Abstract
A reduction of GABAergic markers in postmortem tissue is consistently found in schizophrenia. Importantly, these alterations in GABAergic neurons are not global, which means they are more prevalent among distinct subclasses of interneurons, including those that express the calcium binding protein parvalbumin.
A decreased expression of parvalbumin in the hippocampus is a consistent observation not only in postmortem human schizophrenia patients, but also in a diverse number of rodent models of the disease.
Meanwhile, previously we reported that the congenital hyperbilirubinemia model rats (Gunn rats), which is a mutant of the Wistar strain, showed behavioral abnormalities, for instance, hyperlocomotor activity, deficits of prepulse inhibition, inappropriate social interaction, impaired recognition memory similar with several rodent models of schizophrenia. Several animal studies linked the importance of palvalbumin in relation to abnormal hippocampal activity and schizophrenia-like behavior.
Here, we show that parvalbumin positive cell density was significantly lower in the CA1, CA3 and the total hippocampus of Gunn rats (congenital hyperbilirubinemia model rats) compared to Wistar control rats. The correlations between serum UCB levels and loss of PV expression in the hippocampus were also detected. The decreases in the PV-expression in the hippocampus might suggest an association of the behavioral abnormalities as schizophrenia-like behaviors of Gunn rats, compared to the Wistar control rats.
Keywords: Biochemistry, Anatomy, Cell biology, Developmental biology, Immunology, Molecular biology, Neuroscience, Parvalbumin, GABA, Schizophrenia, Hyperbilirubinemia, Gunn rat
1. Introduction
Schizophrenia is a neuropsychiatric disease affecting up to 1% of the population (Bhurga, 2005; Saha et al., 2005). It is known that the disease is a heterogenous group of mental illnesses with a pathogenesis resulting from multiple factors, including genetic, biological and environmental factors (Sawa and Snyder, 2002). This heterogeneity has led to a number of distinct hypotheses of schizophrenia, however as of yet, the specific neuropathology underlying this disease has not been conclusively determined.
Among a number of studies of schizophrenia, GABAergic neurons have been the subject of focus. Clinical data from postmortem studies of schizophrenia have provided some consistent observations, including an altered expression of GABAergic markers in some cortical and hippocampal regions (Hashimoto et al., 2003; Konradi et al., 2011; Lewis et al., 2005). Meanwhile, regarding the subclasses of the GABAergic interneurons, there are some markers of the GABAergic interneurons, for example, parvalbumin (PV), calbindin (CB), calretinin (CR). Among these, a decreased expression of PV-positive GABAergic interneuron (PVGI) in the hippocampus, also in prefrontal and cingulate cortices is a consistent observation in postmortem studies also in rodent models of schizophrenia (Eyles et al., 2012; Abdul-Monim et al., 2007; Behrens et al., 2007; Francois et al., 2009; Konradi et al., 2011; Lewis et al., 2005; Harte et al., 2007; Lodge et al., 2009).
It is also said that hippocampal GABAergic interneuron deficits are implicated in the pathophysiology of schizophrenia (Konradi et al., 2011). Increasing evidence has demonstrated the importance of PVGI are in relation to abnormal hippocampal activity and schizophrenia-like behaviors (Konradi et al., 2011).
From the standpoint of the heterogeneity of schizophrenia, previous studies have indicated a close association between unconjugated bilirubin (UCB) and schizophrenia (Miyaoka et al., 2000; Radhakrishnan et al., 2011). Previous studies reported that schizophrenic patients have a significantly higher frequency of hyperbilirubinemia compared to patients with other psychiatric disorders and also in comparison to the general healthy population (Miyaoka et al., 2000; Radhakrishnan et al., 2011).
We have reported that the Gunn rat has a potential as an animal model of schizophrenia (Hayashida et al., 2009). The Gunn rat, which is a mutant of the Wistar strain (Gunn, 1944), has a genetic deficiency in glucuronyltransferase leading to congenitally unconjugated hyperbilubinemia.
Our previous study showed behavioral abnormalities in the Gunn rat, for instance, hyperlocomotor activity, inappropriate social interaction, impairment of recognition memory in the novel object recognition test (NORT) and in the object-location test (OLT) and deficits in prepulse inhibition (PPI) which are representative of hippocampal dysfunction (Hayashida et al., 2009; Tsuchie et al., 2013; Furuya et al., 2013; Liaury et al., 2014; Limoa et al., 2016).
