Abstract
Background
Clearance of coronary arterial thrombosis is necessary in patients with acute ST-segment elevation myocardial infarction (STEMI) undergoing urgent percutaneous coronary intervention (PCI). There is currently no highly-recommended method of thrombus removal during interventional procedures. We describe a new method for opening culprit vessels to treat STEMI: intracoronary arterial retrograde thrombolysis (ICART) with PCI.
Methods & Results
Eight patients underwent ICART. The guidewire was advanced to the distal coronary artery through the occlusion lesion. Then, we inserted a microcatheter into the distal end of the occluded coronary artery over the guidewire. Urokinase (5–10 wu) mixed with contrast agents was slowly injected into the occluded section of the coronary artery through the microcatheter. The intracoronary thrombus gradually dissolved in 3–17 min, and the effect of thrombolysis was visible in real time. Stents were then implanted according to the characteristics of the recanalized culprit lesion to achieve full revascularization. One patient experienced premature ventricular contraction during vascular revascularization, and no malignant arrhythmias were seen in any patient. No reflow or slow flow was not observed post PCI. Thrombolysis in myocardial infarction flow grade and myocardial blush grade post-primary PCI was 3 in all eight patients. No patients experienced bleeding or stroke.
Conclusions
ICART was accurate and effective for treating intracoronary thrombi in patients with STEMI in this preliminary study. ICART was an effective, feasible, and simple approach to the management of STEMI, and no intraprocedural complications occurred in any of the patients. ICART may be a breakthrough in the treatment of acute STEMI.
Keywords: ST elevation myocardial infarction, Therapeutic thrombolysis, Thrombus, Urokinase
1. Introduction
Acute ST-segment elevation myocardial infarction (STEMI) is a clinical syndrome involving a series of events: rupture of a coronary atheromatous plaque followed by platelet aggregation and activation of blood coagulating mechanisms, then subsequent rapid thrombosis leading to acute coronary arterial occlusion.[1],[2] Vascular recanalization of the infarct-related coronary artery is the crucial treatment for STEMI.[3]–[5] Currently, the major options for recanalization include intravenous injection of thrombolytic, primary percutaneous coronary intervention (PPCI), and emergent coronary artery bypass grafting (CABG).[6],[7] Intravenous thrombolytic injection has the advantage of prompt treatment before hospitalization; however, this technique can increase the risk of hemorrhagic events. The likelihood of achieving thrombolysis in myocardial infarction (TIMI) grade 3 blood flow is relatively low, while the risk of reinfarction is high.[8]–[12] Once patients are transferred to hospitals offering interventional treatment, intravenous thrombolysis is superseded by PPCI, which is more clinically efficacious.[6],[13] In the past, transcatheter intracoronary antegrade thrombolysis was performed in patients with STEMI. However, this therapy requires a full dose of thrombolytic, a long time to open the occlusion, and greatly increases the possibility of hemorrhage, all of which interfere with subsequent PCI therapy and long-term prognosis. At the same time, transcatheter coronary thrombolysis is not recommended according to the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines.[7] The increasing use of PCI has led to the clinical observation that although many patients demonstrate patency in major blood vessels below the epicardium, and blood flow is able to reach TIMI grade 3, a large proportion of patients still experience serious adverse cardiovascular events during or after the intervention. This can be attributed to a failure to achieve complete perfusion of myocardial microcirculation.[14] Clinically, such a defect in myocardial microcirculation is termed no reflow or slow flow and involves tissue edema, ischemia–reperfusion injury, endothelial dysfunction, and thromboembolism in the distal vasculature; the last of which is the main contributing factor.
Because thrombus formation is the crucial event in the pathogenesis of acute coronary arterial occlusion, reperfusion therapy must involve clearing the thrombus. Failure to remove the thrombus before balloon angioplasty of the coronary artery can lead to unfavorable outcomes, such as no reflow or slow flow. Currently, there are two methods to manage intracoronary thrombi. One is physical, involving thrombus aspiration and the use of a distal protection device,[15],[16] and the another one is chemical, involving the use of pharmacotherapeutics fenhao gaiwei; such as antiplatelet, anticoagulant, and thrombolytic medications.[17] These thrombolytic provide direct clearance of the thrombus, and conventional thrombolytic therapy is performed antegrade to the direction of blood flow and can be administered intravenously or transcatheter. To achieve sufficient blood drug concentrations, these thrombolytic strategies require a full dose of thrombolytic. For example, the required dose of urokinase is 1.5–2 million units; thus, there is a high likelihood of adverse side effects. However, the drug action directly on the intracoronary thrombus accounts for only a small fraction of the entire administered dose, and a large amount of drug is wasted in the systemic circulation making antegrade thrombolysis an inefficient method. To address these shortcomings, we investigated how to achieve maximal coronary patency using a minimal amount of thrombolytic; how to minimize adverse side effects of thrombolytic therapy; and how to perform thrombolytic therapy safely, which can provide a favorable basis for PCI therapy and result in improved efficacy of the intervention.
In the current study, we designed a novel approach to open an occluded vessel in STEMI: intracoronary arterial retrograde thrombolysis (ICART). This protocol restores patency in the occluded sections of a coronary artery and simultaneously clears thrombi in the coronary microcirculatory vasculature using a thrombolytic dose of 1/40–1/20 that of conventional intravenous thrombolytic dosing. After removing the thrombus burden, stents are placed to complete the revascularization, resulting in restoration of myocardial perfusion.
