Abstract
Background
Below-ground bud banks have experienced much recent interest due to discoveries that they (1) account for the majority of seasonal population renewal in many communities, (2) are crucial to regeneration following disturbance, and (3) have important consequences for plant population dynamics and plant and ecosystem function across a number of habitats.
Scope
This review presents an overview of the role of bud banks in plant population renewal, examines bud bank life history, summarizes bud bank traits and their potential ecological implications, synthesizes the response of bud banks to disturbance, and highlights gaps to guide future research. The characteristics and life history of buds, including their natality, dormancy, protection and longevity, provide a useful framework for advancing our understanding of bud banks. The fate of buds depends on their age, size, type, location, and biotic and abiotic factors that collectively regulate bud bank dynamics. A bud bank can provide a demographic storage effect stabilizing population dynamics, and also confer resistance to disturbance and invasion. Regeneration capacity following disturbance is determined by interactions among the rates of bud natality, depletion and dormancy (meristem limitation), and the resources available to support the regeneration process. The resulting response of plants and their bud banks to disturbances such as fire, herbivory and anthropogenic sources determines the community’s regenerative capacity.
Conclusions
Vegetation responses to environmental change may be mediated through changes in bud bank dynamics and phenology. Environmental change that depletes the bud bank or prohibits its formation likely results in a loss of vegetation resilience and plant species diversity. Standardization of bud sampling, examination of bud banks in more ecosystems and their response to environmental variation and disturbance regimes, employment of stage-structured bud bank modelling and evaluation of the cost of bud bank construction and maintenance will benefit this expanding field of research.
Keywords: Bud, bud bank, below-ground organs, clonal, disturbance, meristem, plant traits, population dynamics
INTRODUCTION
In his classic treatise, Population biology of plants, John Harper (1977) devoted a major portion of his discussion to seed reproduction and the population dynamics of seed banks. In addition to seeds, Harper briefly noted that dormant meristems associated with a variety of plant organs, such as rhizomes, corms, bulbs, bulbils and tubers, may also accumulate below ground. He coined the term ‘bud bank’ for this population of vegetative propagules in the soil and he described two fundamental differences between seed and bud banks that could have important ecological and evolutionary consequences. A seed bank produced via sexual recombination ‘conserves as yet untested genotypes’ and thus is characterized by higher genetic diversity than buds that are produced clonally from a single genet (Harper, 1977). Secondly, unlike seeds, which are independent from the parent plant, buried buds are usually attached to the parent. Thus, they may be maintained dormant by processes of correlative inhibition within the genet so that integrated control of bud dormancy and outgrowth are possible, and shoot outgrowth can be supported by resources from the parent. Harper also recognized the potential importance of bud longevity, the contrasting roles of buds in the annual renewal of shoots and in the regeneration of plants following injury or disturbance, and the potential stabilizing effects of a bud bank on plant population dynamics. Although Harper championed the demographic approach to the study of plant ecology and coined the term ‘bud bank’, the importance of below-ground populations of buds and their potential consequences for above-ground population and community dynamics were not immediately recognized. At that time, only a couple of studies had documented the longevity of below-ground buds or bud development and morphology of a few species (e.g. McIntyre, 1970; Hughes, 1974).
Three subsequent developments revealed the importance of bud banks and prompted their increased study in recent years. Detailed studies of plant demography in some communities revealed that successful recruitment from seed is rare and episodic, and the contribution of buds to the annual renewal of above-ground shoot populations is substantial, sometimes exceeding the contribution of seeds by an order of magnitude (Silvertown et al., 1993; Benson and Hartnett, 2006; Alfonso-Corrado et al., 2007; Latzel et al., 2008; Vítová et al., 2017). A second development was the increasing understanding of stochastic processes and the role of natural disturbance in plant population dynamics. Populations of dormant buds were recognized for their important role in the regeneration of lost plant tissue following disturbances such as fire, herbivory, soil disturbances or extreme climatic events. For example, buds enable the resprouter strategy in plants of fire-prone habitats (e.g. Clarke et al., 2013), and outgrowth of dormant buds is a key component of the regeneration of many perennial plants following herbivory, drought or agricultural management. General patterns have emerged in relationships between bud bank size and the frequency of disturbance, and between the vertical distribution of buds (sensuRaunkiaer, 1934) and the intensity of disturbance (Vesk et al., 2004; Klimešová and Klimeš, 2007).
Finally, increasing research on clonal plant species examined the patterns, controls and consequences of the production of new ramets and the lateral spread of plants via vegetative reproduction. This research showed the important role of below-ground buds in influencing the architecture, structure and population dynamics of clonal plants (e.g. Jackson et al., 1985; Hutchings and Bradbury, 1986). The number, spatial arrangement and activity of below-ground buds became recognized as determinants of key aspects of clonal growth strategies, such as resource foraging and habitat selection, phalanx versus guerrilla clonal expansion patterns and local neighbourhood interactions (de Kroon and van Groenendael, 1997).
It has now become recognized that bud banks play an important role in terrestrial plant populations and communities across a number of polar, temperate and tropical habitats (e.g. Ayukawa et al., 2001; Lee, 2004; Hartnett et al., 2006; Zhang et al., 2009) as well as in freshwater and marine habitats (Cellot et al., 1998; Abernathy and Willby, 1999; Combroux et al., 2002). This review builds upon our base knowledge of bud bank traits of individual species and focuses on (1) identifying bud bank characteristics and the below-ground demographic processes that regulate bud bank size and dynamics, (2) the influence of the environment and disturbance on these processes and resulting bud bank structure and composition, and (3) the ecological implications of maintaining a bud bank for individuals, populations and communities.
An earlier review by Klimešová and Klimeš (2007) emphasized the ubiquity of buds, the high capacity for bud initiation from a variety of plant organs, and the range and variety of bud bank traits. We seek to build upon this previous work, describe bud bank dynamics, synthesize our current understanding of the dynamics, traits and ecological roles of bud banks, and suggest avenues for future research. We confine our review to the ecology of bud banks in natural habitats and do not extensively discuss bud banks in agricultural and aquatic environments.
