Abstract
Objectives:
To determine the relationship of coronary artery calcium (CAC) scores with subsequent cardiovascular disease (CVD) events in DCCT/EDIC participants.
Background:
The CAC score has been validated to improve risk stratification in general populations; however, this association has not been well-studied in type 1 diabetes (T1DM).
Methods:
Computed tomography (CT) to measure CAC was performed in 1,205 DCCT/EDIC participants with a mean age of 42.8 years during EDIC 7-9 years after the end of DCCT. We analyzed the association of CAC with time to the first subsequent CVD event or to the first major adverse cardiac event (MACE), a follow-up of 10-13 years. CAC was categorized as: 0, >0–100, >100–300, or >300 Agatston units.
Results:
Of 1156 at risk of subsequent CVD, 105 had an initial CVD event (8.5 per 1,000 patient-years); and of 1187 at risk of MACE, 51 had an initial MACE event (3.9 per 1,000 patient-years). Event rates among those with zero scores (n=817, 70.7%) were very low for CVD (5.6 per 1000 patient years). CAC scores >100-300 (HR=4.17, 5.40) and >300 (HR=6.06, 6.91) were associated with higher risks of CVD and MACE, respectively, compared to CAC=0 (p<0.0001). CAC scores >0-100 were nominally associated with CVD (HR=1.71, p=0.0415) but not with MACE (HR=1.11, p=0.8134). Similar results were observed when also adjusted for mean HbA1c and traditional CVD risk factors. The increment in the AUC due to CAC was modest.
Conclusions:
CAC scores greater than 100 Agatston units were significantly associated with an increased risk of the subsequent occurrence of CVD and MACE in DCCT/EDIC cohort.
Trial Registration:
Keywords: Type 1 diabetes, Coronary artery calcification, Cardiovascular disease, Major Adverse Cardiovascular Event
INTRODUCTION
Type 1 diabetes mellitus (T1DM) is associated with increased risk of cardiovascular complications including myocardial infarction, stroke, congestive heart failure, and unstable angina (1). In addition to glycemia (2), numerous traditional risk factors (age, hypertension, hyperlipidemia, etc.) and unknown hereditary, genetic and environmental factors may be involved. Risk factors for cardiovascular disease (CVD) are well established in type 2 diabetes mellitus (T2DM) but less well established in T1DM, perhaps due to the difference in duration of diabetes, lower body weights and lower prevalence of these traditional risk factors. The coronary artery calcium (CAC) score provides an assessment of calcified coronary artery plaques, a marker of atherosclerotic burden (3). An elevated CAC score has been shown to be predictive of clinical outcomes in several cohorts that include various proportions of participants with diabetes (4-8). However, the association of CAC scores in individuals with T1DM is not well studied (9).
The Diabetes Control and Complications Trial (DCCT) (10) enrolled 1441 T1DM individuals, most of whom enrolled in the follow-up study, the Epidemiology of Diabetes Interventions and Complications (EDIC) (11) study. After ~7 years of EDIC follow-up, CAC scores were measured in 1,205 participants with a mean age of 42.8 years; prior intensive treatment during DCCT was associated with lower CAC scores (12). Herein we present additional analyses to assess the association of CAC scores with the development of initial CVD events in DCCT/EDIC participants who were followed over a subsequent 10 to 13 years.
RESEARCH DESIGN AND METHODS
Subjects
The Diabetes Control and Complications Trial (DCCT), a randomized controlled clinical trial (13), compared the effects of intensive (N=711) versus conventional (N=730) diabetes therapy on long-term diabetes complications. During 1983-1989, 1441 individuals aged 13-39 years old were enrolled: 726 into the primary prevention cohort (diabetes duration 1-5 years and no evidence of microvascular complications), and 715 into the secondary intervention cohort (1-15 years duration and minimal retinopathy or nephropathy complications). After the end of the DCCT (1993), participants in the conventional treatment group were instructed in intensive therapy and referred to their personal physicians for continued diabetes care.
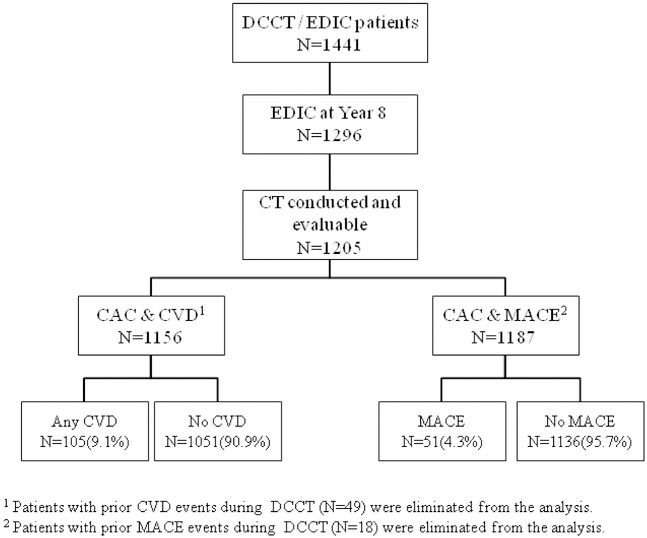
The Epidemiology of Diabetes Interventions and Complications (EDIC) (11) study, an observational follow-up of the DCCT cohort, started in 1994; 1394 (98.2%) of 1420 surviving DCCT participants enrolled. Of these, 1205 of the1296 survivors (93%) consented to undergo Coronary Artery Calcium Computed Tomography (CT) 7-9 years after completion of DCCT (EDIC year 7 between 2001 and 2002). Of these, 49 participants had a prior cardiovascular disease (CVD) event and were excluded from these analyses, leaving 1156 participants with CT who were at risk of an initial CVD event. Likewise, 18 participants who had a prior major adverse cardiac event (MACE) were excluded, leaving 1187 participants at risk for an initial MACE event. Figure 1 depicts the EDIC participants available for the subsequent CVD and MACE analyses.
