Abstract
Study Objectives
Sleep disturbances and sleep apnea are associated with increased vulnerability to age-related disease, altering molecular pathways affecting biological aging. Telomere length captures one component of biological aging. We evaluated whether objectively assessed sleep and sleep apnea relate to leukocyte telomere length (LTL) in the Multi-Ethnic Study of Atherosclerosis (MESA).
Methods
Men and women aged 44–84 years (n = 672) from the MESA Stress and MESA Sleep studies underwent polysomnography and 7 day actigraphy (at Exam 5) and assessment of LTL (at baseline [Exam 1] and about 10 years later [Exam 5]).
Results
General linear models adjusting for age, sex, race/ethnicity, BMI, physical activity, and smoking found that severe obstructive sleep apnea (OSA; apnea–hypopnea index > 30) was cross-sectionally associated with shorter LTL (p = 0.007). Modest associations of shorter LTL with less rapid eye movement sleep, more stage 1 sleep, wake after sleep onset >30 min, and long sleep duration were found, but these effects were diminished after adjusting for lifestyle and OSA. Exploratory analyses found that higher arousal index at Exam 5 was associated with greater LTL decline over the prior 10 years (p = 0.004).
Conclusions
OSA was associated with shorter LTL. Individuals with high-arousal frequency had greater leukocyte telomere attrition over the prior decade. These findings suggest that sleep apnea and sleep fragmentation are associated with accelerated biological aging.
Keywords: biomarkers, aging, obstructive sleep apnea, psychoneuroimmunology, actigraphy, epidemiology, telomere length, sleep
Statement of Significance.
Severe obstructive sleep apnea (OSA) was related to shorter leukocyte telomere length (LTL). When compared with individuals without severe OSA, average LTL in those with OSA was comparable to telomere length differences of 10 years of immune cell aging. Exploratory analyses show that higher cortical arousals at night was related to greater LTL attrition over the prior decade. This study provides evidence that OSA and associated sleep fragmentation are associated with biological aging. Future research should confirm and further dissect the causal mechanisms linking sleep disturbances to biological aging and address whether improving sleep may attenuate accelerated aging and incidence of age-related chronic disease.
Introduction
Sleep disturbances may accelerate age-related disease. Disruption of sleep continuity, frequent arousals, and poor sleep quality are associated with increased vulnerability to declines in physical and mental functioning, predict age-related disease morbidity and mortality risk [1–8], and alter molecular pathways related to inflammation and biological aging [9, 10]. Mechanistically, these associations are supported by experimental studies showing that disruption of sleep results in increased cellular stress responses [11–13] and cellular injury, including DNA damage [14, 15], and increased expression of a marker of aging, p16INK4a [16]. Similar effects have been observed in animal models in response to experimental hypoxemia [17, 18], a cardinal feature of obstructive sleep apnea (OSA). OSA is characterized by recurrent apneas and hypopneas associated with both sleep fragmentation and hypoxemia, and there is evidence that OSA may accelerate aging via these pathways [19]. Likewise, OSA has been shown to be associated with an increased prevalence or incidence of cardiometabolic diseases as well as increased all-cause cardiovascular disease (CVD)-specific and cancer-specific mortality rates [20–23], possibly through elevations in inflammation and oxidative stress [24–28], which drive biological aging [29]. However, epidemiological studies have had scant data on molecular markers of aging, limiting the understanding of how objective measures of sleep, including OSA, sleep duration, and sleep fragmentation may relate to biological aging in community samples.
Telomere length, a hallmark of aging, captures one component of the biological aging process [10, 29–33]. Telomeres are DNA-protein complexes with repeat sequence of DNA and associated proteins at the end of chromosomes that protect coding DNA from being lost during cell division. Critically short telomeres initiate cell senescence, a process thought to drive cellular aging [32, 34]. These senescent cells are a source of inflammatory factors, contributing to a rise in inflammation with increasing age [10, 30, 33, 34]. Telomeric ends shorten over the lifespan [35–38], with epidemiological evidence suggesting an association of shorter leukocyte telomere length (LTL) with increasing chronological age [39–41]. Furthermore, LTL serves as a biomarker of cellular aging of the hematopoietic stem cells and circulating lymphocytes [42, 43], and shorter LTL predicts age-related diseases such asCVD [44, 45], earlier mortality [46–51], and in some cases elevated risk for cancer incidence [52, 53].
Several cross-sectional findings have reported OSA to be associated with shorter LTL [19, 24, 54–58], although most studies used questionnaire-based assessments of OSA [54, 55] or case–control designs [24, 56–58]. A recent meta-analysis of this literature reported shorter LTL in OSA cases compared with controls across 6 of 8 studies reviewed, with an estimated mean difference of −.03 (CI = −.06, −.01). As noted by the authors, these estimates were limited by study heterogeneity and a predominance of data from case–control study designs [59]. Likewise, cross-sectional analyses have found that self-reported short sleep duration and poor sleep quality associated with shorter LTL, although findings vary, with the majority of reports not including careful evaluation for co-morbid OSA [60–66], which might account for these effects. In contrast, we recently reported that the diagnosis of insomnia, without OSA, was associated with significant declines in LTL among older individuals [67]. However, further research that objectively examines multiple dimensions of sleep and addresses both OSA and objective measures of sleep continuity and sleep architecture, along with insomnia symptoms, is needed to better understand the relationship between sleep disturbances and biological aging.
In an ethnically diverse sample of men and women aged 45–84 (n = 672) at baseline, the current study addresses these gaps by objectively assessing sleep duration, sleep fragmentation, sleep continuity, sleep architecture, and OSA as they relate to LTL measured at the same time. Furthermore, given that LTL was also measured a decade earlier, this study explores whether these sleep parameters are associated with LTL attrition over the prior decade.
