Abstract
Purpose:
To investigate correlations of oxygen (pO2) in the ocular anterior segment of human eyes and aqueous humor antioxidant levels of ascorbate (AsA) and total reactive antioxidant potential (TRAP) with glaucoma and vitreous status.
Methods:
This prospective, cross-sectional study stratified patients (n=288 eyes) by lens and vitreous status and presence of primary open angle glaucoma for statistical analyses. Intraocular pO2 measurements with a fiberoptic probe were made in patients at the beginning of planned glaucoma and/or cataract surgery. Aqueous humor specimens were obtained for antioxidant analysis (AsA, TRAP).
Results:
Following prior pars plana vitrectomy, pO2 was significantly higher compared to the reference group (cataract; CAT) in the anterior chamber (AC) angle (16.2 ± 5.0 vs. 13.0 ± 3.9 mmHg, P=.0171) and in the posterior chamber (7.6 ± 3.1 vs. 3.9 ± 2.7 mmHg, P<.0001). AsA and TRAP levels were significantly lower (1.1 ± 0.4 vs. 1.4 ± 0.5 mM; 403.3 ±116.5 vs. 479.0 ± 146.7 Trolox unit; P=.004, P=.024, respectively) in patients following vitrectomy surgery. In patients with an intact vitreous, neither pO2 nor antioxidant status correlated with lens status or glaucoma.
Conclusions:
Increased pO2 and antioxidant depletion following vitrectomy suggests alteration of the intraocular oxidantantioxidant balance. Our studies link physiologic factors, increased pO2 in the AC angle and posterior chamber, to decreased antioxidant levels in aqueous humor following vitrectomy surgery. Oxidative stress/damage to the trabecular meshwork in such post-vitrectomy cases may contribute to intraocular pressure elevation and increased risk of glaucoma.
INTRODUCTION
The precise pathogenesis of primary open angle glaucoma (POAG) has not been fully elucidated. It likely represents a variety of different pathologies, genetic predispositions and contributing environmental factors. Alterations of the local environs of the trabecular meshwork (TM), the main pathway for the conventional outflow of aqueous humor, may also affect its function, leading to increased intraocular pressure (IOP), an important risk factor for glaucoma. Understanding that ocular structures are “interconnected” is not a new idea. For example, feedback mechanisms of IOP regulation exist via nitric oxide synthesis in the TM,1 cyclic mechanical stress yields alterations in conventional outflow facility,2 and TM cells undergo contractile changes, potentially via Rho-kinase mediated signaling.3 In addition to these physiologic mechanisms, the concept of intraocular surgical procedures modifying this environment in a potentially deleterious manner has been previously noted.4,5
Pars plana vitrectomy (PPV) now represents the third most common intraocular procedure performed in the United States, with overall rates in the Medicare population increasing 31% from 2001–2012.6 This increase is likely related to improvements in the safety profile of the procedure, improved success of vision-saving surgical interventions, decreased utilization of alternative surgical therapies, and earlier intervention for non-vision threatening pathologies (e.g. “floaterectomy”). Evaluation of adverse events of this procedure between the years of 1994 to 2005 indicated, however, that rates of severe complications such as endophthalmitis, suprachoroidal hemorrhage and retinal detachment remained stable, but rates of less-severe complications such as glaucoma increased with the prevalence of vitrectomy.7
The vitreous humor is an important ocular structure that plays a prominent role in maintaining biochemical homeostasis, consuming molecular oxygen and protecting the lens from oxidative damage.8 Shui and colleagues found that gel vitreous, in comparison to liquefied vitreous due to myopia, aging, or surgical removal, has a higher concentration of ascorbate (AsA) and consumes oxygen at a faster rate. Previous studies indicated that antioxidant levels, specifically AsA and glutathione, are present in high concentrations in the vitreous humor.9,10 The discovery of this role of gel vitreous to maintain the physiologic hypoxic environment around the lens is important. Initial studies of patients undergoing long-term hyperbaric oxygen therapy noted a 50% incidence of nuclear cataract development within 1 to 3 years.11 In patients undergoing PPV, it has been observed that a nuclear sclerotic cataract develops and progresses rapidly in the ensuing 12–18 months following vitrectomy, with 37–95% of patients requiring cataract extraction within 2 years.12–18 Vitrector gauge size did not influence these results.19 Increased vitreous liquefaction increases the risk of nuclear cataract development.20 Identification of increased oxygen (pO2) levels within the vitreous cavity and at the posterior surface of the lens following vitrectomy surgery led to the proposal that increased oxygen exposure leads to oxidative damage to the lens and nuclear cataract formation.21
Besides the lens, other ocular structures are continuously exposed to a broad spectrum of pO2 levels, ranging from hyperoxic to markedly hypoxic. Cells exposed to high pO2 levels as well as ultraviolet light (e.g. corneal epithelium) contain nuclear ferritin,22,23 AsA,24 glutathione,25 superoxide dismutase, and catalase and otherantioxidants.26,27 Ocular cells that function at low physiologic pO2 are unlikely to adapt to altered (i.e. higher) levels of oxygen exposure. Oxygen either is consumed by functioning cells and/or antioxidants, remains in its molecular form, or is transformed into other potentially unstable reactive oxygen species (ROS), capable of causing damage to RNA, DNA and proteins. “Oxidative stress” is defined as an increase over physiologic values in the intracellular concentrations of ROS, which include superoxide anion, hydrogen peroxide, hydroxyl radical, peroxyl radical and singlet oxygen. Such ROS may be detrimental to cellular structures, destroying membrane lipids as well as structural and enzymatic proteins and DNA, contributing to cell senescence and potentially genetically programmed cell death or apoptosis. Cellular dysfunction results from decreased mitochondrial respiratory function and protein degradation.28 Increases in intracellular ROS may be the result of increased endogenous production by mitochondrial respiration or decreased antioxidant capacity. Increased oxidative stress has been identified as a contributing factor to the pathogenesis of several age-related ocular diseases including glaucoma.29,30
Evidence supporting such oxidative damage to the TM was initially reported by Alvarado in 1981,31 as he first suggested that aging and oxidative stress underlie the degeneration of TM cells in patients with glaucoma. Cell senescence has been shown to increase ROS generation leading to reduced number and function of mitochondria.32 As a result of this exposure to oxidative stress, changes occur in TM protein expression that affect extracellular matrix turnover. For example, in vivo perfusion of calf anterior segments with hydrogen peroxide (H2O2) following depletion of glutathione in the TM increases outflow resistance.33 TM tissue, as compared to corneal and iris tissue, was found to be most sensitive to oxidative damage induced by H2O2 exposure.34 Subsequent studies provide evidence of oxidative damage and reduced resistance to oxidative stress in the outflow pathway.35,36 Oxidative damage to DNA is greater in TM cells in glaucoma patients compared to controls.37 and correlates with IOP level and visual field loss.38 Levels of 8-hydroxy-2’-deoxyguanosine (8-OHdG), an established biomarker of oxidative DNA damage, are significantly higher in both aqueous humor and serum in glaucoma patients compared to controls.39 Increased levels of 8-OHdG have also been found in TM specimens of POAG patients.38
Importantly, PPV has also been associated with increased oxygen exposure to the microenvironment of the TM and outflow pathways40 and increased risk of developing open angle glaucoma in several retrospective clinical studies,41–46 and a recent population based study.47 The present study was undertaken to provide further understanding of the impact of exposure to increased pO2 and/or its metabolites in the local environment of the aqueous outflow pathways following vitrectomy surgery. We hypothesize that increased pO2 in these cases may contribute to alterations of oxidant-antioxidant balance leading to increased oxidative stress/damage of the TM. To assess these conditions in human subjects, we measured in vivo levels of pO2, total reactive antioxidant potential (TRAP) activity, and AsA levels in aqueous humor of eyes undergoing glaucoma and/or cataract surgery to determine associations with glaucoma and vitreous status.
METHODS
STUDY DESIGN
This prospective, cross-sectional study was approved by the Institutional Review Board of the Washington University School of Medicine, in compliance with the tenets of the Declaration of Helsinki and HIPAA guidelines. Informed consent was obtained from subjects after explanation of the nature and possible consequences of the study. This study was designed to measure oxygen distribution within the anterior segment of the eye and to collect aqueous humor for measurement of antioxidants in patients undergoing cataract and/or glaucoma surgery in an academic clinical practice. Patients were excluded from the study if there was evidence of corneal endothelial dysfunction, ischemic ocular disease including diabetic retinopathy, anterior chamber angle closure, inflammatory or traumatic ocular disease, ocular neoplasia, requirement for general anesthesia, or monocular status.
PATIENTS AND pO2 MEASUREMENTS
A complete general medical and ophthalmic history and comprehensive ophthalmic examination, including Lens Opacities Classification System III (LOCS III) analysis for quantitative and qualitative assessment of lens opacities, were performed prior to surgical intervention. The use of topical glaucoma medications within one month of the surgical procedure was verified preoperatively. Patients with a diagnosis of POAG (based on optic nerve and visual field criteria) were classified by glaucoma severity as mild, moderate, or severe (Hodapp Parrish Anderson criteria).48 Central corneal thickness was measured by ultrasound technique (DGH 55 Pachmate, DGH Technology Inc., Exton, Pennsylvania, USA), and axial length measurements were recorded (IOLMaster 500, Carl Zeiss Meditec, Inc., Germany) for patients undergoing cataract extraction. Racial background was based on self-report as indicated on a standardized registration questionnaire.
