Abstract
Objective
Dengue has become a serious public health problem in southern China particularly with a record-breaking outbreak in 2014. Serological evidence from areas with no known dengue cases reported prior to 2014 could provide information on possible unrecognized circulation of dengue virus (DENV) before this outbreak.
Method
Between March and May 2015, we performed a cross-sectional serosurvey using a stratified random sampling method among individuals aged 1–84 years-old in 7 communities in Guangzhou with no reported dengue cases before 2014. Sera of subjects were initially screened with the indirect DENV IgG enzyme-linked immunosorbent assay, and positive samples were further tested by the indirect immunofluorescence assay to identify specific serotypes.
Results
A total of 850 subjects had complete information available. The overall seroprevalence against DENV was 6.59% (56 of 850; 95% CI, 4.92%–8.26%). The seroprevalence increased with age in general (3.86%, 4.58%, 8.72%, 7.22%, and 10.69% among participants in ≤14, 15–29, 30–44, 45–59 and ≥60 years age group, respectively). Living in rural or peri-urban communities and longer years of residence therein were risk factors for higher seroprevalence, whereas wearing long sleeves and pants when outdoors was associated with lower seroprevalence. Of the total subjects, 55.36% (31 of 56) sera were successfully identified with specific serotypes, with 12.90% (4 of 31) being coinfected with 2 serotypes.
Conclusions
Dengue transmission in the study communities had occurred prior to the 2014 massive outbreak, possibly for many years, but went undiagnosed and unreported. A proportion of the study population experienced secondary infection as different serotypes of DENV increased the risk for severe diseases. Active surveillance and education of both healthcare providers and the general population should be conducted in areas at risk for dengue emergence in order to better reduce disease burden.
Keywords: China, dengue, rural areas, seroprevalence, underestimation
The 2015 cross-sectional, stratified random sampling serosurvey in areas with no reported cases before the large outbreak in 2014 in Guangzhou indicated dengue transmission occurred prior to the 2014 for many years, but went undiagnosed and unreported.
INTRODUCTION
Dengue is the most rapidly spreading mosquito-borne disease globally, with approximately 390 million infections and 96 million symptomatic cases occurring annually, mainly in tropical and subtropical regions [1]. Dengue virus (DENV) is classified into 4 serotypes (DENV-1, DENV-2, DENV-3, and DENV-4) and transmitted by female Aedes mosquitoes. DENV infection either can be asymptomatic or lead to a broad spectrum of clinical presentations ranging from mild symptoms known as dengue fever characterized by fever, chills, and muscle aches to more severe or even life-threatening forms, such as dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) [2]. After primary infection, secondary infection by a heterologous DENV serotype may result in severe disease due to the effect of antibody-dependent enhancement [3]. There is no specific treatment for DENV infections and a preventive vaccine is still limited in use and under further evaluation at present.
DENV may have been circulating in an area for some time before it is genuinely identified. Infections from DENV that are asymptomatic or that only develop mild to moderate symptoms normally go undiagnosed as infected individuals are unlikely to visit a doctor. Misdiagnosis also may occur when physicians do not consider dengue in the differential diagnosis and order inappropriate diagnostic tests for those who seek medical care. These unidentified infections, which fail to be captured by passive surveillance systems, may play a key role in amplifying the disease and eventually causing an epidemic. Serosurvey as a main approach can help determine virus exposure and related risk factors [4]. However, the prevalence of DENV infections during the pre-epidemic period has not been well studied.
In recent years, southern China has been experiencing a high frequency of transmission and recurring epidemics of dengue, especially in Guangzhou, which accounts for 69.2% of reported cases in mainland China [5]. Currently, there has been a change of prevalent serotypes over time from 1 predominant serotype, DENV-1, to multiple serotypes cocirculating [6], which can increase the risk of developing severe diseases, like DHF or DSS. Reported indigenous transmission of dengue in Guangzhou is mainly confined to urban and peri-urban areas. Dengue was very rare in rural areas prior to the unprecedented outbreak in 2014 in Guangzhou. Of the 31 predominantly rural communities with no dengue cases recorded before 2014, all but 5 experienced the outbreak in 2014 [5]. The experience of the 2014 massive outbreak, with cases reported from areas where no known dengue cases had been reported previously, prompted us to conduct the first-ever seroprevalence study in these areas to determine whether cases may have been unrecognized in the past. In 2015, we performed a cross-sectional, community-based serological survey to assess the population seroprevalence of DENV and to identify important risk factors for dengue in communities where no cases had been reported before 2014.
