Abstract
Deciding between increased cancer screening or prophylactic surgery and the timing of such procedures can be a difficult and complex process for women with BRCA mutations. There are gaps in our understanding of involvement of others in the decisionmaking process for women with BRCA mutations. This study evaluated the management decision-making process of women with BRCA mutations, focusing on the involvement of others. Grounded theory was used to analyze and code risk management decision-making information from interviews with 20 BRCA mutation carriers. Unaffected at-risk participants with a BRCA mutation, those under age 40, and those with no children described having a difficult time making risk management decisions. Physicians were an integral part of the decision-making process by providing decisional support and management recommendations. Family members and other mutation carriers filled similar yet distinct roles by providing experiential information as well as decisional and emotional support for carriers. Participants described genetic counselors as short-term providers of risk information and management recommendations. The study findings suggest that unaffected at-risk women, women under 40, and those who do not have children may benefit from additional support and information during the decision-making process. Genetic counselors are well trained to help women through this process and connect them with resources, and may be underutilized in long-term follow-up for women with a BRCA mutation.
Keywords: Cancer risk management, Decision-making, HBOC, BRCA1, BRCA2, Genetic counselor
Introduction
Due to the increased risk for breast and ovarian cancers associated with BRCA1 and BRCA2 mutations, the National Comprehensive Cancer Network (NCCN) recommends that women with BRCA mutations have earlier and more frequent breast cancer screening with annual mammography and breast MRI. The NCCN also recommend risk-reducing salpingo-oo-phorectomy (RRSO) preferably between the ages of 35–45 upon the completion of childbearing. Women with a mutation may also consider risk-reducing mastectomy (RRM; National Comprehensive Cancer Network 2016). These guidelines aim to either prevent cancer via prophylactic surgery or detect cancer at an early and more treatable stage through screening, thus reducing cancer mortality. Despite the guidelines, there is a wide range in uptake of prophylactic surgeries and surveillance by women with BRCA mutations. Studies have estimated that 46% of mutation carriers have an RRM by age 70, 71–86% have a RRSO by age 50 (Chai et al. 2014), and 41–46% rely mostly on surveillance with no active prevention by surgery or chemoprevention (Flippo-Morton et al. 2015; Metcalfe et al. 2008).
Deciding if and/or when to have a risk-reducing surgery can be a very complex and difficult decision for many women with BRCA1 and BRCA2 mutations (Hamilton et al. 2009; Leonarczyk and Mawn 2015; Ray et al. 2005). Despite the benefits of prophylactic surgery related to mortality and risk reduction, women must also consider the many possible adverse outcomes associated with these procedures (Gahm et al. 2010). For unaffected women found to have mutations in BRCA1 or BRCA2, studies often describe the decision-making process as a journey that requires weighing pros and cons over time (Leonarczyk and Mawn 2015). Some women describe the decision-making process as empowering, especially if they have support from friends and family (Hesse-Biber 2014). The type of decision-making experience women have is important since experiencing difficulty or uncertainty (with regard to RRSO, specifically) has been found to be associated with lower decisional satisfaction (Westin et al. 2011).
Some women make their medical management decisions in isolation, while others get support or feel pressure to make a certain decision from people in their social networks (Hoskins and Werner-Lin 2013). Family, friends, online relationships, support group members, and healthcare providers can all play a role in the decision-making process (Hesse-Biber 2014; Hoffman et al. 2014; Hoskins and Werner-Lin 2013; Howard et al. 2011; Leonarczyk and Mawn 2015).
Research has shown that family can provide information and advice for decision-making but may also put too much pressure to make a certain decision on these women, especially as they reach the age when their family members were diagnosed with cancer (Hoskins and Werner-Lin 2013; Howard et al. 2011). Studies have shown that family members and friends are involved in the decision-making process to help the carrier answer questions, such as whether or not to have surgery and when to undergo prophylactic surgery, and to provide input aside from the clinical expertise from healthcare providers (Klitzman and Chung 2010). However, women with a BRCA mutation might exclude family and friends from the decision-making process completely if they are unsupportive and/or have unpleasant responses (Howard et al. 2011).
According to the literature, many women with a BRCA mutation perceive the involvement of physicians (e.g., surgeons, oncologists, gynecologists, and primary care physicians, in surgical decision-making) to be less useful than hoped. A qualitative study of women regarding RRM and/or RRSO decision-making by Klitzman and Chung (2010) found that women often turn to physicians for input with decision-making. Some wished their physician provided more guidance and was more directive, while others thought their physician was too directive and felt pressure from them to make a certain decision. Many participants also felt like their physicians were forceful and insensitive in the manner in which they provided guidance and information. This lack of information and decisional support from healthcare providers can impede the decision-making process, possibly making it more difficult. Although not as common in the literature, there are examples of a few women with BRCA mutations who feel their physician provided appropriate information, support, and decision help (Howard et al. 2011; Leonarczyk and Mawn 2015).
In addition to physicians, genetic counselors are involved in the risk-management decision-making process. A prospective, descriptive, cross-sectional study of 62 women at increased risk for breast or ovarian cancer showed that the information provided by genetic counselors, such as information about genetic testing and cancer risk, was helpful in surgical decision-making by providing clients with risk information that they can incorporate into their risk perception (Ray et al. 2005). A large prospective study also showed that genetic counseling can promote surveillance, preventive surgeries, and early cancer detection (Scheuer et al. 2002). However, while a qualitative study of women at increased risk for breast and/or ovarian cancer who had genetic counseling and underwent a RRM showed that 7 of the 15 participants were satisfied with the factual information they received from genetic counseling, only 4 participants were satisfied with the psychological support they received (Josephson et al. 2000).
