Abstract
Purpose:
To report a case of bilateral and repetitive corneal perforations following corneal cross-linking (CXL) for keratoconus in a woman harboring potentially pathogenic variants in the ZNF469 gene, and to characterize the keratoconus phenotype in this woman and her daughter who shared the same ZNF469 mutations.
Methods:
Clinical characterization of the proband and her daughter followed by sequencing of the genes associated with brittle cornea syndrome, ZNF469 and PRDM5, in both individuals.
Results:
An Ashkenazi Jewish woman in her sixth decade presented with diffuse corneal thinning and progressive steepening consistent with keratoconus. Following CXL, epithelium-off in the first eye and epithelium-on in the second, she developed spontaneous corneal perforations in each eye. Her daughter in her fourth decade demonstrated a similar pattern of diffuse corneal thinning and progressive corneal steepening but did not undergo CXL and did not develop corneal perforation. Screening of the ZNF469 and PRDM5 genes revealed three missense ZNF469 variants (c.2035G>A, c.10244G>C, and c.11119A>G) in cis arrangement on one allele of ZNF469 in both the proband and her daughter. Although the three variants share low (< 0.01) global minor allele frequencies (MAF), each has significantly higher MAFs (0.01 ~0.03) in the Ashkenazi Jewish population, leading to uncertainty regarding a pathogenic role for the identified variants.
Conclusion:
Corneal cross-linking may be associated with the development of corneal perforation in particular at-risk individuals with keratoconus. Identifying clinical and genetic risk factors, including screening of ZNF469 and PRDM5, may be useful in the prevention of significant complications following CXL.
Keywords: keratoconus, corneal ectasia, collagen cross-linking, perforation, ZNF469
INTRODUCTION
The corneal ectasias are progressive non-inflammatory corneal thinning disorders in which compromised stromal collagen matrix leads to the loss of corneal biomechanical integrity and subsequent corneal protrusion. Common examples of corneal ectasia include keratoconus including posterior keratoconus, pellucid marginal degeneration, and post-LASIK ectasia.1 Rare conditions classified as corneal ectasia include keratoglobus, posterior polymorphous corneal dystrophy,2 brittle cornea syndrome,3 and Ehler-Danlos syndrome type VI.4
Corneal cross-linking (CXL) is a photopolymerization technique to increase the biomechanical strength of the cornea.5, 6 The main indication for CXL is to retard the progression of keratoconus,7 while CXL has also been used prophylactically or as treatment for iatrogenic ectasia after LASIK,8–10 PRK,8 and radial keratotomy.11, 12 CXL can be performed using the standard Dresden protocol after epithelium debridement (epi-off CXL)13 or via a trans-epithelial approach (epi-on CXL).14 The therapeutic CXL procedure is considered relatively safe given currently available data with complication rates between 1–10%.15 While the most common complication of epi-off CXL is delayed re-epithelialization resulting in corneal haze, more significant complications have been observed, including perforation.15, 16 Perforation has been reported in only nine eyes following CXL: three cases of microbial keratitis after CXL for keratoconus17, 18; and six cases of sterile keratolysis: both eyes of an individual with Down syndrome in which baseline thickness was less than 400 μm; two cases with an acute inflammatory response after CXL; and two cases without significant inflammation following CXL.19–23
We report a case of a woman in her sixth decade with progressive keratoconus who developed recurrent and spontaneous corneal perforations involving both eyes after epi-off CXL in right eye and epi-on CXL in left eye. Extensive phenotypic and genotypic characterization of the proband and her affected daughter were performed to identify risk factors that may be used to identify individuals at increased risk of corneal perforation following CXL.
MATERIALS AND METHODS
The authors followed the tenets of the Declaration of Helsinki in the treatment of the subjects reported herein. This study was approved by the Institutional Review Board at The University of California, Los Angeles (UCLA IRB # 11–000020) and was performed after obtaining informed, written consent from the proband, her daughter and her daughter’s father.
Clinical Evaluation
Serial slit lamp biomicroscopic and corneal tomographic (Oculus Pentacam®, Wetzlar, Germany) evaluations of the proband and her daughter were performed to asses for abnormalities of corneal curvature, clarity and thickness and to detect progression. Central corneal thickness was measured in each eye, while paracentral and peripheral thickness measurements were obtained as the mean value of the corneal thickness measured at radii of 4mm and 8mm from the center, respectively.
