, & Coenye, 2006); 3, Amantichitinum (Moß et al., 2013); 4, Crenobacter (Dong et al., 2015); 5, Rivicola (Sheu et al., 2014). +, Positive reaction; ‒, negative reaction; Q, quinone; ND, not determined.
4.1. Description of Aquella gen. nov
Aquella gen. nov. (A.qu.el'la. L. fem. dim. n. aquella, water, referring to the source of isolation of the novel organism).
Cells are Gram‐negative, aerobic, nonmotile, rod‐shaped. Cells are oxidase and catalase negative. Q‐8 is the only quinone type. Major fatty acids (>10%) are C14:0, iso‐C15:0, and summed feature 3 (C16:1 ω7c/C16:1 ω6c). The main polar lipids consist of uncharacterized aminolipid, phosphatidylglycerol, and phosphatidylethanolamine. The G + C content of the DNA of the type strain of the type species is 36.43 mol%. Based on 16S rRNA sequence analyses, P08T belongs to the Betaproteobacteria. The type species is Aquella oligotrophica P08T.
4.2. Description of Aquella oligotrophica sp. nov
Aquella oligotrophica (ol.i.go.tro.phi'ca. Gr. adj. oligos, few; Gr. adj. trophikos, nursing, tending or feeding; N.L. fem. adj. oligotrophica, eating little, referring to the bacterium not growing in rich media).
Exhibits the following properties in addition to those given in the genus description. Cell sizes range from 0.7–1.4 μm in length and 0.3–0.5 μm in width (Figure 5). Cells occur singly, in pairs, in short chains or in irregular clusters. On R2A agar, colonies (<1 mm in diameter) are milky white, circular, and convex with entire margin. Growth occurs at 12–43 °C (optimal growth at 35–40 °C) and at pH 5.0–8.0 (optimal growth at pH 5.0–7.0). Growth occurs in the presence of NaCl up to 1% (w/v). Negative for oxidase and catalase tests. Positive for hydrolysis of casein and Tween 20, 40, 60, and 80, but negative for hydrolysis of starch, CM‐cellulose, urea, and DNA. Based on testing with API 20NE and API 20E, positive reactions towards 4‐nitrophenyl‐β‐D‐galactopyranoside, 2‐nitrophenyl‐β‐D‐galactopyranoside, and sodium pyruvate. Negative reactions toward potassium nitrate, L‐tryptophane, D‐glucose, L‐arginine, urea, esculin, ferric citrate, gelatin (bovine origin), D‐glucose, L‐arabinose, D‐mannose, D‐mannitol, N‐acetyl‐glucosamine, D‐maltose, potassium gluconate, capric acid, malic acid, trisodium citrate, phenylacetic acid, L‐lysine, L‐ornithine, sodium thiosulfate, inositol, D‐sorbitol, L‐rhamnose, D‐sucrose, D‐melibiose, and amygdalin. Based on testing with API ZYM kit, C4 esterase, C8 esterase lipase, leucine arylamidase, acid phosphatase, naphthol‐AS‐BI‐phosphohydrolase, β‐galactosidase, and N‐acetyl‐β‐glucosaminidase activities are detected but negative for activities of C14 lipase, valine arylamidase, trypsin, α‐chymotrypsin, β‐glucuronidase, α‐glucosidase, β‐glucosidase, α‐mannosidase, and α‐fucosidase. Weak enzymatic activities of alkaline phosphatase, cystine arylamidase, and α‐galactosidase are detected. The major fatty acids (> 5%) are C14:0, iso‐C15:0, C16:0, summed feature 3 (C16:1 ω7c/C16:1 ω6c), and summed feature 8 (C18:1 ω7c). The following compounds are utilized as sole carbon sources in the GEN III microplate: D‐galactose, D‐glucuronic acid, D‐mannose, glucuronamide, glycyl‐L‐proline, inosine, L‐alanine, L‐arginine, L‐aspartic acid, L‐glutamic acid, L‐serine, methyl pyruvate, N‐acetyl‐D‐glucosamine, α‐D‐glucose, and β‐hydroxy‐D,L‐butyric acid. All other substrates in the GEN III microplate are not utilized. Sensitive to ampicillin/sulbactam (20 μg), chloramphenicol (30 μg), nalidixic acid (30 μg), rifampicin (5 μg), sulfamethoxazole (23.75 μg) plus trimethoprim (1.25 μg), and tetracycline (30 μg).