Methods
Study design
A comprehensive review of the study design of Multi-Ethnic Study of Atherosclerosis (MESA) has been published elsewhere [68]. Designed as a prospective study to examine risk factors for the development of clinical CVD in a multiethnic community-based sample, a total of 6814 men and women (45–84 years of age) free of known CVD at the time of enrollment were recruited from six communities across the United States between 2000 and 2002, with subsequent exams every 2 years. Exam 1 occurred in 2000–2002, and Exam 5 (9–11 years after the first visit) occurred between 2010 and 2013. MESA Stress, an ancillary study, recruited African American, Caucasian, and Hispanic individuals (excluded Asian American) from the MESA cohort from three of the sites (Baltimore, MA; Los Angeles, CA; and New York) to examine the influence of stress on CVD [69]. MESA Stress included the assessment of LTL from Exam 1 on 978 participants. Subsequent DNA samples were collected from MESA Stress participants during the Exam 5 visit. Both DNA samples derived from Exam 1 and Exam 5 were assayed for LTL in one batch in the laboratory at UCSF, to provide consistency across exams. Initial sociodemographic factors as they relate to LTL change in MESA Stress has been reported previously [70]. A second ancillary study, MESA Sleep, was also performed on a subset of the MESA cohort. For MESA Sleep, participants in MESA Exam 5 who did not report regular use of oral devices, nocturnal oxygen, or nightly positive airway pressure (PAP) devices were invited to participate. Recruitment details, protocol, and screening can be found elsewhere [71]. Of the 4077 participants invited initially, 2261 completed the sleep exam. The in-home examination included overnight polysomnography (PSG), 7 consecutive days of wrist actigraphy, and the administration of several validated sleep questionnaires. MESA Sleep participants with available LTL data from either Exam 1 or Exam 5 comprise 717 men and women, including 43.9% Hispanic, 29.3% African American, and 26.8% Caucasian. Individuals who have LTL data at both Exam 1 and Exam 5 include 672 participants (44.7% Hispanic, 29.2% African American, and 26.1% Caucasian) of which actigraphy data are available on 631, and PSG recordings on 616. Informed consent was obtained from all participants and all study procedures were approved by the Institution Review Board at each participating institution.
Measures
Polysomnography
As detailed previously [71], at home PSG was performed using a 15-channel PSG portable monitor (Somte, Compumedics, Abbotsville, AU) with recording of electroencephalography (EEG), electrooculography (EOG), chin electromyography (EMG), electrocardiography (ECG), abdominal and thoracic respiratory inductance plethysmography, airflow (by thermocouple and nasal pressure), finger pulse oximetry, and bilateral limb movements (piezoelectric sensors). Studies were scored blinded to all other data by trained research polysomnologists following published guidelines [72], generating summary measurements of overnight hypoxemia, apneas, hypopneas, limb movements, sleep stages, and arousals [73, 74].
Sleep architecture data were used to quantify four variables: (1) % sleep time spent in rapid eye movement sleep (NREM); (2) % time spent in stage 1 sleep (N1); (3) % time spent in slow-wave sleep (SWS or N3); (4) the Arousal Index, the average number of EEG cortical arousals, defined as abrupt changes in EEG frequency lasting ≥3 s and preceded by at least 10 s of sleep, per hour of sleep (see scoring guidelines for further detail [75]). Given skewed distributions, variables were recoded into dummy variables with risk category (coded = 1) as follows: highest quartiles for % Stage 1; highest quartile of the Arousal Index; lowest quartiles for % REM; and lowest quartile for % SWS.
OSA was characterized using the apnea–hypopnea index (AHI), representing the average number of apneas plus hypopnea events, each associated with a ≥4% desaturation, per hour of sleep. A three-category measure was created to define OSA severity using conventional criteria: <15 was classified as no or mild OSA, 15–30 was classified as moderate OSA, and greater than 30 as severe OSA. Overnight hypoxemia was quantified as the percentage of sleep time spent in <90% oxygen saturation, with 5% or greater identifying approximately the highest quintile of hypoxic exposure during sleep (code = 1).
7 Day actigraphy
Actiwatch Spectrum wrist actigraphs (Philips Respironics, Murrysville, PA) were given to participants to wear for 7 consecutive days on their nondominant wrist. Data were collected in 30 s epochs and scored as sleep or wake by Actiware-Sleep version 5.59 software (Mini-Mitter Co, Inc, Bend, OR). Additional details of the scoring methods can be found elsewhere [71]. Briefly, data derived from the actigraphy device included objectively defined: wake after sleep onset (WASO) > 30 min vs 30 or less; sleep maintenance efficiency (SME; the proportion of the period between sleep onset and awakening spent asleep) of <85% vs. 85% or more; and sleep duration, with short, below normal, normal or long sleep defined as <6, 6–7, >7–8, and >8 hr, respectively, as was reported previously [71].
Self-report scales
The Women’s Health Initiative Insomnia Rating Scale (WHIIRS) assesses multiple dimensions of self-reported sleep disturbances [76]. The scale produces an overall sleep disturbances score ranging from 0 to 20, higher scores reflect sleep disturbance, and a WHIIRS score cutoff of >10 indicates significant sleep disturbance [76, 77]. Sleepiness was determined from the Epworth Sleepiness Scale (ESS) [78] and captures self-perceived sleepiness experienced in various common settings. ESS was scored with >10–16 as moderate sleepiness, >16 as clinical impairment, and ≤10 as the reference group.
Covariates
Covariates in adjusted models include chronological age in years, sex, race/ethnicity (i.e. African American, Caucasian, and Hispanic), time of sample collection relative to date of assay, body mass index (BMI; kg/m2), physical activity (estimated as the metabolic equivalent to task (MET) for moderate and vigorous activity reported, as described previously) [68, 79, 80] and smoking (nonsmoker; pack years < 10; pack years ≥ 10). In models examining the relationship of sleep duration, sleep disturbances, and sleep architecture with LTL, we additionally adjust for OSA. Educational attainment, perceived stress scale (PSS) [81], and the Center for Epidemiologic Studies–Depression (CES-D) [82] were used in models to control for educational attainment, current stress, and depressive symptoms.
Leukocyte telomere length in MESA Exam 1 and Exam 5
LTL was determined by a standard assay protocol. Supplementary Material provides extensive details. Assay methods were employed to determine relative LTL adapted from Cawthon [83] and reported previously [64]. Briefly, using a standardized real-time quantitative polymerase chain reaction (qPCR) methodology, matching subject samples from each time point were run together on the same plate, following previously published protocol [64]. Estimated concentration of the telomere (T) repeat and single copy (S) gene is estimated using the standard curve method and then used to calculate the T/S value. The interassay CV is 2.9% ± 2.1%. Lab personnel were blind to patient information.
