Abstract
As clinical applications for chimeric antigen receptor T cell (CART) therapy extend beyond early phase trials, commercial manufacture incorporating cryopreservation steps becomes a logistical necessity. The effect of cryopreservation on CART characteristics is unclear. We retrospectively evaluated the effect of cryopreservation on product release criteria and in vivo characteristics in 158 autologous CART products from 6 single-center clinical trials. Further, from 3 healthy donor manufacturing runs, we prospectively identified differentially expressed cell surface markers and gene signatures among fresh versus cryopreserved CARTs. Within 2 days of culture initiation, cell viability of the starting fraction (peripheral blood mononuclear cells [PBMNCs]) decreased significantly in the cryo-thawed arm compared to the fresh arm. Despite this, PBMNC cryopreservation did not affect final CART fold expansion, transduction efficiency, CD3%, or CD4:CD8 ratios. In vivo CART persistence and clinical responses did not differ among fresh and cryopreserved final products. In healthy donors, compared to fresh CARTs, early apoptotic cell-surface markers were significantly elevated in cryo-thawed CARTs. Cryo-thawed CARTs also demonstrated significantly elevated expression of mitochondrial dysfunction, apoptosis signaling, and cell cycle damage pathways. Cryopreservation during CART manufacture is a viable strategy, based on standard product release parameters. The clinical impact of cryopreservation-related subtle micro-cellular damage needs further study.
Keywords: CAR T cells, cryopreservation, PBMNC, gene expression profiling, early apoptotic cells, chimeric antigen receptor T cells, viability, leukemia, multiple myeloma, lymphoma
Panch et al. demonstrate that CART cryopreservation during manufacture minimally affects final product characteristics, in vivo levels, persistence, and clinical response. However, increases in early apoptotic markers and cell damage pathways in cryo-thawed CARTs require careful consideration to better formulate dosing strategies.
Introduction
In a historic action in 2017, the US Food and Drug Administration (FDA) ushered in 2 new gene therapies for the treatment of hematologic malignancies in the United States.1, 2 As living drugs, these reprogrammed autologous chimeric antigen receptor T cells (CARTs) represent a new age of innovative cancer treatment. Concurrently, they present unique manufacturing3 and commercialization4 challenges that differ from those of traditional pharmaceuticals. Most CARTs are manufactured from autologous peripheral blood mononuclear cells (PBMNCs) collected by leukapheresis, followed by T cell selection, activation, gene modification, and expansion.5 The expanded CARTs are then formulated for infusion into the patient. Each semi-automated processing step above may introduce inter-product variability.
Commercial large-scale manufacturing of autologous CARTs further requires cell transportation to and from centralized processing facilities, and cell cryopreservation becomes a logistical necessity. As a result of the time required for safety and potency testing that includes assessing transduction efficiency (TE), sterility testing, and testing for the absence of replication-competent virus, most commercial manufacturers have elected to cryopreserve the final CART product. The autologous PBMNC starting material is also often cryopreserved to allow for flexibility in scheduling manufacturing.6
While hematopoietic stem cells survive cryopreservation and thawing well, and cryopreserved hematopoietic stem cell transplantation has been performed for many years,7, 8 the effects of the cryopreservation on cultured T cells is less certain. The data available suggest that the overall recovery of cryo-thawed T cells is marginally worse than that of hematopoietic stem cells.9, 10 Recently, one institution reported that CARTs cultured over prolonged periods (9–14 days), particularly in fully automated systems, may have worse post-thaw recovery11, 12 than other cultured T cells (Zhu et al., 2019, Transplantation & Cellular Therapy, annual meeting).
As a center manufacturing CARTs for early phase clinical trials since 2012, we often manufacture CARTs from cryopreserved PBMNCs, and we cryopreserve the final CART products. However, given the advanced nature of the hematologic malignancies of many patients and the need for urgent treatment, fresh, non-cryopreserved PBMNCs have been used to manufacture the CARTs, and the final CART products have been infused fresh immediately after the completion of manufacturing.
The primary objective of this study was to determine the impact of cryopreservation on CARTs. We evaluated factors used in current clinical practice for product safety, purity, potency, and consistency ascertainment. These included post-thaw CART recovery and post-thaw changes in TE, CD3%, and CD4:CD8 ratios. In a subset of patients who received fresh or cryopreserved final products at a standard dose, we compared in vivo CART levels, persistence over time, and clinical response. We also investigated the effects of cryopreserved autologous PBMNCs on the manufacturing process. Specifically, we studied post-thaw PBMNC recovery, fold expansion (FE), TE, and CD4:CD8 ratios during the manufacturing process. In addition, in 3 healthy volunteer donor CART manufacturing runs, we prospectively examined more subtle markers of cryopreservation-related cell damage.
Results
Patient and Product Characteristics
Data were obtained on 145 patients with a total of 158 consecutive autologous CART cultures; 12 patients received more than one infusion in the same protocol. The total numbers of infusions in 6 clinical trials of different CART types were as follows: CD19 CART pediatric (P) trial, n = 58; GD2 CARTs, n = 14; BCMA CARTs, n = 21; CD22 CARTs, n = 42; CD19 adult (A) CART trial, n = 21; and CD30 CARTs, n = 2. After discarding 11 products due to manufacturing failure during culture, 147 CARTs were prepared for infusion into pediatric or adult patients with hematologic malignancies (135, 91.8%) or solid tumors (12, 8.2%). The mean age was 27.7 years (±19.9) and weight was 60.7 kg (±26.3), with a median CART dose of 1 × 106 CAR-transduced viable CD3 ± cells/kg (range: 1 × 105–1 × 107). Gamma-retroviral or lentiviral vectors were used for CAR delivery in 88 (59.9%) and 59 (40.1%) infusates, respectively. See Table 1 for demographics.
Table 1.
Demographics
| Protocol | 12-C-0112 | 14-C-0059 | 14-C-0168 | 15-C-0029 | 16-C-0054 | 17-C-0048 | Total |
|---|---|---|---|---|---|---|---|
| CART type | CD19, CD28 | GD2, CD28, O × 40 | BCMA, CD28 | CD22, 41BB | CD19, CD28 | CD30, CD28 | – |
| Age group | pediatric + adult | pediatric + adult | adult | pediatric + adult | adult | adult | – |
| Diagnosis | ALL, DLBCL | osteosarcoma, neuroblastoma | multiple myeloma | ALL, DLBCL | ALL, DLBCL, MCL, FL, BL |
Hodgkin’s lymphoma | – |
| Infusion, n (%) | 56 (38.1) | 12 (8.2) | 20 (13.6) | 37 (25.2) | 20 (13.6) | 2 (1.4) | 147 (100) |
| Age (years) (mean ± SD) | 14.3 (±6.7) | 17.8 (±5.9) | 56.7 (±4.9) | 18.3 (±7.0) | 56.4 (±11.5) | 35.4(±8.9) | 27.7 (±19.9) |
| Male:female | 45:11 | 10:2 | 10:10 | 25:12 | 14:6 | 2:0 | 106:41 |
| Weight (kg) (mean ± SD) | 46.5 (±20.9) | 60.5 (±21.4) | 89 (±25.7) | 52.6 (±17.8) | 80.3 (±16.4) | 115 (±27.6) | 60.7 (±26.3) |
| CAR-T cell dose (million/kg) (mean ± SD) | 1.2 (±0.6) | 2.9 (±3.9) | 5.4 (±3.8) | 1.1 (±0.7) | 2.5 (±2.1) | 0.3 | 2.0 (±2.5) |
| Delivery vector | gamma-retrovirus | gamma-retrovirus | gamma-retrovirus | lentivirus | lentivirus | lentivirus | – |
| Pre-culture lymphocyte enrichment (n) | elutriation (5), bead enriched (46), both(5) | elutriation (5), bead enriched (7), both (0) | Ficoll (20) | elutriation (7), bead enriched (15), both (13), none (2) | Ficoll (20) | Ficoll (2) | – |
ALL, acute lymphoblastic leukemia; DLBCL, diffuse large B cell lymphoma; MCL, mantle cell lymphoma; FL, follicular lymphoma; BL, Burkitt’s lymphoma.
Of the 147 infusates, the starting fraction (PBMNCs) was cryopreserved for 70 infusions. For 79 infusions, the final harvested CART product was cryopreserved and thawed prior to infusion. For 50 CART products, both the PBMNCs and final CART product were cryopreserved. The median duration of cryopreservation was 6 days (range: 3–868) and 9 days (range: 1–408) for the starting PBMNCs and the final harvested CARTs, respectively. See Figure 1 and Table S1 for overall manufacturing schema and studies described in the Materials and Methods, and see Table S2 for product cryopreservation details by protocol.