Although studies illustrate the ways in which healthcare providers and family members may impede risk management decisions and make the decision-making process more difficult, there is a lack of in-depth information about how individuals can assist in the decision-making process and what roles they may play. Using qualitative methods, we examine the involvement and roles of others (i.e., family, other mutation carriers, physicians, and genetic counselors) in cancer risk management decision-making and explore carriers’ ease of decision-making. By understanding their unique roles, the goal of the present study can help direct support and assistance to women with BRCA mutations experiencing decisional conflict regarding risk-management.
Methods
Participants
Women with a BRCA1 or BRCA2 mutation were identified from a clinical database of patients previously seen for cancer genetic counseling (N =212). The Cancer Institutional Review Board at The Ohio State University approved this study. Eligible participants were between the ages 18 and 60, had undergone genetic counseling within the last 6 years, and were able to read/speak English. Because this study focused on breast cancer risk and decision-making, we excluded women with a previous or current ovarian cancer diagnosis.
Instrumentation and Procedures
Of the 212 eligible participants identified, all 139 individuals who had up-to-date contact information available were contacted by a member of the study team. The first 20 participants to express interest and/or respond to a scripted voicemail left by a member of the study team were scheduled for an interview. Three researchers who had qualitative interviewing experience or genetic counseling experience (including AP and SH) conducted individual interviews (lasting 30–60 min each) in person or over the phone using a semi-structured interview guide. The interviewers had not had any previous interactions or professional relationships with the participants prior to the interview.
We originally designed the interview guide (Online resource 1) to elicit information about preferences for communication of breast cancer risk estimates and how women understand and acclimate to risk over time. The guide was developed by an experienced qualitative interviewer (SH) with input from the study team (including genetic counselors and academic researchers) and was designed to assess recommendations summarized in Lautenbach et al. (2013). Despite not being directly asked, participants naturally discussed risk management decision-making; thus, this study is a secondary analysis of the interview data. All interviews were audio recorded and transcribed verbatim.
Participants received compensation for their participation in the form of a gift card. Table 1 summarizes participant demographic information that was determined from reviewing the interview transcripts and from information that was available in a clinical database.
Table 1.
Demographics of the participants
| Carriers |
||
|---|---|---|
| N =20 | % | |
| Gender | ||
| Female | 20 | 100 |
| Age (year) | ||
| 20–29 | 2 | 10 |
| 30–39 | 8 | 40 |
| 40–49 | 8 | 40 |
| 50+ | 2 | 10 |
| Gene mutated | ||
| BRCA1 | 6 | 30 |
| BRCA2 | 14 | 10 |
| Cancer history | ||
| None | 11 | 55 |
| Breasta | 8 | 40 |
| Other (thyroid) | 1 | 5 |
| Time since genetic testing | ||
| 1 < year | 2 | 10 |
| 1–2 years | 2 | 10 |
| 2–3 years | 3 | 15 |
| 3–4 years | 6 | 30 |
| 4–5 years | 3 | 15 |
| 5+ years | 4 | 20 |
| Have children | ||
| Yes | 12 | 60 |
| No | 3 | 15 |
| Unknown (not mentioned during interview) | 5 | 25 |
| Prophylactic surgeries completed | ||
| Mastectomy only | 4 | 20 |
| Oophorectomy only | 1 | 35 |
| Mastectomy and oophorectomy | 4 | 20 |
| None | 5 | 25 |
Two women undergoing treatment at the time of the interview
Data Analysis
A sample size of 20 participants was adequate to reach thematic saturation in this study and is in line with other, similar qualitative studies (e.g., Hovick et al. 2015; Howard et al. 2011; Leonarczyk and Mawn 2015). Because this was a secondary analysis of the data, a grounded theory methodology was used, which allowed for the development of theory (i.e., propositions or plausible relationships among concepts) regarding surgical decision-making and the role of others in the process (Strauss and Corbett 1994). A constant comparison approach was used (Glaser 1965), whereby an initial set of codes was developed based on topics frequently discussed by participants regarding surgical decision-making during a review of the transcripts. Next, three members of the study team independently coded a subset of transcripts using this initial codebook and then met to compare coding incidences across categories, discuss concepts beginning to emerge from the data, and make additions and modifications to the codebook. Once a final codebook and code definitions were established, two coders coded all transcripts, meeting throughout to discuss coding incidences and discrepancies until they reached 100% agreement for each coding instance. Members of the study team then sorted and organized codes into major themes or categories to develop an overarching set of propositions regarding surgical decision-making.
These methods meet the four criteria of trustworthiness outlined by Lincoln and Guba (1986). For credibility, we used triangulation or cross checking whereby different investigators coded all transcripts and met to review and discuss all coding and discrepancies, as well as concepts and propositions arising from the data. For transferability, we report extensive descriptive data pulled from transcripts regarding our participants (see “Results” and Table 2) and the analysis process so that others may apply our findings. Although a small number of the research team was involved in the data analysis, for dependability and confirmability, study findings were shared among the entire research team and refined. When appropriate, we went back to the data for additional analysis or to better articulate our research findings. We also maintained an audit trail so that we could go back to earlier versions of the codebook and coding documents. NVivo (QSR International, version 10) was used for analysis to help organize coding and more easily interpret major themes. Pseudonyms are used for participants throughout the paper to protect the identity of participants.
Table 2.
