Abstract
Objectives
To describe the adaptability to the patterns in symptoms and quality of life (QoL) during 6 months post low-grade glioma diagnosis by valid and reliable tools; to identify through qualitative interviews patient/provider adaptive techniques and strategies; and to assess associations among patient characteristics, symptoms and QoL, and adaptive techniques or strategies.
Data Sources
Demographic, clinical and pathologic data from medical records. Validated instruments that assess QoL, fatigue, depression, and distress were completed at 2, 4, and 6 months post diagnosis. Qualitative interviews identifying the symptoms, challenges, adaptive techniques and strategies were conducted at 4 and 6 months.
Conclusion
The most frequently used adaptive strategies included: obtaining community support (87%), managing expectations (73%) and support systems (67%), and seeking out knowledge about physical (67%) and behav-ioral symptoms (53%). Seizures were reported with IDH1mut (11%) but not IDH1wildtype. Patients with either IDH1mut or TERTmut consistently reported lower QoL and higher distress, depression, and fatigue scores. IDH1/TERTmut may be related to lower QoL because of IDH1mut-related seizures.
Implications for Nursing Practice
Findings provide a list of adaptive strategies and characteristics to address the problems and symptoms that may improve overall QoL in patients with low-grade glioma.
Keywords: glioma, symptoms, quality of life, adaptive leadership
Historically, the prognosis for the majority of patients diagnosed with a primary brain tumor has been poor, with almost all patients developing progressive and debilitating neurologic deficits prior to their final days. Improving quality of life (QoL) through adaptive mechanisms is an important goal in caring for these patients. Patients with primary brain tumors are confronted with serious challenges to their QoL because they may have difficulty adapting to general symptoms (eg, headache, anorexia, nausea, seizures, and insomnia) and focal neurologic dysfunction (eg, motor sensory problems, personality changes, aphasia, cognitive impairment, and visual problems).1 Glioblastoma (World Health Organization [WHO] grade IV) is the most common and lethal type of brain tumor, with a median survival of less than 15 to 20 months despite standard therapy (consisting of surgery, radiotherapy, and concurrent adjuvant chemotherapy).2 While much attention is given to the intense symptom burden of patients with highly aggressive grade gliomas (HGGs; WHO grade III/grade IV), less is known about the adaptability to chronic symptoms experienced by patients with slow growing low-grade (WHO grade II) tumors which will be the focus of this article. Although low-grade glioma (LGG) consists of only 15% to 20% of all gliomas, these patients survive longer with their ongoing deficits than patients with HGGs.3 The average survival is 7 years, with approximately 20% of patients surviving at least two decades from their original diagnosis.4 Therefore, positively impacting QoL through symptom management and adaptation to challenging LGG deficits is relevant. Furthermore, as survivorship increases, patients with LGG may also need to adapt and manage the potential ongoing morbidities associated with new innovative therapies.
Background
The overall symptom burden and disability for patients with LGG is significant. Adults with LGG experience a range of distressing symptoms including fatigue, sleep disturbances, pain (headache), seizures, cognitive changes, and mood disturbances. These symptoms increase the need for medical interventions and negatively impact coping and QoL.1 In individuals with LGG, some of these symptoms can commonly cause a great deal of distress. These distressing symptoms can persist for many months to years, creating significant difficulties that impact QoL. Thus, individuals must learn to manage and adapt to the ongoing symptoms in the midst of multiple life demands.
The symptoms experienced by patients with LGG result in challenges to the individual and the family’s ability to cope, recuperate, resume activities of daily living, and achieve vocational goals. The key problematic aspects of the symptom experience are the challenges that result from the symptoms. Changing the focus from the symptoms to the resulting challenges will provide the individual and their health care providers with a wider array of adaptive techniques. For example, if one only focuses on the technical seizure management (increasing anticonvulsants) and not the challenges (inability to drive) the symptom (seizures) creates, then one would not address all the available adaptive techniques and strategies (finding a driver) to improve QoL.
Adaptive leadership framework
The adaptive leadership (AL) framework provides a novel and strategic conceptualization of symptom self-management for the person with a LGG because it promotes patient-centered, adaptive approaches to the management of symptoms and the challenges created by this chronic illness.5 Focusing beyond the limitations of the disease and promoting self-management work will enhance and strengthen resources already present or will allow the development of strategies during the process of illness adaption.5 Symptoms associated with a brain tumor are dynamic and may fluctuate over time. As symptoms change, the self-management challenges faced by the individual also change. The AL framework distinguishes between technical and self-management methods and draws attention to strategies required to ameliorate and/or adapt to the challenges. Technical challenges are simple or complicated problems for which an expert (eg, health care provider) can define and offer solutions (eg, alterations in antiemetic treatment regimens to resolve changing nausea and vomiting symptoms).6 Adaptive challenges are complex issues that are often difficult to define and require learning and behavior change by the individual experiencing the problem.6 They are challenges that only the individual and/or family member can address (eg, how to incorporate symptom prevention in daily routines). The individual, family members, and health care providers must collaborate to identify the technical and adaptive challenges over time and jointly develop strategies for the ‘adaptive work’ that the individual with a brain tumor must accomplish.
The trajectory of symptoms for individuals living with a LGG and in particular the adaptive challenges associated with their brain tumor treatment and symptoms over time are not well documented. There is a need to understand the kind of adaptive work patients use to cope with the diagnosis of a LGG and to address their symptoms and challenges over a period of time. Successful adaptation is a key determinant of QoL. The conclusions from this research provide both patients and providers with a list of adaptive methods to address problems/symptoms that can improve overall QoL and may influence how future patients/families adapt to problems and symptoms outside the clinic.