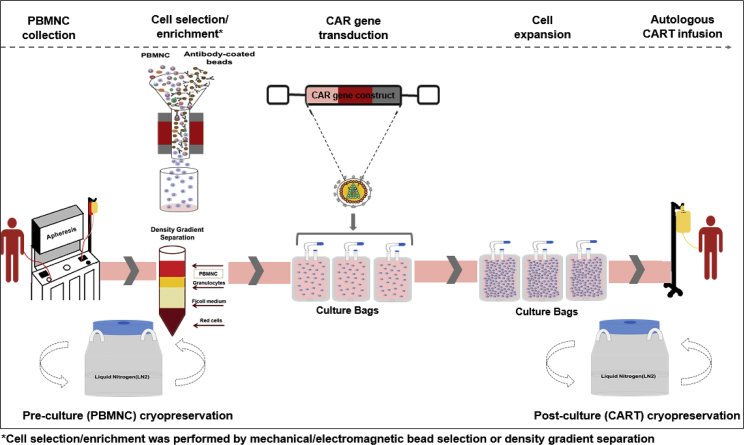
Figure 1.
CART Manufacturing Schema
A simplified diagram of the CART manufacturing process across the various manufacturing protocols at the NIH Center for Cellular Engineering. Starting with collection of PBMNCs by apheresis, the fresh or cryo-thawed cells (PBMNCs) are processed by mechanical or electromagnetic bead selection or density gradient centrifugation. Following the cell selection and enrichment process, CAR gene transduction, cell expansion, and harvest are performed over 7–9 days. After testing for sterility, the final CART product is either released fresh for infusion or cryopreserved again for future thaw and infusion. At the time of release, viability (post-thaw) and counts are repeated on cryo-thawed CARTs for cell dose calculations.
Manufacturing CARTs Using Cryopreserved PBMNCs
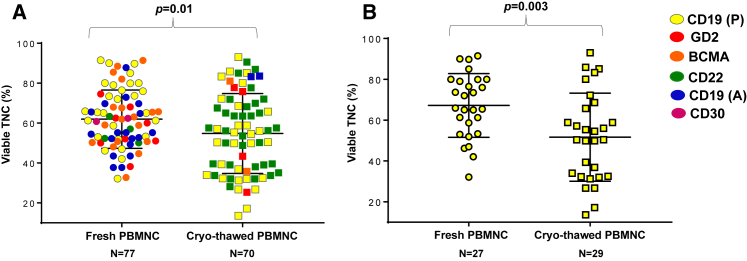
At 2 days after PBMNC culture initiation (stimulation period), viable total nucleated cell (TNC, %) was significantly lower for cultures initiated from cryopreserved PBMNCs compared to fresh cells (Figure 2A). A separate analysis of the processing of CD19 CARTs (P) confirmed this finding (Figure 2B). The CD3% was unavailable for all products on day 2 of T cell culture. Despite the decrease in cell counts on day 2, all products contained the required cell quantity for starting the transduction and expansion process.
Figure 2.
Comparison on Culture Day 2 of Viable TNC Quantities (%) between Fresh and Cryopreserved PBMNCs
The results for PBMNCs from all protocols are shown in (A) [CD19 (P) CARTs, yellow; GD2 CARTs, red; BCMA CARTs, orange; CD22 CARTs, green; CD19 (A) CARTs, blue; and CD30 CARTs, magenta], and the results for PBMNCs from the CD19 CART (P) protocol are shown in (B) (yellow). The scatter dot plots with bars demonstrate mean + SD. p values were calculated for unpaired t tests.
Compared with cultures that were initiated with fresh PBMNCs, cryopreservation-thawed PBMNC cultures did not demonstrate a significant difference in FE, TE, CD3%, or CD4:CD8 ratios at the time of final CART harvest. Data stratified by protocol and all data combined are summarized in Table S4. Figures S1A–S1D demonstrate the lack of a significant correlation between duration of PBMNC cryopreservation and FE, TE, CD3%, and CD4:CD8 ratios. These correlations remained non-significant, even when data were stratified by CART type. The day of final CART harvest varied by type, ranging from 7 to 9 days. We evaluated the same correlations on day 7 for all CART types (data not shown) and identified no difference.
Recovery, Expansion, and Survival of Cryopreserved CARTs
In Vitro Post-thaw Recovery of Cryopreserved CARTs
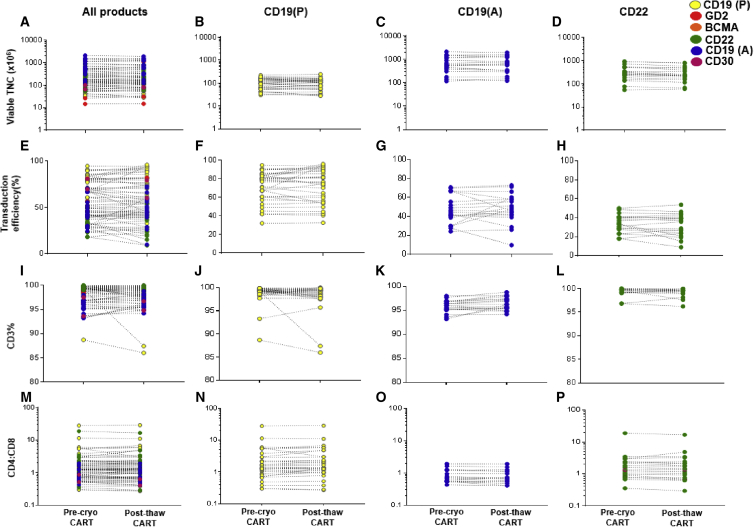
For the 79 cryopreserved CART products, the mean TNC recovery of the thawed fraction was 97% ± 17.4%. Before cryopreservation and after thaw, CART CD3%, TE(%), and CD4:CD8 ratios were 98% ± 2.1% and 98% ± 2.4%, 56% ± 21% and 55% ± 23%, and 2.2 ± 3.9 and 2.3 ± 4.0, respectively. No significant changes were observed in these parameters across the different types of CARTs. CD19(A), CD19(P), and CD22 CARTs with adequate sample sizes were analyzed separately, and no significant changes were identified post-thaw. Mean values and SDs of all the measured parameters varied by protocol, as expected, due to differences in patient population, vector type, and culture conditions (Figure 3).
Figure 3.
Comparisons of CART Characteristics at Cryopreservation and Post-thaw
Viable TNC (row 1), TE (row 2), CD3% (row 3), and CD4:CD8 ratio (row 4) are compared for the final CART product at cryopreservation and following thaw. Column 1 (A, E, I, and M) shows all CART products. Column 2 (B, F, J, and N) shows CD19-CART (P) products, column 3 (C, G, K, and O) shows CD19 CART(A) products, and column 4 (D, H, L, and P) shows CD22-CART products. The p values were calculated for paired t tests.
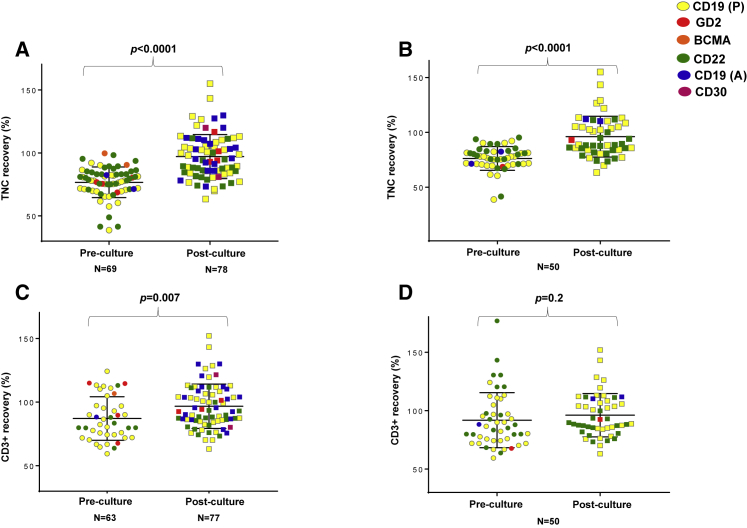
As a control, we compared the post-thaw recovery of 78 CART products with the 69 PBMNC products that had been cryopreserved prior to the start of manufacturing. For these 69 PBMNCs, the viable post-thaw TNC recovery was significantly lower compared to CARTs (77% ± 12% versus 97% ± 17%, p < 0.0001) (Figure 4A). Viable post-thaw CD3% recovery trended lower for PBMNCs compared to CARTs, but the difference was not statistically significant (87% ± 17% versus 97% ± 17%, p = 0.07) (Figure 4C). Overall, CD3% recovery for thawed PBMNCs was better compared to TNC recovery, suggesting that non-CD3+ T cells accounted for most of the cell loss post-thaw.
Figure 4.
Comparison of Post-thaw Characteristics of PBMNCs and CARTs
(A) The post-thaw TNC recovery of all PBMNCs (pre-culture) and all CARTs (post-culture). (B) The post-thaw TNC recovery only for pairs of PBMNCs and CARTs that underwent double-end cryopreservation, i.e., the PBMNCs were cryopreserved prior to culture (pre-culture) and the CARTs were again analyzed post-cryopreservation-thaw (post-culture). (C) The post-thaw CD3+ cell recovery of all PBMNCs (pre-culture) and all CARTs (post-culture). (D) The post-thaw CD3+ cell recovery for pairs of PBMNCs that were cryopreserved prior to culture (pre-culture) and CARTs that were analyzed post-cryopreservation (post-culture). To calculate cell recovery, viability was measured by the standard trypan blue assay as part of clinical release testing. The scatter dot plots with bars demonstrate mean + SD. p values were calculated for unpaired (A and C) and paired t tests (B and D).
