Abstract
Introduction
This study aimed to compare the cytocompatibility and angiogenic potential of two antibiotics (clindamycin [CLIN] and minocycline [MINO]) at distinct concentrations on dental pulp stem cells (DPSCs) and human umbilical vein endothelial cells (HUVECs).
Methods
DPSCs and HUVECs were exposed to cell culture media modified with CLIN or MINO at concentrations ranging from 30 μg/ml to 1000 μg/ml. Cell toxicity and proliferation were investigated using the lactate dehydrogenase (LDH) and tetrazolium reduction (MTS) assays, respectively. A capillary-like tube formation in vitro assay was conducted to determine the angiogenic potential associated with each antibiotic. Additionally, selected morphometric angiogenesis parameters were determined using a dedicated software (WimTube™). All statistical analyses were performed using one-way ANOVA and Tukey’s post-hoc test (α=0.05).
Results
The collected data showed that, compared to the control (cell culture media, α-Minimum Essential Medium Eagle), increasing antibiotics’ concentration significantly decreased cell viability and proliferation of both DPSCs and HUVECs. In terms of angiogenic potential, when tested at 30 μg/ml and 50 μg/ml, CLIN significantly amplified tube formation when compared to MINO with angiogenesis parameters (i.e., tube length and tube number) similar to the effect promoted by exogenous vascular endothelial growth factor (VEGF, 50 ng/ml).
Conclusion
CLIN demonstrated being less cytotoxic when compared to MINO at higher concentrations. Of note, CLIN did not hinder the pro-angiogenic activity induced by VEGF to the same extent as MINO, suggesting that the replacement of MINO by CLIN might translate into positive implications in the overall regenerative outcome.
Keywords: angiogenesis, minocycline, clindamycin, disinfection, pulp, regeneration, stem cells
Introduction
Clinically, the treatment of pulp necrosis in immature permanent teeth is often challenging, mostly due to distinctively thin dentinal walls and wide-open apices (1). Although apexification, using either calcium hydroxide or mineral trioxide aggregate, remains a practical procedure to induce the formation of a calcified apical barrier; it eliminates any further chance for complete root development (2–4), thus increasing the risk of tooth loss upon secondary trauma or reinfection (5).
Recent advances in the field of regenerative endodontics, particularly those associated with using the evoked bleeding (EB) technique, suggest that a biocompatible (i.e., minimal or no deleterious effects on dental stem cells’ survival and function) root canal disinfection, followed by periapical tissue laceration to allow stem cells and growth factors to populate the blood clot derived fibrin matrix, has the potential to induce viable tissue formation in the root canal (1, 7). Worth mentioning, the EB method relies not only on the successful migration of stem cells, but also on a biologically gentle and thorough root canal disinfection (7). Unfortunately, minocycline (MINO), one of the contents of the triple antibiotic paste (TAP) (8–10), has long been known for its potent antiangiogenic activity and is found to inhibit new blood vessel formation (10–12). Another important aspect relates to MINO’s binding ability to Ca+2 ions by chelation and the development of insoluble complexes that increase its substantivity—that is, the ability to maintain its activity over a prolonged period of time (13). In aggregate, one might speculate that the long-term presence of MINO within the root canal system may hamper angiogenesis and, ultimately, the overall regenerative outcome (14).
Clindamycin (CLIN), a bacteriostatic lincosamide, is active against most strains of gram-positive aerobes and most anaerobic organisms responsible for dentoalveolar and endodontic infections (15). In a recent study, polymer-based CLIN-eluting nanofibers were synthesized for use as a potential intracanal drug delivery system (14). Notably, a series of investigations have supported the hypothesis that antibiotic-eluting nanofibers can be used to deliver small, yet effective amounts of antibiotics for root canal disinfection (6, 14, 16–20). In essence, the findings of that research showed that CLIN-eluting nanofibers were able to significantly reduce the growth of endodontic bacteria, such as Enterococcus faecalis and Aggregatibacter actinomycetemcomitans, while maitaining dental pulp stem cells’ (DPSCs) viability (14). Meanwhile, in a different study, the angiogenic activity of human blood mononuclear cells studied under the influence of CLIN showed strong angiogenic response (21). Nonetheless, it is worth mentioning that a rapid increase in the prevalence of CLIN-related bacterial resistance has recently been reported, particularly among organisms in the Bacteroides fragilis group (gram-negative obligated anaerobes) (22); its indication when treating bacteroides with known susceptibilities or other mixed infections (e.g., oral infections) can still be considered (23). Taken together, based on the aforementioned evidence, CLIN might be a good alternative for root canal disinfection prior to regenerative procedures (EB method), particularly when used as an intracanal drug delivery system. Thus, the objectives of this study were twofold: (i) to evaluate the effects of CLIN and MINO on the survival of dental pulp stem cells (DPSC) and human umbilical vein endothelial cell (HUVEC), and (ii) to compare the in vitro angiogenesis by HUVEC between CLIN and MINO. The hypotheses of the study were that CLIN would not only lead to reduced cell toxicity, but more importantly, it would enhance the angiogenesis of HUVEC when compared to MINO.
