Abstract
The aim of the present study was to compare radiation dose received by thyroid gland using different radiotherapy (RT) techniques with or without thyroid dose constraint (DC) for breast cancer patients. Computerized tomography (CT) image sets for 10 patients with breast cancer were selected. All patients were treated originally with opposite tangential field‐in field (FinF) for the chest wall and anteroposterior fields for the ipsilateral supraclavicular field. The thyroid gland was not contoured on the CT images at the time of the original scheduled treatment. Four new treatment plans were created for each patient, including intensity‐modulated radiotherapy (IMRT) and helical tomotherapy (HT) plans with thyroid DC exclusion and inclusion (IMRTDC(−), IMRTDC(+), HTDC(−), and HTDC(+), respectively). Thyroid DCs were used to create acceptable dose limits to avoid hypothyroidism as follows: percentage of thyroid volume exceeding 30 Gy less than 50% (V 30 < 50%) and mean dose of thyroid (TDmean) ≤ 21 Gy. Dose‐volume histograms (DVHs) for TDmean and percentages of thyroid volume exceeding 10, 20, 30, 40, and 50 Gy (V 10, V 20, V 30, V 40, and V 50, respectively) were also analyzed. The Dmean of the FinF, IMRTDC(−), HTDC(−), IMRTDC(+) and HTDC(+) plans were 30.56 ± 5.38 Gy, 25.56 ± 6.66 Gy, 27.48 ± 4.16 Gy, 18.57 ± 2.14 Gy, and 17.34 ± 2.70 Gy, respectively. Median V30 values were 55%, 33%, 36%, 18%, and 17%, for FinF, IMRTDC(−), HTDC(−), IMRTDC(+), and HTDC(+), respectively. Differences between treatment plans with or without DC with respect to D mean and V 30 values were statistically significant (P < 0.05). When thyroid DC during breast cancer RT was applied to IMRT and HT, the TDmean and V 30 values significantly decreased. Therefore, recognition of the thyroid as an organ at risk (OAR) and the use of DCs during IMRT and HT planning to minimize radiation dose and thyroid volume exposure are recommended.
Keywords: breast cancer, dose constraint, supraclavicular radiotherapy, thyroid gland dose
1. INTRODUCTION
Breast cancer is the second most common cancer nowadays, after lung cancer.1 Surgery is one of the most clinically beneficial procedures for treatment of breast cancer. However, it is possible that after surgery the remaining deposits of neoplastic disease locally or at distant sites are present.2 Therefore, radiotherapy (RT) plays an important role in removal of the resident deposit of breast cancer.3, 4 Unfortunately, the side effects of RT are inevitable, particularly on the sensitive organs such as thyroid gland.5, 6
Thyroid gland is very sensitive, important and the largest pure endocrine gland in our body and more importantly its hormones play a very significant role in metabolism, development, growth, overall energy expenditure, and a large number of body organs functions.7, 8 Primary hypothyroidism is a well known side effect of curative RT in patients with head and neck cancer and Hodgkin lymphoma,9, 10, 11, 12 whose RT portals usually encompass the entire thyroid.13, 14, 15, 16 However, limited data are available regarding hypothyroidism in patients with breast cancer treated with locoregional RT wherein the treatment field includes only part of the thyroid.17, 18, 19, 20, 21
Many studies have shown that radiation can cause thyroid gland disorders,7, 8, 11, 22 although the tolerance dose (TD) of the thyroid gland has not been definitively established.23 The minimum thyroid TD5/5 (incidence of clinical hypothyroidism in 5% of patients at 5 yr after treatment) is considered to be 20 Gy when all or part of the gland is irradiated with conventional fractionation.12, 24 Although some studies have reported the occurrence of RT‐induced hypothyroidism at high radiation doses (e.g., ≥30 Gy),21, 25 Dorri et al.3 observed no significant differences in thyroid hormone levels before and after RT in breast cancer patients, further highlighting the contradictory findings regarding RT’s effects on thyroid function.
Our knowledge of radiation‐induced hypothyroidism in patients with breast cancer is limited because the thyroid gland is not routinely considered as an organ at risk (OAR) during the irradiation of breast cancer. There is a growing body of literature examining the relationship between thyroid dose and hypothyroidism development in breast cancer RT.26, 27 However, few studies have compared the effects of different RT techniques on the thyroid dose.
The purpose of this study was to dosimetrically compare locoregional breast treatment plans using tangential field‐in‐field (FinF), intensity‐modulated radiotherapy (IMRT) and helical tomotherapy (HT) techniques in terms of thyroid dose that could potentially predict RT‐induced hypothyroidism risk and to determine whether the use of thyroid dose constraint (DC) is beneficial.
