Abstract
Background
Abnormalities on cardiac imaging (cardiac magnetic resonance imaging [CMR] or positron emission tomography [PET]), left ventricular ejection fraction (LVEF), and electrophysiology study (EPS) all predict ventricular arrhythmias (VA) in patients with cardiac sarcoidosis (CS). We sought to assess the utility of EPS in patients with CS and abnormal cardiac imaging, focusing on those with LVEF >35%.
Methods
We identified all patients treated at our institution from 2000 to 2017 who: 1.) had probable or definite CS; 2.) had either late gadolinium enhancement (LGE) on CMR or abnormal 18-flourodeoxyglucose (FDG) uptake on PET, and 3.) had undergone EPS. The primary endpoint was VA during follow up.
Results
Twenty five patients were included, of whom 10 (40%) had positive EPS. During a mean follow-up of 4.8 +/− 3.4 years, 11 (44%) patients had VA. The positive predictive value (PPV) of EPS for VA was 100% and the negative predictive value (NPV) of EPS for VA was 93%. Among 12 patients with LVEF >35% and no prior VA, the PPV of EPS for VA was 100% and the NPV of EPS for VA was 90%.
Conclusion
EPS may help with risk stratification in patients with CS and abnormal imaging, especially those without conventional indications for ICD placement. Among patients with LVEF >35% and no history of prior VA, a negative EPS has good positive and negative predictive value for future VA events.
Keywords: Cardiac sarcoidosis, Ventricular arrhythmia, Sudden cardiac death, Electrophysiology study, Implantable cardioverter defibrillator
1. Introduction
Sarcoidosis is a granulomatous disease that may affect any organ in the body. Among patients with sarcoidosis, 5–17% have clinically-manifest cardiac involvement (cardiac sarcoidosis, CS) [1,2] and approximately 1% suffer from ventricular arrhythmias (VA) [3]. Ventricular arrhythmias may be the presenting feature of CS [4,5], and are a significant predictor of mortality [6]. Patients with CS and left ventricular ejection fraction (LVEF) >35% may be at increased risk of VA despite their relatively preserved left ventricular (LV) systolic function [[7], [8], [9]]. Therefore, risk stratification techniques are needed to determine which CS patients who do not have conventional indications for implantable cardioverter defibrillator (ICD) placement would benefit from implantation of primary-prevention devices. Abnormalities on cardiac imaging predict VA in patients with CS, including those with LVEF >35% [7,8]. In patients with CS, focal uptake of 18-flourodeoxyglucose (FDG) on cardiac positron emission tomography (PET) represents active, inflammatory disease. When combined with perfusion defects, the presence of focal FDG uptake conferred increased risk of VA events with a hazard ratio of 3.9 in a population with a mean LVEF of 47% [7]. In contrast, the late gadolinium enhancement technique on cardiac magnetic resonance imaging (CMR) delineates areas of irreversible myocardial injury (scar) representing the chronic phase of CS. Among patients with LVEF >50%, the presence of LGE confers increased risk of VA with a hazard ratio of 19.4 [8]. Inducible VA during electrophysiology study (EPS) has also been shown to predict VA in patients with CS [[9], [10], [11]]. However, this has not been well studied in a population of CS patients with abnormal cardiac imaging and LVEF >35%, a group that would not meet conventional indications for primary-prevention ICD placement. Therefore, our aim was to assess the utility of EPS in patients with CS and abnormal cardiac imaging, focusing on those with LVEF >35%.
2. Methods
2.1. Study population
We queried our electronic medical record (EMR) system for patients treated at Johns Hopkins Hospital between 2000 and 2017 who: 1.) had a diagnosis of probable or definite CS according to the criteria set forth in the 2014 Heart Rhythm Society (HRS) Expert Consensus Statement [12]; 2.) had abnormalities on cardiac imaging manifested as either LGE on CMR or FDG uptake on PET, and 3.) had undergone EPS.
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee. Given the retrospective nature of the analysis, the requirement of informed consent was waived.