METHODS
Participant Enrollment
The study protocol and informed consent forms were reviewed and approved by institutional review boards at both the Guangzhou Center for Disease Control and Prevention (GZCDC) and the School of Public Health, Sun Yat-sen University (SYSU). Written informed consent was obtained from all participants enrolled in the study, including children under 18 years-old whose consent was provided by their parents or guardians. All the subject data were de-identified and the data were analyzed anonymously.
Study subjects were enrolled from Guangzhou, the most important epicenter of dengue in mainland China. The estimated population of over 12.84 million makes it one of the most highly populated and urbanized cities in the world. Besides featuring a humid subtropical climate [7], Guangzhou is perfectly suitable for vector reproduction [8]. Over the past 3 decades, Aedes albopictus was observed to be the sole mosquito vector for dengue transmission in Guangzhou and no presence of Aedes aegypti was identified. Specifically, Ae. albopictus made up 5.89% of all adult mosquitoes in Guangdong province, second only to Culex fatigans (89.90%) [9].
A cross-sectional serosurvey was conducted in communities where there was no dengue case reported until 2014. Eligible participants were subjects aged 1–84 years-old in the general population, who had lived in the selected communities for more than 1 year. Sample size was calculated based on a 10% expected prevalence of DENV IgG antibodies with a power of 80% and a type 1 error of 5%. The minimum sample size required to assess the prevalence was 843. We assumed an extra 20% of missing data at the time of analysis, so the final sample size was calculated to be 1011 subjects.
A stratified 2-stage cluster sampling method was applied. In the first stage, 7 of the 26 eligible communities were selected to make sure a range of low to high dengue incidence was included. During the 2014 outbreak, the total incidence of the selected 7 communities was 2.56 per 10 000 population, among which Yuangang had the highest incidence rate (9.15 of 10 000), followed by Jiulong (4.18 of 10 000), Shiling (2.85 of 10 000), Huadong (1.94 of 10 000), Aotou (1.35 of 10 000), Paitan (0.51 of 10 000), and Liangkou (0.30 of 10 000) [5]. In the second stage, the population was stratified by 3 age groups (<5 years, 6–59 years, and ≥60 years) within each selected community and individuals were randomly selected from each strata. All consenting subjects were then requested for a face-to-face interview using a standard questionnaire and the collection of 5 ml of blood. The questionnaire was self-designed to capture information on demographic characteristics, history of clinically diagnosed dengue, living and housing characteristics, and individual behavioral factors, such as the knowledge of transmission vector, using bed nets while sleeping, using mosquito repellents, and wearing long sleeves and pants when outdoors. The survey team comprised 2 medical professionals from the local community’s primary health service center and 1 epidemiologist from GZCDC. The survey was carried out from March to May 2015, which was the pretransmission season for dengue in Guangzhou.
Laboratory Methods
Whole blood specimens were clotted at room temperature for 0.5 hour and then centrifuged at 3000 rpm for 20 minutes to separate serum. The sera were transferred to 3 ml screw-cap containers and stored at -20°C at the community’s primary health centers. Frozen samples from the primary health centers were transferred to GZCDC for further storage -80°C and testing.
All serum specimens initially were screened with indirect DENV IgG enzyme-linked immunosorbent assay (ELISA) to detect the DENV-specific IgG antibody, and positive sera were further tested by indirect immunofluorescence assay to identify the serotype. Specifically, sera first were tested against DENV-specific IgG antibody using indirect ELISA kits (PanBio, Brisbane, Australia) according to the manufacturer’s instructions. This assay has a reported sensitivity of 91.4% for primary infection in nonendemic areas and 97.0% for secondary infection. The specificity is close to 100.0% [10]. Serum dilutions of 1:100 were evaluated, and the cutoffs for IgG positivity were determined based on reactions of the positive control and normal control [11]. Next, indirect immunofluorescence test kits (Mosaic, Euroimmun, Germany), which are designed for the qualitative in vitro determination of human IgG antibody against DENV serotypes 1–4, were used to identify the serotypes of positive sera according to the manufacturer’s instructions. Briefly, DENV-infected Vero cells were incubated with diluted samples. If the specific anti-DENV IgG antibody was present, it attached to the corresponding DENV antigen on the Vero cells. In a second step, the attached antibodies were stained with fluorescein-labeled antihuman antibodies, which were visible under a fluorescence microscope.