LGG Symptoms and Created Challenges
Individuals with LGG cope with the burden of long-term symptoms and the associated challenges created by those ongoing symptoms. In a cross-sectional study, Gustafsson and colleagues7 demonstrated that, in spite of almost all patients with LGG being able to care for themselves, less than half of patients were able to carry out unrestricted, normal activities of daily living and 45% reported low overall QoL. Studies comparing patients with LGG to healthy subjects and low-grade hematologic patients without central nervous system tumors demonstrated that patients with LGG reported more fatigue, cognitive impairment, speech difficulties, pain, sleep problems, and mood disturbances.7–11
Prognostic factors such as age (<40 years), sex (female), Karnofsky performance status (>80%), tumor size (<4 cm), extent of resection (gross total), molecular cytology (1p19q deletion), and molecular biomarkers (IDH mutant) are associated with better survival in patients with LGG.12 Peters and colleagues13 reported that in patients with recurrent HGG, better QoL (as measured by a specific brain cancer subscale, the Functional Assessment of Cancer Therapy-Brain [FACT-Br]) and fatigue were both significant predictors of survival. Furthermore, an increased degree of fatigue provided more information for poorer survival than traditional prognostic factors, such as performance status, age, sex, tumor grade, and number of progressions.13 In the LGG population there is a lack of consistent research on specific symptoms that are prognostic for survival. However, the influence of symptoms on QoL has been investigated.1
The majority of research on symptoms impacting the QoL of glioma populations has been descriptive and focuses on fatigue, sleep, pain, seizures, cognitive impairment, and mood disturbances. Fox et Al14 noted that in HGG, depression, fatigue, sleep disturbance, and cognitive impairment created a correlated cluster of symptoms that explained 29% of the variance in QoL, while depression, fatigue, sleep, pain, and cognitive impairment explained 62% of the variance in functional status. For LGG, a small study demonstrated that fatigue had the strongest relationship with QOL.7 In addition to fatigue, Gustafsson and colleagues determined that the most common symptoms affecting QoL were sleep disturbances (reported in 30% to 50% of patients evaluated) and headache (pain), often a presenting symptom and affecting at least 50% of patients with low-grade brain tumors.7
Seizures are the most common presenting symptom of LGG, occurring in 65% to 85% of patients.12 Uncontrolled seizures in patients with LGG have been reported in approximately 30% of patients, leading to cognitive decline and significant morbidity that can impact QoL.12,15 Though important, the management of seizures with anti-epileptic agents can also contribute to cognitive slowing and decrease QoL. Mood disturbances, primarily described as anxiety, can occur in up to 60% of individuals with gliomas.16,17 Studies have reported depression at rates from 40% to 90% of patients in this population.16−18 As such, a diagnosis of LGG is an important predictor of anxiety and depression.16,17 In a cohort study of LGG, depressive symptoms were noted to be the most important independent predictor of decreased QoL and possibly survival.18–20 Psychological problems can additionally exacerbate cognitive dysfunction (including memory), which may be because of the tumor location (non-dominant left hemisphere), disease progression, and treatment (ie, radiation, chemotherapy, antiepileptics, steroids).8,11
Performance status was strongly correlated with QoL21 and with cognitive function in two large prospective trials in patients with newly diagnosed malignant gliomas.22 The trend in results of an LGG study suggested that physical symptoms had less impact on QoL than cognitive problems.7 In another LGG study of 39 patients, less perceived cognitive impairment (as measured by Functional Assessment of Cancer Therapy-Cognition Function −[ACT-Cog]) significantly correlated with improved performance on neurocognitive tests involving complex tasks, such as executive functioning and attention.23 In 90% of long-term survivors of LGG, cognitive impairment has been described as the most serious challenge to cope with and adapt to post-treatment.12,24 It is interesting to note that even “symptom-free” survivors still have documented cognitive deficits on formal neurocognitive testing.1 However, survivors still report good QoL despite suffering cognitive deficits, thus suggesting that adaptation is achievable.
This descriptive study explored the symptom experience using the AL framework. While previous studies have reported the incidence of symptoms and the technical work (ie, prescribing medications) to manage these symptoms in patients with LGGs, this project described the trajectory of symptoms and adaptive challenges that occur over three time points in the initial 6 months following diagnosis of LGG. Clinical patient characteristics, including new molecular biomarkers that determine prognosis and how LGG are diagnosed, evaluated, and treated, will also be explored in association with symptoms and adaptive strategies. The patterns of symptoms that occur as they relate to the adaptive work used to manage these symptoms have not been well documented. Therefore, these supportive adaptive strategies to manage symptoms and the challenges they create can then be shared with providers and patients to assist in improving QoL.
Methods
The objectives of this pilot project during the first 6 months post-diagnosis with a LLG were to: (1) identify and describe the patterns in symptoms and problems and QoL as measured by valid and reliable tools; (2) identify and describe via qualitative interviews patient adaptive techniques and strategies; and (3) describe association among patient characteristics (including biomarkers), symptoms and QoL, and adaptive techniques/strategies.
This was a descriptive, exploratory study using an innovative mixed-methods design that intended to study the trajectory of symptoms and associated technical and adaptive challenges of individuals with LGG during the first 6 months post diagnosis of LGG at a Southern Academic Brain Tumor Center. The sample size for this exploratory study was 15. Participants were included if they were adults over 18 years of age with a pathologic diagnosis of LGG (WHO grade II). Participants were enrolled in this study within the first 2 months of diagnosis. Individuals who had a Karnofsky performance status below 70% were excluded. Of note, biomarkers were routinely collected as part of the initial clinical work-up of the patients.
Upon determination that a patient’s tumor histology and/or radiographic findings were compatible with the eligibility criteria of this protocol, the clinical team contacted the Study Coordinator to pre-screen for potential eligibility. The study was initially explained to the prospective participant by the patient’s provider. Research nurses or other designated study staff provided protocol education and obtained patient consent to participate in the study. Thirty-one individuals were approached to participate and 17 (including two screen failures) were enrolled in the study, with 15 completing the study.
Data for this study were collected during the first 2 to 6 months following diagnosis because this is theoretically when patients and their caregivers are first involved in adaptive work. During the initial diagnosis phase, there is shock and uncertainty as the patient undergoes surgical intervention to determine the diagnosis. In the first 2 months the focus is on technical work (obtaining the diagnoses, determining medications and treatment, and education about the disease process and prognosis). It is following this initial phase that the patient begins to cope with adjusting to the diagnoses and managing the symptoms as part of their daily life. Thus, our baseline measures were conducted near the 2-month time point, which is theoretically consistent with the beginning of adaptive work, followed by assessments at approximately the 4-month and 6-month time points.
Symptom measures were obtained at the three time points using Functional Assessment of Cancer Therapy (FACT)-General (FACT-G)/Brain (FACT- Br)/Cognition (FACT-Cog),25–27 Beck-Depression-Inventory (BDI)-II,28 National Comprehensive Cancer Network (NCCN) Distress Thermometer and Problem List,29–32 and the Functional Assessment of Chronic Illness Therapy (FACIT) – fatigue (FACIT-F).33 These are reputable instruments with well-established reliability and validity in this patient population, and all are National Cancer Institute common data element tools. These Likert scale questionnaires were completed in 10 to 25 minutes.
Qualitative interviews identifying the symptoms and challenges and adaptive techniques and strategies were conducted at two time points (4 and 6 months). The interviews used a modified version of the questionnaire from the adaptive framework6 and lasted 25 to 40 minutes. All scripted interviews were conducted by two advanced practice nurses, with the same interviewer essentially at both time points for each participant. To ensure consistency, practice interviews were conducted with both interviewers questioning standardized subjects before data collection with study participants. All interviews were audio recorded, transcribed, and coded using a standardized codebook in an Atlas.ti database.
Demographic data were obtained from the participant’s medical record and included age, gender, functional status, pathology with biomarker data (immunohistochemistry, fluorescence in situ hybridization [FISH], and polymerase chain reaction [PCR]), tumor grade, extent of resection, tumor location, and postsurgical treatment. The trajectory of change in symptoms and QoL was measured by the tools noted earlier and were described using means and 95% confidence intervals.