Figure 1. Flow chart of the Epidemiology of Diabetes Interventions and Complications (EDIC).
EDIC Participants with available Computed Tomography (CT) evaluations. At the time CT was performed (EDIC years 7-9), 1205 patients obtained evaluable CT scans. Forty-nine and 18 of the participants were eliminated from the analyses due to the prior CVD and MACE events, respectively.
DCCT/EDIC Covariates
Covariate values were obtained concurrent with or at the last visit prior to the CT evaluation. Smoking, blood pressure, pulse, and body mass index were obtained from an annual follow-up evaluation. The Central Biochemistry Laboratory (CBL) measured hemoglobin A1c (HbA1c) levels quarterly during the DCCT and annually in EDIC. The DCCT/EDIC time-weighted mean HbA1c, with weights of 0.25 for DCCT and 1 for EDIC values, represents the total glycemic exposure during DCCT/EDIC. Fasting lipids and albumin excretion rate (AER) were measured annually during DCCT and in alternate years during EDIC. Microalbuminuria was defined as a history of AER>30 mg/24 hours on at least two consecutive annual visits..
Coronary Artery Calcification (CAC) Assessment
The methods to obtain the CT-derived CAC scores were previously described (12). In brief, CT was performed in 19 scanning sites (see appendix) using a C-150 cardiac-gated electron beam CT scanner (n=9; Imatron, San Francisco, CA), a Lightspeed (n=7; General Electric Medical Systems, Waukesha, WI) or a Volume Zoom (Siemens, Erlanger, Germany) multi-detector CT system, a Lightspeed Marconi MX-8000 (GE), or a Somatom 4+ (Siemens) (n=3). All participants were scanned twice over calibration phantoms of known physical calcium concentration.
CT-scans were read centrally at the Los Angeles Biomedical Research Center Core CT lab (University of California, Los Angeles) to identify and quantify CAC, using the method of Agatston et al. (13) The measurement of coronary artery calcium was assessed by measuring all pixels with density >130 Hounsfield units (HU). The area of the calcium was multiplied by the density factor represented by the peak density in each calcific lesion, with 1=130-199 HU; 2=200-299 HU; 3=300-399 HU; and 4=≥400. The calcium score was obtained by summing all calcific lesions in all 4 major coronary arteries and side branches. The average score from the two scans was used in the analysis. Readers were masked to participant identity and prior DCCT treatment assignment. Coronary Artery Calcium (CAC) was classified into 4 categories: =0, >0–100, >100–300, or >300 Agatston units for the current analysis.
Reading center staff evaluated scan quality based on seven criteria: motion artifact, streak artifact, phantom placement, slice registration, lack of noise, axis coverage, and xy axis coverage. The 19 scanning centers were monitored monthly using these criteria. The intra- and inter-reader precision was evaluated with the use of a set of standard scans that were reread by the same reader and another reader at the reading center. The kappa measure of intra-reader agreement beyond chance for the presence or absence of calcification was 0.81, and the inter-reader kappa was 0.86. The coefficient of reliability for the numerical CAC scores was 0.99 for both inter- and intra-reader as reported previously (12).
Cardiovascular Outcomes
The primary outcome was the time to the first CVD event including either non-fatal myocardial infarction or stroke; death judged to be secondary to cardiovascular disease; subclinical (“silent”) myocardial infarction detected on an annual electrocardiogram; angina confirmed by ischemic changes with exercise tolerance testing or by clinically significant obstruction on coronary angiography; congestive heart failure with paroxysmal nocturnal dyspnea, orthopnea or marked limitation of physical activity caused by heart disease; or revascularization with angioplasty and/or coronary artery bypass. We also evaluated time to the first major adverse cardiovascular event (MACE), including non-fatal MI, non-fatal stroke, or CV death.
Cardiovascular events were captured by participant self-report during the EDIC annual visits, documented by medical records, and centrally adjudicated by the EDIC Mortality and Morbidity Review Committee masked to DCCT treatment assignment, HbA1c, and glucose levels. The analyses reported here only included adjudicated qualifying cardiovascular events that occurred after the CT examination (~2001-2002) through December 31, 2013, a period of 10 to 13 years depending on the time of the CT. This data lock date was selected to provide adequate statistical power for multivariate modeling in the complete cohort. (2).
