Significance
We propose that variation in brain energy expenditure during childhood is an unexplored but important influence on obesity risk. This hypothesis is supported by evidence that the energy required by the developing brain decreases in later childhood as the rate of body weight gain is increasing. The hypothesis is further supported by findings of genetic and brain imaging research indicating a trade-off between the body mass index (BMI) and the volume of cortical and subcortical structures, and inverse associations between BMI and energetically costly executive cognitive functions. Efforts to quantify variability in brain energy use across children could inspire new educational strategies that increase brain energy demands and thereby reduce obesity risk.
Keywords: body composition, cerebral metabolic rate, childhood, neurology, energetics
Abstract
The causes of obesity are complex and multifactorial. We propose that one unconsidered but likely important factor is the energetic demand of brain development, which could constrain energy available for body growth and other functions, including fat deposition. Humans are leanest during early childhood and regain body fat in later childhood. Children reaching this adiposity rebound (AR) early are at risk for adult obesity. In aggregate data, the developing brain consumes a lifetime peak of 66% of resting energy expenditure in the years preceding the AR, and brain energy use is inversely related to body weight gain from infancy until puberty. Building on this finding, we hypothesize that individual variation in childhood brain energy expenditure will help explain variation in the timing of the AR and subsequent obesity risk. The idea that brain energetics constrain fat deposition is consistent with evidence that genes that elevate BMI are expressed in the brain and mediate a trade-off between the size of brain structures and BMI. Variability in energy expended on brain development and function could also help explain widely documented inverse relationships between the BMI and cognitive abilities. We estimate that variability in brain energetics could explain the weight differential separating children at the 50th and 70th BMI-for-age centiles immediately before the AR. Our model proposes a role for brain energetics as a driver of variation within a population’s BMI distribution and suggests that educational interventions that boost global brain energy use during childhood could help reduce the burden of obesity.
As rates of overweight and obesity continue to rise globally, the burden of these conditions among children has risen at an alarming rate (1). In 2016, it was estimated that more than 250 million children were overweight or obese worldwide, with the rate of increase most rapid in lower- and middle-income countries. Childhood obesity imposes social and emotional costs, while increasing the likelihood of being an obese adult who develops disorders that shorten healthy lifespan, including the metabolic syndrome encompassing hypertension, diabetes, and altered lipid profiles (2). The rising global burden of obesity and the severe mental and physical health impacts of these trends underscore the need for additional work aimed at clarifying the origins of excess weight gain during infancy and childhood.
One potential contributor that has not been considered in the nutrition and obesity literatures, but that we think could be important, is brain metabolism. The oft-quoted statistic is that the brain consumes 20% of the body’s daily energy expenditure despite accounting for only 2% of body mass. Although correct, this estimate is specific to adults. A recent study reported that the brain accounts for a lifetime peak of 66% of resting metabolic rate (RMR) or 43% of daily energy requirements (DER) at ∼5 y of age (3). This rate of energy use is two to three times higher than that of the adult brain. This study also reported evidence for a trade-off between brain energetics and the rate of weight gain throughout childhood: there is a close inverse linear relationship between brain metabolism and the rate of weight gain between infancy and puberty, with peak brain energetic demands corresponding with the age of slowest body weight gain, which is also known to be an age of low body fat stores (3). These findings thus suggest that the energy requirements of the developing brain constrain energy devoted to the body during childhood, including fat deposition, and are likely an important influence on the developmental timing of the adiposity rebound and, consequently, later obesity risk (4).
Here we explore possible links between the energetic costs of brain development and risk for excess weight gain, as a complement to the more widely appreciated role of conventional lifestyle factors like diet and physical activity. We begin by briefly reviewing the developmental pattern of body composition changes during childhood and show that the timing of the adiposity rebound roughly coincides with, and therefore may be partly driven by, developmental declines in the brain’s energy requirements. Following from this observation, we propose that factors that shift the developmental timing, magnitude, or duration of the peak in childhood brain energy use could alter age trends in energy balance and body composition. Second, because development of the prefrontal cortex (PFC) is an important contributor to the energy demand of the developing brain in early childhood, we review research that has identified deficits in executive function abilities as a common comorbidity of obesity, in childhood and across the lifespan, as further evidence in support of the concept that energy devoted to the brain constrains energy available for other bodily functions like fat deposition. Third, we review studies of brain structure and genetic evidence for pleiotropic trade-offs between the volume and surface area of a number of cortical and subcortical brain structures, on the one hand, and body fat deposition and the body mass index (BMI) on the other. Recent genome wide association studies (GWAS) predicting BMI identify a prominent role for genes associated with, and expressed in, the central nervous system, including genes associated with energetically costly processes like synaptic function, long-term potentiation, and neurotransmitter signaling (5). Finally, we quantify the potential impact that variability in cerebral metabolism could have on the body’s energy balance and body weight across development. Viewed together, available evidence converges on the idea that variation in the pattern and magnitude of the energy demand of the developing brain in early childhood will have direct effects on weight gain and obesity risk during childhood by impacting the body’s energy balance, thus likely helping explain individual variability in BMI within a population.
Developmental Changes in Body Composition and the Adiposity Rebound
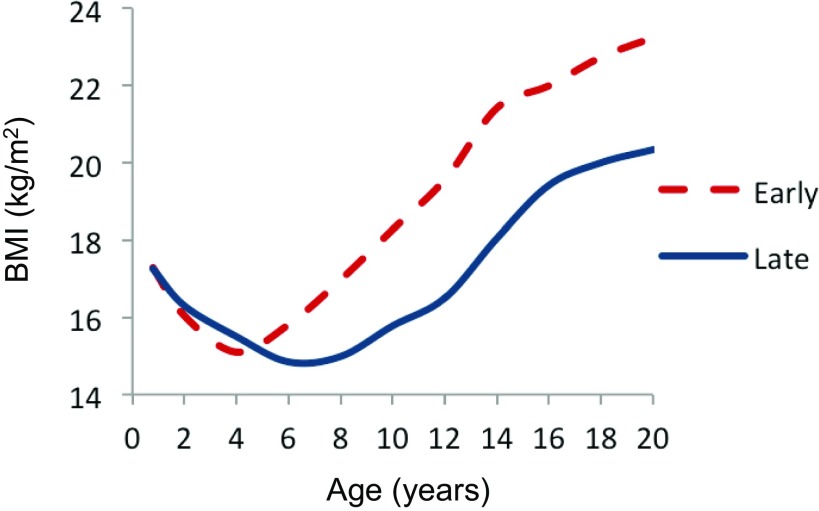
Clues into the underlying drivers of individual variation in childhood risk of overweight and obesity are revealed by the developmental timing of changes in body composition. Healthy infants are born with a large quantity of baby fat, and fat deposition accounts for the majority of the energetic costs of growth during the first 6–9 mo of life (6). The percentage of body weight that is fat usually starts to decline during infancy and eventually reaches a lifetime nadir by early childhood (we define “early childhood” as 3–8 y and with “childhood” ending at adolescence). In most populations, the mean BMI typically reaches its low point and starts to increase by 4–7 y of age (7–10), and the timing of this inflection point is called the adiposity rebound (AR). As shown in Fig. 1 for a French study, children who experience the inflection earlier, and who thus start regaining body weight at a younger age (the dashed line), tend to follow a higher BMI trajectory than their peers and are at increased risk of becoming overweight and obese later in childhood, adolescence, and adulthood (8, 10–12). Other studies report that early rebounders also tend to have a higher BMI, reflecting increased adiposity, at the age of the AR (10).
Fig. 1.
Developmental changes in BMI (kg/m2) related to age at adiposity rebound in a French study. Reprinted from ref. 96, licensed under CC BY-NC-ND 4.0.
Although most studies of the AR use the BMI as a proxy for adiposity, studies that measure body composition directly report variation in the composition of weight that is gained at the AR, with evidence for sex differences in the relative importance of lean and fat tissue (13). In perhaps the largest study of its kind, a German study used bioelectrical impedance to measure body composition in a cross-sectional sample of 3–11 y old children (n = 19,364). The researchers found that both BMI and fat mass experienced a developmental nadir and rebound, whereas lean mass increased linearly with age (14). However, fat mass rebounded 1.6–1.8 y after the rebound as indicated by the BMI. Thus, the common use of the BMI in studies of the AR may mischaracterize the exact age at which the rebound in adiposity occurs.
Children who experience positive centile crossing in weight- or BMI-for-age are likely to experience an early AR as a result, which is thought to help explain why the AR predicts later obesity risk (15). Thus, in a recent commentary, Rolland-Cachera and Cole characterize the AR as “…not itself a critical period, but research on factors responsible for an early AR should help understand the early mechanisms for the development of obesity” (3, 16). Research has demonstrated relationships between the timing of the AR and a range of conventional lifestyle and environmental factors, including physical activity (17), diet (18), and the mother’s prepregnancy BMI (19). This work has generally supported the idea that lifestyle factors during and before the AR, including the gestational environment, can influence the timing of this transition, with long-term impacts on body composition development. Here we suggest that individual variation in the brain’s energy requirements is likely another important factor with direct impacts on age changes in energy balance and body composition.