All serological tests were conducted at GZCDC, where laboratory staff were experienced with DENV detection and characterization and performed good quality control. A random selection of negative (n = 50) and positive (n = 50) serum samples also were tested at the laboratory in the School of Public Health at SYSU for quality control. The participants were informed of the testing results by telephone and written report.
Statistical Methods
All questionnaire data were entered twice by 2 independent staffs from GZCDC using EpiData software (version 3.1, The EpiData Association, Odense, Denmark). We employed R statistical software (version 3.3.3, R Foundation for Statistical Computing, Vienna, Austria) in the analysis, using packages stats, dplyr, ggplot2, reshape2, and lme4 [12]. Univariate and multivariate analysis were conducted using the generalized linear mixed-effects models with binomial link function. The DENV IgG positivity was studied as the response variable. Fixed effects included gender, age group, education, housing type, years of residence, individual behavioral factors, and random effects in communities. The variables with statistical significance in the univariate analysis were included in the multivariate analysis. The statistical significance level was set to 0.05. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for variables listed. In the final analysis, we excluded observations with missing data or no serum collected.
RESULTS
Participants and Seroprevalence
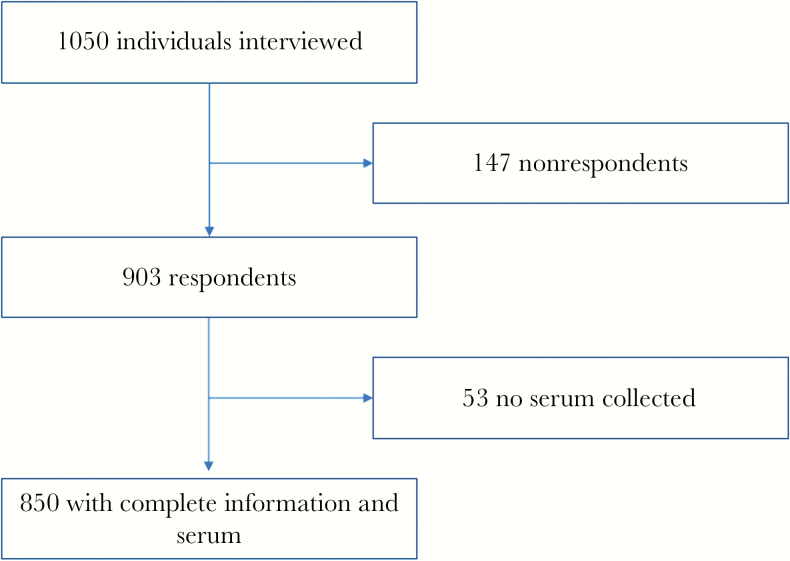
From March to May 2015, using informed consent, we interviewed 1050 individuals and received 903 responses, among which a total of 850 participants had complete questionnaire information and serum samples collected (Figure 1). Among the whole population, the overall seroprevalence against DENV was 6.59% (56 of 850; 95% CI, 4.92%–8.26%). Participants enrolled from different communities demonstrated statistically varied seroprevalence (P < .05) with subjects from Liangkou showing the highest rate of being seropositive (17 of 159, 10.69%), followed by those from Aotou (15 of 160, 9.38%), Huadong (11 of 124, 8.87%), Paitan (6 of 118, 5.08%), Jiulong (2 of 41, 4.88%), Shiling (4 of 123, 3.25%), and Yuangang (1 of 125, 0.80%); see details in Supplementary Table S1. No subject in the survey declared disease history of dengue.
Figure 1.
Flow chart illustrates study subject enrollment, Guangzhou, China, March–May 2015. A total of 850 subjects were included for analysis in this cross-sectional seroepidemiological study.