Results
Patient characteristics
Fifteen patients with newly diagnosed LGG completed the study. Table 1 summarizes the clinical and demographic characteristics of the patients accrued to this study. These demographics data and clinical characteristics are typical of the LGG population. Mean standard deviation [SD] age was 40 (11) years, 73% (11/15) were female, and 93% (14/15 were white.
TABLE 1.
Patient Characteristics
| All Patients (n 15) |
||
|---|---|---|
| N | Percent | |
| Age | ||
| Mean(SD) | 15 | 40 (11) |
| Race | ||
| African American | 1 | 7 |
| White | 14 | 93 |
| Sex | ||
| Female | 11 | 73 |
| Male | 4 | 27 |
| Karnofsky Performance Status at baseline | ||
| 70–80 | 9 | 60 |
| 90–100 | 6 | 40 |
| Diagnosis | ||
| Diffuse astro | 8 | 53 |
| Infiltrating glioma | 1 | 7 |
| Oligodendroglioma (4) | ||
| Well-differentiated oligo (2) | 6 | 40 |
| Tumor location | ||
| Bilateral multifocal | 1 | 7 |
| Left frontal | 3 | 20 |
| Right frontal | 8 | 53 |
| Right parietal | 3 | 20 |
| Surgery extent | ||
| Biopsy | 5 | 33 |
| Gross total resection | 10 | 67 |
| Postsurgical temozolomide | ||
| Yes | 6 | 40 |
Abbreviation: SD, standard deviation.
Sixty percent had a baseline Karnofsky Performance Status between 70% and 80%(which indicates they had some signs or disease symptoms and were able to care for themselves but were unable to carry on normal activity or do active work). Most had right non-dominant, frontal tumors (73%; 11/15) and underwent a gross total resection (67%; 10/15). Pathologic diagnosis con-sisted of 53% (8/15) of enrolled patients with diffuse astrocytoma, 40% (6/15) with oligodendro-glioma, and 7% (1/15) with an infiltrating glioma. Forty percent (40%) of the enrolled subjects received postsurgical treatment with temozolo-mide based on their diagnosis, prognosis, and symptoms.
Biomarkers
In the past, pathologic classification and grading of gliomas had been based on the morphologic appearance and differentiation of tumor cells. However, the 2016 WHO34 guidelines currently include the new molecular advancements in biomarkers that determine prognosis and how LGG are diagnosed, evaluated, and treated. The WHO 2016 LGG pathologic guidelines focus the genetic analysis on four major biomarkers: (1) isocitrate dehydrogenase (IDH), (2) 1p19q chromosomes, (3) telomerase reverse transcriptase (TERT) promoter, and (4) methylguanine methyltransferase (MGMT). The results regarding these biomarkers for this study are described in Tables 2 and 3.
TABLE 2.
Biomarker
| Percent | N | Not Performed (%) | |
|---|---|---|---|
| IDH1 mutation | 60 | 9 | 2/15(13) |
| IDH1 intact (wild type) | 27 | 4 | |
| TERT mutation | 53 | 8 | 3/15 (20) |
| TERT intact (wild type) | 27 | 4 | |
| 1p19q codeleted | 40 | 6 | 3/15 (20) |
| 1p19q intact | 40 | 6 | |
| MGMT methylated | 63 | 10 | 3/15 (20) |
| MGMT unmethylated | 13 | 2 |
Abbreviations: IDH1, isocitrate dehydrogenase mutation; TERT, telomerase reverse transcriptase; MGMT, methylguanine methyltransferase.
TABLE 3.
IDH1 Status by 1p19q and IDH1 Status
| IDH1 molecular diagnostics |
||||||
|---|---|---|---|---|---|---|
| Mutant |
Wild type |
Not performed |
||||
| N | % | N | % | N | % | |
| TOTAL | 9 | 4 | 2 | |||
| 1p19q status | ||||||
| Intact | 4 | 44.4 | 2 | 50.0 | 0 | 0.0 |
| Co-deletion | 5 | 55.6 | 1 | 25.0 | 0 | 0.0 |
| Not performed | 0 | 0.0 | 1 | 25.0 | 2 | 100.0 |
| TERT status | ||||||
| Mutant | 6 | 66.7 | 2 | 50.0 | 0 | 0.0 |
| Wild type | 3 | 33.3 | 1 | 25.0 | 0 | 0.0 |
| Not performed | 0 | 0.0 | 1 | 25.0 | 2 | 100.0 |
Abbreviations: IDH, isocitrate dehydrogenase; TERT, telomerase reverse transcriptase.
As noted in Table 2, the majority of subjects with available data had favorable prognostic biomarkers with IDH mutations (IDHmut −, 60%); TERT mutation (TERTmut −, 53%), and MGMT méthylation (63%). Half of the patients (6/12) who had 1p19q analysis obtained had a favorable 1p19q chromosome co-deletion. Combination of these biomarkers within each patient are required for prognosis and classification. The combination of both IDHmut and TERTmut is considered to be the most favorable prognostic category.35 Table 3 demonstrates a higher percentage of patients who had a more favorable prognostic combination of IDHmut/TERTmut (66.7%) and IDHmut/1p19qco-deletion (55.6 %) than unfavorable combinations (ie, with IDH1 wild type-IDHwildtype). These characteristics are typical of the LGG population.
Symptoms and challenges experienced by patients with LGG
The NCCN Distress Thermometer Problem List is described in Table 4, which outlines the highest frequency of physical symptoms including fatigue (40%) and memory/concentration (40%). Many experienced emotional symptoms reported as depression (40%), nervousness (47%), sadness (40%), worry (60%), and loss of interest in usual activities (40%). These emotional symptoms seem to peak at month 4 from diagnosis. Problems (family/practical challenges) that occur as a result of the LGG symptoms are illustrated in Table 4. It is interesting that challenges with insurance (26%), treatment decision (20%), and dealing with partners (20%) occurred during the first 2 months of diagnosis, whereas work/school (27%) and transportation (20%) challenges occurred during the 4th or 6th month of the diagnosis.
TABLE 4.