Individual participant demographics
| Participant | Age | Personal cancer history | Children | Gene mutation | Length of time since genetic testing (years ) |
Ease of decision making | Individuals involved in decision making | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Physician | Genetic counselor | Family members | Other carriers | |||||||
| Abby | 48 | Breast at 43 | Yes | BRCA1 | 5–6 | - | - | - | - | - |
| Alice | 65 | Breast at 59 | Yes | BRCA2 | 5–6 | BSO decision easy RRM decision hard | X | X | - | - |
| Allison | 36 | None | No data | BRCA2 | 2–3 | Easy | - | - | X | - |
| Brooke | 36 | None | Yes | BRCA2 | <1 | Hard | X | - | - | |
| Christina | 33 | None | No | BRCA2 | 4–5 | Hard | X | - | X | - |
| Cindy | 45 | Thyroid at 42 | No | BRCA2 | 3–4 | BSO decision easy RRM decision hard | - | X | X | - |
| Debbie | 49 | Breast at 45 | Yes | BRCA2 | 3–4 | - | - | - | - | - |
| Diane | 48 | Breast at 45 | No data | BRCA2 | 3–4 | - | X | - | X | - |
| Emily | 30 | None | Yes | BRCA2 | 1–2 | Screening decision easy | X | - | - | X |
| Jessica | 24 | None | No | BRCA1 | 3–4 | Hard | X | X | - | - |
| Kristy | 36 | Breast at 34 | Yes | BRCA1 | 1–2 | Easy | X | - | - | - |
| Lauren | 43 | None | No data | BRCA1 | 5–6 | - | - | - | - | X |
| Maggie | 52 | Breast at 37 | No data | BRCA2 | 3–4 | - | X | X | - | - |
| Nicole | 40 | None | Yes | BRCA1 | 2–3 | BSO decision easy RRM decision hard | X | - | - | - |
| Olivia | 21 | None | No data | BRCA2 | 3–4 | - | - | - | - | - |
| Sarah | 32 | None | Yes | BRCA2 | 4–5 | Hard | X | - | X | - |
| Shannon | 36 | Breast at 33 undergoing treatment | Yes | BRCA2 | 2–3 | - | - | - | - | - |
| Stephanie | 41 | Breast at 41 undergoing treatment | Yes | BRCA2 | <1 | - | - | - | - | - |
| Tiffany | 38 | None | Yes | BRCA1 | 5–6 | Hard | - | - | X | X |
| Tonya | 41 | None | Yes | BRCA2 | 4–5 | - | - | X | - | - |
BSO bilateral salpingo-oophorectomy
Results
Participant Demographics
Table 1 provides a summary of participant demographics. All but two participants discussed having a family history of cancer and/or a known BRCA mutation. More participants with a current or previous breast cancer diagnosis had already undergone or made the decision to undergo prophylactic surgery than those who have not had breast cancer. Of the eight women with a breast cancer diagnosis, four had completed a contralateral prophylactic mastectomy and six a RRSO. Of the twelve women without a personal history of breast cancer, four had a RRM and five had a RRSO. Five of twelve women without a personal history of breast cancer discussed their intention to have a RRM, and three discussed that they intend to have a RRSO in the future. Eight of twelve women who have not had breast cancer discussed undergoing breast and/or ovarian cancer screening during the interview. More participants over the age of 40 had already undergone prophylactic surgery, particularly RRSO; no participants (n = 0 of 2) in their 20s had undergone RRM or RRSO at the time of the interview, while nine (of ten) participants in their 40s and 50s had undergone RRSO at the time of the interview, and four had undergone RRM.
Major Themes
Two major themes arose from the findings regarding decision-making that focused on (a) perceived ease of decision-making and (b) involvement of various individuals in the decision-making process.
Ease of Decision-Making
Of participants who commented on ease of risk management decision-making (n = 14), eight described the process as difficult while six considered it to be a relatively easy process. Those who expressed that the process was easy often described making the decision quickly without much thought. Kristy (36, breast cancer) said, “It was truly, it wasn’t even like I had to make a decision. It was done; it was what I had to do. ” However, the ease of decision-making for participants varied between management for breast cancer and ovarian cancer. More women found the decision to have a RRSO to be simple (n = 3) compared with the RRM decision (n =1), saying that the RRM is/was difficult to prepare for mentally. For example, Nicole (40, no cancer) started crying while discussing the thought of undergoing RRM but described undergoing RRSO as a “no-brainer.”
Unaffected at-risk participants, women in their 20s and 30s, and participants who mentioned that they did not have children often discussed having a difficult time making a decision (i.e., it was tough or complex). As noted by Christina (33, no cancer), “[My decision] was very—it’s been very calculated. It’s been tough, honestly.” Many women who described the surgical decision making as being a difficult process also commonly discussed seeking out information during the decision-making process (n = 7 of 8), including information about cancer risks, surgical procedures and outcomes, others’ personal experiences with cancer, or management options from healthcare providers and other mutation carriers.
Half of the carriers who discussed difficulties making cancer risk management decisions (n = 4 of 8) also expressed negative emotions related to decision-making (e.g., fear, anxiety, and frustration) and were upset by or overwhelmed with the surgical or management options available to them. For example, after her doctors recommended RRSO and RRM, Diane (48, breast cancer) said, “I was really overwhelmed. It went from being, like...’it’s no big deal,’ to now she’s not even giving me a [surgical] choice and saying, ‘Okay. Now you’ve gotta get it all done’.” Three women who experienced difficulty making a decision also discussed concerns about surgical side effects and/or were grieving the future loss of body parts before having undergone prophylactic surgery. Nicole (40, no cancer) said, “I miss [my breasts] already,” with regard to the struggles of the RRM decision-making process, and Allison (36, no cancer) said, “I was really anxious. I went through the grieving process cuz I knew that I would be losing a part of my body.” Other women talked about being scared or concerned about undergoing a major surgery and the pain that may accompany the healing process. Cindy (45, thyroid cancer) said, “It’s really hard for me to then take this next step of having a prophylactic mastectomy … it’s such a major surgery and it’s such a life-changing thing.” Generally, negative emotions about decision-making were more common among participants in their 30s and those without a personal history of cancer.
Individuals Involved in the Decision-Making Process
Fifteen participants spontaneously talked about the involvement of others, particularly physicians, genetic counselors, family members, and other carriers, in their decision-making process. Eight participants discussed the involvement of more than one of these individuals, and they often played different roles. Physicians commonly provided recommendations and decisional reassurance, whereas genetic counselors were short-term providers of information and recommendations. Family members and other carriers/support group members filled similar but distinct roles by providing information about personal experiences as well as various types of support (Fig. 1).