Sanger Sequencing of ZNF469 and PRDM5
Saliva samples were collected using the Oragene saliva collection kits (DNA Genotek, Inc., Ontario, Canada) from the proband, her affected daughter and her daughter’s father. Genomic DNA was extracted from saliva samples using Oragene Purifier (OG-L2P, DNA Genotek, Inc.) and purified with QIAamp DNA micro kit (#56304, Qiagen, Valencia, CA) according to the manufacturer’s instructions. PCR amplification of the 2 exons of ZNF469 and 16 exons of PRDM5 was performed using previously described primers.24, 25 PCR amplification reactions were performed using the KAPA2G Robust PCR Kit (KAPA Biosystems, Wilmington, MA) with annealing temperature of 65 °C (ZNF469 primers) and 63 °C (PRDM5 primers).24, 25 Purified PCR products were purified using 0.5 U of Shrimp Alkaline Phosphatase and 5 U of Exonuclease I (USB Corp., Cleveland, OH) and underwent Sanger sequencing (Laragen, Inc., Culver City, CA). The resulting sequencing reads were compared to the wild-type ZNF469 transcript (NM_001127464.2) and PRDM5 transcript (NM_018699.3). Minor allele frequencies (MAF) were obtained for each identified variant from Trans-Omics for Precision Medicine (TOPMed) Program, Exome Aggregation Consortium (ExAC) database, 1000 Genomes Project and Genome Aggregation Database (gnomeAD). A variant was determined to be rare if the TOPMed MAF was ≤ 0.01.
In silico analysis of identified rare variants
In silico analysis of identified rare coding region variants for their potential effect on ZNF469 protein function was performed using PROVEAN, SIFT and PolyPhen-2.26–28 The Conserved Domain Database from the National Center for Biotechnology Information (NCBI) was used to identify the location of the putative Zinc-finger motifs on the ZNF469 protein and Consurf was used to calculate the evolutionary conservation of the ZNF469 amino acid sequence.29
RESULTS
Clinical Features
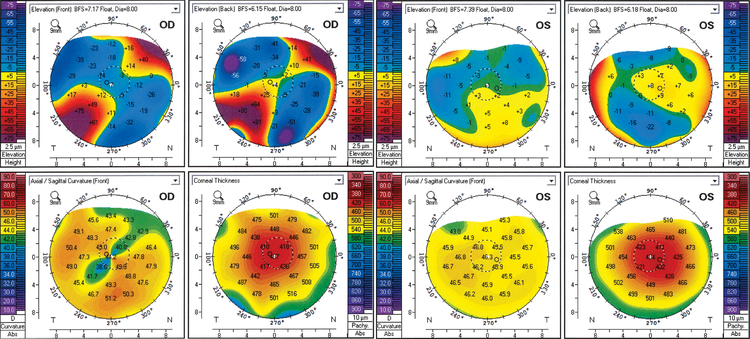
The proband, a woman of Ashkenazi Jewish descent, had a history of rapid myopia progression in her teens and then relatively stable vision with mild astigmatism until her 50s. Her vision was corrected with spectacles and rigid gas permeable (RGP) contact lenses for cosmetic reason in her teens and twenties, and then with glasses and soft contact lenses for the next 30 years. In her 50s, she developed increasing topographic astigmatism in the right eye, which was not fully correctable with glasses. She was diagnosed with keratoconus versus pellucid marginal degeneration at age 58 and when the topographical astigmatism in the right eye reached 6 D, she was referred for evaluation as a candidate for CXL. At that time, corrected distance visual acuities (CDVA) were 20/80 in the right eye (−2.50 −8.00 × 28°) and 20/20 in left eye (−4.25 −5.00 × 130°). Corneal tomographic imaging in the right eye demonstrated significant inferotemporal steepening with a high oblique astigmatism (40.80D/49.00 D × 46.8°, 8.2 D) and diffuse stromal thinning with centrally located minimum thickness of 404 μm (Fig. 1). Corneal tomographic imaging in the left eye demonstrated mild diffuse steepening with low against-the-rule astigmatism (45.50D/46.30 D × 82.5°, 0.8 D) and diffuse stromal thinning with a centrally located minimum thickness of 399 μm (Fig. 1). Based on the corneal tomographic findings, the patient was diagnosed with keratoconus. Neither corneal topographic or tomographic imaging performed prior to referral for CXL were available to assess for progression.
Figure 1:
Corneal tomographic imaging of the proband diagnosed with late-onset keratoconus demonstrating significant inferotemporal steepening and high oblique astigmatism in the right eye with diffuse stromal thinning and centrally located minimum thickness of approximately 400 μm in each eye.
