Abstract
Background:
Transcatheter aortic valve replacement (TAVR) may be associated with less delirium and allow faster recovery than surgical aortic valve replacement (SAVR).
Objective:
To examine the association of delirium and its severity with clinical and functional outcomes after SAVR and TAVR.
Design:
Prospective cohort study
Setting:
An academic medical center
Participants:
187 patients 70 years and older undergoing SAVR (N=77) and TAVR (N=110) in 2014–2016
Measurements:
Delirium was assessed daily using the Confusion Assessment Method (CAM), with severity measured by the CAM-Severity (CAM-S) score (range: 0–19). Outcomes were prolonged hospitalization (≥9 days); institutional discharge; functional status, measured by ability to perform 22 daily activities and physical tasks over 12 months.
Results:
SAVR patients had a higher incidence of delirium than TAVR patients (50.7% vs. 25.5%; p<0.001), despite younger mean age (77.9 vs. 83.7 years) and higher baseline Mini-Mental State Examination score (26.9 vs. 24.7). SAVR patients with delirium had a shorter duration (2.2 vs 3.4 days; p=0.04) with a lower mean CAM-S score than TAVR patients with delirium (4.5 vs 5.7; p=0.01). The risk of prolonged hospitalization in no, mild, and severe delirium was 18.4%, 30.8%, 61.5% after SAVR (p-for-trend=0.009) and 26.8%, 38.5%, 73.3% after TAVR (p-for-trend=0.001), respectively. The risk of institutional discharge was 42.1%, 58.3%, 84.6% after SAVR (p-for-trend=0.01) and 32.5%, 69.2%, 80.0% after TAVR (p-for-trend<0.001), respectively. Severe delirium was associated with delayed functional recovery after SAVR and persistent functional impairment after TAVR at 12 months.
Conclusion:
Less invasive TAVR was associated with lower incidence of delirium than SAVR. Once delirium developed, TAVR patients had more severe delirium and worse functional status trajectory than SAVR patients did.
Registration:
Keywords: Delirium, Functional Status, Aortic Valve Replacement, Transcatheter Aortic Valve Replacement
INTRODUCTION
Delirium is a major postoperative complication after cardiac surgical procedures that develops when patients with predisposing risk factors (e.g., cognitive impairment, frailty) are exposed to acute stress (e.g., surgery, pain).1 Once considered an acute and transient state, contemporary evidence supports long-term consequences of delirium after major cardiac surgery, including mortality,2 functional decline,3 and cognitive impairment.4 Recently, aortic valve replacement volumes have been rising as transcatheter aortic valve replacement (TAVR) has become an established treatment of aortic stenosis (AS) in older adults at intermediate to high risk for surgical aortic valve replacement (SAVR).5 Since TAVR is minimally invasive, with generally shorter procedure time and no need for sternotomy or cardiopulmonary bypass, it imposes overall less physiologic stress than SAVR,6–8 and may cause less delirium and allow faster functional recovery.
Earlier studies of delirium in cardiac surgery were largely conducted in patients undergoing coronary artery bypass graft surgery.2,3 Incidence of delirium in the literature ranges in 25–66% after major cardiac surgery1,9,10 and 5–45% after TAVR.11 This variation in the delirium incidence may be driven by different assessments of delirium (e.g., medical records12 or diagnosis codes13). Although the association of delirium with prolonged hospitalization, non-home discharge and mortality after major cardiac surgery14–16 and TAVR10,12–14,16–19 is well established, it is less well studied how delirium is associated with functional recovery.3,20
We conducted a prospective cohort study to investigate the association of delirium and its severity with clinical and functional outcomes after SAVR and TAVR. We sought insight into the role of predisposing and precipitating factors in development of delirium, by examining the incidence and outcomes of delirium in SAVR and TAVR patients, who have different degrees of preoperative vulnerability and undergo procedures involving different levels of acute stress.
METHODS
FRAILTY-AVR Functional Outcomes Study
This single-center study was conducted as a part of the Frailty Assessment Before Cardiac Surgery and Transcatheter Interventions (FRAILTY-AVR) study, a prospective cohort study comparing various frailty scales in predicting outcomes of aortic valve replacement at 14 centers across Canada, the United States, and France (NCT01845207).21 Patients were eligible if they were aged 70 years and older and undergoing SAVR or TAVR for severe AS. Exclusion criteria were 1) emergent surgery or surgery involving other heart valves or the aorta, 2) clinically unstable (e.g., decompensated heart failure, active myocardial ischemia, or abnormal vital signs), 3) severe neuropsychological impairment (e.g., Mini-Mental State Examination [MMSE] score <15 or active psychosis), or 4) non-English speaking.
The investigators at the Beth Israel Deaconess Medical Center (BIDMC) conducted the FRAILTY-AVR Functional Outcomes Study to determine the longitudinal change in functional status over 12 months.22 Of 446 patients screened between February 2014 and March 2016, we enrolled 246 patients after excluding 200 patients for the following reasons: 1) not meeting the selection criteria (n=96); 2) research team unavailable (n=60); 3) participation declined by patients (n=39); or 4) other reasons (cancelled surgery, withdrawal of consent, or participation in another study) (n=5). In October 2014, we initiated a standardized delirium assessment; 187 consecutive patients who were enrolled since October 2014 are included in this analysis. This study was approved by the Institutional Review Board at BIDMC. All participants provided written informed consent.