2. MATERIALS AND METHODS
2.A. Computerized tomography (CT) imaging
Computerized tomography (CT) image sets for 10 patients with breast cancer were selected from our treatment database. All patients underwent our department's routine procedures for patients with breast cancer. During the CT scan, each patient was in a supine position on a breast board, adjusted to achieve a flat chest wall with the head turned away from the side of treatment and the ipsilateral arm placed above the head.
2.B. Target delineation
The chest wall and ipsilateral supraclavicular field (SCF) were delineated for each patient by an experienced radiation oncologist as a clinical target volume (CTV), along with the contralateral breast, spinal cord, heart, and both lungs. The SCF included the supraclavicular (SC) and level‐1,2,3 axillary nodes. Consensus guidelines of the Radiation Therapy Oncology Group were used to delineate the CTV of the chest wall and SCF. The planning target volume (PTV) was created by adding 5 mm to the CTV. The thyroid gland was not contoured on the CT images at the time of the original scheduled treatment. For this study, the same physician manually contoured the thyroid gland on the CT‐simulated images of all patients.
2.C. Design of the treatment plans
For each of the ten patients, five different plans were created: field‐in‐field (FinF), intensity‐modulated radiotherapy (IMRT) thyroid DC exclusion IMRTDC(−), IMRT thyroid DC inclusion IMRTDC(+), helical tomotherapy (HT) thyroid DC exclusion HTDC(−), and HT thyroid DC inclusion HTDC(+). All patients were treated originally with opposite tangential field‐in‐field (FinF) for the chest wall and anteroposterior fields for the ipsilateral supraclavicular field (SCF). The prescribed dose was 50 Gy in 25 fractions, 5 days per week.
For the FinF technique, the beam arrangement consisted of two parallel opposing tangential beams to ensure the best possible coverage of the chest wall tissue and anteroposterior fields (with 15°–250° gantry angles) for the ipsilateral SCF. A single isocenter was chosen at the level of the match line between the ipsilateral SCF and chest wall below the medial end of the clavicle. Photon energy of 6 MV was used for both the tangential fields and anterior fields of the SCF; 18 MV was used for posterior fields. Shielding blocks were used primarily for spinal cord; no attempt was made to shield thyroid gland itself to prevent any under dosage in SCF.
The IMRT plans consisted of nine coplanar beams. The lateral and medial gantry angles were the same as those used in the FinF approach, while the other seven fields were placed between these fields at equal intervals. The field width, pitch, and modulation factor parameters were assigned as 2.5 cm, 0.287, and 2.0, respectively, for the HT plans. Two virtual structures (constraint‐lung and constraint‐heart) for DCs were contoured for each patient to decrease radiation doses to the lungs and heart. Partial blocking was applied to the contralateral breast.
The FinF and IMRT plans were generated using the Eclipse™ treatment planning system (Varian Medical Systems, Palo Alto, CA) and the HT plans were performed using a tomotherapy Hi‐ART planning system. The dose‐volume constraints used for the IMRT and HT plans are presented in Table 1. While DCs were applied for the heart, ipsilateral lung, contralateral lung, and contralateral breast in the IMRTDC(−) and HTDC(−) plans, thyroid DCs were included in the IMRTDC(+) and HTDC(+) plans in addition to the above constraints.
Table 1.
Target doses and dose constraints (DCs) of the organs at risk (OARs).
| Target or OAR | Goal or constraint dose |
|---|---|
| Planning target volume | 45 or 47.5 Gy |
| Heart | V 20 < 10% |
| İpsilateral lung | V 20 < 35% |
| Contralateral lung | V 5 < 20% |
| Contralateral breast | D max < 10 Gy |
| Thyroid “IMRT DC(+) and HT DC(+) plans with thyroid dose constraint (DC)” | D mean ≤ 21 Gy; V 30 < 50% |
2.D. Dose‐volume histogram data and statistical analysis
The generated treatment plans were compared objectively using dose‐volume histograms (DVHs) for PTVs and different OARs of interest. In the PTV, mean dose (Dmean), conformation number (CN), and homogeneity index (HI) were compared between all five plans. CN is calculated from the following formula:
where TVRI is the target volume covered by the reference isodose (95% of the prescribed dose), TV is the target volume, and VRI is the volume of the reference isodose. The CN ranges from 0 to 1, where 1 is the ideal value.