Statistical analyses
Descriptive data on Exam 5 data are reported followed by analyses of cross-sectional associations of demographics, health parameters, and sleep parameters with LTL at Exam 5. General linear models examined associations of sleep parameters with Exam 5 LTL; Model 1 includes age at visit, sex, race/ethnicity, time of sample collection relative to date of assay, and study site. Model 2 included Model 1 covariates and also adjusted for BMI, physical activity (MET for moderate and vigorous activity) and smoking history. Model 3 adjusted for model 2 covariates and OSA (AHI categories) for models other than those testing AHI/hypoxemia. Next, to explore correlates of change in LTL attrition over the two time points, mixed linear models examined associations of sleep parameters with LTL over time by modeling the two repeat measures of LTL (Exam 1 and Exam 5) as a function of time since baseline, sleep parameters at Exam 5 (treated as a time invariant factor), and interactions between time since baseline and sleep parameters and covariates. Covariates were either time invariant (e.g. sex) or time varying (e.g. age, BMI, and physical activity). We included a random intercept for each person and each site. The sleep-related predictor of interest was entered into each model (tested individually). Unstandardized beta coefficients (B) and standard error (SE) are reported. Interactions between time since baseline and sleep parameters were followed with the least significant difference (LSD) contrast test of means when interactions were p < 0.10. Sensitivity analyses were done to test for the need to additionally adjust for time-varying presence of diabetes and high blood pressure, and for concurrent stress, depressive symptoms, and education, but point estimates were not appreciably influenced by the addition of these health factors.
Results
Descriptive statistics and unadjusted cross-sectional results
Descriptive statistics for the entire sample are shown in Table 1, including demographics, lifestyle, and sleep variables. At Exam 5, Hispanic ethnicity and low physical activity were associated with shorter LTL. In unadjusted analysis of Exam 5 LTL, severe OSA (AHI > 30) and higher percentage time in <90% desaturation were associated with shorter LTL at Exam 5. Lower percentage of time in rapid eye movement (REM) sleep (lowest quartile vs. all others), higher percentage of N1 sleep (highest quartile vs. all others), and high arousal index (highest quartile vs. all others) were also significantly associated with shorter LTL at Exam 5. Modest associations were present with actigraphy-based SME (<85%) and long sleep (>8 hr) with shorter LTL at Exam 5, but these did not reach statistical significance (p < 0.10). Self-report measures of sleep were also examined including the WHIIRS and the Epworth Sleepiness Scale, but were not significantly related to LTL (data shown in Supplementary Table S1).
Table 1.
Descriptive statistics on demographics, lifestyle, and sleep measures and unadjusted estimates of LTL at Exam 5
| Exam 5 | ||
|---|---|---|
| Variable | Mean(SD) [range] or % | Unadjusted estimates of LTL B(SE), p value |
| Age, years | 69.4(9.2) [54–93] | −.004(.001), p < 0.001 |
| Sex, male | 46.5% | −.03(.01), p = 0.01 |
| Race/ethnicity | ||
| African American* | 29.6% | .014(.012), p = 0.23 |
| Caucasian | 26.4% | .016(.012), p = 0.19 |
| Hispanic | 43.9% | −.024(.01), p = 0.02 |
| BMI, kg/m2 | 29.4(5.4) | −.001(.001), p = 0.30 |
| Smoking† | ||
| Nonsmoker | 55.1% | Ref |
| Pack years < 10 | 18.3% | −.01(.014), p = 0.47 |
| Pack years ≥ 10 | 26.6% | −.026(.013), p = 0.04 |
| Physical activity (MET) Lowest quartile |
−.037(.01), p = 0.002 | |
| OSA and hypoxia Exam 5 | ||
| OSA | ||
| AHI ≤15 (ref) | 68.5% | Ref |
| >15–30 | 16.3% | −.014(.015), p = 0.36 |
| >30 | 15.2% | −.041(.019), p = 0.04 |
| Nocturnal hypoxemia‡ | 19.9% | −.04(.014), p = 0.003 |
| Sleep architecture Exam 5§ | ||
| % REM (lowest quartile) | [0–14.2] | −.031(.013), p = 0.01 |
| % N1 (highest quartile) | [17.9–79.3] | −.04(.013), p = 0.002 |
| % SWS (lowest quartile) | [0–1.32] | −.02(.012), p = 0.098 |
| Arousal index (highest quartile) | [27–86] | −.04(.013), p = 0.001 |
| Actigraphy-based measures Exam 5 | ||
| Wake after sleep onset (WASO) > 30 min | 68.5% | −.015(.012), p = 0.19 |
| Sleep maintenance efficiency (SME) < 85% | 23% | −.031(.018), p = 0.08 |
| Sleep duration | ||
| Short sleep, <6 hr | 33.5% | −.015(.014), p = 0.29 |
| Below normal, 6–7 hr | 32.5% | −.009(.014), p = 0.51 |
| Normal sleep, >7–8 hr (ref) | 24.1% | Ref |
| Long sleep, >8 hr | 9.9% | −.033(.02), p = .098 |
*Race/ethnicity variables entered as African American vs non-African American, Caucasian vs. non-Caucasian, and Hispanic vs. non-Hispanic.
†10 Pack year is equivalent to smoking 1 pack a day for 10 years.
‡Nocturnal hypoxemia is present when 5% or greater percentage of sleep time spent in <90% oxygen saturation.
§Range within the quartile (lowest or highest).
Adjusted cross-sectional results
Obstructive sleep apnea and overnight hypoxemia
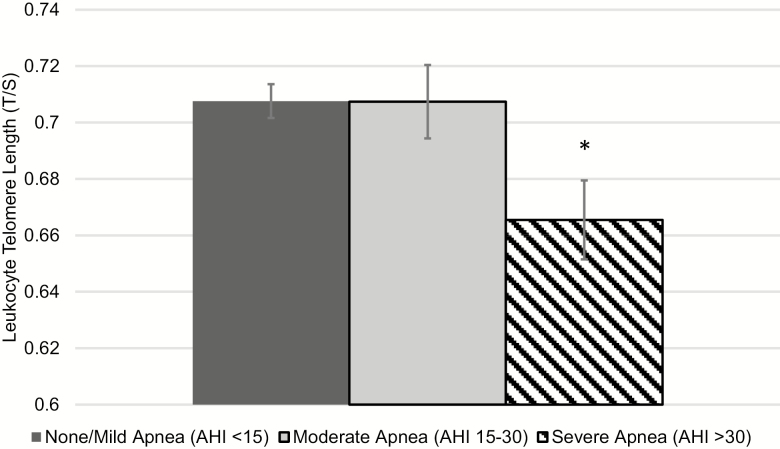
After controlling for age, sex, and race/ethnicity, we found that severe OSA (AHI > 30) was associated with shorter Exam 5 LTL (p = 0.001; Table 2); adjustment for BMI, smoking, and physical activity did not attenuate the association (p = 0.007; Figure 1; d-metric effect size = .29). Sensitivity analyses also adjusted for education, perceived stress, and depressive symptoms, but these factors did not attenuate the association between OSA and LTL (p = 0.004). Nocturnal hypoxemia was also associated with shorter Exam 5 LTL (p = 0.02), but this association was no longer significant after adjustment for BMI, smoking, and physical activity (p = 0.12).