NCCN Distress Thermometer Problem List
| Time (month) |
||||||
|---|---|---|---|---|---|---|
| 2* |
4† |
6 |
||||
| N | % | N | % | N | % | |
| Physical problems | ||||||
| Bathing/dressing | 1 | 6.67 | 1 | 6.67 | 0 | 0.00 |
| Breathing | 3 | 20.00 | 1 | 6.67 | 0 | 0.00 |
| Changes in urination | 2 | 13.33 | 0 | 0.00 | 1 | 6.67 |
| Eating | 0 | 0.00 | 1 | 6.67 | 1 | 6.67 |
| Fatigue | 5 | 33.33 | 5 | 33.33 | 6 | 40.00 |
| Feeling swollen | 2 | 13.33 | 1 | 6.67 | 1 | 6.67 |
| Getting around | 1 | 6.67 | 1 | 6.67 | 1 | 6.67 |
| Indigestion | 2 | 13.33 | 0 | 0.00 | 1 | 6.67 |
| Memory/concentration | 6 | 40.00 | 4 | 26.67 | 5 | 33.33 |
| Mouth sores | 0 | 0.00 | 1 | 6.67 | 0 | 0.000 |
| Nausea | 1 | 6.67 | 0 | 0.00 | 1 | 6.6 |
| Nose dry/congested | 1 | 6.67 | 2 | 13.33 | 1 | 6.67 |
| Pain | 2 | 13.33 | 1 | 6.67 | 3 | 20.00 |
| Sexual | 2 | 13.33 | 0 | 0.00 | 1 | 6.67 |
| Skin dry/itchy | 3 | 20.00 | 1 | 6.67 | 1 | 6.67 |
| Sleep | 2 | 13.33 | 3 | 20.00 | 3 | 20.00 |
| Tingling in hands/feet | 2 | 13.33 | 0 | 0.00 | 2 | 13.33 |
| Emotional problems | ||||||
| Depression | 0 | 0.00 | 6 | 40.00 | 3 | 20.00 |
| Fears | 2 | 13.33 | 4 | 26.67 | 2 | 13.33 |
| Nervousness | 3 | 20.00 | 7 | 46.67 | 4 | 26.67 |
| Sadness | 1 | 6.67 | 6 | 40.00 | 3 | 20.00 |
| Worry | 2 | 13.33 | 9 | 60.00 | 3 | 20.00 |
| Loss of interest in usual activities | 1 | 6.67 | 6 | 40.00 | 1 | 6.67 |
| Appearance | 2 | 13.33 | 1 | 6.67 | 0 | 0.00 |
| Practical/family problems | ||||||
| Housing | 2 | 13.33 | 0 | 0.00 | 0 | 0.00 |
| Insurance/financial | 4 | 26.67 | 2 | 13.33 | 2 | 13.33 |
| Transportation | 1 | 6.67 | 2 | 13.33 | 3 | 20.00 |
| Work/school | 2 | 13.33 | 4 | 26.67 | 1 | 6.67 |
| Treatment decisions | 3 | 20.00 | 2 | 13.33 | 1 | 6.67 |
| Dealing with children | 2 | 13.33 | 0 | 0.00 | 0 | 0.00 |
| Dealing with partner | 3 | 20.00 | 1 | 6.67 | 1 | 6.67 |
| Ability to have children | 0 | 0.00 | 0 | 0.00 | 1 | 6.67 |
| Family health issues | 2 | 13.33 | 2 | 13.33 | 2 | 13.33 |
Abbreviation: NCCN, The National Comprehensive Cancer Network.
One patient missing responses at month 2.
Two patients missing responses at month 4.
Seizures
As previously stated, the most common presenting symptom in individuals with LGG is seizures. Overall, 40% (6/15) of the study patients experienced at least one seizure during the 2- to 6-month period following the initial diagnosis. It is interesting to note that seizures occurred among patients with IDHmut (4/9) but not among patients with IDHwildtype·(0/4), a poorer prognostic factor and is consistent with recent research (Table 5).30,37 Within the IDH mutation group, seizures are seen at each time point, with 44% (4/9) of patients reporting evidence of seizure at 2 months, 22% (2/9) at 4 months, and 22% (2/9) at 6 months from initial diagnosis.
TABLE 5.
Incidence of Seizures
| IDH1 Status |
||||||
|---|---|---|---|---|---|---|
| Mutation (N 9) |
Wild type (N 4) |
Not performed (N 2) |
||||
| VISIT | N | % | N | % | N | % |
| 2 months | ||||||
| Not at all | 4 | 44.4 | 4 | 100.0 | 1 | 50.0 |
| A little bit | 3 | 33.3 | 0 | 0.0 | 0 | 0.0 |
| Quite a bit | 1 | 11.1 | 0 | 0.0 | 0 | 0.0 |
| Very much | 0 | 0.0 | 0 | 0.0 | 1 | 50.0 |
| Missing | 1 | 11.1 | 0 | 0.0 | 0 | 0.0 |
| 4 months | ||||||
| Not at all | 6 | 66.7 | 4 | 100.0 | 0 | 0.0 |
| A little bit | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Quite a bit | 1 | 11.1 | 0 | 0.0 | 0 | 0.0 |
| Very much | 1 | 11.1 | 0 | 0.0 | 1 | 50.0 |
| Missing | 1 | 11.1 | 0 | 0.0 | 1 | 50.0 |
| 6 months | ||||||
| Not at all | 7 | 77.8 | 4 | 100.0 | 0 | 0.0 |
| A little bit | 1 | 11.1 | 0 | 0.0 | 1 | 50.0 |
| Quite a bit | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Very much | 1 | 11.1 | 0 | 0.0 | 1 | 50.0 |
| Missing | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Worst seizure experience reported | ||||||
| Not at all | 5 | 55.6 | 4 | 100.0 | 0 | 0.0 |
| A little bit | 2 | 22.2 | 0 | 0.0 | 1 | 50.0 |
| Quite a bit | 1 | 11.1 | 0 | 0.0 | 0 | 0.0 |
| Very much | 1 | 11.1 | 0 | 0.0 | 1 | 50.0 |
| Missing | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
QoL
Table 6 exhibits QoL scores at 2, 4, and 6 months post-diagnosis. It is noted that QoL scores range from 50% to 75% of the highest possible score at baseline. As indicated in Table 6, there was no overall change in mean QoL (as measured by the FACT-G, FACT-Br, and FACT-Cog) nor distress and depression scores reported over time as measured, respectively, by the NCCN Distress Thermometer and Beck Depression scale. The FACIT-fatigue scale (where higher scores note higher QoL or less fatigue) suggested that there was a clinically important decrease in fatigue (defined as a change in mean score by + 3 in fatigue) over 6 months.33
TABLE 6.