Materials and Methods
Cell Culture
HUVECs (PromoCell, Heidelberg, Germany) were cultured in endothelial growth medium (EGM) supplemented with fetal calf serum, fibroblast growth factor, epidermal growth factors, heparin, and hydrocortisone. Previously isolated and characterized DPSCs were used (24). DPSCs were cultured in alpha minimum essential medium (α-MEM) supplemented with 10% fetal bovine serum. Cells of passage 3–6 were cultured at 37°C in a humidified atmosphere containing 5% CO2 and used for the experiments.
Antibiotic Preparation
MINO and CLIN hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO, USA) and TCI America Inc. (Portland, OR, USA), respectively. The following concentrations (30, 50, 100, 250, 500, 1000 μg/ml) were prepared in α-MEM for angiogenesis and toxicity assays.
Cell Proliferation and Toxicity
DPSCs and HUVECs were seeded at 2×103 cells/well in 96-well plates in the previously mentioned media, with corresponding antibiotic concentrations. Cell proliferation was evaluated using MTS assay (Promega, Madison, WI, USA). The cells were cultured for 24, 48, 72, and 120 h. After each timepoint, 20 μl/well of CellTiter 96R AQueous One Solution was added and incubated for 2 h in a humidified 5% CO2 atmosphere. The absorbance was measured in a microplate reader at 490 nm (Spectra iD3, Molecular Devices LLC, San Jose, CA, USA).
To confirm the cytocompatible character of the antibiotics and check for possible cell membrane damage, the amount of lactate dehydrogenase (LDH) was quantitatively evaluated using CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega) following manufacturer’s guidelines at 24, 48, and 72 h. The high control (total cell death) was generated by adding 1.9 ml of media and a 100 μl lysis solution provided by the manufacturer to detect the maximum LDH release. The low control had a medium from untreated control cells at each period. Absorbance was recorded at 492 nm in a microplate reader. The percentage release of LDH from the treated cells was calculated by comparing it with a maximum release of LDH achieved by the lysis solution used on the control cells. The cytotoxicity percentage was calculated as follows:
Angiogenesis Assay – Capillary-like Tube Formation
The capillary-like tubule formation assay was performed using the PromoKine Angiogenesis Assay Kit (PromoCell). In brief, HUVECs were seeded onto Matrigel-coated plates at a density of 1×105 cells/well in ECGM supplemented with vascular endothelial growth factor (VEGF, 50 ng/ml) with or without CLIN or MINO at a concentration of 30, 50, and 100 μg/ml. After 24 h incubation at 37°C, the plates were carefully washed (2×) with buffer and then stained with calcein (1:200 dilutions of staining dye concentrate in wash buffer). The plates were incubated for 30 min at 37°C and then subjected to light and fluorescence microscopy at distinct magnifications (BZ-X 710, Keyence Corporation of America, Itasca, IL, USA). The morphological features (25) were quantitatively measured to characterize the capillary-like tube structure (CLS) using a dedicated software (WimTube™, Onimagin Technologies SCA, Córdoba, Spain).
Statistical Analysis
All results are reported as the mean ± standard deviation (n=3) of three independent experiments. To compare the difference between means among three or more groups, the statistical significance was analyzed using one-way ANOVA followed by Tukey’s post-hoc test (α=0.05).