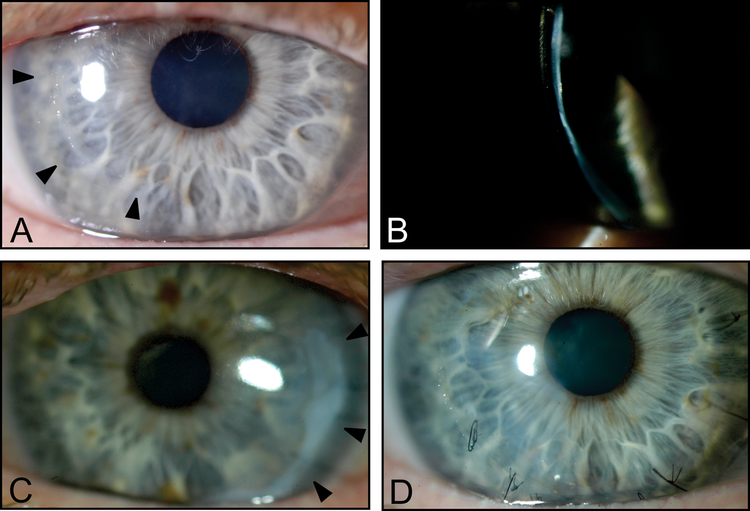
At age 59, the patient underwent epi-off CXL for progressive keratoconus in the right eye. One month and four months after the CXL procedure, spontaneous micro-perforations developed in the right cornea. Slit-lamp examination after the second perforation demonstrated a diffusely thin cornea with a temporal paracentral perforation (Fig. 2A, B). Both perforations were managed conservatively with bandage contact lens placement and aqueous suppression. One year after the second perforation, the vision in the right eye was correctable to 20/60 (−9.00 −6.00 D × 10°) on refraction and to 20/30 with a KeraSoft® IC lens.
Figure 2:
Slit-lamp photos of the proband’s right cornea demonstrating the location of prior temporal paracentral perforations (arrowheads) (A) and diffuse thinning (B). Images obtained 4 months after epi-off CXL. Slit-lamp photos of the proband’s left cornea demonstrating the location of prior temporal paracentral spontaneous perforation that developed 8 days after epi-on CXL (arrowheads) (C). Three months after the third spontaneous perforation in the right cornea, which occurred 3 years after CXL, sutures are present in the location of the corneal perforation (D).
The following year, the patient noted worsening of vision secondary to progressive myopia and astigmatism in the left eye. The topographic astigmatism had progressed to 4.7 D although the CDVA and refractive astigmatism remained relatively stable (20/25 with −4.50 −5.50 D × 136°). Given the history of perforation following epi-off CXL for the right eye, epi-on CXL was performed for the left eye. Eight days after the epi-on CXL, a spontaneous corneal perforation developed in the left eye, which was managed with sutures, amniotic membrane transplantation, and fibrin sealant (Fig. 2C). Seven weeks after CXL, following suture removal in left eye, a leak developed in the area of the initial perforation, which was managed conservatively. Nearly two years after the CXL was performed for the left eye, a spontaneous cornea perforation developed in the right eye that required suturing and amniotic membrane transplantation for closure (Fig. 2D). After recovery, the right eye was fit with a BostonSight® PROSE device, providing 20/40 CDVA. Review of systems and continued follow-up of the proband reveal no history or evidence of immunologic or rheumatologic conditions.
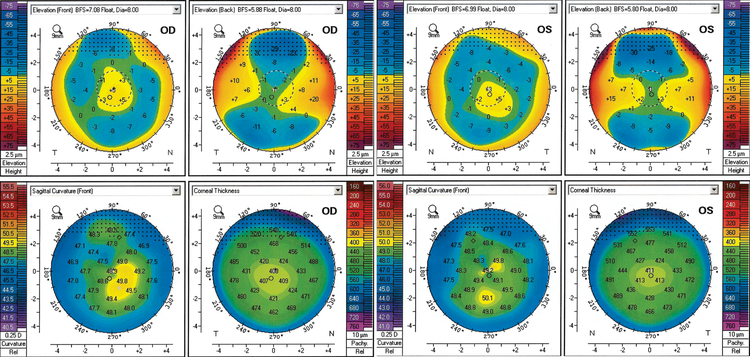
Review of the patient’s 37-year-old daughter’s medical records revealed high myopia with soft contact lens CDVA of 20/25 in both eyes (−9.50 −2.00 × 90° OD, −10.75 sphere OS). Corneal tomographic imaging demonstrated inferior paracentral steepening with low astigmatism in both eyes (OD 48.50D/48.90D × 94.0°, 0.4D, Kmax 50.0D; OS 48.80D/49.20D × 121.5°, 0.4D, Kmax 50.3D) (Fig. 3). Both corneas demonstrated diffuse thinning with centrally located minimum thicknesses of 405 μm (OD) and 410 μm (OS). Serial corneal tomographic imaging performed over the course of five years revealed no progression in the Kmax or topographic astigmatism, although evidence of pachymetric progression was observed in both eyes.