Fig. 1.
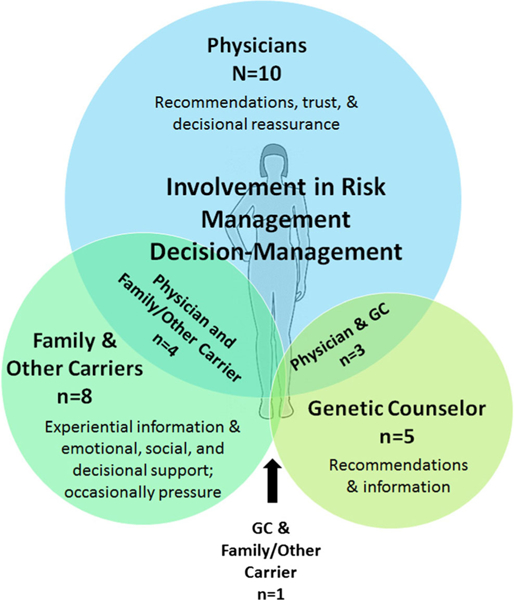
Involvement of Others in the Management Decision-Making Process. Physicians were discussed most often as being involved in the decision-making process (n = 10), followed by family members and other mutation carriers (n = 8), and then genetic counselors (n = 5). Some participants discussed utilizing multiple groups in the decision-making process, with four involving physicians and family members/other carriers, three using physicians and genetic counselors, and one participants discussed involving their genetic counselor and family/ other carriers. GC genetic counselor
One general way that participants (particularly those in their 30s and those with no personal history of cancer) discussed others’ involvement in the decision-making process was by receiving validation and affirmation (from a physician, family member, or other carrier) of their management decision. Some of these women noted that this validation was important to them, such as Brooke (36, no cancer) who said, “For me [support of my decision] helps validate what I’m doing and also gives me more confidence in my decision.” Diane (45, breast cancer) switched doctors to find someone who would affirm what she wanted to do for her cancer treatment/risk management. Other participants, such as Christina (33, no cancer) said, “It didn’t really matter what anyone else thought, honestly,” but then when on to talk about how her family was “totally understanding” of her decision, showing that validation and understanding still had some importance to her.
Physicians
Half of the participants of all ages (n =10 of 20) discussed physicians (oncologists, gynecologists, breast specialists, and plastic surgeons) playing a role in their decision-making process. This was particularly common among women who were struggling with a surgical decision; six of eight women who had a difficult time coming to a decision discussed utilizing their physician in the decision-making process.
The most common way physicians were involved in decision-making was by providing management recommendations. Physicians were able to utilize risk information and published management guidelines to help the women interpret what the risk information means in the context of their own lives. Alice (59, breast cancer) discussed how her physician was important in decision-making because he was able to contextualize risk and surgical information based on her personal history and experiences: “There’s certain things you need. You need to have the statistics, but I can’t imagine somebody not going back to their physician that knows them, not just somebody who’s reporting a statistic.” Helping to interpret the risk information aided these women in determining what they should do in terms of risk-management decision-making. Four participants also discussed trusting their physicians and/or receiving decisional reassurance from them. During the decision-making process, some participants discussed being involved in shared decision-making with their physicians, while others described their physicians as being more directive. For example, Sarah (32, no cancer) said, “[My physician] really was like, ‘Okay, so when do you wanna do this?’ It wasn’t like, ‘If you do this.’ It was ‘When?’” with regard to prophylactic surgery. Despite the different presentations of recommendations from physicians, only one participant (Diane, 45, breast cancer) expressed not being happy with the input and involvement of one of her doctors and switching to a different physician.
Genetic Counselors
Five participants, four of whom were over the age of 40, discussed the involvement of their genetic counselor in decision-making, generally in the short term. For all of these women, the genetic counselor provided information and recommendations, such as risk numbers and management options. While all of these participants discussed receiving recommendations and information from their genetic counselor, participants described differences in emotional support provided by their genetic counselor. Maggie (52, breast cancer), discussed having “a positive conversation” with her genetic counselor and said, “I’m very grateful that [the genetic counselor] was on the other side of the table that day.” She said her genetic counselor “wasn’t doom and gloom” during the results disclosure discussion, providing her with “a gift of this knowledge,” “reassurance,” and “skills” in order to be proactive “through the world of prevention or early detection.” Alternatively, Alice (65, breast cancer) described her experience with the genetic counselor as being very technical and not helpful in the processing of her emotions. She said, “These were very well-intentioned professionals who I think thought they understood the emotional impact, but their behavior showed that they really didn’t,” because the results discussion focused on risk statistics and participation in research studies instead of the emotional processing of the risk information.
Family and Other BRCA1 and BRCA2 Mutation Carriers
Family members and other mutation carriers filled similar but distinct roles in the decision-making process by providing emotional and social support and information about personal experiences. A total of eight participants, mostly unaffected women in their 30s, talked about the involvement of family and/or other carriers in making a risk management decision. Six of these eight women also expressed negative emotions about the decision-making process.
The most common way family members and other carriers were involved in decision-making was by providing social and emotional support during the decision-making process, but often in different ways. The type of support most often provided by family members was decisional reassurance. Allison (36, no cancer) said, “My family overall was very supportive, and completely understood my thought process and my rationale for wanting to do it; very, very supportive.” In contrast, other mutation carriers provided comradery, reassurance that everything will be all right, and normalization of cancer risk management to participants during the decisionmaking process. Emily (30, no cancer) said, “I think people who’ve been though [a risk-reducing surgery] are telling their stories, so that makes it a little bit more relatable. I think it doesn’t seem as alien to me.”