Table 2.
Linear regression analysis of the independent effect of each sleep parameters on leukocyte telomere length at Exam 5
| Predictor | Model 1 Adjusting for age, sex, race/ethnicity, at Exam 5 |
Model 2 Adjusting for Model 1 and BMI, smoking, physical activity |
Model 3 Adjusting for Models 1 and 2 and sleep apnea |
|---|---|---|---|
| Sleep parameter* | B(SE), p value | B(SE), p value | B(SE), p value |
| OSA and hypoxia | |||
| OSA | |||
| AHI ≤ 15 (ref) | – | – | NA |
| >15–30 | −.004(.0143), .77 | .001(.0144), .99 | NA |
| >30 | −.048(.0149), .001 | −.042(.0155), .007 | NA |
| Nocturnal hypoxemia† | −.001(.0005), .020 | −.001(.0005), .12 | NA |
| Sleep architecture | |||
| % REM (Lowest Quartile) | −.021(.0120), .086 | −.017(.0121), .15 | −.011(.0122), .38 |
| % N1 (Highest Quartile) | −.023(.0125), .068 | −.017(.0125), .18 | −.005(.0131), .69 |
| % SWS, Stages 3–4 (Lowest Quartile) | −.009(.0124), .46 | −.007(.0124), .57 | −.002(.0124), .85 |
| Arousal index (Highest Quartile) | −.031(.0122), .012 | −.027(.0124), .026 | −.014(.0136), .32 |
| Actigraphy-assessed sleep | |||
| WASO, actigraphy >30 min | −.021(.0118), .079 | −.021(.0117), .067 | −.022(.0122), .066 |
| SME, actigraphy <85% | −.026(.0162), .11 | −.020(.0162), .21 | −.017(.0166), .30 |
| Sleep duration | |||
| Short sleep, <6 hr | −.011(.0148), .48 | −.005(.0149), .74 | .005(.0154), .75 |
| Below normal, 6–7 hr | −.013(.0138), .34 | −.009(.0191), .53 | −.005(.0142), .71 |
| Normal sleep, >7–8 hr (ref) | – | – | – |
| Long sleep, >8 hr | −.037(.0189), .052 | −.042(.0191), .027 | −.028(.0200), .16 |
*Each sleep parameter was entered as a predictor in separate models.
†Nocturnal hypoxemia is present when 5% or greater percentage of sleep time spent in <90% oxygen saturation.
Figure 1.
Estimated mean and standard error of leukocyte telomere length at Exam 5 by polysomnography determined sleep apnea (AHI score). Estimated mean LTL adjusting for age, sex, race, site, baseline LTL, sleep apnea, obesity, smoking, physical activity, and sample collection time. Statistical tests performed using linear regression. *p < 0.05, none/mild apnea vs. severe apnea.
To explore whether the finding of shorter LTL in those with AHI > 30 persisted after separately adjusting for the arousal index, sleep duration, and nocturnal hypoxemia, models were run entering each separately. Adjusting for the arousal index, OSA (AHI > 30) remained associated with LTL, B(SE) = −0.043(.02), p = 0.01. Similarly, association of severe OSA with LTL at Exam 5 remained after adjusting for either sleep duration [B(SE) = −0.039(.02), p = 0.02] or for nocturnal hypoxemia [B(SE) = −0.038(.02), p = 0.02].
Sleep architecture and sleep fragmentation
Adjusting for age, sex, and race/ethnicity, we found that lower % time in REM (lowest quartile compared with top three quartiles) was not significantly related to shorter LTL at Exam 5 (p = 0.09); adjustment for BMI, physical activity, and smoking further attenuated this association (p = 0.15). Adjustment for OSA further reduced the association between REM time and LTL (p = 0.38), suggesting that health factors, including OSA, explained the association between LTL and lower amounts of REM sleep (Table 2). Additional analyses examined other measures of sleep architecture. In age, sex, and race/ethnicity-adjusted models, percentage time in N1 was not significantly associated with Exam 5 LTL (p = 0.07), and this association was reduced after adjustment for BMI, smoking, and physical activity (p = 0.18), and OSA status (p = 0.38). SWS was unrelated to LTL in adjusted models. Adjusting for age, sex, and race/ethnicity, the arousal index (highest quartile compared with the lowest three quartiles) was associated with shorter LTL at Exam 5 (p = 0.01); this association remained after adjusting for BMI, smoking, and physical activity (p = 0.03). However, this association of the arousal index with LTL became nonsignificant after adjusting for OSA (AHI > 30; p = 0.32).
Actigraphy-estimated sleep associations
General linear model analyses adjusting for age, sex, and race/ethnicity showed no significant association between greater time spent awake after falling asleep (WASO) with shorter LTL at Exam 5 (p = 0.08; Table 2). Models 2 and 3 adjustment of BMI, physical activity, smoking, and OSA did not appreciably change this trend (p = 0.07). Age, sex, and race/ethnicity-adjusted models of sleep duration found a suggestive relationship with long sleep duration, suggesting shorter LTL at Exam 5 in those with long sleep duration (p = 0.05) when compared with normal sleep duration, which was significant after adjusting for BMI, smoking, and physical activity (p = 0.03), but did not retain significance after adjustment for OSA (p = 0.16). SME was not significantly related to Exam 5 LTL.
Questionnaire measures of sleep and LTL at Exam 5
Models controlling for age, sex, and race/ethnicity found no significant relationships of self-reported insomnia symptoms or sleepiness with LTL at Exam 5 (data not shown).
Sensitivity analyses tested whether relationships of sleep parameters as continuous variables were related to LTL more strongly than categorical values. Overall, results were similar, but none of these reached statistical significance in the adjusted models. Adjusting for comorbidities including diabetes and hypertension did not appreciably influence results.
