Abstract
Background
Controversy still exists that whether clopidogrel should add proton pump inhibitors (PPIs) in patients with coronary heart disease after percutaneous coronary intervention (PCI). The aim of this study was to evaluate the efficacy and safety of clopidogrel added proton pump inhibitors (PPIs) vs. clopidogrel for the treatment of patients with coronary heart disease after percutaneous coronary intervention (PCI).
Methods and results
We systematically searched PubMed, EMBASE, Web of Science, the Chinese Biomedical Medical Literature database, and the Cochrane Library for all clinical trials that were published on this topic through October 2018. We specifically selected the clinical trials that evaluated the efficacy and safety of clopidogrel added proton pump inhibitors vs. clopidogrel in the treatment of patients with coronary heart disease after PCI. RevMan 5.0 software was used for quantitative data analyses.
15 randomized controlled trials including 50,366 patients were included. The meta-analysis results showed that compared with the clopidogrel added PPI group, the non-PPI group had significantly less risk of MACE[RR = 0.82,95%CI:0.77–0.88], myocardial infarction recurrence[RR = 0.72,95%CI:0.57–0.90], stent thrombosis[RR = 0.71,95%CI:0.56–0.92], Target vessel revascularization (TVR)[RR = 0.77,95%CI:0.63–0.93] and stroke [RR = 0.72,95%CI:0.67–0.76]. The risks of all cause death [RR = 1.14,95%CI:0.85–1.51], cardiovascular death [RR = 1.14, 95% CI: 0.85–1.52], bleedings events [RR = 1.60,95%CI:0.53–4.81] were similar in the two groups.
Conclusions
The patients in the non-PPI group were observed to be associated with less risk of MACE, myocardial infarction recurrence, stent thrombosis, target vessel revascularization (TVR) and stroke. And the two groups had similar all cause death, cardiovascular death, bleedings events.
Keywords: Clopidogrel, Proton pump inhibitor (PPI), Cardiovascular outcome, Meta-analysis
1. Introduction
Nowadays the dual antiplatelet therapy (DAPE) with aspirin and clopidogrel has been commonly used for the patients with coronary heart disease (CHD) after percutaneous coronary intervention (PCI) [1]. Clopidogrel, a kind of thienopyridine derivatives ticlopidine, could inhibit the platelet P2Y12 adenosine diphosphate receptors irreversibly, so it is widely used to reduce the risk of death and cardiovascular events in the patients with acute coronary syndrome. As DAPE could have some adverse effects such as bleedings, so proton pump inhibitors (PPIs), with strong suppressive effects on gastric acid secretion, are commonly used concomitantly with clopidogrel to reduce the gastrointestinal bleeding risks. Some researches reported that the PPIs could reduce the efficacy of clopidogrel's protect roles in cardiovascular events with the inhibition of the hepatic cytochrome P450(CYP)2 C19 [2], however, whether the PPIs could increase the morbidity is still in the controversy.
Several large meta- analyses have reported that the clinical efficacy is reduced when PPIs are added to the treatment of common cardiovascular patients with or without PCI operations [23,25], whereas there is a lack of large clinical studies that have focused on the efficacy and safety of patients after percutaneous coronary intervention (PCI). The focus of our meta-analysis is “post-PCI patients”, to summarize comprehensive clinical trials that have been conducted to evaluate the efficacy and safety of clopidogrel alone vs. clopidogrel added PPI in the treatment of cardiovascular patients after PCI.
2. Methods
2.1. Search strategy and selection criteria
To identify relevant studies, we conducted a systematic review using PubMed, EMBASE, Web of Science, the Chinese Biomedical Medical Literature database, and the Cochrane Library. Studies that were published through October 2018 were included in the meta-analysis. The following keywords were used in these databases to search for studies: “clopidogrel”, “proton pump inhibitor, PPI”, “OME (omeprazole)”, “Esomeprazole”, “pantoprazole”, “lansoprazole”, and “PCI, percutaneous coronary intervention”. Two reviewers independently searched for and reviewed papers and any disagreements regarding studies to include were discussed and agreed upon by these two reviewers.
