Abstract
Many rodents, including most populations of arctic lemmings (genus Dicrostonyx and Lemmus), have cyclic population dynamics. Among the numerous hypotheses which have been proposed and tested to explain this typical characteristic of some terrestrial vertebrate communities, trophic interactions have often been presented as the most likely drivers of these periodic fluctuations. The possible role of parasites has, however, only seldom been assessed. In this study, we genetically measured the prevalence of two endoparasite taxa, eimerians and cestodes, in 372 faecal samples from collared lemmings, over a five year period and across three distant sites in Northeast Greenland. Prevalence of cestodes was low (2.7% over all sites and years) and this taxon was only found at one site (although in 4 out of 5 years) in adult hosts. By contrast, we found high prevalence for eimerians (77.7% over all sites and years), which occurred at all sites, in every year, for both age classes (at the Hochstetter Forland site where both adult and juvenile faeces were collected) and regardless of reproductive and social status inferred from the characteristics of the lemming nests where the samples had been collected. Prevalence of eimerians significantly varied among years (not among sites) and was higher for juvenile than for adult lemmings at the Hochstetter Forland site. However, higher prevalence of eimerians (Pt) was only associated with lower lemming density (Nt) at one of the three sites and we found no delayed density dependence between Nt and Pt+1 to support the parasite hypothesis. Our results show that there is no clear relation between lemming density and eimerian faecal prevalence in Northeast Greenland and hence no evidence that eimerians could be driving the cyclic population dynamics of collared lemmings in this region.
Keywords: Eimerians, Cestodes, Population dynamics, Faecal prevalence, Greenland, Rodent-parasites interactions
Graphical abstract

Highlights
-
•
Prevalence of eimerians and cestodes was measured in collared lemming in Greenland.
-
•
Prevalence of cestodes was low (2.7%; one site only) compared to eimerians (78%).
-
•
Prevalence of eimerians was higher for juveniles and varied among years.
-
•
Prevalence of eimerians was negatively associated with lemming density at one site.
-
•
lack of delayed density dependence does not support the parasite hypothesis for lemming cycles.
1. Introduction
The cyclic population dynamics of rodent species has long puzzled ecologists (Elton, 1924, 1942). Among the many hypotheses proposed to explain the periodic fluctuations in rodents and especially lemmings (Stenseth and Ims, 1993), trophic interactions (both bottom-up and top-down; Gilg et al., 2003; Oksanen and Oksanen, 2000), in conjunction with climate (Gilg et al., 2009; Kausrud et al., 2008), have regularly been considered as the likely drivers (Ims and Fuglei, 2005; Klemola et al., 2003; Korpimäki et al., 2004; Turchin et al., 2000). On the other hand, parasites and pathogens are other possible candidates but have only seldom been considered in this context (Forbes et al., 2014; Henttonen and Kaikusalo, 1993; Irvine, 2006; Telfer et al., 2010).
Parasites (both macro- and microparasites) are widespread and most animals host many parasites and pathogens in the wild (Poulin, 2007). Parasites can often have negative effects on the hosts’ life history traits (Holmstad et al., 2005; Poulin and Morand, 2000). Indeed, parasites can impact body condition, development or fecundity and can even be lethal in some cases (Beldomenico and Begon, 2009; Hakkarainen et al., 2007; Newey et al., 2004). Parasites can hence have an important influence on some key demographic parameters (e.g., reproduction and survival; Neuhaus, 2003; VanVuren, 1996) and have the potential to drive population cycles by altering the intrinsic growth rate of their host population (Anderson, 1978; Burthe et al., 2006; Irvine, 2006; Redpath et al., 2006). Although theoretical studies support the possible role of parasites on host population dynamics (Anderson and May 1978; May and Anderson, 1978; Turchin, 2003), empirical and experimental evidence are scarce (but see e.g., Watson, 2013 and Møller, 2005 for reviews). This is mainly due to their high and partly unknown diversity, to the many potential confounding factors, as well as to the many obstacles to study pathogen-host interactions at adequate organisational (i.e., community level) and spatiotemporal scales (but see e.g., Laakkonen et al., 1998; Telfer et al., 2007; Turner et al., 2014, for rodents, and Dobson and Hudson, 1992; Redpath et al., 2006, for birds).
Eimeria-like species (i.e., family Eimeriidae, referred to as eimerians) are obligate intracellular protozoan parasites of vertebrates with a worldwide distribution. They exhibit both sexual and asexual phases, generally only needing single hosts to complete their life cycle (monoxenous) and can be responsible for so called coccidiosis diseases (Olsen, 1974). Oocysts, produced during the sexual phase, are released into the host's intestinal tract, expelled with the faeces, and can remain infectious for several months (Jeston et al., 2002). Naïve hosts can become infected orally when ingesting sporulated oocysts released in the faeces of an infected host (Olsen, 1974). The transmission probability of eimerians increases dramatically with host density, as shown in the poultry and sheep industries (Catchpole et al., 1976; Pellerdy, 1974; Soulsby, 1982). Although some species are described as being asymptomatic, in some hosts the presence of eimerians can induce histopathological changes in their intestinal cells. Infested individuals can lose weight and may eventually die in cases of heavy infection (Duszynski and Marquardt, 2003; Newman et al., 2001; Yun et al., 2000). For example, eimerians have been shown to reduce winter survival of male deer mouse (Peromyscus maniculatus; Fuller and Blaustein, 1996). However, although the massive reproduction of eimerians in the intestinal tract of their host can reduce the fitness and thereby impact the population dynamics of the host, the potential role of eimerians as a driver of cyclic changes in population dynamics of rodents has never been clearly demonstrated (Forbes et al., 2014; Laakkonen et al., 1998; Levine, 1951).
Lemmings, which typically follow three to four year population cycles, are known to host a diverse community of gastrointestinal parasites, including eimerians (Laakkonen et al., 2001). Lemmings are also known to reingest their faeces, making them particularly vulnerable to coccidiosis during the high-density phase of their multi-annual population cycle (Hirakawa, 2001). Given these peculiarities, lemmings constitute an ideal biological model to test the assumption that eimerian parasites could contribute to the cyclic population dynamics of their host. We therefore examined the prevalence of eimerian eggs/oocysts in the faeces of collared lemming (Dicrostonyx groenlandicus) in three distinct and asynchronous lemming populations in Northeast Greenland over a five-year period (i.e., covering at least one full lemming cycle, which typically lasts for four years in this region; Gilg et al., 2003; Gilg et al., 2009; Schmidt et al., 2008). Following a pilot study showing that cestode eggs could also be found in the lemming faeces remaining after snowmelt around their winter nests (Richard, 2012), we also investigated the prevalence of this taxon, although it was not our main initial aim. Altogether we tested 372 lemming faecal samples collected between 2010 and 2014 in three distant (>80 km) study areas, to estimate the faecal prevalence of these two parasite taxa. Based on these analyses, we aimed at answering the following questions: (1) does the faecal prevalence of these parasites vary between sites or (2) between years within a site, and (3) is there a negative relationship between eimerian faecal prevalence and lemming population dynamics? Finally, we also investigated (4) if faecal prevalence varied according to previous year's lemming density (since such a delayed density-dependence would suggest a dynamical link between lemming and their parasites), sociability and reproductive activity of the hosts.