Quality of Life Summary (QoL)*
| 2 |
4 |
6 |
||||
|---|---|---|---|---|---|---|
| Mean | ± SD | Mean | ± SD | Mean | ± SD | |
| FACT-G: Physical (range, 0–28) (Higher FACT scores signify increased Q0L)* | 21.95 | 5.10 | 23.07 | 4.07 | 22.73 | 6.50 |
| FACT-G: Social (range, 0–28)* | 20.81 | 5.17 | 20.88 | 4.72 | 19.22 | 4.66 |
| FACT-G: Emotional (range, 0–24)* | 17.20 | 5.5 | 15.64 | 4.96 | 17.87 | 5.36 |
| FACT-G: Functional (range, 0–28)* | 15.80 | 6.65 | 16.29 | 5.95 | 16.73 | 6.86 |
| FACT-G: Total (range, 0–108)* | 75.76 | 18.44 | 75.88 | 16.41 | 76.56 | 20.82 |
| Brain Cancer Subscale (range, 0–76)* | 50.29 | 14.58 | 49.96 | 16.10 | 53.53 | 16.59 |
| FACT-Br: Total (range, 1–184)* | 126.05 | 31.80 | 125.84 | 29.52 | 130.09 | 35.62 |
| FACT-Br: TO1 (range, 0–132)* | 88.04 | 24.67 | 89.32 | 22.84 | 93.00 | 27.99 |
| FAC IT-Fatigue Scale (range, 0.52)* | 30.67 | 13.26 | 32.14 | 12.02 | 34.47 | 12.74 |
| FACT-Cog Perceived Cognitive Impairments Subscale (range, 0–72)* | 49.80 | 18.04 | 48.64 | 12.63 | 46.20 | 19.27 |
| FACT-Cog Impact of Perceived Cog Impairment on QoL (range, 0–16)* | 8.33 | 4.61 | 8.36 | 4.43 | 9.13 | 4.97 |
| FACT-Cog Comments From Others Subscale (range, 0–16)* | 12.67 | 4.70 | 13.71 | 3.41 | 13.80 | 4.31 |
| FACT-Cog Perceived Cognitive Abilities Subscale (range, 0–28)* | 15.73 | 7.68 | 14.57 | 66.66 | 15.53 | 7.74 |
| Beck Depression l-ll (range, 0–63) (higher scores signify increased depression)’ | 15.20 | 12.92 | 16.57 | 12.47 | 13.93 | 11.49 |
| Level of Distress† (higher scores signify increased distress)† | 2.21 | 2.39 | 1.46 | 2.47 | 1.80 | 2.65 |
Abbreviations: SD, standard deviation; FACT-G, Functional Assessment of Cancer Therapy General; FACT-Br, Functional Assessment of Cancer Therapy-Brain; FACIT-Fatigue, Functional Assessment of Chronic Illness Therapy-Fatigue; FACT-Cog, Functional Assessment of Cancer Therapy-Cognition Function.
One patient is missing questionnaires at month 2.
One patient is missing the distress scale at month 2; 2 patients missing at month 4.
QoL reported over time stratified by IDH and TERT status
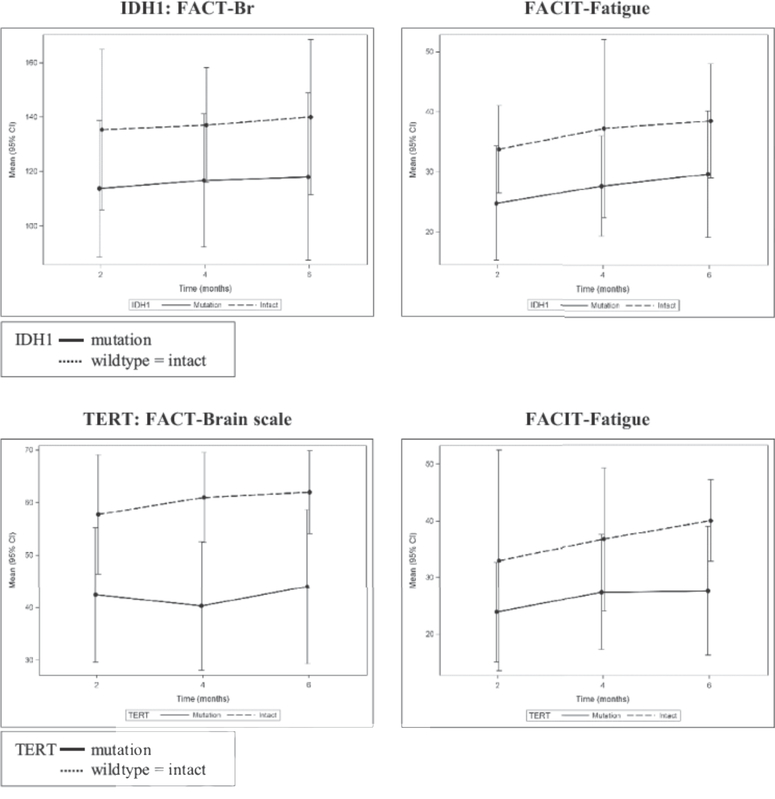
Descriptive analyses suggests that patients with either IDHmut or TERTmut consistently report lower QoL, higher fatigue (Fig. 1), and more depression and distress over time The analysis of the combination of biomarkers as it relates to QoL outcomes was not conducted because of the small sample size.
FIGURE 1.
QoL reported over time stratified by IDH1 and TERT status.
Abbreviations: FACT-Br, Functional Assessment of Cancer Therapy-Brain; FACIT, Functional Assessment of Chronic Illness Therapy.
QoL reported over time stratified by 1p19q and sex
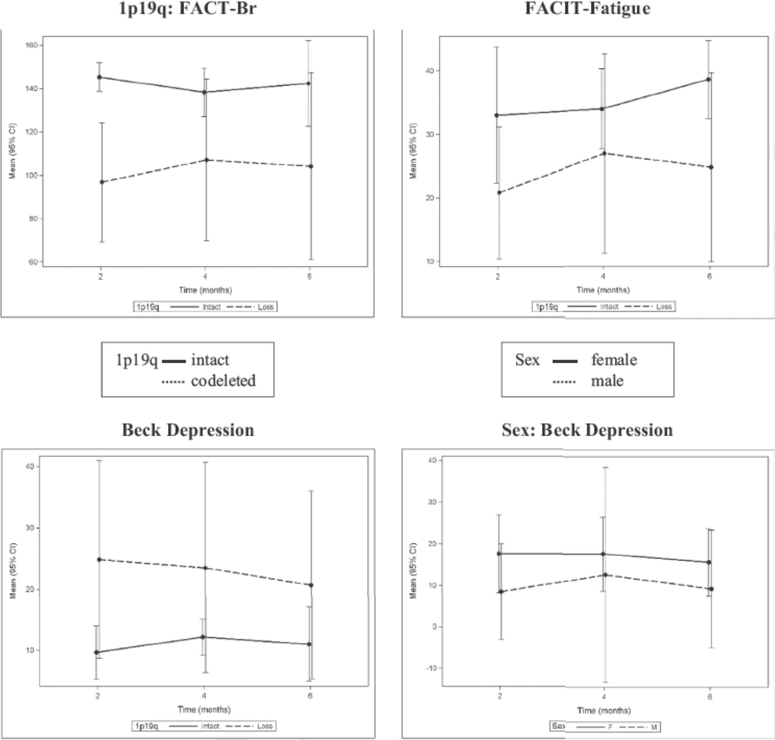
As noted in Fig. 2, patients with 1p19qco-deletion reported lower QoL scores as determined by the specific FACT-Br subscale and less perceived fatigue, but had higher depression. Females reported lower QoL and higher depression scores, which is consistent with the glioma literature.17
FIGURE 2.