The inclusion criteria for studies in the meta-analysis included: (1) RCT published in any form and language; (2) patient population consisting of coronary heart disease patients who have undergone PCI; and (3) intervention measures of clopidogrel plus PPI and clopidogrel only as a comparison. Studies that included the following characteristics were excluded from the meta-analysis:
(1) animal experiments and non-original studies (e.g. review papers, other meta-analyses); (2) use of other antiplatelet drugs or anticoagulation drugs; (3) cross-over studies with self-controls; and (4) significant differences between the groups in the baseline analysis or no mention of baseline analyses.
The quality of the identified studies that met the inclusion criteria for the meta-analysis was evaluated using criteria defined in the Cochrane Review handbook 5.0.2 [3]. We included the high-quality standard.
2.2. Data extraction and quality assessment
The quality of the identified studies that met the inclusion criteria for the meta-analysis was evaluated using criteria defined in the Cochrane Review handbook 5.0.2 [3]. These criteria involve issues related to randomization, allocation concealment, the blinding process, incomplete outcome data, selective outcome reporting, as well as other sources of bias. We evaluated each publication according to these criteria and judged each potential source of bias as “yes”, “no”, or “unclear” (i.e. lack of relevant information or the bias resulting from the criterion was uncertain). This process was conducted by each of two reviewers independently, and disagreements were discussed and resolved.
The primary end point in the meta-analysis was major acute cardiovascular events (MACEs), all-cause death, cardiovascular death and bleeding events. Secondary end points were myocardial infarction recurrence, Target vessel revascularization (TVR), stent thrombosis and stroke.
2.3. Data synthesis and meta-analysis
RevMan5.0 was used for the meta - analysis. The χ2 test was used to analyze heterogeneity between the studies. If Pheterogeneity > 0.1 and I2 < 50%, a fixed effects model was used for the analysis, whereas a random effects model was used if Pheterogeneity < 0.1 and I2 ≥ 50%. Descriptive analyses were conducted as expressed by the mean difference (MD) and 95% CI, and count data were expressed by OR and 95% CI. P < 0.05 was considered statistically significant in all analyses. The flow diagram was in the Fig. 1. The funnel plot was used for the potential publication bias examination.
Fig. 1.
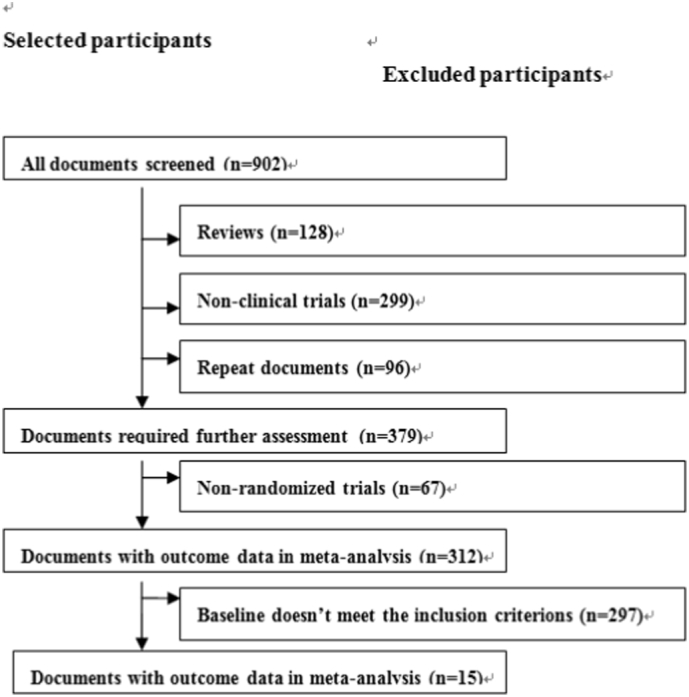
Flow diagram of article screening and selection process.
3. Results
3.1. Literature search and study characteristics
A total of 902 articles were identified using the specified search terms. After a preliminary review of the titles and abstracts of each paper, we eliminated 128 summaries, 299 non-clinical trials, and 96 repeated references across the different databases that were searched. Of the remaining potential studies, we further eliminated 67 non-randomized controlled trials and 297 articles that did not meet our inclusion criteria following a more comprehensive review of the full text of each article. This left a total of 15 clinical trials including 50,366 patients that were included in our final meta-analysis [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18],26], with 29,120 non-PPI patients and 21,246 clopidogrel added PPI patients. The main characteristics and our quality assessments of the included studies are described in Table 1, Table 2.
Table 1.