In our discussion, we delimit the below-ground bud bank following Harper (1977) and Klimešová and Klimeš (2007) with some minor modifications. We consider below-ground bud banks as inclusive of both axillary buds and adventitious buds produced on any below-ground or basal plant organ (Fig. 1). Below-ground space is safe for plants to store reserve meristems and storage compounds in specialized organs adapted to survive severe disturbance or seasonal adversity. We exclude above-ground buds as these mainly play a role in above-ground growth and architecture of woody plants or in regrowth of damaged shoots after mild disturbance. Although a below-ground bud bank can be found in all plant growth forms, it is typical of perennial herbs and many shrubs, which will be the main focus of this review.
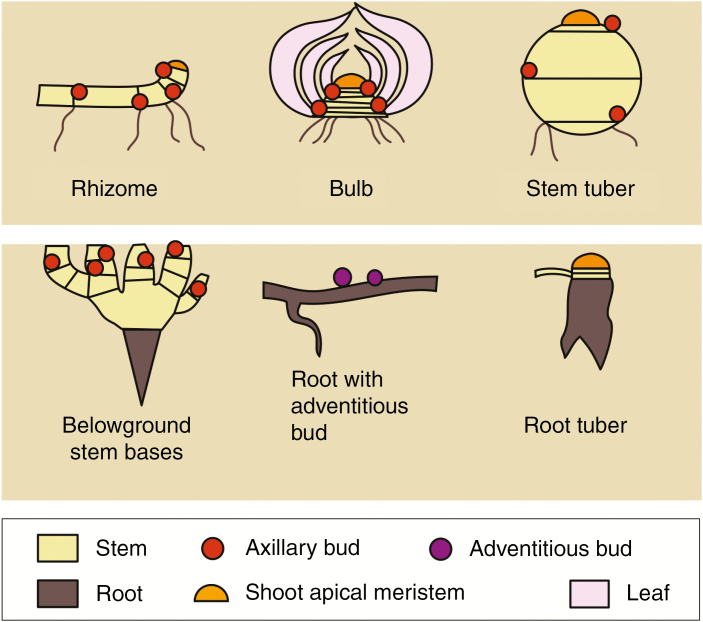
Fig. 1.
Below-ground bud-bearing organs. Buds can occur on roots or stems and in some cases leaf peripheries. Axillary and apical buds only occur on below-ground and above-ground stems and adventitious buds usually occur on roots.
THE ROLE OF BUD BANKS IN PLANT POPULATION RENEWAL AND DYNAMICS
Plant species differ in their relative reliance on seed versus bud banks. In many perennial herbs, the bud bank is an important source of propagules for the annual renewal of above-ground shoot populations, and hence population maintenance and growth. In habitats such as grasslands, forest understorey and arctic tundra, establishment from seeds is rare due to high mortality of seedlings, so that the vast majority of new shoots are recruited from the bud bank. For example, in the tallgrass prairie of North America, where shoot density is high and competition is intense, >99 % of the above-ground stems that emerge each spring are derived from the outgrowth of below-ground buds (Benson and Hartnett, 2006). The low density of seedlings relative to recruits from buds across North American grasslands (Fair et al., 1999; Peters, 2000; Benson and Hartnett, 2006) underscores the importance of the bud bank for population renewal in perennial grasslands. Seedlings experience strong selection pressures, especially from herbivores, drought and fungal attacks, which can lead to high seedling mortality (Moles and Westoby, 2004a, b). Recruitment from the below-ground bud bank can enable plant individuals to persist and the population to expand via clonal reproduction when seedling recruitment is unable to sustain population maintenance.
Annual renewal from a bud bank has some consequences for plant population structure and dynamics that may be more or less similar to the functioning of a seed bank (Table 1). Similar to seed banks, a population of dormant buds provides a demographic storage effect that can buffer population fluctuations and stabilize plant population dynamics. However, there are fundamental differences between buds and seeds in their dispersability and maternal support. Buds are nearly always connected to the parental plants, whereas seeds are independent of the parent once they are dispersed. One important implication is that seeds are provisioned with a small fixed amount of resources, whereas the buds are physiologically integrated with and supported by their parent plant. A second implication is that, once dispersed, the fate of the seed is not controlled by the parent plant, but only by environmental conditions. The fate of buds (dormancy, release, outgrowth pattern) also responds to abiotic conditions, but these responses can be mediated through hormonal signals from the parent plant (Table 1).
Table 1.
Contrasting features of below-ground bud banks and seed banks
| Bud banks | Seed banks |
|---|---|
| Numbers may not exceed adult plants | Numbers may greatly exceed adult plants |
| Time spent in bud bank may or may not exceed time spent as above-ground plant | Time spent in seed bank may greatly exceed time spent as above-ground plant |
| Tried and true genotypes | Source of genetic novelty |
| Direct parental control of dormancy is possible | Parental control of dormancy is very limited |
| More closely resembles above-ground plant species composition | Often little similarity to above-ground species composition |
| Bud densities decline rapidly with depth (most in top 5 cm) | Seed densities decline rapidly with depth (may be much deeper than bud banks) |
| Clumped distribution | Clumped distribution |
| Buffer population dynamics | Buffer population dynamics |
| Dispersal in time (1 to a few years) | Dispersal in time (years to decades) |
| Source of new vegetation if stand is destroyed | Source of new vegetation if stand is destroyed |
Maintenance of a bud bank may also incur a cost by reducing seed reproduction or competitive ability. For example, in fire-prone ecosystems, plant species that rely on a bud bank and below-ground storage to regenerate after fire tend to show lower sexual reproductive success (Bell and Ojeda, 1999). Clonal plants that multiply by producing new shoots from a bud bank produce fewer seeds and show lower seedling establishment than non-clonal plants (Herben et al., 2012, 2015). Resprouters from fire-prone areas relying on a below-ground bud bank are shorter and less competitive than seeders relying on regeneration from seeds after fire (Midgley, 1996).
The relative recruitment from buds versus seeds may have a great effect on the genetic structure of a plant population. Interestingly, even when establishment of new genotypes from seed is negligible, molecular techniques revealed rather high genetic variability in populations of clonal plants (Ellstrand and Roose, 1987; Widén et al., 1994). This may be caused by the initial establishment of populations from genetically diverse seeds and the inability of once-established genets to outcompete each other, and/or by continuous, albeit low, rates of seedling establishment (Watkinson and Powell, 1993). Whether somatic mutations within buds may contribute to the genetic variability of clonal species has not been studied. Additionally, recent studies of epigenetic variation in clonally reproducing species (Latzel and Klimešová, 2010; Ahn et al., 2017) indicate that genetic diversity is not the only factor determining higher or lower flexibility of a plant population in response to environmental fluctuations, but epigenetics plays a role as well.