As per routine surgical protocol, the patient was placed in supine position, intravenous sedation was administered, the eye was prepped and draped and a lid speculum was placed. Supplemental oxygen (21–30%) was provided via nasal cannula and separated from the ocular region by adhesive sterile drape to avoid any additional oxygen exposure. This technique did not impact intraocular oxygen measurements as previously reported.21 Blood oxygen saturation was monitored by continuous pulse oximetry and maintained between 95% and 100%. Topical lidocaine hydrochloride jelly 2% was placed on the ocular surface in the preoperative area. A sub-Tenons injection of 1–3 ml of 2% lidocaine and 0.375% bupivacaine mixture (50/50) was performed to provide additional local anesthesia as indicated. At the beginning of the planned surgical procedure, a 30-gauge needle was utilized for entry through peripheral clear cornea into the anterior chamber (AC) and the Oxylab™ pO2 optical oxygen sensor probe (Optode; Oxford Optronix, Oxford, United Kingdom) was then carefully introduced into the AC without aqueous humor leakage. The instrumentation was calibrated prior to each set of measurements. Under direct visualization with an operating microscope, the tip of the flexible fiberoptic probe was positioned for three measurements in all patients as described in our previous studies: (1) underneath the central corneal endothelium, (2) in the mid-AC, and (3) in the AC angle.49,50 In pseudophakic patients or those scheduled to undergo cataract extraction, two additional measurements were obtained (4) at the central anterior lens surface and (5) in the posterior chamber just behind the iris. These latter two measurements were not performed in patients remaining phakic to avoid risk of lens damage. Approximately 46 seconds (total < 5 minutes) was required for each set of measurements. In order to confirm precise and consistent probe positioning and stabilization of the pO2 level, duplicate testing in the same locations were performed for verification.
AQUEOUS HUMOR SPECIMEN COLLECTION
Following pO2 measurements, the needle entry site in the cornea was slightly enlarged with a 15-degree blade or side port instrument and an aqueous humor sample (50–100 μl) was drawn into a 1-ml tuberculin syringe via a 30-gauge blunt cannula followed by re-inflation of the AC volume with balanced salt solution. Care was taken to avoid contamination of the specimen with blood. The aqueous humor specimen was immediately transferred to a sealed tube, placed on dry ice and transported to storage in the gas phase of a liquid nitrogen tank until analysis. The scheduled surgical procedure was subsequently performed with standard postoperative management, and the patients were monitored for any complications.
AQUEOUS HUMOR ANALYSIS
Ascorbate
AsA concentration was quantified in triplicate, based on its ability to reduce Fe3+ to Fe2+ and the resulting change in the A525 of complexes of Fe2+ with 2,2”-dipyridyl.51 Assay modifications enabled the analysis of 10-μl samples and a standard curve was used for all measurements as in our previous report.8 Gas chromatography-mass spectrometry studies confirmed the specificity of this colorimetric assay. Samples of aqueous humor were mixed with a known amount of carbon 13-labeled ascorbic acid (13C6-ascorbic acid, Omicron Biochemicals, South Bend, Indiana), dried and then reacted with N,O-bis(trimethylsilyl)trifluoroacetamide. The sample was separated on a gas chromatograph (Varian Inc., Palo Alto, CA) using a 30-m, 0.25-mm-internal GC column with a 0.25-μm film (DB-5ms column; P.J. Cobert Associates Inc., St. Louis, MO), maintained at 80°C for 1 minute, and then eluted with a temperature gradient of 80°C to 300°C at 15°C/min. The injection port and transfer line were at 250°C and the source temperature at 200°C of a mass spectrometer (Finnigan MS SSQ7000; Thermo Electron Corp, Waltham, MA) operated in the electron ionization mode at 70 eV. The concentration of AsA was calculated from the ratio of carbon 13- to carbon 12-labeled ascorbate. In order to confirm the specificity of the AsA assay, 2 units of ascorbate oxidase (AO; A 0157, Sigma Chemical, St. Louis, MO) were added to each of the 10-μl samples, mixed well at room temperature and AsA measurements were repeated.
Total reactive antioxidant potential
The TRAP assay is a means to determine the ability of a sample to destroy chemically generated free radicals. A sample is added to a solution containing 2,2’-Azobis(2-amidinopropane) (ABAP; Sigma Aldrich, St. Louis, MO, USA) and 40 μM luminol (3-aminophthalhydrazide, Sigma Aldrich, St. Louis, MO, USA). ABAP combines with oxygen to produce alkyl peroxyl radicals at a constant rate. In the absence of antioxidant, these radicals react with luminol to produce light, which is measured in a scintillation counter. Antioxidants quench luminescence by reacting with the peroxyl radicals. Since ABAP produces radicals at a constant rate, antioxidant activity is measured by the length of time required to quench luminescence. The assay is standardized using Trolox, a water-soluble vitamin E analog and reported as “Trolox units,” with one unit equal to amount of time required to quench luminescence by a sample containing 1 μM Trolox. In addition to measuring TRAP in the aqueous humor samples, samples were treated with ascorbate oxidase to remove AsA as described above. Repeat measurements were then performed in order to differentiate AsA and non-AsA dependent effects on the composite TRAP value.
STATISTICAL ANALYSIS
Multivariate regression analyses were performed with adjustment for all potential confounding variables (P <.1) including age, sex, race, medications, and lens status using SPSS software (Version 24.0, Chicago, Illinois, USA). T-test, one-way ANOVA with multiple comparison analysis (Bonferroni correction), and Spearman correlation analyses were performed with GraphPad Prism (Version 8.0, La Jolla, CA, USA). Results are expressed as mean values ± standard deviation (SD). P values less than .05 were defined as statistically significant.
RESULTS
PATIENT RECRUITMENT AND GROUP ANALYSIS
A total of 288 eyes of 288 patients participated in the study between July 2007 and August 2015. Our initial cohort (July 2007 to July 2010) of 112 eyes of 112 patients were included from a previously published study evaluating intraocular pO2 measurements.49 We extended this work to study total 288 eyes for pO2, AsA, and TRAP measurements, after exclusion of 24 eyes due to inadequate specimen collection. Patients with secondary open angle glaucoma (i.e. pseudoexfoliative or pigmentary) and low tension glaucoma were excluded from this study. Patient characteristics (Table 1), indicate a greater number of females and Caucasian patients in the study. The cataract group (CAT) had no prior history of ocular surgery, glaucoma, or exposure to ocular glaucoma medications. This group served as the reference/control group for select statistical analyses. Consistent with our previously published data,49 patients with a diagnosis of POAG (GL) were subdivided into: a) patients undergoing glaucoma surgery or combined cataract and glaucoma surgery (GL/CAT), and b) pseudophakic patients undergoing glaucoma surgery (GL/IOL). Patients with a history of vitrectomy who had undergone previous pars plana vitrectomy for vitreoretinal conditions including rhegmatogenous retinal detachment, epiretinal membrane and macular hole and excluding proliferative retinopathy comprised the VIT group. All of these patients were either pseudophakic or were scheduled to undergo cataract extraction or glaucoma surgery. Patients in the GL/CAT and GL/IOL groups were older compared to VIT group (P =.0005 and P=.0001, respectively). The GL/IOL patients were also older than the CAT reference group (P=.005). Subgroup analyses were performed to identify correlations of race, age, sex, lens and vitreous status, and ocular medications with pO2 levels and antioxidant status. We randomly selected one eye for the final data analysis in patients who had measurements/specimens from both eyes.
Table 1.
Patient demographic information and group descriptions.
| Groups | Group description | Eyes N= |
Age (yrs) | F / M | Type of surgery | Race | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CAT | GL | Combined | AA | CC | Other | |||||
| CAT | No prior history of eye surgery or POAG (Reference group) | 72 | 68.0 ± 11.4 | 48 / 24 | 72 | 0 | 0 | 26 | 44 | 2 |
| GL/CAT | POAG undergoing glaucoma surgery or combined cataract/glaucoma surgery | 143 | 70.4 ± 10.8 | 80 / 63 | 35 | 30 | 78 | 39 | 102 | 2 |
| GL/IOL | Pseudophakic POAG patients undergoing glaucoma surgery | 39 | 73.8 ± 9.0 | 32 / 7 | 0 | 39 | 0 | 10 | 29 | 0 |
| VIT | Patients who had undergone previous pars plana vitrectomy | 34 | 63.1 ± 13.5 | 18 / 16 | 24 | 10 | 0 | 6 | 27 | 1 |
| Total | 288 | 178 / 110 | 131 | 79 | 78 | 81 | 202 | 5 | ||
Key: POAG = primary open angle glaucoma, GL = glaucoma, CAT = cataract, VIT = prior vitrectomy, IOL = intraocular lens, F= female, M=male, AA=African American, CC=Caucasian.