PVGI in the hippocampus in Gunn rats has to date never been investigated. Therefore, in the current study, we sought to assess the PV-positive cell density within the Gunn rat hippocampus compared to Wistar rats. We assessed the PV-positive cell density in the subregions of the dentate gyrus (DG), the cornu ammonis (CA)1, CA2, CA3 and CA4 of the hippocampus between hyperbilirubinemic Gunn rats and Wistar control rats as described previously in Stansfield et al. (2015).
2. Material and methods
2.1. Animals
Male homozygous (j/j) Gunn rats and male Wistar rats were obtained from Japan SLC Inc. (Shizuoka, Japan); they were 8 weeks old at the time of the experiments (n = 6 in each strain). The rats were housed in plastic cages (39 × 27 × 18 cm), two to a cage, under standard conditions with a room temperature of 23 ± 2 °C, humidity of 55 ± 5%, and a 12 h light, 12 h dark cycle (light phase 7:00 to 19:00). Food was provided in the form of dry pellets, and water was given ad libitum. All procedures were performed with the approval of the Shimane University Animal Ethics Committee, under the guidelines of the National Health and Medical Research Council of Japan.
2.2. Blood sampling
Blood samples were collected into sampling tubes under deep intraperitoneal anesthesia with an anesthetic mixture of three drugs: medetomidine (Domitor, Nippon Zenyaku Kogyo Co., Ltd., Tokyo, Japan), midazolam (dormicum, AstelllasPharma INC., Tokyo, Japan), and butorphanol (Vetorphale, Meiji Seika Pharma Co., Ltd., Tokyo, Japan). We conducted the anesthesia in accordance with our previous studies (Arauchi et al., 2017). Medetomidine 0.15 mg, midazolam 2 mg, and butorphanol 2.5 mg/kg b.w./rat were mixed and added to saline (Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan) to adjust the mixture to a volume of 0.5 ml/100 g b.w./rat. The blood samples were centrifuged at 2500 g for 20 min. Samples were stored at -80 °C until the day of analysis.
2.3. Serum bilirubin determination
Serum unconjugated and total bilirubin concentrations were measured via enzymic methods provided by the SRL Corporation (Tokyo, Japan).
2.4. Brain section preparation
The rats were perfused transcardically with saline, followed by 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB, pH 7.4). The brains were taken out and were post fixed with 4% PFA in 0.1M PB at room temperature (RT) for 4 hours. The brains were immersed in PB containing 10% sucrose at 4 °C overnight and subsequently were immersed in PB containing 20% sucrose at 4 °C for 3 days. Serial frontal sections of the brains were cut at 40 μm thickness with a freezing microtome (Microm HM 430, Thermo Scientific, Germany).
2.5. Immunohistochemistry for PV
The free-floating tissue sections were incubated with 1% H2O2 in 0.1 M PB for 30 min, rinsed with 0.1 M PB, pretreated with a solution containing 0.2% Triton X-100 and 1.5% normal goat serum for 1 h before being incubated with goat anti-PV (1:5000, PVG-214, swant, Bellinzona, Switzerland) at room temperature (RT) overnight. After rinsing with phosphate-buffered saline (PBS, pH 7.4), sections were incubated with biotinylated anti-goat IgG (1:500, Standard ABC kit, Vector Labs, Burlingame, CA, USA) for 1 h at RT followed by incubation with PBS containing avidin–biotin–peroxidase complex (ABC) (Standard ABC Kit, Vector Labs) for 1 hour at RT. After rinsing with PBS, the reaction product was developed by incubating 0.5% diaminobenzidine (DAB) and 0.03% H2O2 in PBS for 10 min at RT. After rinsing with PBS, sections were mounted onto gelatin-coated slides and dried before being coverslipped using mounting medium. Finally, the sections taken from the left hippocampus were examined under a light microscope (Nikon, Eclipse Ci, Japan).
2.6. Immunohistochemistry for NeuN
In order to discuss the cell-type specificity to the PV-positive cells, we observed the neurons stained by NeuN of the hippocampus (n = 3). We modified and followed the immunohistochemistry for PV procedure described as above. The mouse anti-NeuN monoclonal antibody (1:1000, Millipore # MAB 377) was used as primary antibodies. The mouse IgG made in horse (1:500, Vectastain) was used as second antibodies.