QoL reported over time stratified by 1p19q status and sex.
Abbreviations: FACT-Br, Functional Assessment of Cancer Therapy-Brain; FACIT, Functional Assessment of Chronic Illness Therapy.
Adaptive themes and strategic techniques
Patient qualitative interviews were conducted to determine the adaptive themes and strategies described by patients to adapt to LGG symptoms and the challenges they created. The percent of patients with LGG that identified the major themes and strategic techniques are listed in Table 7. The most frequently used strategies include: obtaining community support (87%), managing expectations (73%) and support systems (67%), seeking out knowledge about physical symptoms (67%) and behavioral symptoms (53%), developing memory cues (47%), finding driving support (47%), pacing activities of daily living (47%), and trying restorative activities (47%). It is thought-provoking to note the time point at which the majority of patients reported utilizing these strategies. Most of the adaptive strategies were described at 4 months and/or remained consistent throughout. However, adaptive work completed at 6 months most frequently included pacing activities of daily living, trying restorative activities, and seeking out knowledge about behavior symptoms.
TABLE 7.
Identified Adaptive Themes and Strategic Techniques
| Adaptive Themes | Month |
||||||
|---|---|---|---|---|---|---|---|
| 4 |
6 |
Overall |
|||||
| N | % | N | % | N | % | ||
| Community Support(obtaining support via family, church, neighbor, coworker assistance) | |||||||
| No | 2 | 13.33 | 5 | 33.33 | 2 | 13.33 | |
| Yes | 13 | 86.67 | 10 | 66.67 | 13 | 86.67 | |
| Managing Expectations (realigning goals with physical and mental abilities) | |||||||
| No | 6 | 40.00 | 7 | 46.67 | 4 | 26.67 | |
| Yes | 9 | 60.00 | 8 | 53.33 | 11 | 73.33 | |
| Managing Support (choosing who to help & when, limit setting and hovering) | |||||||
| No | 5 | 33.33 | 8 | 53.33 | 5 | 33.33 | |
| Yes | 10 | 66.67 | 7 | 46.67 | 10 | 66.67 | |
| Physical Knowledge (understanding of disease, prognostic factors, biomarkers via internet) | |||||||
| No | 8 | 53.33 | 8 | 53.33 | 5 | 33.33 | |
| Yes | 7 | 46.67 | 7 | 46.67 | 10 | 66.67 | |
| Behavior Knowledge (engaging therapy, neurocognitive testing, coping, and support techniques) | |||||||
| No | 11 | 73.33 | 9 | 60.00 | 7 | 46.67 | |
| Yes | 4 | 26.67 | 6 | 40.00 | 8 | 53.33 | |
| Driving Support (coordinating rides, driving evaluations) | |||||||
| No | 10 | 66.67 | 12 | 80.00 | 8 | 53.33 | |
| Yes | 5 | 33.33 | 3 | 20.00 | 7 | 46.67 | |
| Memory Cues (creating alarms/text reminders, notes, lists, spread sheets, online scheduling tools, keeping everything in same place, brain games, puzzle) | |||||||
| No | 9 | 60.00 | 10 | 66.67 | 8 | 53.33 | |
| Yes | 6 | 40.00 | 5 | 33.33 | 7 | 46.67 | |
| Pacing Activities of Daily Living (using rest periods, plan/time activities for best cognitive/ physical results) | |||||||
| No | 12 | 80.00 | 9 | 60.00 | 8 | 53.33 | |
| Yes | 3 | 20.00 | 6 | 40.00 | 7 | 46.67 | |
| Restorative Activities (incorporating naps, journaling, yoga, exercise, creative activities) | |||||||
| No | 11 | 73.33 | 9 | 60.00 | 8 | 53.33 | |
| Yes | 4 | 26.67 | 6 | 40.00 | 7 | 46.67 | |
| Employment (changing work hours, using disability leave) | |||||||
| No | 11 | 73.33 | 12 | 80.00 | 9 | 60.00 | |
| Yes | 4 | 26.67 | 3 | 20.00 | 6 | 40.00 | |
| Changes in Living Environment (including caregivers in home; moving closer to support systems) | |||||||
| No | 12 | 80.00 | 12 | 80.00 | 11 | 73.33 | |
| Yes | 3 | 20.00 | 3 | 20.00 | 4 | 26.67 | |
| Monitoring Cues (using code words to prevent inappropriate behavior, provide redirection) | |||||||
| No | 12 | 80.00 | 13 | 86.67 | 11 | 73.33 | |
| Yes | 3 | 20.00 | 2 | 13.33 | 4 | 26.67 | |
Conclusion and Discussion
The overall symptoms that patients with LGG experienced within this study are consistent with the current LGG research. Although the small sample size is a primary limitation of this pilot study, the patients with LGG commonly reported the physical symptoms of seizures and cognitive impairment (ie, memory and concentration issues). The described emotional symptoms of depression and anxiety continued to remain a significant issue throughout the trajectory of their illness. Providers have approached these technical challenges (symptoms) with well-reported management strategies (ie, anticonvulsants, stimulants, antidepressants) that are eloquently reviewed in the companion symptom management articles elsewhere in this issue on glioma.
The unique contribution of this pilot study is the first identification and description of adaptive strategies that patients used when encountering commonly occurring symptoms of LGG and the daily challenges (problems) created by these symptoms. The majority of adaptive strategic work identified by patients with LLG (ie, obtaining community support, managing expectations and support systems, seeking out knowledge about physical and behavioral symptoms, developing memory cues, finding driving support, pacing activities of daily living, and trying restorative activities) were present throughout the 6-month post-diagnosis period. Providing oncology nurses and advanced practice nurses with these identified LGG adaptive strategies and the timing for when they are most utilized will provide a non-pharmacologic patient-centered approach to adjusting to this life-long illness. For example, it is important to suggest early the value of obtaining community support, a predominant adaptive strategy that was used early by 87% of patients. This adaptive strategy can be exemplified by the following patient statements related to memory impairment and the inability to drive:
“There is definitely some absent-mindedness — you know, it’s kind of a big deal and there’s a lot going on, there’s a lot of medications to keep track of … writing down what you need to take and when, because some of them you have to take more as time goes on, and some of them you take less, and it’s really hard to keep track of My dad had to [keep track of the medications] for the first several weeks.”
“Everybody’s been very supportive so far as offering rides… You know, because after I had the seizure, I couldn’t drive for 6 months. So everybody was really ‘oh, do you need me to drive you to the store?’ ‘Do you need me to go get [your daughter]?’… so everybody was really helpful about that sort of thing.”