INTRAOCULAR pO2 MEASUREMENTS
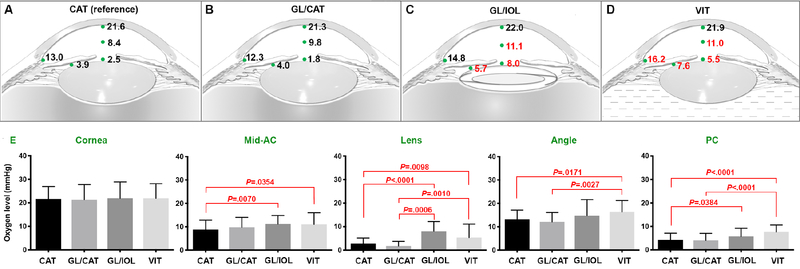
Oxygen measurements at five intraocular locations were analyzed by multiple comparison analysis with Bonferroni correction (Figure 1). Intraocular pO2 measurements were significantly higher following vitrectomy (VIT) compared to the reference (CAT) group in the AC angle (16.2 ± 5.0 vs. 13.0 ± 3.9 mmHg; P=.0171) and posterior chamber (7.6 ± 3.1 vs. 3.9 ± 2.7 mmHg; P<.0001). In the GL/IOL (pseudophakic) group, there were significantly higher levels of pO2 at the anterior lens surface compared to compared to the reference group (8.0 ± 4.1 vs. 2.5 ± 2.4 mmHg; P<.0001), in the mid-AC (11.1 ± 3.8 vs. 8.4 ± 3.9 mmHg; P=.007), and in the posterior chamber (5.7 ± 3.4 vs. 3.9 ± 2.7 mmHg; P=.0384).
Figure 1.
Intraocular pO2 measurements (mmHg) at indicated locations (green dots). A. Cataract (CAT) group used as reference for comparison with other groups. Red numbers indicate P values <.05. B. GL/CAT = glaucoma diagnosis with cataract C. GL/IOL = glaucoma diagnosis with history of prior cataract surgery D. VIT = history of prior pars plana vitrectomy. E. Comparison of pO2 at intraocular locations. (ANOVA with multiple comparison analysis and Bonferroni correction; P values <.05). Error bar: mean mmHg ± standard deviation.
ASCORBATE MEASUREMENTS
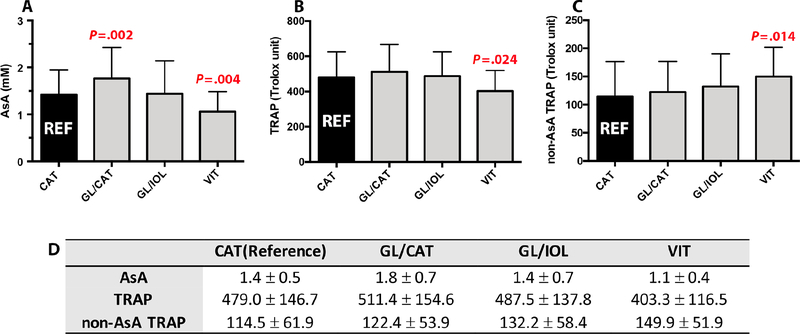
AsA levels were significantly lower in VIT group (1.1 ± 0.4 mM; P=.004) compared to the CAT reference group (1.4 ± 0.5 mM). We further confirmed that AsA is correlated with prior vitrectomy surgery in a multivariate regression model (Beta = −.198, P=.004). AsA levels were increased in phakic patients with POAG diagnosis (GL/CAT; 1.8 ± 0.7 mM; P=.002) as shown in Figures 2A and 2D. Multivariate regression analyses did not identify any correlations of pO2 with AsA following adjustment for race, age, sex, lens status, and presence of glaucoma.
Figure 2.
Comparison of aqueous humor antioxidant levels to reference group (CAT; black bar). A. Ascorbate (AsA) B. Total Reactive Antioxidant Potential (TRAP) C. Non-AsA TRAP D. Table shows mean value ± standard deviation (SD). P values are calculated from unpaired t-test. Bar: SD.
TRAP AND NON-ASA DEPENDENT TRAP
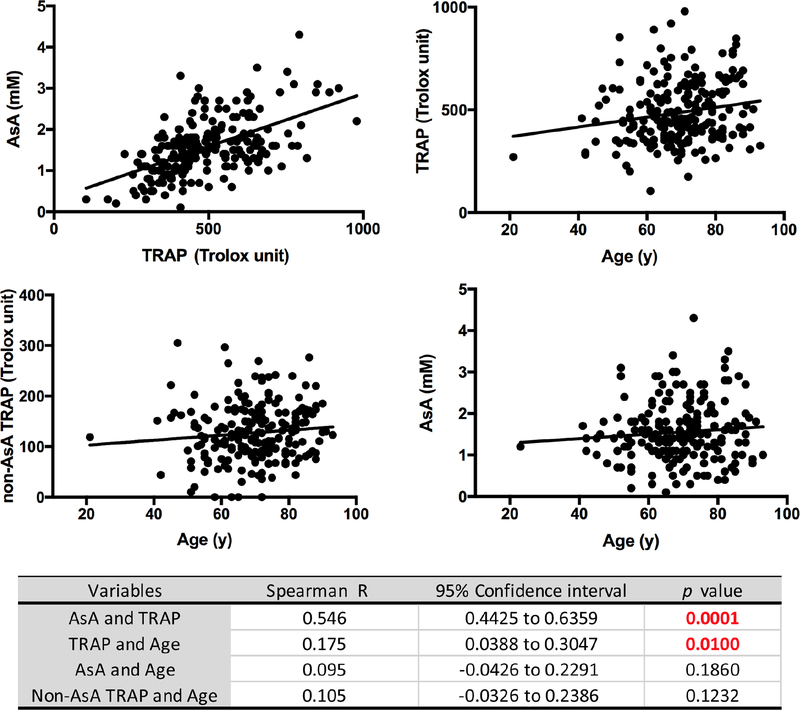
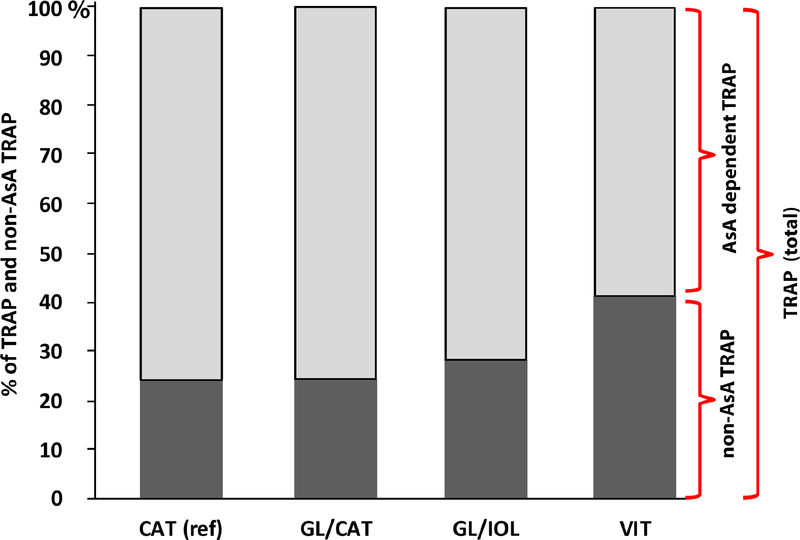
TRAP and its component AsA are highly correlated in all human aqueous humor specimens confirmed by the marked reduction of TRAP values in specimens treated with ascorbate oxidase. We have designated the calculated remainder TRAP value as non-AsA dependent TRAP (non-AsA TRAP) in our subsequent analyses. As shown in Figures 2B, 2C and 2D, there were significantly lower TRAP levels following vitrectomy (VIT; 403.3 ± 116.5 Trolox units; P =.024) in comparison to reference CAT group (479.0 ± 146.7 Trolox unit). Multivariate regression analysis confirmed the correlation between TRAP and post-vitrectomy status (Beta = −.186, P=.007). TRAP is significantly directly correlated with age (rs=.175, P=.01) as indicated in Figure 3. The non-AsA TRAP component percentage was significantly greater in the VIT group (149.9 ± 51.9 Trolox unit, 41.4%) compared to CAT (114.5 ± 61.9 Trolox unit, 24.3%; P=.014). Multivariate regression also showed correlation between non-AsA TRAP and vitreous status (Beta = .135, P=.05). There were no differences between the CAT group and both GL/CAT and GL/IOL TRAP activity. Figure 4 illustrates the comparative contributions of the components of TRAP in each group. AsA contributed 76% of TRAP in CAT group while AsA in the VIT group only contributed 58%. Multivariate regression analyses did not indicate any correlations of pO2 with TRAP in the anterior segment following adjustment for race, age, sex, lens status, and medication use.
Figure 3.
Scatterplot diagrams showing relationships between A. Ascorbate (AsA) and Total Reactive Antioxidant Potential (TRAP), B. TRAP and age, C. non-AsA TRAP and age, D. AsA and age in all cases demonstrating the best linear fit to the data. Table shows Spearman’s rank-order correlations.
Figure 4.
Comparison of ascorbate (AsA) and non-AsA components of Total Reactive Antioxidant Potential (TRAP). TRAP was designated as 100%, and AsA component (light gray) and non-AsA dependent component (dark gray) are shown as percentages of TRAP values in each patient group.