2.2. CMR image acquisition
All CMR images were acquired on a 1.5-T magnetic resonance imaging units (GE Medical Systems, Waukesha, Wis; or Avanto, Siemens, Erlangen, Germany) using electrocardiographic gating and breath holding as previously described [13]. Late gadolinium enhancement (LGE) imaging was performed 10–18 min after injection of 0.2 mmol/kg of gadolinium (gadopentetate dimeglumine; Bayer Healthcare Pharmaceuticals, Montville, NJ, USA). We utilized phase sensitive inversion recovery gradient recall echo sequences (repetition time of 2.5–5.5 ms, echo time of 1.52 ms, flip angle at 10 degrees, in-plane resolution of 1.3 × 1.3, slice thickness of 2.0 mm, and inversion time selected for maximal myocardial nulling, typically 240–290 ms) for the assessment of focal myocardial fibrosis.
2.3. Cardiac PET/CT image acquisition
Prior to PET examination, patients were instructed to follow a high fat, low carbohydrate diet for 1 day followed by 12 h of fasting, as previously described in order to shift myocardial metabolism to fatty acid utilization and suppress uptake of FDG by normal myocardium [14]. Myocardial metabolic imaging was performed with cardiac PET/Computed tomography (CT; Discovery Rx VCT PET/CT [GE Healthcare, Milwaukee, Wisconsin]). An intravenous dose of 0.135 mCi/kg of 18F-fluorodeoxyglucose (FDG) was administered. After approximately 60 min of uptake, cardiac and whole-body FDG PET/CT scans were performed as previously described [15].
2.4. CMR and PET image interpretation
All CMR and PET images were obtained for clinical purposes and were interpreted by experienced clinical imagers. In the case of CMR, the interpretations were performed clinically by experienced radiologists. In the case of PET, the interpretations were performed clinically by experienced nuclear medicine physicians. The results of these clinical interpretations were retrospectively accessed in the EMR. CMR imaging was categorized as either positive or negative for the presence of LGE based on the clinical interpretation. PET imaging was categorized as either positive or negative for focal FDG uptake based on the clinical interpretation.
2.5. Electrophysiology study protocol and interpretation
All EPS were obtained for clinical purposes. Programmed ventricular stimulation was performed with at least 2 drive-cycle lengths (600 ms and 350 ms) from the right ventricular base and apex. In 3 patients, non-invasive programmed stimulation was performed from the right ventricular apex only. Up to three extrastimuli were delivered until ventricular refractoriness. Burst pacing was performed from the right ventricular apex down to a cycle length of 200 ms. These pacing maneuvers were then repeated with infusion of isoproterenol, titrated to a 20% increase in the baseline heart rate. A sustained inducible arrhythmia was considered to be one lasting >30 s or one requiring termination prior to 30 s because of hemodynamic instability. Ventricular tachycardia was defined as a VA with cycle length 600–200 ms. Ventricular flutter was defined as a VA with cycle length <200 ms. Induction of ventricular fibrillation with triple extrastimuli of <220 ms was considered non-specific and was not classified as a sustained inducible arrhythmia. Three studies were performed at outside institutions.
Intracardiac and surface tracings from these studies were accessed from the clinical archive and analyzed by a single observer blinded to arrhythmic outcomes. Electrophysiology studies were classified as positive if one or more VAs were induced that were either sustained or caused hemodynamic instability requiring cardioversion or defibrillation; otherwise they were classified as negative.
2.6. Clinical follow up and arrhythmic outcomes
The EMR was queried using a natural language search capability for clinical VA occurring after the time of EPS, which included sustained ventricular tachycardia, ventricular fibrillation, sudden cardiac death, or any appropriate device tachytherapy. If a device therapy was reported but the underlying rhythm was not documented, the therapy was counted as appropriate. Follow up time was defined as the time from EPS until the last documented clinical encounter in the EMR.
2.7. Statistical analysis
Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools housed at Johns Hopkins University. All analyses were performed using the STATA software system, version 10 (StataCorp, College Station, TX, USA). Continuous data is presented as mean ± SD while categorical variables are presented as percentages. Comparisons between patients who were inducible for VA and non-inducible for VA were performed using the Student t-test (continuous variables) and the Fischer exact test (categorical variables). Difference in VA-free survival between patients who were inducible for VA and non-inducible for VA was assessed with the log-rank test. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics and imaging findings
A total of 393 consecutive patients were referred for evaluation of known or suspected CS by CMR at Johns Hopkins Hospital (Baltimore, MD) between January 1, 2000 and June 23, 2017. Among these, 103 also underwent cardiac PET, and 81 met HRS criteria for probable or definite CS [12]. Among these 81, 76 had abnormal cardiac imaging, and among these 76, 25 underwent EPS.