BUD BANK LIFE HISTORY: FROM BUD FORMATION TO SHOOT OUTGROWTH
Similar to seed banks and populations of above-ground plants, the bud bank has its own structure and dynamics, determined by transition rates among various states (Fig. 2). Like seed bank size, bud bank size is determined by the rate of inputs (bud natality) and outputs (outgrowth, mortality), and its effect on above-ground population dynamics is influenced by the proportion of buds that are dormant or active (Fig. 2). Similar to seed banks of heterocarpic species (Mandák and Pyšek, 2001), bud banks include individual buds having varying roles in population renewal and dynamics (Clarke et al., 2013) (Fig. 1) due to bud origin, size, position and preformation.
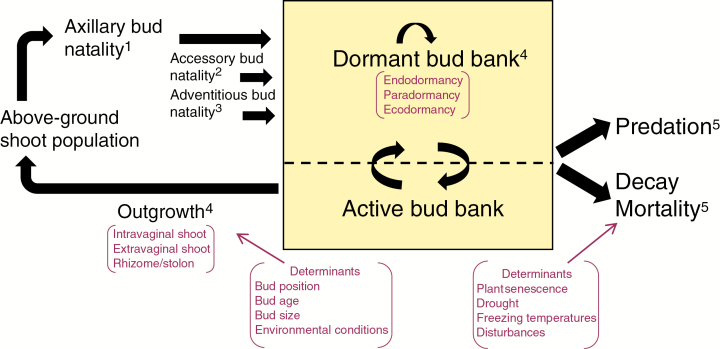
Fig. 2.
Diagrammatic flow chart for the dynamics of below-ground bud populations. (1) The below-ground bud bank is replenished primarily via axillary bud production, although adventitious or accessory buds may contribute. (2) Higher-order branching of existing buds can greatly increase bud bank size. (3) Adventitious bud formation often occurs independently of shoot growth as long as resources are stored or available for bud formation, which is generally a low cost to the plant. (4) The relative proportions of buds that remain dormant or grow out have large effects on both bud bank and above-ground population dynamics. If bud bank size is small and/or outgrowth rates are low, plant population growth may be meristem-limited. (5) Both dormant and active buds can experience mortality or predation.
Bud natality
Bud natality may occur continuously or seasonally as directed by climate, or be triggered by disturbance. The bud bank includes apical, accessory, axillary and adventitious buds. The apical meristem (i.e. bud) found at the tip of stems produces and adds new stem building modules to its body. Each module (also called a metamer in dicotyledonous plants or a phytomer in graminoids) consists of a stem internode and a node with its associated leaf and bud in its axil. Therefore, these new axillary buds are produced as the plant grows. After stem formation, new axillary buds cannot be borne on the stem except for branching of already established buds (accessory buds), as occurs in some herbs. In some grasses, these accessory buds can increase their bud bank size by as much as 4.5-fold (Ott and Hartnett, 2012b). Axillary, accessory or adventitious buds become apical buds once they begin metamer production.
Natality of adventitious buds, which are formed outside of the stem modular structure, on leaves and roots, is not yet fully understood. Adventitious buds are formed endogenously and may remain hidden in root tissue, or form only after plant injury. Consequently, contrary to axillary buds, they can be produced on already developed roots and leaves. This ability has been reported only for ~15 % of temperate species (Bartušková et al., 2017) and is more common in dicotyledonous than monocotyledonous plants, and in trees than in herbs (Evert, 2006; Bartušková et al., 2017).
The bud bank is gradually developed during plant ontogeny. At early developmental stages a plant would need to begin developing its bud bank, which involves producing and placing buds below ground. Below-ground bud bank formation is easy in plants with adventitious buds produced on roots but may be more difficult for plants whose bud-bearing organs are of stem origin (Fig. 3). The simplest way of positioning buds originating on stems below ground is passive burial of the vertical stem base by accumulating litter or soil. Other, more active mechanisms include hypogeic (below-ground) germination of seeds or pulling the basal portion of the shoot downwards by strongly contractile roots (Putz and Sukkau, 2002). Additionally, specialized underground shoots (e.g. rhizomes) that are capable of producing buds may grow positive-geotropically in early plant ontogeny in order to attain their below-ground position.
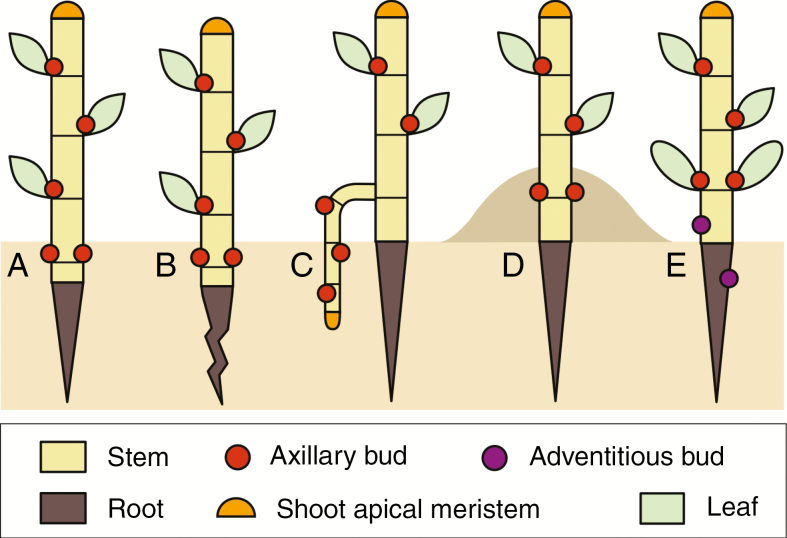
Fig. 3.
Placement of adventitious and axillary buds below ground in the early ontogeny of a plant. There are multiple methods by which axillary buds are placed below ground: (A) hypogeic germination; (B) pulling stem base by root contraction; (C) positive geotropic growth of a young rhizome; (D) partial burial; and (E) adventitious buds on roots.
