Abstract
Background:
Although, both chronic obstructive pulmonary disease (COPD) and rheumatic diseases (RDs) are common, and each has significant impact on patients’ overall health/quality of life, their co-occurrence has received little attention, while 15% of COPD remains undiagnosed in RDs.
Objective:
To update the information regarding the comorbid state of RD/COPD (prevalence, incidence), to examine whether patients with RD have increased risk of developing COPD and vice versa, and what implications this comorbidity has on patients’ outcomes (mortality, hospitalizations, exacerbations).
Methods:
We performed a systematic literature review regarding the comorbidity of an RD (rheumatoid arthritis (RA), ankylosing spondylitis (AS), psoriatic arthritis (PsA), systemic lupus erythematosus (SLE), primary Sjogren syndrome disease (pSS), and systemic sclerosis (SSc)) with COPD. From 2803 reports retrieved, 33 articles were further screened. Finally, 27 articles were included.
Results:
Robust evidence supports that COPD develops up to 68% more frequently in patients with RA, as compared to the general population. Similarly, COPD is increased in every other RD that was studied. Further, self-referred arthritis is more common in COPD patients versus non-COPD controls and a predictor of worst self-rated health status. Patients with inflammatory arthritis/COPD have increased mortality (threefold in RA-COPD, irrespectively of which is first diagnosed), hospitalizations, and emergency visits.
Conclusion:
COPD is more common in patients with RA, AS, PsA, SLE, pSS, and SSc; yet, the association, vice versa, warrants further investigation. Nevertheless, COPD/RDs coexistence has significant prognostic value for worst outcomes; therefore, awareness is required to track early identification, especially in primary care.
Keywords: COPD, rheumatic disease, primary care, epidemiology, multimorbidity
Introduction
Multimorbidity, defined as the coexistence of at least two diseases or psychosocial/somatic risk factors,1 is an emerging and challenging issue, especially at the primary care level. The concept of multimorbidity brings along the notion that the coexisting diseases interact and carry an overall larger impact on the health status of patients.1 Although, sometimes, the term is interchangeably used with comorbidity, multimorbidity is not referred to an index disease, but has a more holistic, patient-centered meaning, as equal significance is attributed to each comorbid condition.1,2
Unfortunately, most current guidelines focus on a single disease treated by a specialist, leaving physicians working at the community health care, without clear advice on when to suspect and how to early identify and manage multimorbid conditions. That is also the case of chronic obstructive pulmonary disease (COPD)/rheumatic diseases (RDs) comorbidity.3,4
More specifically, RDs constitute an heterogeneous group of systemic autoimmune diseases,1 with increasing prevalence and growing burden at individual and societal level.1,5 Comorbidities of RDs, and especially cardiovascular diseases, infections, depression, and cancer, play a significant role in the prognosis of the RDs4 and are highly considered as a cause of the nondeclining mortality trends of the RDs.6 Although a number of studies show that there is an increased frequency of COPD development in RDs and especially in rheumatoid arthritis (RA), the extent and the recognition of their co-occurrence, as a multimorbid state with actual implications for patients, is limited in current guidelines and daily clinical practice.7 Further, COPD is the most common cause of death due to a respiratory reason in RA.8
Similarly, and despite tremendous progress in early diagnosis and management, COPD remains a complex respiratory disease, associated with devastating functional and quality of life impairment of patients.3 Notably, almost every COPD patient is diagnosed with one more comorbid condition but, again, most common comorbidities such as cardiovascular, depression/anxiety, and lung cancer have received most attention.9 Although a number of studies show that there is an increased frequency of arthritis/musculoskeletal symptoms in COPD,10,11 this constitutes a rather neglected comorbidity in clinical routine7 with more than 15% of COPD cases possibly remain undiagnosed in patients with RDs.12
Interesting evidence also emerges regarding common pathogenetic pathways between the two categories of diseases: first, regarding the effects of smoking which is a strong environmental risk factor in both COPD and RDs3,13,14 and second, regarding the anti-citrullinated antibodies (ACPAs)—the hallmark of ACPA-positive RA—which have been found increased in COPD, in both smokers15 and non-smokers.16
The aim of the present review is to update information about the comorbid state that includes COPD and RDs (Rheumatoid Arthritis [RA], ankylosing spondylitis [AS], psoriatic arthritis [PsA], systemic lupus erythematosus [SLE], primary Sjogren syndrome disease [pSS], and systemic sclerosis [SSc]) and more specifically (i) whether COPD is more common in patients with an RD and, vice versa, and (ii) what implications this comorbidity pattern has on patient’ outcomes (mortality, hospitalizations, exacerbations), so that a different approach in daily clinical practice can be proposed.
Methods
Study design
A systematic literature review was performed and reported according to the preferred reporting items for systematic reviews and meta-analyses standardized reporting guidelines.17
Search strategy
A systematic literature search that combined keyword and MeSH terms regarding the diseases of interest was performed on April 7, 2018 across MEDLINE/PUBMED, for studies published from 1950 to April 7, 2018. The search algorithm was the following: (Chronic Obstructive Pulmonary Disease OR Chronic Bronchitis OR emphysema or airway obstruction) AND (Rheumatoid Arthritis OR Ankylosing Spondylitis OR Psoriatic Arthritis OR Arthritis OR Connective Tissue Disease OR Systemic Lupus Erythematosus OR Systemic Sclerosis OR Rheumatic* OR Sjogren Syndrome).