Figure 3:
Corneal tomographic imaging of the proband’s daughter demonstrating mild inferior paracentral steepening with low astigmatism as well as diffuse thinning with centrally located minimum thicknesses of approximately 400 μm in both eyes.
Although the global corneal thinning extended to the limbus in the proband and her daughter, the corneal thickness profiles were within the 95% (± 2 SD) interval around the mean reported for keratoconic corneas at central, paracentral (4 mm) and peripheral (8 mm) locations (with the exception of the peripheral corneal thickness in the proband) with maximal thinning at the apex in both the proband and her daughter (Table 1).30, 31 Based on this, both the proband and her daughter were diagnosed with keratoconus as opposed to keratoglobus.
Table 1.
Corneal thickness profiles of proband and her daughter compared to reported keractoconic cohort.
| Corneal thickness (μm) | Proband (OD / OS) |
Proband’s daughter (OD / OS) |
Keractoconus cohort30, 31 (mean ± SD) |
|---|---|---|---|
| Central | 404 / 399 | 405 / 410 | 428.0 ± 72.0 |
| Paracentral (4 mm) | 452 / 447 | 454 / 467 | 536.5 ± 48.3 |
| Peripheral (8 mm) | 540 / - | 605 / - | 695.6 ± 56.4 |
“-“ indicates corneal thickness cannot be obtained.
Genetic Analysis
The repetitive spontaneous perforations after CXL in both eyes of the proband and the shared diffuse cornea steepening and thinning observed in the proband and her daughter led us to suspect an underlying hereditary connective tissue disorder. We excluded the diagnosis of Ehler-Danlos syndrome type VI (EDSVIB) since neither the proband nor her daughter manifested extraocular features of a connective tissue disorder. Based on the corneal phenotype and clinical course, we suspected an ocular only phenotype of brittle cornea syndrome (BCS).32, 33 Therefore, we screened the coding regions of the BCS-associated genes, PR domain-containing protein 5 (PRDM5) and zinc finger transcriptional factor protein 469 (ZNF469), in the proband and her daughter.24, 25
Screening of PRDM5 in the proband did not reveal any rare or novel variants (Table 2). Screening of ZNF469 in the proband identified three heterozygous, rare, non-synonymous single nucleotide variants (SNVs) (c.2035G>A, c.10244G>C, and c.11119A>G), all with a MAF ≤ 0.01 (Table 2). Screening of ZNF469 in the proband’s affected daughter and the unaffected biological father of the daughter identified the same three variants in the heterozygous state in the daughter but not in the daughter’s father (Table 2). Three common ZNF469 single nucleotide polymorphism (SNPs) that were present in the heterozygous state in proband’s daughter (c.7072G>C, c.8009T>A, c.10888G>C) were present in the homozygous state in her father but absent in the proband, indicating that they were on the opposite allele from the three rare SNVs (c.2035G>A, c.10244G>C, and c.11119A>G) and the three rare SNVs were on the same allele transmitted from the proband to her daughter (Table 2). The presence of all three potentially pathogenic rare SNVs on the same allele effectively excludes the diagnosis of autosomal recessive brittle cornea syndrome.
Table 2.
Identified variants in proband, affected daughter and daughter’s unaffected father
| Proband (Affected Mother) |
Affected Daughter | Unaffected Biological Father of the Daughter | dbSNPs # | TOPMed MAF | Consequence Type | ||
|---|---|---|---|---|---|---|---|
| ZNF469 (NM_001127464.2) | exon1 | het.c.2035G>A | het.c.2035G>A | no variant | rs551591362 | 0.001 | missense |
| exon2 | het.c.3432T>C | het.c.3432T>C, | - | rs111557381 | 0.063 | synonymous | |
| no variant | het.c.7072G>C | homo.c.7072G>C | rs12598474 | 0.229 | synonymous | ||
| no variant | het.c.8009T>A | homo.c.8009T>A | rs3812956 | 0.308 | missense | ||
| homo.c.8520C>T | het.c.8520C>T | - | rs3812953 | 0.432 | synonymous | ||
| het.c.10244G>C | het.c.10244G>C | no variant | rs140056980 | 0.001 | missense | ||
| no variant | het.c.10888G>C | homo.c.10888G>C | rs1105066 | 0.460 | missense | ||
| het.c.11119A>G | het.c.11119A>G | no variant | rs536054902 | 0.0003 | missense | ||
| PRDM5 (NM_018699.3) | exon6 | no variant | het.c.681A>G | - | rs343192 | 0.279 | synonymous |
| intron8 | (intron)het.c.946–60G>C | (intron)het.c.946–60G>C | (intron)het.c.946–60G>C | rs4833677 | 0.225 | intron variant | |
| (intron)het.c.946–41G>C | (intron)het.c.946–41G>C | (intron)het.c.946–41G>C | rs4833676 | 0.225 | intron variant | ||
| intron9 | (intron)het.c.1030+35C>A | (intron)het.c.1030+35C>A | no variant | rs1511310 | 0.151 | intron variant | |
| (intron)het.c.1030+45A>G | (intron)het.c.1030+45A>G | (intron)het.c.1030+45A>G | rs1511309 | 0.278 | intron variant | ||
| exon10 | het.c.1066T>A | het.c.1066T>A | - | rs140634372 | 0.018 | missense | |
| exon11 | het.c.1234T>C | het.c.1234T>C | - | rs12499000 | 0.170 | synonymous | |
Identified rare variants (TOPMed < 0.01) shared between proband and the daughter are bolded. Common SNPs seen only in daughter and father are underlined and italicized. The cells labelled “-” indicate regions not screened.