TOPICAL GLAUCOMA MEDICATIONS
Medications were classified as beta blockers (timolol, betaxolol), carbonic anhydrase inhibitors (dorzolamide, brinzolamide), alpha-2 agonist agents (brimonidine), or prostaglandin analogues (bimatoprost, latanoprost, travoprost). Fixed combination agents (Combigan,® Cosopt,® Simbrinza®) were categorized by their individual medication components. As most patients were on a combination of medications, each of the agents was analyzed individually. There was a significant correlation (Beta 0.274, P=.004) between the use of topical carbonic anhydrase inhibitors (CAIs) and levels of AsA in the aqueous humor of all glaucoma patients (GL/CAT, GL/IOL). Notably, 69 of 146 (47.2%) of this group of patients were utilizing topical CAIs as a component of their medical regimen. Four of 35 (11.4%) of the VIT group were taking CAI agents. Following adjustment for race, age, sex, and lens status, no other medication class was correlated with AsA or TRAP levels. Multivariate regression analysis correcting for this variable resulted in demonstration of this drug’s significant impact on AsA levels (Table 2).
Table 2.
Results of multivariate regression analyses evaluating effect of topical glaucoma medications on ascorbate (AsA) in aqueous humor.
| Dependent Variable | Independent Variable | β | P value |
|---|---|---|---|
| AsA | Alpha | 0.009 | .927 |
| Beta | 0.111 | .306 | |
| CAI | 0.274 | .004 | |
| PG | 0.041 | .696 |
Key: Alpha = Alpha-2 agonists, Beta = Beta blockers, CAI = Carbonic anhydrase inhibitors, PG = prostaglandin analogues.
DISCUSSION
OXYGEN MEASUREMENTS AND HOMEOSTASIS
This prospective, cross-sectional study represents the largest reported cohort of patients undergoing cataract and/or glaucoma surgery in which assessments of both intraocular oxygen levels and aqueous humor antioxidant status were obtained. Precise in vivo measurement techniques of pO2 by our colleagues in rabbits and in human vitreous led to these studies of the anterior segment of the human eye revealing consistent oxygen gradients.21,52,53 Our studies of how oxygen homeostasis is altered by surgical intervention, aging, and disease may reveal important insights of physiology and pathology. As the ocular anterior segment represents a “protected” environment from direct blood flow, it provides an ideal scenario to study homeostatic mechanisms of oxygen metabolism in addition to oxidant-antioxidant balance. Additionally, by excluding patients with ischemic retinal disease and the use of general anesthesia, we aimed to separate effects of decreased retinal blood flow and hyperoxic conditions on intraocular pO2 levels, respectively.
Increased pO2 in the AC angle of post-vitrectomy patients (VIT) compared to reference CAT patients may provide an important source of pro-oxidants leading to increased oxidative stress in the TM. Elevated pO2 levels in the TM region and in the posterior chamber may increase ROS in the aqueous outflow pathway by diffusion from the ciliary body stroma into the aqueous humor at the root of the iris. This movement is consistent with Freddo and colleagues’ description of this pathway facilitating movement of plasma proteins through the TM54 and our previously published hypothesis regarding the correlation of pO2 levels in the AC and posterior chamber.49 Other body tissues exposed to excess levels of molecular oxygen have been shown to accumulate ROS. For example, pulmonary epithelial cells are adapted to much higher oxygen levels than other cells in the body (21% O2 or 160 mmHg), but during prolonged exposure to levels as high at 40% O2 or greater, increased intracellular ROS leads to pulmonary oxygen toxicity.55,56 Physiologic conditions for TM cells are relatively hypoxic as we discovered in the rabbit, monkey and human.40,49,53 Exposure of these specialized cells to elevated pO2 may be “toxic” leading to decreased TM cellularity, altered extracellular matrix formation, and ultimately decreased outflow facility and increased IOP. If the protective mechanisms of the aqueous humor are overwhelmed, then oxidative stress/damage may result. Notably, however, in POAG patients with an intact vitreous, we did not find increased intraocular pO2 in the TM region or posterior chamber, suggesting this may not be an important factor in all glaucoma subtypes.
Adaptation of ocular structures to specific levels of oxygen is revealed in studies of oxidative damage and defense. For example, the basal layer of corneal epithelium is accustomed to high levels of oxygen, essentially equivalent to air with pO2 of 160 mmHg (21% oxygen). In contrast, pO2 in inner retinal tissue and the vitreous adjacent to retinal blood vessels is approximately 20 mmHg, consistent with other body tissues.21,57,58 The environment surrounding the lens is notably hypoxic under normal conditions, measuring approximately 7 mmHg at the posterior surface21 and 3 mmHg at the anterior and lateral surfaces of the lens,49 with oxygen consumption by lens cells further decreasing pO2 within the lens nucleus.59,60 Extraction of the natural lens and replacement with an IOL removes the contributing factor of oxygen consumption by the lens epithelium, thereby increasing pO2 around the lens including the posterior chamber. This pO2 elevation does not reach the oxygen levels following vitrectomy.
OCULAR ANTIOXIDANT STATUS: ASCORBATE AND TRAP
We identified significantly decreased levels of both AsA and TRAP in aqueous humor of patients who had undergone vitrectomy (VIT) compared to the reference group (Figure 2). Vitrectomy surgery, independent of lens status, results in decreased TRAP in comparison to all other groups analyzed. Vitrectomized eyes also displayed an increase in non-AsA TRAP compared to the reference group (Figure 4). Interestingly, we found that patients with POAG diagnosis (GL/CAT, GL/IOL) had higher levels of AsA and no difference in TRAP when compared to the reference group (Figure 2). Lee and coworkers also noted increased aqueous humor AsA in glaucoma patients compared to cataract surgery controls,61 while Ferreira and colleagues found that AsA levels were decreased in patients with both POAG and exfoliation syndrome,62 and TRAP levels from glaucoma patients were significantly lower compared to the cataract group.63
Our present study did not support these findings of decreased AsA in patients with glaucoma compared to cataract controls. Leite and coworkers64 found that AsA levels were significantly lower in secondary aqueous, obtained from patients with history of previous intraocular surgery, compared to primary aqueous in patients with glaucoma and cataract. Our separate analysis of phakic glaucoma patients (GL/CAT) with a history of prior intraocular surgery confirmed this finding of decreased AsA as compared to patients without history of previous surgery (P=.04; data not shown). Confounding variables such as frequent use of CAIs in the glaucoma subgroups significantly correlated with increased AsA and contribute to these contradictory findings. In addition, systemic ascorbate supplementation was not specifically documented in our medication review and may have also impacted our results, especially in cases of high doses of vitamin C (2 grams/day).65
A recently published systematic review and meta-analysis of oxidant-antioxidant stress markers in glaucoma demonstrated decreased total antioxidant status in serum and aqueous humor in glaucoma patients with the exception of two enzymatic antioxidants, superoxide dismutase and glutathione peroxidase.66 These entities may represent a compensatory protective response to oxidative stress reflected in this study as non-AsA TRAP. A study of age-related changes in TRAP plasma levels showed that TRAP increased with age in both females and males.67 However, in males, levels increased only up to the age of 51–74 years when they were noted to decline. Increases of antioxidant potential, especially in response to oxidative stress, were due to unidentified antioxidants which comprise 35% of TRAP in both sexes.
Huang and coworkers described “extreme” exposures to increased pO2 (42.7 ± 12.4 mmHg at the corneal surface) in patients with Fuchs’ dystrophy,68 another ocular condition associated with oxidative stress.69 Aqueous humor levels of AsA and TRAP were significantly lower compared to a cataract reference group (P=.012 and P=.032, respectively; unpublished data). In addition, we previously reported50 increased pO2 in the anterior segment of patients with African American background compared to Caucasians and confirmed in this expanded study cohort (data not shown). These increased levels of pO2 did not correlate with differences in antioxidant status, potentially suggesting alternative mechanisms for this racial group’s increased risk and severity of POAG.
Finally, AsA levels in aqueous humor of patients with cataract have been shown to decrease with age,70 supporting the role of oxidative damage and accumulation of free radicals in cataract development. We did not identify correlations of AsA with age in this study (Figure 3). We acknowledge that the oxidant-antioxidant balance in the eye as reflected in the aqueous humor is undoubtedly highly complex and requires further study.