A software platform (abstrackr),18 http://abstrackr.cebm.brown.edu, was used featuring advanced term recognition within titles or abstracts and keyword search. The software has been shown to have high accuracy, similar to a human screener. Screening of abstracts/titles was not completely done by the software. Manual search was also used to increase accuracy.19
Articles were only included if they were in English language. Editorials, conference abstracts, and case reports were excluded. The screening of the titles and abstracts was performed by a single reviewer (IG). Reference lists of eligible papers and relevant review articles were also screened manually. The full texts of potentially relevant papers that were obtained for further evaluation were independently assessed by two reviewers (IG and IT). Similarly, the final studies that were included were decided by two reviewers (IG and IT). Any disagreements were resolved by consensus.
Criteria for study selection
Screening was performed to identify population-based studies (cohort, prospective or retrospective, cross-sectional, nested studies, or systematic reviews), investigating the comorbidity of COPD and RDs. Reports which studied adult patients with RDs/COPD were only included.
Data extraction
Two reviewers independently extracted data from each selected article. A randomly selected sample (approximately 10%) of the articles was extracted first in order to harmonize and refine the data extraction process. Data points were manually input into a predefined Microsoft Excel form. Risk of bias and methodological quality of the included studies was assessed using the RTI BANK criteria.20
Variables collected and outcomes
The following variables were collected: author details (name of first author and publication year), study characteristics—country, region, study design, settings, data collection period, disease (RA, AS, PA, SLE, pSS, and SSc), identification/case-finding method (i.e. ICD-10, established classification criteria), and main COPD or RD estimates for disease co-occurrence (incidence rate, prevalence, any adjustments that were performed [age groups, gender, other]), disease risk of development (hazard ratio [HR], odds ratio [OR]), implications on outcomes (exacerbations, mortality, hospitalization rates, emergency visits), and if there was smoking, or other risk factor adjustments.
Further meta-analysis was not conducted. The design of our study was a systematic review without a meta-analysis. In practical terms, there was difficulty in pooling our data since we examined many different RDs, with single or just a few studies relevant to each disease with various outcomes examined.
Results
Search results
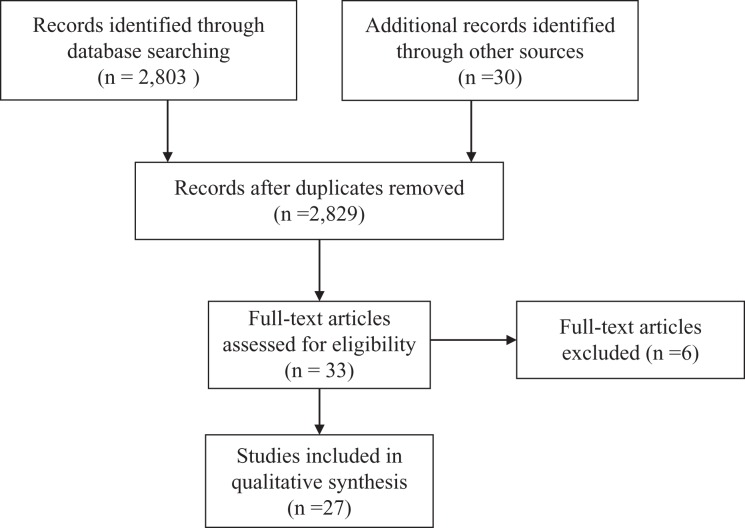
From 2,803 reports retrieved, 33 articles were relevant according to their titles and abstracts to be further screened. Finally, 27 articles were selected for inclusion after reading the full text and adding relevant articles from hand research (Figure 1).
Figure 1.
Flow diagram of search strategy and study selection.
RA and COPD
Increased incidence of COPD in patients with RA versus controls
Most of the articles we retrieved had studied RA (Table 1). A previous relevant systematic review and meta-analysis compared the risk of incident COPD in patients with RA (32,675 patients with RA and 122,204 controls), including four retrospective studies, up to November 2014.21–24 The estimated pooled risk ratio of incident COPD in patients with RA versus controls was 1.99 (95% confidence interval (CI): 1.61–2.45).25
Table 1.
COPD development in patients with inflammatory arthritis.
| First author | Publication year | Country | Design | Sample | Main outcomes |
|---|---|---|---|---|---|
| Sparks et al.26 | 2018 | United States | Prospective cohort | 843 women with RA versus 8,399 controls | Incidence of COPD in RA patients: 8.1% COPD risk in RA: HR 1.68 (95% CI: 1.36–2.07) smoking, BMI, dietary quality and physical activity adjusted |
| Ungprasert et al.25
|
2016 | Systematic meta-analysis21–24 | 32,675 patients with RA versus 122,204 controls | COPD risk in RA: RR 1.99 (95% CI: 1.61–2.45) | |
| Dougados et al.28 | 2013 | International | Cross-sectional | 3,920 patients with RA | COPD prevalence in RA: lower in Asia (Japan, 1.4%; Korea, 1.3%; Taiwan, 0.3%) than in Europe and the United States (Hungary, 8.0%; the United States, 7.5%) |
| Norton et al.27 | 2013 | United Kingdom | Inception cohort | 1,460 patients with RA versus general population | COPD annual incidence in RA: 0.4 (95% CI: 0.3–0.5); 15-year COPD cumulative incidence in RA: 5.0 (95% CI: 3.7–6.8); COPD prevalence in RA: 2.1% (95% CI: 1.4–2.9) |
| Bieber et al.29 | 2013 | Israel | Cross-sectional | 9,039 patients with RA versus 15,070 controls | COPD prevalence in RA: OR 1.98 (95% CI: 1.77–2.21, p = <001), adjustments: age, sex, socioeconomic status, smoking, obesity |
| Sharif et al.39 | 2018 | Israel | Cross-sectional | 4,076 patients with AS versus 20,290 controls | COPD in AS: OR 1.225 (p = 0 03) adjusted for age, gender, and smoking |
| Khraishi et al.38 | 2011 | Canada | Prospective cohort | 165 patients with PsA | COPD prevalence PsA: 11.5% (early PsA). 9.4% (established) |
| Hemminki et al.21 | 2011 | Sweden | Registry-based | 297,300 patients | Incidence of COPD in AS (SIR 1.72; 95% CI: 1.23–2.33) Incidence of COPD in RA (SIR 2.57; 95% CI: 2.21–2.97) |
COPD: chronic obstructive pulmonary disease; RA: rheumatoid arthritis; PsA: psoriatic arthritis; AS: angylosing spondylitis; RR: risk ratio; HR: hazard ratio; IRR: incidence rate ratio; SIR: standardized incidence ratio; BMI: body mass index; OR: odds ratio; CI: confidence interval.