Assessment of Delirium, Severity and Motoric Type
Starting from the first postoperative day, the study geriatricians and trained research assistants interviewed patients, nursing staff and family members if present, daily to assess delirium. Because a hallmark of delirium is fluctuation of symptoms throughout the day, they administered standardized tests of attention, the MMSE, the Delirium Symptom Interview23 and the long-version Confusion Assessment Method (CAM)24 (or CAM for Intensive Care Unit25,26 for intubated patients), during a consistent time of day (12PM −6PM). Our delirium assessment took 15–25 minutes in total. Since patients were discharged in the morning, they were not assessed on the discharge day. According to the CAM algorithm,24 delirium was diagnosed if the patient had “1) acute or fluctuation course of the features of delirium”., 2) inattention, and 3) either disorganized thinking or altered level of consciousness. The CAM has a sensitivity of 94% and specificity 89% against an extensive clinical assessment.27 The inter-rater reliability for delirium status between geriatricians and trained research assistants was high (agreement 95–100%, Cohen’s kappa 0.90–1.00).28
Delirium severity was evaluated using a validated CAM-based severity scale, CAM-S.29 The CAM-S (range: 0–19) assigns scores 0 (absent) or 1 (present) to acute onset or fluctuating course, and 0 (absent), 1 (mild), or 2 (marked) to the remaining 9 features of CAM: inattention, disorganized thinking, altered level of consciousness, disorientation, memory impairment, perceptual disturbances, psychomotor agitation, psychomotor retardation, and sleep-wake cycle disturbance. Higher CAM-S scores indicate greater severity. To enhance clinical interpretability of delirium severity as measured by CAM-S, we classified patients into 3 groups based on the peak delirium severity from all available CAM-S scores for each patient: 1) no delirium (CAM negative for all postoperative days); 2) mild delirium (CAM positive ≥1 day and the highest daily CAM-S score ≤9, which corresponds to the median score of all available assessments in patients with delirium); and 3) severe delirium (CAM positive ≥1 day and the maximum CAM-S score >9). We also classified patients using the mean delirium severity of all CAM-S scores for each patient: 1) no delirium (CAM negative for all postoperative days); 2) mild delirium (CAM positive ≥1 day and the mean CAM-S score ≤4.71, which corresponded to the median mean CAM-S score of all assessments in patients with delirium); and 3) severe delirium (CAM positive ≥1 day and the mean CAM-S score >4.71).
The motoric type of delirium was defined based on all available CAM assessments during the hospitalization: 1) mixed or hyperactive delirium (psychomotor agitation for ≥1 day); 2) hypoactive delirium (psychomotor retardation for ≥1 day without psychomotor agitation); and 3) normoactive delirium (no agitation or retardation throughout the hospitalization).
Outcome Assessment
We assessed the following outcomes: 1) prolonged hospitalization (≥9 days, which corresponded to the 75th percentile of the length of hospitalization in the study population); 2) institutional discharge defined as discharge to any skilled nursing facility or rehabilitation hospital; 3) poor functional recovery at 6 months (based on composite functional status score lower than baseline, as defined below) or death at 6 months; and 4) change in functional status over 12 months. A research assistant conducted telephone interviews at 1, 3, 6, 9 and 12 months after the procedure to assess the ability to perform 22 daily activities and physical tasks: 7 activities of daily living (ADLs) (feeding, dressing, grooming, ambulating, transferring, bathing, toileting), 7 instrumental activities of daily living (IADLs) (using telephone, transportation, shopping, preparing meals, housework, taking medications, managing money) and 8 physical tasks (pulling/pushing large objects, stooping/crouching/kneeling, lifting 10 lbs, reaching arms above the shoulder level, writing/handling small objects, walking up and down a flight of stairs, walking half a mile, doing heavy housework).30,31 The composite functional status score (range: 0–22) was calculated as the number of activities that one can perform without personal help. Higher scores indicate better function.
Other Measurements
A research assistant interviewed patients in the ambulatory preoperative testing center or hospital ward before their procedure to collect information on demographic characteristics, social support and primary residence; assessed self-reported ability to perform 22 daily activities and physical tasks listed above; and conducted MMSE and 5-meter walk test to assess gait speed. A study geriatrician reviewed medical records to obtain information on procedures, comorbidities and laboratory tests; and calculated the Society of Thoracic Surgeons (STS) predicted risk of mortality32 and Charlson Comorbidity Index (CCI).33
Statistical Analysis
Baseline characteristics of SAVR and TAVR patients and their delirium outcomes were compared using the t-test, chi-square test and Fisher’s exact test. Because there were considerable differences in patient characteristics and procedure-related stress between SAVR and TAVR, we analyzed each cohort separately. Within each cohort, the risks of prolonged hospitalization, institutional discharge, and poor functional recovery or death at 6 months were calculated by delirium status and severity (as both a categorical variable and continuous scale). We estimated a minimum clinically important difference (MCID) for CAM-S scores using poor functional recovery or death at 6 months as an anchor. For each cohort, MCID was determined as the difference in the mean of the maximum CAM-S score between those who experienced the outcome and those who did not. Logistic regression was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) of these outcomes associated with mild and severe delirium compared with no delirium after adjusting for age, sex, and CCI. We did not adjust for baseline frailty or cognitive impairment, which are strong risk factors for delirium and outcomes, because this may lead to overadjustment and model overfitting in our cohort of a modest sample size. We also analyzed the CAM-S score as a continuous variable. A linear trend between the delirium severity and outcomes was assessed. To assess the longitudinal change in functional status over 12 months by delirium status, we used a linear mixed-effects model that included delirium status, time indicators, and their interaction terms, as well as age, sex, and CCI as covariates. The analysis was repeated for the delirium severity category. All statistical analyses were performed in Stata Release 14 (StataCorp, College Station, TX). A 2-sided p-value <0.05 was considered statistically significant.