Exam 1 to Exam 5 LTL change and Exam 5 sleep
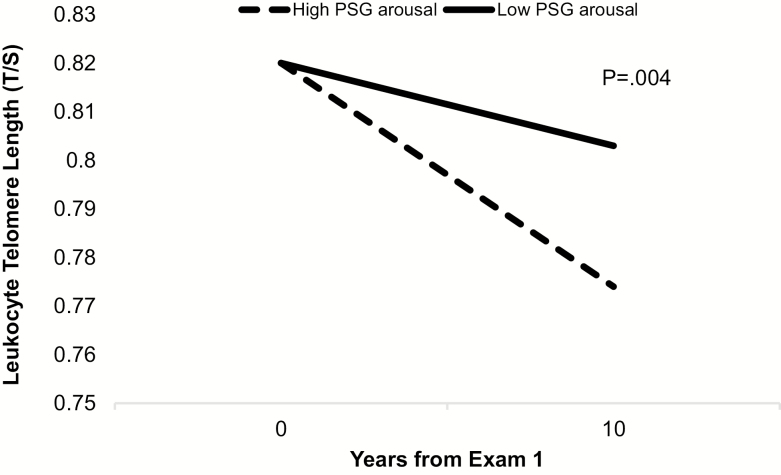
In exploratory analyses, we tested whether the longitudinal change in LTL from Exam 1 to Exam 5 [LTL attrition M = −0.20 (SD = 0.18)] was related to sleep parameters obtained at Exam 5. There were no significant associations between change in LTL between Exams 1 and 5 and AHI category (pinteraction = 0.56) or nocturnal hypoxemia (pinteraction = 0.19) measured at Exam 5. The relationships of WASO, SME, and sleep duration with change in LTL from Exam 1 to Exam 5 also were not significant. In contrast, the arousal index was associated with LTL attrition from Exam 1 to Exam 5 in models adjusting for all covariates, prior to adjusting for OSA (using AHI categories), and continued to be present after adjusting for OSA (AHI categories). The contrast test (LSD) of means at Exam 5 shows shorter LTL in individuals with higher arousal indices when compared with individuals with lower arousal indices (p = 0.004; Cohen’s d = .10; Figure 2; test for interaction between time and arousal index, p = 0.06). Sensitivity analyses testing whether excluding individuals with severe OSA would influence the results revealed no change in the interaction (pinteraction = 0.06), suggesting that the detected differences in LTL change by arousal index score was not driven by individuals with severe OSA. The difference in LTL by arousal remained significant in additional sensitivity analyses adjusted for education, perceived stress, and depressive symptoms (p = 0.03). No other significant relationships of sleep architecture with LTL over time were found (Table 2). In all models, we also tested for the possible interaction of sex and race with sleep and found no significant interaction effects. To estimate the relative magnitude of LTL difference between severe OSA and none to mild OSA as it relates to estimates of average attrition observed with chronological aging, we compared estimates between groups (average LTL in severe OSA was .04 shorter than none to mild OSA), with estimates of mean LTL shortening each year of chronological age of −0.004 (SD = .001). This model estimated that individuals aged 40 would have a telomere .04 longer than individuals aged 50. In other words, the estimated LTL shortening for OSA is comparable to what was estimated for a 10 year age increase in chronological age.
Figure 2.
Estimated mean leukocyte telomere length at year 0 (Exam 1) and year 10 (Exam 5) by polysomnography-determined arousal during the night, high (top quartile) arousal vs. lower (bottom 3 quartiles) arousal. Estimated mean LTL derived after adjusting for age, sex, race, site, baseline LTL, sleep apnea, obesity, smoking, physical activity, and sample collection time. Comparison of means at year 10 between high and low PSG arousal, p = 0.004.
Discussion
In the present sample of racially/ethnically diverse middle aged to late life men and women from multiple cities in the United States, we examined objective and subjective sleep measurements obtained from overnight polysomnography, 7 day actigraphy, and questionnaires, and tested the relationships of OSA, sleep disturbances, and sleep duration with LTL. In cross-sectional analyses, shorter LTL was significantly associated with severe OSA (AHI > 30), overnight hypoxemia, reduced stages N1 and REM sleep, higher arousal index, and long sleep duration in unadjusted analyses. However, in analyses adjusted for age, sex, race/ethnicity, BMI, smoking, and physical activity, the associations with OSA and arousal index persisted, whereas associations of LTL with sleep stages and hypoxemia were attenuated. Further adjustment for OSA in models testing whether sleep duration and arousal index were associated with LTL revealed that OSA explained these associations. The association of LTL with severe OSA remained significant after adjusting for the arousal index, hypoxemia, or sleep duration. Questionnaire-based assessments of sleepiness or insomnia were not associated with LTL in cross-sectional analyses. These findings suggest that severe OSA is associated with accelerated biological aging.
In a large multiethnic sample, we provide several new insights into the association between OSA and shorter LTL. First, we showed that this association was only significant for those defined by severe (AHI > 30) levels of OSA. Second, we showed that this association persisted even after adjusting for demographic and lifestyle factors. Third, associations did not appreciably attenuate in models that individually adjusted for sleep duration, arousal index, or overnight hypoxemia, suggesting that none of these sleep disturbance indices alone mediate this link. Finally, our models estimate that the magnitude of LTL difference between severe OSA and none to mild OSA was equivalent to the average amount of LTL attrition observed over a 10 year period using estimates of T/S decreasing per chronological year from the current cross-sectional analyses. These findings are consistent with prior reports that were questionnaire or medical record based OSA evaluations [54, 55] and or case–control designs [24, 56–58], providing further insights into threshold levels of OSA associated with shorter LTL and address potential confounding by other sleep disturbances. Our findings differ from one recent case–control study that showed longer LTL in moderate-to-severe OSA compared with mild-to-moderate OSA and that did not find differences between the control group and OSA subjects [58]. Differences in study design include a much smaller percentage of females, younger ages of the participants, potential selection biases, and a nonethnically/racially diverse sample in this prior report, all of which may contribute to differences in our results.
In our exploratory analyses examining whether change in LTL from Exam 1 to Exam 5 was related to Exam 5 measures of sleep, the only sleep index that was related to LTL attrition over this prior decade was the arousal index. This analysis showed that individuals with a higher number of cortical arousals had greater LTL attrition over the prior decade of life, an association that was independent of demographics, lifestyle factors, and OSA status. In these exploratory analyses, OSA was not related to LTL change overtime, but this assessment was limited by the lack of objective sleep data from earlier examinations. Overall, these exploratory results suggest that accelerated aging may occur in those who experience greater sleep fragmentation; however, further longitudinal analyses that include assessments of sleep and OSA at both time points are needed to confirm these effects.