2. Material and methods
2.1. Study areas
The study was conducted at three sites located within the “North and East Greenland National Park”: Hochstetter Forland (75.15°N, 19.70°W), Zackenberg (74.47°N, 20.57°W) and Karupelv Valley (72.50°N, 24.00°W). The three sites belong to the bioclimatic prostrate shrub Tundra subzone (mean July temperature <10 °C; discontinuous vegetation coverage usually between 5 and 50%; Walker et al., 2005) and are located on lowland quaternary deposits (Koch and Haller, 1971). Thirty species of birds, mostly migrating shorebirds and wildfowl, and six mammalian species, form the terrestrial vertebrate community found on these sites. The collared lemming and its four main predators (the arctic fox Vulpes lagopus, the snowy owl Bubo scandiacus, the stoat Mustela erminea, and the long-tailed skua Stercorarius longicaudus) are strongly interacting and follow multiannual population cycles (Gilg et al., 2003, 2006; Schmidt et al., 2012).
2.2. Lemming dynamics and sampling of faeces
The population dynamics of collared lemming have been monitored at Karupelv since 1988, at Zackenberg since 1995, and at Hochstetter since 2010. At each of these sites, the relative abundance of lemmings is monitored annually by counting their winter nests after snowmelt (Sittler, 1995). These hollow ball-shaped nests, ca. 15–25 cm in diameter and predominantly made of graminoids, are used in winter, including for reproduction, and remain on the ground surface after snowmelt in June. When living in these subnivean winter nests (i.e., from September–October to June), lemmings use latrines usually located within 2 m from their nests. When censusing the winter nests we collected a few dozen lemming faeces per nest, from a maximum of 100 nests per site and per year, in latrines located less than 1 m from the sampled nest. Faeces were air-dried if necessary and then stored in 0.5 ml Eppendorf® Safe-Lock™ tubes (one tube for each sampled lemming nest) at room temperature prior to analysis. Among the 3699 fresh winter nests that were found at the three sites during the period 2010–2014, we collected faeces from 880 nests (in years of high lemming abundance, samples were only available or collected for some of the nests discovered) and later analysed a subset of 372 samples (323 from adults and 49 from young) selected in order to cover the entire range of nest aggregation sizes and breeding activity. Faeces of juvenile lemmings (aged according to the pellet size) were only available from one site (Hochstetter; Table 1) and were not used in the analyses unless clearly stated (see Results).
Table 1.
Number of samples (each consisting of 5 mixed faeces) analysed in different sites and years according to winter nest attributes (number in bracket gives the number of sample with presence of eimerians, and cestodes when a second number is given).
| Signs of reproduction |
Lemming age class |
Predation |
Size of the aggregation |
Total number |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| in aggregation |
by stoat |
hosting the sampled winter nest |
of samples |
||||||||||
| No | Yes | Adult | Young | No | Yes | 1 | 2 | 3 | 4 | 5 | >5 | ||
| Hochstetter |
55(35/7) |
125(105/3) |
131(95/10) |
49(45) |
175(135/10) |
5(5) |
84(67/6) |
47(39/3) |
23(18) |
12(10) |
8(4/1) |
6(2) |
180(140/10) |
| 2010 | 15(8/1) | 9(9) | 19(12/1) | 5(5) | 23(16/1) | 1(1) | 12(9) | 8(7) | 3(1) | 1(0/1) | 24(17/1) | ||
| 2011 | 5(5) | 17(14) | 14(14) | 8(5) | 22(19) | 12(11) | 10(8) | 22(19) | |||||
| 2012 | 17(16/2) | 43(43) | 44(43/2) | 16(16) | 56(55/2) | 4(4) | 32(31/2) | 13(13) | 10(10) | 5(5) | 60(59/2) | ||
| 2013 | 15(4/3) | 56(39/3) | 51(24/6) | 20(19) | 71(43/6) | 26(14/3) | 15(11/3) | 10(7) | 7(5) | 7(4) | 6(2) | 71(43/6) | |
| 2014 |
3(2/1) |
3(2/1) |
3(2/1) |
2(2/1) |
1 |
3(2/1) |
|||||||
| Karupelv |
53(40) |
26(19) |
79(59) |
68(50) |
11(9) |
38(31) |
26(17) |
10(8) |
5(3) |
79(59) |
|||
| 2010 | 4(1) | 4(1) | 4(1) | 2(1) | 1 | 1 | 4(1) | ||||||
| 2011 | 1 | 8(7) | 9(7) | 6(5) | 3(2) | 3(1) | 5(5) | 1(1) | 9(7) | ||||
| 2012 | 26(18) | 7(5) | 33(23) | 29(20) | 4(3) | 14(11) | 11(7) | 4(3) | 4(2) | 33(23) | |||
| 2013 | 18(17) | 10(7) | 28(24) | 26(22) | 2(2) | 19(16) | 9(8) | 28(24) | |||||
| 2014 |
4(4) |
1 |
5(4) |
3(2) |
2(2) |
3(3) |
2(1) |
5(4) |
|||||
| Zackenberg |
88(70) |
25(20) |
113(90) |
112(89) |
1(1) |
56(45) |
33(25) |
12(10) |
11(9) |
1(1) |
113(90) |
||
| 2010 | 2 | 4(2) | 6(2) | 6(2) | 4(1) | 2(1) | 6(2) | ||||||
| 2011 | 10(5) | 3(3) | 13(8) | 13(8) | 6(4) | 4(2) | 2(1) | 1(1) | 13(8) | ||||
| 2012 | 37(31) | 8(8) | 45(39) | 44(38) | 1(1) | 23(20) | 12(11) | 5(5) | 5(3) | 45(39) | |||
| 2013 | 26(23) | 8(5) | 34(28) | 34(28) | 15(13) | 9(6) | 5(4) | 4(4) | 1(1) | 34(28) | |||
| 2014 |
13(11) |
2(2) |
15(13) |
15(13) |
8(7) |
6(5) |
1(1) |
15(13) |
|||||
| Total number of samples | 196(145/7) | 176(144/3) | 323(244/10) | 49(45) | 355(274/10) | 17(15) | 178(143/6) | 106(81/3) | 45(36) | 28(22) | 8(4/1) | 7(3) | 372(289/10) |
For each winter nest we also recorded the following parameters: (1) signs of reproduction (i.e., presence of small juvenile faeces in distinct latrines and eventually of a thin insulating layer of “woven” lemming hairs at the nest entrance) (2) evidence of winter predation by the stoat (i.e., piles of stoat faeces within ca. 2 m of the winter nest, or a thick layer of “plucked” lemming hair tufts insulating all the walls of the nest chamber; the presence of lemming skulls with squashed cranium could also be used as an additional evidence of stoat predation in some nests), and (3) the size of the aggregation the nest belonged to (i.e., all nests less than 10–15 m apart are considered to be part of the same aggregation (for additional description of lemming winter nest counts and their predation by small mustelids see: Fuller et al., 1975; Fuller et al., 1977; Maclean et al., 1974; Maher, 1967; Sittler, 1995). All winter nests were mapped with hand-held GPS and destroyed after census to prevent double counting in the following year.
