Introduction
Real world data (RWD) generated during clinical care of children provides the opportunity to “upcycle,” producing something more valuable through pharmacokinetic and pharmacodynamic (PK/PD) research (Figure 1). Generating reliable real world evidence (RWE) from RWD requires great care to address challenges not faced when analyzing data collected specifically for research.1 Herein we outline benefits and barriers to using RWD for pediatric PK/PD research and propose standardized data processing to overcome many of these barriers.
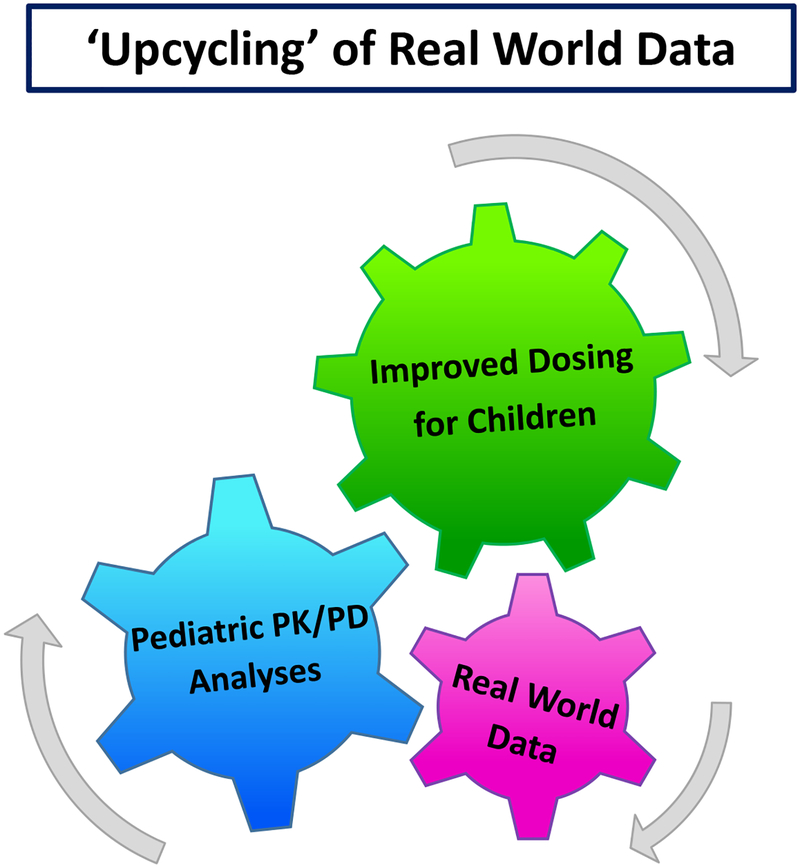
Figure 1. Virtuous Cycle of Real World Evidence.
Pharmacokinetic/pharmacodynamic (PK/PD) analyses are performed incorporating real world data. As a result, empirically-informed dosing can be implemented into clinical practice. Post-implementation real world data can then be analyzed to determine the impact of improved dosing strategies on therapeutic outcomes for children, creating another layer of real world evidence.
Potential impact of RWD/RWE on pediatric dosing
Pediatricians routinely make dosing decisions based on little or no empiric evidence. Pediatric dosing has often been extrapolated from adults, without fully accounting for the changes in body composition, physiology, pathology, and ontogeny of children as they grow. Ideal dosing for children should go beyond “mg per kg” calculations, taking developmental changes into account, maximizing efficacy and minimizing toxicity.
There are several barriers to performing traditional PK/PD studies to support dose optimization in pediatric populations.2 Since children represent a small and healthy population, the number of children with drug exposures for all but the most common conditions are very small. These small target populations reduce incentives for drug companies, dampen support for funding agencies, and limit the ability of individual academic centers to accrue sufficiently large cohorts for study. Drugs commonly used in children were often approved for adult use decades ago, further reducing enthusiasm for PK/PD research. There are also practical barriers, including aversion to painful procedures such as blood sampling in children, limitation on the volume of blood samples that can be obtained from small patients, and ethical complexities of any exposure of healthy children to therapeutics with any potential for short-term or long-term toxicity.
Thus, in the current era of widespread electronic health records (EHRs) use, there is great motivation and opportunity to use RWD to generate RWE for pediatric dosing via PK/PD analysis. In addition to filling the pediatric knowledge gaps, analysis of data from clinical settings has potential advantages over traditional PK/PD studies. RWD by design includes patients clinically exposed to the drug; this reduces sample selection bias in comparison to randomized clinical trials with strict inclusion/exclusion criteria by including “all comers.” Thus, RWE from RWD is more generalizable to the target population. Analysis of RWD also minimizes additional drug exposures in children and, if remnant clinical samples or clinically generated data are used, also minimizes additional sampling.