Another index for evaluating the plan is the HI, which takes into the homogeneity of the dose distribution within the target. HI is calculated from the following formula:
where D 98 for the PTV is the corresponding dose for 98% of the target volume measured on DVH, and D 2 is the corresponding dose for 2% of the volume on the DVH. HI formula shows that lower HI values indicate a more homogeneous target dose.
Based on each patient's dose‐volume histograms (DVHs), TDmean values and the percentage of thyroid gland volume that received 10 Gy (V 10), 20 Gy (V 20), 30 Gy (V 30), 40 Gy (V 40), and 50 Gy (V 50) were analyzed. Additionally, when using DC to the thyroid gland in the IMRTDC(+) and HTDC(+) plans, V 45 of the SC node, which is very close to the thyroid, was evaluated.
Statistical Package for the Social Sciences (SPSS) version 18 for Windows software was used for statistical analysis. Post hoc ANOVA was used to compare parametric data; nonparametric data were analyzed with Kruskal‐Wallis tests. For paired group comparisons of quantitative data, the Bonferroni modified test was applied for parametric data, while the Mann‐Whitney U test was used for nonparametric data. Differences were considered significant at P ≤ 0.05.
3. RESULT
The doses of planning target volume and OAR according to five different plans are summarized in Table 2.
Table 2.
Comparision of target coverage metrics for the planning target volume (PTV) and organs at risk (OAR) dose‐volume metrics as a function of plan modality ( ± SD).
| Metric | FinF | IMRTDC(−) | IMRTDC(+) | HTDC(−) | HTDC(+) | P‐Value |
|---|---|---|---|---|---|---|
| PTV | ||||||
| D mean (Gy) | 51.56 ± 1.00 | 51.24 ± 0.37 | 51.31 ± 0.37 | 50.83 ± 0.21 | 50.88 ± 0.21 | 0.005 |
| CN | 0.61 ± 0.09 | 0.76 ± 0.04 | 0.76 ± 0.04 | 0.80 ± 0.03 | 0.80 ± 0.03 | <0.001 |
| HI | 0.12 ± 0.05 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 | <0.001 |
| Heart | ||||||
| D mean (Gy) | 4.30 ± 2.22 | 8.42 ± 2.51 | 8.49 ± 2.52 | 4.17 ± 0.78 | 4.25 ± 0.77 | <0.001 |
| V 20 (%) | 5.2 ± 4.32 | 2.1 ± 1.65 | 2.15 ± 1.64 | 0.1 ± 0.14 | 0.12 ± 0.14 | <0.001 |
| Ipsilateral lung | ||||||
| D mean (Gy) | 7.35 ± 2.42 | 12.24 ± 2.21 | 12.29 ± 2.20 | 5.18 ± 1.35 | 5.24 ± 1.34 | <0.001 |
| V 20 (%) | 12.65 ± 4.80 | 15.10 ± 5.37 | 15.15 ± 5.36 | 7.20 ± 2.30 | 7.27 ± 2.31 | <0.001 |
| Contralateral lung | ||||||
| D mean (Gy) | 0.40 ± 0.20 | 4.21 ± 1.10 | 4.23 ± 1.11 | 2.52 ± 0.86 | 2.55 ± 0.87 | <0.001 |
| V 5 (%) | 0.0 ± 0.0 | 21.75 ± 14.43 | 21.81 ± 14.40 | 19.16 ± 11.60 | 19.21 ± 11.53 | <0.001 |
| Contralateral breast | ||||||
| D max (Gy) | 2.82 ± 0.70 | 9.10 ± 3.32 | 9.15 ± 3.31 | 9.88 ± 2.06 | 9.86 ± 2.05 | <0.001 |
PTV, Planning Target Volume; D max, max dose; D mean, mean dose; V x, volume (%) receiving × dose (Gy) or higher; , mean dose; sd, standart deviation; CN, conformation number; HI, homogeneity index.
The mean thyroid gland volume of 10 patients was 11.9 cm3 (6.3–19.8 cm3). Detailed dosimetric results for the thyroid glands and SC nodes for the five different plans are presented in Table 3.
Table 3.