Basic characteristics of studies included in the Meta-Analysis
| Included studies | Groups |
Male |
Ages |
Hypertension |
Diabetes mellitus |
Patients type | Country | Outcomes | Follow-up | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No PPI use | PPI use | No PPI use | PPI use | No PPI use | PPI use | No PPI use | PPI use | No PPI use | PPI use | |||||
| 1 Burkard T 2011 | 692 | 109 | 79.9% | 68.8% | 63.3 ± 11.3 | 66.5 ± 10.5 | 65.0% | 72.5% | 17.2% | 29.6% | Post-PCI patients | Switzerland | MACE, death, MI, stent thrombosis, Target vessel revascularization (TVR) | 3 years |
| 2 Rolf P. Kreutz 2010 | 9862 | 6828 | 73.9% | 62% | 65.2 ± 10.6 | 67.5 ± 10.4 | 46.5% | 50.6% | 22.7% | 25.9% | Post-PCI patients | USA | Stroke, revascularization, cardiovascular death, MI, unstable angina | 12 months |
| 3 Robert a Rossini-2010 | 170 | 1158 | 81.2% | 75.6% | 63 ± 11 | 64 ± 11 | 65.2% | 63.6% | 28.0% | 27.1% | Post-PCI patients | Italy | MACE, major bleeding, minor bleeding, death, thrombosis | 1 years |
| 4 Ekta G 2010 | 243 | 72 | NG | NG | 62 ± 0.7 | 61.7 ± 1.2 | 68% | 76% | 30% | 36% | Post-PCI patients | USA | MACE, death, TVR,TVF | 50 months |
| 5 Evanchan 2009 | 4425 | 1369 | NG | NG | 62.9 | 63.5 | 64% | 61% | 36% | 46% | Post-PCI patients | USA | AMI | 4 years |
| 6 Hiroshi Y 2009 | 135 | 108 | 82.2% | 66.7% | 67 | 71 | NG | NG | NG | NG | Post-PCI patients | Japan | bleeding | 2 years |
| 7 Kishore J 2011 | 1902 | 751 | 72% | 62% | 64 ± 12 | 66 ± 11 | 65% | 73% | 27% | 30% | Post-PCI patients | USA | MACE, death, MI, TVR, stent thrombosis | 6 months |
| 8 Michael 2010 | 502 | 318 | 64.1% | 61.9% | 63.7 ± 11.6 | 63.8 ± 11.6 | 74.8% | 78.5% | 33.1% | 36.3% | Post-PCI patients | USA | Myocardial infarction, bleeding, MACE, Death, TVR, stent thrombosis | 1-year |
| 9 Takeo-2010 | 119 | 103 | 72.4% | 67.0% | 67.4 ± 10.1 | 69.0 ± 9.6 | 64.8% | 64.1% | 39.7% | 35.0% | Post-PCI patients | Japan | Bleeding, MACE, cardiovascular death, thrombosis, TVR | 3 years |
| 10 O' Donoghue 2009 | 4538 | 2257 | 74.7% | 70.3% | 60 | 62 | 63.5% | 65.9% | 22.5% | 24.2% | Post-PCI patients | USA | All cause death, cardiovascular death, MI, stent thrombosis, major bleeding, minor bleeding | 1 year |
| 11 Rahel H 2012 | 631 | 87 | 74.3% | 70.1% | 64 | 68 | 62.1% | 79.3% | 15.8% | 12.6% | Post-PCI patients | Switzerland | Bleeding | 23 months |
| 12 Jian Jun zou-2014 | 1456 | 6188 | 73.9% | 73.5% | 65.7 ± 10.6 | 66.2 ± 10.2 | 70.4% | 71.3% | 23.6% | 25.8% | Post-PCI patients | China | MACE, stent thrombosis, MI, death, TVR | 5 years |
| 13 Subhash 2010 | 3678 | 867 | 98.3% | 98.2% | 64.5 ± 10.3 | 63.8 ± 9.9 | 92.4% | 88.9% | 51.4% | 45.1% | Post-PCI patients | USA | MACE, death, revascularization | 6 years |
| 14 Zarris 2009 | 248 | 340 | 81.9% | 82.4% | 61.7 ± 10.8 | 62.1 ± 10.5 | 46.4% | 50.9% | 26.2% | 30.0% | Post-PCI patients | Greece | Death | 12 months |
| 15 Jolanta M-2008 | 519 | 691 | 72.6% | 65.4% | 64.44 ± 11.87 | 64.11 ± 12.42 | 78.2% | 73.3% | 26% | 18.7% | Post-PCI patients | Austria | All-cause death, cardiovascular death, stent thrombosis, MACE | 12 months |
Table 2.