2. Methods
2.1. Patient selection
From March 1, 2013 to November 6, 2013, we selected eight patients with acute STEMI to participate in this study (Table 1). Appropriate permissions to perform ICART in these patients were granted by the medical ethics committee of the Chinese PLA General Hospital. All procedures were performed by a single operator (TIAN JW) under full informed consent.
Table 1. Baseline patients' characteristics.
| ID | Gender | Age | Hypertension | Hyperlipidemia | Diabetes | Smoking | Family history | Previous MI | Killip classification | STEMI |
| 1 | M | 51 | N | Y | N | Y | N | N | 1 | Y |
| 2 | M | 49 | Y | Y | Y | Y | Y | N | 2 | Y |
| 3 | F | 62 | Y | N | N | N | N | N | 1 | Y |
| 4 | M | 58 | N | Y | N | N | N | N | 1 | Y |
| 5 | M | 74 | Y | N | N | N | N | N | 1 | Y |
| 6 | F | 78 | Y | N | Y | N | N | Y | 2 | Y |
| 7 | M | 49 | N | Y | Y | Y | N | N | 1 | Y |
| 8 | M | 49 | Y | Y | Y | Y | Y | N | 2 | Y |
F: female; M: male; MI: myocardial infarction; N: no; STEMI: ST-segment elevation myocardial infarction; Y: yes.
2.2. Patient assessment
Patient evaluation mainly refers to the inclusion and exclusion criteria of patients. If patients were diagnosed as having acute STEMI, were candidates for PPCI according to the American College of Cardiology Foundation/American Heart Association STEMI therapeutic guidelines,[18] and were able to provide informed consent before undergoing the intervention; coronary angiographic imaging of the culprit vessel confirmed complete thrombotic occlusion were included in our study. Participants with the following situations were excluded: (1) patient's age was more than 80 years old; (2) patients had uncontrolled hypertension (systolic pressure > 180 mmHg or diastolic pressure > 110 mmHg); (3) patients experienced mechanical complications resulting from acute myocardial infarction (i.e., interventricular septal perforation, papillary muscle rupture); (4) patients had a known history of allergy to antiplatelet drugs; (5) patients suffered a gastrointestinal tract ulcer within the past 3 months; (6) patients had a recent history of major surgery, trauma, hemorrhagic disease, cerebrovascular accident, or thrombocytopenia; (7) patients had an allergy to urokinase; (8) patients underwent post thrombolytic rescue PCI before hospitalization; (9) patients received previous CABG; (10) patients had incomplete coronary arterial occlusion on coronary angiography; or (11) patients with a serum creatinine level > 2.0 mg/dL.
2.3. Preparation of retrograde thrombolytic
Thrombolytic was prepared by dissolving 10 wu urokinase in 15 mL physiological saline and adding 5 mL iopromide as a contrast medium to create a 20 mL mixed injection.
2.4. ICART with PCI
Upon diagnosis, all patients immediately received oral aspirin (300 mg) and clopidogrel (600 mg). Patients were asked to chew the tablets before swallowing. Intravenous heparin (100 IU/kg) was administered before PCI. If the PCI duration exceeded one hour, a suitable dose of heparin was administered to maintain activated coagulation time in the range of 250–300 s.
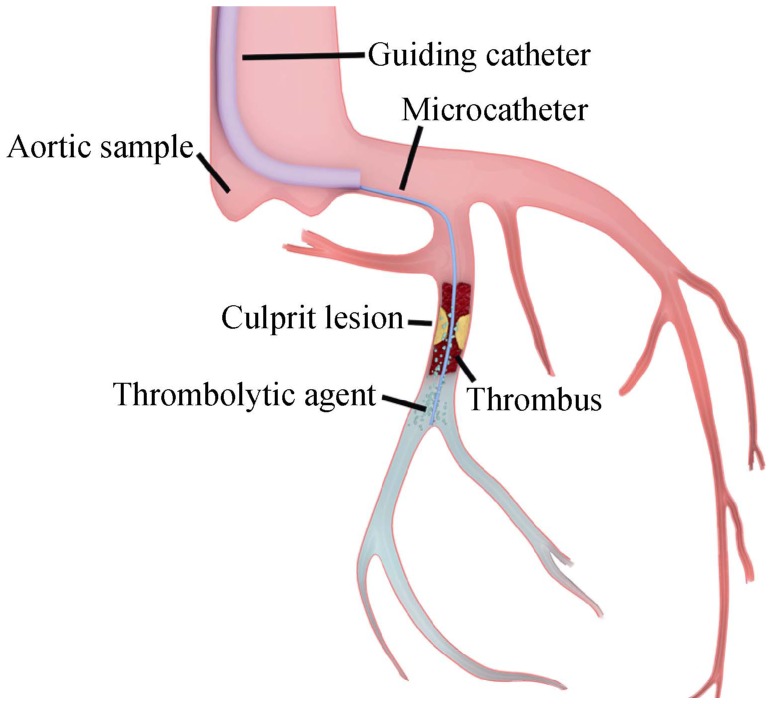
For patients fulfilling the inclusion criteria and without contraindications, conventional coronary arterial imaging was performed to locate culprit vessels, followed by ICART. The flow diagram for the ICART system is shown in Figure 1. A suitable guidewire was chosen and was advanced through the culprit vessel to the distal end of the occluded coronary artery. A microcatheter was inserted over the guidewire and through the stenotic section of the coronary artery until the distal end of the stenosis was reached, and the guidewire was then withdrawn. Then, 1 mL of the thrombolytic solution was bolus-injected through the catheter and repeated every 30 s. The mixed thrombolytic injection was not injected when fluoroscopy showed contrast media retention. During injection, retained contrast medium allowed clear visualization of distal occluded vessels, indicating that the thrombolytic was filling the occluded vessel and exerting thrombolytic action. Thrombolysis occurred in three directions simultaneously: toward the distal end of the occluded main coronary artery; toward the microcirculation, where microthrombi in the microcirculatory vasculature could be dissolved as they were accessed by the thrombolytic; and retrograde dissolution of thrombi in the coronary arterial trunk. When the thrombi in the trunk and microcirculation were completely dissolved, the occluded blood vessels were clearly visible, and antegrade blood flow recovered; we then precisely selected and implanted an appropriate stent.