Figure 5.

Scanning electron micrographs of strain P08T
The type strain Aquella oligotrophica P08T (=LMG 29629T =DSM100970T) was isolated from laboratory tap water collected at University of Malaya, Malaysia.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
AUTHORS CONTRIBUTION
KGC supervised the project. LSL carried out the experiments. WSST and LSL wrote the manuscript with support from KGC, KOC, AP, KMG, KWH, and WFY. WSST and LSL analyzed the data.
ETHICS STATEMENT
None required.
Supporting information
ACKNOWLEDGEMENT
This work was supported by University of Malaya—Ministry of Higher Education High Impact Research Grant (UM‐MOHE HIR Grant UM.C/625/1/HIR/MOHE/CHAN/14/1, Grant No. H‐ 50001‐A000027) and University of Malaya Research Grants (GA001‐2016, GA002‐2016) awarded to Kok‐Gan Chan and Postgraduate Research Grant (PPP) (Grant No. PG124‐2016A) awarded to Li Sin Lee. Kian Mau Goh thanks UTM GUP grant 15H50. Wah‐Seng See‐Too thanks Bright Sparks Unit of University of Malaya for the scholarship support. The authors thank Jian‐Woon Chen from High Impact Research Institute of University of Malaya in providing the GEN III service and Dr. Aidan Parte for his help in defining the Latin etymology of the novel taxon scientific name.
Chan K‐G, See‐Too W‐S, Chua K‐O, et al. Aquella oligotrophica gen. nov. sp. nov.: A new member of the family Neisseriaceae isolated from laboratory tap water. MicrobiologyOpen. 2019;8:e793 10.1002/mbo3.793
DATA ACCESSIBILITY
The assembled and annotated genome of P08T described in this paper has been deposited in GenBank (accession number: CP024847.1) and JGI portal (GOLD ID: Gp0293937; IMG Taxon ID: 2,770,939,448).
REFERENCES
- Adeolu, M. , & Gupta, R. S. (2013). Phylogenomics and molecular signatures for the order Neisseriales: Proposal for division of the order Neisseriales into the emended family Neisseriaceae and Chromobacteriaceae fam. nov. Antonie Van Leeuwenhoek, 104(1), 1–24. 10.1007/s10482-013-9920-6 [DOI] [PubMed] [Google Scholar]
- Aziz, R. K. , Bartels, D. , Best, A. A. , DeJongh, M. , Disz, T. , Edwards, R. , A., … Kubal, M. (2008). The RAST Server: Rapid annotations using subsystems technology. BMC Genomics, 9(1), 75 10.1186/1471-2164-9-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge, T. J. , Lawrence, J. R. , & Murray, R. G. (2007). Sampling and staining for light microscopy. In Methods for General and Molecular Microbiology (Third ed., pp. 19‐33). Washington, DC: American Society of Microbiology. [Google Scholar]
- Bligh, E. G. , & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37(8), 911–917. 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bush, K. , & Jacoby, G. A. (2010). Updated functional classification of β‐lactamases. Antimicrobial Agents and Chemotherapy, 54(3), 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerny, G. (1978). Studies on the aminopeptidase test for the distinction of gram‐negative from gram‐positive bacteria. European Journal of Applied Microbiology and Biotechnology, 5(2), 113–122. 10.1007/BF00498805 [DOI] [Google Scholar]
- Chin, C.‐S. , Alexander, D. H. , Marks, P. , Klammer, A. A. , Drake, J. , Heiner, C. , … Eichler, E. E. (2013). Nonhybrid, finished microbial genome assemblies from long‐read SMRT sequencing data. NatureMethods, 10(6), 563–569. 10.1038/nmeth.2474 [DOI] [PubMed] [Google Scholar]