Table 1 summarizes the baseline characteristics of the study population. A total of 25 patients (mean age 50 +/− 10 years; 44% female) were included in the final study cohort. The mean LVEF was 51 +/− 16%. All 25 patients underwent CMR, which showed LGE in 21 (84%); 18 patients underwent both CMR and PET, which showed LGE in 14 (78%), FDG uptake in 13 (72%), and both LGE and FDG uptake in 9 (50%). All 25 patients had either LGE on CMR or FDG uptake on PET.
Table 1.
Baseline characteristics.
| All (n = 25) | EPS+ (n = 10) | EPS− (n = 15) | p-Value | |
|---|---|---|---|---|
| Age (years) | 50 +/− 10 | 49 +/− 10 | 51 +/− 11 | 0.60 |
| Male sex (n(%)) | 14 (56) | 8 (80) | 6 (40) | 0.10 |
| Caucasian (n(%)) | 16 (64) | 10 (100) | 6 (40) | 0.003 |
| CAD (n(%)) | 1 (4) | 0 (0) | 1 (7) | 1.00 |
| Immunosuppression (n(%)) | 22 (88) | 9 (90) | 13 (87) | 1.00 |
| Beta blockers (n(%)) | 19 (76) | 10 (100) | 9 (60) | 0.05 |
| Antiarrhythmics (n(%)) | 9 (36) | 7 (70) | 2 (13) | 0.009 |
| LGE (n(%)) | 21 (84) | 10 (100) | 11 (73) | 0.13 |
| FDG (n(%)) | 13 (72) | 4 (40) | 9 (60) | 0.43 |
| LVEF (%) | 51 +/− 16 | 51 +/− 11 | 52 +/− 15 | 0.53 |
| Prior VA (n(%)) | 9 (36) | 7 (70) | 2 (13) | 0.009 |
| Prior CA (n(%)) | 2 (8) | 2 (20) | 0 (0) | 0.15 |
| Prior ICD (n(%)) | 3 (12) | 3 (30) | 0 (0) | 0.05 |
| Follow up (yrs) | 4.8 +/− 3.4 | 5.5 +/− 3.4 | 4.3 +/− 3.3 | 0.42 |
CA = catheter ablation; CAD = coronary artery disease; FDG = 18-flourodeoxyclucose uptake on cardiac positron emission tomography; ICD = implantable cardioverter defibrillator; LGE = late gadolinium enhancement on cardiac magnetic resonance imaging; LVEF = left ventricular ejection fraction; VA = ventricular arrhythmia.
3.2. Electrophysiology study and arrhythmic outcomes
Electrophysiology study showed inducible VA in 10 (40%) patients. Table 1 compares baseline characteristics between patients with and without inducible VA. Those with inducible VA were significantly more likely to be Caucasian (100% vs 40%, p = 0.003), and to have had prior VA (70% vs 13%, p = 0.009) and treatment with antiarrhythmic therapy (70% vs 13%, p = 0.009). There was a trend towards greater treatment with beta blockers (100% vs 60%, p = 0.05) and prior ICD therapy (30% vs 0%, p = 0.05) among patients with inducible VA.
There were no significant differences between those with and without inducible VA with respect to age, use of corticosteroids or other immunosuppressant agents, LVEF or follow-up time.
At a mean follow up of 4.8 +/− 3.4 years, 11 patients (44%) had VA. All patients who had VA had ICDs in situ, and all events were episodes of appropriate device tachytherapy. Nine of these were ICD defibrillations and 2 were antitachycardia pacing events.