Log regressions between winter nest counts and live trapping were used to estimate lemming densities at snowmelt. For Karupelv, where winter nests are annually recorded over a large (1500ha) study area, including large areas of unfavourable lemming habitats, we used a published regression (eqn (1); Gilg et al., 2006), slightly updated in order to include all available live trapping data (1998–2009; eqn (1a)). At the Zackenberg site, where lemming winter nests are only recorded over a smaller (106ha) area of favourable habitats, we used a modified equation taking into account these differences in habitat suitability (eqn (2); Gilg et al., 2009). Finally, a similar but specific regression was produced for the Hochstetter site (eqn (3)) where winter nests are monitored along a 17 km long transect (for more details on winter nest counts, live trapping and density estimates, see Gilg, 2002; Gilg et al., 2009; Gilg et al., 2006; Schmidt et al., 2008; Sittler, 1995). Gilg et al. (2006), updated from Gilg et al. (2006), Gilg et al. (2009), Gilg unpublished
| Ln(N) = 1.35Ln(x) + 1.15 | (1) |
| Ln(N) = 1.3751Ln(x) + 1.1823 | (1a) |
| Ln(N) = 1.3751Ln(x/2.55) + 1.1823 | (2) |
| Ln(N) = 0.9366Ln(x’) −4.257 | (3) |
where N is the estimated lemming density at snow melt, x is the number of winter nests per ha and x’ is the number of winter nests counted along a 17 km transect.
2.3. Parasite faecal prevalence
Each analysed sample consisted of five faecal pellets (mixed together) collected from the same lemming winter nest, i.e., from the nest itself or a latrine located within 1 m of the nest. Prior to DNA extraction, 1 ml of ASL Buffer, a stool lysis buffer (Qiagen, Hilden, Germany), was added to each sample. Samples were kept overnight at +4 °C to ensure soaking. Pellets were then squashed with a sterile toothpick and shaken for 2 h at 56 °C. Subsequently, 100 μL from each sample was used for DNA extraction, using the DNeasy Blood and Tissue Kit (Qiagen) and following the manufacturer's instructions, as described in Soininen et al. (2015). The DNA extracts were recovered in an elution volume of 200 μL and stored at −20 °C until DNA amplification.
PCR amplifications were performed with the generic PCR primers F1E (5’ - TACCCAATGAAAACAGTTT - 3′) and R2B (5’ – CAGGAGAAGCCAAGGTAGG - 3’), targeting the 18S rRNA gene of eimerid species (Cyclospora and Eimeria species, referred to as eimerians; Reiman et al., 1996). The PCR amplifications were conducted in a reaction mixture (25 μL) consisting of 2 μL of DNA extract, 1 x HotStarTaq Master Mix (Qiagen), 0.2 μM of each primer, and PCR-grade water. A negative control (PCR-grade water) was included for every 20 samples. The amplification cycling program consisted of an activation step of 15 min at 95 °C followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 60 s, and primer extension at 74 °C for 90 s. A final extension was performed at 72 °C for 10 min.
We used the primers COX-F (5′ - GATGTTTTCTTTACATTTATCTGGTG - 3′) and COX-R (5′ - GCCACCACAAATCAAGTATC-3′) to amplify a 641 bp fragment of the mitochondrial cytochrome c oxidase subunit I (COI) in anoplocephaline cestodes (Haukisalmi et al., 2004). The PCR mixture (25 μL) comprised 4 μL of DNA extract, 1 x HotStarTaq Master Mix (Qiagen), 0.2 μM of each primer, and PCR-grade water. A negative control (PCR-grade water) was included for every 20 samples. The amplification cycling program consisted of an activation step of 15 min at 95 °C followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 53 °C for 60 s, and primer extension at 74 °C for 90 s. A final extension was performed at 72 °C for 10 min.
All the PCR products were separated and visualized using the QIAxcel device and a QIAxcel DNA high-resolution kit (Qiagen). Samples yielding a positive result for PCR were confirmed by sequencing. The amplified products were purified using the QIAquick PCR Purification Kit (Qiagen) according to the manufacturer's instructions. Direct sequencing of the PCR products was performed with an automated sequencer (Applied Biosystems 3130 Genetic Analyzer) using the primers employed for the PCR reactions. A homology search of the sequences generated in this study was then performed by conducting an online search with the NCBI Basic Local Alignment Search Tool for nucleotides (BLASTN) in the GenBank database (http://blast.ncbi.nlm.nih.gov/Blast.cgi), to confirm similarity with eimerian or cestode species.
2.4. Statistical analyses
2.4.1. Determinant of cestode faecal prevalence changes
Given the low faecal prevalence of cestodes, we only tested for differences in faecal prevalence between high and low lemming years (see below) and between nests with and without evidence of reproduction, using Fischer's exact tests.
2.4.2. Variation of eimerian prevalence among years and sites
Regarding eimerians, we first tested variation in their faecal prevalence among sites and across years using generalized linear models (GLM), with a logit link function and a binomial error distribution. We also considered potential interaction between years and sites in a model selection framework. We ranked candidate models using the Akaike Information Criterion for small sample sizes (AICc) as implemented in the R package “AICcmodavg” (Mazerolle, 2017) and calculated ΔAICc and AICc weights. Models with ΔAICc ≤2 were considered equivalent (Burnham and Anderson, 2002).
2.4.3. Association between eimerian prevalence and host density
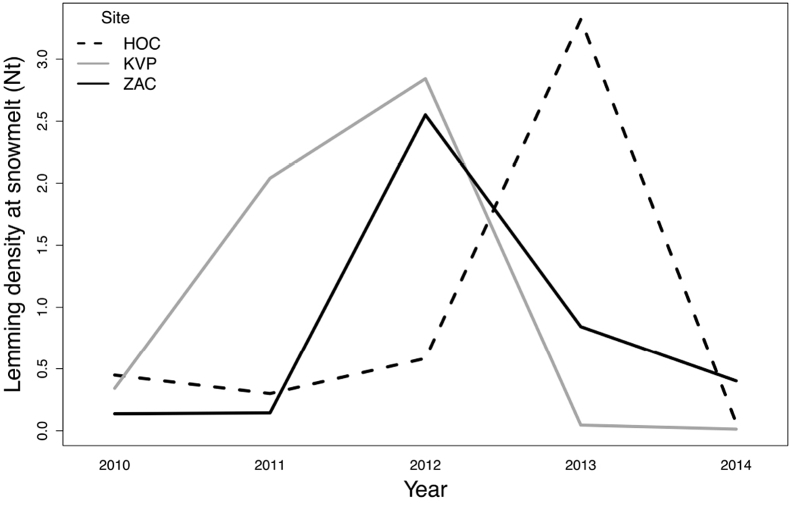
We then used the Cochran-Mantel-Haenszel χ2 test with continuity correction to test whether lemming population density at snowmelt (Nt) was related to eimerian faecal prevalence in winter nests, within each of the three sites (Hochstetter Forland, Karupelv Valley, and Zackenberg). Given the variation in lemming densities observed at the three sites over the study period (Fig. 1), we categorized continuous density estimates in low lemming density when <1 lemmings/ha and high lemming density when >2 lemmings/ha were observed. Based on the functional and numerical responses of lemming predators, lemming density of 1–2 individual per ha clearly distinguishes lemming peaks from lemming low phases in this region (Gilg et al., 2006, 2009) and in other arctic areas hosting similar communities of terrestrial vertebrates (Bilodeau et al., 2013; Therrien et al., 2014). We tested the null hypothesis that the variables lemming density (Nt) and eimerian faecal prevalence are independent at each site. This assumes that there is no three-way interaction among lemming density, eimerian faecal prevalence and studied sites. We however predicted that the association between lemming density and eimerian faecal prevalence differs among studied sites (Fig. 1, Fig. 2). Therefore, we examined odds ratios for each site and tested a 3-way association by using a Woolf-test on homogeneity of odds ratios. Similarly, we also considered a delayed impact of eimerians on lemming density, testing the association between eimerian prevalence (Pt) on lemming density in the following year (Nt+1). All analyses were performed using R 3.3.3 (R Development Core Team, 2017).