Comparison of thyroid gland and supraclavicular (SC) node dosimetric parameters as a function of treatment plans.
| Metric | FinF | IMRTDC(−) | HTDC(−) | IMRTDC(+) | HTDC(+) | P‐value |
|---|---|---|---|---|---|---|
| D mean (Gy) | 30.56 ± 5.38 | 25.56 ± 6.66 | 27.48 ± 4.16 | 18.57 ± 2.14 | 17.34 ± 2.7 | <0.001 |
| V 10 (%) | 67 ± 10.51 | 92 ± 13.82 | 96 ± 5.93 | 76 ± 11.92 | 70 ± 10.55 | <0.001 |
| V 20 (%) | 60 ± 10.03 | 56 ± 19.58 | 66 ± 16.31 | 31 ± 7.03 | 28 ± 11.22 | <0.001 |
| V 30 (%) | 55 ± 10.81 | 33 ± 16.81 | 36 ± 14.27 | 18 ± 7.09 | 17 ± 9.74 | <0.001 |
| V 40 (%) | 51 ± 11.76 | 22 ± 16.41 | 21 ± 14.2 | 8 ± 6.48 | 7 ± 8.92 | <0.001 |
| V 50 (%) | 30 ± 15.59 | 7 ± 7.64 | 4 ± 6.04 | 1 ± 3.08 | 2 ± 4.64 | <0.001 |
| SC Node V 45 (%) | 100 | 99.2 ± 0.53 | 100 | 98.6 ± 0.83 | 97.9 ± 0.66 | <0.001 |
D mean, mean dose; Gy, Gray; V x, volume (%) receiving × dose (Gy) or higher. Values in bold font are statistically significant. Mean ± SD values are presented.
Significant differences were observed between plans with respect to TDmean (P < 0.001). The TDmean ± standard deviation values for the FinF, IMRTDC(−), HTDC(−), IMRTDC(+), and HTDC(+) plans were 30.56 ± 5.38 Gy, 25.56 ± 6.66 Gy, 27.48 ± 4.16 Gy, 18.57 ± 2.14 Gy, and 17.34 ± 2.70 Gy, respectively.
The TDmean for the FinF, IMRTDC(2212), and HTDC(‐) plans was >21 Gy, while the TDmean for the IMRTDC(+) and HTDC(+) plans was <21 Gy. Figure 1 shows the isodose distribution for the IMRTDC(−), IMRTDC(+), HTDC(‐) and HTDC(+) plans in axial plane for a representative patient. The color‐wash threshold was set to 21 Gy. IMRTDC(−) and HTDC(−) plans reduced TDmean values from those used in FinF, while IMRTDC(+) and HTDC(+) further reduced the TDmean.
Figure 1.
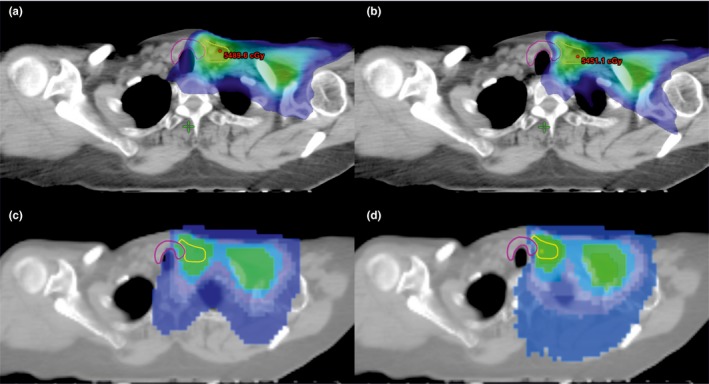
The isodose distribution for the four plans in axial plane for a representative patient. Color‐wash threshold was set to 21 Gy. (a) IMRTDC(−); (b) IMRTDC(+); (c) HTDC(−); and (d) HTDC(+). IMRT, intensity modulated radiotherapy; HT, helical tomotherapy.
There was no statistically significant difference between the three plans [FinF, IMRTDC(−), and HTDC(−)] with respect to TDmean (P> 0.05; Table 4). However, the TDmean values for the IMRTDC(+) and HTDC(+) plans were significantly lower than those of the other three plans. Differences between IMRTDC(+) and HTDC(+) TDmean values were not statistically significant (P = 0.958); in contrast, TDmean difference significantly between the DC(−) and DC(+) plans (P < 0.001). An illustrative DVH comparison for thyroid gland for a representative patient is shown in Fig. 2.
Table 4.