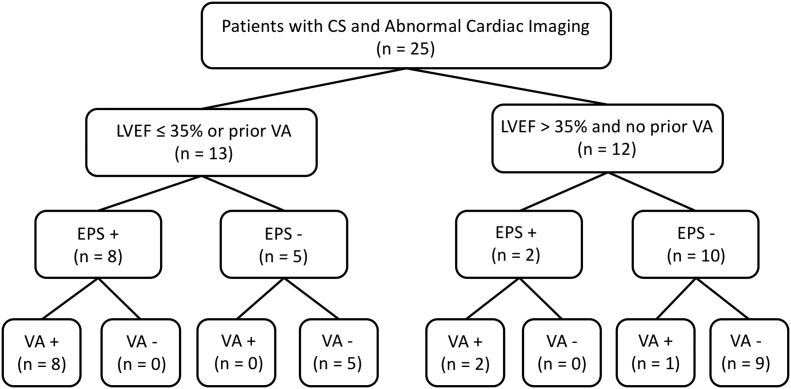
The mean time from EPS to VA event was 1.7 +/− 2.2 years. All VA events occurred within the mean follow-up time of the group that did not have VA events. Fig. 1 provides a flow chart stratifying VA events first by presence or absence of traditional indications for ICD placement and second by positive or negative VA. Among the 56 patients who did not undergo EPS, none had VA events during follow up.
Fig. 1.
Flow chart of study population. Ventricular arrhythmic events are stratified first by presence or absence of a traditional indication for ICD placement (LVEF ≤35% or prior VA) and second by positive or negative EPS.
3.3. Predictive value of EPS
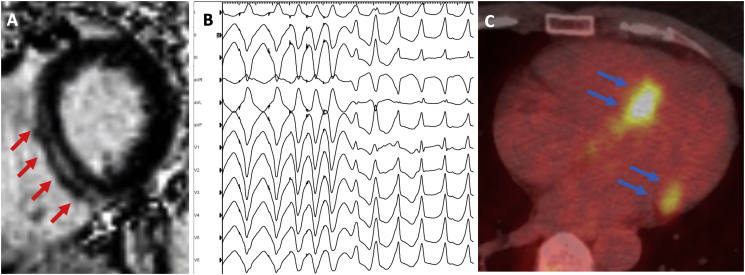
The positive predictive value (PPV) of EPS for VA was 100%; the negative predictive value (NPV) of EPS for VA was 93%; the sensitivity (Sns) of EPS for VA was 91%; and the specificity (Spc) of EPS for VA was 100%. Using the log-rank test, patients with negative EPS demonstrated significantly greater VA-free survival than patients with positive EPS (p < 0.0001). Among 12 patients with no conventional indication for ICD placement, the PPV of EPS for VA was 100%; the NPV of EPS for VA was 90%; the Sns of EPS for VA was 67%; and the Spc of EPS for VA was 100%. Among 17 patients with ICDs in place, the PPV of EPS for VA was 100%; the NPV of EPS for VA was 86%; the Sns of EPS for VA was 91%; and the Spc of EPS for VA was 100%. Among 7 patients with ICDs in place but no conventional indication for ICD placement, the PPV of EPS for VA was 100%; the NPV of EPS for VA was 86%; the Sns of EPS for VA was 67%; and the Spc of EPS for VA was 100%. Table 2 summarizes the predictive value of EPS among patients in our study. Fig. 2 shows an example of a patient with CS and LVEF of 68% who had midmyocardial septal LGE on CMR, inducible monomorphic VT on EPS, and ultimately experienced an appropriate ICD shock (Panels A and B); as well as an example of a different patient with focal FDG uptake on cardiac PET (Panel C).
Table 2.
Predictive value of EPS in CS.
| VA+ | VA− | Raw | Percent | Raw | Percent | |||
|---|---|---|---|---|---|---|---|---|
| All patients (n = 25) | ||||||||
| EPS+ | 10 | 0 | PPV | 10/10 | 100 | Sns | 10/11 | 91 |
| EPS− | 1 | 14 | NPV | 14/15 | 93 | Spc | 14/14 | 100 |
| Patients with LVEF >35% and no prior VA (n = 12) | ||||||||
| EPS+ | 2 | 0 | PPV | 2/2 | 100 | Sns | 2/3 | 67 |
| EPS− | 1 | 9 | NPV | 9/10 | 90 | Spc | 9/9 | 100 |
| Patients with ICDs in situ (n = 17) | ||||||||
| EPS+ | 10 | 0 | PPV | 10/10 | 100 | Sns | 10/11 | 91 |
| EPS− | 1 | 6 | NPV | 6/7 | 86 | Spc | 6/6 | 100 |
| Patients with LVEF >35%, no prior VA, and ICDs in situ (n = 7) | ||||||||
| EPS+ | 2 | 0 | PPV | 2/2 | 100 | Sns | 2/3 | 67 |
| EPS− | 1 | 4 | NPV | 4/5 | 80 | Spc | 4/4 | 100 |
EPS = electrophysiology study; LVEF = left ventricular ejection fraction; ICD = implantable cardioverter defibrillator; NPV = negative predictive value; PPV = positive predictive value; Sns = sensitivity; Spc = specificity; VA = ventricular arrhythmia.