ASCORBATE METABOLISM
Ocular exposure to ultraviolet and visible light irradiation is greater than any other organ except skin. Consequently, this organ requires protective mechanisms against ROS generation. As the TM represents the target tissue of glaucoma in the anterior segment, understanding the role of antioxidants in the trabecular tissue,71,72 as well as in the aqueous humor in which it bathes, is critical to our comprehension of oxidant-antioxidant balance and its role in glaucoma development. The “pecking order” of aqueous humor antioxidants is affected by both the concentration and electrochemical activity of several low molecular weight water soluble species,73,74 including AsA, L-tyrosine, L-cysteine, uric acid and glutathione. Antioxidant enzymes, such as superoxide dismutase, glutathione peroxidase,75 and catalase have also been identified in aqueous humor. In contrast to nocturnal animals, the corneal epithelium,76,77 lens,78 vitreous9 and aqueous humor79,80 of diurnal animals contain extremely high levels of AsA as compared to plasma.81 Shui and colleagues noted that metabolism of molecular oxygen in the vitreous gel occurs in an AsA-dependent manner, without an exogenous catalyst and independent of light, revealing the significance of AsA as a primary regulator of intraocular molecular oxygen.8
AsA is actively concentrated from the plasma via the sodium-dependent vitamin C (SVC2) transporter located in the pigmented epithelial cell layer of the ciliary body with uptake of dehydroascorbic acid (dAsA) via glucose transporter (GLUT1) receptors in the non-pigmented epithelial layers.82 AsA secretion into aqueous humor has been described in animals and humans with subsequent dAsA recycling back to AsA. However, neither the transporters implicated in the uptake of AsA and its metabolites nor other transporters of key antioxidants such as glutathione have been elucidated to provide specific information about antioxidant protection of the aqueous humor and TM.83
Review of the literature has led us to propose that oxygen is consumed by AsA in aqueous humor via a two-step reaction.84–88 These reactions gradually decrease oxygen levels as AsA is converted to dAsA:
Direct in vivo measurements of ROS are problematic due to their reactivity and transient nature.89 As a result, quantification of antioxidants is frequently performed as a surrogate marker of oxidant-antioxidant balance. Using gas chromatography–mass spectrometry, we have measured dAsA byproducts in ocular fluids and identified 2,3-diketogulonate and L-threonate (data not shown). 2,3-diketogulonate reacts with H2O2, producing L-threonate. This provides indirect evidence that H2O2, an important ROS,2 exists in aqueous humor.90
Some have suggested that elevated oxygen may produce enough H2O2 to exceed the ability of catalase to remove it,41,91 potentially increasing exposure of the aqueous outflow pathway to this toxic metabolite. Oxidative damage or death of TM endothelial cells could result as a consequence of this exposure, as observed in glaucoma patients with decreased cellularity of the TM.31,92–94 If increased oxygen oxidizes AsA and other antioxidants, one would expect antioxidant molecules to be depleted in the aqueous humor of patients with elevated oxygen. Our findings of decreased AsA and TRAP levels in eyes following vitrectomy and IOL implantation support this theory.
VITRECTOMY AND RISK OF OPEN ANGLE GLAUCOMA
Increased pO2 in the AC angle and altered antioxidant status may be clinically significant. Alternate mechanisms of TM damage and physiologic responses may be represented in these vitrectomized patients compared to other forms of POAG. As indicated in several retrospective studies with varying inclusion/exclusion criteria and follow-up periods, the prevalence (2–19.2%) of ocular hypertension and glaucoma following vitrectomy surgery and subsequent lens extraction is inconsistent,42–45 with contrary conclusions in some studies.46,95,96 The mechanisms of post-vitrectomy glaucoma likely represent a multifactorial process with various yet unidentified genetic and/or environmental risk factors.
Lelazary and colleagues recently completed the Prospective Retinal and Optic Nerve Vitrectomy Evaluation (PROVE) study.97 The three- year data98 (Patel S, et al. AAO, 2016; AAO abstract P0562) revealed a significant increase of mean IOP in eyes having undergone both vitrectomy and cataract surgery with IOL implantation compared to baseline (P<.05) and compared to the fellow eye (P<.05), consistent with original reports by Chang.41 A recently published retrospective, population-based cohort study confirmed these findings of increased 10-year risk of POAG in post-vitrectomy eyes (10%; 95% CI) and following vitrectomy combined with scleral buckle (17.5%; 95% CI) compared to the nonoperative group (1%; 95% CI).47
Of the patients in the VIT group who underwent glaucoma surgery, 5/10 (50%) had a history of controlled glaucoma prior to PPV surgery and subsequent lens extraction. The mean time from PPV to glaucoma surgery was 51.3 ± 39.4 months (range =12–118 months). Delayed onset of elevated IOP and protective effects of the crystalline lens have been reported, consistent with our data.42,43 Our findings of further increases of pO2 in the AC angle and posterior chamber in these cases following cataract extraction provides additional support for the theory of prolonged oxidative stress causing TM damage.
Future recruitment of a subgroup of patients who have undergone PPV and lens extraction without a diagnosis of ocular hypertension or POAG may provide additional information. We performed longitudinal assessments of aqueous and vitreous humor oxidant-antioxidant balance in an older monkey model of PPV with subsequent lensectomy.40 Our results indicated progressive decrease of both TRAP and AsA as well as increased 8-OHdG, a marker of oxidative damage, in both aqueous and vitreous specimens following each surgical procedure.
ANTIOXIDANT PROPERTIES OF TOPICAL GLAUCOMA MEDICATIONS
An interesting finding in this study was the correlation of topical carbonic anhydrase inhibitors (CAIs) with AsA levels in aqueous humor from collected from human patients in vivo (Table 2). CAIs administered topically or systemically to rabbits resulted in increased concentrations of AsA in the aqueous of the posterior chamber, but not the AC.99 These findings confirmed Becker’s prior studies and were noted to be a reflection of decreased aqueous production and flow in this region.100 However, these measurements were based on acute therapy with systemic carbonic anhydrase inhibitors, and may not be reproduced with chronic topical use, a common component of glaucoma therapy.
Our findings of significantly higher AsA concentrations in patients on topical CAIs may represent a potential secondary mechanism of action, as revealed in the reduction of free radical formation in glaucoma patients taking topical dorzolamide.101 Timolol, a beta blocker, has also been shown to exert direct antioxidant protection of human endothelial cells in culture.102 Metipranolol, in addition to its active metabolite, desacetylmetipranolol, also exhibits antioxidant properties in vitro.103 Brimonidine, an alpha-2 adrenergic agonist, has been shown to exert a neuroprotective effect on rat retinal ganglion cells in the presence of glutamate, oxidative and hypoxic stress,104 but no changes in antioxidant levels of the anterior segment. Pre-incubation of cultured human TM cells with prostaglandin analogues followed by exposure to H2O2 has been shown to reduce glaucomatous TM changes in these cells.105 Further studies of potential antioxidant effects of glaucoma therapies may be warranted.
STUDY LIMITATIONS
The cross-sectional design of this study and others identified in our literature review limits our understanding of how responses to oxidative stress occur over time in a given patient. Future longitudinal analyses may aid to understand questions surrounding progressive TM damage and glaucoma development. As in any human study, individual patient variation may affect group mean data analysis. Dependence on patient’s historical information regarding medication (e.g. antioxidant supplements) and social history (e.g. tobacco use) may significantly alter results, especially with limited sample size within each of the study groups. Our results did not confirm previously published findings of decreased antioxidant protection in glaucoma versus cataract patients, in spite of similar protocol techniques. Although we designated patients undergoing cataract surgery as reference/controls for our comparisons, it is important to note that these are not “normal controls” as they do have condition(s) associated with oxidative damage (cataract and aging). Differences in cataract type and glaucoma severity may have had an impact on the results, as well as our observation of the effect of specific glaucoma medications on AsA in aqueous humor. Given our limited number of VIT subjects, recruitment of additional post-vitrectomy subjects (with and without glaucoma) could be informative since vitreoretinal pathology may independently influence antioxidant levels. Since both patients and specimen quantities are limited, assays of multiple antioxidants cannot be performed for all patients depending on volumes required. We identified AsA and TRAP as the most promising agents given their biochemical reactions with oxygen as the dominant measure of antioxidant potential, but other molecules may play a significant role in antioxidant defense (i.e. non-AsA TRAP).
CONCLUSIONS
Our observation of increased pO2 levels in the anterior segment and decreased levels of AsA and TRAP in the group of patients who had undergone PPV compared to the reference group may provide important insights into how this surgery may increase oxidative stress and glaucoma risk in select patients. We propose increased intraocular pO2 levels in these patients could be a potential source of pro-oxidants for generation of ROS, decreasing antioxidant defenses in the ocular anterior segment (Figure 5). Further understanding of this surgical intervention’s impact on oxygen homeostatic mechanisms, antioxidant balance, and oxidative stress is vital, and these investigations may lead to future therapies targeted to this specific population as well as to other individuals afflicted with this leading cause of irreversible blindness.