Figure 1. Pattern diagram of intracoronary artery retrograde thrombolysis system.
3. Results
3.1. Patients' characteristics
Patients' baseline demographic characteristics and risk factors are summarized in Table 1. The eight enrolled patients (six men and two women, age: 58.75 ± 10.95 years, range: 49–78 years) all experienced obvious chest pain. Electrocardiography indicated that all patients had STEMI [four anterior myocardial infarction (MI), two lateral MI, and two inferior MI]. Five patients had hypertension and hyperlipidemia, four patients had diabetes, and two had a family history of coronary arterial disease. Four patients had a history of smoking, and previous MI had occurred in one patient. Killip classification ranged from I to II (five patients with Killip I, and three patients with Killip II).
3.2. Procedural data and angiographic results
Three patients had bradycardia preoperatively, and temporary pacemakers were implanted. Angiography showed that all culprit vessels were completely occluded. ICART was successful in the occluded coronary arteries in all eight patients: four left anterior descending, two left circumflex, and two right coronary arteries (Table 2). Typical left anterior descending, left circumflex, and right coronary arterial occlusion, retrograde thrombolysis, and stent implantation were shown in Figures 2 & 3 & 4. We used an intra-aortic balloon pump in two patients, no patients underwent thrombus aspiration. The mixed thrombolytic–contrast agent injection was retained for only 1.5 s via antegrade thrombolysis (Figure 2B); however, the injection persisted and dissolved the thrombus using retrograde thrombolysis (Figures 2C & 3B & 4B). Retrograde thrombolysis and simultaneous angiography clearly showed the degree and length of occlusion (Figure 2D).
Table 2. Baseline procedural and angiographic characteristics.
| Variables | ID-1 | ID-2 | ID-3 | ID-4 | ID-5 | ID-6 | ID-7 | ID-8 |
| Angiographic degree of CAD | 3 | 1 | 2 | 1 | 2 | 1 | 1 | 1 |
| Culprit vessel | ||||||||
| LAD/RCA/LCX | LAD | LAD | RCA | LAD | LCX | RCA | LCX | LAD |
| Total occlusion | Y | Y | Y | Y | Y | Y | Y | Y |
| TIMI flow grade | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Treatment of a non-culprit vessel during the same procedure | N | N | N | N | N | N | N | N |
| Preoperative arrhythmia | N | N | Y | N | N | Y | Y | N |
| ICART procedure | ||||||||
| Thrombolysis time, min | 5 | 5 | 6 | 15 | 17 | 3 | 5 | 6 |
| Urokinase quantity, wu | 5 | 10 | 6 | 10 | 10 | 10 | 10 | 6 |
| Arrhythmia | N | N | N | N | N | N | N | N |
| Nausea and vomiting | N | N | Y | N | N | N | N | N |
| Blood pressure drop | Y | N | Y | N | N | N | N | N |
| Post-ICART | ||||||||
| TIMI flow grade | 2 | 3 | 3 | 1 | 3 | 3 | 2 | 2 |
| Initial MLD, mm | 0.9 | 0.3 | 0.3 | 0.15 | 2.1 | 0.15 | 0.14 | 0.15 |
| Initial RLD, mm | 3 | 3 | 3 | 3 | 3 | 3 | 2.75 | 3 |
| Ruptured plaque | Y | Y | N | N | Y | N | N | N |
| Residual thrombus | Y | N | N | N | N | Y | N | Y |
| Pre-dilation | N | N | Y | Y | N | Y | Y | Y |
| Post-PCI QCA | ||||||||
| Final TIMI flow grade | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Final MLD, mm | 3 | 3 | 2.85 | 3 | 3 | 3 | 2.61 | 2.85 |
| Final RLD, mm | 3 | 3 | 3 | 3 | 3 | 3 | 2.75 | 3 |
| Number of stent per lesion | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Stent diameter, mm | 3 | 3 | 3 | 3 | 3 | 3 | 2.75 | 3 |
| Stent length, mm | 24 | 18 | 24 | 18 | 15 | 24 | 24 | 24 |
| Max inflation pressure, atm | 8 | 18 | 12 | 16 | 18 | 16 | 20 | 8 |
| Inflation time, s | 10 | 10 | 10 | 10 | 10 | 10 | 12 | 10 |
| IABP use | Y | N | N | Y | N | N | N | N |
| Pacing use | N | N | Y | N | N | Y | Y | N |
| Thrombus aspiration use | N | N | N | N | N | N | N | N |
| Procedural success | Y | Y | Y | Y | Y | Y | Y | Y |
| Cardiogenic shock | N | N | N | N | N | N | N | N |
CAD: coronary artery disease; IABP: intra-aortic balloon pump; ICART: intracoronary arterial retrograde thrombolysis; LAD: left anterior descending branch; LCX: left circumflex branch; MLD: minimum lumen diameter; N: no; PCI: percutaneous coronary intervention; QCA: quantitative coronary analysis; RCA: right coronary artery; RLD: reference lumen diameter; TIMI: thrombolysis in myocardial infarction; Y: yes.