A more recent prospective study by Sparks et al.26 was performed within the prospective Nurses’ Health Study (n = 121,701 women followed from 1976 to 2014, 843 of them with RA vs. 8,399 comparators). Their main finding was that COPD has developed in 8.1% of RA patients versus 5.5% of comparators. After adjustment for confounders, including smoking, RA was significantly associated with COPD (HR 1.68, 95% CI: 1.36–2.07). Further, a registry-based study from Sweden21 (n = 64,834 persons-years) also suggested that incidence of COPD in RA was increased (standardized incidence ratio [SIR]: 2.57 [95% CI: 2.21–2.97]) in comparison with controls.
Last, in a previous study by Norton et al. (n = 1,460 patients with RA diagnosed from 1986 to 1998), the rate of COPD was found increased only in males, as compared to the general population (standardized incidence ratio (SIR): 1.63; 95% CI: 1.17–2.26).27 The overall SIR was estimated to be 1.15 (95% CI: 0.84–1.45); thus there was a small but non-significant increase in the incidence of COPD in RA.27
Increased prevalence of COPD in patients with RA
We next focused on data regarding COPD prevalence in RA. COMORA (COMOrbidities in RA) is an international cross-sectional study (2011–2012) which found a 3.5% prevalence of COPD in RA patients.28 Interestingly, a range of estimates were observed across countries: In particular, COPD was less common in Asian countries RA cohorts (Japan: 1.4%; Korea: 1.3%; Taiwan: 0.3%) than in European countries or the United States (Hungary: 8.0%; United States: 7.5%).28 In the United Kingdom, the baseline prevalence of COPD in RA patients was estimated to be 2.1% with 95% CI (1.4–2.9).27 Additionally, Bieber et al.29 in a cross-sectional analysis using data from the largest healthcare provider organization in Israel (n = 9,039 RA cases vs. n = 15,070 controls) revealed that COPD was significantly higher in RA patients than controls (8.6 vs. 4.4%, p < 0.01, OR 2.06, 95% CI: 1.85–2.29). In the same report, the association of COPD prevalence in RA remained significant, after controlling for confounders, including age, sex, socioeconomic status, smoking, and obesity (adjusted OR 1.98, 95% CI: 1.77–2.21).29 Last, a recent study estimated the prevalence of COPD in patients with newly diagnosed cases with RA was 10.4%.30
The incidence of RA is not increased in patients diagnosed with COPD
Although, as described above, new cases of COPD are increased in patients already diagnosed with RA, the opposite trend has not been shown in two reports that we retrieved. Notably, a population-based study (time period 1974–1992, including 22,444 men and 10,902 women from Sweden) did not reveal a higher risk of RA, in patients with established COPD. More specifically, there was no association between mild COPD (stage I; OR 1.35, 95% CI: 0.68, 2.66) or moderate to very severe COPD (stages II–IV; OR 1.22, 95% CI: 0.67, 2.22) and subsequent development of RA.31
Similarly, a cross-sectional study, using data from the Korean National Health (2010–2012; 744 COPD cases and 3,313 controls), reported that the age-adjusted OR for RA development in COPD patients was 0.92 (95% CI: 0.40–2.12, p = 0.85), an estimation that corresponded to 1.13% of RA prevalence in COPD patients.32
Prevalence of RA in COPD
The prevalence of RA was estimated at baseline of International collaborative effort on chronic obstructive lung disease exacerbation risk index cohorts, a prospective study, which involved COPD patients aged ≥ 40 years (n = 409) from the Netherlands and Switzerland, with GOLD stages II–IV. In this group, RA prevalence was estimated as high as 4%33 but no comparison with the general population was provided, as the main objective of the study was different.
Sex differences in COPD-RA comorbidity
A national prospective study from Sweden,34 which included patients over 50 years who were starting long-term oxygen therapy for COPD (n = 8,712 patients; 55% women), found a double prevalence of RA in women (2%) versus men (1%). The corresponding adjusted OR (for age, smoking history, and year of starting long-term oxygen therapy) for women compared to men was estimated as high as 1.56 (95% CI: 1.10–2.20).34 On the contrary, a small cross-sectional study by Ekstrom et al.34 (70 RA patients of each sex included)24 reported that COPD in RA patients was less prevalent in women versus men (2.8% vs. 17.1%, respectively, p < 0.01), with no significant differences in current smokers among two genders, yet more “ever smokers” among men.34
Risk of adverse outcomes (mortality, hospital admissions, and exacerbations) of RA-COPD comorbidity
A recent study which included 31,333 patients with RA (10.4% of whom had a diagnosis of COPD) and 9,706 RA patients without COPD estimated that the mortality risk in RA patients with COPD versus RA patients without COPD was 4.5% and 1.5% within the first 6 months after RA diagnosis (adjusted hazard rate ratios (aHRRs) = 3.0, 95% CI: 2.3–3.9), and 59.3% and 39.8% within next 10 years (aHRR = 2.1, 95% CI: 1.9–2.1), adjusted with birth year, age at RA diagnosis, and gender (Table 3).30
Table 3.