Statistical Analysis
The Contingency Chi-Square test assessed differences in categorical characteristics among the four CAC score groups, and the Cochran-Armitage trend test assessed a linear trend (increasing or decreasing) in proportions among the ordered groups. The Kruskal-Wallis test compared quantitative characteristics among groups, and the ANOVA linear trend test assessed linear trends in the means (14).
The Kaplan-Meier estimate of the cumulative incidence function of a CVD event within the four CAC score groups is presented, and the differences among groups tested using the log-rank test. (15) Cox proportional hazards models assessed differences among the CAC score groups in the risk of subsequent CVD events adjusted for known risk factors. Hazard ratios (HR), 95% confidence intervals (CI) and Wald test p-values (p) were reported. (16) Cox proportional hazards models with CAC score group number as a quantitative covariate provided a Wald test of linear trend among CAC score groups.
Cox Model A was minimally adjusted for scanning site, age and gender, while Model B further adjusted for DCCT cohort (primary prevention vs. secondary intervention cohort), mean HbA1c, systolic blood pressure, antihypertensive medication, LDL cholesterol, HDL cholesterol, and smoking at the time of the CT (EDIC years 7-9) and DCCT baseline family history of MI. (2). The association between CAC and the subsequent risk of any CVD and MACE in these multivariable models was assessed using Wald chi-square tests with 3df. Area under the ROC curve (AUC) was calculated using 4 CAC categories for each Cox model based on the Gonen-Heller approach (17), and confidence intervals for difference in AUCs were obtained using bootstrapping. The AUC describes the predictive accuracy of a model, with an AUC=0.5 corresponding to random predictions and an AUC=1 to corresponding to perfect predictions. The predictive value of a biomarker is represented by the increase in AUC (if any) when the marker is added to the model with the other covariates.
Results nominally significant at p < 0.05 (two-sided) are cited. All analyses were performed using SAS software (version 9.3; SAS Institute, Cary, NC) and R.
RESULTS
Of the 1156 participants at risk of an initial CVD event, 817 (70.7%) had a CAC score of 0, 221(19.1%) a CAC score >0-100, 65 (5.6%) a CAC score >100-300 and 53 (4.6%) a CAC score >300 Agatston units. Table 1 presents participant characteristics at the time of the CT within the four CAC score groups; mean age was 42.8 years, mean systolic blood pressure (SBP) was 122 mm Hg, 27.5% were using anti-hypertensive medication and 47.2% were females. Women had lower CAC scores than men. Based on the trend test, increasing CAC scores were also associated with older age, longer duration of diabetes, smoking, higher SBP (but not DBP), lower HDL cholesterol, higher triglycerides, and history of microalbuminuria. Also, the mean non-HDL cholesterol differed significantly among the CAC categories, but without a significant linear trend, with the mean in the CAC score 0 group being less than that in the higher CAC score groups, after adjustment for gender. Higher CAC scores were also associated with increasing anti-hypertensive and lipid-lowering medication use. Interestingly, CAC score was not associated with the current HbA1c, DCCT/EDIC time-weighted mean HbA1c, LDL or total cholesterol.
Table 1.
Characteristics prior to or at CT scan
| Characteristics* | All Participants |
CAC Score group |