Bud dormancy
Once a bud is formed, it remains dormant for a period of time. The length of dormancy varies depending on the individual plant and the environmental conditions. We can recognize three types of bud dormancy (Fig. 4): (1) endodormancy, due to cues located inside the bud; (2) paradormancy, due to signals within the plant but outside the bud; and (3) ecodormancy, due to external environmental factors acting on the whole plant (Lang et al., 1987; Horvath et al., 2003; Waldie et al., 2010). Buds can be under multiple dormancy controls at the same time, as the environment and internal factors may act independently of one another (Horvath et al., 2003) and consequently buds on one bud-bearing organ could have different responses to the same stimuli.
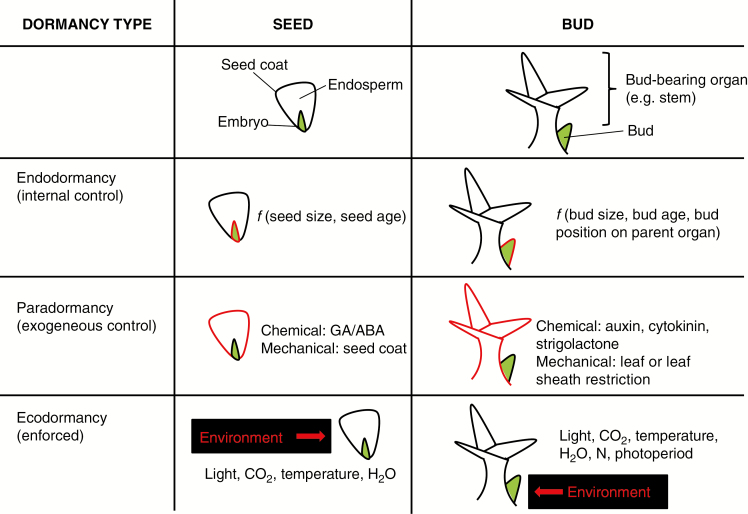
Fig. 4.
General dormancy types of seeds and buds. Green indicates the active embryo or meristem. Red highlights the location of the factor affecting dormancy for each dormancy type. f= ‘function of’. Age and size of seeds and buds can be factors in their endodormancy. Paradormancy can be conferred by mechanical or chemical means. For example, seeds and buds can be restrained by their respective seed coat and leaf sheath or they can be dormant due to hormonal signals involved in the endosperm for seeds or the shoot apical meristem for buds. The environment can impact bud and seed dormancy directly and/or indirectly through affecting paradormancy factors and mechanisms.
Endodormancy, whether caused by an inhibitor or by lack of promoters, may be influenced by bud age, size and position. Buds that have not fully developed are likely unable to grow out because their vascular development may be insufficient to receive the necessary nutrients and signals (Mueller and Richards, 1986). In monocots, below-ground buds increase in size acropetally along the stem base (e.g. Mueller and Richards, 1986; Busso et al., 1989), and distal buds near the apical meristem are the most likely to grow out (McIntyre, 1970; but see also Mueller and Richards, 1986; Hendrickson and Briske, 1997). Therefore, the youngest, most-developed and usually the largest buds grow out. When external conditions promote bud outgrowth, it may still be prevented by endodormancy. Similar to the stages of innate, induced and enforced dormancy in seeds (Harper, 1977), buds can fluctuate between being in dormant or responsive (active) states in response to favourable outgrowth signals and environmental conditions (Waldie et al., 2010).
Paradormancy can be maintained by mechanical or physiological mechanisms. Grass buds can be physically restrained by tightly enclosing leaf sheaths until leaf senescence begins, as observed in wheat (Williams and Langer, 1975). Apical dominance and correlative inhibition mediated by hormonal signals impose paradormancy on buds (Waldie et al., 2010). Physiological paradormancy is caused by the interaction of three primary hormones: auxin, cytokinin and strigolactone (Cline, 1997; Waldie et al., 2010). Auxin transported downwards from the apical meristem inhibits axillary bud outgrowth by blocking cytokinin reception of the bud or cytokinin synthesis in the roots. Strigolactone regulates auxin transport and thus may control the effect of auxin on cytokinin synthesis or its action on axillary buds. For a bud to be released from dormancy, strigolactone must inhibit auxin transport, allowing cytokinin to be translocated to the bud to stimulate its outgrowth (McSteen, 2009; Waldie et al., 2010). In general, axillary rhizome buds are more likely to remain dormant than apical rhizome buds or buds at the base of the parent plant (Zhang et al., 2009; Ott and Hartnett, 2015a). Environmental factors such as temperature, light wavelength and intensity, photoperiod, and water and nutrient availability can affect bud dormancy directly (ecodormancy) or indirectly via influencing paradormancy mechanisms. Ecodormancy is typically caused by unfavourable environmental conditions such as freezing temperatures, drought, low light or nutrient deficiencies (e.g. Tomlinson and O’Connor, 2004).
Very little information is available on the controls of dormancy in adventitious buds. Plants with spontaneous root sprouting probably have regulated bud dormancy similar to plants with axillary buds. Different controls are probably involved in adventitious buds that are formed or released from dormancy by injury, although we know that nutrient availability, plant ontogeny and phenology also play a role (Klimešová and Martínková, 2004; Bartušková and Klimešová, 2010). Therefore, assessing the effect of injury on bud dormancy is necessary for understanding the control of sprouting from adventitious buds.
Buds are considered dormant when they exhibit negligible growth despite being metabolically active (Lang et al., 1987; Shimizu-Sato and Mori, 2001). A few studies on herbs have assessed bud metabolic activity using the triphenyl tetrazolium chloride assay for cellular respiration in conjunction with Evans blue staining to detect necrosis. By using these tests to differentiate between buds that are respiring and buds that are alive but not detectably respiring, only small percentages of perennial grass buds were classified as dormant (Busso et al., 1989; Hendrickson and Briske, 1997; Russell et al., 2015). Metabolically active buds could technically still be considered dormant as long as they show no outgrowth, as is usually observed in field studies (Zhang et al., 2009; Williamson et al., 2012; Ott and Hartnett, 2012a; Liew et al., 2013). It seems that metabolic activity is not a suitable indicator of bud dormancy and other methods (e.g. expression of certain mRNAs) have been proposed (Stafstrom et al., 1998).