RESULTS
Patient Characteristics
Patients who underwent SAVR had lower burden of predisposing risk factors for delirium than those having TAVR (Table 1). In particular, SAVR patients were younger than TAVR patients (77.9 vs 83.7 years) and had lower mean STS predicted risk of mortality (2.7% vs 5.2%) and mean CCI (2.3 vs 3.6). SAVR patients had higher mean MMSE scores (26.9 vs 24.7 points), faster mean gait speed (0.91 vs 0.57 m/sec), and less disability in ADLs (3.9% vs 17.3%) and IADLs (49.4% vs 82.7%).
Table 1.
Characteristics of Patients Undergoing Aortic Valve Replacement
| Characteristics | SAVR (N=77) | TAVR (N=110) | P value |
|---|---|---|---|
| Age, years, mean ± SD | 77.9 ± 5.3 | 83.7 ± 6.0 | <0.001 |
| Male, n (%) | 44 (57.1) | 53 (48.2) | 0.24 |
| Non-white race, n (%) | 5 (6.5) | 2 (1.8) | 0.13 |
| Living alone, n (%) | 28 (36.4) | 39 (35.5) | 0.90 |
| Living at home, n (%) | 76 (98.7) | 107 (97.3) | 0.64 |
| Concurrent coronary artery bypass grafting, n (%) | 31 (40.3) | NA | |
| STS predicted risk of mortality, %, mean ± SD | 2.7 ± 1.4 | 5.2 ± 2.5 | <0.001 |
| Charlson comorbidity index, mean ± SD | 2.3 ± 1.8 | 3.6 ± 2.3 | <0.001 |
| MMSE score, points, mean ± SD | 26.9 ± 2.5 | 24.7 ± 3.3 | <0.001 |
| Gait speed, m/sec, mean ± SD | 0.91 ± 0.33 | 0.57 ± 0.21 | <0.001 |
| ADL disability, n (%) | 3 (3.9) | 19 (17.3) | 0.005 |
| IADL disability, n (%) | 38 (49.4) | 91 (82.7) | <0.001 |
Abbreviations: ADL, activities of daily living; IADL, instrumental activities of daily living; MMSE, Mini-Mental State Examination; NA, not applicable; SAVR, surgical aortic valve replacement; SD, standard deviation; STS, Society of Thoracic Surgeons; TAVR, transcatheter aortic valve replacement
Incidence, Severity, and Types of Delirium
Incidence of delirium was higher in SAVR patients than TAVR patients (50.7% vs. 25.5%; p<0.001) (Table 2). However, SAVR patients with delirium had a shorter duration (2.2 vs 3.4 days; p=0.04) and lower severity of delirium as measured by lower mean CAM-S score than TAVR patients with delirium (4.5 vs 5.7; p=0.01). Maximum CAM-S scores were not statistically significantly different between SAVR and TAVR patients (8.4 vs 9.5; p=0.09).
Table 2.
Characteristics of Patients with Postoperative Delirium After Aortic Valve Replacement
| Characteristics | SAVR (N=77) | TAVR (N=110) | P value |
|---|---|---|---|
| Postoperative delirium, n (%) | 39 (50.7) | 28 (25.5) | <0.001 |
| Delirium duration,* days, mean ± SD | 2.2 ± 1.5 | 3.4 ± 2.8 | 0.04 |
| Delirium severity*,† | 0.10 | ||
| Mild delirium, n (%) | 26 (66.7) | 13 (46.4) | |
| Severe delirium, n (%) | 13 (33.3) | 15 (53.6) | |
| Maximum CAM-S score,*, ‡ mean ± SD | 8.4 ± 2.2 | 9.5 ± 3.1 | 0.09 |
| Mean CAM-S score,*, ‡ mean ± SD | 4.5 ± 1.7 | 5.7 ± 2.2 | 0.01 |
| Delirium motoric type* | 0.43 | ||
| Mixed or hyperactive type, n (%) | 7 (17.9) | 7 (25.0) | |
| Hypoactive type, n (%) | 23 (59.0) | 12 (42.9) | |
| Normoactive type, n (%) | 9 (23.1) | 9 (32.1) |
Abbreviations: CAM, Confusion Assessment Method; CAM-S, Confusion Assessment Method-Severity; SAVR, surgical aortic valve replacement; SD, standard deviation; TAVR, transcatheter aortic valve replacement
Data were presented for patients who developed postoperative delirium.
Delirium severity was defined as mild if CAM-S maximum score ≤9 and severe if CAM-S maximum score >9 (CAM-S score 9 was the median score of all available assessments in patients with delirium).
The maximum and mean CAM-S scores were calculated from all available assessments in patients with delirium.
The motoric type of delirium was not different between the cohorts (p=0.43). Hypoactive delirium was the most common (59.0% of SAVR, 42.9% of TAVR), whereas mixed or hyperactive type was the least common.