Only two participants discussed unsupportive involvement of family members in their decision-making process. Cindy (45, thyroid cancer) decided not to have a RRM, but her sister continued to pressure her to undergo a RRM, which has created a lot of worry about cancer risk for Cindy. Sarah (32, no cancer) decided to have a RRM and RRSO and said, “[My dad is] not happy that I’ve made a decision to just get rid of everything.”
Family members and/or other carriers were often valued for the experiential information they can provide. Participants described the experience of watching family members going through a cancer diagnosis as providing valuable insight and acting as an impetus to be proactive and make a decision to prevent cancer. Participants also described gaining information about the surgical process, healing time, and side effects from people who have already gone through a mastectomy or oophorectomy as being important. Sarah (32, no cancer) said, “I know exactly what’s in store for me for the recovery. I don’t obviously know how painful it’s going to be because my mom couldn’t articulate that, but I know how tough it’s going to be.” Christina (33, no cancer) spoke with other carriers at support groups about RRSO side effects: “I’ve actually talked with other women in some of these support groups, and they’re like, ‘Oh my God. It was a nightmare. I was clinically depressed for six months,’ or ‘My sex life has never been the same’.” This feedback from other carriers has played a role in her considering having just her fallopian tubes removed rather than a complete RRSO.
Discussion
Our findings elucidate some of the aspects and complexities of the risk-management decision-making process for BRCA1 and BRCA2 mutation carriers, as well as coalesce findings from previous studies regarding this process and the roles others play. Our study and others (Dean and Davidson 2016; Kenen et al. 2007) illustrate that women utilize many different strategies during the decision-making process, including seeking out information and management recommendations, and that women receive support from a variety of sources (including physicians, genetic counselors, family members, and other mutation carriers). Some participants described experiencing a more difficult decision-making process than others, and our findings suggest a subgroup of women with BRCA mutations (i.e., those with no personal history of breast cancer, those under the age of 40, and those who said they do not have children) may benefit from additional decisional assistance to ease the decision-making process. Genetic counselors are well suited to be more involved in providing some of this decisional assistance, especially since our study suggests they are often not utilized long term in risk management decision-making.
Physicians were discussed most often as being involved in decision-making, commonly by providing recommendations and information to women. It has been shown that women with a BRCA mutation seek input from physicians regarding risk-management decision-making (Klitzman and Chung 2010), but unlike previous studies, our participants did not commonly express disappointment in the support provided by their physician. Women in our study described having trust in their physician and receiving reassurance from them in their decisions. However, our findings may be due to the fact that our participants were content with the input from their physicians, or because they were not explicitly asked to discuss their full range of thoughts and feelings related to physician involvement in decision-making. We postulate that physician involvement in decision-making is likely both helpful and disappointing at different points throughout the decisionmaking process. Research is needed to validate this hypothesis and better understand the role of physicians and other health providers at each stage of risk-management decision-making.
In contrast with physicians, participants generally described genetic counselors as being involved short term in the decision-making process by providing information and recommendations. While research has shown that women who received a genetics consultation for HBOC felt more prepared to make management decisions and had low decisional conflict after pre-test counseling and results disclosure (Connors et al. 2014), information from genetic counseling sessions can also be helpful in surgical decision-making (Ray et al. 2005). However, the role of the genetic counselor is not just to provide information, but to also provide psychosocial counseling to patients (Resta et al. 2006). While research with more women is needed, our findings and the findings of Josephson et al. (2000) suggest that some women are satisfied with the psychosocial support they receive from genetic counselors while others may be less satisfied.
Part of the difference in the involvement of physicians and genetic counselors in the decision-making process may have to do with the timing of which each becomes involved with women with a BRCA mutation, as well as their mode of communication (i.e., over the telephone or in person). The participants in this study often received their BRCA genetic test results from their genetic counselor over the telephone. These telephone calls are unscheduled, which means that participants are often not expecting the result disclosure call when it occurs, and despite reviewing possible result outcomes in pre-test counseling, they may be surprised by their result. Participants then initiate in-person discussions with their physicians at a later date and likely enter the discussion with a clear goal or set of questions. Additional research is needed to determine if the timing and mode of communication of the risk discussions with the genetic counselor impact the outcomes studied here, but it may be beneficial for genetic counselors to follow up with patients who test positive for mutations at a later date to provide ongoing support. A study by Underhill and Crotser (2014) also concluded that long-term follow-up with genetics professionals may be beneficial for women after the identification of a BRCA mutation because they found that the risk management decision-making process is not static, but rather changes over time. Examples of service delivery models with genetic counseling follow up in multidisciplinary high-risk cancer clinics have been reported (Arden-Jones and Eeles 2004; Bancroft et al. 2010; Engel et al. 2012; Pichert et al. 2010) and have been successful (Firth et al. 2011; Pichert et al. 2010).
Outside of healthcare providers, other carriers and family members filled similar yet distinct roles for participants during the decision-making process. Other studies have had similar findings to ours; Klitzman and Chung (2010) and Kenen et al. (2007) described support groups providing experiential information, emotional support, and a sense of community for women with a BRCA mutation, which is consistent with the type of support our participants described from other carriers. While Werner-Lin (2008) found that support groups provide normalization, validation, and comradery for women with BRCA mutations, similar to our findings, that study showed support from family is not always beneficial due to their emotional involvement and pressure to take certain management measures. Indeed, we similarly found that family members do not always provide the desired support to women with a BRCA mutation during the risk-management decision-making process, either by applying too much pressure to make a certain decision, or by not being reassuring of a decision the woman has already made (Klitzman and Chung 2010).