Methods and results of included studies.
| Included studies | Randomization | Allocation concealment | Blinding | Incomplete outcome data | Selective outcome reporting | Other sources of bias |
|---|---|---|---|---|---|---|
| 1 Burkard T 2011 | Retrospective | Unclear | Unclear | No | No | No |
| 2 Rolf P. Kreutz 2010 | Retrospective | Unclear | Unclear | No | No | No |
| 3 Robert a Rossini-2010 | Yes | Unclear | No | No | No | No |
| 4 Ekta G 2010 | Retrospective | Unclear | Unclear | No | No | No |
| 5 Evanchan 2009 | Retrospective | Unclear | No | No | No | No |
| 6 Hiroshi Y 2009 | Retrospective | Unclear | Unclear | No | No | No |
| 7 Kishore J 2011 | Yes | Unclear | Unclear | No | No | No |
| 8 Michael 2010 | Yes | Unclear | Unclear | No | No | No |
| 9 Takeo-2010 | Retrospective | Unclear | Unclear | No | No | No |
| 10 O' Donoghue 2009 | Yes | Unclear | Unclear | No | No | No |
| 11 Rahel H 2012 | Retrospective | Unclear | No | No | No | No |
| 12 Jian Jun zou-2014 | Retrospective | Unclear | Unclear | No | No | No |
| 13 Subhash 2010 | Yes | Unclear | No | No | No | No |
| 14 Zarris 2009 | Yes | Unclear | Unclear | No | No | No |
| 15 Jolanta M-2008 | Yes | Unclear | Unclear | No | No | No |
3.2. MACEs
The risk of a MACE was evaluated in 9 clinical trials for 2179/9359 individuals in the non-PPI group and for 1539/10,257 individuals in the clopidogrel added PPI group after PCI. The meta-analysis of these data showed RR = 0.82,95%CI:0.77–0.88, which indicated that the non-PPI group had less MACE risk in comparison with clopidogrel added PPI group.
3.3. Death
The risk of all-cause death risk was evaluated in 9 clinical trials for 834/9419 individuals in the non-PPI group and for 459/10,494 individuals in the Clopidogrel added PPI group after PCI. The meta-analysis of these data showed RR = 1.00, 95% CI: 0.88–1.13, indicating that risk of all-cause death was not statistically different between the non-PPI group and the clopidogrel added PPI group. The risk of cardiovascular death risk was evaluated in 4 clinical trials for 139/15,107 individuals in the non-PPI group and for 71/9879 individuals in the clopidogrel added PPI group after PCI. The meta-analysis of these data showed RR = 1.14, 95% CI: 0.85–1.51, indicating that risk of cardiovascular death was not statistically different between the non-PPI group and the clopidogrel added PPI group. And the total results showed RR = 1.02, 95% CI: 0.91–1.15, so the results showed that the death risk was not statistically different between the non-PPI group and the Clopidogrel added PPI group.
3.4. Myocardial infarction recurrence
The myocardial infarction recurrence risk was evaluated in 6 clinical trials for 1671/21,647 individuals in the non-PPI group and for 1145/17,418 individuals in the clopidogrel added PPI group after PCI. The meta-analysis of these data showed RR = 0.72,95%CI:0.57–0.90, which indicated that the non-PPI group had lower risk of myocardial infarction recurrence than the Clopidogrel added PPI group after PCI (Table 3).
Table 3.
The meta-analysis results.