Estimated P‐values for the compared treatment plans.
| Metric | Thyroid | SC Node V 45(%) | |||||
|---|---|---|---|---|---|---|---|
| D mean | V 10(%) | V 20(%) | V 30(%) | V 40(%) | V 50(%) | ||
| FinF vs IMRTDC( 2212 ) | 0.576 | <0.001 | 0.950 | 0.002 | 0.004 | 0.010 | 0.012 |
| FinF vs IMRTDC(+) | <0.001 | 0.304 | <0.001 | <0.001 | <0.001 | 0.002 | 0.004 |
| FinF vs HTDC(−) | 0.846 | <0.001 | 0.984 | 0.007 | <0.001 | 0.004 | <0.001 |
| FinF vs HTDC(+) | <0.001 | 0.946 | <0.001 | <0.001 | <0.001 | 0.002 | <0.001 |
| IMRTDC(−) vs IMRTDC(+) | <0.001 | 0.015 | 0.041 | 0.047 | 0.232 | 0.439 | 0.408 |
| IMRTDC(−) vs HTDC(−) | 0.997 | 0.915 | 0.634 | 0.989 | 1.000 | 0.982 | 0.012 |
| IMRTDC(−) vs HTDC(+) | 0.035 | <0.001 | 0.049 | 0.043 | 0.185 | 0.646 | 0.002 |
| IMRTDC(+) vs HTDC(−) | <0.001 | <0.001 | <0.001 | 0.014 | 0.235 | 0.960 | 0.004 |
| IMRTDC(+) vs HTDC(+) | 0.958 | 0.743 | 0.998 | 1.000 | 1.000 | 1.000 | 0.577 |
| HTDC(−) vs HTDC(+) | <0.001 | <0.001 | <0.001 | 0.012 | 0.189 | 0.997 | <0.001 |
Values in bold font are statistically significant.
Figure 2.
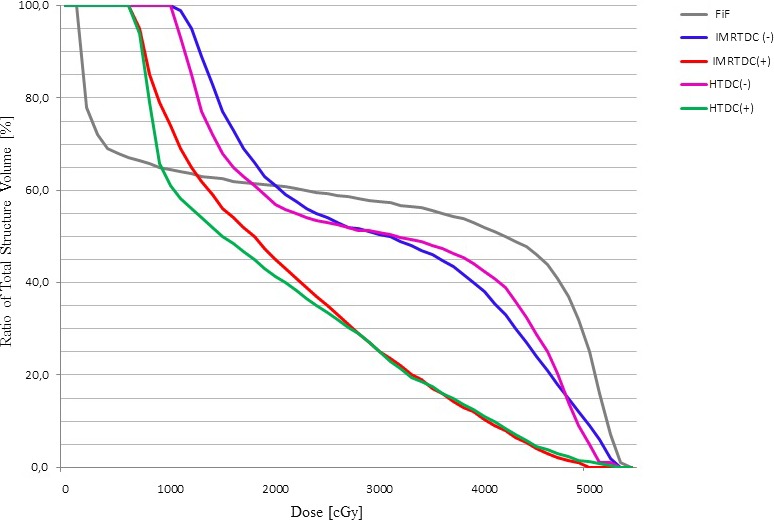
Dose‐volume histograms (DVH) comparison of the thyroid gland using FinF, IMRTDC(−), HTDC(−), IMRTDC(+), and HTDC(+) in a representative patient. IMRT, intensity modulated radiotherapy; HT, helical tomotherapy.
The low dose‐volume (V10) in the thyroid gland was larger for the IMRT and HT plans compared with the FinF plan. It was found that the volume percentage of the thyroid absorbing ≥30 Gy was above 50% in seven of 10 in patients in FinF and two of ten both IMRTDC(−) and HTDC(−).
However, the mean dose for V 30 was <50% for the IMRTDC(−) and HTDC(−) plans (33% and 36%, respectively). When DC was applied for both IMRT and HT, V 30 ≥ 50% was not observed for any patient. The differences between the DC(−) and DC(+) plans were statistically significant for V 10, V 20, and V 30. The V 30 values for the IMRTDC(+) and HTDC(+) plans were significantly lower than the other three plans. There were no statistically significant differences between V 10, V 20, V 30, V 40, and V 50 values for the IMRTDC(+) and HTDC(+) plans.
We found no statistically significant difference between the IMRTDC(−) and IMRTDC(+) plans with respect to the SC node V45 value. Although the SC node V45 values were significantly different in the HTDC(−) and HTDC(+) plans, 97.9% of the SC node volume was covered by 90% of the prescribed dose (45 Gy) for HTDC(+) plans (Table 3).