We also evaluated these differences in the post-thaw recovery of CARTs and PBMNCs using 50 cases where both the PBMNCs and CART products were cryopreserved (n = 50). Of these products cryopreserved at both ends, most (47, 94%) were manufactured for the CD19(A) and CD22 CART clinical trials. These CARTs also demonstrated higher TNC recovery at thaw (96% ± 19%) compared to the starting PBMNCs at thaw (76% ± 11%, p < 0.001) (Figure 4B). However, these differences were not as evident in CD3% recovery: 96% ± 19% for CARTs versus 92% ± 24% for PBMNCs, p = 0.2 (Figure 4D).
Comparison of Expansion, Survival, and Clinical Response to CARTs Infused Fresh and/or Post-cryopreservation
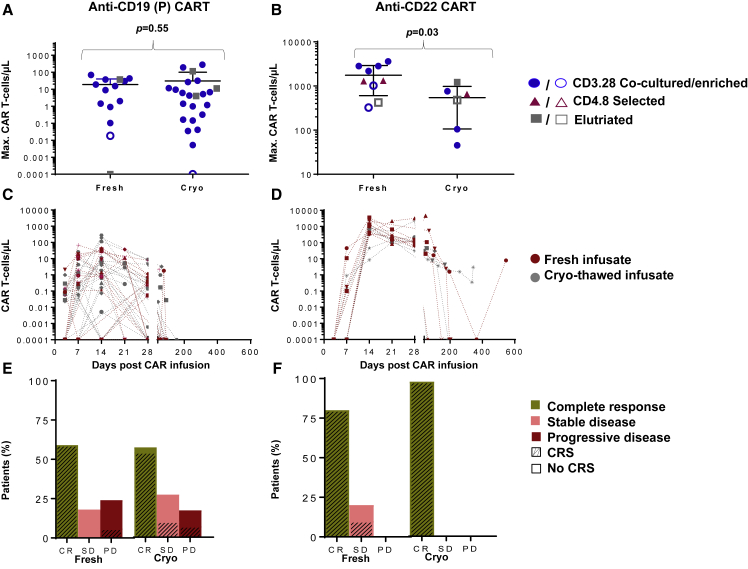
In vivo comparisons between fresh and cryo-thawed CART infusions were feasible for 2 protocols with adequate numbers in each group, at a pre-specified dose level. At a dose level of 1 × 106 transduced viable CD19 CART(P) cells/kg, maximum CART levels, CART persistence in vivo, clinical responses, and occurrence of cytokine release syndrome were not significantly different between patients receiving fresh CARTs (n = 17) and cryo-thawed CARTs (n = 30) (Figures 5A, 5C, and 5E). For CD22 CARTs, the mean maximum CART levels and mean persistence (in days) in vivo were greater than those seen with the CD19 CART(P), which was likely due to differences in the vector and co-stimulatory domains, manufacturing methods, and patient-related differences.
Figure 5.
Comparison of In Vivo Levels and Persistence of CARTs Infused Fresh or after Cryopreservation
(A and B) The clinical risk stratification (solid shape, high disease burden; outline shape, low disease burden) and maximum levels of peripheral blood T cells expressing CD19 CARTs (A, n = 47) and CD22 CARTs (B, n = 15) in patients who received a first infusion of fresh or cryo-thawed product at a dose of 1 × 106 cells/kg. (C and D) The post-infusion persistence of CARTs in the peripheral blood of patients receiving CD19 CARTs (C) and CD22 CARTs (D). All patients received a dose of 1 × 106 CART cells/kg. Patients receiving fresh CARTs are indicated by pink shapes (CD19 CARTs, n = 17 and CD22 CARTs, n = 9). Patients receiving cryopreserved CARTs are indicated by gray shapes (CD19 CARTs, n = 30 and CD22 CARTs, n = 6). The limit of CART detection by FACS in the peripheral blood was determined for each protocol. For CD19 CARTs and CD22 CARTs, the limits of detection were defined as CART cell fractions of 0.01% and 0.001% of total T cells in the peripheral blood, respectively. In patients receiving fresh or cryopreserved CD19 CARTs (E) and CD22 CARTs (F), clinical response at day 28 and proportion of patients experiencing any grade CRS are shown. All patients received a dose of 1 × 106 CARTs/kg. A total of 17 patients received fresh and 30 received cryopreserved CD19 CARTs and 9 patients received fresh and 7 cryopreserved CD22 CARTs. CR, complete response; SD, stable disease; PD, progressive disease; CRS, cytokine release syndrome. p values were calculated for non-parametric tests, where applicable.
Maximum CD22 CART levels in vivo were higher in patients receiving fresh CARTs compared to cryo-thawed CARTs (p = 0.03) (Figure 5B); however, no significant differences were identified in the persistence of CARTs between fresh and cryo-thawed CD22 CARTs (Figure 5D). Upon further evaluation, the number of patients who received CD22 CARTs with high disease burden was greater in the cryo-thawed CART group (5/6, 83%) compared to the fresh group (6/9, 67%). This difference was not statistically significant (p = 0.5). Different cell selection methods used to manufacture these products are also illustrated in Figures 5A and 5B. In this clinical trial as well, clinical responses and occurrence of cytokine release syndrome were no different between patients receiving fresh and cryo-thawed CARTs (Figures 5E and 5F).
For the CD19 CART(P), the proportion of patients that did not show any CARTs in the peripheral blood post-infusion (i.e., had CARTs below the limit of detection [<0.01% of T cells] for the protocol by fluorescence-activated cell sorting [FACS] analysis at all times after infusion) was higher among cryopreserved CD19 CARTs (85.7%, 6 of 7) compared to the fresh infused products (14.3%, 1 of 7; p = 0.01). None of the CD22 CART products demonstrated a complete absence of detection in vivo post-infusion.
Role of Cryopreservation on Manufacturing Failures
Of the original 158 products, 11 (6.9%) failed manufacture and were not infused. These were non-uniformly distributed across CART types. The causes for manufacturing failure were variable. Of the 11 that failed, 5 cultures were initiated from a cryopreserved starting parent product and 6 from fresh PBMNCs. Overall, 5 of 75 (6.7%) cryopreserved PBMNCs and 6 of 83 (7.2%; p = 0.9) fresh PBMNCs failed manufacturing. Following failure, remanufacture from a cryopreserved PBMNC fraction was attempted for 6 cases, and all 6 resulted in the successful production and infusion of a CART product. These 6 remanufacturing cases included 3 that were originally manufactured from cryopreserved PBMNCs (Table S5).
Phenotypic Evaluation of Fresh versus Cryopreserved CARTs in Healthy Donor Samples
To study T cell phenotypic changes prospectively, 3 healthy donor PBMNCs were used to manufacture CART using 4 different manufacturing schemes, as described in the Materials and Methods. As with the clinical products analyzed previously, mean CART FE, TE, CD4%, and CD8% were no different across the 4 schemes in healthy donor samples (Figures S2A–S2E). Viable (7-amino actinomycin D [7AAD]neg, 7AADdim) CD3+ T cell percentage was similar in the 4 manufacturing arms (Figures 6A, 6C, 6E, 6G, and 6I). This population also corresponded to the viable cells identified by trypan blue staining (data not shown). However, after eliminating early apoptotic cells (Annexin-V positive and/or Helix NP positive) destined for eventual cell death, viability was significantly lower in the arms where the final CART product was cryopreserved (Figures 6F, 6H, and 6J) compared to the arms where the final CARTs were tested fresh (Figures 6B, 6D, and 6J). This result was independent of the state (cryo-thaw or fresh) of the starting PBMNCs. Analysis of the CD4+ and CD8+ cells within the CART products revealed that the proportion of naive, central memory, and effector memory T cells did not differ significantly across the 4 manufacturing arms (Figure S3).
Figure 6.
Viability and Apoptosis Analysis of Fresh and Cryopreserved CARTs
CARTs were prepared from fresh or cryopreserved PBMNCs and were analyzed fresh or following cryopreservation. CARTs were prepared from 3 healthy subjects and were transduced (TR) with murine stem cell virus (MSCV)-CAR1922-woodchuck hepatitis virus (WHP) posttranscriptional regulatory element (WPRE) vector. Representative dot plots of fresh CARTs from donor 1 prepared from fresh PBMNCs (Fresh TR Fresh) for 7AAD expression is shown in (A) and for Annexin-V, Helix NP in (B). Dot plots of fresh CARTs from donor 1 prepared from cryopreserved PBMNCs (Cryo TR Fresh) for 7AAD expression is shown in (C) and for Annexin-V, Helix NP in (D). Dot plots of cryopreserved CARTs from donor 1 prepared from fresh PBMNCs (Fresh TR Cryo) for 7AAD expression is shown in (E) and for Annexin-V, Helix NP in (F). Dot plots of cryopreserved CARTs from donor 1 prepared from cryopreserved PBMNCs (Cryo TR Cryo) for 7AAD expression is shown in (G) and for Annexin-V, Helix NP in (H). The 7AADdim population in (A), (C), (E), and (G) was also the cells that were Annexin-V bright in the corresponding adjacent figures, likely representing early apoptotic cells. However, in column 1, the combined viable cell fraction, which included 7AADbright and 7AADdim populations, corresponded with the trypan blue viability used to test clinical products. (I and J) The results for combined analysis CARTs from all 3 donors tested and prepared under the 4 conditions are summarized. The results of the expressions of (I) 7AAD and (J) Annexin-V, Helix NP are shown. Bars in (I) and (J) represent means + SD.