Univariate Analysis
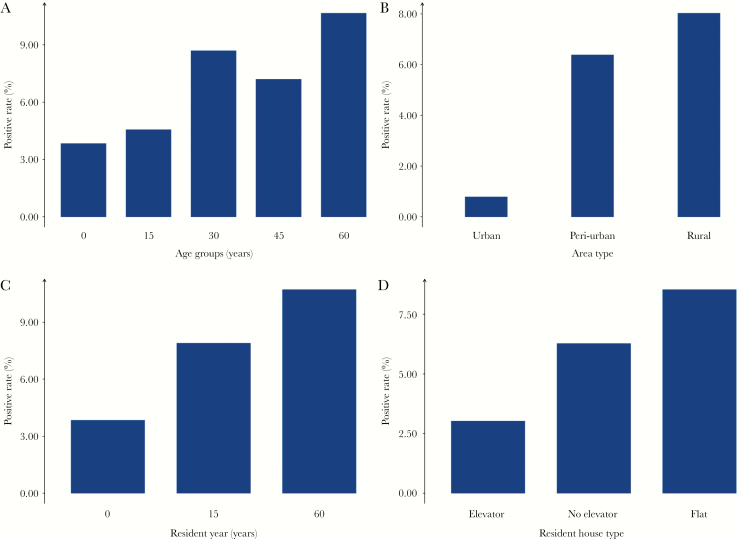
In the univariate analysis, the prevalence of DENV IgG antibody increased with age in general, with the ≥60 years age group demonstrating the highest positive rate (10.69%), followed by the 30–44 years age group (8.72%); both were significantly higher than the ≤14 years age group (3.86%) (P < .05) (Figure 2A). Males seemed to have a greater prevalence than females (7.89% vs 5.47%), and DENV seropositivity appeared to be more common among subjects with middle school and lower (6.61%) or high school (7.50%) education level compared to those with college degree and higher education level (2.94%). However, none of the above characteristics were statistically significant (Table 1).
Figure 2.
The distribution of DENV seroprevalence by demographic and other epidemiological characteristics, Guangzhou, China, March–May 2015. The 4 bar charts graphs the distribution of DENV seroprevalence by (A) age group, (B) community type, (C) years of residence, and (D) housing type, respectively.
Table 1.
Risk Factors for Being Seropositive Against DENV, Using Univariate Generalized Linear Mixed-Effects Modeling, Among Study Participants, Guangzhou, China, March–May 2015
| Variables | N | n | Rate (%) | P value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Female | 457 | 25 | 5.47 | Ref. | ||
| Male | 393 | 31 | 7.89 | .14 | 1.52 | (0.88, 2.62) |
| Age group (years) | ||||||
| ≤14 | 259 | 10 | 3.86 | Ref. | ||
| 15–29 | 131 | 6 | 4.58 | .42 | 1.54 | (0.54, 4.42) |
| 30–44 | 149 | 13 | 8.72 | .03a | 2.53 | (1.08, 5.96) |
| 45–59 | 180 | 13 | 7.22 | .09 | 2.07 | (0.89, 4.83) |
| ≥60 | 131 | 14 | 10.69 | .01a | 2.84 | (1.23, 6.58) |
| Community type | ||||||
| Urban | 125 | 1 | 0.80 | Ref. | ||
| Peri-urban | 203 | 13 | 6.40 | .04a | 8.54 | (1.06, 69.19) |
| Rural area | 522 | 42 | 8.05 | .02a | 10.63 | (1.39, 81.19) |
| Highest level of education completed | ||||||
| Middle school and lower | 696 | 46 | 6.61 | .68 | 1.53 | (0.20, 11.87) |
| High school | 120 | 9 | 7.50 | .48 | 2.11 | (0.26, 17.35) |
| College degree and higher | 34 | 1 | 2.94 | Ref. | ||
| Housing type | ||||||
| Multistory building with elevator | 33 | 1 | 3.03 | Ref. | ||
| Multistory building without elevator | 653 | 41 | 6.28 | .46 | 2.21 | (0.29, 16.57) |
| Single-story building | 164 | 14 | 8.54 | .45 | 3.08 | (0.39, 24.25) |
| Years of residence in the community | ||||||
| ≤14 | 363 | 14 | 3.86 | Ref. | ||
| 15–59 | 366 | 29 | 7.92 | .05a | 1.94 | (0.98, 3.82) |
| ≥60 | 121 | 13 | 10.74 | .02b | 2.57 | (1.14, 5.84) |
| Know the transmission vector of DENV | ||||||
| Yes | 735 | 48 | 6.53 | Ref. | ||
| No | 115 | 8 | 6.96 | .83 | 1.02 | (0.45, 2.30) |
| Used bed nets while sleeping | ||||||
| Yes | 767 | 51 | 6.65 | Ref. | ||
| No | 83 | 5 | 6.02 | .77 | 0.87 | (0.33, 2.28) |
| Used mosquito repellents when outdoors | ||||||
| Yes | 423 | 25 | 5.91 | Ref. | ||
| No | 427 | 31 | 7.26 | .74 | 1.11 | (0.60, 2.06) |
| Wore long sleeves and pants when outdoors | ||||||
| Yes | 258 | 9 | 3.49 | Ref. | ||
| No | 592 | 47 | 7.94 | .04a | 2.23 | (1.03, 4.80) |
Abbreviations: N, the number of respondents; n, the number of subjects being seropositive against DENV; OR, odds ratio; P value, P value for coefficients calculated by t test; rate, rate (%) of being seropositive against DENV; Ref., reference group; 95% CI, 95% confidence interval for odds ratio.