Impact of the comorbidity COPD-RDs on patients outcomes.
| First author | Publication year | Country | Design | Sample | Main outcomes |
|---|---|---|---|---|---|
| Hyldgaard et al.30 | 2018 | Denmark | Population-based | 31,333 patients with RA (10.4% of them with COPD) versus 9,706 RA patients without COPD | Mortality risk in RA patients with COPD versus RA patients without COPD: aHRR = 3.0 (95% CI: 2.3–3.9) and aHRR = 2.1 (95% CI: 1.9–2.1) in 0–6 months and in first 10 years after RA diagnosis |
| McGuire et al.35 | 2017 | British Columbia | Retrospective cohort | 24,625 patients with RA versus 25,396 controls | Risk of COPD hospitalization in RA patients: IRR 1.47 (95% CI: 1.24–1.74) |
| Pieringer et al.36 | 2017 | Austria | Cross-sectional | 74 patients with RA versus 74 controls | Association of COPD/RA with ICU admission: OR 2.89 (95% CI: 1.10–7.54; p = 0.03) |
| Han and Han43 | 2017 | United States | Registry-based | 1,680 total visits with SLE | COPD leads to 6.9% of hospitalizations in male and 3.7% in female SLE patients >60 years |
| Nannini et al.22 | 2013 | Rochester, Minnesota | Population-based cohort | 594 patients with RA versus 596 controls | Worse survival in RA with COPD or asthma versus without respiratory comorbidities: HR 2.09 (95% CI: 1.47–2.97), adjusted for age, sex, smoking, and alcohol |
COPD: chronic obstructive pulmonary disease; SLE: systemic lupus erythematosus; RA: rheumatoid arthritis; aHRR: adjusted hazard rate ratio; HR: hazard ratio; ICU: intensive care unit; IRR: incidence rate ratio; OR: odds ratio; CI: confidence interval.
Further, a population-based study by McGuire et al.35 revealed that individuals with RA had a 47% greater risk of COPD hospitalization compared to the general population, after adjusting for potential confounders (incidence ratio 1.47; 95% CI: 1.24–1.74). Notably, the increased risk remained significant after adjustment for smoking and alternative COPD definitions.35 Additionally, a strong association of COPD presence was reported with intensive care unit (ICU) admissions in patients diagnosed with RA (OR 2.89, 95% CI: 1.10–7.54; p = 0.03).36 COPD coexistence was not found to be significantly associated with RA exacerbations.37 Last, the presence of COPD or asthma in patients with RA was significantly associated with worse survival, compared to RA without respiratory comorbidities, after adjustment for age, sex, smoking, and alcohol use (HR 2.09; 95% CI: 1.47–2.97) (Table 3).22
PsA and COPD
Increased prevalence of COPD in patients with PA
Regarding PA, a study by Khraishi et al.38 suggests that patients with early (less than 2 years from the diagnosis) and established PsA had significantly higher age- and gender-adjusted prevalence of COPD, as compared to the general population (COPD prevalence: 11.5%, 9.4%, and 9.7%, in early and established PsA and total cohort, respectively) (Table 1).
Ankylosing Spondylitis (AS) and COPD
Increased incidence of COPD in patients with AS
Our literature review included a study which analyzed data from the Swedish Hospital Discharge Register, comparing patients hospitalized for autoimmune disease (from 1964 to 1999) versus controls, with regard to COPD development.21 It was revealed that in patients with AS, COPD standardized incidence ratio (SIR) was 1.72 (95% CI: 1.23–2.33).21 Similarly, a report from Israel (n = 4,076 AS cases, n = 4,076 controls)39 suggested that AS was independently associated with COPD (OR 1.22, p = 0.03) after age, gender, and smoking adjustment (Table 1).
COPD and arthritis
Increased prevalence of unspecified, self-referred arthritis in COPD patients versus non-COPD patients
The present review retrieved a number of studies which examined the association of inflammatory arthritis (including collectively different types i.e. RA, PsA, or/and AS) and COPD. Characteristically, the prevalence of self-reported arthritis is 54.6% in patients with COPD versus 36.9% in patients without COPD (p < 0.001) in adults ≥45 years.40 Again, self-referred arthritis was more frequent in COPD than non-COPD controls (13% vs. 9%; p < 0.01) in adults >67 years.11
Finally, in a sample of patients from the United States, with mean age of 56.6 (±16.4) years, arthritis in COPD patients ranked the most common out of five age-adjusted comorbidities10 (6.49%; 95% CI: 6.42 6.55). Notwithstanding, arthritis remained an independent predictor of self-rated health in COPD patients (OR 1.69, 95% CI: 1.13–2.52) in a study using the 2001–2008 National Health and Nutrition Examination Survey data.41
SLE and COPD
Increased incidence of COPD in patients with SLE
A large population-based study from Taiwan (n = 10,623 patients with SLE) found that the overall incidence rate of COPD was 1.73-fold higher in SLE patients than controls (17.4 vs. 10.1 per 10,000 person-years, 95% CI: 1.62–1.84) corresponding to a sex- and age-adjusted HR (1.92, 95% CI: 1.50–2.44).42 Further analysis revealed that both sexes were equally susceptible in developing COPD (adjusted HR for female—2.10, 95% CI: 1.55–2.83 vs. for males—1.88, 95%: CI 1.24–2.86). Finally, increased incidence of COPD was reported in hospitalized patients diagnosed with lupus21 (SIR 2.29, 95% CI: 1.57–3.21).