Overall, our findings are not entirely consistent with existing reports in cross-sectional analyses of shorter sleep duration and shorter LTL. However, prior reports were based on self-reported short sleep duration, which correlates only modestly with actigraphy estimated sleep [84] and has been reported to over-estimate sleep in some samples [60–62, 65]. Other reasons for the discrepant findings may relate to characteristics of the current sample that differ from previous reports, including its inclusion of middle-aged to older aged men and women recruited from a community sample with racial/ethnic heterogeneity. Our finding of an association between longer actigraphy sleep and shorter LTL, however, is consistent with a large body of literature showing that long sleep duration is a marker for increased mortality and other chronic health problems [85, 86]. Our findings suggest that an association between long sleep duration and accelerated biological aging is partially confounded by OSA, pointing to a possible contribution of OSA in the link between long sleep and health risk.
Mechanisms
Our results link severe OSA with shorter LTL and estimate its effect as equivalent to 10 years of biological aging. OSA is characterized by repeated episodes of breathing disruptions that fragment sleep, cause intermittent drops in oxygen saturation, and trigger activation of the sympathetic nervous system. These stressors, individually or together, have been implicated in OSA-related increased risk for hypertension, diabetes, CVD, cancer, cognitive declines, and mortality [19]. Our findings showing a significant association between severe OSA and shorter LTL are consistent with an adverse effect of severe OSA on numerous physiological systems as described in a recent review [19]. Severe OSA, but not moderate OSA, was linked to shorter LTL, consistent with epidemiological data that demonstrate that more severe levels of OSA are associated with increased risk of stroke and mortality, possibly reflecting effects of more severe sleep disruption and hypoxemia on sympathetic activation and inflammation [87]. Our exploratory analyses also suggest that increased arousals are associated with greater LTL shortening over time. Sleep fragmentation may promote accelerated aging via numerous pathways of biological aging, including effects on sympathetic nervous system, cortisol, inflammation, and oxidative stress. Although our cross-sectional findings suggest that OSA was more significantly associated with LTL than measures of sleep fragmentation or hypoxemia, it is possible that alternative metrics of hypoxic burden or sleep disruption may further clarify the components of OSA that may drive accelerated aging.
Our data are also consistent with animal research that has shown that experimental disruption of sleep resulted in increased cellular stress responses [11–13] and cellular injury, including DNA damage [14, 15], and the promotion of cellular aging via increased expression of p16INK4a, a marker of cell senescence [16]. In tandem, these sleep disruptions activate inflammation [19, 88], also a key aspect of aging [33]. Repeated interruptions to sleep likely influence system repair processes that remove damage accumulated over the waking hours, resulting in gradual accumulation of damage and inflammation. This phenomenon has been observed in the brain [89–92], but likely also occurs systemically. Repeated interruptions to sleep or failure of the brain to enter and maintain deep sleep (such as what can occur during OSA) could result in an accumulation of waste that accelerates the aging process both in the brain and systemically.
Strengths and limitations
Several strengths of the current report include the large and ethnically diverse sample size, the high use of standardized objectively obtained assessment of sleep, including rigorous and objective assessment of OSA using established guidelines [72], and information on a large number of potential confounders. The current ancillary studies were able to leverage the ongoing project to add sleep measures as well as longitudinal changes in LTL, spanning 10 years. This study represents the first time objectively obtained sleep characteristics have been examined in relationship to LTL and changes in LTL over a decade. That said, some limitations should also be noted. Our a priori hypotheses were tested using multiple tests raising the possibility for type I error; however, our primary findings with OSA (p = 0.001) and the arousal index (p = 0.004) have p values well below 0.05, suggesting that these associations are likely not false positives. The assessment of sleep parameters occurred at Exam 5 and may not completely capture sleep dimensions occurring in the prior years and may also be influenced by survival bias, thus making interpretation of change in LTL over the prior decade as it relates to sleep, difficult to interpret. Circadian disruptions, such as in shift work, may have influenced the findings, and due to limited measurement, we cannot rule this out. Further research is warranted to disentangle possible effects of shift work and other circadian disruptions. Other undetermined factors might contribute to sleep problems and plausibly serve as a primary driver of accelerated aging. Future work should obtain prospective data on sleep parameters as they change and then also tease apart potential dimensions of health and functioning, and include other biological measurements of aging as well as information on stress and psychological factors that may alter sleep patterns. This work would help identify whether sleep is a driver of accelerated cellular aging or merely a proxy indicator of other dimension of health that contributes to accelerated LTL shortening.
Conclusion
We found that severe OSA was significantly associated with shorter LTL after controlling for demographic and lifestyle factors. Clinically, shortened LTL is an indicator of aging of the hematopoietic stem cells and circulating lymphocytes [42, 43], CVD [44, 45], and early mortality [46–51]. The magnitude of the difference in LTL between those with severe OSA compared with those without OSA or with mild OSA was comparable to LTL differences of 10 years of chronological aging. We also found evidence that sleep fragmentation, as measured by the arousal index, was associated with shorter LTL. Although cross-sectional analysis indicated that this association was partially explained by OSA, an exploratory analyses of change in LTL over a decade suggested that individuals with a high frequency of cortical arousals during sleep might have accelerated leukocyte telomere shortening over the prior decade of life. Further work in longitudinal cohorts is warranted to clarify these relationships further. Intervention studies are needed to assess the impact of improving sleep continuity and treating OSA on biological aging.
Funding
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. MESA Stress was supported by R01HL076831 and R01HL101161 (PI: Ana Diez-Roux). MESA Sleep was supported by grant R01HL098433 (PI: Susan Redline). Dr. Redline also was supported by HL R35HL135818. The manuscript preparation was supported by grant K01-AG044462. Additional support provided by R01-AG034588, R01-AG026364, R01 CA160245-01, CA195637-01, and R01-CA119159 (to M.R.I.), R01HL142051 (to A.A.P.), and UCLA CTSI UL1TR000124 (www.ctsi.ucla.edu/) and the Cousins Center for Psychoneuroimmunology, UCLA (http://www.semel.ucla.edu/cousins). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Conflict of interest statement. None for J.E.C. and A.V.D.-R. S.R. reports grant support from Jazz Pharmaceuticals and Beckman Couter, unrelated to this study. Jue Lin is a co-founder of Telomere Diagnostics Inc., and serves on its scientific advisory board; the company plays no role in the current study. Institutional Review Board approved this research. All subjects provided informed consent.