|||||
|---|---|---|---|---|---|---|---|
| 0 | >0 – 100 | >100 – 300 | > 300 | Difference p-value |
Trend p-value |
||
| N | 1156 | 817 | 221 | 65 | 53 | ||
| Female (%) | 47.2 | 54.1 | 33.5 | 27.7 | 22.6 | < .0001 | < .0001 |
| Race (%White) | 96.5 | 96.3 | 95.9 | 98.5 | 100.0 | .3954 | .1792 |
| Intensive group (%) | 49.9 | 50.8 | 49.8 | 46.2 | 41.5 | .5477 | .1704 |
| Primary Cohort (%) | 50.6 | 54.4 | 46.2 | 36.9 | 28.3 | < .0001 | < .0001 |
| Age, mean (SD), years | 42.8 (6.8) | 41.4 (6.7) | 45.2 (6.0) | 47.1 (5.9) | 48.9 (5.1) | < .0001 | < .0001 |
| Duration of T1DM, mean (SD), years | 21.0 (4.9) | 20.6 (4.7) | 21.4 (5.0) | 23.3 (5.6) | 23.0 (4.)5 | < .0001 | < .0001 |
| Current cigarette smokers (%) | 14.6 | 12.0 | 19.5 | 23.1 | 24.5 | .0009 | .0001 |
| Body Mass Index, mean (SD), kg/m2 | 27.5 (4.4) | 27.4 (4.5) | 27.8 (5.1) | 27.8 (3.8) | 27.6 (4.0) | .3251 | .7474 |
| Systolic Blood pressure, mean (SD), mm Hg | 122 (14) | 121 (14) | 124 (14) | 125 (13) | 129 (15) | < .0001 | < .0001 |
| Diastolic Blood pressure, mean (SD), mm Hg | 76 (9) | 77 (9) | 76 (9) | 76 (9) | 78 (9) | .5037 | .2881 |
| Anti-Hypertensive medication (%) | 27.5 | 22.9 | 33.9 | 49.2 | 45.3 | < .0001 | < .0001 |
| HbA1c (%) | 7.9 (1.3) | 7.9 (1.3) | 8.0 (1.4) | 8.0 (1.2) | 8.1 (1.4) | .1960 | .1776 |
| Weighted mean HbA1c, mean (SD) | 8.1 (1.1) | 8.1 (1.1) | 8.2 (1.0) | 8.2 (1.2) | 8.2 (1.0) | .1431 | .2727 |
| HDL Cholesterol, mean (SD), mg/dl | 56 (15) | 57 (15) | 53 (14) | 53 (12) | 53 (15) | .0001 | .0271 |
| Non-HDL Cholesterol, mean (SD), mg/dl | 130 (34) | 128 (34) | 133 (36) | 135 (28) | 136 (33) | .0175 | .1098 |
| LDL Cholesterol, mean (SD), mg/dl | 112 (29) | 111 (29) | 114 (30) | 116 (28) | 115 (29) | .2448 | .2751 |
| Total Cholesterol, mean (SD), mg/dl | 186 (34) | 186 (34) | 187 (35) | 188 (29) | 188 (36) | .6624 | .5617 |
| Triglyceride, mean (SD), mg/dl | 89 (61) | 85 (53) | 96 (75) | 106 (80) | 107 (79) | .0071 | .0059 |
| Lipid-lowering medication (%) | 21.0 | 14.8 | 31.2 | 43.1 | 47.2 | < .0001 | < .0001 |
| Microalbuminuria ‡(%) | 24.6 | 22.3 | 24.4 | 36.9 | 45.3 | .0002 | < .0001 |
N(%) for categorical variables or Mean ± SD for continuous variables.
p-value is based on Contingency Chi- Square test (df=3) of any difference among CAC score groups and Cochran-Armitage trend test (df=1) for categorical variables; and the Kruskal-Wallis test (df=3) of any difference and ANOVA linear trend test (df=1) for continuous variables.
Microalbuminuria defined as a history of Albumin Excretion Rate (AER) > 30 mg/24h at least two consecutive visits during DCCT/EDIC.
During the 10 to13 years of follow-up after the CT examination (through 12/31/2013), 105 of the 1156 participants at risk over 12,350 patient-years had an initial CVD event (8.5 per 1,000 patient years, 95% CI: 7.0,10.3), and 51 of 1187 at risk over 13,006 patient-years had an initial MACE event (3.9 per 1,000 patient years; CI: 3.0,5.2).
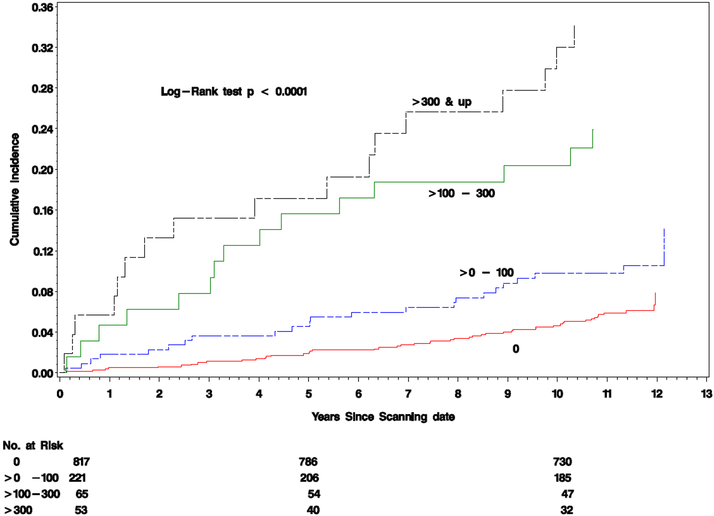
Table 2 shows that the crude incidence of any CVD among the 4 CAC score groups increased as the CAC score category increased with values of 6.1%, 10.4%, 23.1% and 32.1%, respectively. The corresponding CVD event rates in the four CAC categories were 5.6, 9.9, 24.4, and 37.9 per 1000 patient years. Figure 2 presents the cumulative incidence of any CVD following the CT examination within the four CAC score groups. Increasing calcium scores were strongly associated with higher risks of CVD events (p<0.0001).
Table 2.
