Abstract
This article comments on:
Guillaume G. Cossard, Melissa A. Toups and John R. Pannell. 2019. Sexual dimorphism and rapid turnover in gene expression in pre-reproductive seedlings of a dioecious herb. Annals of Botany 123(7): 1119–1131.
Dioecious plants have separate male and female individuals which produce male and female flowers. Although it is impossible with the naked eye to differentiate between male and female Mercurialis annua plants until they flower, Cossard et al. (2019) find that the herb exhibits sexual dimorphism at the molecular level as early as the seedling stage. This finding is surprising for two reasons. The first is because plants, unlike animals, do not have reproductive organs during the first stages of their development. Therefore, one naïvely expects sexual dimorphism to appear only late in development, just before flowering. Second, selection for viability is strong at the seedling stage and could favour an optimal phenotype common to males and females, preventing early sexual dimorphism.
Sex is determined genetically in M. annua, with XY males and XX females. Males and females share most of their genome, with the exception of a small male-specific non-recombining region on the Y chromosome (Cossard et al., 2019). Consequently, in M. annua and other organisms, sexual dimorphism mainly arises from the regulation of sex-specific gene expression (Grath and Parsch, 2016). One way to characterize gene expression divergence between the sexes is through the study of RNA-seq data, allowing the identification of sex-biased genes (that is, genes that differ in transcript abundance between the sexes). Such studies have become common in animals (Grath and Parsch, 2016) and are becoming more common in plant flowers and vegetative tissues (Robinson et al., 2014; Harkess et al., 2015; Zemp et al., 2016; Muyle et al., 2017; Darolti et al., 2018; Sanderson et al., 2019). The study of Cossard et al. (2019) marks a point where there is data from a sufficient number of plants to identify patterns of sex-biased gene expression (hereafter SBGE) that are specific to plants and also patterns that are common to both plants and animals.
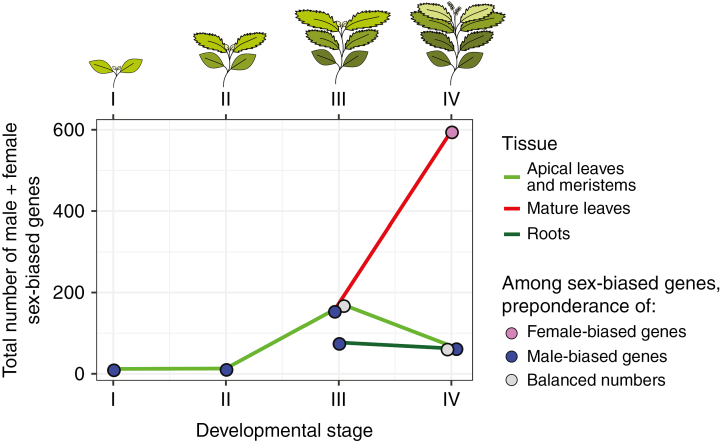
The analysis of Cossard et al. (2019) is the first to look at SBGE in seedlings and throughout plant development (Figure 1). Although only twelve genes have SBGE at the seedling stage I, these genes are sometimes sex-biased in stage II, but not in later stages III and IV. This suggests a specific role for these genes in making seedlings sexually dimorphic or in preparing for the later onset of sexual dimorphism. M. annua males are known to germinate and flower earlier than females and have a lower above ground biomass compared to females (Barrett and Hough, 2013). This is presumably due to a higher investment of males into roots in order to provide nitrogen for the production of pollen. In contrast, females invest more into above-ground biomass to fuel seed and fruit production with carbohydrates. It is possible that the SBGE detected in seedlings by Cossard et al. (2019) is linked to the early differentiation of the root/shoot ratio between males and females. However, functional analyses that manipulate the expression level of the genes will be necessary to confirm this conjecture.
Fig. 1.
Total number of male plus female sex-biased genes (y axis) in Mercurialis annua throughout development (x axis) in three different tissues (apical leaves and meristems in light green, mature leaves in red and roots in dark green) as found by Cossard et al. (2019). Each tissue and developmental stage is plotted with a pink dot if female-biased genes are more numerous than male biased genes, blue if male-biased genes are more numerous and grey if numbers are balanced.