Global Gene Expression Analysis of Fresh versus Cryopreserved CARTs in Healthy Donor Samples
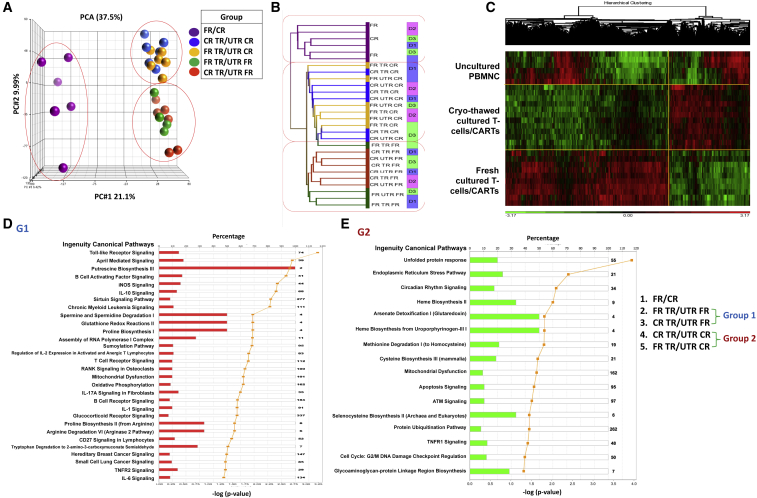
Global gene expression changes were also studied prospectively in the manufacturing arms above (categories described in the Materials and Methods). A total of 30 samples was analyzed. Of these, 6 samples were uncultured PBMNCs (fresh or cryo-thawed). 12 others were CARTs from the 3 healthy donors, with each donor sample subjected to one of the 4 manufacturing schemes detailed above. As controls, 12 more samples were cultured simultaneously using all the same conditions, except they were not transduced. Principal-component analysis (PCA) of 28 samples was available for further evaluation. Two samples were eliminated from analysis due to sample-processing discrepancies. All the samples clustered into 3 groups. One group contained all of the PBMNCs that were not cultured, the second group contained fresh final CARTs and cultured T cells that were analyzed fresh, and the third contained all cryo-thawed final CARTs and cryo-thawed cultured T cells (Figure 7A). Unsupervised hierarchical clustering analysis also grouped the samples into the same 3 categories (Figures 7B and 7C). As expected, the effect of cryopreservation eclipsed the effects of vector transduction and inter-donor variability.
Figure 7.
Gene Expression Analysis of Fresh and Cryopreserved CARTs
CARTs were manufactured from 3 healthy subjects, and fresh (C-FR) and cryopreserved (C-CR) CARTs were analyzed by global gene expression analysis. The CARTs were produced from both fresh PBMNCs (P-FR) and cryopreserved PBMNCs (P-FR), and they were transduced (TR) with MSCV-CAR1922-WPRE vector. Untransduced (UTR) fresh and cryopreserved PBMNCs and cultured PBMNCs were also analyzed as a control. The 28 samples were available for global gene expression profiling, and the results were analyzed by principal-component analysis (A) and unsupervised hierarchical clustering analysis (B, cluster dendrogram; C, hierarchical clustering heatmap). (D and E) Ingenuity pathway analysis of genes expressed by cryopreserved and fresh CARTs and cultured T cells are shown. A comparison of the transcriptome of cryopreserved CARTs (n = 6) and cultured T cells (n = 6) with that of fresh CARTs (n = 6) and cultured T cells revealed 2,124 differentially expressed genes. The results of ingenuity pathway analysis of 1,139 genes whose expression was greater in cryopreserved cells is shown in (D) and ingenuity pathway analysis of 985 genes whose expression was greater in fresh cells is shown in (E).
Eliminating the untransduced control samples from the analysis decreased sample size significantly. PCA of the smaller sample set demonstrated inter-donor variability alone as a significant factor (data not shown).
Further, a total of 2,124 genes were differentially expressed between the fresh CARTs and cultured T cells that were analyzed fresh (group 1) and the cryo-thawed CARTs and cryo-thawed cultured T cells (group 2). The 1,139 genes overexpressed in cryo-thawed CARTs were more likely to belong to apoptotic and cell cycle damage pathways (unfolded protein response, endoplasmic reticulum [ER] stress pathways, mitochondrial dysfunction, apoptosis signaling, protein ubiquitination, and cell cycle damage pathways) (Figure 7D). The 985 genes overexpressed in fresh CARTs and culture-expanded T cells were more likely to belong to signaling pathways specific to T cell cytolysis (Toll-like receptor signaling, B cell-activating factors, and leukemia-signaling pathways) (Figure 7E).
Discussion
More than 70% of therapeutic agents that demonstrate success in early phase clinical trials for refractory solid organ and hematologic malignancies fail efficacy endpoints in larger trials.13, 14 Further, with cell therapies, the conversion rate from a phase III study to regulatory approval is estimated to be at 14.3%, which is considerably lower than the conversion rate (48.7%) of mature pharmaceutical drug classes that demonstrate new drug application success with the FDA.15 This is due in part to the fact that small changes in manufacturing practices during the scale-out of cell therapies can greatly impact final product safety, purity, and potency.16, 17 While smaller single institution studies often use fresh PBMNCs for CART manufacturing and/or fresh final CART products for autologous infusion, the short stability and shelf life of liquid-stored T cells makes cryopreservation essential for advancement to multicenter trials and licensure, with centralized manufacturing. Hence, it is essential to verify that cryopreserved products are equivalent in safety and efficacy to fresh CARTs, prior to commercialization.
Despite the optimized use of intracellular cryoprotective cocktails,18 slow cooling in controlled rate freezers,19 and quick-thaw processes prior to cell use, some decrease in post-thaw cell viability is inevitable.20 This limitation may be overcome by increasing cell numbers used for cryopreservation. Other issues, including phenotypic and functional drifts in cell subtypes, occur following the freeze-thaw process.21 Consequently, the biophysical and physiologic properties of thawed mature cells cultured ex vivo may vary from those for freshly isolated cells that encounter the same stressors.22, 23 To better understand the clinical impact of these phenomena, we studied relevant phenotypic and functional cellular alterations during and after the manufacture of CARTs cryo-thawed at either or both ends of cell manufacturing.
The results of this study suggest that the CARTs that are cryopreserved at the end of manufacturing and thawed immediately before infusion are as clinically effective as CARTs given fresh. Furthermore, cryopreserved CD19 CARTs have a similar in vivo survival and peak in vivo levels as CD19 CARTs that are infused fresh.
It is not certain if the dose of CARTs administered should be increased if cryopreserved cells are given. We administered CARTs as transduced viable (based on post-thaw trypan blue viability) T cells per kilogram of recipient weight, and we used the same dose for fresh and cryopreserved CARTs. While the in vivo recovery and survival of fresh and cryopreserved CD19 CARTs was similar, in a small number of CD22 CARTs the survival of cryopreserved cells was less than that of fresh cells. Further, based on in vitro data from healthy volunteer donor CART products, despite similar viability (based on traditional viability assays) between fresh and cryo-thawed CARTs, using markers for early apoptosis and including a novel nucleic acid-binding dye24 identified a significantly larger number of cells among the viable cells post-thaw, which were destined for eventual demise. Gene expression profiling also identified an increased expression of pathways involved in cell cycle damage and apoptosis in the cryo-thawed CART products.