4. DISCUSSION
The thyroid gland is very sensitive to radiation and a large number of studies showed that radiation can cause disorders such as hypothyroidism, Graves' disease, and thyroid cancer.7, 8, 11 Although the dose of radiation is a significant factor for predicting thyroid dysfunction,21, 28, 29, 30, 31 few investigators have performed clinical thyroid‐associated DVH analysis after RT.12, 32, 33 Most of these investigations were studied in patients with head and neck cancer patients treated with RT doses higher than those used in RT for breast cancer.12, 26, 31
Hypothyroidism is one of the late toxicities of curative RT to the neck region, and the incidences of hypothyroidism that have been reported range from 20% to 52%.9, 10, 11, 12, 29 Unfortunately, our knowledge of radiation‐induced hypothyroidism in breast cancer patients is limited because the thyroid gland is not routinely considered as an OAR during breast cancer RT. As a result, radiation‐induced hypothyroidism in these patients has been investigated in only a few studies, which reported varying incidence rates (6%–21%) in patients with breast cancer.17, 19, 20, 21, 34
The correlation between radiation dose and hypothyroidism was demonstrated by Kuten et al.9 and Yoden et al.,28 who used DVHs to evaluate the relationship between the volume of the thyroid receiving radiation and thyroid function. Their results indicated that the thyroid volume receiving doses V 10 to V 30 significantly impacted the peak level of thyroid‐stimulating hormone. Similarly, Cella et al.32 and Akgun et al.29 reported that V30 was a statistically significant predictor for the development of hypothyroidism. According to Kanyılmaz et al.,27 Dmean was the only factor that accurately predicted hypothyroidism, with 21 Gy as the threshold value. Additionally, Tunio et all.26 showed that the risk of hypothyroidism in breast cancer patients after SC‐RT depends on the thyroid gland volume and V 30 > 50%.
In contrast, Diaz et al.35 reported that the D mean and V 10 to V 70 were not associated with hypothyroidism. Alterio et al.12 also showed that D mean, V 10, V 30, and V 50 were not associated with hypothyroidism, and Dorri et al.3 found no significant difference in thyroid hormone levels before and after RT in breast cancer patients.
Although radiation‐induced thyroid disorders remain underestimated and study results are often contradictory, the current consensus is that RT causes hypothyroidism, and V 30 and D mean values have the most predictive value for development of hypothyroidism in patients with breast cancer. Therefore, in our study, these two parameters were used as a reference for DC of the thyroid gland.
In the present study, all treatment plans provided adequate coverage of the planning target volume. Our results of IMRTDC(−), HTDC(−), IMRTDC(+), and HTDC(+) plans presented similar dosimetric results as the previous studies with respect to critical organs (e.g., contralateral breast, heart, and both lungs). The TDmean > 21 Gy and the V 30 was >50% for the FinF technique, which was not planned to include a special shield to reduce the dose to the thyroid gland. In the IMRTDC(−) and HTDC(−) plans, the TDmean was >21 Gy, while the V30 was <50%. For the IMRTDC(+) and HTDC(+) plans, we were able to achieve the dose limits to the thyroid gland that we set for V 30 and D mean.
In addition to dose‐volume parameters, other factors have been identified as predictors for thyroid dysfunction such as thyroid gland volume. Thus, accurate estimation of the size and localization of the thyroid is critical for evaluating dose‐volume parameters and management of thyroid disorders. Therefore, it is recommended that the thyroid gland is contoured by experienced radiation oncologists, and contrast‐enhanced CT may be beneficial.
One of the important challenges to address during breast RT is secondary cancer risk. Various reports have shown that increased low doses may increase the risk of secondary malignancy development.36, 37, 38 The move from three‐dimensional conformal RT to intensity‐modulated techniques involves more fields, and the dose‐volume histograms show that, as a consequence, a larger volume of normal tissue is exposed to lower doses. In addition, the number of monitor units is increase. Both factors will tend to increase the risk of development of secondary cancers. In this study, low dose‐volume (V 10) was significantly larger in the IMRTDC(−) and HTDC(−) plans than in the FinF, IMRTDC(+), and HTDC(+) plans. According to some authors, V 10 was not associated with hypothyroidism.12, 35 However, it should not be ignored that larger low dose‐volume may be a risk factor for the development of secondary thyroid cancer in breast cancer patients with long life expectancies.
5. CONCLUSION
The use of intensity‐modulated techniques with thyroid DC(+) significantly reduce the dose to the thyroid gland when compared with DC(−) for the breast patients with SCF irradiation; therefore, it is recommended that recognition of the thyroid as an OAR and the use of DCs during IMRT and HT planning to minimize radiation dose and thyroid volume. Future clinical studies are needed to confirm this dosimetric results.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this article.