Incidence of any CVD and of MACE 10-13 years after the CT examination.
| All Participants |
CT group |
||||||
|---|---|---|---|---|---|---|---|
| 0 | > 0 – 100 | > 100 – 300 | > 300 | Difference p-value |
Trend p-value |
||
| Any CVD Event | |||||||
| N participants at risk | 1156 | 817 | 221 | 65 | 53 | ||
| First Event, n(%) | 105 (9.1) | 50 (6.1) | 23 (10.4) | 15 (23.1) | 17 (32.1) | < .0001 | < .0001 |
| Patient years (PY) | 12,350 | 8,952 | 2,334 | 615 | 449 | ||
| Rate per 1000 PY | 8.5 | 5.6 | 9.9 | 24.4 | 37.9 | ||
| 95% Confidence Limits | (7.0, 10.3) | (4.2, 7.4) | (6.6, 14.8) | (14.5, 41.0) | (23.2, 61.8) | ||
| MACE Event | |||||||
| N participants at risk | 1187 | 827 | 226 | 72 | 62 | ||
| First Event, n(%) | 51 (4.3) | 23 (2.8) | 7 (3.1) | 10 (13.9) | 11 (17.7) | < .0001 | < .0001 |
| Patient years (PY) | 13,006 | 9,200 | 2,479 | 722 | 605 | ||
| Rate per 1000 PY | 3.9 | 2.5 | 2.8 | 13.9 | 18.2 | ||
| 95% Confidence Limits | (3.0, 5.2) | (1.7, 3.8) | (1.3, 5.9) | (7.4, 26.1) | (10.1, 32.7) | ||
The difference p-value is obtained from a Cox PH model adjusted for the scanning site using the 4 CAC score groups as a class effect on 3 degrees of freedom (df). The trend p-value was obtained from a Cox PH model adjusted for the scanning site using the 4 CAC score groups as a quantitative covariate on 1 df.
Figure 2. Cardiovascular Events by coronary artery calcium Score.
Cumulative incidence of the first cardiovascular event by coronary artery calcium group (CAC) scores: 0, >0-100, >100-300, >300 Agatston units. P-value from Log-Rank test was < 0.0001.
Using the annual rate per year (Table 2), the absolute risks (cumulative incidences, Figure 2) of any CVD over the 5 years following the CAC evaluation of CAC = 0, >0-100, >100-300, and >300 are 2.8%, 4.8%, 11.5% and 17.3%, respectively. Thus, the 5-year risk of any CVD with a calcium score >300 is 1.5 times greater than with a calcium score of >100-300, and the latter is 2.4 times greater than a calcium score of 0-100 Agatston units.
The incidence of MACE similarly increased significantly (p<0.0001) over the four CAC score categories with respective values of 2.8%, 3.1%, 13.9% and 17.7% and corresponding event rates of 2.5, 2.8, 13.9 and 18.2 per 1000 patient years, respectively. The corresponding 5- year absolute risks of MACE for four CAC categories, CAC = 0, >0-100, >100-300, and > 300 are 1.2%, 1.4%, 6.7% and 8.7%, respectively. Thus, the 5-year risk of subsequent MACE with a calcium score >300 is 1.3 times greater than with a calcium score of >100-300, and the latter is 4.8 times greater than with a calcium score of >0-100 Agatston units.
CAC was highly associated with the subsequent risk of CVD and MACE in Cox models A (3df Wald chi-square values of 38.68 and 20.86, respectively, p<0.0001 for both) and B (3df Wald chi-square values of 30.53 and 27.38, respectively, p<0.0001 for both).
Table 3 presents hazard ratios (HRs) for any CVD and for MACE estimated from Cox proportional hazards models comparing the upper 3 CAC score groups to the first category (CAC score 0), with adjustment for other covariates. Adjusted only for the scanning site, gender, and age (Model A), CAC scores of >100-300 (CVD: HR=4.17, 95%CI (2.23, 7.80); MACE: HR=5.40, 95%CI (2.37, 12.27))and >300 (CVD: HR=6.06, 95%CI (3.22, 11.40); MACE: HR=6.91, 95%CI (2.99, 15.97)) had higher risks of both any CVD and MACE compared with CAC 0 (p<0.0001), while the HR for a CAC score of >0-100 was significant for any CVD (HR=1.71, 95%CI (1.02, 2.88), p=0.0415) but not for MACE (HR=1.11, 95%CI (0.46, 2.66), p=0.8134). The increased risks for CAC scores of >100-300 and >300, but not >0-100, remained significant after further adjustment for mean HbA1c and other traditional risk factors (Model B, see Methods). The same findings were observed when the mean HbA1c value was substituted for treatment group (data not shown), and when Models B was further adjusted for use of lipid lowering medications, ACE inhibitor, or T1DM duration (data not shown). . In addition, the interactions between CAC and gender (p=0.4922), between CAC and mean HbA1c (p=0.2431), and between CAC and intensive vs. conventional treatment group (p=0.3693) were not significant.
Table 3.