Despite the above-mentioned studies, we know little about what is happening inside of closed buds except that they enlarge in size. This is typical especially for buds that will serve as renewal buds for new shoot initiation in the next suitable growing season. The renewal buds are usually the highest buds located on the shoot base from the previous season, such as those at the top of the crown or the tip of a new rhizome branch. Renewal buds may preform differentiated structures before outgrowth. This preformation is either whole, including an inflorescence, or partial, including only the vegetative parts of the next year’s shoot, or even less advanced when a bud contains only partially preformed basal vegetative portions of next season’s shoot (Geber et al., 1997). Growth of preformed parts of seasonal shoots is fast and is typical of woodland herbs and plants of arctic and alpine communities – habitats with short growing seasons (Diggle, 1997; Geber et al., 1997). Plants with bud preformation are not flexible when responding to disturbance and loss of renewal buds may result in growth retrogression or whole-plant dormancy (Shefferson et al., 2014).
The fraction of buds maintained in a dormant versus active stage is a key determinant of bud and above-ground plant population dynamics (Bonser and Aarssen, 1996, 2006) and has important ecological implications. A low fraction, or lack of bud dormancy, results in high rates of bud outgrowth in favourable conditions, allowing some plant species to quickly respond to resource pulses and increase in abundance, but this may incur a cost by limiting the number of buds available to persist during unfavourable periods (Shefferson et al., 2014). Maintaining a high ratio of the bud population in a dormant state would limit increases in shoot populations during favourable periods (Ott and Hartnett, 2012a, 2015a, b), but at the same time would enhance the storage effect, stabilizing plant populations during unfavourable conditions and conferring high resilience by allowing population recovery. In some perennial grasslands and shrublands, this buffering effect of dormant buds causes plant population densities and primary production in a given year to be strongly correlated with past (e.g. 3–5 years) rather than current conditions (Anderson and Inouye, 2001; Wiegand et al., 2004). This is allowed by a low turnover rate of carbohydrate and also by an accumulated bud bank that provides a population dynamic memory enabling stabilization of temporal variation in above-ground productivity (Hendrickson and Briske, 1997; Wiegand et al., 2004; Ott and Hartnett, 2012a; Šťastná et al., 2012; VanderWeide et al., 2014).
In extreme cases, plant individuals can maintain 100 % of their buds in a dormant state for one or more years (Shefferson, 2009; Shefferson et al., 2014). During these periods of absence above ground, plants may remain viable below ground through the utilization of energy reserves provided by storage tissue or by mycorrhizal fungi (Bidartondo et al., 2004; Gremer et al., 2010). This extreme case of dormancy is considered an adaptation to periods of low resource availability or stress, when bud outgrowth and above-ground sprouting may incur high physiological and demographic costs, or it may represent a cost of sexual reproduction, as it often occurs more frequently following periods of high fecundity (Primack and Stacy, 1998; Shefferson et al., 2014). On the other hand, there are plants that use all buds for seasonal regrowth and therefore do not possess any buds for part of the season and would not be able to regenerate after injury (Klimešová and Klimeš, 2007). Although this strategy has not yet been properly studied, it may be an adaptation for conditions when resprouting is unwanted, such as in environments with a short growing season (e.g. spring geophytes of temperate forests) where vegetative regeneration would result in delayed vegetative growth, leading to a mismatch with suitable growing conditions.
Bud longevity, mortality and turnover
Below-ground bud longevity is limited by the longevity of the organ to which it is attached but may be much shorter than that of its parent organ. In some species whole bud-bearing organs undergo yearly turnover and such plants are called pseudo-annuals (Krumbiegel, 2001). In other species bud-bearing organs accumulate both stored carbohydrates and buds over years (Suzuki and Mutchings, 1997). Although some rhizomes can live for decades, we have only limited knowledge about the longevity of buds attached to them. In some grass species, the bud bank undergoes rapid turnover. In several caespitose grasses, bud viability depends on the presence of live tillers, and buds usually die within 6 months of their parent tiller’s death (Ott and Hartnett, 2012a, 2015b). On the other hand, rhizomatous and stoloniferous grasses maintain buds for a year or more after their parent tiller dies (e.g. Hendrickson and Briske, 1997; Ott and Hartnett, 2012a, 2015a). Bud longevity influences bud bank age structure, and accumulation of long-lived buds from multiple recruitment events can result in mixed-age bud banks (e.g. Busso et al., 1989; Ott and Hartnett, 2012a, 2015b; Reichmann et al., 2013). In grasses, however, bud viability tends to decline with age (Hendrickson and Briske, 1997) and most shoot recruitment comes from younger buds.
Bud mortality can be caused by numerous factors, including plant senescence, drought, freezing temperatures, herbivory or other disturbances (see Bud Banks and Disturbance section below). Harsh conditions or disturbances could cause direct bud mortality, or may reduce the parent plant’s capability of supporting the bud, causing indirect mortality. Above-ground herbivory and/or fungal and bacterial disease may reduce resources provided to buds below a survival threshold, while below-ground herbivory by insect larvae can directly kill buds. While some organs have no difficulty keeping their below-ground position, others (e.g. those dependent on contractile roots) may lose the ability to position buds below the soil surface, resulting in bud death during the adverse season (Klimešová et al., 2015).
Bud outgrowth
Signals for bud outgrowth are those that break bud dormancy (see above). These signals ensure that sprouting is properly timed in respect to climatic seasonality, higher resource availability is used to produce more vigorous growth, and damaged biomass is replaced by new shoots. For example, studies of several grass species have shown that bud release from dormancy depends on temperature (e.g. Mitchell, 1953), and suggest that the synchronous spring recruitment from overwintering buds could be due to warming temperatures (e.g. Ott and Hartnett, 2012a). Concerning bud outgrowth as a response to optimal local conditions, an integrated model for grasses was developed by Tomlinson and O’Connor (2004). In their model, soil available nitrogen and light spectral composition (red light) are the two key environmental factors that interact to stimulate bud outgrowth and tillering by mediating hormonal controls and carbon dynamics within the plant. The model is supported by empirical observations of positive effects of increased nitrogen availability on bud release from dormancy in many grass species (Derner and Briske, 1999; Tomlinson and O’Connor, 2004; Dalgleish et al., 2008). Also, the effect of light on bud outgrowth has been repeatedly reported for buds situated at the soil surface (e.g. Skálová and Krahulec, 1992) but not for buds on below-ground bud-bearing organs (Murphy and Briske, 1994; Williamson et al., 2012; Xie et al., 2014). After biomass damage, bud outgrowth is initiated by their release from correlative inhibition, leading to higher proportions of buds sprouting than in programmed seasonal regrowth, which may lead to meristem limitation under certain conditions (Klimešová et al., 2014).