REFERENCES
- 1. Ferlay J, Héry C, Autier P, Sankaranarayanan R. Global burden of breast cancer In: Li C, ed. Breast Cancer Epidemiology. New York, NY: Springer Inc; 2010:1–19. [Google Scholar]
- 2. Weinberg R, Hahn W, Watnick R, et al. Rules governing the creation of human tumor cells. Int J Cancer. 2002;100:1. [Google Scholar]
- 3. Dorri Giv M, Bahreini Toosi MH, Aghamiri SMR, Akbari F, Taeb S. Calculation of thyroid dose with planner system and evaluation of thyroid function after radiotherapy for patients with breast cancer. J Biomed Phys Eng. 2016;6:229–234. [PMC free article] [PubMed] [Google Scholar]
- 4. Early Breast Cancer . Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15‐year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. [DOI] [PubMed] [Google Scholar]
- 5. Ozawa H, Saitou H, Mizutari K, Takata Y, Ogawa K. Hypothyroidism after radiotherapy for patients with head and neck cancer. Am J Otolaryngo. 2007;28:46–49. [DOI] [PubMed] [Google Scholar]
- 6. Hermann R, Henkel K, Christiansen H, et al. Testicular dose and hormonal changes after radiotherapy of rectal cancer. Radiother Oncol. 2005;75:83–88. [DOI] [PubMed] [Google Scholar]
- 7. Hancock SL, McDougall IR, Constine LS. Thyroid abnormalities after therapeutic external radiation. Int J Radiat Oncol Biol Phys. 1995;31:1165–1170. [DOI] [PubMed] [Google Scholar]
- 8. Beck‐Peccoz P, Amr S, Menezes‐Ferreira MM, Faglia G, Weintraub BD. Decreased receptor binding of biologically inactive thyrotropin in central hypothyroidism. Effect of treatment with thyrotropin‐releasing hormone. N Engl J Med. 1985;312:1085–1090. [DOI] [PubMed] [Google Scholar]
- 9. Kuten A, Lubochitski R, Fishman G, Dale J, Stein ME. Postradiotherapy hypothyroidism: radiation dose response and chemotherapeutic radiosensitization at less than 40 Gy. J Surg Oncol. 1996;61:281–283. [DOI] [PubMed] [Google Scholar]
- 10. Koc M, Capoglu I. Thyroid dysfunction in patients treated with radiotherapy for neck. Am. J Clin Oncol. 2002;32:150–153. [DOI] [PubMed] [Google Scholar]
- 11. Jereczek‐Fossa BA, Alterio D, Jassem J, Gibelli B, Tradati N, Orecchia R. Radiotherapy‐induced thyroid disorders. Cancer Treat Rev. 2004;30:369–384. [DOI] [PubMed] [Google Scholar]
- 12. Alterio D, Jereczek‐Fossa BA, Franchi B, et al. Thyroid disorders in patients treated with radiotherapy for head‐and‐neck cancer: a retrospective analysis of seventy‐three patients. Int J Radiat Oncol Biol Phys. 2007;67:144–150. [DOI] [PubMed] [Google Scholar]
- 13. Garcia‐Serra A, Amdur RJ, Morris CG, Mazzaferri E, Mendenhall WM. Thyroid function should be monitored following radiotherapy to the low neck. Am J Clin Oncol. 2005;28:255–258. [DOI] [PubMed] [Google Scholar]
- 14. Chow LM, Nathan PC, Hodgson DC, et al. Survival and late effects in children with Hodgkin’s lymphoma treated with MOPP/ABV and low‐dose, extended‐field irradiation. J Clin Oncol. 2006;24:5735–5741. [DOI] [PubMed] [Google Scholar]
- 15. Metzger ML, Hudson MM, Somes GW, et al. White race as a risk factor for hypothyroidism after treatment for pediatric Hodgkin’s lymphoma. J Clin Oncol. 2006;24:1516–1521. [DOI] [PubMed] [Google Scholar]
- 16. Norris AA, Amdur RJ, Morris CG, , Mendenhall WM. Hypothyroidism when the thyroid is included only in the low neck field during head and neck radiotherapy. Am J Clin Oncol. 2006;29:442–445. [DOI] [PubMed] [Google Scholar]
- 17. Joensuu H, Viikari J. Thyroid function after postoperative radiation therapy in patients with breast cancer. Acta Oncol. 1986;25:167–170. [DOI] [PubMed] [Google Scholar]
- 18. Bonato C, Severino RF, Elnecave RH. Reduced thyroid volume and hypothyroidism in survivors of childhood cancer treated with radiotherapy. J Pediatr Endocrinol Metab. 2008;21:943–949. [DOI] [PubMed] [Google Scholar]
- 19. Smith GL, Smith BD, Giordano SH, et al. Risk of hypothyroidism in older breast cancer patients treated with radiation. Cancer. 2008;112:1371–1379. [DOI] [PubMed] [Google Scholar]