As the proband is of Ashkenazi Jewish decent, we further determined the MAFs of the three rare ZNF469 SNVs within the Ashkenazi Jewish population (Supplemental Table 1). In the gnomAD database, the MAFs of c.2035G>A, c.10244G>C, and c.11119A>G in the Ashkenazi Jewish population were 0.0320, 0.0167, and 0.0265 respectively, significantly higher than the global MAFs (0.001, 0.001 and 0.0003) and failed to meet the selected MAF for rare variants (MAF ≤ 0.01) (Supplemental Table 1).
To predict the potential impact of the three missense variants on ZNF469 protein function, in silico analysis of the resulting amino acid substitutions was performed (Supplemental Table 2). Located within the first proline-rich domain of the ZNF469 protein (Supplemental Fig. 1), c.2035G>A (p.(Glu679Lys)) leads to the replacement of the conserved acidic glutamic acid (Consurf conservation score 7) with a basic lysine and is predicted to be “Damaging” to ZNF469 protein function by SIFT. The p.(Gly3415Ala) change secondary to c.10244G>C variant is located in the last of 7 Zinc-finger binding domains and is predicted to be “Possibly Damaging” by PolyPhen-2. None of the programs predicted the polar to nonpolar amino acid substitution p.(Ser3707Gly) resulting from c.11119A>G to be damaging.
DISCUSSION
We here report a case of repetitive spontaneous corneal perforations after CXL in a patient with atypical keratoconus to increase the awareness of this potential complication among corneal specialists. Careful review of the patient and her affected daughter’s clinical and imaging records revealed several atypical features for keratoconus that might be associated with progressive corneal thinning following CXL: stable refraction during early adulthood, in contrast to the typical progression of keratoconus; topographic evidence of progressive corneal steepening noted in the sixth decade of life, resulting in a late diagnosis of keratoconus; and diffuse stromal thinning, rather than being localized to the area of corneal ectasia. In addition, progression of steepening was observed after CXL, in contrast to the reports of minimal corneal topographical changes after CXL in multiple clinical trials.34
Genetic analysis of the proband and the daughter revealed three potentially pathogenic heterozygous ZNF469 SNVs in cis, indicative of a potential underlying genetic predisposition to progressive corneal thinning and perforation after CXL. Encoding a protein that is involved in corneal extracellular matrix development and maintenance25, ZNF496 is reported as the gene most strongly associated with central corneal thickness (CCT) in multiple genome-wide association studies (GWAS) that include various ethnic populations.35–37 While homozygous frameshift or truncating ZNF469 mutations are associated with BCS,4, 24, 33 heterozygous missense mutations are associated with a keratoconic phenotype, as seen in the proband and her daughter.35, 38, 39 Therefore, given the association of ZNF496 with decreased central corneal thickness, BCS and keratoconus, it is possible that one, two or all of the three heterozygous ZNF469 SNVs identified in the proband and her daughter are causative of the atypical keratoconic presentation in both and corneal perforation following CXL in the proband. Evidence for the pathogenicity of the three identified SNVs include: each is associated with a MAF < 0.01; in silico analysis predicted c.2035G>A to be “Damaging” and c.10244G>C to be “Possibly Damaging”; c.2035G>A was identified in the heterozygous state in two affected siblings with keratoconus;38 a variant involving the same nucleotide as c.10244G>C SNV (c.10244G>T) was identified in a family and two sporadic cases with keratoconus; and c.11119A>G was submitted by Illumina Clinical Services Laboratory to NCBI with a clinical diagnosis of “corneal fragility keratoglobus, blue sclerae and joint hypermobility (BCS1)”.40 However, evidence against the pathogenicity of the three identified SNVs includes: the fact that the MAF of each in the Ashkenazi Jewish population was significantly higher than the global MAFs; the c.2035G>A was associated with a MAF ≥ 0.001 in ethnically-matched controls38; and the c.10244G>T variant was associated with a MAF of 0.1304 in ethnically-matched controls.40