Supplementary Material
References
- 1. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. [DOI] [PubMed] [Google Scholar]
- 2. Center for Disease Control. CDC Data & Statistics | Feature: Insufficient Sleep Is a Public Health Epidemic 2011. http://www.cdc.gov/Features/dsSleep/. Accessed August 4, 2011.
- 3. Colten HR, et al. ; Research I of MUS. C on SM and. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington, DC: National Academies Press; 2006. http://books.google.com/books?hl=en&lr=&id=KexmhhRu84MC&pgis=1. Accessed August 4, 2011. [PubMed] [Google Scholar]
- 4. Motivala SJ. Sleep and inflammation: psychoneuroimmunology in the context of cardiovascular disease. Ann Behav Med. 2011;42(2):141–152. [DOI] [PubMed] [Google Scholar]
- 5. Ensrud KE, et al. Sleep disturbances and risk of frailty and mortality in older men. Sleep Med. 2012;13(10):1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kripke DF, et al. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59(2):131–136. [DOI] [PubMed] [Google Scholar]
- 7. Li Y, et al. Association between insomnia symptoms and mortality: a prospective study of U.S. men. Circulation. 2014;129(7):737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parthasarathy S, et al. Persistent insomnia is associated with mortality risk. Am J Med. 2015;128(3):268–75.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carroll JE, et al. Partial sleep deprivation activates the DNA damage response (DDR) and the senescence-associated secretory phenotype (SASP) in aged adult humans. Brain Behav Immun. 2016;51:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carroll JE, et al. Epigenetic aging and immune senescence in women with insomnia symptoms: findings from the women’s health initiative study. Biol Psychiatry. 2017;81(2):136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Naidoo N, et al. Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. J Neurosci. 2008;28(26):6539–6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naidoo N. Cellular stress/the unfolded protein response: relevance to sleep and sleep disorders. Sleep Med Rev. 2009;13(3):195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown MK, et al. The UPR and the anti-oxidant response: relevance to sleep and sleep loss. Mol Neurobiol. 2010;42(2):103–113. [DOI] [PubMed] [Google Scholar]
- 14. Everson CA, et al. Cell injury and repair resulting from sleep loss and sleep recovery in laboratory rats. Sleep. 2014;37(12):1929–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andersen ML, et al. Distinct effects of acute and chronic sleep loss on DNA damage in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(3):562–567. [DOI] [PubMed] [Google Scholar]
- 16. Carreras A, et al. Chronic sleep fragmentation induces endothelial dysfunction and structural vascular changes in mice. Sleep. 2014;37(11):1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Snyder B, et al. Chronic intermittent hypoxia induces oxidative stress and inflammation in brain regions associated with early-stage neurodegeneration. Physiol Rep. 2017;5(9):e13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zygmunt A, et al. Hypoxia stimulates p16 expression and association with cdk4. Exp Cell Res. 2002;278(1):53–60. [DOI] [PubMed] [Google Scholar]
- 19. Gaspar LS, et al. Obstructive sleep apnea and hallmarks of aging. Trends Mol Med. 2017;23(8):675–692. [DOI] [PubMed] [Google Scholar]
- 20. Lurie A. Metabolic disorders associated with obstructive sleep apnea in adults. Adv Cardiol. 2011;46:67–138. [DOI] [PubMed] [Google Scholar]
- 21. Lavie P, et al. Cardiovascular morbidity and mortality in obstructive sleep apnea. Curr Pharm Des. 2008;14(32):3466–3473. [DOI] [PubMed] [Google Scholar]
- 22. McNicholas WT, et al. ; Management Committee of EU COST ACTION B26. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29(1):156–178. [DOI] [PubMed] [Google Scholar]
- 23. Jun J, et al. Metabolic consequences of sleep-disordered breathing. ILAR J. 2009;50(3):289–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tempaku PF, et al. The effect of the severity of obstructive sleep apnea syndrome on telomere length. Oncotarget. 2016;7(43):69216–69224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lavie L. Sleep-disordered breathing and cerebrovascular disease: a mechanistic approach. Neurol Clin. 2005;23(4):1059–1075. [DOI] [PubMed] [Google Scholar]
- 26. Hatipoğlu U, et al. Inflammation and obstructive sleep apnea syndrome pathogenesis: a working hypothesis. Respiration. 2003;70(6):665–671. [DOI] [PubMed] [Google Scholar]
- 27. Lavie L, et al. Cardiovascular aspects in obstructive sleep apnea syndrome–molecular issues, hypoxia and cytokine profiles. Respiration. 2009;78(4):361–370. [DOI] [PubMed] [Google Scholar]
- 28. Kohler M, et al. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol. 2010;7(12):677–685. [DOI] [PubMed] [Google Scholar]
- 29. López-Otín C, et al. The hallmarks of aging. Cell. 2013;153(6):1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Demaria M, et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 2017;7(2):165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–522. [DOI] [PubMed] [Google Scholar]
- 32. Blackburn EH. Telomere states and cell fates. Nature. 2000;408(6808):53–56. [DOI] [PubMed] [Google Scholar]
- 33. Franceschi C, et al. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69 (Suppl 1):S4–S9. [DOI] [PubMed] [Google Scholar]
- 34. Campisi J, et al. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–740. [DOI] [PubMed] [Google Scholar]
- 35. Erusalimsky JD. Vascular endothelial senescence: from mechanisms to pathophysiology. J Appl Physiol (1985). 2009;106(1):326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Houben JM, et al. Telomere length assessment: biomarker of chronic oxidative stress? Free Radic Biol Med. 2008;44(3):235–246. [DOI] [PubMed] [Google Scholar]
- 37. Jiang H, et al. Telomere shortening and ageing. Z Gerontol Geriatr. 2007;40(5):314–324. [DOI] [PubMed] [Google Scholar]
- 38. von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339–344. [DOI] [PubMed] [Google Scholar]
- 39. Calado RT, et al. Telomere diseases. N Engl J Med. 2009;361(24):2353–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Frenck RW Jr, et al. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci USA. 1998;95(10):5607–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Müezzinler A, et al. A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev. 2013;12(2):509–519. [DOI] [PubMed] [Google Scholar]
- 42. Aviv A. Genetics of leukocyte telomere length and its role in atherosclerosis. Mutat Res. 2011;730(1-2):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lansdorp PM. Telomeres, stem cells, and hematology. Blood. 2008;111(4):1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Farzaneh-Far R, et al. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: data from the Heart and Soul Study. Arterioscler Thromb Vasc Biol. 2008;28(7):1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fitzpatrick AL, et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165(1):14–21. [DOI] [PubMed] [Google Scholar]
- 46. Bakaysa SL, et al. Telomere length predicts survival independent of genetic influences. Aging Cell. 2007;6(6):769. [DOI] [PubMed] [Google Scholar]
- 47. Cawthon RM, et al. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361(9355):393–395. [DOI] [PubMed] [Google Scholar]