Successful use of RWD/RWE for pediatric PK/PD research
There are several examples of the successful use of RWD for pediatric PK/PD studies. The Pediatric Trials Network (PTN) is an NIH-funded program including >100 clinical sites with the goal of increasing drug safety and efficacy for children. The PTN has identified use of RWD as a strategy for ethical, efficient, and translatable pediatric therapeutic research. For example, the Pharmacokinetics of Understudied Drugs Administered to Children Per Standard of Care (POPS) trial (Clinicaltrials.gov NCT01431326) will enroll thousands of children who are administered one of the 32 understudied target drugs. POPS uses an opportunistic study design, including enrollment of patients exposed to target drug as part of their routine clinical care and use of laboratory results obtained as part of the clinical course. Additional blood samples for drug concentration measurement are obtained, but these are collected at the same time as blood samples required for the patient’s clinical care, avoiding additional procedures. The recently established PTN EHR data repository promises to accelerate pediatric therapeutic research through the analysis of RWD.
At our own center, we have used RWD without any additional sampling to perform population PK analysis of fentanyl in children after cardiac surgery.3 Over the course of 23 months, we collected 1321 fentanyl drug concentration measurements from 130 patients/participants through the analysis of plasma leftover from clinical testing. This study is embraced with enthusiasm by the parents/guardians whose children are eligible for the study. The consent rate for this study is greater than 80%, and over the 11 total years of the study, only 1 individual has withdrawn from the study. As of December 20, 2018, our dataset includes 913 children and 7086 plasma specimens, in which we have measured the concentration of 16 commonly used sedatives and analgesics. Insofar as we can extract meaningful endpoints from EHRs, this dataset provides the opportunity to study not only pediatric PK, but also PD.
Barriers to widespread use of RWE/RWD for PK and PD studies in children
Although the above examples provide evidence of the progress made in using RWD to generate RWE for PK/PD in children, published studies are limited and the approach is not yet well established. Among challenges and issues that have been discussed,4 ensuring data quality is fundamental in using RWD. Specifically, for PK/PD studies, there are several barriers to generating valid and usable datasets from EHRs (Figure 2).
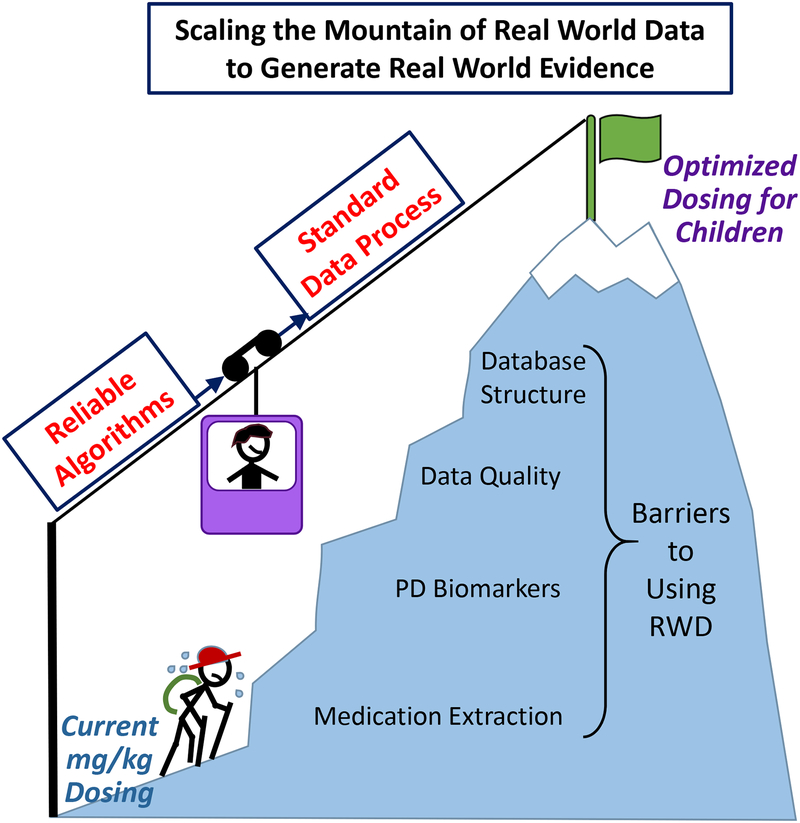
Figure 2. Overcoming the Barriers to using Real World Data to Achieve Optimized Dosing for Children.
Currently, pediatric dosing relies on mg/kg calculations. In order to use real world data (RWD) to achieve optimized pediatric dosing, several barriers must be overcome. Developing reliable algorithms and establishing standardized data processing will assist in overcoming these barriers.