- 20. Reinertsen KV, Cvancarova M, Wist E, et al. Thyroid function in women after multimodal treatment for breast cancer stage II/III: comparison with controls from a population sample. Int J Radiat Oncol Biol Phys. 2009;75:764–770. [DOI] [PubMed] [Google Scholar]
- 21. Johansen S, Reinertsen KV, Knutstad K, Olsen DR, Fosså SD. Dose distribution in the thyroid gland following radiation therapy of breast cancer—a retrospective study. Radiat Oncol. 2011;6:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bassiri RM, Utiger RD. Thyrotropin‐releasing hormone in the hypothalamus of the rat. Endocrinology. 1974;94:188–197. [DOI] [PubMed] [Google Scholar]
- 23. Weissler MC, Berry B. Thyroid‐stimulating hormone levels after radiotherapy and combined therapy for head and neck cancer. Head Neck. 1991;13:420–423. [DOI] [PubMed] [Google Scholar]
- 24. Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. [DOI] [PubMed] [Google Scholar]
- 25. Laway BA, Shafi KM, Majid S, et al. Incidence of primary hypothyroidism in patients exposed to therapeutic external beam radiation, where radiation portals include a part or whole of the thyroid gland. Indian J Endocrinol Metab. 2012;16:329–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tunio MA, Al Asiri M, Bayoumi Y, Stanciu LG, Al Johani N. Al Saeed EF Is thyroid gland an organ at risk in breastcancer patients treated with locoregional radiotherapy? Results of a pilot study. J Cancer Res Ther. 2015;11:684–689. [DOI] [PubMed] [Google Scholar]
- 27. Kanyilmaz G, Aktan M, Koc M, Demir H, Demir LS. Radiation‐induced hypothyroidism in patients with breast cancer: a retrospective analysis of 243 cases. Med Dosim. 2017;42:190–196. [DOI] [PubMed] [Google Scholar]
- 28. Yoden E, Soejima T, Maruta T, et al. Hypothyroidism after radiotherapy to the neck. Nihon Igaku Hoshasen Gakkai Zasshi. 2004;64:146–150. [PubMed] [Google Scholar]
- 29. Akgun Z, Atasoy BM, Ozen Z, et al. V30 as a predictor for radiation‐induced hypothyroidism: a dosimetric analysis in patients who received radiotherapy to the neck. Radiat Oncol. 2014;2:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim MY, Yu T, Wu HG. Dose‐volumetric parameters for predicting hypothyroidism after radiotherapy for head and neck cancer. Jpn J Clin Oncol. 2014;44:331–337. [DOI] [PubMed] [Google Scholar]
- 31. Fujiwara M, Kamikonya N, Odawara S, et al. The threshold of hypothyroidism after radiation therapy for head and neck cancer: a retrospective analysis of 116 cases. J Radiat Res. 2015;56:577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cella L, Conson M, Caterino M, et al. Thyroid V30 predicts radiation‐induced hypothyroidism in patients treated with sequential chemo‐radiotherapy for Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 2012;82:1802–1808. [DOI] [PubMed] [Google Scholar]
- 33. Diaz R, Jaboin JJ, Morales‐Paliza M, et al. Hypothyroidism as a consequence of intensity‐modulated radiotherapy with concurrent taxane‐based chemotherapy for locally advanced head‐and‐neck cancer. Int J Radiat Oncol Biol Phys. 2010;77:468–476. [DOI] [PubMed] [Google Scholar]
- 34. Bruning P, Bonfrèr J, De Jong‐Bakker M, Nooyen W, Burgers M. Primary hypothyroidism in breast cancer patients with irradiated supraclavicular lymph nodes. Br J Cancer. 1985;51:659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Diaz R, Jaboin JJ, Morales‐Paliza M, et al. Hypothyroidism as a consequence of intensity‐modulated radiotherapy with concurrent taxane‐based chemotherapy for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2010;77:468–476. [DOI] [PubMed] [Google Scholar]
- 36. Dracham CB, Shankar A, Madan R. Radiation induced secondary malignancies: a review article. Radiat Oncol J. 2018;36:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Paganetti H. Assessment of the risk for developing a second malignancy from scattered and secondary radiation in radiation therapy. Health Phys. 2012;103:652–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ron E, Modan B, Boice JD Jr, et al. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988;319:1033–1039. [DOI] [PubMed] [Google Scholar]