Fig. 1.
Collared lemming densities (individuals per ha) at the three sites in Northeast Greenland (HOC: Hochstetter Forland, ZAC: Zackenberg, KVP: Karupelv Valley) during 2010–2014.
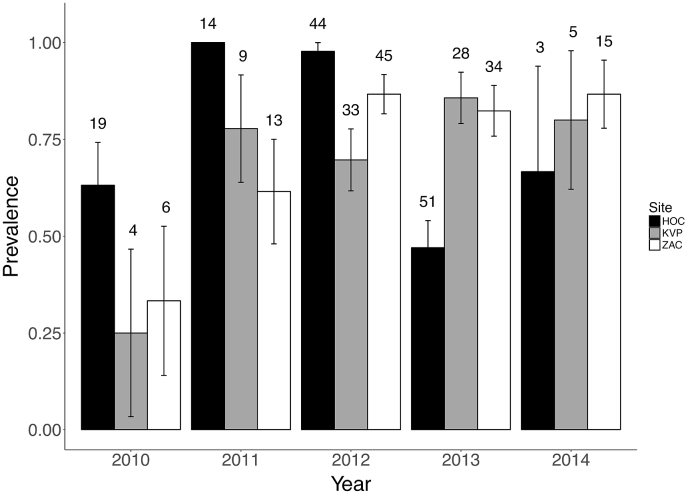
Fig. 2.
Eimerian faecal prevalence in adult collared lemming droppings from three sites in Northeast Greenland (HOC: Hochstetter Forland, ZAC: Zackenberg, KVP: Karupelv Valley), during 2010–2014. Numbers above bars give the sampling size and error bars show standard errors (estimated as SE = sqrt [p·(1-p)/n]; with p being the prevalence).
2.4.4. Effect of host density on eimerian faecal prevalence
In a complementary approach, we used generalized linear mixed-effect models (GLMM) to test the causal effects of (1) lemming density at snowmelt (Nt), in interaction with (2) sites (Hochstetter Forland, Karupelv Valley, or Zackenberg), (3) nest aggregation size (1 ≤ n ≤ 5), and (4) indices of reproduction in the nest aggregation, as fixed effects, on the probability of lemming droppings being infected by eimerians in the following winter (Pt+1), with 1 = presence and 0 = absence of eimerians in droppings. We fitted GLMMs with a binomial distributed error structure and a logit link function, using the “glmer” function implemented in the package “lme4” (Bates et al., 2015) of R 3.3.3 (R Development Core Team, 2017). We did not correct for over dispersion since the c-hat was close to one (c-hat = 0.965). Year was added as a random factor in all the models to account for annual variation in lemming density. We ranked candidate models using the Akaike Information Criterion for small sample sizes (AICc) (see above).
3. Results
3.1. Determinant of cestode faecal prevalence
Cestodes were relatively rare (10 out of the 372 samples) and only found at the Hochstetter site in adult faeces (Table 1). In these faeces, prevalence was 5% in low and 12% in high lemming years and fewer nests had signs of reproduction in aggregations where cestodes were found (30% versus 56% for non-infested nests) but, given the small sample sizes, these differences were not significant (all Fisher exact test p-values > 0.10). Due to a low DNA recovery after PCR, the species identification of anoplocephaline cestodes was only possible for one of the 10 PCR-positive samples, which matched with Microcephaloides krebsi. This is a Nearctic species that has already been found in collared lemmings (Dicrostonyx groenlandicus and D. hudsonius) collected in Canada, Greenland and on the Eastern Siberian Wrangel Island (Haukisalmi et al., 2001, 2008). Due to their low faecal prevalence, we did not include cestodes in the following analyses.
3.2. Variation of eimerian prevalence among years and sites
Faecal prevalence (adult samples only) of eimerians varied from 25 to 30% in 2010 at the Karupelv Valley and Zackenberg sites, to 95–100% in 2011–2012 at the Hochstetter Forland site (Fig. 2). Although the interaction between year and site was highly significant in the GLM (Dev(df=8, 302): 39.10, p-value < 0.001), eimerian faecal prevalence varied among years (Dev(df=4, 310): 18.81, p-value < 0.001) but not among sites (Dev(df=2,314): 0.86, p-value = 0.62), suggesting asynchronous variations of eimerian faecal prevalence among sites (Fig. 2).
Faecal prevalence found at Hochstetter in the 49 samples from young lemmings (92%) was higher than in the 131 adult samples from the same site (73%; Fisher's exact test, p-value < 0.01).
3.3. Association between eimerian prevalence and host density
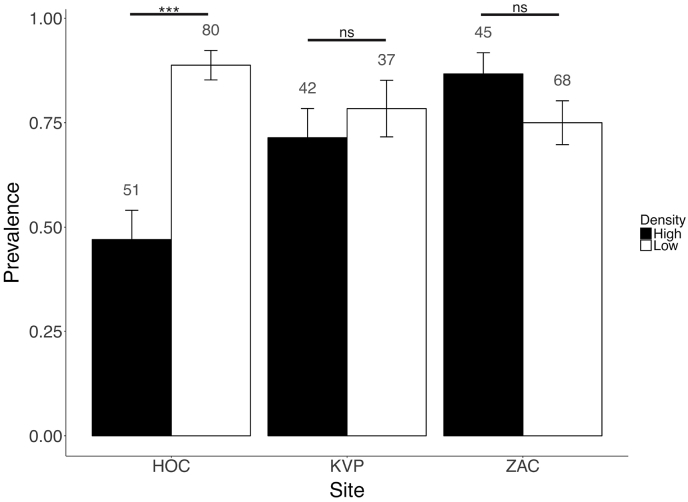
Overall, we found evidence for a weak association between eimerian faecal prevalence in winter nests and lemming density at snowmelt (Nt), when adjusted for sites (Cochran-Mantel-Haenszel χ2 = 5.81, df = 1, p-value = 0.016). However, odds ratios greatly differed between the three sites (Woolf-test χ2MH = 17.39, df = 2, p-value < 0.001). We found that eimerian faecal prevalence had a negative relationship with lemming density at Hochstetter only (Fig. 3). Odds ratios were indeed significantly different from 1 only at Hochstetter (odds ratioHOC = 7.90 (95% CI = 3.20/19.46); β = 2.06 ± 0.46, z = 4.48, p-value <0.001) in comparison with Karupelv (odds ratioKVP = 1.45 (95% CI = 0.52/4.06); β = 0.37 ± 0.53, z = 0.71, p-value = 0.479) and Zackenberg (odds ratioZAC = 0.45 (95% CI = 0.16/1.26); β = -0.79 ± 0.52, z = −1.53, p-value = 0.123).
Fig. 3.
Eimerian faecal prevalence in adult collared lemming droppings according the phase of the lemming cycle, in three sites in Northeast Greenland (HOC: Hochstetter Forland, ZAC: Zackenberg, KVP: Karupelv Valley). Numbers above bars give the sample size and error bars show standard errors (estimated as in Fig. 2). Significance tested from odds ratios (see §3.3 in Results).