| Items | Non-PPI | Clopidogrel added PPI | RR (95% CI)/MH(95% CI) | P |
|---|---|---|---|---|
| 1 MACE | 2179/9359 | 1539/10,257 | RR = 0.82,95%CI:0.77–0.88 | P < 0.01 |
| 2 Death | 973/24526 | 530/20373 | RR = 1.14,95%CI:0.85–1.51 | P > 0.05 |
| 3 Myocardial infarction | 1671/21647 | 1145/17418 | RR = 0.72,95%CI:0.57–0.90 | P < 0.01 |
| 4 Stent thrombosis | 172/9976 | 168/11676 | RR = 0.71,95%CI:0.56–0.92 | P < 0.01 |
| 5 Target vessel revascularization (TVR) | 214/4749 | 471/7469 | RR = 0.77,95%CI:0.63–0.93 | P < 0.01 |
| 6 Bleeding | 170/6175 | 109/4815 | RR = 1.60,95%CI:0.53–4.81 | P > 0.05 |
| 7 Stroke | 1766/9862 | 1710/6328 | RR = 0.72,95%CI:0.67–0.76 | P < 0.01 |
3.5. Stent thrombosis
The stent thrombosis recurrence risk was evaluated in 8 clinical trials for 172/9976 individuals in the non-PPI group and for 168/11,575 individuals in the clopidogrel added PPI group after PCI. The meta-analysis of these data showed RR = 0.71,95%CI:0.56–0.92, which indicated that the non-PPI group had lower risk of stent thrombosis than the Clopidogrel added PPI group.
3.6. Target vessel revascularization (TVR)
Target vessel revascularization risk was evaluated in 5 clinical trials for 214/4749 individuals in the non-PPI group and for 471/7469 individuals in the clopidogrel group after PCI. The meta-analysis of these data showed RR = 0.77,95%CI:0.63–0.93, which indicated that the non-PPI group had lower risk of target vessel revascularization than the clopidogrel added PPI group.
3.7. Bleeding
The risk of bleeding was evaluated in 6 clinical trials for 170/6175 individuals in the Clopidogrel added PPI group and for 109/4815 individuals in the clopidogrel added PPI group after PCI. The meta-analysis of these data showed RR = 1.60,95%CI:0.53–4.81, which indicated that the risk of bleeding was not statistically different between the non-PPI and clopidogrel added PPI group.
3.8. Stroke
Stroke was evaluated in 1 clinical trial for 1766/9862 individuals in the non-PPI group and for 1710/6828 individuals in the clopidogrel added PPI group after PCI. The meta-analysis of these data showed RR = 0.72,95%CI:0.67–0.76, which indicated that non PPI group had less stroke incidence compared with the clopidogrel group added PPI group.
3.9. Publication bias
According to the funnel plots, there was no significant publication bias existed.
4. Discussion
The meta-analysis supports that the non-PPI patients showed less incidence in MACE, myocardial infarction recurrence, stent thrombosis, target vessel revascularization (TVR) and stroke compared to patients who received clopidogrel added PPI following PCI procedures. And the two groups had similar all cause death, cardiovascular death and bleedings events. And these results were inconsistent with several published papers [9,10].
Clopidogrel is a common antiplatelet drug that metabolized through the hepatic cytochrome P450(CYP)2 C19 enzyme. The biological activity of clopidogrel is binding the ADP receptor P2Y12 to prevent the fibrinogen polymerization. This process mainly relies on the CYP2C19 enzyme. At the same time, proton pump inhibitors PPIs also relies on CYP2C19 to irreversibly bind with proton cytoplasmic pumps in the gastric wall to reduce gastric acid secretion, so the coadministration PPIs could influence the activity of CYP2C19 by competitive inhibition and cause medicine interaction, which could lead to the reduced protective role in death and cardiovascular events, so the results of this meta-analysis supported this possibility as patients who received PPIs along with clopidogrel had an increased risk of MACE, myocardial infarction recurrence, stent thrombosis, target vessel revascularization (TVR) and stroke. The risk of MACE, which was a multi-event composite endpoint including myocardial infarction, stroke, target vessel revascularization and stent thrombosis, also showed less risk in the non-PPI group.
Some published papers illustrated that the clopidogrel concomitant with the PPIs could increase the cardiovascular risks [12,13]. Kreutz et al. [6] reported the large sample of 16,690 patients under PCI with stent implantation, the results showed clopidogrel concomitant with PPI was associated with a higher risk of MACE one year after stent placement [OR:1.51, 95%CI:1.39 to 1.64]. And Hulot JS et al.'s research [18] found that carriers of loss-of-function CYP2 C19*2 allele could displayed a 30% increase in the MACE risk compared with the noncarriers. And the single gene variant could also be associated with the higher risk in death, stent thrombosis. These results demonstrated that the clopidogrel could increase the risks of cardiovascular events and death through the CYP metabolic enzymes. And Hulot et al. [19] also carried out meta-analysis about the clinical outcome of the clopidogrel concomitant with the PPIs, and the results showed the PPI users displayed increased risk of MACE[21.8% vs. 16.7%, OR:1.41, 95%CI:1.34 to 1.48] and mortality [12.7% vs. 7.4%, OR:1.18, 95%CI:1.07 to 1.30] in comparison with the non-PPI users. These results were in consistent with ours. However, the difference between Hulot JS et al.'s research [19] and our research were the research population, that our research focused on the post-PCI patients. So clopidogrel added PPIs could increase the death and cardiovascular events risk, regardless of PCI or not-PCI. And Gilard M et al. [20] reported that through the vasodilator-stimulated phosphoprotein (VASP) phosphorylation test, the results showed omeprazole could significantly decreased clopidogrel's inhibitory effect on platelet P2Y12, which could be a mechanism illustration that PPIs influence clopidogrel.