a P < .05.
b P < .01.
In terms of community type, individuals living in both rural areas and peri-urban areas had much greater prevalence than those living in urban areas of being seropositive against DENV (P < .05) (Figure 2B). The prevalence rose with the number of years of residence in that community, with the highest positive rate (10.74%, P < .05) in those living in a certain community for more than 60 years (Figure 2C). Although the differences were not statistically significant, participants staying in single-story houses were more likely to be seropositive compared to those living in multistory buildings equipped with or without elevators (Figure 2D). As shown in Table 1, the behavior of wearing long sleeves and pants when outdoors was negatively associated with DENV seropositivity while no statistically significant differences were observed for other factors, such as knowing the transmission vector of DENV, using bed nets while sleeping, and using mosquito repellents when outdoors.
Multivariate Analysis
The final multivariate model suggested that living in rural areas, living in peri-urban areas, elder age, and not wearing long sleeves and pants when outdoors were statistically important risk factors for being seropositive against DENV in general population (P < .05) (Table 2).
Table 2.
Selected Risk Factors for Being Seropositive Against DENV, Using Multivariate Generalized Linear Mixed-Effects Modeling, Among Study Participants, Guangzhou, China, March–May 2015
| Variables | P value | OR | 95% CI |
|---|---|---|---|
| Peri-urban community type | .01b | 24.02 | (2.18, 264.06) |
| Rural community type | .01b | 27.56 | (2.80, 271.02) |
| Age (years) | .02a | 1.04 | (1.01, 1.07) |
| Years of residence in the community | .08 | 0.97 | (0.94, 1.01) |
| Did not wear long sleeves and pants when outdoors | .02a | 2.51 | (1.19, 5.27) |
Abbreviations: OR, odds ratio; P value, P value for coefficients calculated by t test; 95% CI, 95% confidence interval for odds ratio.
a P < .05.
b P < .01.
DENV Serotype Identification
Of all 56 serum samples positive for DENV IgG antibody, 31 (55.36%) sera were further successfully identified with specific serotypes using indirect immunofluorescence assay (Table 3). All 4 DENV serotypes were found in the study population, including 15 DENV-1, 11 DENV-2, 7 DENV-3 and 2 DENV-4. Of note, there were 3 different combinations of coinfection identified in 4 serum samples; they included 2 subjects seropositive against both DENV-3 and 4, 1 subject seropositive against both DENV-1 and 4, and 1 subject seropositive against both DENV-1 and 2.
Table 3.
DENV Serotypes Identified by Indirect Immunofluorescence Assays in Different Study Communities, Guangzhou, China, March–May 2015
| Communities | DENV-1 | DENV-2 | DENV-3 | DENV-4 |
|---|---|---|---|---|
| Aotou | 1 | 3 | 7 | 3 |
| Liangkou | 2 | 5 | 0 | 0 |
| Huadong | 2 | 1 | 0 | 0 |
| Shiling | 3 | 1 | 0 | 0 |
| Jiulong | 2 | 0 | 0 | 0 |
| Paitan | 5 | 1 | 0 | 0 |
| Yuangang | 0 | 0 | 0 | 0 |
| Total | 15 | 11 | 7 | 3 |
Several subjects were found to be seropositive against 2 DENV serotypes (2 subjects in Aotou were seropositive against both DENV-3 and -4, 1 subject in Aotou was seropositive against both DENV-1 and -4, and 1 subject in Liangkou was seropositive against both DENV-1 and -2).