Most patients described adapting to their physical and mental limitations at 6 months through pacing activities (described as taking more rest periods and by planning activities to ensure the best cognitive and physical functioning). Trying restorative activities was another common theme that included incorporating naps, journaling, yoga, exercise, and creative activities into their daily routine to improve their mental well-being. Seeking out knowledge about behavior symptoms through neurocognitive testing, coping techniques, and therapy sessions were reported to be helpful in ameliorating emotional challenges. Many providers may initiate antidepressants and recommend therapy in this LGG population because it Ls well known that the diagnosis of LGG and female sex are associated with anxiety and depression.16,17 An anecdotal observation that multiple neuro-oncology investigators verbalized during the study was that subjects benefited from the actual interviewing process and by answering adaptation questions as a way of expressing their thoughts and feelings. Some seemed to be more receptive and adaptive to medication and therapy. This qualitative observation that emerged from the study underscored the importance of cognitive therapy as a way of addressing the emotional needs of a patient with a LGG. Oncology nurses could educate patients with LGG and their families earlier about some of these learned adaptive techniques to assist in emotional needs to maintain/improve QoL.
The results of the NCCN Distress Thermometer further elucidated the challenges with LGG treatment decision and dealing with partners that were mentioned upfront when subsequent adaptive strategies about seeking out physical knowledge and managing support (ie, choosing who to help and when, setting limits, and reducing the hovering of others) were developed. Transportation issues were also NCCN-reported concerns that occurred during the 4 to 6 months from diagnosis. Driving support through coordinating rides was described as a frequent and important adaptive strategy for those who were unable to drive possibly because of seizures, cognitive impairment, or weakness. Educating providers, patients, and families of these common adaptive themes may allow patients to adapt to the new LGG diagnosis with the hope of improving knowledge, relationships, mobility, and safety.
An important clinical objective for the glioma population is to improve overall QoL. Providers should be aware that patients within this LGG study population (and in the glioma population in general) frequently reported low baseline QoL scores compared with the average QoL data known for the general cancer population. For example, the normative FACT-G total QoL mean score for a large sample of patients with mixed cancer diagnoses is higher (80.9 [SD ± 17]) compared with the mean score of 75.6 (SD ± 18.44) for these LGG study subjects. Brucker and colleagues38 indicate a score >2 points is a meaningful important difference and is clinically significant in patients with LGG. The FACT-G domain subscales that contributed to the total low LGG QoL score in this pilot were social, emotional, and functional well-being. Overall, there was essentially no change in low QoL scores during the 6 months post diagnoses except the FACIT-fatigue scale, which demonstrated an improvement in fatigue over the 6-month study period.
Biomarker data for this LGG population were thought-provoking because patients with either 1p19qco-deletion, IDHmut, or TERTmut reported lower QoL. Although IDHmut is a better prognostic indicator than IDHwildtype, it is suggested that the lower QoL scores are possibly because of the seizure experience (and antiepileptic treatment) associated with the IDHmut study group. A recent meta-analysis demonstrated a significant association with (preoperative) seizures among patient with IDHmut LGG (WHO grade II), but not among patients with HGGs (WHO grade III–IV), possibly because of increased neuronal activity caused by an IDHmut product called D2HG.36,37 Further investigation should be conducted to determine if certain combinations of biomarkers are predictive of QoL and the adaptation to LGG symptoms and challenges.
This descriptive study explored the symptom and challenges (problems) experienced by patients with LGG using the AL framework. While previous studies have reported the incidence of symptoms and the technical work to manage these symptoms in patients with low LGG (ie, prescribing anticonvulsants for seizure prevention), this project was one of the first to describe the trajectory of symptoms and the challenges the symptoms created 6 months following a diagnosis of LGG. The patterns of symptoms that occur and the adaptive work individuals do to manage these symptoms and challenges have not been well documented in the LGG population. The supportive adaptive mechanisms identified in this pilot can be shared with providers, nurses, and patients to assist in improving the quality of everyday life of patients with LGG. Future larger intervention studies to explore identified adaptive strategies to these glioma challenges should be pursued.
Acknowledgments
The authors would like to acknowledge Karen Hawkins, BA and Olivia Kohrman for their transcription, coding, and data entry; Elizabeth S. Miller, BA for her regulatory and editing efforts; Natalie Ammarell, PhD for her coding and data assistance; and Wendy Gentry for her development of the tables and figures.
This project has been funded in part by the ADAPT Center (Center for Adaptive Leadership in Symptom Science) which is funded by National Institutes of Health / National Institute of Nursing Research award 1P30 NR014139 (MPI: R.A. Anderson with S.L. Docherty).
Contributor Information
Mary Lou Affronti, Associate Professor, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center; Duke University School of Nursing; Duke Cancer Institute; and Department of Neurosurgery, Duke University..
Dina Randazzo, Assistant Professor, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center; Duke Cancer Institute; and Department of Neurosurgery, Duke University..
Eric S. Lipp, Clinical Research Coordinator, Senior, Lead, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center; and Department of Neurosurgery, Duke University.
Katherine B. Peters, Associate Professor, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center; Duke Cancer Institute; and Department of Neurosurgery, Duke University.
Susan C. Herndon, Adult Nurse Practitioner, Duke University School of Nursing.
Sarah Woodring, Clinical Research Coordinator, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center; and Duke Cancer Institute..
Patrick Healy, Statistician Duke Cancer Institute..
Christina K. Cone, The Preston Robert Tisch Brain Tumor Center, Duke University Medical Center; and Department of Neurosurgery, Duke University.
James E. Herndon, II, Professor of Biostatistics, Biostatistics and Bioinformatics, Duke University Medical Center..
Susan M. Schneider, Associate Professor, Lead Faculty, Oncology Nursing Specialty, Duke University School of Nursing; and Department of Neurosurgery, Duke University Durham, NC.