Our results showed there were no significant difference in clopidogrel added PPIs and clopidogrel alone groups. PPIs are usually used for bleeding precaution in the clinical practice, however several included studies didn't find the clopidogrel added PPI group could significantly reduce the bleeding events. We consider the reasons could be attributed to reasons as follows: the bleeding events mainly refer to the gastrointestinal bleeding and puncture bleeding events. However, only a small number of the patients who under the clopidogrel treatment has a high risk in gastrointestinal bleeding and most patients didn't bleed when they used clopidogrel, so the bleeding events were small in all patients and didn't show significant difference between the clopidogrel added PPI and clopidogrel alone group. In addition, competitive drug interactions also existed between clopidogrel and PPI, so when used together, the PPIs' pharmaceutical activity may weaken by the drug interactions of clopidogrel, which could also reduce the PPI's gastrointestinal protective roles [[24], [25], [26]].
Furthermore, Roberta et al. [7] found that pantoprazole, which is not CYP 250 dependent PPIs, had lower bleeding events in (1.1%, 2/178) in comparison with the CYP 250 dependent PPIs such as omeprazole (7.1%, 9/125), with significant difference. And some published papers also had similar results. Frelinger et al. [21] carried out a randomized study to assess the effects that the different PPIs on the pharmacokinetics of clopidogrel, and the results showed the coadministration of dexlansoprazole or lansoprazole with clopidogrel could less inhibit clopidogrel's activation metabolite and less influence the platelet function than by the coadministration of esomeprazole or omeprazole, so the results further expounded the mechanism that the drug competitive interaction could influence the pharmaceutical activity.
A “black box warning” [22] was issued by the Food and Drug Administration(FDA) in March 2010 to announce that if the patients identified as CYP2C19 poor metabolizers, the clopidogrel should not be recommended to use. As omeprazole and esomeprazole is irreversible metabolism-dependent inhibitors (MDIs) of CYP2C19, however lansoprazole and pantoprazole are not, so lansoprazole and pantoprazole could have less drug interaction with clopidogrel and could be consider for gastrointestinal bleeding precaution in the clinical practice. The American FDA recommends the clopidogrel should avoid concomitant use of omeprazole or esomeprazole.
Several potential limitations existed in this study. First, some of the included studies didn't report on the subgroups of CYP2C19 enzyme dependent PPIs and CYP2C19 enzyme independent PPIs, so we could not further divide the groups into the more specific groups. Secondly, MACE is composited by several indicators such as death, myocardial infarction, target vessel revascularization, recurrence of heart failure, and arrhythmia, not all of which were evaluated in the included studies. The included studies only reported on some of the MACE indicators such as death, myocardial infarction, and target vessel revascularization, but did not analyze the recurrence of heart failure or arrhythmia, so we could not analyze these indicators.
5. Conclusions
In summary, our meta-analysis showed non-PPI group were observed to be associated with less risk of MACE, myocardial infarction recurrence, stent thrombosis, target vessel revascularization (TVR) and stroke. And the two groups had similar all cause death, cardiovascular death, bleedings events.
Sources of funding
This work was partially supported by the Natural Science Foundation of China [NO.81760071], The Traditional Chinese Medicine, Chinese Medicine Science and Technology Research in Guizhou Province[NO. QZYY-2016-076], Science and Technology Fund of Guizhou Health and Family Planning Commission [NO. gzwjkj2018-1-025], National Clinical Key Specialty Construction Project of China [NO. (2013)544], Clinical Research Center Project of Department of Science and Technology of Guizhou Province [NO. (2017)5405], Provincial Science and Technology Fund Project of Guizhou Province.Basic Research Projects of Guizhou Province [Guizhou Foundation in Scientific cooperation (2019) 1190].