Participants in our study who discussed involving family members also commonly expressed negative emotions related to decision-making. We do not know for certain if women who experience more negative emotions regarding decision-making were more likely to turn to family members for support or whether the involvement of their family members led to more negative emotions (such as worry). Our findings and others (Klitzman and Chung 2010; Werner-Lin 2008) suggest that if the involvement of family members is not helpful, support groups and other mutation carriers may be able to act as a surrogate for support and experiential information for some women. Thus, there may be a role for genetic counselors to be more involved in the decision-making process to gauge support from family member and provide support resources and psychosocial counseling when needed.
Some participants of this study, particularly those who had not had cancer, were under the age of 40, and/or did not have children, expressed having a difficult time with the management decision-making process. Those who expressed difficulty with risk-management decision-making often discussed negative emotions related to the risk-management decisionmaking process as well. Our results support findings that unaffected individuals feel less prepared to make a decision regarding cancer risk management and have more decisional conflict than individuals with a personal history of cancer (Connors et al. 2014) and that younger individuals with a BRCA mutation are more likely to report more need for additional support after learning about the mutation (Metcalfe et al. 2000). However, unlike the Connors etal. (2014) study, which included women with and without a BRCA mutation, we focused only on women with an identified mutation.
The difficulty of and emotions related to the decision-making process for women with a BRCA mutation are important because studies have shown they can affect decision satisfaction later. Women who feel like RRSO is a difficult decision and who feel a lot of uncertainty about the surgery are less satisfied with their decision than women who do not feel that it was a difficult decision, no matter the decision that they made (Westin et al. 2011). Because risk-management decisions cannot only increase negative emotions for women with a BRCA mutation during the actual decision-making process, but influence long-term satisfaction with the decision as well, it is important to help women through this process of making a risk management decision.
Our findings suggest that physicians, family members, and other mutation carriers seem to play an especially important role in the decision-making process of a surgical decision with which that woman is struggling, a finding which (to our knowledge) has not been described elsewhere. Thus, women struggling with the risk-management decision-making process (particularly those who have not had cancer, are under the age of 40, and/or do not have children, since this group most commonly discussed struggling with the process and having negative emotions surrounding decision-making) may benefit from additional assistance with the decision-making process.
Identifying women with a BRCA mutation who struggle with the risk-management decision-making process early on can allow for decisional assistance, which may in turn improve decision satisfaction since the decision-making process affects satisfaction with decision (Westin et al. 2011). Our data suggest genetic counselors are currently involved short term. However, genetic counselors are specially trained to help individuals through the process of making a difficult decision, psychosocially assess patients, provide psychosocial counseling, and identify and help mobilize support resources for patients (Accreditation Council for Genetic Counseling 2015). Furthermore, studies have shown that individuals with a BRCA mutation who received extensive genetic counseling were well connected to psychosocial and medical resources compared with those who did not receive genetic counseling (Werner-Lin 2008). Due to the skillset of genetic counselors, they are well suited to play a more active role throughout the decision-making process for women with a BRCA mutation since our data suggest that they currently are generally involved short term.
Even though cancer genetic counselors generally do not have long-term follow-up with women beyond results disclosure and they are currently viewed as short-term providers, they may have a role in providing longer-term assistance for this group (Underhill and Crotser 2014). For this reason, follow-up genetic counseling (either in-person or over the phone) to help with the risk-management decision-making process may be helpful after results have been disclosed, or after women have undergone one prophylactic surgery, such as RRSO, and are considering a second (given that women in our study found the RRM decision to be more difficult than the decision to undergo RRSO). A study by Metcalfe et al. (2000) found that approximately one third of women with a BRCA mutation felt as though they needed a follow-up genetic counseling appointment after results disclosure. There are a few reports of multidisciplinary high-risk cancer clinics (primarily in the UK and one in the USA) that provide ongoing follow-up for individuals with a BRCA mutation with various providers such as breast surgeons, oncologists, genetic counselors, and psychologists (Arden-Jones and Eeles 2004; Bancroft et al. 2010; Engel et al. 2012; Firth et al. 2011; Pichert et al. 2010). One study from a London clinic found high satisfaction with the multidisciplinary model and suggested that it provided help in the risk management decision-making process (Firth et al. 2011). More research is needed regarding the impact and benefit of longer-term follow-up genetic counseling for women with a BRCA mutation, especially as new genetic counseling service delivery models are being examined.
Study Limitations
As this is a qualitative study of participants who received genetic counseling from one institution, the findings from this study are not generalizable to the broader HBOC population, but rather help provide a better understanding ofthe decisional supports needed by women with BRCA1 and BRCA2 mutations. This study was not originally designed to analyze decision-making among mutation carriers. Therefore, these findings may not provide a comprehensive assessment of the decision-making process, as questions were not specifically asked to probe about the process and experience. Because of this, the same information, such as ease of decision-making and physician involvement in decision-making, was not obtained for every participant. However, since these women naturally brought up this topic without being specifically asked, our findings suggest that the presence of support for decisionmaking is of concern for participants. Finally, women who decided to participate in this study may represent highly motivated individuals or those who may have had a particularly striking genetic testing and decision-making experience that they wanted to talk about. Thus, they may not be representative of women with BRCA mutations in general.
Practice Implications
Women with a BRCA mutation who have not had cancer, are under age 40, and/or do not have children tend to have a more difficult time during the risk-management decision-making process, which could influence their satisfaction with the management decision they make (Westin et al. 2011). Therefore, these individuals may benefit from additional decisional assistance through information and support. Due to the training and skillset of genetic counselors, they are well-suited to be more involved in the decision-making process by providing women with additional risk and management information and psychosocial support, referring them to information resources and healthcare providers who can assist with management decisions, helping to introduce the potential benefit of support from family or other mutation carriers, and/or referring them to support groups. It may be helpful for genetic counselors to establish follow-up phone calls or visits with women found to have a BRCA mutation after some time has passed following result disclosure. Additional research is necessary to determine whether and how this improves outcomes.