Furthermore, we did not find evidence for an association between eimerian faecal prevalence (Pt) and lemming density during the following year (Nt+1) (Cochran-Mantel-Haenszel χ2 = 3.36, df = 1, p-value = 0.07). However, once again, odds ratios significantly differed among sites (Woolf-test χ2MH = 13.663, df = 2, p-value < 0.01), with a positive relation (i.e., low eimerian faecal prevalence associated with low lemming density) at Hochstetter only (odds ratioHOC = 0.4 (95%CI = 0.00/0.29), β = -3.26 ± 1.04, z = −3.15, p-value = 0.002; odds ratioKVP = 2.12 (95%CI = 0.60/7.47), β = 0.75 ± 0.64, z = 1.18, p-value = 0.24; odds ratioZAC = 2.81 (95%CI = 0.82/9.61), β = 1.03 ± 0.63, z = 1.65, p-value = 0.09).
3.4. Effect of host density on eimerian faecal prevalence
The GLMM selection procedure indicated that the first-ranked models testing the effect of lemming density at snowmelt (Nt) on the probability of lemming faeces to be eimerian positive over the subsequent winter (Pt+1) were considered equivalent to the null model, based on the ΔAICc ≤2 criteria (Table 2). Aggregation size and reproduction had no significant effect on the probability to be infected by eimerians (aggregation: β = −0.21 ± 0.15; 95%CI: 0.51/0.08 and reproduction: β = −0.58 ± 0.37; 95%CI: 0.15/1.31).
Table 2.
Model selection of the effects of lemming density (Nt), site, nest aggregation size and reproduction on the probability to be infected by eimerians the following year (Pt+1). For each model, differences in AICc are compared to the lowest-scoring model (ΔAICc). Number of parameters (k), AICc weight (ωi) and cumulative weight (cum. ωi) are given. Bold type highlights the best model (ΔAICc≤2). All models include nest identity and year as random factor. When an interaction (“x”) was used, the corresponding fixed effects were also incorporated in the model.
| Models | k | AICc | ΔAICc | ωi | cum. ωi |
|---|---|---|---|---|---|
| Nt x Site + aggregation + reproduction | 9 | 315.38 | 0.00 | 0.17 | 0.17 |
| Nt x Site | 7 | 315.81 | 0.43 | 0.14 | 0.31 |
| Nt x Site + reproduction | 8 | 315.95 | 0.56 | 0.13 | 0.45 |
| Nt + Site + aggregation + reproduction | 8 | 316.86 | 1.48 | 0.08 | 0.53 |
| Nt x Site + aggregation | 7 | 316.87 | 1.49 | 0.08 | 0.61 |
| Nt | 3 | 316.95 | 1.57 | 0.08 | 0.69 |
| Null | 2 | 317.16 | 1.78 | 0.07 | 0.76 |
| aggregation | 3 | 317.50 | 2.12 | 0.06 | 0.82 |
| Nt + Site + reproduction | 6 | 317.95 | 2.57 | 0.05 | 0.87 |
| reproduction | 3 | 318.03 | 2.65 | 0.05 | 0.92 |
| Nt + Site | 5 | 318.23 | 2.85 | 0.04 | 0.96 |
| Nt + Site + aggregation | 6 | 319.07 | 3.68 | 0.03 | 0.99 |
| Site | 4 | 320.65 | 5.27 | 0.01 | 1.00 |
4. Discussion
Parasites can regulate the population dynamics of their hosts through effects on reproduction and survival (Anderson and May 1978; Burthe et al., 2006; May and Anderson, 1978). Levine (1951) first hypothesized the role that eimerian parasites could play in explaining the cyclic dynamics of lemmings. However, evidence for parasite-induced cyclic dynamics of wild rodent populations has rarely been demonstrated (see Smith et al., 2008) and the results of previous studies are contrasted. For example, Fuller and Blaustein (1996) showed that eimerians impacted the overwinter survival rate of male deer mice and concluded that these parasites could potentially influence the population dynamics of wild rodents. Similarly, Hakkarainen et al. (2007) found that eimerians, by having a negative effect on individuals’ body conditions, could contribute to the decline of small insular bank vole (Myodes glareolus) populations. In contrast, Laakkonen et al. (1998) found that the temporal and spatial variations in eimerian prevalence and intensity in cyclic vole populations did not support this hypothesis. Our own results similarly suggest that there is no general relation between lemming density and eimerian faecal prevalence (which is site dependent) at our study sites, and hence no evidence of any eimerian-induced delayed density dependence driving the cyclic population dynamics of collared lemmings in northeast Greenland.
Large variations in eimerian prevalence (i.e., between 25 and 100%) found in our three distinct lemming populations and over several successive years are similar to those found in previous studies for other rodent species. For example, Laakkonen et al. (1998) found prevalence from 8 to 85% in three vole species in Finland and Ball and Lewis (1984) between 23 and 56% in five British rodent species. Despite the large variations in eimerian faecal prevalence found in our study, these were not associated with variations in lemming density, except at the Hochstetter site. Hence, the results of our study are not consistent with the hypothesis that eimerian faecal prevalence alone is a driver of the cyclic population dynamics of collared lemmings in this region. However, this lack of direct relation between eimerian faecal prevalence and lemming densities does not mean that eimerians cannot negatively impact lemming population dynamics.
Firstly, coccidian parasites (including eimerians) are only one of the many groups of gastrointestinal parasites known to infest lemming (Laakkonen and Henttonen, 2000; Quinn et al., 1987), others being nematodes, trematodes and cestodes (Haukisalmi and Henttonen, 2001; Rausch, 1952; Wickström et al., 2001). Since different groups of lemming parasites could have synergetic impacts on their hosts’ population dynamics (Telfer et al., 2010; van Baalen and Sabelis, 1995), investigating the prevalence of eimerians alone may be insufficient to identify the specific contribution of this group to the population dynamics of lemmings, and the prevalence measured at the (parasite) community level might have shown different results. However, Forbes et al. (2014), in an experimental study in which they analysed the combined role of two gastrointestinal parasites groups (nematodes and eimerians) on the population dynamics of vole populations in winter, also concluded that it was unlikely that parasites contributed to the cyclic winter decline of boreal vole populations. In our study, faecal prevalence in adult lemming faeces at Hochstetter (the only site where cestodes were found) was not different from the faecal prevalence of eimerians alone (75 and 73% respectively).
Secondly, prevalence, as quantified in our study, does not necessarily relate to the intensity of infection and the latter is more likely to be associated with changes in population dynamics of the host species (see e.g., Albon et al., 2002) and specific age groups. However, given the large variation in eimerian prevalence found in rodent populations (see above) and the high number of faeces analysed in our study (i.e., 372 samples of 5 faeces each), we assume that both faecal prevalence and intensity of infection were probably correlated in our study areas (as they were in Laakkonen et al., 1998). In the case of cyclic populations of collared lemmings, as in this study, it would have been impossible to sample and analyse enough individuals through classical trapping methods since densities usually decline below 1 individual per km2 in summer during the low phase of their cycles (Gilg et al., 2006). During this period, which typically lasts for 2 or 3 years, only lemming winter nests can easily be used in Greenland to infer their relative abundance or collect faecal samples (Sittler, 1995).