The first buds to resprout are those located at the highest position along the vertical axis (Raunkiaer, 1934), the youngest, largest and the most preformed buds of the bud bank. Considering bud morphological origin, below-ground apical buds maintain active growth of a shoot and potentially transition into above-ground vegetative or generative structures, axillary buds often remain dormant unless needed to replace their apical meristem, and adventitious buds resprout only when no axillary bud is preserved after plant damage (Bartušková and Klimešová, 2010). Such gradual bud activation may serve as insurance for a plant experiencing recurrent disturbance. Nevertheless, bud outgrowth, either seasonal or regenerative, may result in the exhaustion of the bud bank, temporal bud limitation, and therefore difficulties in resprouting.
In perennial plants, the capacity for shoot population renewal may be limited by bud (meristem) availability, by resource availability in the environment, or by stored resources within the plant. The role of meristem limitation at the individual level was first noted in the context of resource allocation patterns in plants (e.g. Watson and Casper, 1984; Bonser and Aarssen, 1996, 2006), and was also recognized as a potentially important factor constraining primary productivity at the system level (e.g. Knapp and Smith, 2001; Dalgleish and Hartnett, 2006). Systems can be meristem-limited by overall bud bank density per area, which is driven by plant density, whereas individuals are meristem-limited by the number of buds per plant. There remains ongoing debate regarding the roles of meristem limitation versus resource limitation in regulating plant growth capacity (Wise and Abrahamson, 2007; Paula and Ojeda, 2011). Some evidence for the relative importance of buds versus stored carbohydrate comes from the morphology of below-ground organs themselves. Some organs, such as xylopodia, have very high bud density but low storage capacity, suggesting greater dependence on buds than on stored resources, whereas others, such as bulbs, have high storage capacity but only a single bud, suggesting greater dependence on stored reserves (Fidelis et al., 2014). However, the size of perennating organs does not always provide a clear indication. For example, lignotubers were considered to be specialized for storage, but recent studies suggest that they may play a more important role in enlarging the bud bank (Bell and Ojeda, 1999; Paula et al., 2016). Meristem limitation may be further compounded by bud dormancy as buds may be present but internal dormancy patterns prevent them from outgrowth under favourable conditions.
In summary, the population of buds in a bud bank is not uniform, and the fate and role of a bud depends on a number of characteristics, including its age, size, protection, position, developmental stage (e.g. preformation) and origin (apical, axillary, accessory and adventitious buds), and the type and longevity of its bud-bearing organ. The fate and role of buds is also influenced by biotic and abiotic factors such as nutrient availability, competition, environmental stress and disturbance. These intrinsic and extrinsic factors collectively regulate the dynamics, size and spatial structure of the bud bank (i.e. bud bank traits), and hence its role in plant population renewal or regeneration.
BUD BANK TRAITS
As evident from the overview of bud bank life history above, each bud has specific characteristics (age, dormancy, position) often determined by the circumstances of its formation (e.g. origin determines position) and its environment (e.g. dormancy). Collectively, the characteristics of all the buds can be used to evaluate bud bank dynamics and identify bud bank traits that can be used to compare plant species (Table 2). In addition to describing the general prerequisites of vegetative regeneration of species, we can study the intraspecific variability of these traits and how these traits vary according to ecosystem, disturbance type or experimental manipulation for individual species as well as the overall plant community. A bud bank trait can describe a static point at a key life history stage (e.g. bud bank size at the time of shoot renewal) or the dynamics occurring within the bud bank due to seasonal climate or disturbance (e.g. annual fluctuation in bud bank size). Certain bud bank traits may be more relevant to specific growth forms or regions. For example, bud protection by bark is important for woody plants in fire-prone areas (Clarke et al., 2013), and bud number per unit rhizome length is unique to rhizomatous species and especially relevant in temperate grasslands (Carter and VanderWeide, 2014). Many bud bank traits are self-explanatory (Table 2) but a few are given further review below.
Table 2.
Bud bank traits. Most bud bank traits can be applied to plant individuals or the plant community as a whole. Example units of measurement are not an exhaustive list
| Bud bank traits | Example units of measurement |
|---|---|
| Morphological type of bud-bearing organ (BBO) | Proportion of buds on rhizomes |
| BBO longevity | Range or average longevity of BBO |
| Bud longevity | Range or average longevity of buds |
| Bud bank size | Number per stem, per plant or per soil volume |
| Bud preformation | Proportion of performed buds for generative purposes |
| Bud protection | Proportion of buds protected by bark |
| Age structure of buds in the bud bank | Number of bud cohorts at a given point in time; proportion of buds according to age group; single- or mixed-aged bud banks |
| Size/stage structure of buds in the bud bank | Number of size or stage classes at a given point in time; proportion of buds according to size/stage classes; single- or multi-staged bud banks |
| Dormancy within the bud bank | Ratio of dormant to active buds |
| Seasonal fluctuation of bud numbers | Maximum relative change in bud bank size; timing of major changes in bud bank size |
| Vertical distribution of bud bank | Range of bud depths; average bud depth |
| Spatial distribution of bud bank | Proportion of buds on horizontal rhizomes versus at the base of vertical stems; proportion of buds on roots versus buds on stems |
| Meristem:BBO support ratio | Bud number per amount of storage carbohydrates; bud bank size per biomass of bud-bearing organ |
Type and longevity of the bud-bearing organ
Below-ground bud banks can be found on organs with diverse morphologies, including bulbs, corms, rhizomes, stem collars, xylopodia, tuberous roots and rhizophores (Klimešová and Klimeš, 2008; Pausas et al., 2018). The classification of bud-bearing organs is not uniform as different morphological schools in different regions use different categories and names. For example, classifications of bud-bearing organs differ between the CLO-PLA database describing temperate herbs (Klimešová and Klimeš, 2008) and that used by Pausas et al. (2018) for fire-prone tropical grasslands of Brazil. We will need cautious morphological analyses that will result in only a few comparable categories to be able to standardize the trait descriptions of such different regions (Filartiga et al., 2017). For example, lignotubers bear numerous buds and store carbohydrates, conferring high resprouting capacity after injury by fire (Paula et al., 2016). They are typical bud-bearing organs of fire-prone areas, having no counterpart in temperate regions. On the other hand, roots that are able to create buds after fragmentation and regenerate when soil is disturbed by ploughing (Klimešová et al., 2017b) can be found in both regions.