Results
Cell Proliferation and Cytotoxicity
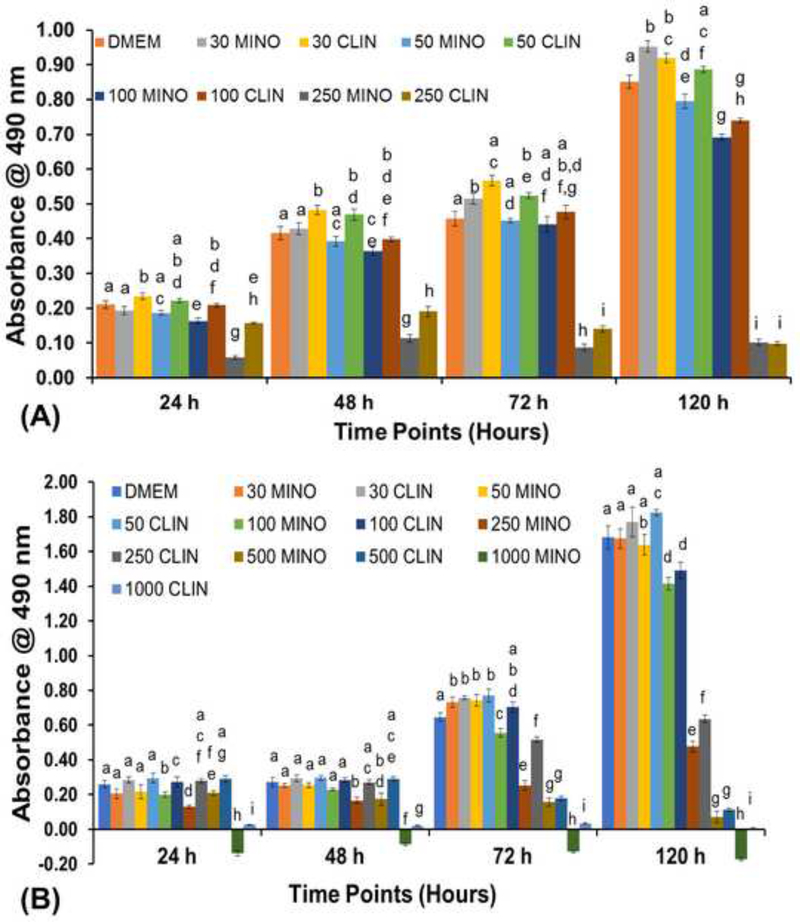
Figure 1 shows the antibiotics-induced effects on DPSCs’ and HUVECs’ proliferation, as assessed by the MTS assay. Following CLIN and MINO exposure, HUVEC proliferation decreased in a dose-dependent manner. After 24 h, HUVEC proliferation was significantly reduced at 250 μg/ml, compared to the untreated control. CLIN showed significantly higher proliferation compared to MINO at 250 μg/ml at all timepoints tested except at 120 h for HUVEC. The lower concentration of CLIN (i.e., 30 and 50 μg/ml) showed enhanced proliferation compared to MINO at a similar concentration for up to 72 h for HUVEC. However, there was no significant difference in the proliferation of DPSC compared to the untreated control and at lower concentrations of CLIN and MINO. The concentrations, ranging from 100 to 500 μg/ml of CLIN, showed significantly higher proliferation for up to 48 h. Nevertheless, 1000 μg/ml of MINO showed negative cell proliferation, indicating cell death at all timepoints tested.
Figure 1.
Cell proliferation for both cell types after incubation with different antibiotics’ concentrations. A) HUVEC and B) DPSC. MINO and CLIN at 250 μg/ml showed a decrease in cell proliferation for HUVEC; whereas, DPSC showed decreased proliferation at 500 μg/ml. The same letters indicate a nonsignificant difference compared with the results on the same day.