Prolonged Hospitalization, Institutional Discharge, and Poor Functional Recovery or Death
Higher delirium severity was associated with a greater risk of prolonged hospitalization after SAVR (no delirium 18.4%, mild delirium 30.8%, severe delirium 61.5%; p-for-trend=0.009) and after TAVR (26.8%, 38.5%, 73.3%, respectively; p-for-trend=0.001) (Table 3). It was also positively associated with institutional discharge after SAVR (no delirium 42.1%, mild delirium 58.3%, severe delirium 84.6%; p-for-trend=0.01) and after TAVR (32.5%, 69.2%, 80.0%, respectively; p-for-trend<0.001). Increasing delirium severity was associated with a greater incidence of poor functional recovery or death at 6 months (no delirium 46.6%, mild delirium 50.0%; severe delirium 80.0%; p=0.03) in TAVR patients, but not in SAVR patients (30.0%, 44.0%, 38.5%, respectively; p-for-trend=0.72). The results were similar when the delirium severity was analyzed as a continuous variable (ORs per one CAM-S point ranging from 1.19 to 1.24 in SAVR and from 1.22 to 1.37 in TAVR) (Table 2) and defined using the mean CAM-S score (Supplementary Table 1).
Table 3.
Outcomes of Aortic Valve Replacement According to the Delirium Status and Severity*
| Delirium severity | Prolonged Hospitalization | Institutional Discharge | Poor Functional Recovery or Death at 6 Month |
|||
|---|---|---|---|---|---|---|
| n / N (%) | OR (95% CI)† | n / N (%) | OR (95% CI)† | n / N (%) | OR (95% CI)b | |
| SAVR | 23 / 77 (29.9) | 41 / 75 (54.7) | 25 / 68 (36.8) | |||
| No delirium | 7 / 38 (18.4) | Reference | 16 / 38 (42.1) | Reference | 9 / 30 (30.0) | Reference |
| Mild delirium | 8 / 26 (30.8) | 2.1 (0.63, 7.4) | 14 / 24 (58.3) | 1.4 (0.40, 5.0) | 11 / 25 (44.0) | 1.5 (0.5, 5.1) |
| Severe delirium | 8 / 13 (61.5) | 7.1 (1.7, 30.3) | 11 / 13 (84.6) | 14.4 (2.0, 105.7) | 5 / 13 (38.5) | 1.2 (0.3, 5.0) |
| P-for-trend | P=0.009 | P=0.01 | P=0.72 | |||
| CAM-S score (per point) | 1.19 (1.01,1.39) | 1.24 (1.04,1.48) | 1.22 (0.91,1.63) | |||
| TAVR | 38 / 110 (34.6) | 47 / 108 (43.5) | 52 / 100 (52.0) | |||
| No delirium | 22 / 82 (26.8) | Reference | 26 / 80 (32.5) | Reference | 34 / 73 (46.6) | Reference |
| Mild delirium | 5 / 13 (38.5) | 1.8 (0.52, 6.4) | 9 / 13 (69.2) | 5.3 (1.4, 19.9) | 6 / 12 (50.0) | 1.1 (0.3, 3.9) |
| Severe delirium | 11 / 15 (73.3) | 7.7 (2.2, 27.4) | 12 / 15 (80.0) | 9.6 (2.3, 39.9) | 12 / 15 (80.0) | 4.7 (1.2, 18.4) |
| P-for-trend | P=0.001 | P<0.001 | P=0.03 | |||
| CAM-S score (per point) | 1.22 (1.08,1.40) | 1.37 (1.17,1.61) | 1.22 (1.07,1.39) | |||
Abbreviations: CI, confidence interval; OR, odds ratio; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement
Delirium severity was defined as mild if CAM-S maximum score ≤9 and severe if CAM-S maximum score >9 (A CAM-S maximum score of 9 was the median CAM-S maximum score of patients with delirium).
Adjusted for age, sex, and Charlson Comorbidity Index.
The mean maximum CAM-S score for SAVR patients who experienced poor recovery or death at 6 months was 8.0 versus 5.7 for those who had not. This corresponds to a MCID of 2.3. By comparison, the mean maximum CAM-S score for TAVR patients who experienced poor recovery or death at 6 months was 6.9 versus 4.3 for those who had not, corresponding to an MCID of 2.6.
Longitudinal Change in Functional Status Over 12 Months
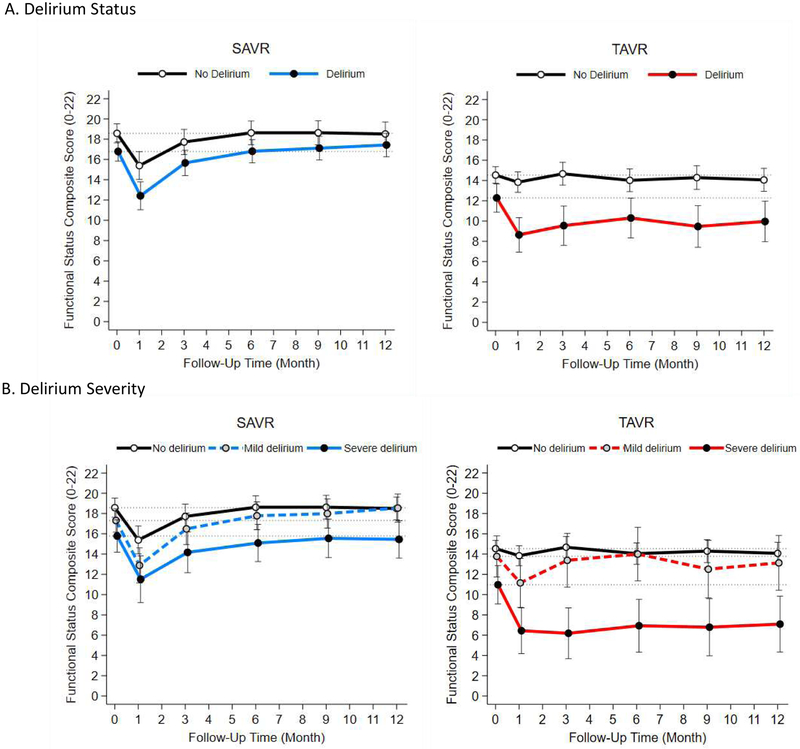
Over 12 months, 3 SAVR (3 with delirium) and 18 TAVR patients (7 with delirium) died. Patients who developed delirium after SAVR had lower baseline mean functional status scores than those without delirium (16.5 vs 18.8) and up to 6 months after surgery (16.5 vs 19.0) Figure Panel A and Table 4). The difference was not statistically significant after 6 months. Although patients with mild delirium declined at 1 month, they recovered by 3 months and became comparable to those without delirium at 12 months (Figure Panel B and Supplementary Table 3). Patients with severe delirium recovered baseline function by 9 months, but their functional level remained lower than that of patients without delirium throughout the year.