Although the type of below-ground organ largely defines the resprouting and/or clonal growth capacity of a species, resprouting, shoot population renewal and clonal growth also depend on traits such as organ storage capacity, longevity and branching intensity (Klimeš et al., 1997). Thus, community responses to resource limitation or disturbance may be partially driven by differences in the types of below-ground bud-bearing organs that dominate (Klimešová and Klimeš, 2008; Rusch et al., 2011). For example, in alpine communities on fertile soils, bud-bearing organs are diverse and include bulbs, root and stem tubers, and rhizomes, whereas plants on nutrient-poor soils primarily have rhizomes (Klimeš, 2008; Rusch et al., 2011). Wetland communities with stable hydrology have mainly rhizomes, whereas more disturbed or open water wetlands have root-derived bud-bearing organs (Sosnová et al., 2010). How these differences translate to bud bank functioning is not known. Bud-bearing organs have numerous functions apart from providing plants with a bud bank for seasonal renewal and regeneration after disturbance. Bud-bearing organs play a role in clonal growth, nutrient acquisition and competitive ability. This multi-functionality of bud-bearing organs makes understanding their specific role in the maintenance of a bud bank very difficult and will require innovative research strategies to disentangle them.
Number of buds
The number of buds per shoot is the most often studied bud bank trait and strongly depends on plant architecture. For example, higher numbers of buds occur in rhizomatous plants than in species where shoots have no horizontal below-ground parts. As the modular structure of a plant is more or less regular, the number of new buds born per shoot is usually constant for a species or ecotype despite varying environmental conditions, as was shown for several grasses (Table 3) (Hendrickson and Briske, 1997; Dalgleish et al., 2008; Ott and Hartnett, 2012a; Carter and VanderWeide, 2014). Flowering tillers may have a higher and less variable number of buds per tiller than vegetative tillers because vegetative tillers can be in a range of developmental stages due to variation in the timing of tiller initiation or environmental effects on tiller development (Ott and Hartnett, 2011). Although these data are based on direct counts of buds, the regular modularity of plant growth would allow general bud number estimation using indirect indices, as done in the CLO-PLA clonal plant trait database (Klimešová and Klimeš, 2007).
Table 3.
Bud production per tiller (ramet) of various dominant perennial grasses
| Species | Growth form | Photosynthetic pathway | MAP (mm) | Location | Time of year | Undisturbed buds/tiller | Fire buds/ tiller | Grazing buds/ tiller |
|---|---|---|---|---|---|---|---|---|
| Agropyron desertorum | Caespitose | C3 | 420 | Utah, USA | May, June | 4.5–5.61,2 | – | – |
| Agropyron spicatum | Caespitose | C3 | 420 | Utah, USA | May, June | 6.7–7.01,2 | – | – |
| Andropogon gerardii var. paucipilus | Rhizomatous | C4 | 437 | Nebraska, USA | October | 4.5–5.23 | – | – |
| Andropogon gerardii | Rhizomatous | C4 | 499 | South Dakota, USA | Autumn | 6.34 | – | – |
| 835 | Kansas, USA | September–November | 8.25 | |||||
| Aristida diffusa | Caespitose | C4 | 425 | Khama, Botswana | March, May | 1.36 | – | – |
| Aristida junciformis | Caespitose | C4 | 340 | Khutse, Botswana | March, May | 2.66 | – | – |
| Aristida meridionalis | Caespitose | C4 | 425 | Khama, Botswana | March, May | 1.37 | – | – |
| Aristida purpurea | Caespitose | C4 | 295 | Eastern Montana, USA | August, September | 11.58 | 6.5–7.08 | – |
| Aristida stipitata | Caespitose | C4 | 500 | Kumakwane, Botswana | March | 2.17 | – | – |
| Bouteloua curtipendula | Rhizomatous | C4 | 439 | Texas, USA | November | 3.5–4.39 | – | 4.2–4.4*9 |
| 835 | Kansas, USA | October | 3.510 | – | – | |||
| Bouteloua eriopoda | Stoloniferous | C4 | 264 | Jornada, New Mexico, USA | October | 1.0–1.511 | – | – |
| Bouteloua gracilis | Rhizomatous | C4 | 339 | Eastern Montana, USA | March, September, November | 3.9–5.212 | 3.6–8.612 | – |
| Brachiaria nigropedata | Caespitose | C4 | 425 | Khama, Botswana | March, May | 2.86 | – | – |
| Calamovilfa longifolia | Rhizomatous | C4 | 437 | Nebraska, USA | October | 1.33 | – | – |
| Cymbopogon pospischilii | Caespitose | C4 | 425 | Khama, Botswana | March, May | 1.96 | – | – |
| Dichanthelium oligosanthes | Caespitose | C3 | 835 | Kansas, USA | November–December | 4.65 | – | – |
| Digitaria californica | Caespitose | C4 | 394 | Arizona, USA | Winter | 3.313 | – | – |
| Digitaria eriantha | Caespitose | C4 | 425 | Khama, Botswana | March, May | 2.66 | – | – |
| Eragrostis lehmanniana | Caespitose | C4 | 500 | Kumakwane, Botswana | March | 2.17 | – | – |
| Hesperostipa comata | Caespitose | C3 | 339 | Eastern Montana, USA | March, September, November | 1.4–4.312 | 1.4–3.912 | – |
| 499 | Western South Dakota, USA | Autumn | 2.114 | |||||