Fig. 2.
Imaging and electrophysiology findings in patients with CS. A. Cardiac magnetic resonance imaging in a patient with LVEF 68%. Short axis slice at the basal level showing midmyocardial septal scar (red arrows). B. Electrophysiology study in the same patient showing induction of sustained monomorphic ventricular tachycardia with a right-bundle-branch-block pattern and a left inferior axis with double extrastimuli. C. Cardiac positron emission tomography in a different patient showing focal FDG avidity in the basal septum and lateral wall (blue arrows).
4. Discussion
Many patients with known or suspected CS undergo CMR and/or cardiac PET, both of which have prognostic value in predicting VA [7,8]. We sought to assess the utility of EPS in patients with CS and abnormal cardiac imaging, focusing on those without conventional indications for ICD placement. We showed that among patients with CS and LGE on CMR, FDG on PET, or both, EPS has a good PPV and NPV for VA. Since there is already a clear role for ICD placement in patients with LVEF <35% or a prior history of VA, we focused our analysis on patients without conventional indications for ICD placement. We showed that among patients with LVEF >35% and no prior history of VA, EPS similarly has a good PPV and NPV for VA. In order to address concern that patients with inducible VA on EPS may be more likely to undergo ICD placement and may, in turn, be more likely to have arrhythmic events detected, we separately analyzed those patients with ICDs in situ. In this population, EPS had a good PPV and modest NPV for VA.
Current guidelines support the use of CMR for risk stratification in patients with CS [12]. Areas of LGE on CMR correlate well with areas of low-voltage on electroanatomical mapping [16]. The presence of LGE on CMR predicts arrhythmic outcomes in patients with CS, including those with LVEF >35 [8]. Coleman and coworkers carried out a meta-analysis of 10 studies including 760 patients with known or suspected CS undergoing CMR [8]. The mean follow up duration was 3 years and the average LVEF was 58%. Among patients with an LVEF >50%, the presence of LGE predicted VA or death with an odds ratio of 19.43 [7.62, 49.56]. At the same time, among patients with LVEF <50%, the presence of LGE did not appear to predict VA or death (odds ratio 2.16 [0.53, 8.75]).
While its use is not yet supported by guidelines, cardiac PET may also be useful in predicting VA in patients with CS, including those with LVEF >35%. Blankstein and coworkers studied 118 patients with suspected CS undergoing 18-flourodeoxyglucose (FDG) cardiac PET [7]. The mean LVEF was 47 +/− 16%. Among these patients, the presence of focal FDG uptake on PET, indicating active inflammation, was highly predictive of VT or death. At a medium follow up of 1.5 years, the combined presence of perfusion defects and focal FDG uptake predicted VT or death with a hazard ratio of 3.9 (1.5–10.3). While the analysis was not further stratified by LV systolic function, among patients with adverse events, the mean LVEF was 40 +/− 15% and only 31% had a prior history of sustained VT.
Although the presence LGE on CMR or uptake of FDG on PET predict VA in patients with CS, the majority of patients with these imaging abnormalities do not have VA. There is therefore a need for further risk stratification among patients with CS and abnormal cardiac imaging. The 2014 HRS Expert Consensus Statement on the Diagnosis and Management of Arrhythmias Associated With Cardiac Sarcoidosis recommends EPS for further risk stratification in these patients [12]. However, the data on utility of EPS for this purpose is limited.