- Ding, W. , Baumdicker, F. , & Neher, R. A. (2018). panX: Pan‐genome analysis and exploration. Nucleic Acids Research., 46(1), e5 10.1093/nar/gkx977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, L. , Ming, H. , Zhou, E.‐M. , Yin, Y.‐R. , Liu, L. , Feng, H.‐G. , … Li, W.‐J. (2015). Crenobacter luteus gen. nov., sp. nov., isolated from a hot spring. International Journal of Systematic and Evolutionary Microbiology, 65(1), 214–219. 10.1099/ijs.0.060996-0. [DOI] [PubMed] [Google Scholar]
- Felsenstein, J. (1981). Evolutionary trees from DNA sequences: A maximum likelihood approach. Journal of Molecular Evolution, 17(6), 368–376. 10.1007/BF01734359 [DOI] [PubMed] [Google Scholar]
- Felsenstein, J. (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution, 39(4), 783–791. 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Fitch, W. M. (1971). Toward defining the course of evolution: Minimum change for a specific tree topology. Systematic Zoology, 20(4), 406–416. 10.2307/2412116. [DOI] [Google Scholar]
- Garrity, G. , Staley, J. T. , Boone, D. R. , DeVos, P. , Goodfellow, M. , Rainey, F. A. , & Schleifer, K.‐H. (2006). Bergey's Manual® of Systematic Bacteriology: Volume Two: The Proteobacteria (G. Garrity Ed.) New York, NY: Springer Science & Business Media. [Google Scholar]
- Henriksen, S. , & Bøvre, K. (1976). Transfer of Moraxella kingae Henriksen and Bøvre to the genus Kingella gen. nov. in the family Neisseriaceae . International Journal of Systematic and Evolutionary Microbiology, 26(4), 447–450. 10.1099/00207713-26-4-447. [DOI] [Google Scholar]
- Jackson, F. , & Goodman, Y. E. (1972). Transfer of the facultatively anaerobic organism Bacteroides corrodens Eiken to a new genus. Eikenella. International Journal of Systematic Bacteriology, 22(2), 73–77. 10.1099/00207713-22-2-73. [DOI] [Google Scholar]
- Kimura, M. (1980). A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution, 16(2), 111–120. 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Konstantinidis, K. T. , & Tiedje, J. M. (2005). Towards a Genome‐Based Taxonomy for Prokaryotes. Journal of Bacteriology, 187(18), 6258–6264. 10.1128/JB.187.18.6258-6264.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuykendall, L. , Roy, M. , O'neill, J., & Devine, T., (1988). Fatty acids, antibiotic resistance, and deoxyribonucleic acid homology groups of Bradyrhizobium japonicum . International Journal of Systematic and Evolutionary Microbiology, 38(4), 358–361. 10.1099/00207713-38-4-358. [DOI] [Google Scholar]
- Kwong, W. K. , & Moran, N. A. (2013). Cultivation and characterization of the gut symbionts of honey bees and bumble bees: Description of Snodgrassella alvi gen. nov., sp. nov., a member of the family Neisseriaceae of the Betaproteobacteria, and Gilliamella apicola gen. nov., sp. nov., a member of Orbaceae fam. nov., Orbales ord. nov., a sister taxon to the order ‘Enterobacteriales’ of the Gammaproteobacteria . International Journal of Systematic and Evolutionary Microbiology, 63(6), 2008–2018. 10.1099/ijs.0.044875-0. [DOI] [PubMed] [Google Scholar]
- Lagesen, K. , Hallin, P. , Rødland, E. A. , Stærfeldt, H.‐H. , Rognes, T. , & Ussery, D. W. (2007). RNAmmer: Consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Research, 35(9), 3100–3108. 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeron, M. (1923). Les Oscillariees parasite du tube dogestif de l’homme et des animaux. Annales De Parasitologie Humaine Et Comparée, 1(2), 113. [Google Scholar]