Implications of COPD comorbidity in SLE patients: Increased hospitalizations and emergency visits
Interestingly, Han and Han reported that comorbid COPD in SLE patients was one of the most common causes of emergency department visits and hospitalizations.43 In particular, COPD accounted for 6.9% and 3.7% of hospitalizations in male and female of 60 and over years, respectively. COPD was also a leading cause of emergency room visits (6.3% in men over 60 years and 2.4% in women in the age group of 40–59 with SLE) (Table 3).43
pSS and COPD
Increased incidence of COPD in patients with pSS
Regarding the association of pSS with COPD, a study from Taiwan (n = 3,013 women with pSS vs. 12,052 controls) estimated that the adjusted HR for developing COPD in pSS was as high as 1.39 (95% CI: 1.10–1.75, p = 0.007).44 Notably, the incidence of COPD was higher in female over 50 years as compared with the age group of 20–49 (adjusted HR 4.24; 95% CI: 3.06–5.88, p = < 0.001) (Table 2).44
Table 2.
COPD development in patients with connective tissue diseases.
| First author | Publication year | Country | Design | Sample | Main outcomes |
|---|---|---|---|---|---|
| Shen et al.42 | 2014 | Taiwan | Retrospective cohort | 10,623 patients with SLE | Incidence of COPD in SLE: sex- and age-adjusted HR: 1.92, (95% CI: 1.50–2.44) |
| Strevens Bolmgren et al.45 | 2016 | Sweden | Cross-sectional | 51 patients with pSS versus 80 controls | COPD prevalence: 1.4-fold higher in pSS versus controls |
| Shen et al.44 | 2015 | Taiwan | Cohort | 3.013 female with pSS versus 12,052 controls | Incidence of COPD in pSS: aHRR of 1.39 (95% CI: 1.10–1.75, p = 0.007) |
| Nilsson et al.47 | 2015 | Sweden | Cross-sectional | 51 patients with pSS versus 186 controls | COPD prevalence: 41% of patients with pSS 30% of never-smoking patients with pSS |
| Mandl et al.46 | 2012 | Sweden | Cross-sectional | 41 female with pSS | COPD prevalence: 37% of patients with pSS,73% of ever-smoking patients with pSS and 15% in never-smokers |
| Hemminki et al.21 | 2011 | Sweden | Registry-based | Incidence of COPD in SLE (SIR 2.29, 95% CI: 1.57–3.21) Incidence of COPD in SS (SIR 1.89, 95% CI: 1.12–2.99) Incidence of COPD in SSc (3.08, 95% CI: 1.11–6.74) |
COPD: chronic obstructive pulmonary disease; SLE: systemic lupus erythematosus; SSc: systemic sclerosis; pSS: primary Sjogren syndrome disease; SIR: standardized incidence ratio; CI: confidence interval; aHRR; adjusted hazard rate ratio; HR: hazard ratio.
Finally, a Swedish study reported that in hospitalized patients with SS (8,661 persons-years), the standardized incidence rate for COPD was 1.89 (95% CI: 1.12–2.99).21
Increased prevalence of COPD in patients with pSS even in nonsmokers
Strevens Bolmgren et al. estimated that COPD prevalence was 1.4-fold higher in the pSS group than in the non-pSS group.45 COPD diagnosis was more prevalent in ever-smoking (73%) than in never-smoking pSS patients (15%) (Table 2).46 Nilsson et al.47 also reported that COPD was common in patients with pSS, even among never-smoking patients (30% of never-smoking patients with pSS had also COPD).
SSc and COPD
Increased incidence of COPD in patients with SSc
Last, an analysis from the Swedish Hospital Discharge Register controls revealed a triple increase in COPD development (standardized incidence ratio: 3.08, 95% CI: 1.11–6.74) in patients with SSc (Table 2).21
Discussion
Summary of findings
To the best of our knowledge, this is the first review to highlight the important association of the most common RDs and COPD. Among various RDs examined, the strongest evidence exists for patients, especially women, diagnosed with RA, who have a substantially elevated risk of developing COPD as compared to controls (mainly, patients with no-RDs). This comorbid state leads to more hospitalizations and ICU admissions and decreases survival, highlighting the need for early detection and management.
Additionally, all of the studies that included other categories of inflammatory arthritis, such as PA and angylosing spondylitis, report increased COPD prevalence and/or incidence. Similarly, connective tissue disorders, such as SLE, Sjogren disease, and SSc, that we included in our review present higher occurrence of COPD.
Possible mechanisms of increased COPD risk in RDs
Although there are a few possible explanations for the higher risk of COPD in patients with RDs, yet, there is no strong evidence whether the observed association is a causal one or it might result from confounding factors such as smoking, which is a well-established risk factor for both COPD and RDs development and course13,14,48 or others risk factors, such as diet and obesity.8 The main areas that research has been focused regarding shared mechanisms are the role of citrullination, autoimmunity, and systemic inflammation. In particular, ACPAs, a hallmark of RA, is consistently found increased in tobacco- or non tobacco-induced COPD.15,16 At this point, it is important to highlight that Sparks et al.8 reported that seropositivity (defined as positive rheumatoid factor or anti-cyclic citrullinated peptide antibodies) does not have a significant effect on COPD risk.8
It is also considered that chronic systemic inflammation or/and local respiratory lesions may predispose patients with RDs to COPD dysfunction47; thus, it can be hypothesized that in addition to cigarette smoke, RDs may be itself a determining factor for incidence of COPD and/or facilitate shortening of the time course for developing COPD.