2.7. Stereological cell counting of parvalbumin-positive cells
Previous assessments for the stereological cell counting in the hippocampus was modified and followed (Stansfield et al., 2015; Limoa et al., 2016). We selected the first slice located at bregma -3.8 mm. A few unclear slices were excluded, resulting in a remaining ten sequential slices (every 40 μm) from the left hippocampus section of each animal used for analysis. As a result, the selected slices were located between at least bregma -3.3 mm to -3.8 mm. The selected slices contained clearly the dentate gyrus (DG), cornu ammonis (CA) 1, CA2, CA3 and CA4 regions of the pyramidal cell layer. All images were captured with a digital Nikon 1 J1 camera using a 20× objective lens. Overall, a total of 50 images per each rat were captured. The intensity of PV-positive neuron immunoreactivity in the DAB staining of each slice was measured by a computer-assisted image analysis program (Image J 1.47v, Wayne Rasband, National Institutes of Health, MD). Using the intensity of PV positive neuron immunoreactivity, we calculated the total PV labeled positive cell volume by summing the results of the sequential 10 slices (Dorph-Petersen and Lewis, 2011; Stansfield et al., 2015).
2.8. Statistical analysis
Statistical analysis of the data was carried out using SPSS software (IBM for Windows Version 22 SPSS Japan Inc., Japan). The data are presented as the mean ± standard error of the mean (SEM).
To confirm the higher levels of unconjugated bilirubin of Gunn rats, we compared serum bilirubin levels of Gunn and Wistar rats. A two-sample t-test was used to detect difference in serum bilirubin levels of the both strains.
We used a stereological count of the subregion specific PV-positive cell density of Gunn and Wistar rats. A two-way Analysis of variance was used to detect differences in PV-positive cell density in specific hippocampus regions (the CA1, CA2, CA3, CA4 and the DG). A post hoc Tukey-HSD test was used for the pairwise comparisons. A two-sample t-test was used to detect difference in the total mean PV-positive cell density in the hippocampus between both of the strains. Pearson correlation coefficients were calculated in regards to the serum UCB in the both rats to assess whether the serum UCB levels are related to total PV cell density in the dorsal hippocampus. Significance for the result was set at p < 0.05.
3. Results
3.1. Serum bilirubin level
The serum bilirubin levels of Gunn and Wistar rats are shown in Table 1. Both the serum unconjugated bilirubin (UCB) and the total bilirubin levels in the Gunn rats were significantly higher than in the Wistar rats (n = 6 in each strain: *** p < 0.0001).
Table 1.
Bilirubin level of Gunn and Wistar rats (mg/dl).
| Gunn | Wistar | p | |
|---|---|---|---|
| UCB | 4.450 ± 0.216 | 0.550 ± 0.224 | 8.135 × 10−6 *** |
| Total bilirubin | 5.167 ± 0.209 | 0.667 ± 0.333 | 2.691 × 10−6 *** |
Data represent mean ± SEM.
N = 6 subjects in each strain.
UCB: unconjugated bilirubin.