While we routinely measure the post-thaw viability of CARTs and calculate cells based on viable transduced CD3+ cells, this measure is of limited accuracy. Since cells were infused immediately post-thaw, delayed onset cell death (DOCD) could not be assessed. Furthermore, the in vivo expansion of CARTs is also dependent on tumor burden in addition to cell dose. Patients enrolled in 2 clinical protocols received fresh or cryo-thawed final products at the same dose level (1 × 106 transduced viable CARTs/kg). In both protocols, CART persistence did not differ by cryopreservation status. This aligned with data from another recent retrospective analysis.25 In 1 of the 2 protocols, maximum CART numbers were higher for the fresh infusions compared to cryo-thawed infusions. However, differences in patient disease burden at baseline and variability in cell selection methods during manufacture, which are known to impact in vivo CART levels,26, 27, 28 precluded definitive conclusions on this protocol. Of the infusions that failed to demonstrate any CART appearance in vivo, 86% and 0% were cryo-thawed in the CD19 and CD22 CART protocols, respectively. Testing, validation, and routine use of commercially available current good manufacturing practice (cGMP) grade cryoprotectant solutions may possibly minimize inter-product variability seen with home-brewed counterparts.29, 30
We also found that CARTs can be manufactured from cryopreserved PBMNCs. We did observe a reduction of T cells over the first 2 days of culture for cryopreserved, but not fresh, PBMNCs. This DOCD is known to occur between 6 and 48 h post-thaw, and it may occur due to a transcriptional upregulation of key apoptotic markers, including the proteolytic activation of caspase-3 post-thaw. Immediately post-thaw these early apoptotic cells destined for secondary necrosis are not identified by the standard dye-exclusion methods (such as the trypan blue assay used for product release), which only identify losses in structural integrity. Despite this difference in the quality of cells early in culture, we had a sufficient quantity of cells to transduce, and we had similar numbers of CARTs at the end of manufacturing for both fresh and cryopreserved PBMNCs. As with the PBMNCs post-thaw and within the initial culture period, DOCD likely occurs hours to days in vivo after CART administration, followed by a partially compensatory expansion.20 The in vivo levels of cryopreserved CARTs may have been higher had a greater dose of cells been administered.
The cryo-thawed starting fraction, PBMNCs, demonstrated significantly lower TNC recovery than the final cryo-thawed CART product, TNCs. This may be attributable to a higher number of non-lymphocytic mononuclear cells, including granulocytes and monocytes, in the starting fraction. These cells are known to have lower post-thaw viability (∼7% and 36%, respectively) compared to lymphocytes alone (74%).31 This finding is consistent with what was previously reported for TNC recovery in stem cell products, wherein global TNC recovery was recognized to be lower than that of the more resilient CD34+ cell subsets (post-thaw viability ∼80%).32, 33 Upon further analysis of CD3+ T cell subsets, viable post-thaw recovery of T cells in the starting fraction was not significantly different from that in the final manufactured CARTs, which were harvested and cryo-thawed prior to infusion. This was in contrast to a previous report suggesting lower viability of transduced T cells cultured over a period of 7 days in patients with immune deficiencies.23
Cryopreservation of PBMNCs and/or of the final manufactured CARTs did not impact TE, CD3%, or CD4:CD8 ratios at harvest or before infusion, respectively. Neither were the proportion of T cell subsets, including naive, effector, and central memory T cells, impacted significantly in our prospective donor cell analysis. Prior studies of other cell types identified phenotypic changes in T cell subsets with cryopreservation34 and functional impairments due to transient warming events, which occurred during prolonged hypothermic storage.35 While we were unable to evaluate the frequency and duration of transient warming events in our study, we noticed no significant effect of duration of cryopreservation on T cell phenotype and functional characteristics, during or after manufacture. Further, of the products that failed manufacture, none was specifically related to cryopreservation.
Efforts are underway to add inhibitors of apoptosis and secondary necrosis (zVAD-fmk, p38 mitogen-activated protein kinase [MAPK], and ROCK inhibitors) to cryopreservation cocktails and culture media.20, 36 Further, cryobiologists are evaluating the role of epigenetic changes and phenotypic and functional drifts in cryo-thawed cells, either due to cryopreservation itself or from a proliferative stress imposed on the surviving cells post-thaw.37 The need to study DOCD and obtain accurate viable cell counts in the immediate post-thaw period is critical after manufacture, as dose calculations for cell infusion rely on viable transduced T cell counts. To our knowledge, our study presents the most detailed assessment to date of phenotypic, functional, and gene expression alterations in manufactured CARTs.
In summary, cryopreservation at either end of CART manufacture is a viable strategy. Specifically, the cryo-thaw process does not significantly affect clinically relevant CART phenotypic and/or functional parameters, including FE, TE, CD3%, and CD4:CD8 ratios. Further, manufacturing failures, which accounted for approximately 7% of all products in our study, were unrelated to cryopreservation. In vivo CART levels and persistence may not be affected by cryopreservation. However, larger controlled studies are necessary to further understand functional and phenotypic alterations with cryopreservation at a molecular level and to assess the occurrence of and mechanisms underlying DOCD in vitro and in vivo. These studies will inform the need for standardized dose adjustments that may be required to account for the structurally viable albeit early apoptotic cells in cryo-thawed CARTs prepared for infusion into patients. With an increasing demand for large-scale manufacture of these autologous CART therapies of high logistical and regulatory complexity, standardizing and optimizing cryopreservation steps are pivotal to maintaining downstream cell quantity and quality and to enhancing patient outcomes.
Materials and Methods
Study Design
From January 2012 to July 2017, a retrospective data analysis was performed on all CARTs manufactured at the NIH, Center for Cellular Engineering. Clinical protocols on which data were obtained are listed in Table S1. All subjects were enrolled in protocols approved by the National Cancer Institute (NCI) institutional review board (IRB). Protocols comprised phase I dose escalation studies using the various CART constructs against antigens overexpressed in hematologic malignancies or solid tumors. Data on products that failed manufacture were collected separately.
CART manufacturing on the clinical protocols followed 1 of 2 methods. In the protocols treating adult patients (B cell maturation antigen [BCMA], CD19[A], and CD30 CARTs), fresh or cryo-thawed PBMNCs collected by apheresis underwent density gradient separation. T cell stimulation was performed with interleukin-2 (IL-2) and soluble anti-CD3 monoclonal antibody (OKT3) in culture bags, followed by gamma retroviral or lentiviral transduction and expansion, as shown in Figure 1 and Table S3. Between days 7 and 9, cells were harvested, concentrated, and infused fresh or cryopreserved for thaw and infusion at a later time. In the pediatric patient trials (CD19[P], GD2, and CD22 CARTs), fresh or cryo-thawed PBMNC concentrates underwent cell enrichment steps (CD4/8 selection, monocyte depletion by flask [plastic] adherence, elutriation, and/or CD3/CD28 paramagnetic bead enrichment and stimulation). This was followed by gamma-retroviral transduction and expansion with culture harvest between days 7 and 11 for fresh or cryo-thawed infusion (Figure 1; Table S4). See the Supplemental Materials and Methods I for additional CART manufacturing details.38, 39, 40, 41, 42
Further, to study T cell phenotypic changes and gene expression prospectively, 3 healthy donor PBMNCs were used to manufacture CARTs using 4 different manufacturing schemes: (1) starting manufacturing with fresh PBMNCs and analysis of fresh CARTs, (2) starting with fresh PBMNCs and analysis of cryopreserved CARTs, (3) starting with cryopreserved PBMNCs and analysis of fresh CARTs, and (4) starting with cryopreserved PBMNCs and analysis of cryopreserved CARTs.
Cryopreservation and Thaw
Products that were cryopreserved used a controlled rate freezer (Kryosave, Integra, Planer, Sunbury-on-Thames, UK) and 5% DMSO and 6% pentastarch with 4% human serum albumin as a cryoprotectant. The cryopreserved polyfluoroethylene bags (KryoSure, American Fluroseal, Gaithersburg, MD) or vials (Nunc Cryotube Vials, Roskilde, Denmark) were stored in both the vapor and liquid phases of liquid nitrogen. Thaw was performed in a water bath at 37°C. Immediately after thawing, the products were diluted to a final required volume with Plasma-Lyte A (Baxter Healthcare) containing 10 U/mL preservative-free heparin.
Laboratory Assays
Flow cytometry staining included CD3, CD45, 7-AAD (BD Biosciences), cell surface CAR expression (biotin-labeled protein L, GenScript), Annexin-V (BD Biosciences), and Helix NP (BioLegend). T cell subset analysis used CCR7 and CD45RA (BD Biosciences). RNA purification and microarray data analysis were done as described previously.43
Statistical Analysis
Non-parametric Wilcoxon signed-rank tests were used to estimate strength of effects. Multiple linear regression analysis was performed to examine the effect of cryopreservation on the CART production, adjusting for other factors such as different protocols, cell manipulation methods, and infusion dose levels. Statistical analyses were performed using SAS version (v.)9.4 software (Cary, NC). See the Supplemental Materials and Methods II.
Author Contributions
S.R.P., S.K.S., N.E., N.N.S., and D.F.S. designed the study and wrote the manuscript. A.M., S.L., P.J., S.L.H., and P.D. contributed to data collection and laboratory experiments and data analysis. J.N.K., T.J.F., C.L.M., and D.L. recruited patients in the clinical trials from which data were obtained. They also contributed to study design and manuscript preparation.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
We wish to thank Aby J. Mathew, PhD (Biolife Solutions), for critical review of the manuscript. We thank the Center for Cellular Engineering technical staff, DTM, NIH for their critical role in product development, cell manufacturing, cell characterization, and assay development. The Intramural Research Program of the NIH Clinical Center supported this research (in part). The authors duly acknowledge the support of the NHLBI flow core facility in flow cytometric data generation and analysis.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2019.05.015.