To this end, as recent studies use more appropriate control measures for any confounding factors, the hypothesis that it maybe also RA itself8 (or other connective tissue diseases),47 that trigger COPD development, independently of smoking, is gaining ground. This is in accordance with findings supporting that there is possibly an autoimmune component of COPD with persistent airway inflammation even among never smokers.49,50
Strengths and limitations and comparison with the existing literature
One previous systematic review has already shown increased risk of COPD in RA patients, although there were methodological limitations of the studies included and, especially, the lack of smoking confounding control, in many of them. We updated the relevant literature, including more recent studies with better smoking adjustment, so that the message is more clear. Our review also examined the extent and implications of COPD-RDs and it is the first to include most of the RDs and not only RA. Although for the rest RDs the studies were less in number, the trend remains the same, thus there is an increased risk of developing COPD in patients with an RD. Nevertheless, and this is one limitation of our study, the individual studies regarding RDs’ development in patients already diagnosed with COPD were limited and no strong conclusion could be made. However, the finding that more than half of the patients with COPD complain about musculoskeletal problems/arthritis underlies the great need to identify such symptoms and whether they are part of a systematic RD or a non inflammatory one.
Implications for policy, clinical practice, and research
Putting the results into a broader context, COPD-RDs association should be seen under the concept of multimorbidity. Up to date, the majority of the current guidelines do not provide explicit guidance on treatment of patients with the specific disease combination.51
In overall, the implications of our work emerge from the unmet need to assess multimorbidity especially in primary care settings. Primary care physicians (PCPs) should enforce their clinical skills and try to incorporate into their practice a plan for early diagnosis of COPD, in patients with RDs. The use of structured questionnaires and micro-spirometry, especially for patients who smoke or are over 35 years old and present respiratory symptoms, would be of great value for a rigorous case finding.52 Notably, PCPs should enhance their clinical skills toward examination of “arthritis” or other relevant complaints in COPD patients to ensure the differential diagnosis of inflammatory arthritis/RD versus other musculoskeletal problems and adapt to a proper management plan. Importantly, in COPD/RD coexistence, PCPs and specialists should be aware of the possibility of worse long-term health outcomes and act promptly.
Undoubtedly, any non-pharmacological co-management plan of COPD-RDs should include smoking cessation53,54 which is extremely important for reducing symptoms and improving the long-term outcomes in both diseases.55,56 Further, although the role of PCPs is critical so that the use of fragmented health-care services and associated risks may be reduced,57 a multidisciplinary team is probably needed, as well as close collaboration with specialists (rheumatologists, pulmonologists) to avoid further complications and decrease the overall burden of diseases. Illustratively, one important part of the COPD management is the pulmonary rehabilitation,58 which is relevant also for rheumatic disorders; therefore, joint plans are strongly suggested toward individualized rehabilitation programs that will take patients’ comorbidities and tailored needs into consideration.53 For the future research agenda, RDs and COPD cohorts, with longitudinal follow-up, are needed to investigate the effects of the comorbidity on diseases activity/exacerbations and long-term respiratory or overall outcomes. Finally, more studies are required to unfold the exact mechanisms under these diseases’ coexistence and, of course, to investigate the best possible ways that this increased COPD risk should be addressed in patients with arthritis/connective tissue disease (and vice versa) in routine clinical settings.
Conclusion
In the present review, our main findings are that COPD is more common in all RDs that we studied (RA, PsA, ankylosing spondylitis, SLE, pSS, and SSc) as compared to controls with no RD or the general population. In case of RA, this comorbid pattern carries greater risk of mortality, hospitalizations, and emergency visits. On the other hand, arthritis is increased in COPD patients, although more evidence is needed to clarify the exact associations.
Footnotes
Authors’ contributions: Dr Irini Gergianaki performed the literature search, screening of the full manuscripts, data collection, and interpretation and wrote the manuscript. Dr Ioanna Tsiligianni conceived the idea of the study, performed the full manuscripts screening, supervised the review, and edited the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Le Reste JY, Nabbe P, Rivet C, et al. The European general practice research network presents the translations of its comprehensive definition of multimorbidity in family medicine in ten European languages. PloS one 2015; 10: e0115796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Catalá-López F, Alonso-Arroyo A, Page MJ, et al. Mapping of global scientific research in comorbidity and multimorbidity: a cross-sectional analysis. PloS one 2018; 13: e0189091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burkes RM, Donohue JF. An update on the global initiative for chronic obstructive lung disease 2017 guidelines with a focus on classification and management of stable COPD. Respir Care 2018; 63: 749–758. [DOI] [PubMed] [Google Scholar]
- 4. Baillet A, Gossec L, Carmona L, et al. Points to consider for reporting, screening for and preventing selected comorbidities in chronic inflammatory rheumatic diseases in daily practice: a EULAR initiative. Ann Rheum Dis 2016; 75: 965–973. [DOI] [PubMed] [Google Scholar]