***: p < 0.001.
3.2. Identification and stereological cell counting of PV-positive GABAergic interneurons
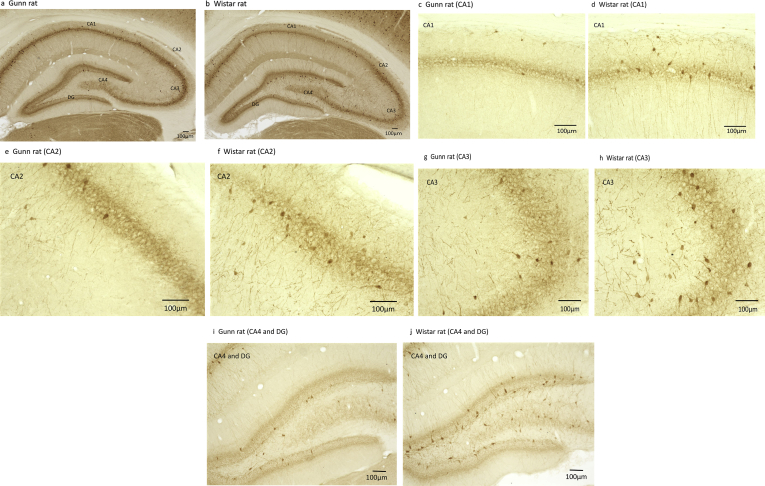
PV-positive cells were identified as shown in Fig. 1. Stereological cell counting of PV-positive cells was performed as shown in Table 2. Strain (F (1, 50) = 18.381, ***p < 0.001), subregion (F (4, 50) = 35.729, ***p < 0.001) were revealed to affect the counts. The interaction of strain and subregion (F (4, 50) = 1.828 p = 0.138) were not revealed. That indicated that a significant strain effect in the PV-positive cell density was present in the subregions which was significantly lower in the Gunn rats relative to Wistar rats (***p < 0.001). As shown in Table 2, further analysis revealed that, PV-positive cell density was significantly lower in the CA1 and CA3 of Gunn rats compared with Wistar rats (CA1: **p < 0.001; CA3: *p < 0.05). The CA1 exhibited the most significant difference (reduction of 39.6%) between Gunn and Wistar rats. The total mean PV- positive cell density in the hippocampus of the Gunn rats was significantly lower than those of the Wistar rats as shown in Table 2 (reduction of 36. 7%).
Fig. 1.
Parvalbumin (PV)-positive cells in the hippocampus. (a.c.e.g.i: Gunn rat; b.d.f.h.j: Wistar rat).
Table 2.
PV-positive cell density in the pyramidal and granule cell layers of the hippocampus (cells mm−3).
| Gunn | Wistar | p | |
|---|---|---|---|
| CA1 | 1508.94 ± 246.2 | 2499.65 ± 171.56 | 0.000175*** |
| CA2 | 182.22 ± 42.22 | 363.63 ± 50.45 | 0.4643 |
| CA3 | 1173.30 ± 97.19 | 1726.70 ± 291.98 | 0.0275* |
| CA4 | 276.70 ± 41.17 | 480.02 ± 91.28 | 0.4087 |
| DG | 893.97 ± 163.21 | 1309.42 ± 269.96 | 0.0966 |
| Total | 807.71 ± 111.71 | 1275.91 ± 169.37 | 0.025* |
Data represent mean ± SEM.
N = 6 subjects in each strain.
PV: parvalbumin.
***: p < 0.001 *: p < 0.05.
3.3. Identification of NeuN-positive cell
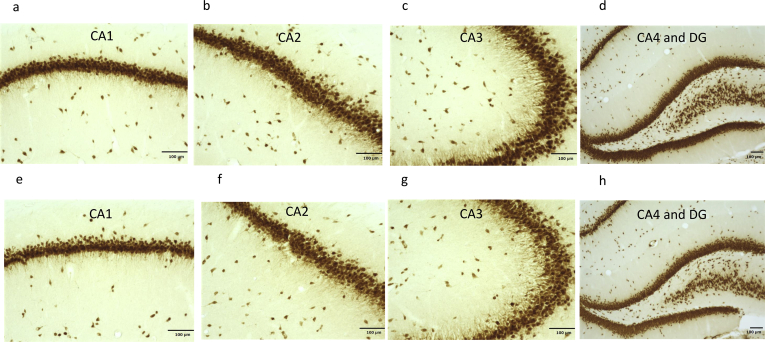
The images of the immunohistochemistry of staining by NeuN in the dorsal hippocampus were shown in the Fig. 2. Decreases in the number of neurons were not shown in in the DG, CA1, CA2, CA3 andCA4 in the Wistar-Gunn rats compared to Wistar rats.
Fig. 2.
NeuN-positive cells in the hippocampus. (a.b.c.d: Gunn rat, e.f.g.h: Wistar rat).
3.4. Correlation between serum UCB and PV-positive cell density
Significant Pearson correlation coefficients between serum UCB levels and PV-positive cell density were detected in the CA1 (r = -0.741, **p = 0.006), and CA3 (r = -0.708, *p = 0.01). Meanwhile, no significant correlation coefficients between serum UCB levels and PV-positive cell density were detected in the CA2 (r = -0.515, p = 0.086), CA4 (r = -0.526, p = 0.079), either in DG (r = -0.402, p = 0.195). Significant Pearson correlation coefficients between serum UCB and total PV-positive cell density were also detected (r = -0.643, * p = 0.024).