Supplemental Information
References
- 1.O’Leary M.C., Lu X., Huang Y., Lin X., Mahmood I., Przepiorka D., Gavin D., Lee S., Liu K., George B. FDA Approval Summary: Tisagenlecleucel for Treatment of Patients with Relapsed or Refractory B-Cell Precursor Acute Lymphoblastic Leukemia. Clin. Cancer Res. 2019;25:1142–1146. doi: 10.1158/1078-0432.CCR-18-2035. [DOI] [PubMed] [Google Scholar]; O’Leary, M.C., Lu, X., Huang, Y., Lin, X., Mahmood, I., Przepiorka, D., Gavin, D., Lee, S., Liu, K., George, B., et al. (2019). FDA Approval Summary: Tisagenlecleucel for Treatment of Patients with Relapsed or Refractory B-Cell Precursor Acute Lymphoblastic Leukemia. Clin. Cancer Res. 25, 1142-1146. [DOI] [PubMed]
- 2.Mullard A. Second anticancer CAR T therapy receives FDA approval. Nat. Rev. Drug Discov. 2017;16:818. doi: 10.1038/nrd.2017.249. [DOI] [PubMed] [Google Scholar]; Mullard, A. (2017). Second anticancer CAR T therapy receives FDA approval. Nat. Rev. Drug Discov. 16, 818. [DOI] [PubMed]
- 3.Ruella M., Xu J., Barrett D.M., Fraietta J.A., Reich T.J., Ambrose D.E., Klichinsky M., Shestova O., Patel P.R., Kulikovskaya I. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat. Med. 2018;24:1499–1503. doi: 10.1038/s41591-018-0201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ruella, M., Xu, J., Barrett, D.M., Fraietta, J.A., Reich, T.J., Ambrose, D.E., Klichinsky, M., Shestova, O., Patel, P.R., Kulikovskaya, I., et al. (2018). Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat. Med. 24, 1499-1503. [DOI] [PMC free article] [PubMed]
- 4.Iyer R.K., Bowles P.A., Kim H., Dulgar-Tulloch A. Industrializing Autologous Adoptive Immunotherapies: Manufacturing Advances and Challenges. Front. Med. (Lausanne) 2018;5:150. doi: 10.3389/fmed.2018.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]; Iyer, R.K., Bowles, P.A., Kim, H., and Dulgar-Tulloch, A. (2018). Industrializing Autologous Adoptive Immunotherapies: Manufacturing Advances and Challenges. Front. Med. (Lausanne) 5, 150. [DOI] [PMC free article] [PubMed]
- 5.Vormittag P., Gunn R., Ghorashian S., Veraitch F.S. A guide to manufacturing CAR T cell therapies. Curr. Opin. Biotechnol. 2018;53:164–181. doi: 10.1016/j.copbio.2018.01.025. [DOI] [PubMed] [Google Scholar]; Vormittag, P., Gunn, R., Ghorashian, S., and Veraitch, F.S. (2018). A guide to manufacturing CAR T cell therapies. Curr. Opin. Biotechnol. 53, 164-181. [DOI] [PubMed]
- 6.Wang X., Rivière I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol. Ther. Oncolytics. 2016;3:16015. doi: 10.1038/mto.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wang, X., and Riviere, I. (2016). Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol. Ther. Oncolytics 3, 16015. [DOI] [PMC free article] [PubMed]
- 7.Aziz J., Morris G., Rizk M., Shorr R., Mercer D., Young K., Allan D. Cryopreservation of adult unrelated donor products in hematopoietic cell transplantation: the OneMatch experience and systematic review of the literature. Transfusion. 2017;57:2782–2789. doi: 10.1111/trf.14360. [DOI] [PubMed] [Google Scholar]; Aziz, J., Morris, G., Rizk, M., Shorr, R., Mercer, D., Young, K., and Allan, D. (2017). Cryopreservation of adult unrelated donor products in hematopoietic cell transplantation: the OneMatch experience and systematic review of the literature. Transfusion 57, 2782-2789. [DOI] [PubMed]
- 8.Pavlů J., Auner H.W., Szydlo R.M., Sevillano B., Palani R., O’Boyle F., Chaidos A., Jakob C., Kanfer E., MacDonald D. Analysis of hematopoietic recovery after autologous transplantation as method of quality control for long-term progenitor cell cryopreservation. Bone Marrow Transplant. 2017;52:1599–1601. doi: 10.1038/bmt.2017.113. [DOI] [PubMed] [Google Scholar]; Pavlů, J., Auner, H.W., Szydlo, R.M., Sevillano, B., Palani, R., O’Boyle, F., Chaidos, A., Jakob, C., Kanfer, E., MacDonald, D., et al. (2017). Analysis of hematopoietic recovery after autologous transplantation as method of quality control for long-term progenitor cell cryopreservation. Bone Marrow Transplant. 52, 1599-1601. [DOI] [PubMed]
- 9.Berens C., Heine A., Müller J., Held S.A., Mayer K., Brossart P., Oldenburg J., Pötzsch B., Wolf D., Rühl H. Variable resistance to freezing and thawing of CD34-positive stem cells and lymphocyte subpopulations in leukapheresis products. Cytotherapy. 2016;18:1325–1331. doi: 10.1016/j.jcyt.2016.06.014. [DOI] [PubMed] [Google Scholar]; Berens, C., Heine, A., Muller, J., Held, S.A., Mayer, K., Brossart, P., Oldenburg, J., Potzsch, B., Wolf, D., and Ruhl, H. (2016). Variable resistance to freezing and thawing of CD34-positive stem cells and lymphocyte subpopulations in leukapheresis products. Cytotherapy 18, 1325-1331. [DOI] [PubMed]
- 10.Fisher V., Khuu H., David-Ocampo V., Byrne K., Pavletic S., Bishop M., Fowler D.H., Barrett A.J., Stroncek D.F. Analysis of the recovery of cryopreserved and thawed CD34+ and CD3+ cells collected for hematopoietic transplantation. Transfusion. 2014;54:1088–1092. doi: 10.1111/trf.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fisher, V., Khuu, H., David-Ocampo, V., Byrne, K., Pavletic, S., Bishop, M., Fowler, D.H., Barrett, A.J., and Stroncek, D.F. (2014). Analysis of the recovery of cryopreserved and thawed CD34+ and CD3+ cells collected for hematopoietic transplantation. Transfusion 54, 1088-1092. [DOI] [PMC free article] [PubMed]
- 11.Zhu F., Shah N.N., Schneider D., Xu H., Chaney K., Luib L., Keever-Taylor C., Dropulic B., Orentas R., Hari P., Johnson B.D. Automated Manufacturing of CD20.19 Bi-Specific Chimeric Antigen Receptor T (CAR-T) Cells at an Academic Center for a Phase I Clinical Trial in Relapsed, Refractory NHL. Biol. Blood Marrow Transplant. 2019;25:S62. (Suppl) [Google Scholar]; Zhu, F., Shah, N.N., Schneider, D., Xu, H., Chaney, K., Luib, L., Keever-Taylor, C., Dropulic, B., Orentas, R., Hari, P., and Johnson, B.D. (2019). Automated Manufacturing of CD20.19 Bi-Specific Chimeric Antigen Receptor T (CAR-T) Cells at an Academic Center for a Phase I Clinical Trial in Relapsed, Refractory NHL. Biol. Blood Marrow Transplant. 25 (Suppl), S62.
- 12.Zhu F., Shah N.N., Schneider D., Xu H., Chaney K., Luib L., Keever-Taylor C.A., Dropulic B., Orentas R., Hari P., Johnson B. Point-of-Care Manufacturing of CD20.19 Bi-Specific Chimeric Antigen Receptor T (CAR-T) Cells in a Standard Academic Cell Processing Facility for a Phase I Clinical Trial in Relapsed, Refractory NHL. Blood. 2018;132(Suppl 1):4553. [Google Scholar]; Zhu, F., Shah, N.N., Schneider, D., Xu, H., Chaney, K., Luib, L., Keever-Taylor, C.A., Dropulic, B., Orentas, R., Hari, P., and Johnson, B. (2018). Point-of-Care Manufacturing of CD20.19 Bi-Specific Chimeric Antigen Receptor T (CAR-T) Cells in a Standard Academic Cell Processing Facility for a Phase I Clinical Trial in Relapsed, Refractory NHL. Blood 132 (Suppl 1), 4553.