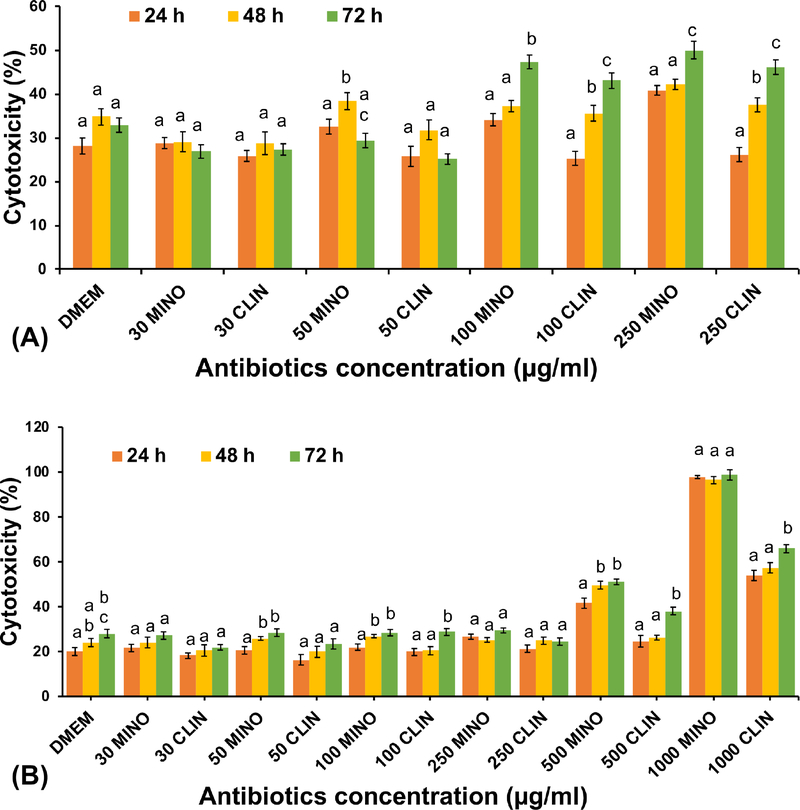
Cell membrane integrity was assessed at 24, 48, and 72 h of incubation with antibiotics using the LDH assay (Figure 2). For HUVECs, the concentration of 100 and 250 μg/ml caused significant toxicity compared with 30 and 50 μg/ml concentrations and the untreated control. For DPSCs, there was no significant difference in concentrations ranging from 30 up to 250 μg/ml in terms of toxicity compared with the untreated control at the same timepoint tested. LDH activity increased twofold for DPSC compared with the corresponding control cells at 500 μg/ml for MINO; and, in the case of CLIN, it was fairly similar to the control at 24 h and 48 h. MINO and CLIN at 1000 μg/ml showed approximately 100% and 60% toxicity, respectively, at all timepoints.
Figure 2.
Cell toxicity measured by LDH release for both cell types after incubation with different antibiotics’ concentrations. A) HUVEC and B) DPSC. MINO and CLIN showed approximately a 50% toxicity at 100 μg/mL for HUVEC; whereas, for DPSC, it was 500 μg/mL. The same letters indicate a non-significant difference compared with the results on different day.
Capillary-like Tube Formation
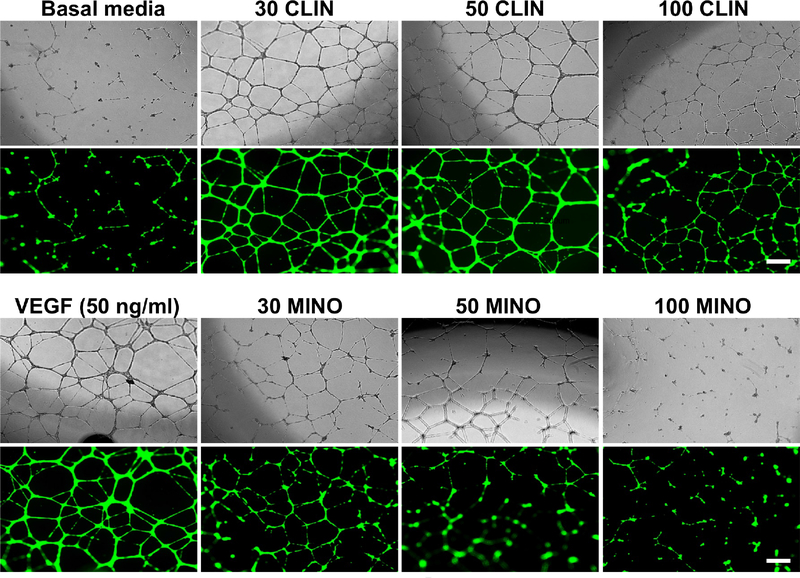
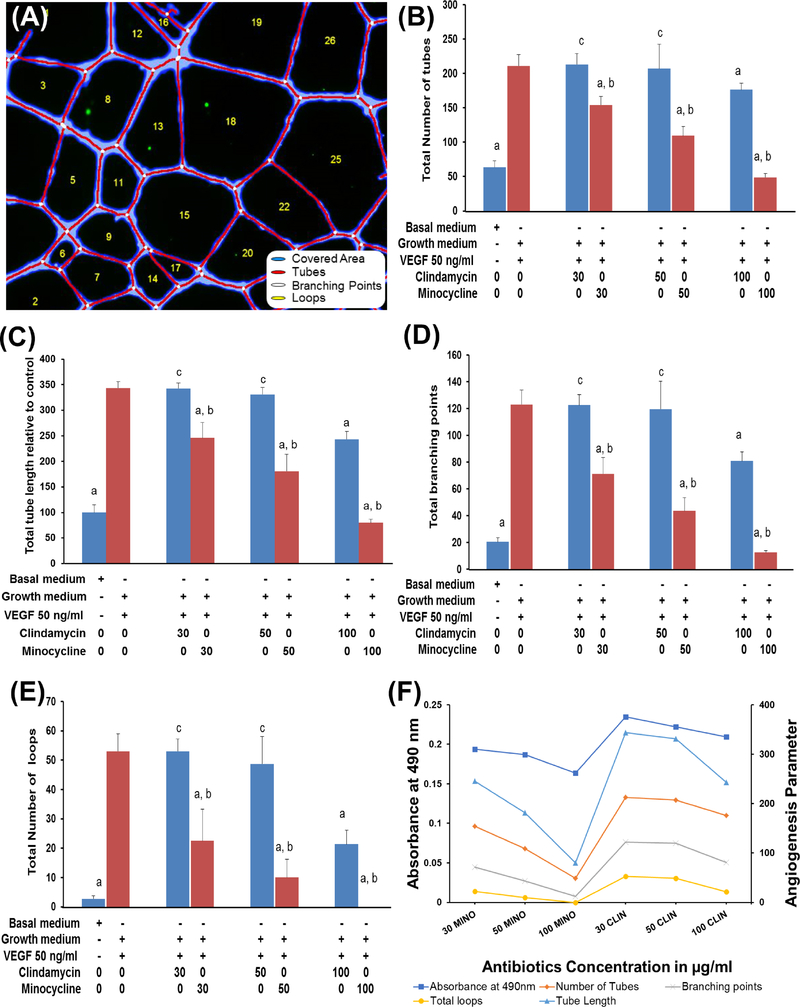
In the previous section, we showed that 100 μg/ml of antibiotics decreased proliferation and are toxic to HUVEC; hence, the angiogenic capability of HUVECs seeded in the presence of exogenous VEGF (50 ng/ml) were tested with antibiotics concentrations that varied from 30 to 100 μg/ml. As evidenced by Figure 3, the formation of CLS by HUVECs was more prominent in CLIN at all concentrations compared to MINO. In fact, a lower concentration of MINO showed a distorted CLS, which was more prominent at higher concentrations. From a quantitative perspective, the effects of antibiotic concentration on the formation of a CLS were characterized by the most widely used morphometric parameters (Figure 4A). CLIN shows longer capillary tube lengths and tube numbers similar to exogenous VEGF, implying that CLIN does not inhibit HUVEC angiogenic ability in inducing capillary-like tube formation. In contrast, the addition of MINO resulted in an inhibition of tube length and number (Figure 4B–C). Compared to CLIN, MINO at 100 μg/ml exhibited the complete absence of loops identified by empty regions of the field surrounded by cell clusters/tubules and a smaller number of branching points, resulting in very few network structures that were not interconnected (Figure 4D–E). Furthermore, the CLS features of MINO and CLIN lessen with a decrease in cell proliferation as the concentration of antibiotics increase (Figure 4F). The tube formation efficiency of MINO showed a more dramatic decrease than CLIN with an increase in the antibiotics’ concentration.