4. Discussion
In summary, we demonstrated the decreased PV-positive cell density in the CA1, CA3 and total areas in the hippocampus. Meanwhile, we observed the NeuN- positive cells in the hippocampus suggesting no reduction in neurons in Gunn rats. Furthermore, we detected an association between serum UCB levels and PV-positive cell density in these areas as well.
It is said that hippocampal network activity is generated by a complex interplay between excitatory pyramidal cells and inhibitory interneurons including PV (Hertle and Yeckel, 2007). It means that decreased activity of the PV interneuron leads to disinhibition of hippocampal pyramidal cells altering the excitatory potential, as a result controlling the pyramidal cells alters the excitatory drive to hippocampal disruptive hyperactivity, disruption of hippocampal dependent cognition and dysregulation of the mesolimbic dopamine system (Moghaddam and Javitt, 2012; Nakazawa et al., 2012; Kato et al., 2000; Seeman, 2009; Behrens et al., 2007; Bickel and Javitt, 2009). Therefore, as a next step, it is worth examining each of the regions of the hippocampal pyramidal cells that alter the hippocampal function between Gunn rats and Wistar control rats. It has also been suggested that the distinctness of the hippocampus may correlate with different functional regions (Bast and Feldon, 2003; Fenselow and Dong, 2010). The amount of evidence suggested spatial learning and memory have been demonstrated to be more susceptible to dorsal hippocampal lesions than to those in the ventral hippocampus (Bast and Feldon. 2003; Fenselow and Dong, 2010). Previously, we demonstrated the cognitive deficits in the novel object recognition test (NORT) and in the object-location test (OLT) shown by Gunn rats compared to the Wistar control rats (Liaury et al., 2014; Furuya et al., 2013). The decreases of PV-labeled cells in the dorsal hippocampus in the current study might be consistent with the cognitive deficits shown in Gunn rats. We have also reported the tendency of hyperlocomotor activity and deficits of PPI shown by Gunn rats (Hayashida et al., 2009; Limoa et al., 2016), which has been suggested to be associated more with the ventral hippocampus than with the dorsal hippocampus (Bast and Feldon, 2003; Fenselow and Dong, 2010). Gunn rats have also shown the tendency of an inappropriate social interaction (Hayashida et al., 2009) in reference to a dysfunction of the prefrontal cortex (Bicks et al., 2015). Therefore, PV-positive cell density in the ventral hippocampus and the frontal cortex should also be examined.
We demonstrated the association between serum UCB levels and PV-positive cell density in the CA1, CA3 and total areas in the hippocampus. The bilirubin level of Gunn rats peak at two weeks postnatal and falls to lower but still elevated levels throughout the rest of their lives (Schutta and Johnson, 1969). According to previous literatures, the reduction of PV-positive cells bas the relation between Glutamatergic hypothesis of schizophrenia including N-methyl-D-aspartate (NMDA) receptor hypofunction (Coyle et al., 2012; Benneyworth et al., 2011). Furthermore, increasing evidence suggests that in rodent models of schizophrenia, enhanced neuroinflammation and oxidative stress were demonstrated to mediate the loss of PV-positive cells (Ji et al., 2015). Previously we demonstrated the neuroinflammation due to microgliosis and astrogliosis in the hippocampus in Gunn rats (Liaury et al., 2012; Limoa et al., 2016). Evidence suggests that the exposure of microglia and astrocytes to elevated levels of UCB in the brain initiates an inflammatory response with the release of proinflammatory cytokines and induces an accumulation of extracellular glutamate (Fernandes and Brites, 2009; Falcão et al., 2006). The mechanism of UCB-induced neurotoxicity is related to the toxic accumulation of extracellular glutamate (Brito et al., 2004; Falcão et al., 2006). The prolonged presence of glutamate in the synaptic creft induces disruptive overstimulation of NMDA receptors which consequently leads to oxidative stress inducing neurotoxicity (Falcão et al., 2006). It is also suggested that oxidative stress is a hallmark of UCB-induced neurotoxicity as well (Seubert et al., 2002). Recent findings indicate that neuroinflamation and oxidative stress are mechanistically interdependent and contribute to a common schizophrenia-associated pathology (Boveris et al., 2002; Hardingham and Do, 2016). From the neurodevelopmental viewpoint, it is also demonstrated that there is a certain susceptible period of the neonatal period to elevated UCB toxicity. Such a susceptibility of the exposure to excess UCB is supposed as an important determinant of the neurodevelopmental brain lesions (Shapiro, 2003). Therefore, elevated serum UCB levels in the postnatal period might result in the decrease of PV interneuron in the hippocampus at the age of 8 weeks old. However, the current study regards cross-sectional correlations and therefore, the causality should be elucidated in a future study.