- 5. Simoes D, Araujo FA, Monjardino T, et al. The population impact of rheumatic and musculoskeletal diseases in relation to other non-communicable disorders: comparing two estimation approaches. Rheumatol Int 2018; 38(5): 905–915. [DOI] [PubMed] [Google Scholar]
- 6. Nikiphorou E, Nurmohamed MT, Szekanecz Z. Editorial: comorbidity burden in rheumatic diseases. Front Med 2018; 5: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hyldgaard C, Ellingsen T, Bendstrup E. COPD: an overlooked cause of excess mortality in patients with rheumatoid arthritis. Lancet Respir Med 2018; 6(5): 326–327. [DOI] [PubMed] [Google Scholar]
- 8. Sparks JA, Chang SC, Liao KP, et al. Rheumatoid arthritis and mortality among women during 36 years of prospective follow-up: results from the Nurses’ Health Study. Arthrit Care Res 2016; 68: 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Putcha N, Drummond MB, Wise RA, et al. Comorbidities and chronic obstructive pulmonary disease: prevalence, influence on outcomes, and management. Sem Resp Crit Care M 2015; 36: 575–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamour A, David M, Kanotra S. Prevalence and comorbidities of chronic obstructive pulmonary disease among adults in Kentucky across gender and area development districts, 2011. Chronic Obstr Pulm Dis 2015; 2: 296–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Craig BM, Kraus CK, Chewning BA, et al. Quality of care for older adults with chronic obstructive pulmonary disease and asthma based on comparisons to practice guidelines and smoking status. BMC Health Serv Res 2008; 8: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daien CI, Tubery A, Beurai-Weber M, et al. Relevance and feasibility of a systematic screening of multimorbidities in patients with chronic inflammatory rheumatic diseases. Joint Bone Spine 2018. 10.1016/j.jbspin.2018.03.016. [DOI] [PubMed]
- 13. Hedstrom AK, Stawiarz L, Klareskog L, et al. Smoking and susceptibility to rheumatoid arthritis in a Swedish population-based case-control study. Eur J Epidemiol 2018; 33(4): 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barbhaiya M, Tedeschi SK, Lu B, et al. Cigarette smoking and the risk of systemic lupus erythematosus, overall and by anti-double stranded DNA antibody subtype, in the Nurses’ Health Study cohorts. Ann Rheum Dis 2017; 77(2): 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruiz-Esquide V, Gomara MJ, Peinado VI, et al. Anti-citrullinated peptide antibodies in the serum of heavy smokers without rheumatoid arthritis. A differential effect of chronic obstructive pulmonary disease? Clin Rheumatol 2012; 31: 1047–1050. [DOI] [PubMed] [Google Scholar]
- 16. Sigari N, Moghimi N, Shahraki FS, et al. Anti-cyclic citrullinated peptide (CCP) antibody in patients with wood-smoke-induced chronic obstructive pulmonary disease (COPD) without rheumatoid arthritis. Rheumatol Int 2015; 35: 85–91. [DOI] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wallace BC, Small K, Brodley CE, et al. Deploying an interactive machine learning system in an evidence-based practice center: abstrackr. In: Proceedings of the 2nd ACM SIGHIT international health informatics symposium, 28–30 January, 2012, Miami, Florida USA: ACM, 2012, pp. 819–824. [Google Scholar]
- 19. Rathbone J, Hoffmann T, Glasziou P. Faster title and abstract screening? Evaluating Abstrackr, a semi-automated online screening program for systematic reviewers. Syst Rev 2015; 4: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Margulis AV, Pladevall M, Riera-Guardia N, et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa Scale and the RTI item bank. Clin Epidemiol 2014; 6: 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hemminki K, Liu X, Ji J, et al. Subsequent COPD and lung cancer in patients with autoimmune disease. Eur Respir J 2011; 37: 463–465. [DOI] [PubMed] [Google Scholar]
- 22. Nannini C, Medina-Velasquez YF, Achenbach SJ, et al. Incidence and mortality of obstructive lung disease in rheumatoid arthritis: a population-based study. Arthrit care res 2013; 65: 1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ursum J, Nielen MMJ, Twisk JWR, et al. Increased risk for chronic comorbid disorders in patients with inflammatory arthritis: a population based study. BMC Fam Pract 2013; 14: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shen TC, Lin CL, Chen CH, et al. Increased risk of chronic obstructive pulmonary disease in patients with rheumatoid arthritis: a population-based cohort study. QJM: Monthly journal of the Association of Physicians 2014; 107: 537–543. [DOI] [PubMed] [Google Scholar]
- 25. Ungprasert P, Srivali N, Cheungpasitporn W, et al. Risk of incident chronic obstructive pulmonary disease in patients with rheumatoid arthritis: a systematic review and meta-analysis. Joint, Bone, Spine: Revue Du Rhumatisme 2016; 83: 290–294. [DOI] [PubMed] [Google Scholar]
- 26. Sparks JA, Lin TC, Camargo CA, Jr, et al. Rheumatoid arthritis and risk of chronic obstructive pulmonary disease or asthma among women: a marginal structural model analysis in the Nurses’ Health Study. Semin Arthritis Rheu 2018; 47: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Norton S, Koduri G, Nikiphorou E, et al. A study of baseline prevalence and cumulative incidence of comorbidity and extra-articular manifestations in RA and their impact on outcome. Rheumatology (Oxford, England) 2013; 52: 99–110. [DOI] [PubMed] [Google Scholar]
- 28. Dougados M, Soubrier M, Antunez A, et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis 2014; 73: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bieber V, Cohen AD, Freud T, et al. Autoimmune smoke and fire-coexisting rheumatoid arthritis and chronic obstructive pulmonary disease: a cross-sectional analysis. Immunol Res 2013; 56: 261–266. [DOI] [PubMed] [Google Scholar]
- 30. Hyldgaard C, Bendstrup E, Pedersen AB, et al. Increased mortality among patients with rheumatoid arthritis and COPD: a population-based study. Resp Med 2018; 140: 101–107. [DOI] [PubMed] [Google Scholar]
- 31. Bergstrom U, Jacobsson LT, Nilsson JA, et al. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford, England) 2011; 50: 2005–2013. [DOI] [PubMed] [Google Scholar]