Figure 3.
Brightfield and fluorescence images of capillary-like tube formation. Prior to the start of the assay, the endothelial cells were treated with calcein AM to visualize the cells using fluorescence microscopy. Increasing concentrations of antibiotics resulted in decreases in the capillary-like network structure. However, for MINO, even at lower concentrations, cells showed apoptosis, and tubes detach from the matrix and break apart (bar = 200 μm).
Figure 4.
(A) Morphological parameters of angiogenesis estimated by processing the binary skeleton using WimTubeTM angiogenesis analysis. (B-F) The quantitative evaluation of morphological features of a capillary-like network structure after treating HUVECs with different concentrations of antibiotics were calculated in 4 different images using WimTubeTM angiogenesis image analysis. (B) Total tube length relative to the control (C) Total number of tubes (D) Total branching point (E) Total number of loops (F) Relationship of cell proliferation and angiogenesis.
Discussion
To date, there is no consensus on the use of TAP and its clinical implication in revascularization of the root canal system to achieve true regeneration of the pulp-dentin complex (7, 26–29). MINO, due to its high substantivity, remains in the root canal system for a long time and can induce deleterious effects on the survivability of cells and angiogenesis (7, 30). Several other antibiotics or the use of DAP has been suggested as a replacement for MINO to reduce tooth discoloration; however, none of these antibiotics have been examined for their potential (positive or negative) effects on angiogenesis (14, 31). Indeed, for successful regenerative endodontic treatment, it is crucial to establish an environment that allows stem cell survival and supports the formation of new blood vessels (1–4, 30). Hence, the wise selection of antibiotics that display effective antimicrobial activity against endodontic bacteria, while preserving the proliferation potential of dental stem cells and angiogenic activity, is key for endodontic regeneration.