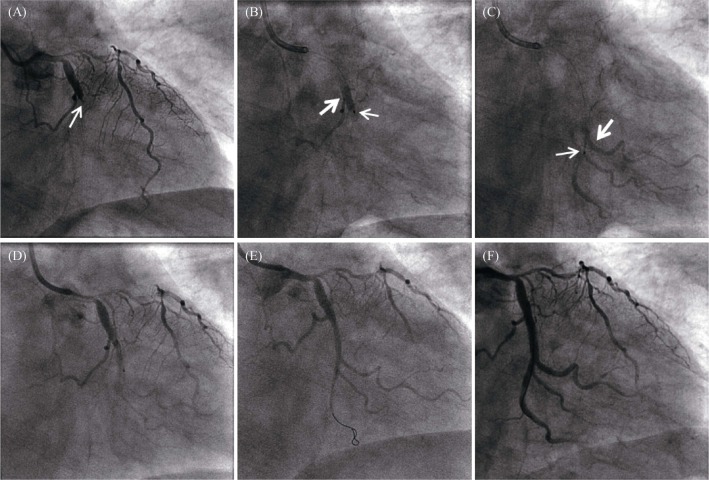
Figure 2. Comparison of antegrade thrombolysis vs. retrograde thrombolysis in acute circumflex arterial occlusion.
(A): The baseline angiogram shows total occlusion of the circumflex artery. The arrow showed the occlusion; (B): During transcatheter antegrade thrombolysis, the thrombolytic agent filled the vessel proximal to the stenosis. Thrombolytic agent injected antegrade was completely cleared after 1.5 seconds, the occlusion showed no change. The fine arrow indicated the tip of the microcatheter, and the coarse arrow showed the thrombolytic agent with contrast agent to fill the lumen; (C): In retrograde thrombolysis, the thrombolytic agent remained visible in the vessel distal to the occlusion. The fine arrow indicated the tip of the microcatheter, and the coarse arrow showed the thrombolytic agent with contrast agent to fill the lumen; (D): Using retrograde thrombolysis, antegrade angiography was used to clearly display the lesion; (E): The culprit lesion is seen in the distal segment of the LCX; and (F): Stent was placed in the circumflex branch to achieve revascularization. LCX: left circumflex branch.
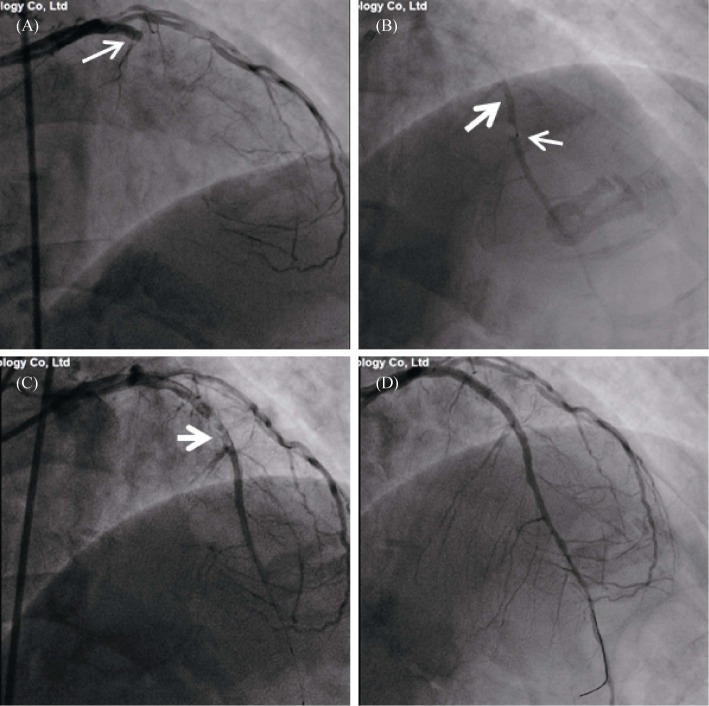
Figure 3. Coronary angiogram in acute anterior myocardial infarction.
(A): The basal angiogram showed total occlusion of the left anterior descending artery proximal segment with thrombus image. The arrow showed the occlusion; (B): The procedure of intracoronary artery retrograde thrombolysis through the microcatheter. The distal thrombus was gradually dissolved. The fine arrow indicated the tip of the microcatheter, and the coarse arrow showed the thrombolytic agent with contrast agent to fill the lumen; (C): After thrombolysis, the culprit lesion (arrow) was observed in the proximal segment of the descending branch; and (D): A stent was implanted in the anterior descending branch to achieve revascularization.