Thirdly, parasites can act in synergy with other factors like competition for food or predation (see Forbes et al., 2015; Forbes et al., 2014 for an experimental approach to investigate the effects of both food and intestinal parasites). For example, Hakkarainen et al. (2007) only found an effect of parasites on small populations of insular bank voles, where competition for space and resources was higher than on larger islands. On the smaller islands, presence of eimerians was associated with lowered body condition in mothers and offspring. Hence, parasites could reduce survival rates only for some age or sex classes. Similarly, several studies have shown that eimerians can increase the predation rate of voles (Kavaliers and Colwell, 1994) through behavioural changes in the host (Simeonovska-Nikolova and Golemansky, 2015; Vorisek et al., 1998). This is particularly advantageous for parasites relying on trophic transmission to complete their life cycles and some are known to manipulate their host's behaviour in order to increase the susceptibility of the latter to predation (Lagrue et al., 2007; Quinn et al., 1987). If eimerians could increase the predation rate of infested lemmings, they could hence also play an indirect role in the predator-prey population dynamics. In our study, however, less than 5% of the analysed samples (i.e., 17 out of 372 samples) were collected in aggregations where predation by stoats was documented. Eimerian faecal prevalence found for these nests (89%) was high, but not significantly higher than in other nests (Fisher's exact test; two-sided p-value = 0.39). Including this information in an alternative model did not improve the quality of its predictive value to explain lemming dynamics.
In line with Laakkonen et al. (1998) and Forbes et al. (2014), our study suggests that eimerians are unlikely to contribute to the observed cyclic dynamics of lemming populations in Greenland. Indeed, to be considered as a possible driver of the lemming cycles, eimerian faecal prevalence should show a delayed response to lemming densities (i.e., association between Nt and Pt+1), which is not the case, for any of the sites (see GLMM results). Association between eimerians and lemming density was, however, clearly significant at one study site (Hochstetter; Fig. 3), where eimerian faecal prevalence (Pt) was high at low lemming densities (Nt < 2 ind./ha) and where a delayed relationship was found between Pt and Nt+1. Hence, under some specific biotic or abiotic conditions, eimerians might impact the population dynamics of its lemming host to some extent, even if they do not explain the cyclic nature of their dynamics.
Conflicts of interest
We have no conflict of interest.
Acknowledgements
This study was supported by the French Polar Institute-IPEV [Program “Interactions 1036”] and analyses were funded by the Fondation de Coopération Scientifique of the Université de Bourgogne Franche-Comté [grant “BQR”]. Greenland Ecosystem Monitoring is thanked for access to Zackenberg data. Dimitrios Thanos helped collecting lemming droppings at KVP and Michael Buckle helped to edit the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2019.06.011.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Albon S.D., Stien A., Irvine R.J., Langvatn R., Ropstad E., Halvorsen O. The role of parasites in the dynamics of a reindeer population. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2002;269:1625–1632. doi: 10.1098/rspb.2002.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.M. The regulation of host populations growth by parasite species. Parasitology. 1978;76:119–157. doi: 10.1017/s0031182000047739. [DOI] [PubMed] [Google Scholar]
- Anderson R.M., May R.M. Regulation and stability of host-parasite population interactions. 1. Regulatory processes. J. Anim. Ecol. 1978;47:219–247. [Google Scholar]
- Ball S.J., Lewis D.C. Eimeria (Protozoa, Coccidia) in wild populations of some British rodents. J. Zool. 1984;202:373–381. [Google Scholar]
- Bates D., Machler M., Bolker B.M., Walker S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:1–48. [Google Scholar]
- Beldomenico P.M., Begon M. Disease spread, susceptibility and infection intensity: vicious circles? Trends Ecol. Evol. 2009;25:21–27. doi: 10.1016/j.tree.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Bilodeau F., Gauthier G., Berteaux D. Effect of snow cover on the vulnerability of lemmings to mammalian predators in the Canadian Arctic. J. Mammal. 2013;94:813–819. [Google Scholar]
- Burnham K.P., Anderson D.R. Springer Verlag; New York: 2002. Model Selection and Multimodel Inference: a Practical Information-Theoretic Approach. [Google Scholar]
- Burthe S., Telfer S., Lambin X., Bennett M., Carslake D., Smith A., Begon M. Cowpox virus infection in natural field vole Microtus agrestis populations: delayed density dependence and individual risk. J. Anim. Ecol. 2006;75:1416–1425. doi: 10.1111/j.1365-2656.2006.01166.x. [DOI] [PubMed] [Google Scholar]
- Catchpole J., Norton C.C., Joyner L.P. Experiments with defined multispecific coccidial infections in lambs. Parasitology. 1976;72:137–147. doi: 10.1017/s0031182000048447. [DOI] [PubMed] [Google Scholar]
- Dobson A.P., Hudson P.J. Regulation and stability of a free-living host-parasite system: trichostrongylus tenuis in red grouse. II. Population models. J. Anim. Ecol. 1992;61:487–498. [Google Scholar]
- Duszynski D.W., Marquardt W.C. Coccidia in the mammary glands of shrews (Order: insectivora) J. Parasitol. 2003;89:609–611. doi: 10.1645/GE-3141RN. [DOI] [PubMed] [Google Scholar]
- Elton C.S. Periodic fluctuations in the numbers of animals: their causes and effects. J. Exp. Biol. 1924;2:119–163. [Google Scholar]
- Elton C.S. Clarendon Press; Oxford: 1942. Voles, Mice and Lemmings. [Google Scholar]
- Forbes K.M., Henttonen H., Hirvelä-Koski V., Kipar A., Mappes T., Stuart P., Huitu O. Proceedings of the Royal Society of London B: Biological Sciences 282. 2015. Food provisioning alters infection dynamics in populations of a wild rodent. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes K.M., Stuart P., Mappes T., Henttonen H., Huitu O. Food resources and intestinal parasites as limiting factors for boreal vole populations during winter. Ecology. 2014;95:3139–3148. [Google Scholar]
- Fuller C.A., Blaustein A.R. Effects of the parasite Eimeria arizonensis on survival of deer mice (Peromyscus maniculatus) Ecology. 1996;77:2196–2202. [Google Scholar]
- Fuller W.A., Martel A.M., Smith R.F.C., Speller S.W. High-arctic lemmings, Dicrostonyx groenlandicus. II. Demography. Can. J. Zool. 1975;53:867–878. [Google Scholar]
- Fuller W.A., Martel A.M., Smith R.F.C., Speller S.W., Bliss L.C. University of Alberta Press; Alberta: 1977. Biology and Secondary Production of Dicrostonyx groenlandicus on Truelove Lowland, Truelove Lowland, Devon Island, Canada: A High Arctic Ecosystem; pp. 437–459. [Google Scholar]