Early Attempts to Measure Brain Energetics across Development
The potential importance of the brain as an influence on energy balance during childhood is underscored by the organ’s high, and developmentally dynamic, energy costs. The majority of the brain’s energy expenditure is related to glucose metabolism for neuronal signaling, synapse formation, and information processing (20, 21). The energy requirements of neuronal activity are dominated by synaptic potentials and action potentials, which account for roughly half of the brain’s adenosine triphosphate (ATP) consumption, with postsynaptic receptors and the maintenance and restoration of resting potentials being substantial consumers of energy (22–24). Although most work on the energetic costs of the brain has focused on the adult brain, here we are specifically concerned with how those costs change across early development, when synaptic densities and other parameters shaping brain energy requirements reach their lifetime peak.
Several methods have been used to measure cerebral metabolic rate (CMR), reflecting the brain’s global metabolic expenditure. The first direct quantification of the energy costs of the human brain was conducted among adults in the mid-20th century using the nitrous oxide method (25). This method is invasive as it involves measuring the gradient of an inert gas (nitrous oxide, N2O) between the arteries and veins servicing the brain, which allows estimation of oxygen extraction by the brain. This method led to the frequently cited estimate that the brain consumes 20% of the body’s oxygen uptake at rest, which is a value far higher than in most other mammals, for which 2–4% is more typical (6).
It was widely noted by anthropologists interested in the evolutionary implications of human brain energetics that the brain is even more costly in a relative sense early in life, given the much higher brain-to-body size ratio, which is maximal at birth (26–28). Building from this assumption, it was proposed that the large energy requirements of human brain development might help explain why, during childhood, humans gain body weight at a rate that is 30–100 times slower than other nonprimate mammals of our size. This places human growth on the growth allometry of reptiles, which have very low energy expenditure as a result of being cold-blooded (28). This slow growth, in turn, is understood as helping explain other unusual features of the human lifecycle, including the extension of the preadult years to include the unique developmental stage of childhood, and the deferment of major growth costs until after brain metabolic requirements are reduced, leading to a particularly pronounced pubertal growth spurt in our species (29).
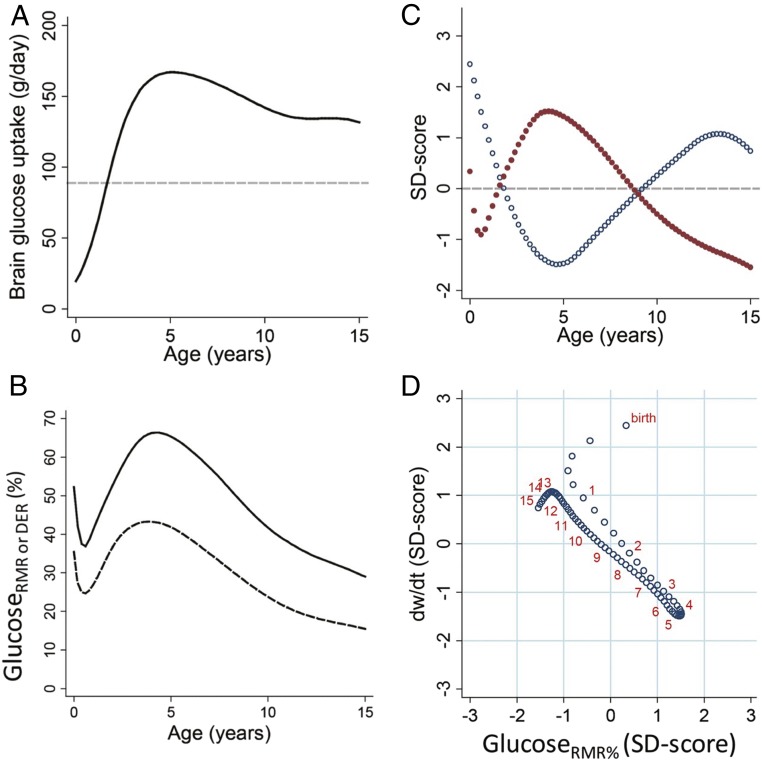
Efforts to characterize the evolutionary impacts of human brain development have been hampered by a lack of direct measures of the brain’s global energetic costs across human development. Although the N2O method has been used in children and yielded estimates of CMRO2 considerably higher than in adults (30), relatively few data points are available across development (five boys and four girls spanning 3–11 y of age), which were collected among a highly selected sample in which the refusal rate was also very high. Until recently, the one attempt to calculate developmental changes in brain energetics across different ages of human development assumed that brain metabolism was proportionate to brain mass during infancy and childhood, and estimated brain expenditure was divided by estimates of the body’s resting metabolic rate (RMR; e.g., kcal expended per day) at that age (31). The limitation with this approach is that it assumed that the per-gram metabolic expenditure of the brain is stable across early development, which direct measures of glucose uptake rates using positron emission tomography (FDG-PET) have shown is not the case (32). A recent study used a unique age series of PET data (32), along with MRI volumetric data, to estimate the total energy expenditure of the brain and how this changes developmentally (3). These estimates showed that the costs of the human brain reach a peak in both relative and absolute terms around 4–5 y of age (Fig. 2 A and B), when the brain accounts for 66.3% and 65.0% of RMR, in males and females, respectively, and ∼43% of DER in both sexes. Notably, this is an age when brain growth is nearly complete but when synaptic densities are at or near their lifetime maxima in PFC and other regions, as executive function abilities that support higher-order thinking, and that organize and regulate behavior, are developing (33).
Fig. 2.
Glucose use by age and trade-offs with body weight growth rate (all males): (A) daily grams of glucose used by the brain, (B) brain glucose use as a fraction of RMR (upper line) and daily energy requirements (lower line), (C) sample z scores of %RMR to the brain (red) and body weight velocity (blue) by age, and (D) body weight velocity vs. %RMR to the brain (both sample z scores) showing linear trade-off between the two. Weight velocity and glucose uptake values are predicted at intervals of one-fifth of a year, with red numbers marking each birthday for reference. Reprinted from ref. 3.
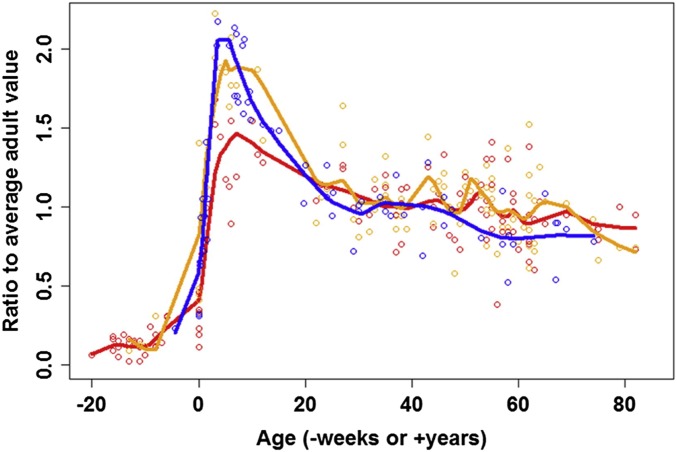
A similar developmental pattern of brain metabolism was recently replicated using data on total cerebral blood flow and aortic blood flow in individuals spanning early infancy to adulthood (34). The ratio of these two measures can be interpreted as reflecting the percentage of cardiac output destined for the brain and is thus a second metric reflecting the brain’s relative dominance of the body’s metabolism. This approach yielded a similar curve to the findings of the PET and MRI-based study and most notably confirmed that the brain reaches a peak in relative metabolism (accounting for ∼50% of total cardiac output) during early childhood, followed by a gradual decline to adult levels that are about one-third those of the childhood peak. Finally, another recent study compiled published data on glucose uptake rates, cerebral metabolic rate of oxygen, and cerebral blood flow by age and again demonstrated a similar childhood peak in brain energy requirements using all three measures (35).
Linking Brain Energetics, Weight Gain, and Body Composition
The studies described above use several methods to show that the brain does not consume the largest fraction of the body’s metabolism at birth, when relative brain size is largest, but in early childhood, when energetically costly processes of synapse proliferation and synapse elimination, and synaptic densities, are at lifetime peaks (36). This finding is of strong interest for research on obesity risk because it shows that the brain’s peak demand for energy coincides developmentally with the age of slowest weight gain, which also roughly corresponds with lifetime lowest body fat stores and the AR (9). Processes of synapse formation and elimination continue throughout childhood and into adolescence (37), however, and CMRglc is elevated relative to adult values from birth through adolescence, although at reduced levels relative to the childhood peak at approximately age 5 y (Fig. 3). The study of Kuzawa and colleagues (3), discussed above, explicitly addressed the links between brain energetics and weight gain and found strong evidence that brain energy use constrains the rate of weight gain (Fig. 2 C and D). The authors reported that the age of peak brain energetics is also the age of slowest weight gain and with a clear inverse linear relationship between the percent of RMR (%RMR) accounted for by the brain and weight velocity between infancy and puberty. The %RMR required for brain development is reduced in later childhood and adolescence, allowing for a larger proportion of the body’s total energy budget to be devoted to the body, including a more rapid pace of body growth and increased fat deposition.