- Lee, I. , Kim, Y. O. , Park, S.‐C. , & Chun, J. (2015). OrthoANI: An improved algorithm and software for calculating average nucleotide identity. International Journal of Systematic and Evolutionary Microbiology, 66(2), 1100–1103. 10.1099/ijsem.0.000760. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Xue, H. , Sang, S.‐Q. , Lin, C.‐L. , & Wang, X.‐Z.‐J.‐P. (2017). Phylogenetic analysis of family Neisseriaceae based on genome sequences and description of Populibacter corticis gen. nov., sp. nov., a member of the family Neisseriaceae, isolated from symptomatic bark of Populus× euramericana Canker. PLoS ONE, 12(4), e0174506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liolios, K. , Chen, I.‐M.‐A. , Mavromatis, K. , Tavernarakis, N. , Hugenholtz, P. , Markowitz, V. M. , & Kyrpides, N. C. (2010). The Genomes On Line Database (GOLD) in 2009: Status of genomic and metagenomic projects and their associated metadata. Nucleic Acids Research, 38(suppl 1), D346–D354. 10.1093/nar/gkp848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, P. , Sly, L. , Pham, A. , & Davis, G. (1981). Characterization of Morococcus cerebrosus gen. nov., sp. nov. and comparison with Neisseria mucosa . International Journal of Systematic and Evolutionary Microbiology, 31(3), 294–301. 10.1099/00207713-31-3-294. [DOI] [Google Scholar]
- Ludwig, W. , Strunk, O. , Klugbauer, S. , Klugbauer, N. , Weizenegger, M. , Neumaier, J. , … Schleifer, K. H. (1998). Bacterial phylogeny based on comparative sequence analysis. Electrophoresis, 19(4), 554–568. 10.1002/elps.1150190416. [DOI] [PubMed] [Google Scholar]
- Markowitz, V. M. , Mavromatis, K. , Ivanova, N. N. , Chen, I.‐M.‐A. , Chu, K. , & Kyrpides, N. C. (2009). IMG ER: A system for microbial genome annotation expert review and curation. Bioinformatics, 25(17), 2271–2278. 10.1093/bioinformatics/btp393 [DOI] [PubMed] [Google Scholar]
- Miller, L. T. (1982). Single derivatization method for routine analysis of bacterial whole‐cell fatty acid methyl esters, including hydroxy acids. Journal of Clinical Microbiology, 16(3), 584–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moß, K. S. , Hartmann, S. C. , Müller, I. , Fritz, C. , Krügener, S. , Zibek, S. , … Rupp, S. (2013). Amantichitinum ursilacus gen. nov., sp. nov., a chitin‐degrading bacterium isolated from soil. International Journal of Systematic and Evolutionary Microbiology, 63(1), 98–103. 10.1099/ijs.0.034447-0. [DOI] [PubMed] [Google Scholar]
- Nokhal, T.‐H. , & Schlegel, H. G. (1983). Taxonomic study of Paracoccus denitrificans . International Journal of Systematic and Evolutionary Microbiology, 33, 26–37. 10.1099/00207713-33-1-26. [DOI] [Google Scholar]
- Overbeek, R. , Olson, R. , Pusch, G. D. , Olsen, G. J. , Davis, J. J. , Disz, T. , … Shukla, M. (2014). The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Research, 42(D1), D206–D214. 10.1093/nar/gkt1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parte, A. C. (2013). LPSN—list of prokaryotic names with standing in nomenclature. Nucleic Acids Research, 42(D1), D613–D616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattengale, N. D. , Alipour, M. , Bininda‐Emonds, O. R. , Moret, B. M. , & Stamatakis, A. (2010). How many bootstrap replicates are necessary? Journal of Computational Biology, 17(3), 337–354. 10.1089/cmb.2009.0179 [DOI] [PubMed] [Google Scholar]