Research Recommendations
Studies assessing ways to identify women with a BRCA mutation who are likely to struggle with the decision-making process and benefit from additional decisional assistance are important in order to be able to pre-emptively help these women and improve decisional satisfaction. Tools for this process may include surveys assessing how they have made big decisions in the past and/or demographic data such as age, cancer history, and parity. More research is also needed regarding the best way to assist women in the risk-management decision-making process. Decision aids are sometimes used to help individuals make decisions. A recent randomized controlled trial of a decisional aid for breast cancer prevention with women who carry a BRCA mutation found that the tool decreased cancer distress but did not have an effect on decisional conflict (Metcalfe et al. 2017). Work can be done to create a decision aid that also improves decisional conflict. However, some women may benefit more from other methods such as use of support groups, receiving emotional support, and/or finding a physician they can trust. Studies looking at identifying what decisional assistance methods would be most beneficial for individual women would allow for tailored, individualized care. More research is also needed to determine if longer-term genetic counseling follow-up after results disclosure to help with the risk-management decision-making process, such as through a multidisciplinary clinic, improves outcomes.
Supplementary Material
Acknowledgements
This research was conducted as part of Athena Puski’s genetic counseling training. We thank Jessica Bachman and Phokeng Dailey for their assistance in coding and data collection.
Funding Information This project was funded in part by Nationwide Children’s Hospital, grant P30 CA016058 from the National Cancer Institute, Bethesda, MD and by Award Number UL1TR001070 from the National Center for Advancing Translational Sciences (NCATS).
Footnotes
Disclaimer The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NCATS or the National Institutes of Health.
Compliance with Ethical Standards
Conflict of Interest Athena Puski, Shelly Hovick, and Amanda Ewart Toland declare that they have no conflict of interest. Leigha Senter is a consultant for AstraZeneca and received a speaker honorarium from Ambry Genetics.
Human Studies and Informed Consent All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Animal Studies No animal studies were carried out by the authors for this article.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10897–018-0254–4) contains supplementary material, which is available to authorized users.
References
- Accreditation Council for Genetic Counseling (2015). Practice-based competencies for genetic counselors. http://www.gceducation.org/Documents/ACGC%20Core%20Competencies%20Brochure_15_Web.pdf
- Arden-Jones A, & Eeles RA (2004). Development in clinical practice: follow up clinic for BRCA mutation carriers: a case study highlighting the “virtual clinic”. Heredity Cancer Clinical Practice, 2,77–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft EK, Lock I, Arden-Jones A, D’Mello L, McReynolds K, Lennard F, et al. (2010). The carrier clinic: an evaluation of a novelclinic dedicated to follow-up of BRCA1 and BRCA2 carriers-implications for oncogenetics practice. Journal of Medical Genetics, 47(7), 486–491. [DOI] [PubMed] [Google Scholar]
- Chai X, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, Garber JE, Neuhausen SL, Matloff E, Eeles R, Tung N, Weitzel JN, Couch FJ, Hulick PJ, Ganz PA, Daly MB, Olopade OI, Tomlinson G, Blum JL, Domchek SM, Chen J, & Rebbeck TR (2014). Use of risk-reducing surgeries in a prospective cohort of 1,499 BRCA1 and BRCA2 mutation carriers. Breast Cancer Research and Treatment, 148(2), 397–406. 10.1007/s10549-014-3134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors LM, Voian N, Shi Y, Lally RM, & Edge S (2014). Decision making after BRCA genetic testing down the road of transition. Clinical Journal of Oncology Nursing, 18(3), E58–E63. 10.1188/14.CJON.E58-E63. [DOI] [PubMed] [Google Scholar]
- Dean M, & Davidson L (2016). Previvors’ uncertainty management strategies for hereditary breast and ovarian cancer. Health Communication, 33, 1–9. 10.1080/10410236.2016.1250187. [DOI] [PubMed] [Google Scholar]
- Engel N, Gordon P, Thull D, Dudley B, Herstine J, Jankowitz R, & Zorn K (2012). A multidisciplinary clinic for individualizing management of patients at increased risk for breast and gynecologic cancer. Familial Cancer, 11, 419–427. [DOI] [PubMed] [Google Scholar]
- Firth C, Jacobs C, Evison M, Pichert G, Izatt L, & Hunter MS (2011). Novel one-stop multidisciplinary follow-up clinic for BRCA1/2 carriers: Patient satisfaction and decision making. Psycho-Oncology, 20(12), 1301–1308. [DOI] [PubMed] [Google Scholar]
- Flippo-Morton T, Walsh K, Chambers K, Amacker-North L, White B, Sarantou T, Boselli D, & White R (2015). Surgical decision making in the BRCA-positive population: Institutional experience and comparison with recent literature. The Breast Journal, 22(1), 35–44. 10.1111/tbj.12521. [DOI] [PubMed] [Google Scholar]
- Gahm J, Wickman M, & Brandberg Y (2010). Bilateral prophylactic mastectomy in women with inherited risk of breast cancer—prevalence of pain and discomfort, impact on sexuality, quality of life and feelings of regret two years after surgery. The Breast, 19(6), 462–469. 10.1016/j.breast.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Glaser B (1965). The constant comparative method of qualitative analysis. Social Problems, 12(4), 436–445. 10.2307/798843. [DOI] [Google Scholar]