Keratoconus has been reported to occur in a higher percentage of the Jewish population than in other populations,41 although the prevalence in the Ashkenazi Jewish population has not been reported. If this prevalence were known, it would facilitate our judgement in assigning significance to the three identified SNVs. While we observed a positive association between keratoconus prevalence and the MAFs of each of the three ZNF469 variants in specific ethnic groups, including Jewish, Middle Eastern and South Asian populations,42–45 the MAFs were above the threshold for rare variants (MAF ≤ 0.01) (supplemental table 1), decreasing the likely pathogenicity of these variants in regards to keratoconus. However, if the identified SNVs are in fact associated with keratoconus, the higher MAFs of the three variants in these ethnic groups would explain the observed multiple-fold higher prevalence of keratoconus in these ethnic groups.41, 45
Low frequency SNVs with MAFs between 0.01 – 0.05 can be pathogenic in the setting of a complex disease such as keratoconus and a quantitative trait such as CCT. There is an inverse relationship between a variant’s “regression effect size” (the strength of the relationship between pathogenic variant and disease’s quantitative trait phenotype on a numeric scale) and its frequency in the population.46 Trait inheritability is contributed by common variants (MAF > 0.05) of very weak effect and low frequency (MAF 0.01 – 0.05) and rare variants (MAF < 0.01) of small to modest effect, or a combination of both.47 Isolated populations are particularly helpful in the identification of low-frequency disease-causing variant(s) affecting complex traits, because the founding event and genetic drift can cause an increase in allele frequency that can significantly increase the power to detect an association. The prevalence of keratoconus in Jewish (2.2%, CI 1.2 – 3.3% in Israeli Jewish41) and Maori/Polynesian45, 48 (26.9%, CI 21.0 – 33.7% of Maori/Polynesian teenagers are topographically keratoconus suspect45) populations is multiple-fold higher than in non-isolated populations (e.g. 0.054% in Minnesota, USA49). These two populations would be very helpful in deciphering the role of ZNF469 SNVs in keratoconus if one could document keratoconus phenotype as a quantitative trait, so statistical regression models could be applied to estimate the effect size of candidate SNVs on keratoconus phenotype.
In summary, we report a patient with a delayed onset of corneal ectasia diagnosed as keratoconus who developed spontaneous bilateral corneal perforation after CXL and was subsequently found to have thee potentially pathogenic heterozygous ZNF496 missense variants on the same allele, all of which have been previously implicated in the pathogenesis of corneal ectasia.
Supplementary Material
Supplemental Figure 1: Schematic of ZNF469 indicating location of functional domains and missense mutations identified in the proband and her daughter.
Supplemental Table 1. Minor Allele Frequencies (MAF) of three rare ZNF469 variants identified in the proband and her daughter.
Supplemental Table 2. In silico analysis of identified rare SNPs in ZNF469.
Funding:
National Eye Institute grants R01EY022082 (A.J.A.) and P30EY000331 (core grant to the Stein Eye Institute), an unrestricted grant from Research to Prevent Blindness (Stein Eye Institute)
Footnotes
Conflicts of interest: None
REFERENCES
- 1.Ziaei M, Barsam A, Shamie N, et al. Reshaping procedures for the surgical management of corneal ectasia. J Cataract Refract Surg 2015;41:842–872. [DOI] [PubMed] [Google Scholar]
- 2.Aldave AJ, Ann LB, Frausto RF, et al. Classification of posterior polymorphous corneal dystrophy as a corneal ectatic disorder following confirmation of associated significant corneal steepening. JAMA Ophthalmol 2013;131:1583–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ticho U, Ivry M, Merin S. Brittle cornea, blue sclera, and red hair syndrome (the brittle cornea syndrome). Br J Ophthalmol 1980;64:175–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Hussain H, Zeisberger SM, Huber PR, et al. Brittle cornea syndrome and its delineation from the kyphoscoliotic type of Ehlers–Danlos syndrome (EDS VI): Report on 23 patients and review of the literature. Am J Med Genet A 2004;124A:28–34. [DOI] [PubMed] [Google Scholar]
- 5.Wollensak G Crosslinking treatment of progressive keratoconus: new hope. Curr Opin Ophthalmol 2006;17:356–360. [DOI] [PubMed] [Google Scholar]