- 32. Jo YS, Choi SM, Lee J, et al. The relationship between chronic obstructive pulmonary disease and comorbidities: a cross-sectional study using data from KNHANES 2010-2012. Resp Med 2015; 109: 96–104. [DOI] [PubMed] [Google Scholar]
- 33. Yu T, ter Riet G, Puhan MA, et al. Physical activity and risk of comorbidities in patients with chronic obstructive pulmonary disease: a cohort study. NPJ Prim Care Respir Med 2017; 27: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ekstrom MP, Jogreus C, Strom KE. Comorbidity and sex-related differences in mortality in oxygen-dependent chronic obstructive pulmonary disease. PloS one 2012; 7: e35806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McGuire K, Avina-Zubieta JA, Esdaile JM, et al. Risk of incident chronic obstructive pulmonary disease in rheumatoid arthritis: a population-based cohort study. Arthritis Care Res 2017. Epub ahead of print 19 October 2017. DOI: 10.1002/acr.23410 [DOI] [PubMed] [Google Scholar]
- 36. Pieringer H, Hintenberger R, Pohanka E, et al. RABBIT risk score and ICU admission due to infection in patients with rheumatoid arthritis. Clin Rheumatol 2017; 36: 2439–2445. [DOI] [PubMed] [Google Scholar]
- 37. Curtis JR, Greenberg JD, Harrold LR, et al. Influence of obesity, age, and comorbidities on the multi-biomarker disease activity test in rheumatoid arthritis. Semin Arthritis Rheu 2018; 47: 472–477. [DOI] [PubMed] [Google Scholar]
- 38. Khraishi M, MacDonald D, Rampakakis E, et al. Prevalence of patient-reported comorbidities in early and established psoriatic arthritis cohorts. Clin Rheumatol 2011; 30: 877–885. [DOI] [PubMed] [Google Scholar]
- 39. Sharif K, Watad A, Tiosano S, et al. The link between COPD and ankylosing spondylitis: a population based study. Eur J Intern Med 2018; 53: 62–65. [DOI] [PubMed] [Google Scholar]
- 40. Schnell K, Weiss CO, Lee T, et al. The prevalence of clinically-relevant comorbid conditions in patients with physician-diagnosed COPD: a cross-sectional study using data from NHANES 1999-2008. BMC Pulm Med 2012; 12: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Putcha N, Puhan MA, Hansel NN, et al. Impact of co-morbidities on self-rated health in self-reported COPD: an analysis of NHANES 2001–2008. Copd 2013; 10: 324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shen TC, Lin CL, Chen CH, et al. Increased risk of chronic obstructive pulmonary disease in patients with systemic lupus erythematosus: a population-based cohort study. PloS one 2014; 9: e91821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Han GM, Han XF. Comorbid conditions are associated with emergency department visits, hospitalizations, and medical charges of patients with systemic lupus erythematosus. Journal of Clinical Rheumatology: Practical Reports on Rheumatic and Musculoskeletal Diseases 2017; 23: 19–25. [DOI] [PubMed] [Google Scholar]
- 44. Shen TC, Wu BR, Chen HJ, et al. Risk of chronic obstructive pulmonary disease in female adults with primary Sjogren syndrome: a nationwide population-based cohort study. Medicine 2016; 95: e3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Strevens Bolmgren V, Olsson P, Wollmer P, et al. Respiratory symptoms are poor predictors of concomitant chronic obstructive pulmonary disease in patients with primary Sjogren’s syndrome. Rheumatol Int 2017; 37: 813–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mandl T, Diaz S, Ekberg O, et al. Frequent development of chronic obstructive pulmonary disease in primary SS—results of a longitudinal follow-up. Rheumatology 2012; 51: 941–946. [DOI] [PubMed] [Google Scholar]
- 47. Nilsson AM, Diaz S, Theander E, et al. Chronic obstructive pulmonary disease is common in never-smoking patients with primary Sjogren syndrome. J Rheumatol 2015; 42: 464–471. [DOI] [PubMed] [Google Scholar]
- 48. Nguyen UDT, Zhang Y, Lu N, et al. Smoking paradox in the development of psoriatic arthritis among patients with psoriasis: a population-based study. Ann Rheum Dis 2018; 77: 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lamprecht B, McBurnie MA, Vollmer WM, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest 2011; 139: 752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rutgers S, Postma D, ten H, et al. Ongoing airway inflammation in patients with COPD who do not currently smoke. Thorax 2000; 55: 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lugtenberg M, Burgers JS, Clancy C, et al. Current guidelines have limited applicability to patients with comorbid conditions: a systematic analysis of evidence-based guidelines. PloS one 2011; 6: e25987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Price DB, Tinkelman DG, Halbert RJ, et al. Symptom-based questionnaire for identifying COPD in smokers. Respiration; International Review of Thoracic Diseases 2006; 73: 285–295. [DOI] [PubMed] [Google Scholar]
- 53. Hillas G, Perlikos F, Tsiligianni I, et al. Managing comorbidities in COPD. Int J Chronic Obstr 2015; 10: 95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fragoso E, Andre S, Boleo-Tome JP, et al. Understanding COPD: a vision on phenotypes, comorbidities and treatment approach. Rev Port Pneumol 2016; 22: 101–111. [DOI] [PubMed] [Google Scholar]
- 55. Van Schayck OCP, Williams S, Barchilon V, et al. Treating tobacco dependence: guidance for primary care on life-saving interventions. Position statement of the IPCRG. NPJ Prim Care Respir Med 2017; 27: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Roelsgaard IK, Thomsen T, Ostergaard M, et al. The effect of an intensive smoking cessation intervention on disease activity in patients with rheumatoid arthritis: study protocol for a randomised controlled trial. Trials 2017; 18: 570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Walunas TL, Jackson KL, Chung AH, et al. Disease outcomes and care fragmentation among patients with systemic lupus erythematosus. Arthrit Care Res 2016; 69(9): 1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Spruit MA, Singh SJ, Garvey C, et al. An official American thoracic society/European respiratory society statement: key concepts and advances in pulmonary rehabilitation. Am J Resp Crit Care 2013; 188: e13–e64. [DOI] [PubMed] [Google Scholar]