DISCUSSION
When an emerging disease spreads into new areas, it can be unrecognized. In the case of dengue, individuals with unapparent infection or only mild to moderate symptoms may not visit a doctor. And those patients who seek medical care may not be readily diagnosed if the diagnosis of dengue is not considered and appropriate diagnostic tests are not ordered. Severe dengue (DHF and DSS), which leads to immediate medical attention and appropriate diagnostic testing, is usually less common in initial dengue infections. Our study strongly suggested that this phenomenon occurred in Guangzhou prior to the unprecedented outbreak of dengue in 2014.
Three lines of reasoning contribute to the conclusion that DENV had been circulating in these areas prior to 2014. First, the increase of DENV seroprevalence by age in a dose-response pattern suggested that elderly people probably had experienced DENV exposure already in past years, because there was no alternative explanation for why they would be more likely to be exposed just in the year of 2014. Second, DENV seroprevalence also increased with the number of years of residence in a certain community; although, this was not seen in the multivariate analysis. Similarly, if these areas just experienced 1 year of epidemic, the seroprevalence would have not shown significant differences among different age groups and years of residence. Finally, 4 different DENV serotypes were identified in our study. However, the predominant serotype in 2014 outbreak was DENV-1 and no other serotype was identified in the reported cases. The diversity of DENV serotype demonstrated that all 4 serotypes had existed in the general population before 2014.
The case data typically used alone for illustrating the actual epidemiological pattern can be biased because of its large dependence on reported cases, which is only a fraction of cases captured by passive surveillance systems [13]. There were no recorded dengue epidemics in the study communities before 2014. However, indigenous cases were reported from all 7 communities in the 2014 outbreak. Using a serosurvey with participants randomly selected from an age-stratified population, our results demonstrated the actual DENV infection burden in the study areas was substantially underestimated [14]. Three key factors may cause the underestimation of DENV transmission. The main reason for underdiagnosis and underreporting of dengue cases in the 7 communities we studied was probably due to the rural nature of these communities and their corresponding primary health care systems being relatively underdeveloped. Moreover, people living in rural areas normally had less access to health education campaigns and were less willing to seek health care compared to those living in urban areas. Physicians in these areas may not have enough awareness of dengue symptoms and related diagnostic methods. Finally, laboratory testing for dengue may not be as readily available as for other diseases, such as influenza. Due to the inability of the primary medical care system in rural areas to diagnose dengue, DENV infections always were substantially underreported in these areas.
The picture of Yuangang community helps to illustrate the above observation. Of the 7 communities we studied, Yuangang was the most urbanized town and had the highest reported incidence of dengue in the 2014 outbreak, which also reflected that its healthcare system was able to quickly diagnose dengue cases. Meanwhile, Yuangang also had the lowest DENV seroprevalence among the 7 communities in our study, which suggested that the town was able to implement earlier control measures to contain the spread of the epidemic. This partly could be attributed to united control effort and well-organized medical institutes in this area, which helped to strengthen early identification of cases and early enforcement of control measures [8]. In fact, the city-wide mosquito vector surveillance and control program implemented from April in each year in Guangzhou played a key role in dengue control. However, this was mainly well put into practice in urban areas. The higher risk of being seropositive against DENV also might be correlated with the relatively weaker implementation strength in rural and peri-urban areas.
The age pattern of disease could affect trends in transmission hazard and age distribution of cases reported. In Thailand, the shift in age pattern of severe dengue occurred and transformed from children to adults, and the same situation was seen in other countries in Southeast Asia [15]. Due to the highest incidence and seroprevalence, the elderly population had the highest risk for developing severe dengue and death. Elderly people usually had more outdoors activities in common public areas, such as the park, markets, etc., and time spent outdoors during the daytime during epidemic periods of Ae. albopictus activity [16], which increased their opportunity of being exposed to infected mosquitoes or individuals [5]. This age pattern also was close to the situation in other endemic areas, like the Caribbean [17].