- 6.McCall AS, Kraft S, Edelhauser HF, et al. Mechanisms of corneal tissue cross-linking in response to treatment with topical riboflavin and long-wavelength ultraviolet radiation (UVA). Invest Ophthalmol Vis Sci 2010;51:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol 2003;135:620–627. [DOI] [PubMed] [Google Scholar]
- 8.Richoz O, Mavrakanas N, Pajic B, et al. Corneal collagen cross-linking for ectasia after LASIK and photorefractive keratectomy: long-term results. Ophthalmology 2013;120:1354–1359. [DOI] [PubMed] [Google Scholar]
- 9.Zhang ZY, Zhang XR. Prevention of ectasia for laser in situ keratomileusis with simultaneous corneal crosslinking. J Cataract Refract Surg 2012;38:2206–2207; author reply 2207–2208. [DOI] [PubMed] [Google Scholar]
- 10.Mackool RJ. Crosslinking for iatrogenic keratectasia after LASIK and for keratoconus. J Cataract Refract Surg 2008;34:879; author reply 879. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira TB, Marques EF, Filipe HP. Combined corneal collagen crosslinking and secondary intraocular lens implantation for keratectasia after radial keratotomy. J Cataract Refract Surg 2014;40:143–147. [DOI] [PubMed] [Google Scholar]
- 12.Mazzotta C, Baiocchi S, Denaro R, et al. Corneal collagen cross-linking to stop corneal ectasia exacerbated by radial keratotomy. Cornea 2011;30:225–228. [DOI] [PubMed] [Google Scholar]
- 13.Richoz O, Hammer A, Tabibian D, et al. The Biomechanical Effect of Corneal Collagen Cross-Linking (CXL) With Riboflavin and UV-A is Oxygen Dependent. Transl Vis Sci Technol 2013;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raiskup F, Spoerl E. Corneal crosslinking with riboflavin and ultraviolet A. I. Principles. Ocul Surf 2013;11:65–74. [DOI] [PubMed] [Google Scholar]
- 15.Kohlhaas M [Complications and postoperative therapeutic strategies in cross-linking]. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft 2017. [DOI] [PubMed] [Google Scholar]
- 16.Asri D, Touboul D, Fournié P, et al. Corneal collagen crosslinking in progressive keratoconus: Multicenter results from the French National Reference Center for Keratoconus. J Cataract Refract Surg 2011;37:2137–2143. [DOI] [PubMed] [Google Scholar]
- 17.Maharana PK, Sahay P, Sujeeth M, et al. Microbial Keratitis After Accelerated Corneal Collagen Cross-Linking in Keratoconus. Cornea 2017. [DOI] [PubMed] [Google Scholar]
- 18.Rana M, Lau A, Aralikatti A, et al. Severe microbial keratitis and associated perforation after corneal crosslinking for keratoconus. Cont Lens Anterior Eye 2015;38:134–137. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed-Noriega K, Butrón-Valdez K, Vazquez-Galvan J, et al. Corneal Melting after Collagen Cross-Linking for Keratoconus in a Thin Cornea of a Diabetic Patient Treated with Topical Nepafenac: A Case Report with a Literature Review. Case Rep Ophthalmol 2016;7:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gokhale NS, Vemuganti GK. Diclofenac-induced acute corneal melt after collagen crosslinking for keratoconus. Cornea 2010;29:117–119. [DOI] [PubMed] [Google Scholar]
- 21.Labiris G, Kaloghianni E, Koukoula S, et al. Corneal melting after collagen cross-linking for keratoconus: a case report. J Med Case Rep 2011;5:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goktug Demirci AO. Case of Corneal Perforation as a Complication after Uneventful CXL without Infection. Int J Ker Ext Corneal Dis 2013;2:5. [Google Scholar]
- 23.Faschinger C, Kleinert R, Wedrich A. [Corneal melting in both eyes after simultaneous corneal cross-linking in a patient with keratoconus and Down syndrome]. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft 2010;107:951–952, 954–955. [DOI] [PubMed] [Google Scholar]
- 24.Abu A, Frydman M, Marek D, et al. Deleterious mutations in the Zinc-Finger 469 gene cause brittle cornea syndrome. Am J Hum Genet 2008;82:1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burkitt Wright Emma MM, Spencer Helen L, Daly Sarah B, et al. Mutations in PRDM5 in Brittle Cornea Syndrome Identify a Pathway Regulating Extracellular Matrix Development and Maintenance. Am J Hum Genet 2011;88:767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi Y, Sims GE, Murphy S, et al. Predicting the Functional Effect of Amino Acid Substitutions and Indels. PLoS One 2012;7:e46688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 2009;4:1073–1081. [DOI] [PubMed] [Google Scholar]