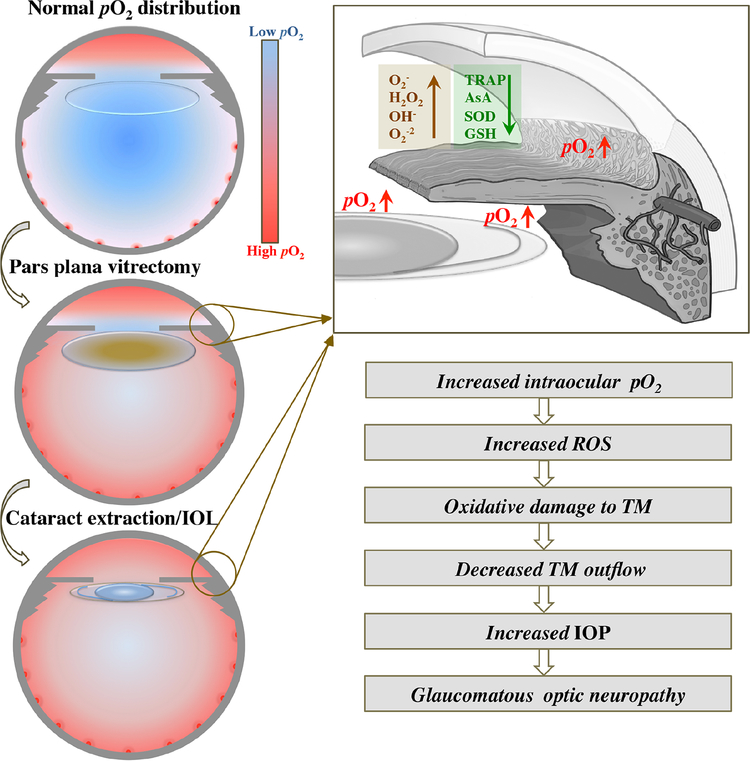
Figure 5.
Proposed mechanism for development of post-vitrectomy glaucoma. Upper left image: Low levels of pO2 in vitreous cavity, surrounding clear lens and in anterior chamber (AC) angle. Oxygen diffuses across cornea into the AC and from retinal blood vessels into the vitreous cavity. Center left image: Following pars plana vitrectomy surgery, pO2 increases in the vitreous cavity, around lens, in AC angle and posterior chamber with development of nuclear sclerotic cataract. Lower left image: Following cataract extraction and intraocular lens implantation (IOL), pO2 increases in the AC angle, posterior chamber and mid-AC. Upper right image: Expanded view of AC angle illustrating alterations in aqueous humor including increased pO2 surrounding lens implant and in AC angle leading to increased reactive oxygen species formation (brown; ROS) and decreased antioxidants (green). Lower right image: Proposed cascade of events following vitrectomy and IOL implantation ultimately leading to increased intraocular pressure (IOP) and risk of glaucoma.
O2− = superoxide anion, H2O2 = hydrogen peroxide, OH− = hydroxyl ion, O2−2 = peroxide, TRAP = total reactive antioxidant potential, AsA = ascorbate, SOD= superoxide dismutase, GSH= glutathione, TM = trabecular meshwork
ACKNOWLEDGMENTS
A. Funding/Support:
NEI EY021515, NEI EY015863, NEI P30EY02687 (WU), Grace Nelson Lacy Glaucoma Research Grant, American Health Assistance Foundation/BrightFocus Foundation- National Glaucoma Research Grant, Shaffer Grant- Glaucoma Research Foundation, unrestricted grant from Research to Prevent Blindness (New York, New York) to the Washington University Department of Ophthalmology and Visual Sciences. The funding organizations had no role in the design or conduct of this research.
B. Financial Disclosures:
CJS- Allergan Inc.- Lecture fees
YBS- No financial disclosures
D. Other Acknowledgments: David C. Beebe, PhD (deceased) for his inspiration and passion to bring this scientific investigation to life, Andrew Huang, MD for his guidance and contribution of patients to the study, and Fang Bai, MD for her assistance with the aqueous humor assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schneemann A, Leusink-Muis A, van den Berg T, Hoyng PF, Kamphuis W. Elevation of nitric oxide production in human trabecular meshwork by increased pressure. Graefes Arch Clin Exp Ophthalmol. 2003;241(4):321–326. [DOI] [PubMed] [Google Scholar]
- 2.Ramos RF, Stamer WD. Effects of cyclic intraocular pressure on conventional outflow facility. Invest Ophthalmol Vis Sci. 2008;49(1):275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramos RF, Sumida GM, Stamer WD. Cyclic mechanical stress and trabecular meshwork cell contractility. Invest Ophthalmol Vis Sci. 2009;50(8):3826–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lutjen-Drecoll E, Barany EH. Functional and electron microscopic changes in the trabecular meshwork remaining after trabeculectomy in cynomolgus monkeys. Invest Ophthalmol. 1974;13(7):511–524. [PubMed] [Google Scholar]
- 5.Johnson DH, Matsumoto Y. Schlemm’s canal becomes smaller after successful filtration surgery. Arch Ophthalmol. 2000;118(9):1251–1256. [DOI] [PubMed] [Google Scholar]
- 6.Wubben TJ, Talwar N, Blachley TS, et al. Rates of Vitrectomy among Enrollees in a United States Managed Care Network, 2001–2012. Ophthalmology. 2016;123(3):590–598. [DOI] [PubMed] [Google Scholar]
- 7.Stein JD, Zacks DN, Grossman D, Grabe H, Johnson MW, Sloan FA. Adverse events after pars plana vitrectomy among medicare beneficiaries. Arch Ophthalmol. 2009;127(12):1656–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shui YB, Holekamp NM, Kramer BC, et al. The gel state of the vitreous and ascorbate-dependent oxygen consumption: relationship to the etiology of nuclear cataracts. Arch Ophthalmol. 2009;127(4):475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takano S, Ishiwata S, Nakazawa M, Mizugaki M, Tamai M. Determination of ascorbic acid in human vitreous humor by high-performance liquid chromatography with UV detection. Curr Eye Res. 1997;16(6):589–594. [DOI] [PubMed] [Google Scholar]
- 10.Rose RC, Richer SP, Bode AM. Ocular oxidants and antioxidant protection. Proc Soc Exp Biol Med. 1998;217(4):397–407. [DOI] [PubMed] [Google Scholar]
- 11.Palmquist BM, Philipson B, Barr PO. Nuclear cataract and myopia during hyperbaric oxygen therapy. Br J Ophthalmol. 1984;68(2):113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novak MA, Rice TA, Michels RG, Auer C. The crystalline lens after vitrectomy for diabetic retinopathy. Ophthalmology. 1984;91(12):1480–1484. [DOI] [PubMed] [Google Scholar]
- 13.Melberg NS, Thomas MA. Nuclear Sclerotic Cataract after Vitrectomy in Patients Younger Than 50 Years of Age. Ophthalmology. 1995;102(10):1466–1471. [DOI] [PubMed] [Google Scholar]
- 14.de Bustros S, Thompson JT, Michels RG, Enger C, Rice TA, Glaser BM. Nuclear sclerosis after vitrectomy for idiopathic epiretinal membranes. Am J Ophthalmol. 1988;105(2):160–164. [DOI] [PubMed] [Google Scholar]
- 15.Cherfan GM, Michels RG, de Bustros S, Enger C, Glaser BM. Nuclear sclerotic cataract after vitrectomy for idiopathic epiretinal membranes causing macular pucker. Am J Ophthalmol. 1991;111(4):434–438. [DOI] [PubMed] [Google Scholar]
- 16.Thompson JT, Glaser BM, Sjaarda RN, Murphy RP. Progression of nuclear sclerosis and long-term visual results of vitrectomy with transforming growth factor beta-2 for macular holes. Am J Ophthalmol. 1995;119(1):48–54. [DOI] [PubMed] [Google Scholar]
- 17.Panozzo G, Parolini B. Cataracts associated with posterior segment surgery. Ophthalmol Clin North Am.2004;17(4):557–568, vi. [DOI] [PubMed] [Google Scholar]
- 18.Feng H, Adelman RA. Cataract formation following vitreoretinal procedures. Clin Ophthalmol. 2014;8:1957–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almony A, Holekamp NM, Bai F, Shui YB, Beebe D. Small-gauge vitrectomy does not protect against nuclear sclerotic cataract. Retina. 2012;32(3):499–505. [DOI] [PubMed] [Google Scholar]
- 20.Harocopos GJ, Shui YB, McKinnon M, Holekamp NM, Gordon MO, Beebe DC. Importance of vitreous liquefaction in age-related cataract. Invest Ophthalmol Vis Sci. 2004;45(1):77–85. [DOI] [PubMed] [Google Scholar]
- 21.Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol. 2005;139(2):302–310. [DOI] [PubMed] [Google Scholar]
- 22.Nurminskaya MV, Talbot CJ, Nurminsky DI, Beazley KE, Linsenmayer TF. Nuclear ferritin: a ferritoidferritin complex in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2009;50(8):3655–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai C, Ching A, Lagace C, Linsenmayer T. Nuclear ferritin-mediated protection of corneal epithelial cells from oxidative damage to DNA. Dev Dyn. 2008;237(10):2676–2683. [DOI] [PubMed] [Google Scholar]
- 24.Brubaker RF, Bourne WM, Bachman LA, McLaren JW. Ascorbic acid content of human corneal epithelium. Invest Ophthalmol Vis Sci. 2000;41(7):1681–1683. [PubMed] [Google Scholar]
- 25.Ganea E, Harding JJ. Glutathione-related enzymes and the eye. Curr Eye Res. 2006;31(1):1–11. [DOI] [PubMed] [Google Scholar]
- 26.Cejkova J, Stipek S, Crkovska J, Ardan T. Changes of superoxide dismutase, catalase and glutathione peroxidase in the corneal epithelium after UVB rays. Histochemical and biochemical study. Histol Histopathol. 2000;15(4):1043–1050. [DOI] [PubMed] [Google Scholar]