As a limitation, other regions of the brain were not examined in the present study. And, although we observed the NeuN-positive cells in the hippocampus in both of the Gunn and Wistar rats suggesting no reduction in neurons in Gunn rats, other types of cells, for example, those labeled by calcium-binding protein, as calbindin and the hippocampal pyramidal cells were not examined.
5. Conclusion
The present study showed that that the hippocampal decreases in PV expression was detected in Gunn rats. This neuropathology might be suggested to have the association of the behavioral abnormalities as schizophrenia-like behaviors of Gunn rats, compared to the Wistar control rats.
Declarations
Author contribution statement
Maiko Hayashida, Jun Horiguchi, Masatoshi Inagaki, Shoko Miura: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Tsuyoshi Miyaoka, Sadayuki Hashioka, Rei Wake: Conceived and designed the experiments.
Keiko Tsuchie, Muneto Izuhara, Ryosuke Arauchi: Performed the experiments.
Tomoko Araki: Performed the experiments; Analyzed and interpreted the data.
Misako Kanayama: Conceived and designed the experiments; Analyzed and interpreted the data.
Michiharu Nagahama, Koji Otsuki: Analyzed and interpreted the data.
Muhammad Alim Jaya, Ilhamuddin Abdul Azis, Rostia Abdullah, Toshiko Tsumori: Contributed reagents, materials, analysis tools or data.
Arata Oh-Nishi: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was partially supported by Grants-in-Aid for Scientific Research on Priority Areas Nos. 15H04894, supported by the Ministry of Education, Science, Sports, and Culture of Japan.
Competing interest statement
The authors declare the following conflicts of interest: A. O-N is RESVO Inc. CTO and has more than 5% RESVO Inc. shares, but had no role in the study design data collection and analysis, decision to publish, or preparation of the manuscript. Other authors have no Competing Interests.
Additional information
No additional information is available for this paper.
References
- Abdul-Monim Z., Meill J.C., Reynolds G.P. Sub-chronic psychotomimetic phencyclidine induces deficits in reversal learning and alterations in parvalbumin-immunoreactive expression in the rat. J. Psychopharmacol. 2007;21(2):198–205. doi: 10.1177/0269881107067097. [DOI] [PubMed] [Google Scholar]
- Arauchi R., Hashioka S., Tsuchie K., Miyaoka T., Tsumori T. Gunn rats with glial activation in the hippocampus showed prolonged immobility time in the forced swimming test and suspension test. J Brain Behav. 2017;8(8) doi: 10.1002/brb3.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast R., Feldon J. Hippocampal modulation of sensorimotor processes. Prog. Neurobiol. 2003;70(4):319–345. doi: 10.1016/s0301-0082(03)00112-6. [DOI] [PubMed] [Google Scholar]
- Behrens M.M., Ali S.S., Dao D.N., Lucero J., Shekhtman G. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Benneyworth M.A., Roseman A.S., Basu A.C., Coyle J.T. Failure of NMDA receptor hypofunction to induce a pathological reduction in PV-positive GABAergic cell markers. Neurosci. Lett. 2011;488:267–271. doi: 10.1016/j.neulet.2010.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhurga D. The global prevalence of schizophrenia. PLoS Med. 2005;2(5) doi: 10.1371/journal.pmed.0020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicks L.K., Koike H., Akbarian S., Morishita H. Prefrontal cortex and social cognition in mouse and man. Front. Psychol. 2015;6:1805. doi: 10.3389/fpsyg.2015.01805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel S., Javitt D.C. Neurophysiological and neurochemical animal models of schizophrenia: focus on glutamate. Behav. Brain Res. 2009;204:352–362. doi: 10.1016/j.bbr.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Alvarez S., Navarro A. The role of mitochondrial nitric oxide synthase in inflammation and septic shock. Free Radic. Biol. Med. 2002;33:1186–1193. doi: 10.1016/s0891-5849(02)01009-2. [DOI] [PubMed] [Google Scholar]