Temporal data extraction:
Obtaining sufficiently detailed medication dosing and outcome data is a challenging step, as precise temporal information is mandatory for PK/PD analysis. Temporal data for dosing may be available from medication administration records for drugs administered to inpatients, but outpatient drug exposure data often need to be extracted from clinical notes in EHRs or obtained through linking with external databases such as pharmacy dispense records. Likewise, PD outcomes such as pain scores (analgesics), blood pressures (anti-hypertensives and pressors), and coagulation testing results (anticoagulants) are routinely documented, but careful attention must be paid to the temporal relationship between drug dose and outcome measurement. The large number of individuals with multiple records required for population PK/PD studies prohibits manual extraction, warranting automated data extraction algorithms. Currently available natural language processing systems for data extraction from clinical text have not been validated to the degree of accuracy required for PK/PD analysis and may need to be improved.
Algorithmically defined PD biomarkers:
Research in pediatric PK has outpaced that of PD. Some biomarkers for PD analyses are routinely documented as part of clinical care (as listed above) but require transformation to account for changes in physiology and development (e.g., normalization of blood pressure for age and gestational age). Other PD outcomes are not available as structured data in the EHR, necessitating sophisticated phenotyping algorithms for extraction. Development of standardized, validated, shareable programs for data transformation and phenotyping will facilitate pediatric PD studies.
Data quality and data building process:
Medication data, clinical variables, and PK/PD outcomes extracted from the EHRs are “messy,” requiring several data processing steps. For example, duplicate data should be removed, outliers should be identified and investigated, time-varying data need to be matched or aligned, and some data may require imputation. Furthermore, the data building process becomes more complicated as RWD gets bigger. Manual data processing and construction of PK/PD datasets requiring a specific form with typically very large sample size is labor intensive and error prone; thus, reliable data building programs for PK/PD analyses are also needed to facilitate efficient and reproducible PK/PD research. Data validation is also required after each step to ensure fidelity and completeness.
Common database structure:
Ideally, pediatric PK/PD data will include data from multiple sites to increase sample size, support generalizability of the findings, provide validation sets, and facilitate incorporation of the results into clinical care. Variability of EHR systems, both across and within EHR vendors, provides another challenge to consolidating data. The pediatric pharmacology community must continue to participate in ongoing efforts to build common database structures for research to ensure that the specific needs for PK/PD studies in children are addressed.5
Future directions
Using RWD to generate RWE provides a great opportunity to improve pediatric drug dosing but has substantial challenges to overcome. In addition to the data challenges outlined above, for many drugs valid measures of drug effect are not consistently documented (e.g., therapeutic response to medications for attention deficit hyperactivity disorder or depression) or not available for the full spectrum of pediatric patients. Across all drugs, reliable documentation of adverse drug events is incomplete and represents an area of need for complete PD analysis. Long-term funding for multi-site collaborative networks is required to address the challenges, pool data, and validate findings. Generation of high-quality, generalizable, and validated data will facilitate subsequent clinical implementation. Furthermore, the same approach can be used to test the hypothesis that these clinical implementations result in measurable benefit for pediatric patients, including increased time in therapeutic range, increased drug efficacy, decreased drug toxicity, decreased length of stay, decreased costs, decreased mortality, or increased quality of life. In order to overcome the challenges to RWD use and achieve common goals, we strongly recommend standardizing the whole data generation process so that reliable RWD can be obtained from EHRs. This standardization will facilitate transparent and reproducible RWE generation and foster efficient collaborations across institutions by enabling them to share and combine datasets more easily. Ultimately this will promote widespread use of RWD for PK/PD studies and result in improved care of children.
Funding:
Supported in part by Doris Duke Clinical Scientist Development Award 2017075, Burroughs Wellcome Fund Innovation in Regulatory Science Award 1015006, and NIH/R01 GM124109.
Footnotes
Conflict of Interest: The authors declared no competing interests for this work.
References
- 1.Miksad RA & Abernethy AP Harnessing the Power of Real-World Evidence (RWE): A Checklist to Ensure Regulatory-Grade Data Quality. Clin. Pharmacol. Ther 103, 202–205 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thakkar N, Salerno S, Hornik CP & Gonzalez D Clinical Pharmacology Studies in Critically Ill Children. Pharm. Res 34, 7–24 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Driest SL et al. Pragmatic pharmacology: population pharmacokinetic analysis of fentanyl using remnant samples from children after cardiac surgery. Br J Clin Pharmacol 81, 1165–1174 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auffray C et al. Making sense of big data in health research: Towards an EU action plan. Genome Med 8, 71 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trifirò G et al. Combining multiple healthcare databases for postmarketing drug and vaccine safety surveillance: why and how? J. Intern. Med 275, 551–561 (2014). [DOI] [PubMed] [Google Scholar]