Fig. 3.
CMRglc (blue), CMRO2 (red), and CBF (orange) plotted across the lifespan as normalized proportions of average adult values. Reprinted from ref. 35. Copyright (2014), with permission from Elsevier.
This finding confirmed the long-standing hypothesis posed by anthropologists that the unusually slow pace of body growth during human childhood evolved in part to help free up energy to subsidize the brain’s high energy costs (27–29). Both the extended postnatal period of brain development and the slow pace of physical growth are atypical aspects of development in our species (26–28) that this recent work shows are tightly linked. A recent paper by a separate group independently replicated a key finding from this analysis (38). In a large sample of 8–20 y olds, CBF was used to estimate %RMR to the brain, and a cross-sectional estimate of weight velocity was calculated. The authors found that age changes in average %RMR to the brain and average weight velocity were inversely related before puberty, consistent with decreasing brain energy use allowing an increase in growth rate in late childhood and early adolescence.
Hypothesis: Individual Variation in Brain Developmental Energetics Will Be Inversely Related to Individual Variation in Weight Gain and Obesity Risk
The foregoing findings have potentially important but as yet unexplored implications for understanding the rate and pattern of weight gain during infancy and childhood. Although there are abundant data on individual variability in weight gain and BMI, little is presently known about variability in the energy demand of the developing brain between children. Obesity is a complex condition in which factors like diet and physical activity are clearly implicated. However, several observations lead us to propose that variation in the energy demands of the developing brain is likely an important additional influence on individual patterns of weight gain and changes in BMI during childhood, which could thereby have long-term impacts on weight trajectories and risk for overweight and obesity.
First, and perhaps most obviously, the brain accounts for a large fraction of energy expenditure during childhood, whether measured at rest or in relation to total expenditure. Much of this increased expenditure is likely accounted for by processes of neurogenesis and synaptogenesis. Rapid increases followed by gradual decreases in cortical thickness and surface area in gray matter, and in the density of gray matter synapses, account for a large proportion of the high energetic cost of brain development. Increases in white matter in somatosensory, motor, and visual cortices in the first year, followed by more gradual myelination in frontal and temporal cortices, also contribute to the energy demand of the developing brain (39, 40). In these energy-intensive processes, glial cells likely play a substantial role in powering brain development. Astrocytes support synapse formation and elimination (41), neurovascular coupling to regulate energy supply to regions where neurons are active (24), and supply lactate to oligodendrocytes for myelin formation to establish white matter tracts (42). The role of glia in the brain’s energy requirements is highlighted by the finding that although white matter synapses require only one-third the energy of gray matter synapses, myelination results in no net energy savings in the brain given that the energy required to maintain resting potentials in oligodendrocytes offsets any energy savings associated with the greater efficiency of white matter neural transmission (43). Furthermore, astrocytes and oligodendrocytes primarily, but by no means exclusively, derive energy through glycolysis which is at a lifetime peak in early childhood (∼3–6 y; Fig. 3). In addition, glycolysis is associated with gene expression related to energetically costly synapse formation and growth, throughout the brain but particularly strongly in PFC where synaptic plasticity is maintained into adulthood (35). As such, factors that modify patterns of synaptic proliferation and elimination, the development of white matter tracts, and neurovascular coupling could alter the magnitude of the peak, along with its timing or duration, thereby impacting patterns of energy expenditure, energy balance, and fat deposition.
Second, and relatedly, the timing of the adiposity rebound—when the body begins depositing excess energy in fat deposits—does appear to roughly coincide developmentally with the age of decreasing brain energy demand. As indicated in Fig. 2, the Kuzawa et al. (3) paper shows clearly that in the pooled data used in that analysis, the average rate of weight gain is increasing at ages when brain developmental energetics are on the decline. Further, we know that the growth dynamics around the AR include a rebounding of fat deposition (14). Thus, children’s bodies begin to accrue excess fat at an age when the brain’s energy requirements are decreasing with development. Given that fat tissue is literally stored energy, we feel it unlikely that this correspondence is a coincidence.
The above considerations suggest that any changes in brain developmental energetics—whether in peak energy demands or their developmental timing—could impact energy balance and, thereby, the rate, composition, and timing of weight gain. The absolute magnitude of expenditure could be important, as could the timing: if children experience the peak early or late, this presumably reflects age-specific patterns of metabolic expenditure and, thereby, the likelihood that there are excess calories to be laid down as fat. The changing energy demand of the brain in early childhood might vary in (i) the timing of onset, (ii) the rate (slope) of rise, (iii) the magnitude of the peak, (iv) the duration of the peak, and (v) rate of the decline.
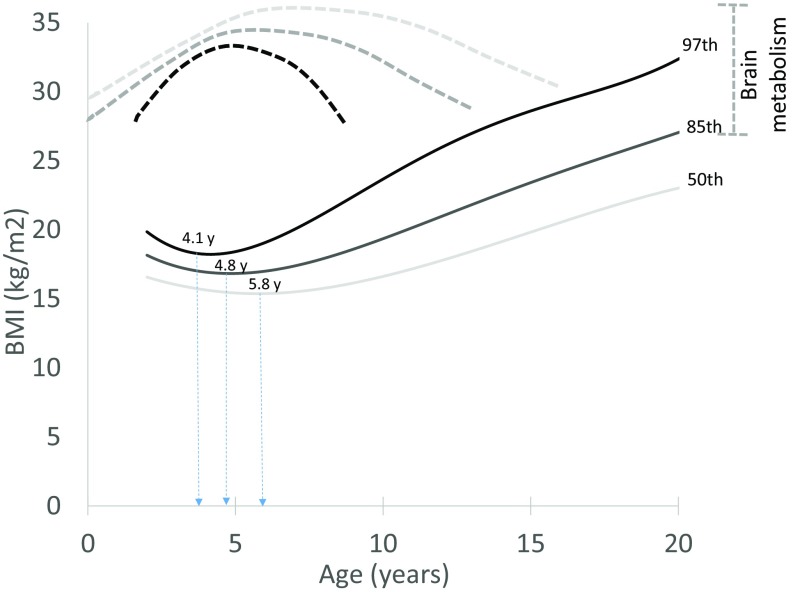
Fig. 4 plots BMI-for-age growth curves (CDC) for individuals at the 97th, 85th, and 50th percentiles (44). Above the chart, we overlay speculative brain energy use curves that we hypothesize correspond with, and could help explain, this population variation in BMI trajectories. It is important to acknowledge that the brain is only one potential influence on energy balance and body composition change, with variation in factors like diet and physical activity important influences on the balance between intake and expenditure. This caveat aside, we hypothesize that, holding other influences on BMI equal, the child with lower peak brain energy demand in early childhood, or for whom brain energy demand peaks earlier or is of shorter duration, will experience an earlier AR and thus be at a higher BMI for age relative to a child with greater peak brain energy demand or for whom brain energy demand peaks later and is of longer duration.
Fig. 4.
The hypothesized role of variation in brain developmental energetics as an influence on the timing and pattern of body fat gain during childhood. Solid lines show age percentiles for BMI (CDC), with the age of adiposity rebound noted for each. Dotted lines show the patterns of brain developmental energetics that are hypothesized to correspond with each BMI growth pattern.
It is important to emphasize that essentially no data are available to address the hypothesis graphically depicted in Fig. 4. Such data could readily be acquired using several noninvasive proxies (CBF and MRI-based CMRO2, discussed below), but measurement of variability in brain energy demand has not been a scientific priority, possibly owing to an assumption that this variability is unrelated to other aspects of child development. Despite a lack of direct measures of variability in brain energy use, several literatures, reviewed below, are independently converging on evidence consistent with the hypothesis that individuals with higher brain energy expenditure are lighter, as reflected in a lower BMI. These findings include evidence for inverse relationships between the BMI and cognitive functions and between the BMI and the size of anatomic structures in the brain and, finally, a growing literature on the genetic architecture of obesity that is revealing widespread pleiotropically mediated trade-offs between the size of brain structures and the BMI.