- 27.Kovaceva J, Platenik J, Vejrazka M, et al. Differences in activities of antioxidant superoxide dismutase, glutathione peroxidase and prooxidant xanthine oxidoreductase/xanthine oxidase in the normal corneal epithelium of various mammals. Physiol Res. 2007;56(1):105–112. [DOI] [PubMed] [Google Scholar]
- 28.Lee HC, Wei YH. Mitochondrial alterations, cellular response to oxidative stress and defective degradation of proteins in aging. Biogerontology. 2001;2(4):231–244. [DOI] [PubMed] [Google Scholar]
- 29.Sacca SC, Izzotti A. Oxidative stress and glaucoma: injury in the anterior segment of the eye. Prog Brain Res. 2008;173:385–407. [DOI] [PubMed] [Google Scholar]
- 30.Izzotti A, Bagnis A, Sacca SC. The role of oxidative stress in glaucoma. Mutat Res. 2006;612(2):105–114. [DOI] [PubMed] [Google Scholar]
- 31.Alvarado J, Murphy C, Polansky J, Juster R. Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci. 1981;21(5):714–727. [PubMed] [Google Scholar]
- 32.Crouch PJ, Cimdins K, Duce JA, Bush AI, Trounce IA. Mitochondria in aging and Alzheimer’s disease. Rejuvenation Res. 2007;10(3):349–357. [DOI] [PubMed] [Google Scholar]
- 33.Kahn MG, Giblin FJ, Epstein DL. Glutathione in calf trabecular meshwork and its relation to aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 1983;24(9):1283–1287. [PubMed] [Google Scholar]
- 34.Izzotti A, Sacca SC, Longobardi M, Cartiglia C. Sensitivity of ocular anterior chamber tissues to oxidative damage and its relevance to the pathogenesis of glaucoma. Invest Ophthalmol Vis Sci. 2009;50(11):5251–5258. [DOI] [PubMed] [Google Scholar]
- 35.Green K Free radicals and aging of anterior segment tissues of the eye: a hypothesis. Ophthal Res. 1995;27 Suppl 1:143–149. [DOI] [PubMed] [Google Scholar]
- 36.Chen JZ, Kadlubar FF. A new clue to glaucoma pathogenesis. Am J Med. 2003;114(8):697–698. [DOI] [PubMed] [Google Scholar]
- 37.Izzotti A, Sacca SC, Cartiglia C, De Flora S. Oxidative deoxyribonucleic acid damage in the eyes of glaucoma patients. Am J Med. 2003;114(8):638–646. [DOI] [PubMed] [Google Scholar]
- 38.Sacca SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005;123(4):458–463. [DOI] [PubMed] [Google Scholar]
- 39.Sorkhabi R, Ghorbanihaghjo A, Javadzadeh A, Rashtchizadeh N, Moharrery M. Oxidative DNA damage and total antioxidant status in glaucoma patients. Mol Vis. 2011;17:41–46. [PMC free article] [PubMed] [Google Scholar]
- 40.Siegfried CJ, Shui YB, Tian B, Nork TM, Heatley GA, Kaufman PL. Effects of Vitrectomy and Lensectomy on Older Rhesus Macaques: Oxygen Distribution, Antioxidant Status, and Aqueous Humor Dynamics. Invest Ophthalmol Vis Sci. 2017;58(10):4003–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang S LXII Edward Jackson lecture: open angle glaucoma after vitrectomy. Am J Ophthalmol. 2006;141(6):1033–1043. [DOI] [PubMed] [Google Scholar]
- 42.Koreen L, Yoshida N, Escariao P, et al. Incidence of, Risk Factors for, and Combined Mechanism of Late-Onset Open-Angle Glaucoma after Vitrectomy. Retina-J Ret Vit Dis. 2012;32(1):160–167. [DOI] [PubMed] [Google Scholar]
- 43.Luk FOJ, Kwok AKH, Lai TYY, Lam DSC. Presence of Crystalline Lens as a Protective Factor for the Late Development of Open Angle Glaucoma after Vitrectomy. Retina-J Ret Vit Dis. 2009;29(2):218–224. [DOI] [PubMed] [Google Scholar]
- 44.Toyokawa N, Kimura H, Matsumura M, Kuroda S. Incidence of late-onset ocular hypertension following uncomplicated pars plana vitrectomy in pseudophakic eyes. Am J Ophthalmol. 2015;159(4):727–732. [DOI] [PubMed] [Google Scholar]
- 45.Wu L, Berrocal MH, Rodriguez FJ, et al. INTRAOCULAR PRESSURE ELEVATION AFTER UNCOMPLICATED PARS PLANA VITRECTOMY Results of the Pan American Collaborative Retina Study Group. Retina-J Ret Vit Dis. 2014;34(10):1985–1989. [DOI] [PubMed] [Google Scholar]
- 46.Mi CW, Thompson JT. Long-Term Follow-up of Intraocular Pressure after Vitrectomy in Eyes without Preexisting Glaucoma. Retina-J Ret Vit Dis. 2015;35(12):2543–2551. [DOI] [PubMed] [Google Scholar]
- 47.Mansukhani SA, Barkmeier AJ, Bakri SJ, et al. The Risk of Primary Open-Angle Glaucoma Following Vitreoretinal Surgery-A Population-based Study. Am J Ophthalmol. 2018;193:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hodapp EPRI, Anderson DR. Clinical decisions in glaucoma. St. Louis: The CV Mosby Co; 1993. [Google Scholar]
- 49.Siegfried CJ, Shui YB, Holekamp NM, Bai F, Beebe DC. Oxygen Distribution in the Human Eye: Relevance to the Etiology of Open-Angle Glaucoma after Vitrectomy. Invest Ophthalmol Vis Sci. 2010;51(11):5731–5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siegfried CJ, Shui YB, Holekamp NM, Bai F, Beebe DC. Racial differences in ocular oxidative metabolism: implications for ocular disease. Arch Ophthalmol. 2011;129(7):849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kampfenkel K, Van Montagu M, Inze D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem. 1995;225(1):165–167. [DOI] [PubMed] [Google Scholar]
- 52.Holekamp NM, Shui YB, Beebe D. Lower intraocular oxygen tension in diabetic patients: possible contribution to decreased incidence of nuclear sclerotic cataract. Am J Ophthalmol. 2006;141(6):1027–1032. [DOI] [PubMed] [Google Scholar]
- 53.Shui YB, Fu JJ, Garcia C, et al. Oxygen distribution in the rabbit eye and oxygen consumption by the lens. Invest Ophthalmol Vis Sci. 2006;47(4):1571–1580. [DOI] [PubMed] [Google Scholar]
- 54.Freddo TF, Bartels SP, Barsotti MF, Kamm RD. The source of proteins in the aqueous humor of the normal rabbit. Invest Ophthalmol Vis Sci. 1990;31(1):125–137. [PubMed] [Google Scholar]
- 55.Fridovich I Oxygen toxicity: a radical explanation. J Exp Biol. 1998;201(Pt 8):1203–1209. [DOI] [PubMed] [Google Scholar]
- 56.Jackson RM. Pulmonary oxygen toxicity. Chest. 1985;88(6):900–905. [DOI] [PubMed] [Google Scholar]
- 57.Alder VA, Cringle SJ. Vitreal and retinal oxygenation. Graefes Arch Clin Exp Ophthalmol. 1990;228(2):151–157. [DOI] [PubMed] [Google Scholar]
- 58.Sakaue H, Tsukahara Y, Negi A, Ogino N, Honda Y. Measurement of vitreous oxygen tension in human eyes. Jpn J Ophthalmol. 1989;33(2):199–203. [PubMed] [Google Scholar]
- 59.Giblin FJ, Quiram PA, Leverenz VR, Baker RM, Dang L, Trese MT. Enzyme-induced posterior vitreous detachment in the rat produces increased lens nuclear pO(2) levels. Exp Eye Res. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McNulty R, Wang H, Mathias RT, Ortwerth BJ, Truscott RJW, Bassnett S. Regulation of tissue oxygen levels in the mammalian lens. J Physiol (Lond). 2004;559(3):883–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee P, Lam KW, Lai M. Aqueous humor ascorbate concentration and open-angle glaucoma. Arch Ophthalmol. 1977;95(2):308–310. [DOI] [PubMed] [Google Scholar]
- 62.Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. Antioxidant status in the aqueous humour of patients with glaucoma associated with exfoliation syndrome. Eye (Lond). 2009;23(8):1691–1697. [DOI] [PubMed] [Google Scholar]
- 63.Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol. 2004;137(1):62–69. [DOI] [PubMed] [Google Scholar]
- 64.Leite MT, Prata TS, Kera CZ, Miranda DV, de Moraes Barros SB, Melo LA Jr. Ascorbic acid concentration is reduced in the secondary aqueous humour of glaucomatous patients. Clin Exp Ophthalmol. 2009;37(4):402–406. [DOI] [PubMed] [Google Scholar]
- 65.Hah YS, Chung HJ, Sontakke SB, et al. Ascorbic acid concentrations in aqueous humor after systemic vitamin C supplementation in patients with cataract: pilot study. BMC Ophthalmol. 2017;17(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benoist d’Azy C, Pereira B, Chiambaretta F, Dutheil F. Oxidative and Anti-Oxidative Stress Markers in Chronic Glaucoma: A Systematic Review and Meta-Analysis. PLoS One. 2016;11(12):e0166915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aejmelaeus RT, Holm P, Kaukinen U, et al. Age-related changes in the peroxyl radical scavenging capacity of human plasma. Free Radic Biol Med. 1997;23(1):69–75. [DOI] [PubMed] [Google Scholar]