We have previously reported the use of CLIN as a substitute for MINO, based on its broad antibacterial spectrum and stain-free properties (14). Besides, it is the second-choice antibiotic for patients who are allergic to penicillin (32, 33). To the best of our knowledge, this study is the first to present a head-to-head comparison of the angiogenesis activity of MINO and CLIN. Moreover, the current investigation demonstrated the dose-dependent effects of MINO and CLIN on DPSCs’ and HUVECs’ viability and proliferation. Our results agree with previously published literature for MINO and CLIN in terms of cell toxicity and effects on angiogenesis (14, 21, 31, 34, 35). Recent studies have shown 30–60% cell viability for stem cells from the apical papilla (SCAPs) and DPSCs when treated with 25 μg/ml of MINO, a concentration lower than that used in endodontic regeneration (8). Our group previously reported that DPSC treated with aliquots of CLIN nanofibers led to ∼ 70% cell viability compared to CLIN-modified triple antibiotic nanofibers, which showed ∼ 52% at day 1 (14). In this study, differences were detected in the safest concentrations of MINO and CLIN as determined by the MTS and LDH assays. The proliferation of DPSC was inhibited at 250 μg/ml concentration after 72 h, compared to lactate production, which was similar for both MINO and CLIN; whereas, in contrast, lactate production of HUVEC was approximately 50% at 100 μg/ml after 72 h and there was no inhibition of the proliferation. This difference in proliferation and lactate production can be explained by high metabolic activity of dental pulp stem cells compared with HUVECs (36).
Dental pulp is a complex, highly vascularized tissue. Well-developed microvasculature and microcirculation systems are critical for successful regeneration of the dentin-pulp complex (37). Many recent histologic findings of teeth treated with TAP have shown that newly generated tissue is not pulp tissue (26, 27). It seems that the high concentration of antibiotics in TAP affects not only cell survival and proliferation, but also angiogenesis. VEGF is known to promote the proliferation, migration, and capillary-like tube formation of endothelial cells (38). MINO, one of the antibiotics used in TAP, is known to inhibit angiogenesis by suppression of the hypoxia-induced VEGF expression and migration of human aortic smooth muscle cells (10, 11). Here, HUVECs treated with different concentrations of MINO in the presence of VEGF strongly reduced chemotaxis migration and capillary-like tube formation as compared with CLIN with VEGF-treated HUVEC. Interestingly, CLIN showed prominent capillary-like tube networks at 30 and 50 μg/ml concentrations with a higher number of branching points and tube lengths compared to MINO, suggesting that CLIN possesses an angiogenic ability to induce endothelial cell differentiation. Meanwhile, MINO-treated HUVECs were desensitized to stimulation by angiogenic factors. In sum, CLIN did not hinder the pro-angiogenic activity induced by VEGF to the same extent as MINO, suggesting that the replacement of MINO by CLIN might translate into positive implications in the overall regenerative outcome. Future studies in preclinical animal models are warranted to determine the role of CLIN in the revascularization of the root canal and as a potential substitute for MINO in TAP.
Highlights.
MINO and CLIN displayed dose-dependent cytotoxicity on DPSCs and HUVECs
CLIN is less cytotoxic when compared to MINO.
CLIN enhanced HUVECs proliferation and capillary-like tube formation.
CLIN (30 and 50 μg/ml) displayed angiogenic effects similar to exogenous VEGF.
Statement of Clinical Relevance.
Our findings shed new light on the possible use of CLIN as a viable alternative to MINO to improve stem cells survival and support angiogenesis during regenerative endodontics procedures.