Evidence Linking BMI and Obesity with Cognitive Development and Executive Function Abilities
Because brain development is itself energetically costly, measures of cognitive development during childhood, and of cognitive function in adults, provide indirect proxies for brain energy use. Normatively speaking, changes in cognitive ability, primarily the development of executive functions, the emergence of which coincide with steep increases in brain energy demand and the timing of the adiposity rebound, represent a key developmental transition that marks the end of early childhood before typical school entry at age 6 y (45). By the age of ∼5–7 y, children are typically capable of regulating behavior and reason and to think abstractly in ways that allow for engagement in formal learning activities (46). The area of the brain primarily associated with these changes in cognitive ability—the PFC—is characterized by high energy demand during the child’s first 4–6 y. A period of synaptic proliferation in early childhood is followed by synaptic pruning that is particularly rapid during late childhood but continues into the third decade of life (47, 48). Available data suggest that peak synaptic spine density in pyramidal neurons in layers III and V of PFC is reached at ∼5 y of age (47), thus corresponding with the peak in brain energetics. Given that white matter has few synapses which collectively account for a small fraction of the energy required by gray matter synapses (22, 43), the timing of peak gray matter thickness during childhood is one factor that likely helps explain why the brain is more energetically costly in early childhood than at any other time in the lifespan.
Because the temporary spike in energy-intensive synaptic and related processes largely accounts for the childhood brain energetics peak, individual variation in those developmental processes might serve as a proxy for brain energy use across children. In this light, it is notable that a recent metaanalysis (49) as well as systematic reviews of the literature comparing cognitive abilities of obese versus healthy weight individuals (50, 51) indicates deficits in learning and memory and executive function in children and adults in a majority of studies across a variety of assessments. Overall, a recent metaanalysis (49) found deficits in performance of moderate effect size (0.33–0.44, e.g., explaining 10–20% of variance) in obese vs. normal weight comparisons for each aspect of executive function examined, including inhibitory control, working memory, cognitive flexibility, decision making, verbal fluency, and planning. However, relatively few studies included child participants exclusively, and many used BMI as the sole indicator of body composition. Notably, one of the methodologically stronger studies in the metaanalysis that included child participants found that BMI and fat mass measured by dual-energy X-ray absorptiometry (DXA) were both inversely related to measures of executive function and academic achievement in a sample of 126 children spanning 7–9 y of age (52). Further, a follow-up to this study with a larger sample (n = 233) of 7–9 y olds, also using DXA, found that whole body adiposity was negatively related to executive function even when controlling for aerobic fitness relative to fat-free mass (53).
As perhaps even more direct evidence for an energetic trade-off between cognitive development and body fat gain, follow-ups of two longitudinal samples of very young children have demonstrated that deficits in executive function and the ability to delay gratification are associated with an earlier timing and steeper trajectory of adiposity increase in childhood. In one sample (n = 1,061), children exhibiting difficulty with delay of gratification tasks at ages 3 and 5 had a steeper trajectory of BMI increase between 3 and 12 y of age (54). In a second longitudinal study (n = 195), children exhibiting difficulty with self-regulation at age 2 had higher BMI at age 10 and were more likely to be obese (55).
Conventional Explanations Cannot Fully Account for BMI-Cognitive Function Relationships
Traditional explanations for findings of executive function deficits in relation to higher BMI have focused on several pathways and consider the potential for bidirectional links between the brain and excess body fat. One straightforward explanation is that children with reduced delay of gratification ability and poor impulse control are more likely to consume high-fat foods when available to them. A more complex association is likely, however, as excess body fat deposition, whether diet-induced or otherwise, can result in low-grade inflammation and alter production of hormones that have reciprocal impacts on neuronal structure and function, potentially further eroding appetite control. For instance, a number of metabolic and immunological changes precipitated by excess adiposity, or the often correlated behavior of high fat intake, have deleterious impacts on neuronal function (56, 57). Adipocytes produce proinflammatory cytokines and other factors that stimulate systemic as well as central inflammation (58). One well-studied target of central inflammation is the hypothalamus (59), which plays a key role in regulating hormones (60), eating behavior, and executive functions (61, 62); as such, hypothalamic damage can lead to further weight gain.
The behavioral effects of impaired cognitive and executive function, and the reciprocal effects of excess weight gain on the brain, almost certainly help explain why measures of executive functions tend to decline with increasing BMI. However, many studies have now reported inverse relationships between the BMI and brain or executive function that are not contingent upon obesity and its metabolic sequelae. For instance, a particularly compelling PET study of 21 healthy adults found that resting glucose metabolism in frontal cortex was positively correlated with performance on multiple measures of executive function but negatively correlated with BMI (63). Importantly, only three study participants were obese, and the relationship was present across the full range of BMI. Another example in adults is seen in a study in which cortical thickness in superior frontal gyrus was inversely related to BMI, and higher BMI and reduced frontal cortical thickness were associated with poor inhibitory control (64). A third example is seen in a large study (n = 521) with older adults (60–80 y) in which BMI in the normal range was associated with lower default mode functional connectivity in posterior cingulate cortex and precuneus and lower performance on an executive function battery (65).
Neuroimaging Evidence That Decreased Size of Brain Structures Is Associated with Higher BMI
A second and more direct line of evidence that reduced expenditure on the brain is associated with excess weight comes from MRI-based studies of brain structure. A growing neuroimaging literature documents reductions in energetically costly aspects of the brain, notably including regional volumes and the proportion of the brain that is gray matter, across the full range of BMI in healthy adults, adolescents, and children (66–69). Reduced gray matter volume in relation to BMI in children is consistently observed in frontal cortex, but associations have been demonstrated in most cortical regions and in whole brain gray matter volume as well as the volume of subcortical structures. These findings are generally consistent with neuroimaging studies comparing obese vs. normal weight controls in which reductions in cortical thickness and surface area are found to be specific to orbitofrontal cortex, anterior cingulate cortex, and hippocampus and correlated with performance on measures of executive function abilities (70–72). Two studies that included large independent samples for internal replication examined variation in brain structure as a function of variation in BMI within the typical range (73, 74). These studies indicated incremental reductions in brain structure with incremental increases in BMI, including inverse associations of prefrontal gray matter volume in orbital frontal cortex (73) and reduced white matter integrity with BMI (74). These observations were not limited to overweight or obese individuals or to individuals suffering from the metabolic dysregulations that often accompany obesity (the metabolic syndrome).
Evidence for Genetic Pleiotropy Linking Reduced Size of Brain Structures to the BMI
Related work is showing that inverse relationships between the brain and the BMI have at least a partial genetic basis, with genes mediating pleiotropic trade-offs between the size of brain structures and body weight or fat deposition. Genome-wide association studies (GWAS) identify the genetic architecture of complex traits, like BMI, by measuring single nucleotide polymorphisms (SNPs) sampled across the genome in large human samples. These studies have identified a growing list of gene variants associated with excess body weight in humans. Building on findings from a prior smaller analysis (75), a recent metaanalysis of the largest sample analyzed up to that time (n = 339,224) indicated a dominant role for genes associated with central nervous system (CNS) function and neuronal development, as much as or more so than genes associated with energy homeostasis and the regulation of appetite (5). The authors capitalized on the large sample to identify biological pathways and mechanisms through which identified genes might affect BMI. Using a variety of approaches to identify cell types and tissues in which genes near SNPs associated with BMI are highly expressed, this analysis found converging evidence indicating that BMI-associated genes are largely expressed in the CNS. Using publicly available gene expression microarray data, enrichment was observed in brain areas associated with appetite regulation, hypothalamus and pituitary, but was even stronger in brain areas associated with learning and memory, the hippocampus and limbic structures. Further, by examining BMI-associated variants with five regulatory marks found in most of the selected cell types, strongest enrichment was found in midfrontal lobe, anterior caudate, astrocytes, and substantia nigra. Second, using predefined gene sets reconstituted from coexpression data, enriched gene sets were identified relating to synaptic function, long-term potentiation, and neurotransmitter signaling, glutamate most prominently but also monoamines and GABA. Finally, by conducting manual review of all 405 genes within 500 kb of BMI-associated SNPs and identifying biological categories associated with those genes, this analysis found that the largest category comprised genes involved in neuronal processes, including genes involved in hypothalamic function and energy homeostasis and also neuronal transmission and development.
The authors hypothesize that these findings reflect the importance of functions like neuronal plasticity to obesity-relevant behaviors like impulse control and appetite regulation (5). Although by no means mutually exclusive, we hypothesize that there are also direct effects of these polymorphisms on obesity risk operating through their impact on energetically costly neuronal processes, which in turn will moderate the brain’s contribution to the body’s energy use. It is particularly notable that the list of significant identified genes included many involved with energetically costly synaptic processes that are thought to account for a sizeable fraction of cerebral metabolic rate, including those that drive the large childhood increase in cerebral metabolism over that of the adult that coincides with the nadir in childhood weight gain and that precedes onset of the adiposity rebound.
Indeed, the analysis (73) that identified an association between reduced gray matter volume in PFC and BMI in two large independent samples, discussed above, also found that the polygenic risk score for obesity, based upon the Locke et al. (5) GWAS metaanalysis, was strongly positively related to BMI, as expected, but equally strongly and inversely related to prefrontal gray matter volume, thus providing evidence of individual variation in a genetically mediated trade-off between cortical volume in PFC and BMI. Another study reported that each 1 kg/m2 increase in the BMI predicted a 1–1.5% volume reduction throughout the frontal, temporal, parietal, and occipital lobes, with additional reductions in subcortical structures like the brainstem and cerebellum (76). This analysis also found that carriers of a common obesity-risk polymorphism at the FTO gene were heavier but also had 8 and 12% volume reductions, respectively, in the frontal and occipital lobes.