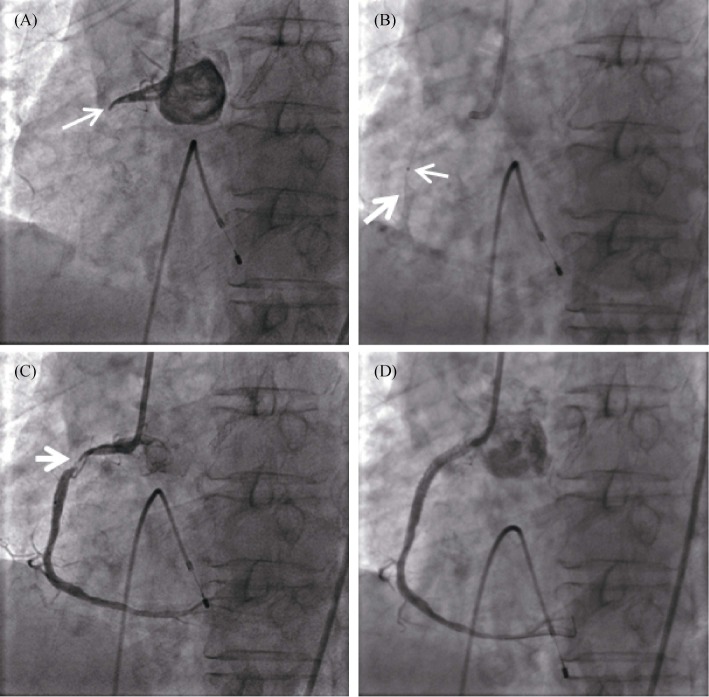
Figure 4. Coronary angiogram in acute right coronary artery myocardial infarction.
(A): The basal angiogram showed total occlusion of the RCA proximal-middle segment with thrombus image. The arrow showed the occlusion; (B): The process of intracoronary artery retrograde thrombolysis through the microcatheter. The fine arrow indicated the tip of the microcatheter, and the coarse arrow showed the thrombolytic agent with contrast agent to fill the lumen; (C): After thrombolysis, the culprit lesion (arrow) was observed in the proximal-middle segment of the RCA; and (D): A stent was implanted in the RCA to achieve revascularization. RCA: right coronary artery.
During ICART, the mean thrombolysis time was 7.75 ± 4.87 min (range: 3–17 min), and the dose of urokinase was 8.38 ± 2.26 wu (5–10 wu). Patients experienced no obvious arrhythmia or cardiogenic shock; one patient experienced nausea and vomiting, and two patients had a mild drop in blood pressure during ICART. The TIMI flow grade in all eight patients was 0 before ICART and 2.38 ± 0.70 post ICART (one TIMI 1, three TIMI 2, and four TIMI 3). Plaque rupture and residual thrombus occurred in three patients, and five patients underwent predilation (Table 2). After ICART, all occluded vessels were opened and had a post ICART minimum luminal diameter of 0.52 ± 0.64 mm (range: 0.14–2.10 mm).
After stent implantation, blood flow in all eight patients was restored to TIMI grade 3. The minimal luminal diameter after stent implantation was 2.91 ± 0.13 mm (range: 2.61–3.00 mm). One stent was implanted in each culprit vessel, and the mean stent length was 21.38 ± 3.74 mm (range: 15–24 mm) (Table 2).
3.3. End points of the ICART procedure
As shown in Table 3, direct stenting was performed in all eight patients. Amazingly, no patients experienced distal embolization or side branch occlusion, no patients developed any no reflow or slow flow post PCI, and no hemorrhage or stroke occurred during and after the operation. The myocardial blush grade post PPCI was 3, which was very high. The corrected TIMI frame count post PPCI was 12.18 ± 3.90 frames (range: 7.05–19 frames).
Table 3. End points of the ICART procedure.
| Variables | ID-1 | ID-2 | ID-3 | ID-4 | ID-5 | ID-6 | ID-7 | ID-8 |
| Direct stenting | Y | Y | Y | Y | Y | Y | Y | Y |
| Distal embolization | N | N | N | N | N | N | N | N |
| Composite angiographic end-point (distal embolization, slow flow, or no reflow) | N | N | N | N | N | N | N | N |
| No reflow | N | N | N | N | N | N | N | N |
| Slow flow | N | N | N | N | N | N | N | N |
| Side branch occlusion | N | N | N | N | N | N | N | N |
| Hemorrhage | N | N | N | N | N | N | N | N |
| Stroke | N | N | N | N | N | N | N | N |
| CTFC post-PPCI | 13 | 8.82 | 9 | 10.58 | 17 | 19 | 13 | 7.05 |
| Myocardial blush grade post-PPCI | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Peak of TnT mass, ng/mL | 31.2 | 0.185 | 4.09 | 6.59 | 33.1 | 3.33 | 14.8 | 5.7 |
| Peak of NT Pro-BNP, pg/mL | 3667 | 46.18 | 1625 | 4784 | 1391 | 4738 | 511 | 632 |
CTFC: corrected thrombolysis in myocardial infarction frame count; ICART: intracoronary arterial retrograde thrombolysis; N: no; NT Pro-BNP: N-terminal pro-B-type natriuretic peptide; PPCI: primary percutaneous coronary intervention; TnT: troponin T; Y: yes.
3.4. Thirty-day major adverse event rate
No death occurred in the catheterization lab or post PCI during the one-month follow-up (Table 4). No reinfarction, left ventricular failure, stroke, target lesion revascularization, or major adverse events occurred.