- 28.Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods 2010;7:248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashkenazy H, Abadi S, Martz E, et al. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res 2016;44:W344–W350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmadi Hosseini SM, Mohidin N, Abolbashari F, et al. Corneal thickness and volume in subclinical and clinical keratoconus. Intl Ophthalmol 2013;33:139–145. [DOI] [PubMed] [Google Scholar]
- 31.Ambrosio R Jr., Alonso RS, Luz A, et al. Corneal-thickness spatial profile and corneal-volume distribution: tomographic indices to detect keratoconus. J Cataract Refract Surg 2006;32:1851–1859. [DOI] [PubMed] [Google Scholar]
- 32.Micheal S, Khan MI, Islam F, et al. Identification of Mutations in the PRDM5 Gene in Brittle Cornea Syndrome. Cornea 2016;35:853–859. [DOI] [PubMed] [Google Scholar]
- 33.Khan AO, Aldahmesh MA, Alkuraya FS. Brittle cornea without clinically-evident extraocular findings in an adult harboring a novel homozygous ZNF469 mutation. Ophthalmic Genet 2012;33:257–259. [DOI] [PubMed] [Google Scholar]
- 34.Li W, Wang B. Efficacy and safety of transepithelial corneal collagen crosslinking surgery versus standard corneal collagen crosslinking surgery for keratoconus: a meta-analysis of randomized controlled trials. BMC Ophthalmol 2017;17:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lechner J, Porter LF, Rice A, et al. Enrichment of pathogenic alleles in the brittle cornea gene, ZNF469, in keratoconus. Hum Mol Genet 2014;23:5527–5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu Y, Dimasi DP, Hysi PG, et al. Common genetic variants near the Brittle Cornea Syndrome locus ZNF469 influence the blinding disease risk factor central corneal thickness. PLoS Genet 2010;6:e1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vithana EN, Aung T, Khor CC, et al. Collagen-related genes influence the glaucoma risk factor, central corneal thickness. Hum Mol Gen 2011;20:649–658. [DOI] [PubMed] [Google Scholar]
- 38.Davidson AE, Borasio E, Liskova P, et al. Brittle cornea syndrome ZNF469 mutation carrier phenotype and segregation analysis of rare ZNF469 variants in familial keratoconus. Invest Ophthalmol Vis Sci 2015;56:578–586. [DOI] [PubMed] [Google Scholar]
- 39.Yildiz E, Bardak H, Gunay M, et al. Novel Zinc Finger Protein Gene 469 (ZNF469) Variants in Advanced Keratoconus. Curr Eye Res 2017;42:1396–1400. [DOI] [PubMed] [Google Scholar]
- 40.Vincent AL, Jordan CA, Cadzow MJ, et al. Mutations in the zinc finger protein gene, ZNF469, contribute to the pathogenesis of keratoconus. Invest Ophthalmol Vis Sci 2014;55:5629–5635. [DOI] [PubMed] [Google Scholar]
- 41.Millodot M, Shneor E, Albou S, et al. Prevalence and associated factors of keratoconus in Jerusalem: a cross-sectional study. Ophthalmic Epidemiol 2011;18:91–97. [DOI] [PubMed] [Google Scholar]
- 42.Pearson AR, Soneji B, Sarvananthan N, et al. Does ethnic origin influence the incidence or severity of keratoconus? Eye (London, England) 2000;14 ( Pt 4):625–628. [DOI] [PubMed] [Google Scholar]
- 43.Owens H, Gamble G. A Profile of Keratoconus in New Zealand. Cornea 2003;22:122–125. [DOI] [PubMed] [Google Scholar]
- 44.Millodot M, Shneor E, Albou S, et al. Prevalence and Associated Factors of Keratoconus in Jerusalem: A Cross-sectional Study. Ophthalmic Epidemiol 2011;18:91–97. [DOI] [PubMed] [Google Scholar]
- 45.Owens H, Gamble GD, Bjornholdt MC, et al. Topographic indications of emerging keratoconus in teenage New Zealanders. Cornea 2007;26:312–318. [DOI] [PubMed] [Google Scholar]
- 46.Park JH, Gail MH, Weinberg CR, et al. Distribution of allele frequencies and effect sizes and their interrelationships for common genetic susceptibility variants. Proc Natl Acad Sci U S A 2011;108:18026–18031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agarwala V, Flannick J, Sunyaev S, et al. Evaluating empirical bounds on complex disease genetic architecture. Nat Genet 2013;45:1418–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jordan CA, Zamri A, Wheeldon C, et al. Computerized corneal tomography and associated features in a large New Zealand keratoconic population. J Cataract Refract Surg 2011;37:1493–1501. [DOI] [PubMed] [Google Scholar]
- 49.Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol 1986;101:267–273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Schematic of ZNF469 indicating location of functional domains and missense mutations identified in the proband and her daughter.
Supplemental Table 1. Minor Allele Frequencies (MAF) of three rare ZNF469 variants identified in the proband and her daughter.
Supplemental Table 2. In silico analysis of identified rare SNPs in ZNF469.