Further evidence for genetic pleiotropy linking increased BMI with reduced brain structures comes from a large family-based Mexican American study (n = 839) of adults that identified negative genetic correlations between BMI and the size of structures throughout the brain (77). Specifically, the authors found evidence for negative genetic correlations between the BMI and cortical surface area in parietal (inferior parietal and supramarginal gyrus), temporal (middle temporal gyrus), occipital (cuneus), and frontal cortex (orbitofrontal, middle frontal, and inferior frontal). BMI was also inversely correlated with the volumes of subcortical structures including the accumbens area and ventral diencephalon.
Summary of Existing Evidence.
Although no study to our knowledge has quantified variability in global brain energy use across individuals, three independent literatures are revealing inverse relationships between the BMI and measures that are plausible correlates of brain energy use. Inverse relationships between cognitive functions and the BMI are present across the full BMI distribution, and also among young kids, showing that metabolic derangements like inflammation, although potentially important among the obese, need not be present for these relationships to exist. In both children and adults, neuroimaging studies similarly show that the BMI across the normal range is inversely related to the size of anatomical structures throughout the brain and not limited to regions that regulate appetite-relevant behaviors. Finally, work on the genetic architecture of obesity is showing that obesity-promoting genes are overwhelmingly expressed in the brain, while neuroimaging studies are documenting negative genetic correlations between the size or volume of brain structures and the BMI. Collectively, these findings show that the BMI tends to be higher among individuals with measures consistent with reduced brain energy expenditure. If such effects do in fact reflect at least a partial energetic component, as we propose, it remains uncertain what magnitude of effect this might plausibly have on body composition. We now return to our model to consider the potential quantitative effects of individual variation in developmental brain energetics on body weight and the BMI.
How Large of an Effect Might Individual Differences in Brain Energetics Have on Weight Gain and Obesity Risk?
Weight gain includes both fat mass and fat free mass, and the latter contributes to an increase in total energy expenditure. As such, as rising dietary intake leads to an increase in body weight, the impact of the increment in lean mass on energy expenditure must be taken into account when calculating energy balance. In addition, as the body’s energy flux increases, the increase in available energy will be divvied across many (perhaps all, to varying degrees) of the body’s systems and functions, such as immunity or reproduction, and not just to weight gain or fat deposition. As a result, only a fraction of each kilocalorie of energy saved through reduced brain expenditure will be deposited as fat. The method of Swinburn et al. (78) links variation in measured body weight to measured variation in total energy expenditure (TEE) and thus allows estimation of the net impact that a sustained change in intake or expenditure will have on a new stable body weight or settling point. Using information on total energy expenditure and energy intake in a sampling of population studies of children, they estimate that, holding age, height, and sex constant, a population of children that increases its energy flux (intake in excess of expenditure) by 10% will stabilize on a body weight that is 4.5% heavier. Here we use this method to estimate the potential impacts of individual variation in brain metabolism on body weight and BMI by age.
As discussed above, the brain accounts for 66% of RMR at 4 y of age, when it is estimated to consume more than 500 kcal each day. This large level of expenditure underscores the potential for variability in brain energetics to have important impacts on individual differences in energy balance and body weight in the years that lead up to the adiposity rebound. Estimating the magnitude of this effect is unfortunately hindered by the extreme paucity of published information on variability in cerebral metabolic rate across children of the same age. Such variability will result from differences in both the size of the brain or specific brain structures and differences in the rate of energy use per unit volume or mass of that tissue. While little is known about variability in global brain energetics during development, there clearly is substantial variability in brain volume and mass and in the size and thickness of specific cortical structures by age (79, 80). PET data illustrate individual variation in the rate of glucose uptake per unit of tissue mass, which will also contribute to variability in brain energetics that is independent of brain size. As one illustration from the data of Chugani and colleagues (32), the per-gram rate of glucose uptake in the cerebrum of two children with very similar ages (∼7 y old) was found to be 40.6 and 51.6, the latter reflecting a 27% higher rate of glucose consumption per gram of tissue among individuals of the same age. Estimates of the percentage of total cardiac output accounted for by the brain also illustrate interindividual variability in age-specific cerebral metabolism (34): of three individuals measured at roughly 3 y of age (all female), the highest value for TCBF/AAo, which reflects the fraction of cardiac output used by the brain, is nearly 40% higher than that of the lowest (0.55 vs. 0.4). Although the paucity of currently available data must be emphasized, the few data that are available point to potentially significant and biologically important variability in the degree to which brain energetics might dominate the body’s energy budget during childhood.
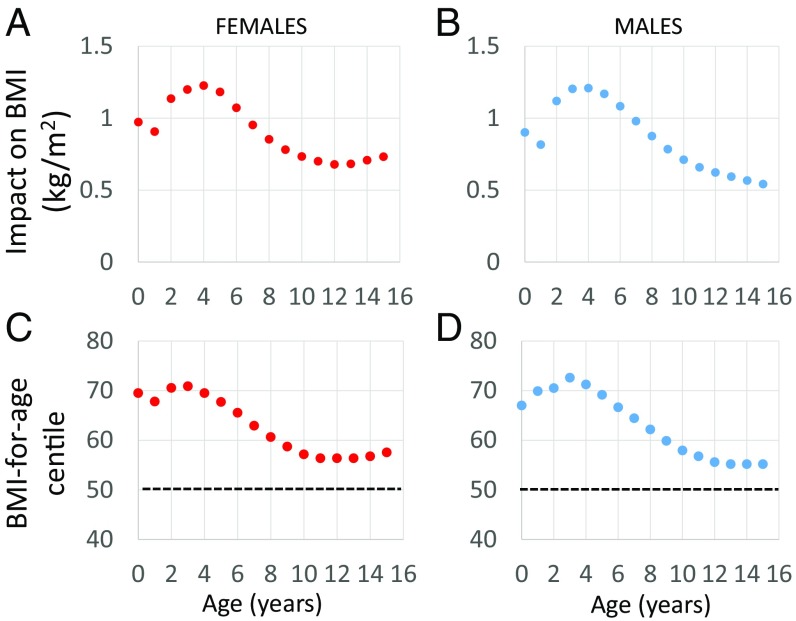
Fig. 5 uses data on daily energy requirements, brain energy use, and body weights in humans (ref. 3, supplemental information), and the method of Swinburn et al. (78), to estimate the effect on body weight of moving from 20% above to 20% below the mean (a 33% reduction) for brain energetics for that age. We assume that the energy savings of moving from high to low brain expenditure is the caloric equivalent of becoming more sedentary. Fig. 5 A and B show that this 33% change in brain energetics would add about 1.2 BMI (kg/m2) units at the peak in brain energy demand at 3–4 y of age. That is, relative to the child whose brain energy requirement is 20% above the mean, the child whose brain energy requirement is 20% below the mean will have meaningfully higher BMI, holding other factors like diet and activity constant.
Fig. 5.
The predicted impact on BMI of moving from 20% above the mean for brain energy use to 20% below the mean for brain energy use (a reduction of 33%). Median height and weight for age (CDC) were used to estimate the BMI. An estimate of additional weight gained as a result of energy saved from reduced brain expenditure was calculated using the method of Swinburn et al. (78), from which a second BMI was calculated. The plotted values are the differences between the two BMI estimates in (A) females and (B) males. The BMI centile predicted after that level of energetic savings in (C) females and (D) males (dotted lines reflect the starting point of the 50th centile).
Although this effect may appear modest, the age of the adiposity rebound is also a point in the lifecycle when population variability in BMI is attenuated. Thus, a 1.2 unit change in BMI would be the rough equivalent of moving between the 50th and 70th centile for BMI at that age (Fig. 5 C and D). The age of maximal effect of brain metabolism on weight status occurs in the years immediately preceding the adiposity rebound, when one’s BMI centile is predictive of future obesity risk. To the extent that any effects of childhood brain metabolism on BMI population centile are stable and track into adulthood, these estimates point to potentially large impacts of the energy demand of the developing brain on long-term body weight trajectories.
Discussion: Policy Implications of Links between Brain Energetics and Fat Deposition
We have argued that developmental changes in body composition during infancy and childhood, and the nadir in body fat stores called the adiposity rebound, are very likely a partial result of the shifting dominance of the brain’s energy use to the body’s energy expenditure, which will influence energy available for other bodily functions like fat deposition. Recent work showing that average developmental changes in brain energy use are tightly, inversely linked with normative changes in the rate of childhood weight gain suggests that individual variation in the energy demand of the developing brain likely accounts for some proportion of concurrent changes in body fat deposition. In support of this idea, we reviewed evidence indicating that the energetic cost of brain development in childhood, starting in late infancy, is high and that these costs covary with developmental changes in the rate of weight gain (9, 14, 26). We also reviewed evidence that BMI in the normal range is inversely related to a range of cognitive functions, including but not limited to executive functions, and also with surface area and/or thickness in multiple cortical and subcortical regions. Evidence is emerging for a strong link between the genetic architecture of BMI and of brain development as seen in a suite of neuronal processes that are energetically costly, such as those governing the biology of synapse formation and neuronal transmission (10, 26). There is now substantial evidence for pleiotropically mediated trade-offs between the BMI and the thickness or volume of structures throughout the cerebral cortex and other brain regions, pointing to the potential for direct genetically mediated energetic trade-offs between the brain and fat deposition (40, 41).