- 68.Huang AJ, Shui YB, Han YP, Bai F, Siegfried CJ, Beebe DC. Impact of Corneal Endothelial Dysfunctions on Intraocular Oxygen Levels in Human Eyes. Invest Ophthalmol Vis Sci. 2015;56(11):6483–6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buddi R, Lin B, Atilano SR, Zorapapel NC, Kenney MC, Brown DJ. Evidence of oxidative stress in human corneal diseases. J Histochem Cytochem. 2002;50(3):341–351. [DOI] [PubMed] [Google Scholar]
- 70.Canadananovic V, Latinovic S, Barisic S, Babic N, Jovanovic S. Age-related changes of vitamin C levels in aqueous humour. Vojnosanit Pregl. 2015;72(9):823–826. [DOI] [PubMed] [Google Scholar]
- 71.Freedman SF, Anderson PJ, Epstein DL. Superoxide dismutase and catalase of calf trabecular meshwork. Invest Ophthalmol Vis Sci. 1985;26(10):1330–1335. [PubMed] [Google Scholar]
- 72.Ammar DA, Hamweyah KM, Kahook MY. Antioxidants Protect Trabecular Meshwork Cells From Hydrogen Peroxide-Induced Cell Death. Transl Vis Sci Technol. 2012;1(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300(2):535–543. [DOI] [PubMed] [Google Scholar]
- 74.Richer SP, Rose RC. Water soluble antioxidants in mammalian aqueous humor: interaction with UV B and hydrogen peroxide. Vision Res. 1998;38(19):2881–2888. [DOI] [PubMed] [Google Scholar]
- 75.Huang W, Akesson B. Radioimmunoassay of glutathione peroxidase in human serum. Clin Chim Acta. 1993;219(1–2):139–148. [DOI] [PubMed] [Google Scholar]
- 76.Ringvold A In vitro evidence for UV-protection of the eye by the corneal epithelium mediated by the cytoplasmic protein, RNA, and ascorbate. Acta Ophthalmol Scand. 1997;75(5):496–498. [DOI] [PubMed] [Google Scholar]
- 77.Ringvold A, Anderssen E, Kjonniksen I. Distribution of ascorbate in the anterior bovine eye. Invest Ophthalmol Vis Sci. 2000;41(1):20–23. [PubMed] [Google Scholar]
- 78.Reddy VN, Giblin FJ, Lin LR, Chakrapani B. The effect of aqueous humor ascorbate on ultraviolet-B-induced DNA damage in lens epithelium. Invest Ophthalmol Vis Sci. 1998;39(2):344–350. [PubMed] [Google Scholar]
- 79.De Berardinis E, Tieri O, Polzella A, Iuglio N. The chemical composition of the human aqueous humour in normal and pathological conditions. Exp Eye Res. 1965;4(3):179–186. [DOI] [PubMed] [Google Scholar]
- 80.Kodama T, Kabasawa I, Tamura O, Reddy VN. Dynamics of ascorbate in the aqueous humor and tissues surrounding ocular chambers. Ophthalmic Res. 1985;17(6):331–337. [DOI] [PubMed] [Google Scholar]
- 81.Duarte TL, Lunec J. Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic Res. 2005;39(7):671–686. [DOI] [PubMed] [Google Scholar]
- 82.Ma N, Siegfried C, Kubota M, et al. Expression Profiling of Ascorbic Acid-Related Transporters in Human and Mouse Eyes. Invest Ophthalmol Vis Sci. 2016;57(7):3440–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Umapathy A, Donaldson P, Lim J. Antioxidant delivery pathways in the anterior eye. Biomed Res Int. 2013;2013:207250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsuoka Y, Yamato M, Yamada K. Fluorescence probe for the convenient and sensitive detection of ascorbic acid. J Clin Biochem Nutr. 2016;58(1):16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nimse SB, Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. Rsc Adv. 2015;5(35):27986–28006. [Google Scholar]
- 86.Jiang D, Li X, Liu L, Yagnik GB, Zhou F. Reaction rates and mechanism of the ascorbic acid oxidation by molecular oxygen facilitated by Cu(II)-containing amyloid-beta complexes and aggregates. J Phys Chem B. 2010;114(14):4896–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011;51(5):1000–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boatright WL. Oxygen dependency of one-electron reactions generating ascorbate radicals and hydrogen peroxide from ascorbic acid. Food Chem. 2016;196:1361–1367. [DOI] [PubMed] [Google Scholar]
- 89.Pryor WA, Godber SS. Noninvasive measures of oxidative stress status in humans. Free Radic Biol Med. 1991;10(3–4):177–184. [DOI] [PubMed] [Google Scholar]
- 90.Linster CL, Van Schaftingen E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 2007;274(1):1–22. [DOI] [PubMed] [Google Scholar]
- 91.Spector A, Ma W, Wang RR. The aqueous humor is capable of generating and degrading H2O2. Invest Ophthalmol Vis Sci. 1998;39(7):1188–1197. [PubMed] [Google Scholar]
- 92.Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res. 2005;24(5):612–637. [DOI] [PubMed] [Google Scholar]
- 93.Grierson I, Howes RC. Age-related depletion of the cell population in the human trabecular meshwork. Eye (Lond) 1987;1 (Pt 2):204–210. [DOI] [PubMed] [Google Scholar]
- 94.Sacca SC, Izzotti A, Rossi P, Traverso C. Glaucomatous outflow pathway and oxidative stress. Exp Eye Res. 2007;84(3):389–399. [DOI] [PubMed] [Google Scholar]
- 95.Yu AL, Brummeisl W, Schaumberger M, Kampik A, Welge-Lussen U. Vitrectomy does not increase the risk of open-angle glaucoma or ocular hypertension--a 5-year follow-up. Graefes Arch Clin Exp Ophthalmol. 2010;248(10):1407–1414. [DOI] [PubMed] [Google Scholar]
- 96.Lalezary M, Kim SJ, Jiramongkolchai K, Recchia FM, Agarwal A, Sternberg P Jr. Long-term trends in intraocular pressure after pars plana vitrectomy. Retina. 2011;31(4):679–685. [DOI] [PubMed] [Google Scholar]
- 97.Lalezary M, Shah RJ, Reddy RK, et al. Prospective Retinal and Optic Nerve Vitrectomy Evaluation (PROVE) study: twelve-month findings. Ophthalmology. 2014;121(10):1983–1989. [DOI] [PubMed] [Google Scholar]
- 98.Patel SKS, Lalezary M, Shah RJ, Kuchtey RW, Joos KM, Kammer JA, Cherney EF. IOP Changes: Three-Year Findings of the Prospective Retinal and Optic Nerve Vitrectomy Evaluation (PROVE) Study. American Academy of Ophthalmology Annual Meeting; 2016; Chicago, IL. [Google Scholar]
- 99.Bar-Ilan A, Pessah NI, Maren TH. The effects of carbonic anhydrase inhibitors on aqueous humor chemistry and dynamics. Invest Ophthalmol Vis Sci. 1984;25(10):1198–1205. [PubMed] [Google Scholar]
- 100.Becker B The effects of the carbonic anhydrase inhibitor, acetazoleamide, on the composition of the aqueous humor. Am J Ophthalmol. 1955;40(5 Pt 2):129–136. [DOI] [PubMed] [Google Scholar]
- 101.Zanon-Moreno V, Garcia-Medina JJ, Gallego-Pinazo R, Vinuesa-Silva I, Moreno-Nadal MA, Pinazo-Duran MD. Antioxidant status modifications by topical administration of dorzolamide in primary open-angle glaucoma. Eur J Ophthalmol. 2009;19(4):565–571. [DOI] [PubMed] [Google Scholar]
- 102.Izzotti A, Sacca SC, Di Marco B, Penco S, Bassi AM. Antioxidant activity of timolol on endothelial cells and its relevance for glaucoma course. Eye (Lond). 2008;22(3):445–453. [DOI] [PubMed] [Google Scholar]
- 103.Melena J, Osborne NN. Metipranolol attenuates lipid peroxidation in rat brain: a comparative study with other antiglaucoma drugs. Graefes Arch Clin Exp Ophthalmol. 2003;241(10):827–833. [DOI] [PubMed] [Google Scholar]
- 104.Lee KY, Nakayama M, Aihara M, Chen YN, Araie M. Brimonidine is neuroprotective against glutamate-induced neurotoxicity, oxidative stress, and hypoxia in purified rat retinal ganglion cells. Mol Vis. 2010;16:246–251. [PMC free article] [PubMed] [Google Scholar]
- 105.Yu AL, Fuchshofer R, Kampik A, Welge-Lussen U. Effects of oxidative stress in trabecular meshwork cells are reduced by prostaglandin analogues. Invest Ophthalmol Vis Sci. 2008;49(11):4872–4880. [DOI] [PubMed] [Google Scholar]