Disclosures
None.
References
- 1.Faggioni M., Baber U., Chandrasekhar J. Use of prasugrel vs clopidogrel and outcomes in patients with and without diabetes mellitus presenting with acute coronary syndrome undergoing percutaneous coronary intervention. Int. J. Cardiol. 2018 doi: 10.1016/j.ijcard.2018.10.071. 10.S0167-5273(18)34231-1. [DOI] [PubMed] [Google Scholar]
- 2.Zocca P., Kok M.M., van der Heijden L.C. High bleeding risk patients with acute coronary syndromes treated with contemporary drug-eluting stents and Clopidogrel or Ticagrelor: Insights from CHANGE DAPT. Int. J. Cardiol. 2018;10:11–17. doi: 10.1016/j.ijcard.2018.03.116. [DOI] [PubMed] [Google Scholar]
- 3.Simon T., Steg P.G., Gilard M. Clinical events as a function of proton pump inhibitor use, clopidogrel use, and cytochrome P450 2C19 genotype in a large nationwide cohort of acute myocardial infarction: results from the French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) registry. Circulation. 2011;8:474–482. doi: 10.1161/CIRCULATIONAHA.110.965640. [DOI] [PubMed] [Google Scholar]
- 5.Burkard T., Laiser C.A., Rocca H. Brunner-La. Combined clopdogrel and proton pump inhibitor therapy is associated with higher cardiovascular event rates after percutaneous coronary intervention: a report from the BASKET trail. J. Intern. Med. 2011;271:257–263. doi: 10.1111/j.1365-2796.2011.02423.x. [DOI] [PubMed] [Google Scholar]
- 6.Kreutz R.P., Stanek E.J., Aubert R. Impact of proton pump inhibitors on the effectiveness of clopidogrel after coronary stent placement: the clopidogrel Medco outcomes study. Pharmacotherapy. 2010;30:787–796. doi: 10.1592/phco.30.8.787. [DOI] [PubMed] [Google Scholar]
- 7.Rossini R., Capodanno D., Musumeci G. Safety of clopidogrel and proton pump inhibitors in patients undergoing drug-eluting stent implantation. Coron. Artery Dis. 2011;22:199–205. doi: 10.1097/MCA.0b013e328343b03a. [DOI] [PubMed] [Google Scholar]
- 8.Gupta Ekta, Bansal Darpan, Sotos John. Risk of Adverse clinical outcomes with concomitant use of clopidogrel and proton pump inhibitors following percutaneous coronary intervention. Dig. Dis. Sci. 2010;55:1964–1968. doi: 10.1007/s10620-009-0960-8. [DOI] [PubMed] [Google Scholar]
- 9.Evanchan Jason, Donnally Michael R., Binkley Phillip. Recurrence of acute myocaridial infarction in patietns discharged on clopidogrel and a proton pump inhibitor after stent placement for acute myocardial infarction. Clin. Cardiol. 2010;33:168–171. doi: 10.1002/clc.20721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasuda H., Yamada M., Sawada S. Upper gastrointestinal bleeding in patients receiving dual antiplatelet therapy after coronary stenting. Intern. Med. 2009;48:1725–1730. doi: 10.2169/internalmedicine.48.2031. [DOI] [PubMed] [Google Scholar]
- 11.Harjai K.J., Shenoy C., Orshaw P. Clinical outcomes in patients with the concomitant use of clopidogrel and proton pump inhibitors after percutaneous coronary intervention. Circ Cardiovasc Interv. 2011;1:162–170. doi: 10.1161/CIRCINTERVENTIONS.110.958884. [DOI] [PubMed] [Google Scholar]
- 12.Gaglia M.A., Jr., Torguson R., Hanna N. Relation of proton pump inhibitor use after percutaneous coronary intervention with drug-eluting stents to outcomes. Am. J. Cardiol. 2010;15:833–838. doi: 10.1016/j.amjcard.2009.10.063. [DOI] [PubMed] [Google Scholar]
- 13.Yasu T., Ikee R., Miyasaka Y. Efficacy and safety of concomitant use of rabeprazole during dual-antiplatelet therapy with clopidogrel and aspirin after drug-eluting stent implantation: a retrospective cohort study. Yakugaku Zasshi. 2010;30:1743–1750. doi: 10.1248/yakushi.130.1743. [DOI] [PubMed] [Google Scholar]
- 14.O'Donoghue M.L., Braunwald E., Antman E.M. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trails. Lancet. 2009;374:989–997. doi: 10.1016/S0140-6736(09)61525-7. [DOI] [PubMed] [Google Scholar]