Aizer and coworkers assessed 32 patients with CS undergoing EPS [10]. Among 12 who had spontaneous or inducible VA, all underwent placement of ICDs and 9 (75%) had appropriate device therapy during follow up. Among 20 who had neither spontaneous nor inducible VA, none underwent placement of ICDs and 2 (10%) had VA during follow up. The positive predictive value of EPS for VA in this study was 75%, while the negative predictive value of EPS was 90%. Mehta and colleagues subsequently assessed 76 patients with probable CS undergoing EPS [11]. Among 8 who had inducible VA, 6 (75%) had VA during a median follow up of 5 years. Among 68 who had no inducible VA, 1 (1.5%) had VA during follow up. The positive predictive value of EPS for VA was 75%, while the negative predictive value of was 98.5%. However, this study did not assess the additive utility of EPS to LVEF in predicting VA. Indeed, patients with a positive EPS had a mean baseline LVEF of 36%, and only 1 patient with a preserved LVEF had a positive EPS.
The present study builds upon the exiting literature by showing that the predictive utility of EPS is preserved in CS patients with abnormal cardiac imaging and LVEF >35%. This may help to prevent unnecessary ICD implantation, which, in turn, may help to prevent procedural complications and inappropriate device therapies, which occur in up to 17% of patients with CS [17]. In our study, among patients with 12 patients with no conventional indications for ICD therapy, 10 had negative EPS, among whom 1 eventually had VA. It is important to note that this patient, despite having an initially preserved LVEF, suffered deterioration of the LVEF to <15% over the 7 years between the negative EPS and the VA event. This underscores the importance of continued clinical monitoring after a negative EPS, and suggests a role for repeat EPS in the case of a decline in LVEF.
Finally, among patient with ICD in place, our study suggests that there may be a role for EPS in predicting appropriate tachytherapies. This may help to guide the use of antiarrhythmic drugs or catheter ablation in the management of patients with CS and ICDs.
4.1. Study limitations
This study had several limitations. First, because of the retrospective nature of the study design, not all patients were followed in a uniform manner. The possibility cannot be excluded that some patients had VA events that were not documented in our EMR. Similarly, the method of EMR query for the identification of VA events in a retrospective fashion has not been independently validated, nor assessed for sensitivity or specificity. Our separate analysis of patients with ICDs in situ was specifically designed to address this concern. Additionally, our results may have been subject to referral bias in that out of 76 patients meeting HRS criteria for CS with abnormal cardiac imaging, only 25 underwent EPS. We believe this reflects evolving practice patterns and practice variability among referring physicians. However, among the 51 patients not undergoing EPS, none had VA events on follow up. This suggests that the group undergoing EPS represents a higher-risk cohort. Lastly, our study is limited by a small sample size. Despite being a large referral center, due to the rare nature of the disease being studied, our experience was limited to the patient population reported above.
5. Conclusions
Electrophysiology study may help with clinical risk stratification in patients with CS and abnormal imaging, including those with LVEF >35% and those without conventional indications for ICD therapy. Among patients with LVEF >35% and no history of prior VA, a negative EPS has good positive and negative predictive value for future VA events, and may help to prevent unnecessary ICD implantation. Among patients with ICDs, positive EPS may predict appropriate shocks and may guide the use of antiarrhythmic drug therapy and/or catheter ablation.
Grant support
None.
Conflicts of interest
None.