- Brito M.A., Brites D., Butterfield D.A. A link between hyperbilirubinemia, oxidative stress and injury to neocortical synaptosomes. Brain Res. 2004;1026:33–43. doi: 10.1016/j.brainres.2004.07.063. [DOI] [PubMed] [Google Scholar]
- Coyle J.T., Basu A., Benneyworth M., Balu D., Konopaske G. Glutamatergic synaptic dysregulation in schizophrenia: therapeutic implications. Handb. Exp. Pharmacol. 2012:267–295. doi: 10.1007/978-3-642-25758-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorph-Petersen K.A., Lewis D.A. Stereological approaches to identifying neuropathology in psychosis. Biol. Psychiatry. 2011;69:113–126. doi: 10.1016/j.biopsych.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles D.W., Feldon J., Meyer U. Schizophrenia: do all roads lead to dopamine or is this where they start? Evidence from two epidemiologically informed developmental models. Transl. Psychiatry. 2012;2(2):e81. doi: 10.1038/tp.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcão A.S., Fernandes A., Brito M.A., Silva R.F., Brites D. Bilirubin-induced immunostimulant effects and toxicity vary with neural cell type and maturation state. Acta Neuropathol. 2006;112:95–105. doi: 10.1007/s00401-006-0078-4. [DOI] [PubMed] [Google Scholar]
- Fernandes A., Brites D. Contribution of inflammatory processes to nerve cell toxicity by bilirubin and efficacy of potential therapeutic agents. Curr. Pharmaceut. Des. 2009;15:2915–2926. doi: 10.2174/138161209789058165. [DOI] [PubMed] [Google Scholar]
- Fenselow M.S., Dong H.W. Are the Dorsal and Ventral Hippocampus functionally distinct structures? Neuron. 2010;65(1):7. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois J., Ferrandon A., Koning E., Angst M.J., Sandner G., Nhlig A. Selective reorganization of Gabaergic transmission in neonatal ventral hippocampus –lesioned rats. Int. J. Neurosci. 2009;26(10):2767–2776. doi: 10.1017/S1461145709009985. [DOI] [PubMed] [Google Scholar]
- Furuya M., Miyaoka T., Tsumori T., Liaury K., Hashioka S. Yokukansan promotes hippocampal neurogenesis associated with the suppression of activated microglia in Gunn rat. J. Neuroinflammation. 2013;10:145. doi: 10.1186/1742-2094-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn C.K. Hereditary Acholuric Jaundice in the rat. Can. Med. Assoc. J. 1944;50:230–237. [PMC free article] [PubMed] [Google Scholar]
- Hardingham G.E., Do K.Q. Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat. Rev. Neurosci. 2016;17:125–134. doi: 10.1038/nrn.2015.19. [DOI] [PubMed] [Google Scholar]
- Harte M.K., Powell S.B., Swerdlow N.R., Geyer M.A., Reynolds G.P. Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. J. Neural Transm. 2007;114:893–898. doi: 10.1007/s00702-007-0627-6. [DOI] [PubMed] [Google Scholar]
- Hertle D.N., Yeckel M.F. Distribution of Inositol-1,4,5-trisphosphate receptor Isotypes and Ryanodine receptor Isotypes during maturation of the rat hippocampu. Neuroscience. 2007;150(3):625–638. doi: 10.1016/j.neuroscience.2007.09.058. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Volk D.W., Eggan S.M., Mirnics K., Pierri J.N. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J. Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida M., Miyaoka T., Tsuchie K., Yasuda H., Wake R. Hyperbilirubinemia-related behavioral and neuropathological changes in rats: a possible schizophrenia animal model. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2009;33:581–588. doi: 10.1016/j.pnpbp.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Ji M.H., Qiu L.L., Tang H., Ju L.S., Sun X.R. Sepsis-induced selective parvalbumin interneuron phenotype loss and cognitive impairments may be mediated by NADPH oxidase 2 activation in mice. J. Neuroinflammation. 2015;12:182. doi: 10.1186/s12974-015-0401-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K., Shishido T., Ono M., Shishido K., Kobayashi M. Effects of phencyclidine on behavior and extracellular levels of dopamine and its metabolites in neonatal ventral hippocampal damaged rats. Psychopharmacology (Berl) 2000;150:163–169. doi: 10.1007/s002130000433. [DOI] [PubMed] [Google Scholar]