- 15.Hauptle R., Weilenmann D., Schneider T. Individualised PPU prescription in patients on combination antiplatelet therapy and upper gastrointestinal events after percutaneous coronary intervention: a cohort study. Wien. Med. Wochenschr. 2012;162:67–73. doi: 10.1007/s10354-012-0056-5. [DOI] [PubMed] [Google Scholar]
- 16.Zou J.J., Chen S.L., Tan J. Increased risk for developing major adverse cardiovascular events in stented Chinese patients treated with dual antiplatelet therapy after concomitant use of the proton pump inhibitor. PLoS One. 2014;8 doi: 10.1371/journal.pone.0084985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banerjee S., Weideman R.A., Weideman M.W. Effect of concomitant use of clopidogrel and proton pump inhibitors after percutaneous coronary intervention. Am. J. Cardiol. 2011;107:871–878. doi: 10.1016/j.amjcard.2010.10.073. [DOI] [PubMed] [Google Scholar]
- 18.Zairis M.N., Tsiaousis G.Z., Patsourakos N.G. The impact of treatment with omeprazole on the effectiveness of clopidogrel drug therapy during the first year after successful coronary stenting. Can J Cardiol. 2010;26:54–57. doi: 10.1016/s0828-282x(10)70008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hulot J.S., Collet J.P., Silvain J. Cardiovascular risk in clopidogrel-treated patients according to cytochrome P450 2C19*2 loss-of-function allele or proton pump inhibitor coadministration: a systematic meta-analysis. J. Am. Coll. Cardiol. 2010;6:134–143. doi: 10.1016/j.jacc.2009.12.071. [DOI] [PubMed] [Google Scholar]
- 20.Gilard M., Arnaud B., Cornily J.C. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J. Am. Coll. Cardiol. 2008;22:256–260. doi: 10.1016/j.jacc.2007.06.064. [DOI] [PubMed] [Google Scholar]
- 21.Frelinger A.L., III, Lee R.D. A randomized, 2-period, crossover design study to assess the effects of dexlansoprazole, lansoprazole, esomeprazole, and omeprazole on the steady-state pharmacokinetics and pharmacodynamics of clopidogrel in healthy volunteers. J. Am. Coll. Cardiol. 2012;3:1304–1311. doi: 10.1016/j.jacc.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Food and Drug Administration. FDA Drug Safety Communication Reduced effectiveness of Plavix (clopidogrel) in patients who are poor metabolizers of the drug. http://www.fda.gov/Drugs/DrugSafety/Postmarket Drug Safety Information for Patients and Providers/ucm203888.htm#ds Available at:
- 23.Kwok C.S., Loke Y.K. Meta-analysis: the effects of proton pump inhibitors on cardiovascular events and mortality in patients receiving clopidogrel. Aliment. Pharmacol. Ther. 2010;31:810–823. doi: 10.1111/j.1365-2036.2010.04247.x. [DOI] [PubMed] [Google Scholar]
- 24.Agewall S., Cattaneo M., Collet J.P. ESC Working Group on Cardiovascular Pharmacology and Drug Therapy and ESC Working Group on Thrombosis.Expert position paper on the use of proton pump inhibitors in patients with cardiovascular disease and antithrombotic therapy. Eur. Heart J. 2013;34:1708–1713. doi: 10.1093/eurheartj/eht042. [DOI] [PubMed] [Google Scholar]
- 25.Huang B., Huang Y., Li Y. Adverse cardiovascular effects of concomitant use of proton pump inhibitors and clopidogrel in patients with coronary artery disease: a systematic review and meta-analysis. Arch. Med. Res. 2012;43:212–224. doi: 10.1016/j.arcmed.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Hulot J.S., Collet J.P., Silvain J. Cardiovascular risk in clopidogrel-treated patients according to cytochrome P450 2C19*2 loss-of-function allele or proton pump inhibitor coadministration: a systematic meta-analysis. J. Am. Coll. Cardiol. 2010;6:134–143. doi: 10.1016/j.jacc.2009.12.071. [DOI] [PubMed] [Google Scholar]