Acknowledgments
The authors deny any conflicts of interest related to this study. M.C.B. acknowledges the National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research (NIDCR) (Grant # DE026578). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod 2004;30:196–200. [DOI] [PubMed] [Google Scholar]
- 2.Diogenes A, Henry MA, Teixeira FB, Hargreaves KM. An update on clinical regenerative endodontics. Endod Topics 2013;28:2–23. [Google Scholar]
- 3.Albuquerque MT, Valera MC, Nakashima M, Nör JE, Bottino MC. Tissue-engineering-based strategies for regenerative endodontics. J Dent Res 2014;93:1222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hargreaves KM, Diogenes A, Teixeira FB. Treatment options: biological basis of regenerative endodontic procedures. J Endod 2013;39(3 Suppl):S30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cvek M Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endod Dent Traumatol 1992;8:45–55. [DOI] [PubMed] [Google Scholar]
- 6.Albuquerque MTP, Nagata JY, Diogenes AR, Azabi AA, Gregory RL, Bottino MC. Clinical perspective of electrospun nanofibers as a drug delivery strategy for regenerative endodontics. Curr Oral Health Rep 2016;3:209–220. [Google Scholar]
- 7.Bottino MC, Pankajakshan D, Nör JE. Advanced scaffolds for dental pulp and periodontal regeneration. Dent Clin North Am 2017;61:689–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuensombat S, Khemaleelakul S, Chattipakorn S, Srisuwan T. Cytotoxic effects and antibacterial efficacy of a 3-antibiotic combination: an in vitro study. J Endod 2013;39:813–819. [DOI] [PubMed] [Google Scholar]
- 9.Park JB. Effects of doxycycline, minocycline, and tetracycline on cell proliferation, differentiation, and protein expression in osteoprecursor cells. J Craniofac Surg 2011;22:1839–1842. [DOI] [PubMed] [Google Scholar]
- 10.Yao JS, Shen F, Young WL, Yang GY. Comparison of doxycycline and minocycline in the inhibition of VEGF-induced smooth muscle cell migration. Neurochem Int 2007;50:524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li CH, Liao PL, Yang YT, Huang SH, Lin CH, Cheng YW, Kang JJ. Minocycline accelerates hypoxia-inducible factor-1 alpha degradation and inhibits hypoxia-induced neovasculogenesis through prolyl hydroxylase, von Hippel–Lindau-dependent pathway. Arch Toxicol 2014;88:659–671. [DOI] [PubMed] [Google Scholar]
- 12.Tamargo RJ, Bok RA, Brem H. Angiogenesis inhibition by minocycline. Cancer Res 1991;51:672–675. [PubMed] [Google Scholar]
- 13.Mohammadi Z, Abbott PV. On the local applications of antibiotics and antibiotic-based agents in endodontics and dental traumatology. Int Endod J 2009;42:555–67. [DOI] [PubMed] [Google Scholar]
- 14.Karczewski A, Feitosa SA, Hamer EI, Pankajakshan D, Gregory RL, Spolnik KJ, Bottino MC. Clindamycin-modified triple antibiotic nanofibers: a stain-free antimicrobial intracanal drug delivery system. J Endod 2018;44:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilad JZ, Teles R, Goodson M, White RR, Stashenko P. Development of a clindamycin-impregnated fiber as an intracanal medication in endodontic therapy. J Endod 1999;25:722–727. [DOI] [PubMed] [Google Scholar]
- 16.Bottino MC, Albuquerque MTP, Azabi A, Münchow EA, Spolnik KJ, Nör JE, Edwards PC. A novel patient-specific three-dimensional drug delivery construct for regenerative endodontics. J Biomed Mater Res B Appl Biomater. 2018. October 3. doi: 10.1002/jbm.b.34250. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albuquerque MTP, Nagata J, Bottino MC. Antimicrobial efficacy of triple antibiotic-eluting polymer nanofibers against multispecies biofilm. J Endod 2017;43:S51–S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pankajakshan D, Albuquerque MT, Evans JD, Kamocka MM, Gregory RL, Bottino MC. Triple antibiotic polymer nanofibers for intracanal drug delivery: effects on dual species biofilm and cell function. J Endod 2016;42:1490–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albuquerque MT, Valera MC, Moreira CS, Bresciani E, de Melo RM, Bottino MC. Effects of ciprofloxacin-containing scaffolds on enterococcus faecalis biofilms. J Endod 2015;41:710–4. [DOI] [PubMed] [Google Scholar]
- 20.Albuquerque MT, Ryan SJ, Münchow EA, Kamocka MM, Gregory RL, Valera MC, Bottino MC. Antimicrobial effects of novel triple antibiotic paste-mimic scaffolds on Actinomyces naeslundii biofilm. J Endod 2015;41:1337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radomska-Leśniewska DM, Skopińska-Różewska E, Malejczyk J. The effect of clindamycin and lincomycin on angiogenic activity of human blood mononuclear cells. Centr Eur J Immunol 2010;35:217–222. [Google Scholar]