- Hamilton R, Williams JK, Bowers BJ, & Calzone K (2009). Life trajectories, genetic testing, and risk reduction decisions in 18–39 year old women at risk for hereditary breast and ovarian cancer. Journal of Genetic Counseling, 18(2), 147–159. 10.1007/s10897-008-9200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse-Biber S (2014). The genetic testing experience of BRCA-positive women: deciding between surveillance and surgery. Qualitative Health Research, 24(6), 773–789. 10.1177/1049732314529666. [DOI] [PubMed] [Google Scholar]
- Hoffman RM, Elmore JG, Fairfield KM, Gerstein BS, Levin CA, & Pignone MP (2014). Lack of shared decision making in cancer screening discussions: results from a national survey. American Journal of Preventive Medicine, 47(3), 251–259. 10.1016/j.amepre.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Hoskins LM, & Werner-Lin A (2013). A multi-case report of the pathways to and through genetic testing and cancer risk management for BRCA mutation-positive women aged 18–25. Journal of Genetic Counseling, 22(1), 27–38. 10.1007/s10897-012-9521-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovick SR, Yamasaki JS, Burton-Chase AM, & Peterson SK (2015). Patterns of family health history communication among older African American adults. Journal ofHealth Communication, 20(1), 80–87. 10.1080/10810730.2014.908984. [DOI] [PubMed] [Google Scholar]
- Howard AF, Balneaves LG, Bottorff JL, & Rodney P (2011). Preserving the self: the process of decision making about hereditary breast cancer and ovarian cancer risk reduction. Qualitative Health Research, 21(4), 502–519. 10.1177/1049732310387798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson U, Wickman M, & Sandelin K (2000). Initial experiences of women from hereditary breast cancer families after bilateral prophylactic mastectomy: a retrospective study. European Journal of Surgical Oncology (EJSO), 26(4), 351–356. 10.1053/ejso.1999.0897. [DOI] [PubMed] [Google Scholar]
- Kenen RH, Shapiro PJ, Friedman S, & Coyne JC (2007). Peer-support in coping with medical uncertainty: discussion of oophorectomy and hormone replacement therapy on a web-based message board. Psychooncology, 16(8), 763–771. [DOI] [PubMed] [Google Scholar]
- Klitzman R, & Chung W (2010). The process of deciding about prophylactic surgery for breast and ovarian cancer: patient questions, uncertainties, and communication. American Journal of Medical Genetics. Part A, 152A(1), 52–66. 10.1002/ajmg.a.33068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenbach DM, Christensen KD, Sparks JA, & Green RC (2013). Communicating genetic risk information for common disorders in the era of genomic medicine. Annual Review of Genomics and Human Genetics, 14, 491–513. 10.1146/annurev-genom-092010-110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonarczyk TJ, & Mawn BE (2015). Cancer risk management decision making for BRCA+ women. Western Journal of Nursing Research, 37(1), 66–84. 10.1177/0193945913519870. [DOI] [PubMed] [Google Scholar]
- Lincoln YS, & Guba EG (1986). But is it rigorous? Trustworthiness and authenticity in naturalistic evaluation. New Directions for Program Evaluation, 1986(30), 73–84. [Google Scholar]
- Metcalfe K, Liede A, Hoodfar E, Scott A, Foulkes W, & Narod S (2000). An evaluation of needs of female BRCA1 and BRCA2 carriers undergoing genetic counselling. Journal of Medical Genetics, 37, 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe KA, Birenbaum-Carmeli D, Lubinski J, Gronwald J, Lynch H, Moller P, & Hereditary Breast Cancer Clinical Study Group. (2008). International variation in rates of uptake of preventive options in BRCA1 and BRCA2 mutation carriers. International Journal of Cancer, 122(9), 2017–2022. 10.1002/ijc.23340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe KA, Dennis CL, Poll A, Armel S, Demsky R, Carlsson L, et al. (2017). Effect of decision aid for breast cancer prevention on decisional conflict in women with BRCA1 or BRCA2 mutation: a multisite, randomized, controlled trial. Genetics in Medicine, 19, 330–336. 10.1038/gim.2016.108. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network (2016). Genetic/familial high-risk assessment: breast and ovarian. National Comprehensive Cancer Network. [Google Scholar]
- Pichert G, Jacobs C, Jacobs I, Menon U, Manchanda R, Johnson M, et al. (2010). Novel one-stop multidisciplinary follow-up clinic significantly improves cancer risk management in BRCA1/2 carriers. Familial Cancer, 9(3), 313–319. [DOI] [PubMed] [Google Scholar]
- Ray JA, Loescher LJ, & Brewer M (2005). Risk-reduction surgery decisions in high-risk women seen for genetic counseling. Journal of Genetic Counseling, 14(6), 473–484. 10.1007/s10897-005-5833-5. [DOI] [PubMed] [Google Scholar]
- Resta R, Biesecker BB, Bennett RL, Blum S, Hahn SE, Strecker MN, & Williams JL (2006). A new definition of genetic counseling: National Society of Genetic Counselors’ Task Force report. Journal of Genetic Counseling, 15(2), 77–83. 10.1007/s10897-005-9014-3. [DOI] [PubMed] [Google Scholar]
- Scheuer L, Kauff N, Robson M, Kelly B, Barakat R, Satagopan J, & Offit K (2002). Outcome ofpreventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 20(5), 1260–1268. [DOI] [PubMed] [Google Scholar]
- Strauss A, & Corbett J (1994). Grounded theory methodology: an overview In Denzin NK & Lincoln YS (Eds.), Handbook of qualitative research (pp. 273–285). Thousand Oaks: Sage. [Google Scholar]
- Underhill ML, & Crotser CB (2014). Seeking balance: decision support needs of women without cancer and a deleterious BRCA1 or BRCA2 mutation. Journal of Genetic Counseling, 23(3), 350–362. 10.1007/s10897-013-9667-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Lin A (2008). Formal and informal support needs of young women with BRCA mutations. Journal ofPsychosocial Oncology, 26(4), 111–133. [DOI] [PubMed] [Google Scholar]
- Westin SN, Sun CC, Lu KH, Schmeler KM, Soliman PT, Lacour RA, & Bodurka DC (2011). Satisfaction with ovarian carcinoma risk-reduction strategies among women at high risk for breast and ovarian carcinoma. Cancer, 117(12), 2659–2667. 10.1002/cncr.25820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