Without question, nonenergetic, and potentially reciprocal, links between excess body fat and cognitive deficits likely play a role in these relationships. Poor inhibitory control and problems with gratification delay can contribute to poor appetite regulation and excess fat intake, which in turn can lead to weight gain, inflammation, and other factors that can further impact brain structure and cognitive function. Despite these pathways, small but meaningful relations among BMI, brain structure, and cognition are present in infancy and early childhood, before the emergence of metabolic dysregulation, and among adults within the normal BMI range, suggesting that a direct energetic role may also be operative. We estimate that plausible levels of variability in brain energy expenditure could lead to the weight gain equivalent of moving a child from the 50th to the 70th BMI-for-age centile in the years immediately preceding the adiposity rebound. Although the potential impact of the brain’s metabolism on fat deposition is greatest during childhood, the logic of our model also applies to adults, albeit likely in an attenuated fashion owing to the adult brain’s smaller relative contribution to the body’s energy expenditure.
Obesity is a complex and multifactorial disease, and we do not see our model as in any way competing with the focus on conventional lifestyle factors like diet or physical activity or on more recently identified influences like the gut microbiome. Indeed, the secular trend toward rising rates of overweight and obesity very likely relate to trends in intake and/or expenditure (81). Adding to this existing complexity, the brain is not only an important contributor to variation in energy expenditure in childhood, but variation in these costs could make nontrivial contributions to population variation in body weight at an age when BMI is predictive of one’s future trajectory of adult overweight and obesity risk. As the societal BMI distribution shifts with lifestyle change, where individuals lie within that distribution will determine who reaches high disease risk cut points earlier. It is here—in explaining variation within a population’s BMI distribution, rather than societal BMI trends through time—that we feel variation in brain energetics could be most important. As discussed above, a growing literature points to reciprocal effects linking cognitive decline and weight gain. Because decreased expenditure on cognitive function involves an energetic savings to the body, thus likely enabling additional weight gain, we view energetic trade-offs between cognitive expenditure and fat deposition as potentially reinforcing the effects of these conventional pathways.
The science that we review hints at novel intervention approaches that could be explored with the intent of reducing the public health burden of overweight and obesity. On the one hand, the strong genetic linkages between neuronal function and obesity, and the pleiotropic trade-offs between these traits, could be interpreted as evidence that important components of obesity risk are inherited at birth and not likely to change. We feel that such an interpretation is not correct, because most genes, and especially those related to, e.g., neuronal and glial cell biology, have phenotypic effects that are contingent upon experience (phenotypic plasticity; refs. 82 and 83). When the phenotypic effect of a gene is contingent upon experience, changes in genes or experiences can lead to comparable phenotypic changes (83). Genetic research shows not only that many obesity-related genes influence energetically costly neuronal functions but that they are also expressed primarily in the central nervous system (5). The functions that these genes contribute to include molecular and cellular processes that likely contribute to the capacity for neuronal plasticity and learning, which are the quintessential examples of human phenotypic plasticity (84). To the extent that genes have effects on the BMI in part by modulating energy expended in the brain, any environmental factors that impact these same systems and pathways could have similar effects on body composition.
In light of this interpretation, educational or other interventions that increase the brain’s demand for metabolic substrate to fuel increased neural activity in response to educational programming could reduce obesity risk. That early educational intervention can reduce obesity risk is suggested by analyses of longitudinal follow-up data on growth and body composition in infancy, early childhood, and adulthood in the intensive early intervention known as the Abecedarian Project (85). Children were randomly assigned to a full day center-based care condition or to a control condition starting at 6 mo of age. Analysis of the early life growth data indicated that males in the control group experienced accelerated weight velocity in infancy and higher BMI at the adiposity rebound relative to participants in the treatment group (85). As such, children receiving the intensive early educational care starting in infancy were at lower risk for overweight across infancy and early childhood. Follow-up of these individuals at age 35 y indicated that male treatment group participants were less likely to exhibit characteristics of the metabolic syndrome, while both males and females showed evidence for reduced body fat or waist circumferences. Similar findings were seen in a follow-up to a trial of an innovative program to enhance parenting practices in low-income families with preschool children (86). Children in the treatment group were less likely to be obese in preadolescence, on average 5 y after the intervention ended.
Such findings suggest that cognitively stimulating activities reduce the risk of obesity associated with an accelerated BMI trajectory in early childhood. Although some of the effect of high-quality care on the development of obesity unquestionably occurs through treatment-related increases in self-regulation, we suggest that a direct energetic effect of enhanced cerebral metabolic activity on body composition is also likely. This suggestion is consistent with the underlying rationale for intensive early educational center-based interventions. Programmatic activities, such as an enriched language environment and opportunities for learning through play and exploration, are designed to stimulate cognitive abilities that are dependent on the development of increasingly complex and elaborated neural circuitry, including but not limited to PFC. The effect of the program on cognitive abilities associated with enriched development in, and connectivity between, specific brain areas would increase cerebral blood flow and the energy demand of the developing brain.
Although few if any early intervention studies include measures of brain structure, separate studies have shown sizeable gradients in energetically costly aspects of brain function and development, including cortical thickness, surface area, and even brain volume, in relation to favorable early rearing environments. Multiple studies have shown that cortical thickness during childhood is increased in a linear fashion in relation to the parents’ level of education (87, 88), which has also been shown to positively predict cortical surface area (88). Another found that parents that were more sensitive as caregivers from 1 to 4 y had children with thicker cortices, increased gray matter volume, and even significantly larger brain volumes (89). Although a contribution of genetic variability to these outcomes cannot be ruled out, the authors of these studies interpret these various findings as evidence that improvements in rearing environments will tend to boost brain growth and cognitive development. All of this points to higher brain energy expenditure in children experiencing high-quality care in infancy and early childhood.
Future Priorities
Brain energetics will only be an important predictor of energy balance and weight gain if it is variable across individuals, and at present we have almost no information on the range of this variability. The Kuzawa et al. (3) study generated a composite brain energetics curve derived from PET, MRI, and brain/body size data obtained from three separate populations. We know almost nothing about how this curve varies in timing, magnitude, or duration, whether considered within or between populations, and there is currently a great scientific need to collect these data. Noninvasive MRI-based approaches to measuring cerebral blood flow (34), and cerebral metabolic rate of oxygen (90), provide opportunities to incorporate proxies of brain metabolism into longitudinal studies of normal healthy child development, which hold promise to help clarify the role of energetics per se in the relationships between BMI and the development of the brain and cognition.
Similarly, we have essentially no information about the association between the energy demand of the developing brain and cognitive development. The neural efficiency hypothesis, primarily investigated in adults, suggests that individuals with higher general mental abilities expend less—not more—brain energy on a given task (91, 92). However, the few studies that have equated task difficulty with general mental ability find that high-ability individuals expend more rather than less energy on difficult cognitive tasks relative to low-ability individuals (93). Our model suggests that the energetic demands of the resting brain will be positively associated with general mental ability, accounting for a larger proportion of the RMR relative to lower-ability individuals. To date, only one study to our knowledge has examined these relationships in adults and found that resting state CBF is—as would be consistent with our predictions—positively associated with a measure of general intelligence (94).
Even if differences in brain energetics help explain population variation in traits like the timing of the adiposity rebound and, to some extent, general intelligence, it is not certain which, if any, experiences have the capacity to modify these relationships. Thus, a second priority is to clarify the sensitivity and responsiveness of brain developmental energetics to modifiable educational and other environmental interventions. We reviewed evidence that such interventions have had some success in reducing overweight and obesity, but a focus on brain energetics as a causal pathway could lead to additional, broader strategies not limited to boosting cognitive functions that impact behaviors like appetite regulation. According to the model outlined here, any boost in global brain energetics, irrespective of the underlying function, should have impacts on the body’s energy balance.