The Association of CAC at EDIC Year 7-9 with subsequent CVD and MACE among those still at risk during EDIC.
| CVD (N=1156) |
MACE (N=1187) |
|||
|---|---|---|---|---|
| Subclinical | Hazard ratio (95% C.I.) |
p-value | Hazard ratio (95% C.I.) |
p-value |
| CAC Score (Agatston units) | ||||
| Model A* | ||||
| 0 | 1[Reference] | 1[Reference] | ||
| >0 – 100 | 1.71 (1.02, 2.88) | .0415 | 1.11 (0.46, 2.66) | .8134 |
| >100 – 300 | 4.17 (2.23, 7.80) | < .0001 | 5.40 (2.37, 12.27) | < .0001 |
| >300 | 6.06 (3.22, 11.40) | < .0001 | 6.91 (2.99, 15.97) | < .0001 |
| Chi-Square Test‡ | 38.68 | <.0001 | 20.86 | <.0001 |
| Model B† | ||||
| 0 | 1[Reference] | 1[Reference] | ||
| >0 – 100 | 1.54 (0.91, 2.60) | −.1060 | 0.93 (0.38, 2.30) | .8770 |
| >100 – 300 | 4.05 (2.14, 7.64) | < .0001 | 6.05 (2.56, 14.30) | < .0001 |
| >300 | 4.73 (2.47, 9.08) | < .0001 | 5.57 (2.33, 13.35) | .0001 |
| Chi-Square Test‡ | 30.53 | <.0001 | 27.38 | <.0001 |
A: Adjusted for scanning site, gender, and age at the time of the CT for each subject during EDIC years 7-9.
3df Wald Chi-Square test of the significance of the 4 categories of CAC in the respective model.
B: Adjusted scanning site, gender, study cohort, log mean HbA1c, age, systolic blood pressure, antihypertensive medication, LDL, HDL, smoking at EDIC year 7-9, and DCCT baseline family history of MI.
In the AUC analysis (On line eTable 1), when CAC score categories were added to the other covariates in Model A for any CVD, the AUC increased from 0.684 without CAC to 0.697 with CAC, 95% CI for the difference (0.0005-0.0292), indicating a significant increase in the AUC. When added to the covariates in Model B for any CVD, the AUC increase was not statistically significant.. The increase in AUC was not significant for MACE.
Statin use (or not) during EDIC was only recorded starting in EDIC year 11, after CT was conducted. The proportion known to be using statins at the time of the CVD event was 44.4% (20/45) among those with CAC score 0, 64.7% (11/17) among those with score >0-100, 45.5% (5/11) with CAC >100-300, and 60.0% (6/10) with CAC>300 Agatston units.
DISCUSSION
Coronary artery calcium has been shown in numerous studies to predict CVD events, most strongly in participants at intermediate risk with T2DM (18, 19). This study demonstrates similar predictive power for CAC among those persons with T1DM. The 10-13 year CVD incidence was 23% among those with CAC >100–300, and 32% for CAC >300 Agatston units.
The largest study prior to this report to evaluate participants with T1DM with CAC measurements was the CACTI (Coronary Artery Calcification in Type 1 Diabetes) study (20). The 656 T1D participants in the CACTI study showed a higher prevalence and extent of CAC than 764 age- and gender-matched control participants without diabetes with no difference between men and women. Prior studies have demonstrated extensive calcification even in young (17 - 28 years old) adults with TIDM (21) and calcification has been associated with factors including genetic polymorphism for hepatic lipoxygenase (LIPC-480 T) (22), smoking, and poor glycemic control (22, 23). A cross sectional study correlated CAC with coronary artery disease in T1DM in 302 men and women in the Pittsburgh Epidemiology of Diabetes Complications Study cohort (mean age of 38.1 +/− 7.8 years). This study concluded that CAC had an 84 and 71% sensitivity for CAD in men and women respectively and a 100% sensitivity for myocardial infarction and obstructive CAD. It also reported that a CAC cut point of 400 was the most efficient coronary calcium correlate of CAD (20).
We did not find an association of the CAC score with mean or time-weighted HbA1c measures. Some studies have demonstrated a relationship between diabetes control and atherosclerosis and others have not (21-24). A recent report from the Diabetes Prevention Program (in persons with T2DM) demonstrated no relationship of CAC or CAC severity with HbA1c, similar to our current report. (24) CAC sore categories did not have a cross-sectional association with HbA1c in our study (Table 1). The prior DCCT/EDIC paper showed significant associations of various measures of HbA1c over DCCT and EDIC with the prevalence of CAC>0 and of CAC>200 as well as with the log (CAC).
Results in our study of T1DM are similar to cohorts that included both asymptomatic (25) and symptomatic persons (26), whereby increasing CAC scores were associated with increasing risk of MACE, and zero scores were generally associated with low event rates. The event rates among the cohort for those with zero scores (n=817, 70.7% of the cohort) were very low at 5 years (2.8%) and at long term follow up (5.6 per 1,000 patient years) for ASCVD. This represents what has been reported as the ‘power of zero’ and potentially affords patients and physicians the potential to avoid more aggressive risk reduction strategies in this very low risk cohort.
Our study showed the lack of gender and CAC interaction, suggesting that type 1diabetes seemed to blunt the age differential for the development of atherosclerosis in men as compared to women. The EDIC CVD risk factor analyses (2) also suggested that gender was not a significant contributor to the final multivariate model when adjusted for other risk factors.