- 13.Hay M., Thomas D.W., Craighead J.L., Economides C., Rosenthal J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 2014;32:40–51. doi: 10.1038/nbt.2786. [DOI] [PubMed] [Google Scholar]; Hay, M., Thomas, D.W., Craighead, J.L., Economides, C., and Rosenthal, J. (2014). Clinical development success rates for investigational drugs. Nat. Biotechnol. 32, 40-51. [DOI] [PubMed]
- 14.Khozin S., Liu K., Jarow J.P., Pazdur R. Regulatory watch: Why do oncology drugs fail to gain US regulatory approval? Nat. Rev. Drug Discov. 2015;14:450–451. doi: 10.1038/nrd4651. [DOI] [PubMed] [Google Scholar]; Khozin, S., Liu, K., Jarow, J.P., and Pazdur, R. (2015). Regulatory watch: Why do oncology drugs fail to gain US regulatory approval? Nat. Rev. Drug Discov. 14, 450-451. [DOI] [PubMed]
- 15.Aijaz A., Li M., Smith D., Khong D., LeBlon C., Fenton O.S., Olabisi R.M., Libutti S., Tischfield J., Maus M.V. Biomanufacturing for clinically advanced cell therapies. Nat. Biomed. Eng. 2018;2:362–376. doi: 10.1038/s41551-018-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Aijaz, A., Li, M., Smith, D., Khong, D., LeBlon, C., Fenton, O.S., Olabisi, R.M., Libutti, S., Tischfield, J., Maus, M.V., et al. (2018). Biomanufacturing for clinically advanced cell therapies. Nat. Biomed. Eng. 2, 362-376. [DOI] [PMC free article] [PubMed]
- 16.Lipsitz Y.Y., Timmins N.E., Zandstra P.W. Quality cell therapy manufacturing by design. Nat. Biotechnol. 2016;34:393–400. doi: 10.1038/nbt.3525. [DOI] [PubMed] [Google Scholar]; Lipsitz, Y.Y., Timmins, N.E., and Zandstra, P.W. (2016). Quality cell therapy manufacturing by design. Nat. Biotechnol. 34, 393-400. [DOI] [PubMed]
- 17.Lipsitz Y.Y., Bedford P., Davies A.H., Timmins N.E., Zandstra P.W. Achieving Efficient Manufacturing and Quality Assurance through Synthetic Cell Therapy Design. Cell Stem Cell. 2017;20:13–17. doi: 10.1016/j.stem.2016.12.003. [DOI] [PubMed] [Google Scholar]; Lipsitz, Y.Y., Bedford, P., Davies, A.H., Timmins, N.E., and Zandstra, P.W. (2017). Achieving Efficient Manufacturing and Quality Assurance through Synthetic Cell Therapy Design. Cell Stem Cell 20, 13-17. [DOI] [PubMed]
- 18.Nicoud I.B., Clarke D.M., Taber G., Stolowski K.M., Roberge S.E., Song M.K., Mathew A.J., Reems J.A. Cryopreservation of umbilical cord blood with a novel freezing solution that mimics intracellular ionic composition. Transfusion. 2012;52:2055–2062. doi: 10.1111/j.1537-2995.2011.03547.x. [DOI] [PubMed] [Google Scholar]; Nicoud, I.B., Clarke, D.M., Taber, G., Stolowski, K.M., Roberge, S.E., Song, M.K., Mathew, A.J., and Reems, J.A. (2012). Cryopreservation of umbilical cord blood with a novel freezing solution that mimics intracellular ionic composition. Transfusion 52, 2055-2062. [DOI] [PubMed]
- 19.Dijkstra-Tiekstra M.J., Setroikromo A.C., Kraan M., Gkoumassi E., de Wildt-Eggen J. Optimization of the freezing process for hematopoietic progenitor cells: effect of precooling, initial dimethyl sulfoxide concentration, freezing program, and storage in vapor-phase or liquid nitrogen on in vitro white blood cell quality. Transfusion. 2014;54:3155–3163. doi: 10.1111/trf.12756. [DOI] [PubMed] [Google Scholar]; Dijkstra-Tiekstra, M.J., Setroikromo, A.C., Kraan, M., Gkoumassi, E., and de Wildt-Eggen, J. (2014). Optimization of the freezing process for hematopoietic progenitor cells: effect of precooling, initial dimethyl sulfoxide concentration, freezing program, and storage in vapor-phase or liquid nitrogen on in vitro white blood cell quality. Transfusion 54, 3155-3163. [DOI] [PubMed]
- 20.Baust J.G., Snyder K.K., Van Buskirk R., Baust J.M. Integrating Molecular Control to Improve Cryopreservation Outcome. Biopreserv. Biobank. 2017;15:134–141. doi: 10.1089/bio.2016.0119. [DOI] [PubMed] [Google Scholar]; Baust, J.G., Snyder, K.K., Van Buskirk, R., and Baust, J.M. (2017). Integrating Molecular Control to Improve Cryopreservation Outcome. Biopreserv. Biobank. 15, 134-141. [DOI] [PubMed]
- 21.Golab K., Grose R., Placencia V., Wickrema A., Solomina J., Tibudan M., Konsur E., Ciepły K., Marek-Trzonkowska N., Trzonkowski P. Cell banking for regulatory T cell-based therapy: strategies to overcome the impact of cryopreservation on the Treg viability and phenotype. Oncotarget. 2018;9:9728–9740. doi: 10.18632/oncotarget.23887. [DOI] [PMC free article] [PubMed] [Google Scholar]; Golab, K., Grose, R., Placencia, V., Wickrema, A., Solomina, J., Tibudan, M., Konsur, E., Ciepły, K., Marek-Trzonkowska, N., Trzonkowski, P., et al. (2018). Cell banking for regulatory T cell-based therapy: strategies to overcome the impact of cryopreservation on the Treg viability and phenotype. Oncotarget 9, 9728-9740. [DOI] [PMC free article] [PubMed]
- 22.Hubel A., Norman J., Darr T.B. Cryobiophysical characteristics of genetically modified hematopoietic progenitor cells. Cryobiology. 1999;38:140–153. doi: 10.1006/cryo.1999.2157. [DOI] [PubMed] [Google Scholar]; Hubel, A., Norman, J., and Darr, T.B. (1999). Cryobiophysical characteristics of genetically modified hematopoietic progenitor cells. Cryobiology 38, 140-153. [DOI] [PubMed]
- 23.Hubel A., Darr T.B., Norman J.A. Freezing characteristics of genetically modified lymphocytes for the treatment of MPS II. Cell Transplant. 1999;8:521–530. doi: 10.1177/096368979900800507. [DOI] [PubMed] [Google Scholar]; Hubel, A., Darr, T.B., and Norman, J.A. (1999). Freezing characteristics of genetically modified lymphocytes for the treatment of MPS II. Cell Transplant. 8, 521-530. [DOI] [PubMed]
- 24.Jiang L., Tixeira R., Caruso S., Atkin-Smith G.K., Baxter A.A., Paone S., Hulett M.D., Poon I.K. Monitoring the progression of cell death and the disassembly of dying cells by flow cytometry. Nat. Protoc. 2016;11:655–663. doi: 10.1038/nprot.2016.028. [DOI] [PubMed] [Google Scholar]; Jiang, L., Tixeira, R., Caruso, S., Atkin-Smith, G.K., Baxter, A.A., Paone, S., Hulett, M.D., and Poon, I.K. (2016). Monitoring the progression of cell death and the disassembly of dying cells by flow cytometry. Nat. Protoc. 11, 655-663. [DOI] [PubMed]
- 25.Chong E.A., Levine B.L., Grupp S.A., Davis M., Siegel D.L., Maude S.L., Gladney W.L., Frey N.V., Porter D.L., June C.H., Schuster S.J. CD19-Directed CAR T-Cell (CTL019) Product Viability and Clinical Outcomes in Non-Hodgkin Lymphomas and B-Cell Acute Lymphoblastic Leukemia. Blood. 2018;132(Suppl 1):197. [Google Scholar]; Chong, E.A., Levine, B.L., Grupp, S.A., Davis, M., Siegel, D.L., Maude, S.L., Gladney, W.L., Frey, N.V., Porter, D.L., June, C.H., and Schuster, S.J. (2018). CD19-Directed CAR T-Cell (CTL019) Product Viability and Clinical Outcomes in Non-Hodgkin Lymphomas and B-Cell Acute Lymphoblastic Leukemia. Blood 132 (Suppl 1), 197.