- 48. Deelen J, et al. Leukocyte telomere length associates with prospective mortality independent of immune-related parameters and known genetic markers. Int J Epidemiol. 2014;43(3):878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fitzpatrick AL, et al. Leukocyte telomere length and mortality in the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2011;66(4):421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Needham BL, et al. Leukocyte telomere length and mortality in the National Health and Nutrition Examination Survey, 1999-2002. Epidemiology. 2015;26(4):528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mons U, et al. Leukocyte telomere length and all-cause, cardiovascular disease, and cancer mortality: results from individual-participant-data meta-analysis of 2 large prospective cohort studies. Am J Epidemiol. 2017;185(12):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Willeit P, et al. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304(1):69–75. [DOI] [PubMed] [Google Scholar]
- 53. Weischer M, et al. Short telomere length, cancer survival, and cancer risk in 47102 individuals. J Natl Cancer Inst. 2013;105(7):459–468. [DOI] [PubMed] [Google Scholar]
- 54. Riestra P, et al. Obstructive sleep apnea risk and leukocyte telomere length in African Americans from the MH-GRID study. Sleep Breath. 2017;21(3):751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Savolainen K, et al. The history of sleep apnea is associated with shorter leukocyte telomere length: the Helsinki Birth Cohort Study. Sleep Med. 2014;15(2):209–212. [DOI] [PubMed] [Google Scholar]
- 56. Barceló A, et al. Telomere shortening in sleep apnea syndrome. Respir Med. 2010;104(8):1225–1229. [DOI] [PubMed] [Google Scholar]
- 57. Kim KS, et al. Oxidative stress-induced telomere length shortening of circulating leukocyte in patients with obstructive sleep apnea. Aging Dis. 2016;7(5):604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Polonis K, et al. Moderate-to-severe obstructive sleep apnea is associated with telomere lengthening. Am J Physiol - Hear Circ Physiol. 2017;313(5). doi: 10.1152/ajpheart.00197.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huang P, et al. The association between obstructive sleep apnea and shortened telomere length: a systematic review and meta-analysis. Sleep Med. 2018;48:107–112. [DOI] [PubMed] [Google Scholar]
- 60. Liang G, et al. Associations between rotating night shifts, sleep duration, and telomere length in women. PLoS One. 2011;6(8):e23462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee KA, et al. Telomere length is associated with sleep duration but not sleep quality in adults with human immunodeficiency virus. Sleep. 2014;37(1):157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jackowska M, et al. Short sleep duration is associated with shorter telomere length in healthy men: findings from the Whitehall II Cohort Study. PLoS One. 2012;7(10):e47292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Prather AA, et al. Shorter leukocyte telomere length in midlife women with poor sleep quality. J Aging Res. 2011;2011:721390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Prather AA, et al. Tired telomeres: poor global sleep quality, perceived stress, and telomere length in immune cell subsets in obese men and women. Brain Behav Immun. 2015;47:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cribbet MR, et al. Cellular aging and restorative processes: subjective sleep quality and duration moderate the association between age and telomere length in a sample of middle-aged and older adults. Sleep. 2014;37(1):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kananen L, et al. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLoS One. 2010;5(5):e10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Carroll JE, et al. Insomnia and telomere length in older adults. Sleep. 2016;39(3):559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bild DE, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. [DOI] [PubMed] [Google Scholar]
- 69. Diez Roux AV, et al. Race/ethnicity and telomere length in the Multi-Ethnic Study of Atherosclerosis. Aging Cell. 2009;8(3):251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Needham BL, et al. Sociodemographic correlates of change in leukocyte telomere length during mid- to late-life: the Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2019;102:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chen X, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep. 2015;38(6):877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Redline S, et al. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med. 2007;3(2):169–200. [PubMed] [Google Scholar]
- 73. Whitney CW, et al. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep. 1998;21(7):749–757. [DOI] [PubMed] [Google Scholar]
- 74. Redline S, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21(7):759–767. [PubMed] [Google Scholar]
- 75. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15(2):173–184. [PubMed] [Google Scholar]
- 76. Levine DW, et al. Reliability and validity of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15(2):137–148. [DOI] [PubMed] [Google Scholar]
- 77. Levine DW, et al. Factor structure and measurement invariance of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15(2):123–136. [DOI] [PubMed] [Google Scholar]
- 78. Deutschman CS, et al. Sepsis: current dogma and new perspectives. Immunity. 2014;40(4):463–475. [DOI] [PubMed] [Google Scholar]
- 79. Carroll JE, et al. Socioeconomic factors and leukocyte telomere length in a multi-ethnic sample: findings from the multi-ethnic study of atherosclerosis (MESA). Brain Behav Immun. 2013;28:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nettleton JA, et al. Dietary patterns, food groups, and telomere length in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2008;88(5):1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cohen S, et al. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 82. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 83. Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cespedes EM, et al. Comparison of self-reported sleep duration with actigraphy: results from the Hispanic Community Health Study/Study of Latinos Sueño Ancillary Study. Am J Epidemiol. 2016;183(6):561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cappuccio FP, et al. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cappuccio FP, et al. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–1492. [DOI] [PubMed] [Google Scholar]
- 87. Geovanini GR, et al. Elevations in neutrophils with obstructive sleep apnea: the Multi-Ethnic Study of Atherosclerosis (MESA). Int J Cardiol. 2018;257:318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Irwin MR, et al. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1):40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Welberg L. Sleep: sleep: not such a waste. Nat Rev Neurosci. 2013;14(12):816–817. [DOI] [PubMed] [Google Scholar]
- 90. Iliff JJ, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123(3):1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Iliff JJ, et al. Is there a cerebral lymphatic system? Stroke. 2013;44(6 Suppl 1):S93–S95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Xie L, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.