The ascertainment of statin use started in EDIC year 11 about 3 years after the CAC measurement. After the year 11 visit, an initial CVD event subsequently occurred in 55 participants, of whom 32 (58%) were using statin at the last EDIC visit prior to the CVD event. These studies were done prior to more widespread use of statin and ACE inhibitor therapies. Clearly a randomized trial of statins in persons with TIDM has not been done, but would be prudent given the increased ASCVD risk associated with higher CAC scores in this study.
In this group of participants with type I DM, higher coronary artery calcium scores were associated with CV events, suggesting that this is an important assessment tool to determine CV risk in participants with type 1 diabetes. Recommendations by the American College of Cardiology/American Heart Association (27) already recommend “In asymptomatic adults with diabetes, 40 years of age and older, measurement of CAC is reasonable for cardiovascular risk assessment.” This was largely based on data from persons with T2 DM in which studies enrolled T2DM participants age >40 years; the current study also strongly supports this IIA recommendation in T1DM participants. In addition, the American Diabetes Association and American Heart Association more recently stated that “it is reasonable to apply the current guidelines for the use of CAC assessment for T1DM as recommended for the general population.” (28). Coronary artery calcium, in persons with T1DM can inform health care providers regarding the management or risk factors for CV disease in patients at risk.
Supplementary Material
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Type I DM, although less well studied in the context of atherosclerosis presence and outcomes, demonstrates similar risk of future CVD with coronary calcium scores as persons with Type 2 DM. This is critical to understand the implications for clinicians, given the increased risk seen with higher CAC scores in these asymptomatic patients.
TRANSLATIONAL OUTLOOK: Prospective clinical trials evaluating CV risk and targeting specific coronary atherosclerotic treatments in Type I DM are necessary to delineate the impact of disease modifying therapies on clinical outcomes.
Acknowledgements:
A complete list of participants in the DCCT/EDIC Research Group is presented in the Supplementary Material published online for the article in N Engl J Med 2015;372:1722-33. The authors acknowledge the data processing and technical assistance of Wanyu Hsu at the Biostatistics Center, the George Washington University.
Funding/Support:
The DCCT/EDIC has been supported by cooperative agreement grants (1982-1993, 2012-2017, 2017-2022), and contracts (1982-2012) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Disease (current grant numbers U01 DK094176 and U01 DK094157), and through support by the National Eye Institute, the National Institute of Neurologic Disorders and Stroke, the General Clinical Research Centers Program (1993-2007), and Clinical Translational Science Center Program (2006-present), Bethesda, Maryland, USA.
Abbreviations:
- CAC
Coronary artery calcium
- CVD
Cardiovascular disease
- CT
Computed tomography
- MACE
Major adverse cardiac event
- T1DM
Type 1 diabetes mellitus
- T2DM
Type 2 diabetes mellitus
- CBL
Central Biochemistry Laboratory
- HbA1c
Hemoglobin A1c
- AER
Albumin excretion rate
Footnotes
A complete list of participants in the DCCT/EDIC Research Group is presented in the Supplementary Material published online for the article in N Engl J Med 2015;372:1722-33.
Industry Support:
Industry contributors have had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), Eli Lilly (Indianapolis, IN), Extend Nutrition (St. Louis, MO), Insulet Corporation (Bedford, MA), Lifescan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MN), Nipro Home Diagnostics (Ft. Lauderdale, FL), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), Perrigo Diabetes Care (Allegan, MI), Roche Diabetes Care (Indianapolis, IN) , and Sanofi-Aventis (Bridgewater, NJ).
Disclosures:
Matthew Budoff has grant support from NIH and General Electric. Philip Raskin is a consultant for Reata Pharmaceutical and UTSW receives grant money in his name from Boehringer-Ingelheim Pharmaceutical and Gan & Lee Pharmaceutical. No other author has any disclosure.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Secrest AM, Becker DJ, Kelsey SF, LaPorte RE, Orchard TJ. Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset Type 1 diabetes. Diabetes. 2010;59:3216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diabetes Control and Complications Trial (DCCT)-Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group, Nathan DM, Bebu I, Braffett BH, Orchard TJ, Cowie CC, et al. Risk factors for Cardiovascular Disease in Type 1 Diabetes. Diabetes. 2016. February 19;65:1370–9.26895792 [Google Scholar]
- 3.Hecht HS. Coronary artery calcium scanning: past, present, and future. JACC Cardiovasc Imaging. 2015. May;8(5):579–96. [DOI] [PubMed] [Google Scholar]
- 4.Coronary artery calcium score prediction of all-cause mortality and cardiovascular events in people with type 2 diabetes: systematic review and meta-analysis. BMJ 2013; 346:f1654. [DOI] [PubMed] [Google Scholar]