References
- 1.Liu R, Page M, Solheim K, Fox S, Chang SM. Quality of life in adults with brain tumors: current knowledge and future directions. Neuro Oncol. 2009;11:330 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thakkar JP, Dolecek TA, Horbinski C, et al. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epi demiol Biomarkers Prev. 2014;23:1985 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(suppl 4):iv1 iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claus EB, Walsh KM, Wiencke JK, et al. Survival and low-grade glioma: the emergence of genetic information. Neurosurg Focus. 2015;38:E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thygeson M, Morrissey L, Ulstad V. Adaptive leadership and the practice of medicine: a complexity-based approach to reframing the doctor-patient relationship. J Eval Clin Pract. 2010;16:1009 1015. [DOI] [PubMed] [Google Scholar]
- 6.Bailey DE Jr., Docherty SL, Adams JA, et al. Studying the clinical encounter with the Adaptive Leadership framework. J HealthcLeadersh. 2012;2012(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gustafsson M, Edvardsson T, Ahlstrom G. The relationship between function, quality of life and coping in patients with low-grade gliomas. Support Care Cancer. 2006;14:1205 1212. [DOI] [PubMed] [Google Scholar]
- 8.Taphoom MJ, Schiphorst AK, Snoek FJ, et al. Cognitive functions and quality of life in patients with low-grade gliomas: the impact of radiotherapy. Ann Neurol. 1994;36:48 54. [DOI] [PubMed] [Google Scholar]
- 9.Klein M, Engelberts NH, van der Ploeg HM, et al. Epilepsy in low-grade gliomas: the impact on cognitive function and quality of life. Ann Neurol. 2003;54:514 520. [DOI] [PubMed] [Google Scholar]
- 10.Laack NN, Brown PD, Ivnik RJ, et al. Cognitive function after radiotherapy for supratentorial low-grade glioma: a North Central Cancer Treatment Group prospective study. Int J Radiat Oncol Biol Phys. 2005;63:1175 1183. [DOI] [PubMed] [Google Scholar]
- 11.Klein M, Heimans JJ, Aaronson NK, et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2002;360:1361 1368. [DOI] [PubMed] [Google Scholar]
- 12.Shields LB, Choucair AK. Management of low-grade gliomas: a review of patient-perceived quality of life and neurocognitive outcome. World Neurosurg. 2014;82:e299 e309. [DOI] [PubMed] [Google Scholar]
- 13.Peters KB, West MJ, Hornsby WE, et al. Impact of health-related quality of life and fatigue on survival of recurrent high-grade glioma patients. J Neurooncol. 2014;120:499 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox SW, Lyon D, Farace E. Symptom clusters in patients with high-grade glioma. J Nurs Scholarsh. 2007;39:61 67. [DOI] [PubMed] [Google Scholar]
- 15.Englot DJ, Berger MS, Barbaro NM, Chang EF. Predictors of seizure freedom after resection of supratentorial low-grade gliomas. A Review J Neurosurg. 2011;115:240 244. [DOI] [PubMed] [Google Scholar]
- 16.Litofsky NS, Farace E, Anderson F Jr., et al. Depression in patients with high-grade glioma: results of the Glioma Outcomes Project. Neurosurgery. 2004;54:358 366. discussion 366–357. [DOI] [PubMed] [Google Scholar]
- 17.Arnold SD, Forman LM, Brigidi BD, et al. Evaluation and characterization of generalized anxiety and depression in patients with primary brain tumors. Neuro Oncol. 2008;10:171 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelletier G, Verhoef MJ, Khatri N, Hagen N. Quality of life in brain tumor patients: the relative contributions of depression, fatigue, emotional distress, and existential issues. J Neuro oncol. 2002;57:41 49. [DOI] [PubMed] [Google Scholar]
- 19.Mainio A, Hakko H, Niemela A, Koivukangas J, Rasanen P. Depression and functional outcome in patients with brain tumors: a population-based 1-year follow-up study. J Neurosurg. 2005;103:841 847. [DOI] [PubMed] [Google Scholar]
- 20.Rooney AG, Carson A, Grant R. Depression in cerebral glioma patients: a systematic review of observational studies. J Natl Cancer Inst. 2011;103:61 76. [DOI] [PubMed] [Google Scholar]
- 21.Klein M, Taphoorn MJ, Heimans JJ, et al. Neurobehavioral status and health-related quality of life in newly diagnosed high-grade glioma patients. J Clin Oncol. 2001;19:4037 4047. [DOI] [PubMed] [Google Scholar]
- 22.Taylor BV, Buckner JC, Cascino TL, et al. Effects of radiation and chemotherapy on cognitive function in patients with high-grade glioma. J Clin Oncol. 1998;16:2195 2201. [DOI] [PubMed] [Google Scholar]
- 23.Peters K, Affronti ML, Woodring S, et al. Neurocognition in low-grade gliomas: associations with measurable and perceived impairments; Paper presented at: The 21st Society for Neuro Oncology Annual Scientific Meeting and Education Day; November 17 20 Scottsdale, AZ Available at: 10.1093/neuonc/now212.507. [DOI] [Google Scholar]
- 24.Taphoom MJ. Neurocognitive sequelae in the treatment of low-grade gliomas. Semin Oncol. 2003;30(suppl 19):45 48. [DOI] [PubMed] [Google Scholar]
- 25.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570 579. [DOI] [PubMed] [Google Scholar]
- 26.Weitzner MA, Meyers GA, Gelke CK, Byrne KS, Cella DF, Levin VA. The Functional Assessment of Cancer Therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer. 1995;75:1151 1161. [DOI] [PubMed] [Google Scholar]
- 27.Lai JS, Butt Z, Wagner L, et al. Evaluating the dimensionality of perceived cognitive function. J Pain Symptom Manage. 2009;37:982 995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893 897. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell AJ, Vahabzadeh A, Magruder K. Screening for distress and depression in cancer settings: 10 lessons from 40 years of primary-care research. Psychooncology. 2011;20:572 584. [DOI] [PubMed] [Google Scholar]
- 30.Tuinman MA, Gazendam-Donofrio SM, Hoekstra-Weebers JE. Screening and referral for psychosocial distress in oncologic practice: use of the Distress Thermometer. Cancer. 2008;113:870 878. [DOI] [PubMed] [Google Scholar]
- 31.Vodermaier A, Linden W, Siu C. Screening for emotional distress in cancer patients: a systematic review of assessment instruments. J Natl Cancer Inst. 2009;101:1464 1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell AJ. Short screening tools for cancer-related distress: a review and diagnostic validity meta-analysis. J Natl Compr Canc Netw. 2010;8:487 494. [DOI] [PubMed] [Google Scholar]
- 33.Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symp tom Manage. 2002;24:547 561. [DOI] [PubMed] [Google Scholar]
- 34.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:8Q3 820. [DOI] [PubMed] [Google Scholar]
- 35.Chan AK, Yao Y, Zhang Z, et al. TERT promoter mutations contribute to subset prognostication of lower-grade gliomas. Mod Pathol. 2015;28:177 186. [DOI] [PubMed] [Google Scholar]
- 36.Phan K, Ng W, Lu VM, et al. Association between IDH1 and IDH2 mutations and preoperative seizures in patients with low-grade versus high-grade glioma: a systematic review and meta-analysis. World Neurosurg. 2018;111:e539 e545. [DOI] [PubMed] [Google Scholar]
- 37.Chen H, Judkins J, Thomas C, et al. Mutant IDH1 and seizures in patients with glioma. Neurology. 2017;88:1805 1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brucker PS, Yost K, Cashy J, Webster K, Cella D. General population and cancer patient norms for the Functional Assessment of Cancer Therapy-General (FACT-G). Eval Health Prof. 2005;8:192 211. [DOI] [PubMed] [Google Scholar]