Housing type was a strong index of subjects’ socio-economic status in this study. People living in single-story houses were more likely to be detected seropositive. In rural areas of southern China, people with higher incomes always build multistory houses, while poorer people still stay in single-story houses with more breeding sites for mosquitoes. Wearing long sleeves and pants when outdoors was a protective factor while using bed nets while sleeping was not statistically important, suggesting that infections outdoors may be the main reason for DENV transmission in Guangzhou, where the transmission vector is Ae. Albopictus. This is different from other endemic areas, like Thailand and Brazil, where DENV infection is thought to occur more in indoor settings and transmitted by Ae. aegypti.
Previous seroepidemiological studies in China’s west Yunnan and Guangzhou provinces showed that the prevalence of DENV IgG antibody was 10.9% and 10.04%, respectively [18]. However, these studies were conducted in areas with outbreaks in the past by convenience sampling method. No study had investigated areas with no dengue cases reported before the 2014 record-breaking outbreak. The present study extended these observations and used random sampling strategy to achieve a more representative study population with adequate sample size.
Furthermore, our study suggested that dengue was still not an endemic issue in these 7 communities in Guangzhou. The DENV IgG prevalence in the study areas was significantly lower than that in endemic areas. In a 2010 serosurvey in Singapore, the prevalence of DENV IgG in the adult population was 56.8%, and the older the age, the higher the positive rate, which was consistent with our study [19]. Similarly high DENV seroprevalence was observed in other endemic countries, such as 49.8% in Puerto Rico in 2007 [20], 77.4% in Venezuela [21], and 76.6% in Mexico in 2011 [22]. The previous infection rate was identified as 40% to 78% on the Texas–Mexico border in 2014 [14]. Our study indicated that dengue epidemics transmitted by Ae. albopictus were deemed as mild and generally short-lived [23]. Therefore, dengue fever transmission in our study areas still did not reach the level of endemic areas, which indicated that dengue was mostly imported to these areas.
Our study had several limitations. First, we may have introduced some selection bias in selecting the 7 communities, which might not represent the whole population. Second, we employed only anti-DENV IgG ELISA and indirect immunofluorescence assays to determine the serotype, without performing neutralization assays to assess specific DENV antibody reactivity. The ELISA in our study also had the limitation of cross-reactivity, especially with Japanese encephalitis virus. Third, the indirect immunofluorescence assays in the study used the international standard virus strains, not native strains, which may have lowered the reactivity. Finally, we were not able to determine how much of the seroprevalence we found was due to infections in the 2014 outbreak or in previous years.
In summary, this study showed that dengue transmission in the investigated communities had occurred at low levels prior to the 2014 massive outbreak, possibly for many years, but such cases went undiagnosed and unreported. These findings have important implications for the design and implementation of dengue prevention and control interventions in areas where dengue remains as an emerging issue. In order to better prevent and control future dengue outbreaks, the general population needs to be educated on the risks of dengue and how to prevent DENV exposure, and medical care providers need to be educated on the symptoms, treatment, diagnosis, and reporting of dengue. Meanwhile, public health officials need to conduct active surveillance and create evaluation measures for controlling mosquito vectors, identify areas of early dengue emergence, and take steps to reduce mosquito density in order to have a chance to prevent an outbreak or blunt its impact. Furthermore, investments in rural infrastructure, economic assistance for air conditioning, and sustained community education on the importance of reducing larval habitat around the house will be essential to control dengue transmission in these areas.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors would like to thank the staff in dengue control from the Guangzhou Center for Disease Control and Prevention, 4 district CDCs and 7 related primary health centers in Conghua, Zengcheng, Tianhe, and Huadu for their assistance in the study.
J.H.L. and Z.C.Y. conceived and designed the experiments. Q.L.J., Y.L.L., J.H.L., Z.Q.C., and G.S.B. analyzed the data. Q.L.J., W.Z.S., and L.Y.J. conducted laboratory testing. Q.L.J., G.S.B., and Z.C.Y. contributed reagents, materials, and analysis tools. Q.L.J., J.H.L., and Z.C.Y. wrote the paper.