Figure 1. Functional Status Over 12 Months After Aortic Valve Replacement According to Delirium Status and Severity*.
Abbreviations: SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
* The functional status composite score ranges from 0 to 22, with higher scores indicating better function. Delirium severity was defined as mild if CAM-S maximum score ≤9 and severe if CAM-S maximum score >9 (CAM-S score 9 was the medial score of patients with delirium). The nodes and vertical bars indicate the means and 95% confidence intervals of the functional status composite score at each assessment that were estimated from a linear mixed-effects regression model that adjusted for age, sex, and Charlson Comorbidity Index.
Table 4.
Functional Status Over 12 Months After Aortic Valve Replacement According to Delirium Status
| Time Since Surgery |
No Delirium | Delirium | Adjusted Differenceb (Delirium-No Delirium) Estimate (95% CI) |
||||
|---|---|---|---|---|---|---|---|
| N | Functional Score* Mean ± SD |
Change from Baseline† Estimate (95% CI) |
N | Functional Score* Mean ± SD |
Change from Baseline† Estimate (95% CI) |
||
| SAVR | |||||||
| Baseline | 38 | 18.8 ± 3.0 | Reference | 39 | 16.5 ± 3.3 | Reference | −1.8 (−3.1 to −0.4) |
| Month 1 | 35 | 15.7 ± 3.7 | −3.2 (−4.5 to −1.8) | 34 | 12.6 ± 4.6 | −4.4 (−5.7 to −3.0) | −3.0 (−4.9 to −1.0) |
| Month 3 | 32 | 17.8 ± 3.6 | −0.9 (−1.9 to 0.2) | 32 | 15.8 ± 3.9 | −1.1 (−2.2 to −0.1) | −2.1 (−3.9 to −0.3) |
| Month 6 | 30 | 19.0 ± 3.5 | 0.1 (−0.9 to 1.1) | 35 | 16.5 ± 3.7 | 0.0 (−1.0 to 1.0) | −1.8 (−3.5 to −0.2) |
| Month 9 | 32 | 18.9 ± 3.3 | 0.1 (−0.9 to 1.1) | 34 | 16.9 ± 4.0 | 0.3 (−0.7 to 1.3) | −1.5 (−3.2 to 0.2) |
| Month 12 | 32 | 18.7 ± 3.6 | −0.1 (−1.2 to 1.1) | 34 | 17.2 ± 3.7 | 0.6 (−0.5 to 1.8) | −1.1 (−2.7 to 0.6) |
| TAVR | |||||||
| Baseline | 82 | 14.6 ± 3.2 | Reference | 28 | 12.3 ± 5.0 | Reference | −2.3 (−3.9 to −0.6) |
| Month 1 | 77 | 13.8 ± 4.2 | −0.7 (−1.5 to 0.1) | 28 | 8.6 ± 5.7 | −3.6 (−5.0 to −2.3) | −5.2 (−7.2 to −3.2) |
| Month 3 | 72 | 14.7 ± 4.4 | 0.1 (−0.8 to 1.1) | 23 | 10.1 ± 6.1 | −2.7 (−4.4 to −1.1) | −5.1 (−7.4 to −2.9) |
| Month 6 | 66 | 14.2 ± 4.5 | −0.5 (−1.4 to 0.4) | 21 | 11.2 ± 5.9 | −2.0 (−3.6 to −0.4) | −3.7 (−6.0 to −1.5) |
| Month 9 | 63 | 14.8 ± 4.4 | −0.3 (−1.2 to 0.7) | 19 | 10.9 ± 6.1 | −2.8 (−4.6 to −1.1) | −4.8 (−7.2 to −2.5) |
| Month 12 | 63 | 14.5 ± 4.2 | −0.5 (−1.5 to 0.6) | 20 | 11.0 ± 6.2 | −2.3 (−4.1 to −0.5) | −4.1 (−6.4 to −1.8) |
Abbreviations: CI, confidence interval; NA, not applicable; SAVR, surgical aortic valve replacement; SD, standard deviation; TAVR, transcatheter aortic valve replacement
The functional status composite score ranges from 0 to 22, with higher scores indicating better function.