References
- 1.ATS Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am. J. Respir. Crit. Care Med. 1999;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 2.Silverman K.J., Hutchins G.M., Bulkley B.H. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58:1204–1211. doi: 10.1161/01.cir.58.6.1204. [DOI] [PubMed] [Google Scholar]
- 3.Te A.L., Lin Y.J., Chen Y.Y., Chung F.P., Chang S.L., Lo L.W., Hu Y.F. Increased risk of ventricular tachycardia in patients with sarcoidosis during the very long term follow-up. Int. J. Cardiol. 2017;228:68–73. doi: 10.1016/j.ijcard.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 4.Muser D., Santangeli P., Pathak R.K., Castro S.A., Liang J.J., Magnani S., Hayashi T. Long-term outcomes of catheter ablation of ventricular tachycardia in patients with cardiac sarcoidosis. Circ. Arrhythm. Electrophysiol. 2016;9(8) doi: 10.1161/CIRCEP.116.004333. [DOI] [PubMed] [Google Scholar]
- 5.Uusimaa P., Ylitalo K., Anttonen O., Kerola T., Virtanen V., Pääkkö E., Raatikainen P. Ventricular tachyarrhythmia as a primary presentation of sarcoidosis. Europace. 2008;10(6):760–766. doi: 10.1093/europace/eun110. [DOI] [PubMed] [Google Scholar]
- 6.Yazaki Y., Isobe M., Hiroe M., Morimoto S., Hiramitsu S., Nakano T., Izumi T., Sekiguchi M. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am. J. Cardiol. 2001;88(9):1006–1010. doi: 10.1016/s0002-9149(01)01978-6. [DOI] [PubMed] [Google Scholar]
- 7.Blankstein R., Osborne M., Naya M., Waller A., Kim C.K., Murthy V.L., Kazemian P. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J. Am. Coll. Cardiol. Feb 4 2014;63(4):329–336. doi: 10.1016/j.jacc.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman G.C., Shaw P.W., Balfour P.C., Jr., Gonzalez J.A., Kramer C.M., Patel A.R., Salerno M. Prognostic value of myocardial scarring on CMR in patients with cardiac sarcoidosis. JACC Cardiovasc. Imaging. 2017;10(4):411–420. doi: 10.1016/j.jcmg.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smedema J.P., van Geuns R.J., Ector J., Heidbuchel H., Ainslie G., Crijns H.J.G.M. Right ventricular involvement and the extent of left ventricular enhancement with magnetic resonance predict adverse outcome in pulmonary sarcoidosis. ESC Heart Fail. 2017;5(1):157–171. doi: 10.1002/ehf2.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aizer A., Stern E.H., Gomes J.A., Teirstein A.S., Eckart R.E., Mehta D. Usefulness of programmed ventricular stimulation in predicting future arrhythmic events in patients with cardiac sarcoidosis. Am. J. Cardiol. 2005;96:276–282. doi: 10.1016/j.amjcard.2005.03.059. [DOI] [PubMed] [Google Scholar]
- 11.Mehta D., Mori N., Goldbarg S.H., Lubitz S., Wisnivesky J.P., Teirstein A. Primary prevention of sudden cardiac death in silent cardiac sarcoidosis: role of programmed ventricular stimulation. Circ. Arrhythm. Electrophysiol. 2011;4(1):43–48. doi: 10.1161/CIRCEP.110.958322. [DOI] [PubMed] [Google Scholar]
- 12.Birnie D.H., Sauer W.H., Bogun F., Cooper J.M., Culver D.A., Duvernoy C.S., Judson M.A. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. Jul 2014;11(7):1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 13.Zghaib T., Ipek E.G., Hansford R., Ashikaga H., Berger R.D., Marine J.E., Spragg D.D. Standard ablation versus magnetic resonance imaging-guided ablation in the treatment of ventricular tachycardia. Circ. Arrhythm. Electrophysiol. 2018;11(1) doi: 10.1161/CIRCEP.117.005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams G., Kolodny G.M. Suppression of myocardial 18F-FDG uptake by preparing patients with a high-fat, low-carbohydrate diet. Am. J. Roentgenol. 2008;190:W151–W156. doi: 10.2214/AJR.07.2409. [DOI] [PubMed] [Google Scholar]
- 15.Kruse M.J., Kovell L., Kasper E.K., Pomper M.G., Moller D.R., Solnes L., Chen E.S. Myocardial blood flow and inflammatory cardiac sarcoidosis. J. Am. Coll. Cardiol. Img. 2017;10:157–167. doi: 10.1016/j.jcmg.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 16.Thajudeen A., Jackman W.M., Stewart B., Cokic I., Nakagawa H., Shehata M., Amorn A.M. Correlation of scar in cardiac MRI and high-resolution contact mapping of left ventricle in a chronic infarct model. Pacing Clin. Electrophysiol. 2015;38(6):663–674. doi: 10.1111/pace.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Betensky B.P., Tschabrunn C.M., Zado E.S., Goldberg L.R., Marchlinski F.E., Garcia F.C., Cooper J.M. Long-term follow-up of patients with cardiac sarcoidosis and implantable cardioverter-defibrillators. Heart Rhythm. 2012;9(6):884–891. doi: 10.1016/j.hrthm.2012.02.010. [DOI] [PubMed] [Google Scholar]