Cossard et al. (2019) also observed that the number of sex-biased genes increased during development (Figure 1), which is consistent with what is known in animals (Grath and Parsch, 2016). In M. annua, the number of sex-biased genes peaked just before and after flowering (stages III and IV, Figure 1), when sexual dimorphism is either incipient or the most pronounced. Above-ground tissues had more sex-biased genes than roots (Figure 1), suggesting that differential resource allocation between males and females is stronger in aerial tissues.
The sex chromosomes of M. annua were enriched in sex-biased genes compared to the autosomes (Cossard et al., 2019), as has been observed in numerous animal and plant species (Grath and Parsch, 2016; Muyle et al., 2017). This may be attributable to the male-specific sequences of the Y chromosome that can facilitate the evolution of sex-biased expression.
Most tissues and developmental stages in M. annua exhibited a higher number of male-biased genes, with the exception of mature leaves (Figure 1). An excess of male-biased genes was also found in other plants (Harkess et al., 2015; Zemp et al., 2016), although in Populus balsamifera female biased genes are more numerous (Sanderson et al., 2019). An excess of male biased genes could be due to the fact that sexual selection is typically stronger in males than in females, due to female mate choice and competition between males to fertilize females. Although female mate choice is more limited in plants than in animals, it can still occur through incompatibility systems and post-fertilization selective embryo abortion (Barrett and Hough, 2013). During the evolution of dioecy from the ancestral bisexual state, sexual selection could drive faster divergence of males than females, which would explain the higher number of observed male-biased genes. To verify this hypothesis, the direction of the changes in expression levels that led to sex-biased expression will have to be inferred. If sexual selection is driving the evolution of more male-biased genes, then expression levels should have increased in males compared to the bisexual ancestor. Such an analysis has been performed in Silene (Zemp et al., 2016), but here a dioecious species was compared to a gynodioecious species (a species with females and hermaphrodites). It is known that hermaphrodites in gynodioecious species can be masculinized and it would be valuable to repeat such an analysis on a dioecious plant with a hermaphrodite outgroup. Salix viminalis had a slightly higher number of female-biased genes (Darolti et al., 2018), which could be due to its ZW sex chromosomes facilitating the evolution of female-biased genes.
Sex-biased genes do not evolve faster than non sex-biased genes in M. annua (Cossard et al., 2019). This provides an emerging pattern in plants, given a similar finding in S. viminalis (Darolti et al., 2018) and P. balsamifera (Sanderson et al., 2019). In contrast, male-biased genes typically evolve faster in animals, either because of higher adaptation rates in Drosophila or due to relaxed selection in animals other than Drosophila (Grath and Parsch, 2016). In animals, male-biased genes tend to have a smaller expression breadth than other genes, which could cause a relaxed selection pressure and increase their evolutionary rate. In plants, it has been suggested that haploid selection could be responsible for the lack of faster evolution of male-biased genes (Darolti et al., 2018). Indeed, male-biased genes expressed in pollen grains are subject to strong purifying selection because deleterious recessive mutations are uncovered and purged in haploids. This could prevent male-biased genes from evolving faster in plants, unlike animals where expression is virtually absent from spermatozoa and haploid selection does not happen. To fully test this hypothesis, diploid and haploid male-biased genes will have to be compared in plants.