- 26.June C.H., Sadelain M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 2018;379:64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]; June, C.H., and Sadelain, M. (2018). Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 379, 64-73. [DOI] [PMC free article] [PubMed]
- 27.Ramos C.A., Rouce R., Robertson C.S., Reyna A., Narala N., Vyas G., Mehta B., Zhang H., Dakhova O., Carrum G. In Vivo Fate and Activity of Second- versus Third-Generation CD19-Specific CAR-T Cells in B Cell Non-Hodgkin’s Lymphomas. Mol. Ther. 2018;26:2727–2737. doi: 10.1016/j.ymthe.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ramos, C.A., Rouce, R., Robertson, C.S., Reyna, A., Narala, N., Vyas, G., Mehta, B., Zhang, H., Dakhova, O., Carrum, G., et al. (2018). In Vivo Fate and Activity of Second- versus Third-Generation CD19-Specific CAR-T Cells in B Cell Non-Hodgkin’s Lymphomas. Mol. Ther. 26, 2727-2737. [DOI] [PMC free article] [PubMed]
- 28.Turtle C.J., Hanafi L.A., Berger C., Gooley T.A., Cherian S., Hudecek M., Sommermeyer D., Melville K., Pender B., Budiarto T.M. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 2016;126:2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]; Turtle, C.J., Hanafi, L.A., Berger, C., Gooley, T.A., Cherian, S., Hudecek, M., Sommermeyer, D., Melville, K., Pender, B., Budiarto, T.M., et al. (2016). CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 126, 2123-2138. [DOI] [PMC free article] [PubMed]
- 29.Woods E.J., Thirumala S., Badhe-Buchanan S.S., Clarke D., Mathew A.J. Off the shelf cellular therapeutics: Factors to consider during cryopreservation and storage of human cells for clinical use. Cytotherapy. 2016;18:697–711. doi: 10.1016/j.jcyt.2016.03.295. [DOI] [PubMed] [Google Scholar]; Woods, E.J., Thirumala, S., Badhe-Buchanan, S.S., Clarke, D., and Mathew, A.J. (2016). Off the shelf cellular therapeutics: Factors to consider during cryopreservation and storage of human cells for clinical use. Cytotherapy 18, 697-711. [DOI] [PubMed]
- 30.Worsham D.N., Reems J.A., Szczepiorkowski Z.M., McKenna D.H., Leemhuis T., Mathew A.J., Cancelas J.A., Biomedical Excellence for Safer Transfusion (BEST) Collaborative Group Clinical methods of cryopreservation for donor lymphocyte infusions vary in their ability to preserve functional T-cell subpopulations. Transfusion. 2017;57:1555–1565. doi: 10.1111/trf.14112. [DOI] [PubMed] [Google Scholar]; Worsham, D.N., Reems, J.A., Szczepiorkowski, Z.M., McKenna, D.H., Leemhuis, T., Mathew, A.J., and Cancelas, J.A.; Biomedical Excellence for Safer Transfusion (BEST) Collaborative Group (2017). Clinical methods of cryopreservation for donor lymphocyte infusions vary in their ability to preserve functional T-cell subpopulations. Transfusion 57, 1555-1565. [DOI] [PubMed]
- 31.Creer M.H., Lemas M.V., Mathew A.J. American Association of Blood Banks; 2015. Practical Handbook of Cell Therapy Cryopreservation. [Google Scholar]; Creer, M.H., Lemas, M.V., and Mathew, A.J. (2015). Practical Handbook of Cell Therapy Cryopreservation (American Association of Blood Banks).
- 32.Laroche V., McKenna D.H., Moroff G., Schierman T., Kadidlo D., McCullough J. Cell loss and recovery in umbilical cord blood processing: a comparison of postthaw and postwash samples. Transfusion. 2005;45:1909–1916. doi: 10.1111/j.1537-2995.2005.00638.x. [DOI] [PubMed] [Google Scholar]; Laroche, V., McKenna, D.H., Moroff, G., Schierman, T., Kadidlo, D., and McCullough, J. (2005). Cell loss and recovery in umbilical cord blood processing: a comparison of postthaw and postwash samples. Transfusion 45, 1909-1916. [DOI] [PubMed]
- 33.Regan D.M., Wofford J.D., Wall D.A. Comparison of cord blood thawing methods on cell recovery, potency, and infusion. Transfusion. 2010;50:2670–2675. doi: 10.1111/j.1537-2995.2010.02803.x. [DOI] [PubMed] [Google Scholar]; Regan, D.M., Wofford, J.D., and Wall, D.A. (2010). Comparison of cord blood thawing methods on cell recovery, potency, and infusion. Transfusion 50, 2670-2675. [DOI] [PubMed]
- 34.Xu H., Cao W., Huang L., Xiao M., Cao Y., Zhao L., Wang N., Zhou J. Effects of cryopreservation on chimeric antigen receptor T cell functions. Cryobiology. 2018;83:40–47. doi: 10.1016/j.cryobiol.2018.06.007. [DOI] [PubMed] [Google Scholar]; Xu, H., Cao, W., Huang, L., Xiao, M., Cao, Y., Zhao, L., Wang, N., and Zhou, J. (2018). Effects of cryopreservation on chimeric antigen receptor T cell functions. Cryobiology 83, 40-47. [DOI] [PubMed]
- 35.Chabot D., Tremblay T., Paré I., Bazin R., Loubaki L. Transient warming events occurring after freezing impairs umbilical cord-derived mesenchymal stromal cells functionality. Cytotherapy. 2017;19:978–989. doi: 10.1016/j.jcyt.2017.04.005. [DOI] [PubMed] [Google Scholar]; Chabot, D., Tremblay, T., Pare, I., Bazin, R., and Loubaki, L. (2017). Transient warming events occurring after freezing impairs umbilical cord-derived mesenchymal stromal cells functionality. Cytotherapy 19, 978-989. [DOI] [PubMed]
- 36.Bissoyi A., Nayak B., Pramanik K., Sarangi S.K. Targeting cryopreservation-induced cell death: a review. Biopreserv. Biobank. 2014;12:23–34. doi: 10.1089/bio.2013.0032. [DOI] [PubMed] [Google Scholar]; Bissoyi, A., Nayak, B., Pramanik, K., and Sarangi, S.K. (2014). Targeting cryopreservation-induced cell death: a review. Biopreserv. Biobank. 12, 23-34. [DOI] [PubMed]
- 37.Chatterjee A., Saha D., Niemann H., Gryshkov O., Glasmacher B., Hofmann N. Effects of cryopreservation on the epigenetic profile of cells. Cryobiology. 2017;74:1–7. doi: 10.1016/j.cryobiol.2016.12.002. [DOI] [PubMed] [Google Scholar]; Chatterjee, A., Saha, D., Niemann, H., Gryshkov, O., Glasmacher, B., and Hofmann, N. (2017). Effects of cryopreservation on the epigenetic profile of cells. Cryobiology 74, 1-7. [DOI] [PubMed]
- 38.Stroncek D.F., Ren J., Lee D.W., Tran M., Frodigh S.E., Sabatino M., Khuu H., Merchant M.S., Mackall C.L. Myeloid cells in peripheral blood mononuclear cell concentrates inhibit the expansion of chimeric antigen receptor T cells. Cytotherapy. 2016;18:893–901. doi: 10.1016/j.jcyt.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Stroncek, D.F., Ren, J., Lee, D.W., Tran, M., Frodigh, S.E., Sabatino, M., Khuu, H., Merchant, M.S., and Mackall, C.L. (2016). Myeloid cells in peripheral blood mononuclear cell concentrates inhibit the expansion of chimeric antigen receptor T cells. Cytotherapy 18, 893-901. [DOI] [PMC free article] [PubMed]
- 39.Fry T.J., Shah N.N., Orentas R.J., Stetler-Stevenson M., Yuan C.M., Ramakrishna S., Wolters P., Martin S., Delbrook C., Yates B. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fry, T.J., Shah, N.N., Orentas, R.J., Stetler-Stevenson, M., Yuan, C.M., Ramakrishna, S., Wolters, P., Martin, S., Delbrook, C., Yates, B., et al. (2018). CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 24, 20-28. [DOI] [PMC free article] [PubMed]
- 40.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lee, D.W., Kochenderfer, J.N., Stetler-Stevenson, M., Cui, Y.K., Delbrook, C., Feldman, S.A., Fry, T.J., Orentas, R., Sabatino, M., Shah, N.N., et al. (2015). T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385, 517-528. [DOI] [PMC free article] [PubMed]
- 41.Ali S.A., Shi V., Maric I., Wang M., Stroncek D.F., Rose J.J., Brudno J.N., Stetler-Stevenson M., Feldman S.A., Hansen B.G. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128:1688–1700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ali, S.A., Shi, V., Maric, I., Wang, M., Stroncek, D.F., Rose, J.J., Brudno, J.N., Stetler-Stevenson, M., Feldman, S.A., Hansen, B.G., et al. (2016). T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 128, 1688-1700. [DOI] [PMC free article] [PubMed]
- 42.Brudno J.N., Somerville R.P.T., Shi V., Rose J.J., Halverson D.C., Fowler D.H., Gea-Banacloche J.C., Pavletic S.Z., Hickstein D.D., Lu T.L. Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation Without Causing Graft-Versus-Host Disease. J. Clin. Oncol. 2016;34:1112–1121. doi: 10.1200/JCO.2015.64.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]; Brudno, J.N., Somerville, R.P.T., Shi, V., Rose, J.J., Halverson, D.C., Fowler, D.H., Gea-Banacloche, J.C., Pavletic, S.Z., Hickstein, D.D., Lu, T.L., et al. (2016). Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation Without Causing Graft-Versus-Host Disease. J. Clin. Oncol. 34, 1112-1121. [DOI] [PMC free article] [PubMed]
- 43.Liu S., de Castro L.F., Jin P., Civini S., Ren J., Reems J.A., Cancelas J., Nayak R., Shaw G., O’Brien T. Manufacturing Differences Affect Human Bone Marrow Stromal Cell Characteristics and Function: Comparison of Production Methods and Products from Multiple Centers. Sci. Rep. 2017;7:46731. doi: 10.1038/srep46731. [DOI] [PMC free article] [PubMed] [Google Scholar]; Liu, S., de Castro, L.F., Jin, P., Civini, S., Ren, J., Reems, J.A., Cancelas, J., Nayak, R., Shaw, G., O’Brien, T., et al. (2017). Manufacturing Differences Affect Human Bone Marrow Stromal Cell Characteristics and Function: Comparison of Production Methods and Products from Multiple Centers. Sci. Rep. 7, 46731. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.