The change from baseline, group difference and their 95% confidence intervals were estimated from a linear mixed-effects regression model that adjusted for age, sex, and Charlson Comorbidity Index.
Patients who developed delirium after TAVR had lower mean functional status scores than those without delirium at baseline and throughout the follow-up period (Figure Panel A and Table 4). Patients with mild delirium declined at 1 month and recovered their baseline level by 3 months with minimal change afterwards (Figure Panel B and Supplementary Table 3). In contrast, patients with severe delirium experienced a large decline at 1 month and remained persistently impaired throughout the year. The results were similar when the delirium severity was defined using the mean CAM-S scores (Supplementary Figure).
A total of 17 patients (9.1%) did not have complete data at 12 months (8 [10.3%] SAVR and 9 [8.1%] TAVR). On average, SAVR patients responded to 4.3 interviews (standard deviation: 1.5) and TAVR patients responded to 4.1 interviews (standard deviation: 1.5). Although patients who died during the follow-up had older age, greater comorbidity burden, poorer functional status and higher rate of delirium than those who survived, those who responded to ≤3 interviews were not sicker than those who responded to 4–5 interviews (Supplementary Table 2).
DISCUSSION
In our study, despite younger age, less cognitive impairment, and less disability at baseline, SAVR patients were more likely to develop delirium than TAVR patients. However, once delirium developed, TAVR patients had more severe and prolonged delirium than SAVR patients did. Severe delirium was associated with prolonged hospitalization and discharge to an institution after both procedures, and with poor functional recovery or death at 6 months after TAVR. Patients who had severe delirium after SAVR and TAVR had lower functional status compared to those with no or mild delirium. In particular, severe delirium after TAVR was associated with persistent functional impairment throughout the year.
Our study sheds light on the role of pre-existing vulnerability (predisposing factors) versus acute stress (precipitating factors) in the development of delirium. Our findings suggest that the occurrence of delirium may be more affected by the magnitude of an the stress imposed by a procedure (i.e., SAVR, which involves open sternotomy and cardiopulmonary bypass is more stressful than minimally invasive TAVR), while the severity and outcomes of delirium are more dependent on pre-existing vulnerability to stressors due to frailty and decreased functional reserve (i.e., TAVR patients have more severe cognitive and functional impairments at baseline than SAVR patients).
The incidence of delirium in our SAVR cohort is comparable to previously reported incidence in SAVR.14,18,34 However, literature in TAVR patients showed the estimated incidence ranging from 5% to 45%.11–14,17–19,34 In a prospective cohort study of 136 patients in which the CAM was administered for 5 days post-procedurally, Eide et al. found higher rates of delirium in SAVR patients than TAVR patients (66% vs 44%; p=0.01).35 Other studies reported a statistically non-significant trend towards increase in the delirium incidence in SAVR patients compared with TAVR patients (21% vs 19%; p=0.6518 and 33% vs 29%; p=0.4014). Because many studies identified delirium based on diagnosis codes13 or subjectively by a nurse or a physician,17 the true incidence of delirium may have been underestimated.
Several studies have found that postoperative delirium is associated with poor short-term outcomes after SAVR and TAVR, including prolonged hospitalization,14,17,19 discharge to a facility,13,19 procedural morbidity,13 and mortality.10,12,14,17–19 One study of IADL disability after major cardiac surgery (largely, coronary artery bypass grafting procedures) demonstrated an association between delirium and functional decline at 1-month (relative risk, 1.9; 95% CI, 1.3 to 2.8) with a similar trend at 12 months.3,36 In a study by Eide et al, patients with delirium after TAVR had worse ADL and IADL disability 1 month after the procedure and a statistically non-significant trend towards lower functional status at 6 months.34 In contrast, SAVR patients with delirium had worse IADL disability at 1 month, with eventual recovery, consistent with our results. Our study adds to their findings by additionally stratifying by delirium severity, with frequent serial measurements of functional status change over 12 months following the procedure, and with clear demonstration of persistent functional impairment among TAVR patients with severe delirium.
The development of delirium portends poor prognosis in patients undergoing TAVR. Patients with delirium, especially severe delirium, may experience greater functional decline due perioperative complications, preventing them from gaining full functional recovery and long-term functional benefits from surgery.37 We demonstrated that the CAM-S, which was previously validated in major non-cardiac surgical patients,29 identifies patients with severe delirium with delayed functional recovery or persistent functional impairment at 12 months postoperatively. We estimated a MCID of 2.3 to 2.6 in the maximum CAM-S score, which needs to be replicated in future studies. Our results underscore the importance of implementing preventive measures and identifying and managing delirium when it occurs, to improve clinical and functional outcomes in older patients undergoing SAVR and TAVR.
Preventing delirium may promote functional recovery and help preserve independence in elderly patients undergoing SAVR or TAVR. Effective strategies for delirium prevention include proactive interdisciplinary collaboration such as geriatrics consultation, frequent reorientation, non-pharmacologic sleep protocols, early mobilization, sensory aids, and minimizing psychoactive medication use.1,38,39 Another preventative strategy being studied is prehabilitation, a multi-modal intervention to optimize a patient’s resilience prior to surgery.40 Further research is warranted to investigate the role of rehabilitative interventions to mitigate the negative long-term functional consequences in patients who develop delirium after these procedures.