Table 4. Thirty-day major adverse events.
| Variables | ID-1 | ID-2 | ID-3 | ID-4 | ID-5 | ID-6 | ID-7 | ID-8 |
| Death | ||||||||
| In the cath lab | N | N | N | N | N | N | N | N |
| After PCI | N | N | N | N | N | N | N | N |
| Reinfarction | N | N | N | N | N | N | N | N |
| Stroke | N | N | N | N | N | N | N | N |
| Target lesion revascularization | N | N | N | N | N | N | N | N |
| Any major adverse event | N | N | N | N | N | N | N | N |
| Left ventricular failure | N | N | N | N | N | N | N | N |
N: no; PCI: percutaneous coronary intervention.
3.5. Procedural complications
ICART was relatively simple and feasible, and no complications occurred intraoperatively in any of the eight patients.
4. Discussion
The pathogenic mechanism of STEMI begins with rupture of an intracoronary plaque, which leads to thrombosis and subsequent acute coronary arterial stenosis, occlusion, and ischemia. Prompt, sustained, effective myocardial reperfusion therapy can significantly decrease the size of the myocardial infarction area and salvage heart function, which lowers the mortality rate and improves the prognosis in patients with STEMI.[19],[20] PPCI is the primary therapy to restore myocardial blood flow;[21] however, percutaneous transluminal coronary angioplasty and/or stenting cannot effectively scavenge the thrombus with high thrombus loads. In contrast, intracoronary intervention can increase the possibility of thrombus shift and embolism in the distal microcirculation, resulting in failure to restore coronary blood flow and tissue perfusion (slow flow and no reflow).[22],[23] Slow flow and no reflow increase the mortality rate and the rate of other adverse cardiac events, which have an incidence rate of 5%–50%.[24]
Thrombosis poses a challenge in intracoronary interventional therapy. Before performing PCI, the thrombus load should be properly managed, and antithrombotic drugs are prerequisites for thrombus management. To reduce the intracoronary thrombus load in patients with STEMI and to prevent no reflow, a number of measures, namely, placing a distal protection device, thrombus aspiration, and glycoprotein IIb/IIIa antagonists, have been suggested for use during PPCI. However, prospective randomized trials of distal protection devices, including the EMERALD, PROMISE, and AIMI trials,[25] have demonstrated that using a conventional distal protection device in PPCI achieved neutral and even negative effects on myocardial perfusion and the final infarct area.
Recent studies demonstrate that routine intracoronary thrombus aspiration before PPCI in patients with STEMI has not been proven to reduce short-term mortality, and does not reduce the rate of death from any cause or the composite of death from any cause, rehospitalization for myocardial infarction, or stent thrombosis at one year.[26] The recommended level of conventional thrombus aspiration has been reduced to TIMI grade 3, and with a high thrombus load, the recommended level of thrombus aspiration has been reduced to TIMI 2b. Aspiration cannot prevent the dislodgement of the thrombus and the resulting microembolism in the distal coronary vasculature caused by microthrombi. Furthermore, mechanical damage and stimulation to the endothelium caused by the negative pressure should not be overlooked; this damage can result in functional disturbance such as endothelial dysfunction and vasospasm. The sudden restoration of coronary patency by negative pressure aspiration is accompanied by a strong chance of reperfusion injury, but incomplete suction or an aspiration catheter pushing the thrombus toward the distal end commonly results in slow flow or no reflow.[16] In this situation, even if imaging reveals patency in the culprit vessel, myocardial tissue perfusion may remain insufficient and adversely affects the clinical efficacy of PCI and the patient's prognosis.[27]
The use of tirofiban is also associated with limitations. Tirofiban is an antiplatelet medication with a limited effect on existing thrombi. High doses of thrombolytics are used in current intravenous and intracoronary antegrade thrombolytic therapies; however, there is a high likelihood of hemorrhage with these therapies. The occurrence of bleeding creates a dilemma regarding therapy, because antiplatelet and anticoagulant medications are contraindicated with bleeding. Therefore, there is an urgent need to develop a novel therapeutic approach that can reduce the incidence of no reflow or slow flow as well as hemorrhagic complications. ICART with emergency PCI offers encouraging results in this regard.
Previous studies have demonstrated that the thrombus in the culprit vessel usually grows from the proximal to the distal end and is wedge-shaped. The initial part of the intracoronary thrombus is mostly white thrombus, composed mainly of multiple platelet deposits and a small amount of fibrin, and forms a continuous thrombus head. The distal end of the thrombus is mostly red thrombus, composed of a large number of red blood cells encapsulated by cellulose. These characteristics determine that the distal red thrombus is more likely to be lysed by thrombolytic agents. Retrograde thrombolysis takes advantage of this characteristic of thrombus formation. Using a microcatheter, the thrombolytic agent can be delivered to the distal portion of the coronary occlusion. The drugs can then act precisely on the location with the heaviest thrombus load and make maximal contact with the freshest portion of the thrombus. This can block the formation of new thrombi, while dissolving the existing thrombus and more effectively clear the thrombus load. In antegrade thrombolysis, the thrombolytic agent cannot be localized in the lesion because of the effect of blood flow; thus, thrombolysis is ineffective.
As shown in Figure 2B, the medication was rapidly cleared from the lesion in antegrade thrombolysis, and the stenosis showed no change after injecting 10 wu urokinase. To enable continued thrombolysis, the thrombolytic injection must be sustained. The approach in the patient in Figure 2B was changed to retrograde thrombolysis, and the thrombolytic agent remained stable at the distal end of the occlusive segment. The coronary artery was opened with less than 10 wu urokinase.