- 22.Solomkin JS, Mazuski JE, Baron EJ, Sawyer RG, Nathens AB, DiPiro JT, Buchman T, Dellinger EP, Jernigan J, Gorbach S, Chow AW, Bartlett J. Guidelines for the selection of anti-infective agents for complicated intra-abdominal infections. Clin Infect Dis 2003;37:997–1005. [DOI] [PubMed] [Google Scholar]
- 23.Hecht DW. Prevalence of antibiotic resistance in anaerobic bacteria: worrisome developments. Clin Infect Dis 2004;39:92–7. [DOI] [PubMed] [Google Scholar]
- 24.Silva GO, Zhang Z, Cucco C, Oh M, Camargo CHR, Nör JE. Lipoprotein receptor-related protein 6 signaling is necessary for vasculogenic differentiation of human dental pulp stem cells. J Endod 2017;43:S25–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varberg KM, Winfree S, Dunn KW, Haneline LS. Kinetic analysis of vasculogenesis quantifies dynamics of vasculogenesis and angiogenesis in vitro. J Vis Exp 2018. January 31;(131). doi: 10.3791/57044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Thibodeau B, Trope M, Lin LM, Huang GT-J. Histologic characterization of regenerated tissues in canal space after the revitalization/revascularization procedure of immature dog teeth with apical periodontitis. J Endod 2010;36:56–63. [DOI] [PubMed] [Google Scholar]
- 27.Yamauchi N, Nagaoka H, Yamauchi S, Teixeira FB, Miguez P, Yamauchi M. Immunohistological characterization of newly formed tissues after regenerative procedure in immature dog teeth. J Endod 2011;37:1636–1641. [DOI] [PubMed] [Google Scholar]
- 28.Shah N, Logani A, Bhaskar U, Aggarwal V. Efficacy of revascularization to induce apexification/apexogensis in infected, nonvital, immature teeth: a pilot clinical study. J Endod 2008;34:919–925. [DOI] [PubMed] [Google Scholar]
- 29.Becerra P, Ricucci D, Loghin S, Gibbs JL, Lin LM. Histologic study of a human immature permanent premolar with chronic apical abscess after revascularization/revitalization. J Endod 2014;40:133–139. [DOI] [PubMed] [Google Scholar]
- 30.Diogenes AR, Ruparel NB, Teixeira FB, Hargreaves KM. Translational science in disinfection for regenerative endodontics. J Endod 2014:S52–7. [DOI] [PubMed] [Google Scholar]
- 31.Ruparel NB, Teixeira FB, Ferraz CC, Diogenes A. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. Journal of endodontics 2012;38(10):1372–1375. [DOI] [PubMed] [Google Scholar]
- 32.Walker CB, Gordon J, Cornwall H, Murphy J, Socransky S. Gingival crevicular fluid levels of clindamycin compared with its minimal inhibitory concentrations for periodontal bacteria. Antimicrobial agents and chemotherapy 1981;19(5):867–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segura-Egea JJ, Martín-González J, Jiménez-Sánchez MdC, Crespo-Gallardo I, Saúco-Márquez JJ, Velasco-Ortega E. Worldwide pattern of antibiotic prescription in endodontic infections. International dental journal 2017;67(4):197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naal FD, Salzmann GM, Von Knoch F, Tuebel J, Diehl P, Gradinger R, et al. The effects of clindamycin on human osteoblasts in vitro. Archives of orthopaedic and trauma surgery 2008;128(3):317–323. [DOI] [PubMed] [Google Scholar]
- 35.Wu H, Dai X, Li H, Lv C. Effect of minocycline on vascular proliferation after corneal alkaline burn: A mechanism study. Biomedical Research 2017;28(17). [DOI] [PubMed] [Google Scholar]
- 36.Bok J-S, Byun S-H, Park B-W, Kang Y-H, Lee S-L, Rho G-J, et al. The Role of Human Umbilical Vein Endothelial Cells in Osteogenic Differentiation of Dental Follicle-Derived Stem Cells in In Vitro Co-cultures. International journal of medical sciences 2018;15(11):1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakashima M, Iohara K, Bottino MC, Fouad AF, Nör JE, Huang GT. Animal models for stem cellbased pulp regeneration: Foundation for human clinical applications. Tissue Eng Part B Rev. 2018. October 4. doi: 10.1089/ten.TEB.2018.0194. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters KG, De Vries C, Williams LT. Vascular endothelial growth factor receptor expression during embryogenesis and tissue repair suggests a role in endothelial differentiation and blood vessel growth. Proceedings of the National Academy of Sciences 1993;90(19):8915–8919. [DOI] [PMC free article] [PubMed] [Google Scholar]