Financial support. This work was supported by a grant from the Natural Science Foundation of Guangdong Province (No. S2013010013637), the Project for Key Medicine Discipline Construction of Guangzhou Municipality (No. 2017-2019-04), the Medical Scientific Research Foundation of Guangdong Province (A2017481), the Collaborative Innovation Project of Bureau of Science and Technology of Guangzhou Municipality (No. 201704020226 and 201803040006), and the Science and Technology Plan Project of Guangzhou (201804010121). The funders had no roles in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guzman MG, Harris E. Dengue. Lancet 2015; 385:453–65. [DOI] [PubMed] [Google Scholar]
- 3. Dejnirattisai W, Jumnainsong A, Onsirisakul N, et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010; 328:745–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Metcalf CJ, Farrar J, Cutts FT, et al. Use of serological surveys to generate key insights into the changing global landscape of infectious disease. Lancet 2016; 388:728–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luo L, Jiang LY, Xiao XC, et al. The dengue preface to endemic in mainland China: the historical largest outbreak by Aedes albopictus in Guangzhou, 2014. Infect Dis Poverty 2017; 6:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang LY, Jing QL, Liu Y, et al. Molecular characterization and genotype shift of dengue virus strains between 2001 and 2014 in Guangzhou. Epidemiol Infect 2017; 145:760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lu L, Lin H, Tian L, et al. Time series analysis of dengue fever and weather in Guangzhou, China. BMC Public Health 2009; 9:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng Q, Jing Q, Spear RC, Marshall JM, Yang Z, Gong P. Climate and the timing of imported cases as determinants of the Dengue outbreak in Guangzhou, 2014: evidence from a mathematical model. PLOS Negl Trop Dis 2016; 10:e0004417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cai S, Duan J, Liu W, Zou Q. Density fluctuation and population composition of mosquitoes in Guangdong Province. Chin J Hyg Insect & Equip 2014; 20:357–359. [Google Scholar]
- 10. Leder K, Mutsch M, Schlagenhauf P, et al. Seroepidemiology of dengue in travellers: a paired sera analysis. Travel Med Infect Dis 2013; 11:210–3. [DOI] [PubMed] [Google Scholar]
- 11. Corbett KS, Katzelnick L, Tissera H, Amerasinghe A, de Silva AD, de Silva AM. Preexisting neutralizing antibody responses distinguish clinically inapparent and apparent dengue virus infections in a Sri Lankan pediatric cohort. J Infect Dis 2015; 211:590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 13. Ash C. Estimating transmission chains for dengue. Science 2017; 355:1277–8. [DOI] [PubMed] [Google Scholar]
- 14. Brunkard JM, Robles Lopez JL, Ramirez J, et al. Dengue fever seroprevalence and risk factors, Texas–Mexico border, 2004. Emerg Infect Dis 2007; 13:1477–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cummings DA, Iamsirithaworn S, Lessler JT, et al. The impact of the demographic transition on dengue in Thailand: insights from a statistical analysis and mathematical modeling. PLOS Med 2009; 6:e1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moro ML, Gagliotti C, Silvi G, et al. ; Chikungunya Study Group Chikungunya virus in North-Eastern Italy: a seroprevalence survey. Am J Trop Med Hyg 2010; 82:508–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leslie T, Martin NJ, Jack-Roosberg C, et al. Dengue serosurvey in Sint Eustatius. PLOS ONE 2014; 9:e95002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guo X, Wu C, Wang P, et al. Serological investigation of dengue in western Yunnan. Chin J Zoonoses 2010; 26:502–4. [Google Scholar]
- 19. Ang LW, Cutter J, James L, Goh KT. Seroepidemiology of dengue virus infection in the adult population in tropical Singapore. Epidemiol Infect 2015; 143:1585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Argüello DF, Tomashek KM, Quiñones L, et al. Incidence of dengue virus infection in school-aged children in Puerto Rico: a prospective seroepidemiologic study. Am J Trop Med Hyg 2015; 92:486–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Velasco-Salas ZI, Sierra GM, Guzmán DM, et al. Dengue seroprevalence and risk factors for past and recent viral transmission in Venezuela: a comprehensive community-based study. Am J Trop Med Hyg 2014; 91:1039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amaya-Larios IY, Martínez-Vega RA, Mayer SV, et al. Seroprevalence of neutralizing antibodies against dengue virus in two localities in the state of Morelos, Mexico. Am J Trop Med Hyg 2014; 91:1057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Issack MI, Pursem VN, Barkham TM, Ng LC, Inoue M, Manraj SS. Reemergence of dengue in Mauritius. Emerg Infect Dis 2010; 16:716–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.