ICART provided satisfactory outcomes in all eight patients participating in this trial and was an effective, feasible, and simple approach for the management of STEMI. Transcatheter retrograde intracoronary administration of small amounts of thrombolytic may produce very high local blood drug concentrations, resulting in the opening of an obstructed coronary artery. In all eight patients, the dose of urokinase was less than 20 wu. In one patient, the dose sufficient to recover patency was only 5 wu, which is the lowest dose of thrombolytic reported in STEMI thrombolytic therapy, a mere 1/40 to 1/20 of the doses used in intravenous and intracoronary antegrade thrombolysis reported in previous studies. The small dose of thrombolytic used with ICART can theoretically lower the risk of hemorrhage in other sites, such as the brain and gastrointestinal tract. No hemorrhage was observed in any of our eight patients.
The time of vascular patency was less than 17 min in all eight patients in our study, with the shortest time being only 3 min. In addition, there was slight blood flow when the guidewire passed through the occluded section. ICART also does not significantly prolong the revascularization time.
The thrombolytic effect was complete, and the drug was localized in the lesion with sufficient concentration and time to exert its action, using ICART. The thrombolytic could effectively dissolve the microthromboemboli in the distal vascular bed, which cannot be reached by mechanical aspiration. TIMI grade 3 blood flow was restored in all patients after the procedure. Re-establishing coronary patency was a gradual process of restored blood flow with decreasing thrombus burden, which resulted in reperfusion preconditioning, unlike the abrupt increase in blood flow caused by aspiration or balloon dilatation, thereby decreasing the incidence of reperfusion injury.
We were able to perform coronary imaging during the thrombolytic process, enabling observation of thrombus dissolution and providing guidance on thrombolytic dosing. The effect of thrombolysis could be observed instantly. After ICART, the lesion could be accurately measured, which is helpful for accurate selection and precisely locating the stent; and also prevents unnecessary or even contraindicated stent implantation in nonsevere stenosis. Thus, the benefit of stenting can be maximized.[28]
4.1. Limitations
Because the ICART protocol is novel, only a small number of patients have been treated using this therapy; and a standardized procedure has not been confirmed. The long-term effects of this therapy await further follow-up, while the safety and efficacy can be confirmed only in trials with larger sample sizes. Future improvements may include the following three items: (1) Determining the optimal choice and concentration of the thrombolytic. The contrast medium helps only with imaging, and may be used at lower concentrations to reduce endothelial irritation. Once the protocol is refined, the volume of contrast medium could be minimized. The ultimate goal is a standardized regimen for the type and concentration of thrombolytic. (2) Standardizing the pushing force and speed of thrombolytic injection. Currently, these parameters were determined by retention of the contrast medium in the trunk and branch vessels. Whether a stronger pushing force results in better perfusion in the vascular bed is still unknown. (3) Confirming the implications of satisfactory imaging results during the operation for genuine clinical benefit using controlled trials involving larger sample sizes and longer follow-up.
4.2. Conclusions
In summary, transcutaneous ICART with PCI is a potential therapy and may become widely used in patients with STEMI. This improved intracoronary thrombolytic protocol is different from facilitated PCI[29] and rescue PCI, and can provide maximal rescue of dying myocardium and prevent adverse effects secondary to high-dose thrombolytic and rapid physical reperfusion.
Acknowledgments
This study was supported by the Hainan Province's Key Research and Development Project (ZDYF 2017096 & ZDYF2018118), National Natural Science Foundation of China (NSFC: 81500202), Beijing Lisheng Cardiovascular Health Foundation Pilot Fund Project (LHJJ201610620), Provincial Key Science and Technology Projects supporting projects in Sanya (2018PT48), Sanya Medical and Health Science and Technology Innovation Project (2017YW10). All authors had no conflicts of interest to disclose.