Our study is distinguished from previous research by prospective use of validated measures for delirium diagnosis and severity. Furthermore, repeated assessments of functional status over 12 months with a high follow-up rate allow for examination of functional recovery trajectories, an important patient-centered outcome. However, in the absence of randomization, outcomes cannot be compared between SAVR and TAVR patients as they are fundamentally different patient populations. TAVR has continued to evolve; at the time of our study all patients underwent general anesthesia, however with updated techniques it is possible to do the procedure under sedation, thus further minimizing the stress of TAVR. We acknowledge that our study was insufficiently powered to find differences in infrequent clinical events, such as mortality, or outcome differences between mild delirium and no delirium. Additionally, we did not collect data on perioperative medications, including narcotics or particular anesthetic agents. As our study included predominantly white patients treated at a high-volume academic center, the rates of clinical outcomes may not be generalizable to patients of different racial or ethnic backgrounds or treated at different centers. However, we believe that the association of delirium with adverse outcomes would be consistent in patients treated at other centers. Functional status was not measured in 5–12% of eligible patients at each interview. Although patients who died during the follow-up were sicker than those who survived, there was no indication that the surviving patients who responded to <3 interviews were sicker than those who responded to 4–5 interviews (Supplementary Table 2). Nonetheless, selective drop-out of sicker patients might have underestimated the negative impact of delirium status and severity.
In conclusion, our study showed that delirium is associated with poor clinical outcomes and delayed functional recovery after SAVR and TAVR, and that severe delirium is associated with worse outcomes and functional impairment. Perioperative strategies to prevent delirium and to mitigate adverse outcomes of delirium should be investigated further. For patients who develop severe delirium, longer rehabilitation and supportive services may be needed to optimize their recovery. As TAVR patients appeared to be particularly vulnerable to the long-term negative outcomes of postoperative severe delirium, it will be crucial to further study and refine delirium prevention strategies in this population.
Supplementary Material
ACKNOWLEDGMENTS
Funding/Support: The FRAILTY-AVR Functional Outcomes Study was conducted with the support of a KL2/Catalyst Medical Research Investigator Training award (an appointed KL2 award) and an additional support from Harvard Catalyst / The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award KL2 TR001100–01 and UL1 TR001102) and the Training Program in Cardiovascular Research award (T32-HL007374) from the National Heart, Lung, and Blood Institute, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Funding Source: Harvard Catalyst, National Institutes of Health
Conflict of Interest Disclosures:
Dr. Lipsitz is supported by grants R01AG025037, R01AG041785, and P30AG031679 from the National Institute on Aging. He holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew SeniorLife.
Dr. C Kim was supported by the Training Program in Cardiovascular Research award (T32-HL007374) from the National Heart, Lung, and Blood Institute, National Institutes of Health.
Dr. Popma reports receiving institutional grants from Medtronic, Boston Scientific, and Edwards Lifesciences, and serves on advisory boards for Boston Scientific and Edwards Lifesciences.
Ms. Guibone is a consultant to Medtronic, Inc.
Dr. Marcantonio is supported by grants R01AG030618, R01AG051658, P30AG031679, R24AG054259 (NIDUS) and a Mid-Career Investigator Award (K24AG035075) from the National Institute on Aging.
Dr. D Kim is supported by the Paul B. Beeson Clinical Scientist Development Award in Aging (K08AG051187) from the National Institute on Aging, the American Federation for Aging Research, the John A. Hartford Foundation, and Atlantic Philanthropies. He is also supported by the Boston Claude D. Pepper Older Americans Independence Center/Pilot and Exploratory Studies Core (P30AG031679) and Boston Roybal Center Pilot Award (P30AG048785). He provides paid consultative services to Alosa Health, a nonprofit educational organization with no relationship to any drug or device manufacturers.
Other authors declare no disclosures.
Footnotes
SUPPLEMENTAL MATERIAL
Delirium Incidence and Functional Outcomes after Transcatheter and Surgical Aortic Valve Replacement
REFERENCES
- 1.Marcantonio ER. Delirium in Hospitalized Older Adults. N Engl J Med. 2017;377(15):1456–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottesman RF, Grega MA, Bailey MM, et al. Delirium after coronary artery bypass graft surgery and late mortality. Annals of neurology. 2010;67(3):338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudolph JL, Inouye SK, Jones RN, et al. Delirium: an independent predictor of functional decline after cardiac surgery. Journal of the American Geriatrics Society. 2010;58(4):643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bureau ML, Liuu E, Christiaens L, et al. Using a multidimensional prognostic index (MPI) based on comprehensive geriatric assessment (CGA) to predict mortality in elderly undergoing transcatheter aortic valve implantation. Int J Cardiol. 2017;236:381–386. [DOI] [PubMed] [Google Scholar]
- 6.Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. New England Journal of Medicine. 2016;374(17):1609–1620. [DOI] [PubMed] [Google Scholar]
- 7.Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2477–2484. [DOI] [PubMed] [Google Scholar]
- 8.Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2017;376(14):1321–1331. [DOI] [PubMed] [Google Scholar]