We estimated that brain energetics have greatest potential to impact the BMI at roughly 3–4 y of age, which are the years of peak brain metabolism that also precede the adiposity rebound. As noted, the timing of the AR, but also the mean BMI at this age, is predictive of later obesity risk. What is less clear is whether an intervention that succeeds in boosting global brain energy use at this early age will merely lead to transient weight reductions, or potentially alter long-term trajectories of overweight and obesity risk. Although it has been argued that the AR itself is not a critical period (15, 16, 95), we know that once gained, weight tends to be difficult to lose, and thus, any weight kept off in childhood could increase the likelihood of remaining thin into later life. Correlations between infancy and childhood BMI and adult BMI (tracking) tend to strengthen with age, and in one longitudinal study the rate of weight gain between 2 and 6 y of age was found to be the strongest predictor of adult BMI, after which correlations with adult BMI were strengthened substantially (10). Such findings hint at the potential for interventions that increase childhood brain energy use to have not only short-term but potentially also long-term impacts on obesity risk. The severity of the public health burden of overweight and obesity underscores the need for future research aimed at clarifying the role of brain energetics as an influence on developmental trajectories of body weight gain.
Acknowledgments
Bernie Crespi, Caleb Finch, Julie Lumeng, Tom O’Connor, Cybele Raver, Chet Sherwood, and Michael Willoughby provided helpful feedback on drafts of this manuscript. The contributions of C.B. were supported by grants 1UH3OD023332 and R01HD081252 from NIH.
Footnotes
The authors declare no conflict of interest.
References
- 1.Abarca-Gómez L., et al. ; NCD Risk Factor Collaboration (NCD-RisC) , Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 390, 2627–2642 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd L. J., Langley-Evans S. C., McMullen S., Childhood obesity and risk of the adult metabolic syndrome: A systematic review. Int. J. Obes. 36, 1–11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuzawa C. W., et al. , Metabolic costs and evolutionary implications of human brain development. Proc. Natl. Acad. Sci. U.S.A. 111, 13010–13015 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuzawa C. W., Blair C., “Is the energy demand of the developing brain related to lifetime obesity risk?” (IPR Working Paper WP-19-06, Institute for Policy Research, Evanston, IL, 2019).
- 5.Locke A. E., et al. ; LifeLines Cohort Study; ADIPOGen Consortium; AGEN-BMI Working Group; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GLGC; ICBP; MAGIC Investigators; MuTHER Consortium; MIGen Consortium; PAGE Consortium; ReproGen Consortium; GENIE Consortium; International Endogene Consortium , Genetic studies of body mass index yield new insights for obesity biology. Nature 518, 197–206 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuzawa C. W., Adipose tissue in human infancy and childhood: An evolutionary perspective. Am. J. Phys. Anthropol. (suppl. 27), 177–209 (1998). [DOI] [PubMed] [Google Scholar]
- 7.Rolland-Cachera M. F., et al. , Adiposity rebound in children: A simple indicator for predicting obesity. Am. J. Clin. Nutr. 39, 129–135 (1984). [DOI] [PubMed] [Google Scholar]
- 8.Rolland-Cachera M. F., et al. , Tracking the development of adiposity from one month of age to adulthood. Ann. Hum. Biol. 14, 219–229 (1987). [DOI] [PubMed] [Google Scholar]
- 9.Siervogel R. M., Roche A. F., Guo S. M., Mukherjee D., Chumlea W. C., Patterns of change in weight/stature2 from 2 to 18 years: Findings from long-term serial data for children in the Fels longitudinal growth study. Int. J. Obes. 15, 479–485 (1991). [PubMed] [Google Scholar]
- 10.Whitaker R. C., Pepe M. S., Wright J. A., Seidel K. D., Dietz W. H., Early adiposity rebound and the risk of adult obesity. Pediatrics 101, E5 (1998). [DOI] [PubMed] [Google Scholar]
- 11.de Kroon M. L., Renders C. M., van Wouwe J. P., van Buuren S., Hirasing R. A., The Terneuzen Birth Cohort: BMI change between 2 and 6 years is most predictive of adult cardiometabolic risk. PLoS One 5, e13966 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes A. R., Sherriff A., Ness A. R., Reilly J. J., Timing of adiposity rebound and adiposity in adolescence. Pediatrics 134, e1354–e1361 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Taylor R. W., et al. , Changes in fat mass and fat-free mass during the adiposity rebound: FLAME study. Int. J. Pediatr. Obes. 6, e243–e251 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Plachta-Danielzik S., et al. , Adiposity rebound is misclassified by BMI rebound. Eur. J. Clin. Nutr. 67, 984–989 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Cole T. J., Children grow and horses race: Is the adiposity rebound a critical period for later obesity? BMC Pediatr. 4, 6 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rolland-Cachera M. F., Cole T. J., Does the age at adiposity rebound reflect a critical period? Pediatr. Obes. 10.1111/ijpo.12467 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Janz K. F., et al. , Fatness, physical activity, and television viewing in children during the adiposity rebound period: The Iowa Bone Development Study. Prev. Med. 35, 563–571 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Rolland-Cachera M. F., Deheeger M., Akrout M., Bellisle F., Influence of macronutrients on adiposity development: A follow up study of nutrition and growth from 10 months to 8 years of age. Int. J. Obes. Relat. Metab. Disord. 19, 573–578 (1995). [PubMed] [Google Scholar]
- 19.Linares J., et al. , The effects of pre-pregnancy BMI and maternal factors on the timing of adiposity rebound in offspring. Obesity (Silver Spring) 24, 1313–1319 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Harris J. J., Jolivet R., Attwell D., Synaptic energy use and supply. Neuron 75, 762–777 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Rusinek H., Convit A., Obesity: Cerebral damage in obesity-associated metabolic syndrome. Nat. Rev. Endocrinol. 10, 642–644 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Attwell D., Laughlin S. B., An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 21, 1133–1145 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Alle H., Roth A., Geiger J. R., Energy-efficient action potentials in hippocampal mossy fibers. Science 325, 1405–1408 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Nortley R., Attwell D., Control of brain energy supply by astrocytes. Curr. Opin. Neurobiol. 47, 80–85 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Kety S. S., Schmidt C. F., The nitrous oxide method for the quantitative determination of cerebral blood flow in man: Theory, procedure and normal values. J. Clin. Invest. 27, 476–483 (1948). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foley R. A., Lee P. C., Ecology and energetics of encephalization in hominid evolution. Philos. Trans. R. Soc. Lond. B Biol. Sci. 334, 223–231, discussion 232 (1991). [DOI] [PubMed] [Google Scholar]
- 27.Leonard W. R., Robertson M. L., Nutritional requirements and human evolution: A bioenergetics model. Am. J. Hum. Biol. 4, 179–195 (1992). [DOI] [PubMed] [Google Scholar]
- 28.Walker R., Hill K., Burger O., Hurtado A. M., Life in the slow lane revisited: Ontogenetic separation between chimpanzees and humans. Am. J. Phys. Anthropol. 129, 577–583 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Bogin B., Patterns of Human Growth (Cambridge University Press, Cambridge, UK, ed. 2, 1999), 472 pp. [Google Scholar]
- 30.Kennedy C., Sokoloff L., An adaptation of the nitrous oxide method to the study of the cerebral circulation in children; normal values for cerebral blood flow and cerebral metabolic rate in childhood. J. Clin. Invest. 36, 1130–1137 (1957). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holliday M. A., “Body composition and energy needs during growth” in Postnatal Growth, Falkner F., Tanner J. M., Eds. (Human Growth: A Comprehensive Treatise, Plenum Press, New York, ed. 2, 1986), vol. 2, pp. 117–139. [Google Scholar]
- 32.Chugani H. T., Phelps M. E., Mazziotta J. C., Positron emission tomography study of human brain functional development. Ann. Neurol. 22, 487–497 (1987). [DOI] [PubMed] [Google Scholar]
- 33.Fuster J. M., The Prefrontal Cortex (Elsevier, Amsterdam, ed. 5, 2015), 444 pp. [Google Scholar]
- 34.Wu C., et al. , Age-related changes of normal cerebral and cardiac blood flow in children and adults aged 7 months to 61 years. J. Am. Heart Assoc. 5, e002657 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goyal M. S., Hawrylycz M., Miller J. A., Snyder A. Z., Raichle M. E., Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab. 19, 49–57 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huttenlocher P. R., Synaptic density in human frontal cortex—Developmental changes and effects of aging. Brain Res. 163, 195–205 (1979). [DOI] [PubMed] [Google Scholar]
- 37.Juraska J. M., Willing J., Pubertal onset as a critical transition for neural development and cognition. Brain Res 1654, 87–94 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandekar S. N., et al. , Sex differences in estimated brain metabolism in relation to body growth through adolescence. J. Cereb. Blood Flow Metab. 39, 524–535 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deoni S. C., Dean D. C. 3rd, Remer J., Dirks H., O’Muircheartaigh J., Cortical maturation and myelination in healthy toddlers and young children. Neuroimage 115, 147–161 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyall A. E., et al. , Dynamic development of regional cortical thickness and surface area in early childhood. Cereb. Cortex 25, 2204–2212 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allen N. J., Eroglu C., Cell biology of astrocyte-synapse interactions. Neuron 96, 697–708 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nave K. A., Werner H. B., Myelination of the nervous system: Mechanisms and functions. Annu. Rev. Cell Dev. Biol. 30, 503–533 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Harris J. J., Attwell D., The energetics of CNS white matter. J. Neurosci. 32, 356–371 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuczmarski R. J., et al. , CDC growth charts: United States. Adv. Data 314, 1–27 (2000). [PubMed] [Google Scholar]