- Gilg O. The summer decline of the collared lemming (Dicrostonyx groenlandicus) in high arctic Greenland. Oikos. 2002;99:499–510. [Google Scholar]
- Gilg O., Hanski I., Sittler B. Cyclic dynamics in a simple vertebrate predator-prey community. Science. 2003;302:866–868. doi: 10.1126/science.1087509. [DOI] [PubMed] [Google Scholar]
- Gilg O., Sittler B., Hanski I. Climate change and cyclic predator-prey population dynamics in the high-Arctic. Glob. Chang. Biol. 2009;15:2634–2652. [Google Scholar]
- Gilg O., Sittler B., Sabard B., Hurstel A., Sané R., Delattre P., Hanski I. Functional and numerical responses of four lemming predators in high arctic Greenland. Oikos. 2006;113:196–213. [Google Scholar]
- Hakkarainen H., Huhta E., Koskela E., Mappes T., Soveri T., Suorsa P. Eimeria-parasites are associated with a lowered mother's and offspring's body condition in island and mainland populations of the bank vole. Parasitology. 2007;134:23–31. doi: 10.1017/S0031182006001120. [DOI] [PubMed] [Google Scholar]
- Haukisalmi V., Hardman L.M., Hardman M., Rausch R.L., Henttonen H. Molecular systematics of the holarctic anoplocephaloides variabilis (douthitt, 1915) complex, with the proposal of Microcephaloides n g (cestoda : anoplocephalidae) Syst. Parasitol. 2008;70:15–26. doi: 10.1007/s11230-008-9129-7. [DOI] [PubMed] [Google Scholar]
- Haukisalmi V., Henttonen H. Biogeography of helminth parasitism in lemmus link (arvicolinae), with the description of paranoplocephala fellmani n. sp. (cestoda: anoplocephalidae) from the Norwegian lemming L. Lemmus (linnaeus) Syst. Parasitol. 2001;49:7–22. doi: 10.1023/a:1010778504559. [DOI] [PubMed] [Google Scholar]
- Haukisalmi V., Wickström L.M., Hantula J., Henttonen H. Taxonomy, genetic differentiation and holarctic biogeography of paranoplocephala spp. (cestoda: anoplocephalidae) in collared lemmings (Dicrostonyx; arvicolinae) Biol. J. Linn. Soc. 2001;74:171–196. [Google Scholar]
- Haukisalmi V., Wickström L.M., Henttonen H., Hantula J., Gubányi A. Molecular and morphological evidence for multiple species within Paranoplocephala omphalodes (Cestoda, Anoplocephalidae) in Microtus voles (Arvicolinae) Zool. Scripta. 2004;33:277–290. [Google Scholar]
- Henttonen H., Kaikusalo A. Lemming movements. In: Stenseth N.C., Ims R.A., editors. The Biology of Lemmings. Academic Press; London: 1993. pp. 157–186. [Google Scholar]
- Hirakawa H. Coprophagy in leporids and other mammalian herbivores. Mamm. Rev. 2001;31:61–80. [Google Scholar]
- Holmstad P.R., Hudson P.J., Skorping A. The influence of a parasite community on the dynamics of a host population : a longitudinal study on willow ptarmigan and their parasites. Oikos. 2005;2:377–391. [Google Scholar]
- Ims R.A., Fuglei E. Trophic interaction cycles in Tundra ecosystems and the impact of climate change. Bioscience. 2005;55:311–322. [Google Scholar]
- Irvine R.J. Parasites and the dynamics of wild mammal populations. Anim. Sci. 2006;82:775–781. [Google Scholar]
- Jeston P.J., Blight G.W., Anderson G.R., Molloy J.B., Jorgensen W.K. Comparison of infectivity of Eimeria tenella oocysts maintained at 4, 12 or 28 degrees C for up to 10 months. Aust. Vet. J. 2002;80:91–92. [PubMed] [Google Scholar]
- Kausrud K.L., Mysterud A., Steen H., Vik J.O., Ostbye E., Cazelles B., Framstad E., Eikeset A.M., Mysterud I., Solhoy T., Stenseth N.C. Linking climate change to lemming cycles. Nature. 2008;456:93–97. doi: 10.1038/nature07442. [DOI] [PubMed] [Google Scholar]
- Kavaliers M., Colwell D.D. Parasite infection attenuates nonopoid mediated predator-induced analgesia in mice. Physiol. Behav. 1994;55:505–510. doi: 10.1016/0031-9384(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Klemola T., Pettersen T., Stenseth N.C. Trophic interactions in population cycles of voles and lemmings: a model-based synthesis. Adv. Ecol. Res. 2003;33:75–160. [Google Scholar]
- Koch L., Haller J. Geological map of East Greenland 72°-76° N. Lat. Meddelelser om Grønland. 1971;183:1–26. (+ 13 maps) [Google Scholar]
- Korpimäki E., Brown P.R., Jacob J., Pech R.P. The puzzles of population cycles and outbreaks of small mammals solved. Bioscience. 2004;54:1071–1079. [Google Scholar]
- Laakkonen J., Haukisalmi V., Niemimaa J., Henttonen H. Parasite diversity of Norwegian lemmings (Lemmus lemmus) J. Zool. Lond. 2001;253:549–553. [Google Scholar]
- Laakkonen J., Henttonen H. Ultrastructure of Frenkelia sp from a Norwegian lemming in Finland. J. Wildl. Dis. 2000;36:362–366. doi: 10.7589/0090-3558-36.2.362. [DOI] [PubMed] [Google Scholar]
- Laakkonen J., Oksanen A., Soveri T., Henttonen H. Dynamics of intestinal coccidia in peak density Microtus agrestis, Microtus oeconomus and Clethrionomys glareolus populations in Finland. Ecography. 1998;21:135–139. [Google Scholar]
- Lagrue C., Kaldonski N., Perrot-Minnot M.J., Motreuil B., Bollache L. Modification of hosts' behavior by a parasite: field evidence for adaptive manipulation. Ecology. 2007;88:2839–2847. doi: 10.1890/06-2105.1. [DOI] [PubMed] [Google Scholar]
- Levine N.D. Eimeria dicrostonicis n. sp., a protozoan parasite of the lemming, and other parasites from arctic rodents. Ill. State Acad. Sci. Trans. 1951;44:205–208. [Google Scholar]
- Maclean S.F., Fitzgerald B.M., Pitelka F.A. Population cycles in arctic lemmings: winter reproduction and predation by weasels. Arctic Alpine Res. 1974;6:1–12. [Google Scholar]
- Maher W.J. Predation by weasels on a winter population of lemmings, banks island, northwest territories. Can. Field Nat. 1967;81:248–250. [Google Scholar]
- May R.M., Anderson R.M. Regulation and stability of host-parasite population interactions. 2. Destabilizing processes. J. Anim. Ecol. 1978;47:249–267. [Google Scholar]
- Mazerolle M.J. 2017. AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c). R Package Version 2. 1-1. [Google Scholar]
- Møller A.P. Parasitism and the regulation of host populations. In: Thomas F., Renaud F., Guegan J.-F., editors. Parasitism and Ecosystems. Oxford University Press; Oxford: 2005. pp. 43–53. [Google Scholar]
- Neuhaus P. Parasite removal and its impact on litter size and body condition in Columbian ground squirrels (Spermophilus columbianus) Proc. R. Soc. B-Biol. Sci. 2003;270:S213–S215. doi: 10.1098/rsbl.2003.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newey S., Thirgood S.J., Hudson P.J. Do parasite burdens in spring influence condition and fecundity of female mountain hares Lepus timidus? Wildl. Biol. 2004;10:171–176. [Google Scholar]
- Newman C., Macdonald D.W., Anwar M.A. Coccidiosis in the European badger, Meles meles in Wytham Woods: infection and consequences for growth and survival. Parasitology. 2001;123:133–142. doi: 10.1017/s0031182001008265. [DOI] [PubMed] [Google Scholar]