References
- 1.Zipes DP, Libby P, Bonow RO, et al. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 7th Edition. Singapore: Elsevier Medicine; 2007. pp. 1068–1090. [Google Scholar]
- 2.Antman EM, Hand M, Armstrong PW, et al. 2007 Focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 writing group to review new evidence and update the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction, writing on behalf of the 2004 writing committee. Circulation. 2008;117:296–329. doi: 10.1161/CIRCULATIONAHA.107.188209. [DOI] [PubMed] [Google Scholar]
- 3.Antman EM. Time is muscle: translation into practice. J Am Coll Cardiol. 2008;52:1216–1221. doi: 10.1016/j.jacc.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Gibson CM, de Lemos JA, Antman EM, et al. Time is muscle in primary PCI: the strength of the evidence grows. Eur Heart J. 2004;25:1001–1002. doi: 10.1016/j.ehj.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 5.Muñoz D, Roettig ML, Monk L, et al. Transport time and care processes for patients transferred with ST-segment-elevation myocardial infarction: the reperfusion in acute myocardial infarction in Carolina emergency rooms experience. Circ Cardiovasc Interv. 2012;5:555–562. doi: 10.1161/CIRCINTERVENTIONS.112.968461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Widimsky P, Wijns W, Fajadet J, et al. Reperfusion therapy for ST elevation acute myocardial infarction in Europe: description of the current situation in 30 countries. Eur Heart J. 2010;31:943–957. doi: 10.1093/eurheartj/ehp492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:485–510. doi: 10.1016/j.jacc.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Lincoff AM, Topol EJ. Illusion of reperfusion. Does anyone achieve optimal reperfusion during acute myocardial infarction? Circulation. 1993;88:1361–1374. doi: 10.1161/01.cir.88.3.1361. [DOI] [PubMed] [Google Scholar]
- 9.Boersma E, Maas AC, Deckers JW, et al. Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour. Lancet. 1996;348:771–775. doi: 10.1016/S0140-6736(96)02514-7. [DOI] [PubMed] [Google Scholar]
- 10.Fibrinolytic Therapy Trialists' Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomized trials of more than 1000 patients. Lancet. 1994;343:311–322. [PubMed] [Google Scholar]
- 11.Tiefenbrunn AJ, Sobel BE. Timing of coronary recanalization: paradigms, paradoxes, and pertinence. Circulation. 1992;85:2311–2315. doi: 10.1161/01.cir.85.6.2311. [DOI] [PubMed] [Google Scholar]
- 12.Holmes DR, Jr, Califf RM, Topol EJ. Lessons we have learned from the GUSTO trial. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries. J Am Coll Cardiol. 1995;25:10S–17S. doi: 10.1016/0735-1097(95)00188-a. [DOI] [PubMed] [Google Scholar]
- 13.Dong J, Ndrepepa G, Schmitt C, et al. Early resolution of ST-segment elevation correlates with myocardial salvage assessed by Tc-99m sestamibi scintigraphy in patients with acute myocardial infarction after mechanical or thrombolytic reperfusion therapy. Circulation. 2002;105:2946–2949. doi: 10.1161/01.cir.0000022604.56986.ff. [DOI] [PubMed] [Google Scholar]
- 14.Rezkalla SH, Kloner RA. Coronary no-reflow phenomenon: from the experimental laboratory to the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 2008;72:950–957. doi: 10.1002/ccd.21715. [DOI] [PubMed] [Google Scholar]
- 15.Sardella G, Mancone M, Bucciarelli-Ducci C, et al. Thrombus aspiration during primary percutaneous coronary intervention improves myocardial reperfusion and reduces infarct size: the EXPIRA (Thrombectomy with export catheter in infarct-related artery during primary percutaneous coronary intervention) prospective, randomized trial. J Am Coll Cardiol. 2009;53:309–315. doi: 10.1016/j.jacc.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Vlaar PJ, Svilaas T, van der Horst IC, et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet. 2008;371:1915–1920. doi: 10.1016/S0140-6736(08)60833-8. [DOI] [PubMed] [Google Scholar]
- 17.Thiele H, Schindler K, Friedenberger J, et al. Intracoronary compared with intravenous bolus abciximab application in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: the randomized Leipzig immediate percutaneous coronary intervention abciximab IV versus IC in ST elevation myocardial infarction trial. Circulation. 2008;118:49–57. doi: 10.1161/CIRCULATIONAHA.107.747642. [DOI] [PubMed] [Google Scholar]
- 18.Kushner FG, Hand M, Smith SC, Jr, et al. 2009 Focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–2241. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 19.Kushner FG, Hand M, Smith SC, Jr, et al. 2009 Focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:2271–2306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 20.Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 21.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarct ion: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 22.Niccoli G, Burzotta F, Galiuto L, et al. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009;54:281–292. doi: 10.1016/j.jacc.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 23.Rezkalla SH, Dharmashankar KC, Abdalrahman IB, et al. No-reflow phenomenon following percutaneous coronary intervention for acute myocardial infarction: incidence, outcome, and effect of pharmacologic therapy. J Interv Cardiol. 2010;23:429–436. doi: 10.1111/j.1540-8183.2010.00561.x. [DOI] [PubMed] [Google Scholar]
- 24.Eeckhout E, Kern MJ. The coronary no-reflow phenomenon: a review of mechanism s and therapies. Eur Heart J. 2001;22:729–739. doi: 10.1053/euhj.2000.2172. [DOI] [PubMed] [Google Scholar]
- 25.Limbruno U, De Caterina R. EMERALD, AIMI, and PROMISE: is there still a potential for embolic protection in primary PCI? Eur Heart J. 2006;27:1139–1145. doi: 10.1093/eurheartj/ehi755. [DOI] [PubMed] [Google Scholar]
- 26.Lagerqvist B, Fröbert O, Olivecrona GK, et al. Outcomes 1 year after thrombus aspiration for myocardial infarction. N Engl J Med. 2014;371:1111–11120. doi: 10.1056/NEJMoa1405707. [DOI] [PubMed] [Google Scholar]
- 27.MA YL, WANG WM, LIU J, et al. Comparison of different strategies to reduce thrombus burden during primary percutaneous coronary intervention in ST-segment elevation myocardial infarction patients. Chin J Intervent Cardiol. 2010;18:9–12. [In Chinese] [Google Scholar]
- 28.Ke D, Zhong W, Fan L, et al. Delayed versus immediate stenting for the treatment of ST-elevation acute myocardial infarction with a high thrombus burden. Coron Artery Dis. 2012;23:497–506. doi: 10.1097/MCA.0b013e328358a5ad. [DOI] [PubMed] [Google Scholar]
- 29.Zimarino M, Sacchetta D, Renda G, et al. Facilitated PCI: rationale, current evidence, open questions, and future directions. J Cardiovasc Pharmacol. 2008;51:3–10. doi: 10.1097/FJC.0b013e3181565e26. [DOI] [PubMed] [Google Scholar]