- 45.Sameroff A. J., Haith M. M., The Five to Seven Year Shift (The University of London, London, 1996). [Google Scholar]
- 46.Blair C., School readiness. Integrating cognition and emotion in a neurobiological conceptualization of children’s functioning at school entry. Am. Psychol. 57, 111–127 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Petanjek Z., et al. , Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. U.S.A. 108, 13281–13286 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huttenlocher P. R., Dabholkar A. S., Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 387, 167–178 (1997). [DOI] [PubMed] [Google Scholar]
- 49.Yang Y., Shields G. S., Guo C., Liu Y., Executive function performance in obesity and overweight individuals: A meta-analysis and review. Neurosci. Biobehav. Rev. 84, 225–244 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Fitzpatrick S., Gilbert S., Serpell L., Systematic review: Are overweight and obese individuals impaired on behavioural tasks of executive functioning? Neuropsychol. Rev. 23, 138–156 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Liang J., Matheson B. E., Kaye W. H., Boutelle K. N., Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. Int. J. Obes. 38, 494–506 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamijo K., et al. , The relation of adiposity to cognitive control and scholastic achievement in preadolescent children. Obesity (Silver Spring) 20, 2406–2411 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chojnacki M. R., et al. , The negative influence of adiposity extends to intraindividual variability in cognitive control among preadolescent children. Obesity (Silver Spring) 26, 405–411 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Francis L. A., Susman E. J., Self-regulation and rapid weight gain in children from age 3 to 12 years. Arch. Pediatr. Adolesc. Med. 163, 297–302 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Graziano P. A., Kelleher R., Calkins S. D., Keane S. P., Brien M. O., Predicting weight outcomes in preadolescence: The role of toddlers’ self-regulation skills and the temperament dimension of pleasure. Int. J. Obes. 37, 937–942 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller A. A., Spencer S. J., Obesity and neuroinflammation: A pathway to cognitive impairment. Brain Behav. Immun. 42, 10–21 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Thaler J. P., et al. , Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122, 153–162 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guillemot-Legris O., Muccioli G. G., Obesity-induced neuroinflammation: Beyond the hypothalamus. Trends Neurosci. 40, 237–253 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Thaler J. P., Schwartz M. W., Minireview: Inflammation and obesity pathogenesis: The hypothalamus heats up. Endocrinology 151, 4109–4115 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller A. L., Lee H. J., Lumeng J. C., Obesity-associated biomarkers and executive function in children. Pediatr. Res. 77, 143–147 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arnsten A. F., Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 10, 410–422 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Kloet E. R., Oitzl M. S., Joëls M., Stress and cognition: Are corticosteroids good or bad guys? Trends Neurosci. 22, 422–426 (1999). [DOI] [PubMed] [Google Scholar]
- 63.Volkow N. D., et al. , Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity (Silver Spring) 17, 60–65 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lavagnino L., et al. , Reduced inhibitory control mediates the relationship between cortical thickness in the right superior frontal gyrus and body mass index. Neuropsychopharmacology 41, 2275–2282 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beyer F., et al. , Higher body mass index is associated with reduced posterior default mode connectivity in older adults. Hum. Brain Mapp. 38, 3502–3515 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alosco M. L., et al. , Body mass index and brain structure in healthy children and adolescents. Int. J. Neurosci. 124, 49–55 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Brain Development Cooperative Group , Total and regional brain volumes in a population-based normative sample from 4 to 18 years: The NIH MRI Study of Normal Brain Development. Cereb. Cortex 22, 1–12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Debette S., et al. , Abdominal obesity and lower gray matter volume: A Mendelian randomization study. Neurobiol. Aging 35, 378–386 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Marqués-Iturria I., et al. , Frontal cortical thinning and subcortical volume reductions in early adulthood obesity. Psychiatry Res. 214, 109–115 (2013). [DOI] [PubMed] [Google Scholar]
- 70.Bauer C. C., et al. , Child overweight and obesity are associated with reduced executive cognitive performance and brain alterations: A magnetic resonance imaging study in Mexican children. Pediatr. Obes. 10, 196–204 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Ross N., Yau P. L., Convit A., Obesity, fitness, and brain integrity in adolescence. Appetite 93, 44–50 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yau P. L., Kang E. H., Javier D. C., Convit A., Preliminary evidence of cognitive and brain abnormalities in uncomplicated adolescent obesity. Obesity (Silver Spring) 22, 1865–1871 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Opel N., et al. , Prefrontal gray matter volume mediates genetic risks for obesity. Mol. Psychiatry 22, 703–710 (2017). [DOI] [PubMed] [Google Scholar]
- 74.Repple J., et al. , Elevated body-mass index is associated with reduced white matter integrity in two large independent cohorts. Psychoneuroendocrinology 91, 179–185 (2018). [DOI] [PubMed] [Google Scholar]
- 75.Willer C. J., et al. ; Wellcome Trust Case Control Consortium; Genetic Investigation of Anthropometric Traits Consortium , Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet. 41, 25–34 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ho A. J., et al. ; Alzheimer’s Disease Neuroimaging Initiative , A commonly carried allele of the obesity-related FTO gene is associated with reduced brain volume in the healthy elderly. Proc. Natl. Acad. Sci. U.S.A. 107, 8404–8409 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Curran J. E., et al. , Identification of pleiotropic genetic effects on obesity and brain anatomy. Hum. Hered. 75, 136–143 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Swinburn B. A., Jolley D., Kremer P. J., Salbe A. D., Ravussin E., Estimating the effects of energy imbalance on changes in body weight in children. Am. J. Clin. Nutr. 83, 859–863 (2006). [DOI] [PubMed] [Google Scholar]
- 79.Dekaban A. S., Sadowsky D., Changes in brain weights during the span of human life: Relation of brain weights to body heights and body weights. Ann. Neurol. 4, 345–356 (1978). [DOI] [PubMed] [Google Scholar]
- 80.Giedd J. N., et al. , Brain development during childhood and adolescence: A longitudinal MRI study. Nat. Neurosci. 2, 861–863 (1999). [DOI] [PubMed] [Google Scholar]
- 81.Popkin B. M., Adair L. S., Ng S. W., Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 70, 3–21 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gottlieb G., Probabilistic epigenesis. Dev. Sci. 10, 1–11 (2007). [DOI] [PubMed] [Google Scholar]
- 83.West-Eberhard M. J., Developmental Plasticity and Evolution (Oxford University Press, Oxford, 2003), 794 pp. [Google Scholar]
- 84.Changeux J. P., Danchin A., Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature 264, 705–712 (1976). [DOI] [PubMed] [Google Scholar]
- 85.Campbell F., et al. , Early childhood investments substantially boost adult health. Science 343, 1478–1485 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brotman L. M., et al. , Early childhood family intervention and long-term obesity prevention among high-risk minority youth. Pediatrics 129, e621–e628 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lawson G. M., Duda J. T., Avants B. B., Wu J., Farah M. J., Associations between children’s socioeconomic status and prefrontal cortical thickness. Dev. Sci. 16, 641–652 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Noble K. G., et al. , Family income, parental education and brain structure in children and adolescents. Nat. Neurosci. 18, 773–778 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kok R., et al. , Normal variation in early parental sensitivity predicts child structural brain development. J. Am. Acad. Child Adolesc. Psychiatry 54, 824–831 e821 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Xu F., Ge Y., Lu H., Noninvasive quantification of whole-brain cerebral metabolic rate of oxygen (CMRO2) by MRI. Magn. Reson. Med. 62, 141–148 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haier R. J., et al. , Cortical glucose metabolic rate correlates of abstract reasoning and attention studied with positron emission tomography. Intelligence 12, 199–217 (1988). [Google Scholar]
- 92.Neubauer A. C., Fink A., Intelligence and neural efficiency. Neurosci. Biobehav. Rev. 33, 1004–1023 (2009). [DOI] [PubMed] [Google Scholar]
- 93.Larson G. E., Haier R. J., LaCasse L., Hazen K., Evaluation of a “mental effort” hypothesis for correlations between cortical metabolism and intelligence. Intelligence 21, 267–278 (1995). [Google Scholar]
- 94.Takeuchi H., et al. , Cerebral blood flow during rest associates with general intelligence and creativity. PLoS One 6, e25532 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dietz W. H., Critical periods in childhood for the development of obesity. Am. J. Clin. Nutr. 59, 955–959 (1994). [DOI] [PubMed] [Google Scholar]
- 96.Rolland-Cachera M. F., Akrout M., Péneau S., “History and meaning of the body mass index. Interest of other anthropometric measurements”in The ECOG’s eBook on Child and Adolescent Obesity, European Childhood Obesity Group, Frelut M. L., Ed. (ECOG, Belgium, 2015), pp 1–20. [Google Scholar]