- 9.Marcantonio ER. Postoperative delirium: a 76-year-old woman with delirium following surgery. Jama. 2012;308(1):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eide LS, Ranhoff AH, Fridlund B, et al. Readmissions and mortality in delirious versus non-delirious octogenarian patients after aortic valve therapy: a prospective cohort study. BMJ open. 2016;6(10):e012683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abawi M, Pagnesi M, Agostoni P, et al. Postoperative Delirium in Individuals Undergoing Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. J Am Geriatr Soc. 2018;66(12):2417–2424. [DOI] [PubMed] [Google Scholar]
- 12.Bagienski M, Kleczynski P, Dziewierz A, et al. Incidence of Postoperative Delirium and Its Impact on Outcomes After Transcatheter Aortic Valve Implantation. Am J Cardiol. 2017;120(7):1187–1192. [DOI] [PubMed] [Google Scholar]
- 13.Soundhar A, Udesh R, Mehta A, et al. Delirium Following Transcatheter Aortic Valve Replacement: National Inpatient Sample Analysis. Journal of cardiothoracic and vascular anesthesia. 2017. [DOI] [PubMed] [Google Scholar]
- 14.Maniar HS, Lindman BR, Escallier K, et al. Delirium after surgical and transcatheter aortic valve replacement is associated with increased mortality. J Thorac Cardiovasc Surg. 2016;151(3):815–823 e812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crocker E, Beggs T, Hassan A, et al. Long-Term Effects of Postoperative Delirium in Patients Undergoing Cardiac Operation: A Systematic Review. The Annals of thoracic surgery. 2016;102(4):1391–1399. [DOI] [PubMed] [Google Scholar]
- 16.Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Annals of surgery. 2009;249(1):173–178. [DOI] [PubMed] [Google Scholar]
- 17.Abawi M, Nijhoff F, Agostoni P, et al. Incidence, Predictive Factors, and Effect of Delirium After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2016;9(2):160–168. [DOI] [PubMed] [Google Scholar]
- 18.Giuseffi JL, Borges NE, Boehm LM, et al. Delirium After Transcatheter Aortic Valve Replacement. American journal of critical care : an official publication, American Association of Critical-Care Nurses. 2017;26(4):e58–e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huded CP, Huded JM, Sweis RN, et al. The impact of delirium on healthcare utilization and survival after transcatheter aortic valve replacement. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2017;89(7):1286–1291. [DOI] [PubMed] [Google Scholar]
- 20.Jansen Klomp WW, Nierich AP, Peelen LM, et al. Survival and quality of life after surgical aortic valve replacement in octogenarians. Journal of cardiothoracic surgery. 2016;11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afilalo J, Lauck S, Kim DH, et al. Frailty in Older Adults Undergoing Aortic Valve Replacement: The FRAILTY-AVR Study. J Am Coll Cardiol. 2017;70(6):689–700. [DOI] [PubMed] [Google Scholar]
- 22.Kim DH, Afilalo J, Shi SM, et al. Changes in Functional Status in the Year Following Aortic Valve Replacement. JAMA internal medicine. 2019;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albert MS, Levkoff SE, Reilly C, et al. The delirium symptom interview: an interview for the detection of delirium symptoms in hospitalized patients. Journal of geriatric psychiatry and neurology. 1992;5(1):14–21. [DOI] [PubMed] [Google Scholar]
- 24.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Annals of internal medicine. 1990;113(12):941–948. [DOI] [PubMed] [Google Scholar]
- 25.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). Jama. 2001;286(21):2703–2710. [DOI] [PubMed] [Google Scholar]
- 26.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Critical care medicine. 2001;29(7):1370–1379. [DOI] [PubMed] [Google Scholar]
- 27.Wong CL, Holroyd-Leduc J, Simel DL, Straus SE. Does this patient have delirium?: value of bedside instruments. Jama. 2010;304(7):779–786. [DOI] [PubMed] [Google Scholar]
- 28.Kim DH, Lee J, Kim CA, et al. Evaluation of algorithms to identify delirium in administrative claims and drug utilization database. Pharmacoepidemiol Drug Saf. 2017;26:945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inouye SK, Kosar CM, Tommet D, et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Annals of internal medicine. 2014;160(8):526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagi SZ. An epidemiology of disability among adults in the United States. The Milbank Memorial Fund quarterly Health and society. 1976;54(4):439–467. [PubMed] [Google Scholar]
- 31.Rosow I, Breslau N. A Guttman health scale for the aged. Journal of gerontology. 1966;21(4):556–559. [DOI] [PubMed] [Google Scholar]
- 32.Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Journal of the American College of Cardiology. 2012;60(15):1438–1454. [DOI] [PubMed] [Google Scholar]
- 33.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 34.Eide LS, Ranhoff AH, Fridlund B, et al. Delirium as a Predictor of Physical and Cognitive Function in Individuals Aged 80 and Older After Transcatheter Aortic Valve Implantation or Surgical Aortic Valve Replacement. J Am Geriatr Soc. 2016;64(6):1178–1186. [DOI] [PubMed] [Google Scholar]
- 35.Eide LS, Ranhoff AH, Fridlund B, et al. Comparison of frequency, risk factors, and time course of postoperative delirium in octogenarians after transcatheter aortic valve implantation versus surgical aortic valve replacement. The American journal of cardiology. 2015;115(6):802–809. [DOI] [PubMed] [Google Scholar]
- 36.Osnabrugge RL, Arnold SV, Reynolds MR, et al. Health status after transcatheter aortic valve replacement in patients at extreme surgical risk: results from the CoreValve U.S. trial. JACC Cardiovasc Interv. 2015;8(2):315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hshieh TT, Saczynski J, Gou RY, et al. Trajectory of Functional Recovery After Postoperative Delirium in Elective Surgery. Annals of surgery. 2017;265(4):647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA internal medicine. 2015;175(4):512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516–522. [DOI] [PubMed] [Google Scholar]
- 40.Wynter-Blyth V, Moorthy K. Prehabilitation: preparing patients for surgery. BMJ. 2017;358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.