- Konradi C., Yang C.K., Zimmerman E.I., Lohmann K.M., Gresch P. Hippocampal interneurons are abnormal in schizophrenia. Schizophr. Res. 2011;131:165–173. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D.A., Hashimoto T., Volk D.W. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Liaury K., Miyaoka T., Tsumori T., Furuya M., Wake R. Morphological features of microglial cells in the hippocampal dentate gyrus of Gunn rat: a possible schizophrenia animal model. J. Neuroinflammation. 2012;9:56. doi: 10.1186/1742-2094-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaury K., Miyaoka T., Tsumori T., Furuya M., Hashioka S. Minocycline improves recognition memory and attenuates microglial activation in Gunn rat: a possible hyperbilirubinemia-induced animal model of schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;50:184–190. doi: 10.1016/j.pnpbp.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Limoa E., Hashioka S., Miyaoka T., Tsuchie K., Arauchi R. Electroconvulsive shock attenuated microgliosis and astrogliosis in the hippocampus and ameliorated schizophrenia-like behavior of Gunn rat. J. Neuroinflammation. 2016;13:230. doi: 10.1186/s12974-016-0688-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge D.J., Behrens M.M., Grace A.A. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J. Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaoka T., Seno H., Itoga M., Iijima M., Inagaki T., Horiguchi J. Schizophrenia-associated idiopathic unconjugated hyperbilirubinemia (Gilbert's syndrome) J. Clin. Psychiatry. 2000;61:868–871. doi: 10.4088/jcp.v61n1110. [DOI] [PubMed] [Google Scholar]
- Moghaddam B., Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Zsiros V., Jiang Z., Nakao K., Kolata S. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62:1574–1583. doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan R., Kanigere M., Menon J., Calvin S., Janish A., Srinivasan K. Association between unconjugated bilirubin and schizophrenia. Psychiatr. Res. 2011;189:480–482. doi: 10.1016/j.psychres.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Saha S., Chant D., Welham J., McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2:e141. doi: 10.1371/journal.pmed.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa A., Snyder S.H. Schizophrenia: diverse approaches to a complex disease. Science. 2002;296:692–695. doi: 10.1126/science.1070532. [DOI] [PubMed] [Google Scholar]
- Schutta H.S., Johnson L. Clinical signs and morphologic abnormalities in Gunn rats treated with sulfadimethoxine. J. Pediatr. 1969;75:1070–1079. doi: 10.1016/s0022-3476(69)80351-3. [DOI] [PubMed] [Google Scholar]
- Seeman P. Dopamine D2 High receptors measured ex vivo are elevated in amphetamine-sensitized animals. Synapse. 2009;63:186–192. doi: 10.1002/syn.20595. [DOI] [PubMed] [Google Scholar]
- Seubert J.M., Darmon A.J., El-Kadi A.O., D'Souza S.J., Bend J.R. Apoptosis in murine hepatoma hepa 1c1c7 wild-type, C12, and C4 cells mediated by bilirubin. Mol. Pharmacol. 2002;62:257–264. doi: 10.1124/mol.62.2.257. [DOI] [PubMed] [Google Scholar]
- Shapiro S.M. Bilirubin toxicity in development in nervous system. Pediatr. Neurol. 2003;29(5):410–421. doi: 10.1016/j.pediatrneurol.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Stansfield K.H., Ruby K.N., Soares B.D., McGlothan Liu X., Guilarte T.R. Early-life lead exposure recapitulates the selective loss of parvalbumin-positive GABAergic interneurons and subcortical dopamine system hyperactivity present in schizophrenia. J Transl Psychiatry. 2015;5:E522. doi: 10.1038/tp.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchie K., Miyaoka T., Furuya M., Liaury K., Ieda M. The effects of antipsychotics on behavioral abnormalities of the Gunn rat (unconjugated hyperbilirubinemia rat), a rat model of schizophrenia. Asian J Psychiatr. 2013;6:119–123. doi: 10.1016/j.ajp.2012.09.007. [DOI] [PubMed] [Google Scholar]