It is now becoming more and more apparent that SBGE is less pronounced in plants than in animals, likely due to the fact that plants are less sexually dimorphic (Barrett and Hough, 2013). The age of separated sexes is often young in angiosperms while it is ancient in animals. Therefore, less time has passed in angiosperms to allow for the evolution of sexual dimorphism (Barrett and Hough, 2013). Also, as already stated, mate choice is limited in plants compared with animals, which should lower the intensity of sexual selection and slow down the evolution of sexual dimorphism in plants compared with animals (Barrett and Hough, 2013). Finally, the evolution of sexual dimorphism is constrained in plants that are pollinated by animals, because pollinators have to recognize males and females to pollinate them both (Barrett and Hough, 2013). In P. balsamifera and S. viminalis, pollination is mediated by wind, which likely allowed the evolution of stronger sexual dimorphism compared with insect-pollinated plants. Accordingly, the percentage of sex-biased genes in P. balsamifera catkins (36.28%, Sanderson et al., 2019) and S. viminalis catkins (43.6%, Darolti et al., 2018) is higher than in the flower buds of S. latifolia (16.95%, Zemp et al., 2016), which is insect-pollinated. Estimates of SBGE in the wind-pollinated M. annua isolated flower buds are lacking. Cossard et al. (2019) estimated that 6.63% of genes are sex-biased in M. annua mature leaves. This is comparable with mature leaves in S. latifolia (2.11%, Zemp et al., 2016) and Asparagus officinalis recently emerged spear tips (1.36% male versus female SBGE, Harkess et al., 2015) but a lot higher than mature leaves in the Salicaceae family with 0.0034% sex-biased genes in P. balsamifera (Sanderson et al., 2019), 0.0065% sex-biased genes in Populus tremula (Robinson et al., 2014) and 0.009% in S. viminalis (Darolti et al., 2018). The known values listed above suggest the age of dioecy is not a preponderant factor for the evolution of vegetative SBGE. Dioecy in Salicaceae is old (~45 My, Muyle et al., 2017) compared to S. latifolia (~5My, Muyle et al., 2017) and M. annua (~1.5My, John Pannell, personal communication). And yet, SBGE is very low in Salicaceae vegetative tissues. The factors that shape the amount of SBGE in plant vegetative tissues remain to be explored.
Acknowledgements
I would like to thank Brandon Gaut and Gabriel Marais for commenting on the manuscript. This work was supported by The International Human Frontier Science Program Organization (HFSPO) fellowship LT000496/2018-L.
Literature cited
- Barrett SCH, Hough J. 2013. Sexual dimorphism in flowering plants. Journal of Experimental Botany 64: 67–82. [DOI] [PubMed] [Google Scholar]
- Cossard GG, Toups MA, Pannell JR. 2019. Sexual dimorphism and rapid turnover in gene expression in pre-reproductive seedlings of a dioecious herb. Annals of Botany 123: 1119–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darolti I, Wright AE, Pucholt P, Berlin S, Mank JE. 2018. Slow evolution of sex-biased genes in the reproductive tissue of the dioecious plant Salix viminalis. Molecular Ecology 27: 694–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grath S, Parsch J. 2016. Sex-biased gene expression. Annual Review of Genetics 50: 29–44. [DOI] [PubMed] [Google Scholar]
- Harkess A, Mercati F, Shan H-Y, Sunseri F, Falavigna A, Leebens-Mack J. 2015. Sex-biased gene expression in dioecious garden asparagus (Asparagus officinalis). The New Phytologist 207: 883–892. [DOI] [PubMed] [Google Scholar]
- Muyle A, Shearn R, Marais GA. 2017. The evolution of sex chromosomes and dosage compensation in plants. Genome Biology and Evolution 9: 627–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KM, Delhomme N, Mähler N, Schiffthaler B, Önskog J, Albrectsen BR, Ingvarsson PK, Hvidsten TR, Jansson S, Street NR. 2014. Populus tremula (European aspen) shows no evidence of sexual dimorphism. BMC plant biology 14: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson BJ, Wang L, Tiffin P, Wu Z, Olson MS. 2019. Sex-biased gene expression in flowers, but not leaves, reveals secondary sexual dimorphism in Populus balsamifera. The New Phytologist 221: 527–539. [DOI] [PubMed] [Google Scholar]
- Zemp N, Tavares R, Muyle A, Charlesworth D, Marais GAB, Widmer A. 2016. Evolution of sex-biased gene expression in a dioecious plant. Nature Plants 2: 16168. [DOI] [PubMed] [Google Scholar]