- Pot, B. , Willems, A. , Gillis, M. , & De Ley, J. (1992). Intrageneric and intergeneric relationships of the genus Aquaspirillum: Prolinoborus, a new genus for Aquaspirillum fasciculus, with the species Prolinoborus fasciculus comb. nov. International Journal of Systematic and Evolutionary Microbiology, 42(1), 44–57. [Google Scholar]
- Pringsheim, E. (1949). The relationship between bacteria and Myxophyceae. Bacteriological Reviews, 13(2), 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, Q.‐L. , Xie, B.‐B. , Zhang, X.‐Y. , Chen, X.‐L. , Zhou, B.‐C. , Zhou, J. , … Zhang, Y.‐Z. (2014). A proposed genus boundary for the prokaryotes based on genomic insights. Journal of Bacteriology, 196(12), 2210–2215. 10.1128/JB.01688-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter, M. , & Rosselló‐Móra, R. (2009). Shifting the genomic gold standard for the prokaryotic species definition. Proceedings of the National Academy of Sciences of the United States of America, 106(45), 19126–19131. 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐R, L. M. , & Konstantinidis, K. T. (2016). The enveomics collection: A toolbox for specialized analyses of microbial genomes and metagenomes. Peer J, 10.7287/peerj.preprints.1900v1. [DOI] [Google Scholar]
- Saitou, N. , & Nei, M. (1987). The neighbor‐joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4(4), 406–425. 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sheu, S.‐Y. , Chen, J.‐C. , Young, C.‐C. , & Chen, W.‐M. (2014). Rivicola pingtungensis gen. nov., sp. nov., a new member of the family Neisseriaceae isolated from a freshwater river. International Journal of Systematic and Evolutionary Microbiology, 64(6), 2009–2016. 10.1099/ijs.0.055285-0. [DOI] [PubMed] [Google Scholar]
- Simons, H. (1922). Saprophytische Oscillarien des Menschen und der Tiere. Zentralbl Bakteriol B, 88, 501–510. [Google Scholar]
- Srinivas, T. , Manasa, P. , Begum, Z. , Sunil, B. , Sailaja, B. , Singh, S. , … Shivaji, S. (2013). Iodobacter arcticus sp. nov., a psychrotolerant bacterium isolated from meltwater stream sediment of an Arctic glacier. International Journal of Systematic and Evolutionary Microbiology, 63(8), 2800–2805. 10.1099/ijs.0.044776-0. [DOI] [PubMed] [Google Scholar]
- Stamatakis, A. (2014). RAxML version 8: A tool for phylogenetic analysis and post‐analysis of large phylogenies. Bioinformatics, 30(9), 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis, A. , Hoover, P. , & Rougemont, J. (2008). A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology, 57(5), 758–771. 10.1080/10635150802429642 [DOI] [PubMed] [Google Scholar]
- Su, W. , Zhou, Z. , Jiang, F. , Chang, X. , Liu, Y. , Wang, S. , … Peng, F. (2013). Iodobacter limnosediminis sp. nov., isolated from Arctic lake sediment. International Journal of Systematic and Evolutionary Microbiology, 63(4), 1464–1470. 10.1099/ijs.0.039982-0. [DOI] [PubMed] [Google Scholar]