- Oksanen L., Oksanen T. The logic and realism of the hypothesis of exploitation ecosystems. Am. Nat. 2000;155:703–723. doi: 10.1086/303354. [DOI] [PubMed] [Google Scholar]
- Olsen O.W. University Park Press; Baltimore: 1974. Animal Parasites: Their Life Cycles and Ecology. [Google Scholar]
- Pellerdy L.P. Akademiai Kiadó; Budapest: 1974. Coccidia and Coccidiosis. Budapest. [Google Scholar]
- Poulin R. second ed. Princeton University Press; Princeton and Oxford: 2007. Evolutionary Ecology of Parasites. [Google Scholar]
- Poulin R., Morand S. The diversity of parasites. Q. Rev. Biol. 2000;75:277–293. doi: 10.1086/393500. [DOI] [PubMed] [Google Scholar]
- Quinn S.C., Brooks R.J., Cawthorn R.J. Effects of the protozoan parasite Sarcocystis raushorum on open-field behavior of its intermediate vertebrate host, Dicrostonyx richardsoni. J. Parasitol. 1987;73:265–271. [PubMed] [Google Scholar]
- R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2017. R Version 3.3.3: A Language and Environment for Statistical Computing. [Google Scholar]
- Rausch R. Studies on the Helminth fauna of Alaska. XI. Helminth parasites of Microtine rodents - taxonomic considerations. J. Parasitol. 1952;38:415–444. [PubMed] [Google Scholar]
- Redpath S.M., Mougeot F., Leckie F.M., Elston D.A., Hudson P.J. Testing the role of parasites in driving the cyclic population dynamics of a gamebird. Ecol. Lett. 2006;9:410–418. doi: 10.1111/j.1461-0248.2006.00895.x. [DOI] [PubMed] [Google Scholar]
- Reiman D.A., Schmidt T.M., Gajadhar A., Sogin M., Cross J., Yoder K., Sethabutr O., Echeverria P. Molecular phylogenetic analysis of cyclospora, the human intestinal pathogen, suggests that it is closely related to Eimeria species. J. Infect. Dis. 1996;173:440–445. doi: 10.1093/infdis/173.2.440. [DOI] [PubMed] [Google Scholar]
- Richard Y. Université de Bourgogne; Dijon: 2012. Détermination du statut parasitaire de trois populations de lemming à collier en relation avec leurs densités. [Google Scholar]
- Schmidt N.M., Berg T.B., Forchhammer M., Hendrichsen D.K., Kyhn L.A., Meltofte H., Høye T.T. Vertebrate predator-prey interactions in a seasonal environment. Adv. Ecol. Res. 2008;40:345–370. [Google Scholar]
- Schmidt N.M., Ims R.A., Høye T.T., Gilg O., Hansen L.H., Hansen J., Lund M., Fuglei E., Forchhammer M.C., Sittler B. Response of an arctic predator guild to collapsing lemming cycles. Proc. Biol. Sci. 2012;279:4417–4422. doi: 10.1098/rspb.2012.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt Pedersen P. Scott Polar Research Institute; Cambridge: 2008. North-east Greenland 1908-1960: the Trapper Era. [Google Scholar]
- Simeonovska-Nikolova D.M., Golemansky V.G. Behavioural response of mound-building mouse, Mus spicilegus (Petenyi, 1882) to coccidian parasites (Eucoccidia:Eimeriidae) Comptes Rendus Acad. Bulg. Sci. 2015;68:1387–1392. [Google Scholar]
- Sittler B. Response of stoat (Mustela erminea) to a fluctuating lemming (Dicrostonyx groenlandicus) population in North East Greenland: preliminary results from a long term study. Ann. Zool. Fenn. 1995;32:79–92. [Google Scholar]
- Smith M.J., White A., Sherratt J.A., Telfer S., Begon M., Lambin X. Disease effects on reproduction can cause population cycles in seasonal environments. J. Anim. Ecol. 2008;77:378–389. doi: 10.1111/j.1365-2656.2007.01328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soininen E.M., Gauthier G., Bilodeau F., Berteaux D., Gielly L., Taberlet P., Gussarova G., Bellemain E., Hassel K., Stenøien H.K., Epp L., Schrøder-Nielsen A., Brochmann C., Yoccoz N.G. Highly overlapping winter diet in two sympatric lemming species revealed by DNA metabarcoding. PLoS One. 2015;10 doi: 10.1371/journal.pone.0115335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulsby E.J.L. seventh ed. Lea and Febiger; Philadelphia: 1982. Helminths, Arthropods and Protozoa of Domesticated Animals. [Google Scholar]
- Stenseth N.C., Ims R.A. Academic Press; London: 1993. The Biology of Lemmings. [Google Scholar]
- Telfer S., Begon M., Bennett M., Bown K.J., Burthe S., Lambin X., Telford G., Birtles R. Contrasting dynamics of Bartonella spp. in cyclic field vole populations: the impact of vector and host dynamics. Parasitology. 2007;134:413–425. doi: 10.1017/S0031182006001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer S., Lambin X., Birtles R., Beldomenico P., Burthe S., Paterson S., Begon M. Species interactions in a parasite community drive infection risk in a wildlife population. Science. 2010;330:243–246. doi: 10.1126/science.1190333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therrien J.F., Gauthier G., Korpimaki E., Bety J. Predation pressure by avian predators suggests summer limitation of small-mammal populations in the Canadian Arctic. Ecology. 2014;95:56–67. doi: 10.1890/13-0458.1. [DOI] [PubMed] [Google Scholar]
- Turchin P. Princeton University Press; Princeton: 2003. Complex Population Dynamics: a Theoritical/Empirical Synthesis. [Google Scholar]
- Turchin P., Oksanen L., Ekerholm P., Oksanen T., Henttonen H. Are lemmings prey or predators? Nature. 2000;405:562–565. doi: 10.1038/35014595. [DOI] [PubMed] [Google Scholar]
- Turner A.K., Beldomenico P.M., Bown K., Burthe S.J., Jackson J.A., Lambin X., Begon M. Host–parasite biology in the real world: the field voles of Kielder. Parasitology. 2014;141:997–1017. doi: 10.1017/S0031182014000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Baalen M., Sabelis M.W. The dynamics of multiple infection and the evolution of virulence. Am. Nat. 1995;146:881–910. [Google Scholar]
- VanVuren D. Ectoparasites, fitness, and social behaviour of yellow-bellied marmots. Ethology. 1996;102:686–694. [Google Scholar]
- Vorisek P., Votypka J., Zvara K., Svobodova M. Heteroxenous coccidia increase the predation risk of parasitized rodents. Parasitology. 1998;117:521–524. doi: 10.1017/s0031182098003242. [DOI] [PubMed] [Google Scholar]
- Walker D.A., Raynolds M.K., Daniëls F.J.A., Einarsson E., Elvebakk A., Gould W.A., Katenin A.E., Kholod S.S., Markon C.J., Melnikov E.S., Moskalenko N.G., Talbot S.S., Yurtsev B.A. The Circumpolar Arctic vegetation map. J. Veg. Sci. 2005;16:267–282. [Google Scholar]
- Watson M.J. What drives population-level effects of parasites? Meta-analysis meets life-history. Int. J. Parasitol.: Parasites Wildl. 2013;2:190–196. doi: 10.1016/j.ijppaw.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickström L.M., Hantula J., Haukisalmi V., Henttonen H. Genetic and morphometric variation in the Holarctic helminth parasite Andrya arctica (Cestoda, Anoplocephalidae) in relation to the divergence of its lemming hosts (Dicrostonyx spp.) Zool. J. Linn. Soc. 2001;131:443–457. [Google Scholar]
- Yun C.H., Lillehoj H.S., Lillehoj E.P. Intestinal immune responses to coccidiosis. Dev. Comp. Immunol. 2000;24:303–324. doi: 10.1016/s0145-305x(99)00080-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.