- 5.McClelland RL, Jorgensen NW, Budoff MJ, Blaha MJ, Post WS, Kronmal RA. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J Am Coll Cardiol. 2015;66:1643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budoff MJ, Yu D, Nasir K, Mehrotra R, Chen L, Takasu J, et al. Diabetes and progression of coronary calcium under the influence of statin therapy. Am Heart J. 2005;149:695–700. [DOI] [PubMed] [Google Scholar]
- 7.Hecht HS, Narula J. Coronary artery calcium scanning in asymptomatic patients with diabetes mellitus: a paradigm shift. J Diabetes. 2012;4:342–50. [DOI] [PubMed] [Google Scholar]
- 8.Budoff MJ, Raggi P, Beller GA, Berman DS, Druz RS, Malik S, et al. Imaging Council of the American College of Cardiology. Noninvasive Cardiovascular Risk Assessment of the Asymptomatic Diabetic Patient: The Imaging Council of the American College of Cardiology. JACC Cardiovasc Imaging. 2016;9(2):176–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burge MR, Eaton RP, Schade DS. The role of a coronary artery calcium scan in type 1 diabetes. Diabetes Technol Ther. 2016;18(9):594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993. September 30;329(14):977–86. [DOI] [PubMed] [Google Scholar]
- 11.The DCCT/EDIC Research Group. Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999. January;22(1):99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cleary PA, Orchard TJ, Genuth S, Wong ND, Detrano R, Backlund JY, et al. The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes. 2006. December;55(12):3556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The DCCT Research Group. The Diabetes Control and Complications Trial (DCCT). Design and methodologic considerations for the feasibility phase. Diabetes. 1986. May;35(5):530–45. [PubMed] [Google Scholar]
- 14.Snedecor GW CW. Statistical methods. 7th ed. Ames: Iowa State University Press; 1980. [Google Scholar]
- 15.JM L, editor. Biostatistical Methods: The Assessment of Relative Risks. 2nd ed. New York: John Wiley and Sons; 2011. [Google Scholar]
- 16.Kalbfleisch JD PR, editor. The Statistical Analysis of Failure Time Data. 2nd ed. New York: John Wiley and Sons; 2002. [Google Scholar]
- 17.Gonen M, Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika. 2005;92(4):965–70. [Google Scholar]
- 18.Malik S, Budoff MJ, Blumenthal RS, Bertoni AG, Nasir K, Szklo M, et al. Impact of Subclinical Atherosclerosis on Cardiovascular Disease Events in Individuals With Metabolic Syndrome and Diabetes: The Multi-Ethnic Study of Atherosclerosis. Diabetes Care. 2011;34:2285–90. PMID: 21844289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malik S, Zhao Y, Budoff MJ, Nasir K, Blumenthal RS, Bertoni AG, et al. Coronary Artery Calcium Score for Long-term Risk Classification in Individuals With Type 2 and Metabolic Syndrome From the Multi-Ethnic Study of Atherosclerosis. JAMA Cardiol. 2017. November 8 DOI: 10.1001/jamacardio.2017.4191. [Epub ahead of print] PMID: 29117273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson JC, Edmundowicz D, Becker DJ, Kuller LH, Orchard TJ. Coronary calcium in adults with Type 1 diabetes: A stronger correlate of clinical coronary artery disease in men than in women. Diabetes. 2000;49:1571–8. [DOI] [PubMed] [Google Scholar]
- 21.Starkman HS, Cable G, Hala V, Hecht H, Donnelly CM. Delineation of prevalence and risk factors for early coronary artery disease by electron beam computed tomography in young adults with type 1 diabetes. Diabetes Care. 2003;26:433–6. [DOI] [PubMed] [Google Scholar]
- 22.Hokanson JE, Cheng S, Snell-Bergeon JK, Fijal BA, Grow MA, Hung C, et al. A common promoter polymorphism in the hepatic lipase gene (LIPC-480C>T) is associated with an increase in coronary calcification in type 1 diabetes. Diabetes. 2002. April;51(4):1208–13. [DOI] [PubMed] [Google Scholar]
- 23.Snell-Bergeon JK, Hokanson JE, Jensen L, MacKenzie T, Kinney G, Dabelea D, et al. Progression of coronary artery calcification in type 1 diabetes: the importance of glycemic control. Diabetes Care. 2003. October;26(10):2923–8. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg RB, Aroda VR, Bluemke DA, Barrett-Connor E, Budoff MJ, Crandall JP, et al. Diabetes Prevention Program Research Group. Effect of Long-term Metformin and Lifestyle in the Diabetes Prevention Program and its Outcome Study on Coronary Artery Calcium. Circulation. 2017. July;136(1):52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joshi PH, Blaha MJ, Budoff MJ, Miedema MD, McClelland RL, Lima JAC, et al. The 10-Year Prognostic Value of Zero and Minimal CAC. JACC Cardiovasc Imaging. 2017. Aug;10(8):957–8. [DOI] [PubMed] [Google Scholar]
- 26.Budoff MJ, Mayrhofer T, Ferencik M, Bittner D, Lee KL, Lu MT, et al. The Prognostic Value of Coronary Artery Calcium in the PROMISE Study. Circulation. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA Guideline for Assessment of Cardiovascular Risk in Asymptomatic Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:50–103. [DOI] [PubMed] [Google Scholar]
- 28.de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2014. October;37(10):2843–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.