- Tamura, K. , Stecher, G. , Peterson, D. , Filipski, A. , & Kumar, S. (2013). MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30(12), 2725–2729. 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatusov, R. L. , Galperin, M. Y. , Natale, D. A. , & Koonin, E. V. (2000). The COG database: A tool for genome‐scale analysis of protein functions and evolution. Nucleic Acids Research, 28(1), 33–36. 10.1093/nar/28.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatusova, T. , DiCuccio, M. , Badretdin, A. , Chetvernin, V. , Nawrocki, E. P. , Zaslavsky, L. , … Ostell, J. (2016). NCBI prokaryotic genome annotation pipeline. Nucleic Acids Research, 44(14), 6614–6624. 10.1093/nar/gkw569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindall, B. (1990a). A comparative study of the lipid composition of Halobacterium saccharovorum from various sources. Systematic and Applied Microbiology, 13(2), 128–130. 10.1016/S0723-2020(11)80158-X. [DOI] [Google Scholar]
- Tindall, B. (1990b). Lipid composition of Halobacterium lacusprofundi . FEMS Microbiology Letters, 66(1–3), 199–202. 10.1111/j.1574-6968.1990.tb03996.x. [DOI] [Google Scholar]
- Tindall, B. J. , Sikorski, J. , Smibert, R. A. , & Krieg, N. R. (2007). Phenotypic characterization and the principles of comparative systematics (Third Ed), Methods for General and Molecular Microbiology (pp. 330–393). Washington, DC: American Society of Microbiology. [Google Scholar]
- Tønjum, T. (2015). Neisseria In B. E. Murray P. R., Pfaller M. A., Tenover F. C., & Yolken R. H. (Ed.), Manual of Clinical Microbiology (5th ed., pp. 1–48). Washington, DC: American Society for Microbiology. [Google Scholar]
- Trevisan, V. (1885). Caratteri di alcuni nuovi generi di Batteriacee. Atti. Accad. Fisio‐medico‐statistica Milano (ser, 4), 3, 92–107. [Google Scholar]
- Vandamme, P. , Holmes, B. , Bercovier, H. , & Coenye, T. (2006). Classification of Centers for Disease Control Group Eugonic Fermenter (EF)‐4a and EF‐4b as Neisseria animaloris sp. nov. and Neisseria zoodegmatis sp. nov., respectively. International Journal of Systematic and Evolutionary Microbiology, 56(8), 1801–1805. 10.1099/ijs.0.64142-0. [DOI] [PubMed] [Google Scholar]
- Vela, A. , Collins, M. , Lawson, P. , García, N. , Domínguez, L. , & Fernández‐Garayzábal, J. (2005). Uruburuella suis gen. nov., sp. nov., isolated from clinical specimens of pigs. International Journal of Systematic and Evolutionary Microbiology, 55(2), 643–647. 10.1099/ijs.0.63346-0. [DOI] [PubMed] [Google Scholar]
- Wertz, J. T. , & Breznak, J. A. (2007). Stenoxybacter acetivorans gen. nov., sp. nov., an acetate‐oxidizing obligate microaerophile among diverse O2‐consuming bacteria from termite guts. Applied and Environment Microbiology, 73(21), 6819–6828. 10.1128/AEM.00786-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, C.‐H. , & Yokota, A. (2005). Phylogenetic analysis of Alysiella and related genera of Neisseriaceae: Proposal of Alysiella crassa comb. nov., Conchiformibium steedae gen. nov., comb. nov., Conchiformibium kuhniae sp. nov. and Bergeriella denitrificans gen. nov., comb. nov. Journal of General and Applied Microbiology, 51(1), 1–10. 10.2323/jgam.51.1. [DOI] [PubMed] [Google Scholar]
- Yoon, S.‐H. , Ha, S.‐M. , Kwon, S. , Lim, J. , Kim, Y. , Seo, H. , & Chun, J. (2017). Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole‐genome assemblies. International Journal of Systematic and Evolutionary Microbiology, 67(5), 1613–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The assembled and annotated genome of P08T described in this paper has been deposited in GenBank (accession number: CP024847.1) and JGI portal (GOLD ID: Gp0293937; IMG Taxon ID: 2,770,939,448).