| Hilaria belangeri | Stoloniferous | C4 | 439 | Texas, USA | November | 1.9–2.79 | – | 1.7–2.3*9 |
| Hyperthelia dissoluta | Caespitose | C4 | 425 | Khama, Botswana | March, May | 2.36 | – | – |
| Koeleria pyramidata | Caespitose | C3 | 835 | Kansas, USA | Grow. Seas. | 1.715 | – | – |
| Leymus chinensis | Rhizomatous | C3 | 471 | Jilin, China | September | 1.5–1.816 | – | – |
| Nassella viridula | Caespitose | C3 | 499 | South Dakota, USA | Autumn | 3.514 | – | – |
| Pascopyrum smithii | Rhizomatous | C3 | 339 | Eastern Montana, USA | March, September, November | 3.4–3.912 | 3.2–6.112 | |
| 499 | Western South Dakota, USA | Autumn | 2.617 | |||||
| Pennisetum macrourum | Caespitose | C4 | 425 | Khama, Botswana | March, May | 3.36 | – | – |
| Piptochaetium napostaense | Caespitose | C3 | 344 | South-east La Pampa, Argentina | July/growing season | 1.6–2.318,19 | 0.6–1.418 | – |
| 400 | August–November | 2.520 | – | 2.0–2.2†20 | ||||
| Poa ligularis | Caespitose | C3 | 280 | AES, Argentina | Growing season | 1.2–2.921 | – | 1.2–2.8†21 |
| 400 | La Pampa, Argentina | August–November | 2.020 | – | 1.2–1.3†20 | |||
| Schizachyrium scoparium | Caespitose | C4 | 835 | Kansas, USA | October | 5.322 | – | – |
| Sorghastrum nutans | Rhizomatous | C4 | 629 | Nebraska, USA | November | 6.023 | – | – |
| 835 | Kansas, USA | November | 7.023‡ | |||||
| 1041‡ | Oklahoma, USA | November | 3.523 | |||||
| Sporobolus heterolepis | Caespitose | C4 | 835 | Kansas, USA | Growing season | 3.415 | – | – |
| Stipa gynerioides | Caespitose | C3 | 344 | South-east La Pampa, Argentina | July | 2.2–2.318 | 0.7–2.118 | – |
| Stipa tenuis | Caespitose | C3 | 344 | La Pampa, Argentina | June, July/growing season | 1.7–2.618,19,24 | 0.7–1.618,24 | – |
| Stipagrostis uniplumis | Caespitose | C4 | 320 | Kang, Botswana | March | 1.820 | – | – |
1 Busso et al., 1989; 2Mueller and Richards, 1986; 3Mullahey et al., 1991; 4Ott and Hartnett, 2015c; 5Ott and Hartnett, 2012a; 6Dalgleish et al., 2012; 7Hartnett et al., 2006; 8Russell and Vermeire, 2015; 9Hendrickson and Briske, 1997; 10N’Guessan, 2007; 11Reichmann and Sala, 2014; 12Russell et al., 2015; 13Cable, 1971; 14Ott and Hartnett, 2015b; 15Dalgleish et al., 2008; 16Li and Yang, 2011; 17Ott and Hartnett, 2015a; 18Pelaez et al., 1997; 19Becker et al., 1997; 20Pelaez et al., 2009; 21Busso et al., 2011; 22N’Guessan and Hartnett, 2011; 23Carter et al., 2014; 24Busso et al., 1993.
*In a grazed pasture; †directly grazed or clipped; ‡sampled in a drought year well below MAP; note that rhizomatous grasses may have buds that were uncounted on their rhizomes.
AES, Agropecuarian Experimental Station near San Carlos de Bariloche; MAP, mean annual precipitation.
Bud bank size is also often studied at the community level as bud density per unit soil volume (Table 4). Whole-community assessment enables examination of climatic gradients (Qian et al., 2017) or comparisons of disturbance regimes (Fidelis et al., 2014) and has the advantage that it can be directly compared with other ecological information obtained at the community level, such as productivity. This was demonstrated by Dalgleish and Hartnett (2006) when they tested whether bud bank densities decreased as water limitation increased and productivity decreased in grasslands (Knapp and Smith, 2001). They measured grass bud bank densities across a precipitation gradient from mesic tallgrass prairie to desert grasslands and found a strong decline in bud densities from the most mesic to most arid grasslands. These results implied that productivity on the dry end of the gradient was limited by water availability and remained low even in exceptionally wet years due to meristem limitations, as it lacked a large bud bank needed to increase biomass production.
Table 4.
Bud bank sizes of grassland communities (number of buds per m2)
| Ambient | Grazed | Burned | Drought | Disturbed soil | |
|---|---|---|---|---|---|
| Campos grassland (Brazil)1 | Total: 302–398 | Total: 2408 | Total: 1122 | – | – |
| Grass: 171 | Grass: 1418 | Grass: 9 | |||
| Forb: 115 | Forb: 978 | Forb: 933 | |||
| Woody:15.8 | Woody: 12 | Woody: 180 | |||
| Tallgrass prairie (USA) | Total: 7332–13 0453 | Total: 27505–13 0403 | Total: 1830–34102,5,6 | Total: 99003 | Total: 12647 |
| Grass: 3642–10 2003,4 | Grass: 25005–99903 | Grass: 978–33002,5,6 | Grass: 89003 | ||
| Forb: 3702–2 9253 | Forb: 2505–30503 | Forb: 23–8572,5,6 | Forb: 10003 | ||
| Southern mixed-grass prairie (USA) | Total: 581–5956 | – | – | – | – |
| Grass: 544–5766 | |||||
| Forb: 19–376 | |||||
| Short-grass steppe (USA) | Total: 7306 | – | – | – | – |
| Grass:7206 | |||||
| Forb: 106 | |||||
| Desert grassland (USA) | Total: 1466 | – | – | – | – |
| Grass: 1376 | |||||
| Forb: 96 | |||||
| Cultivated monospecific stand of Leymus chinensis | Total: 4008 | – | – | – | – |
| Grass: 4008 | |||||
| Forb: 08 | |||||
| Temperate steppe (Inner Mongolia, China) | Total: 1000–30009 | ||||
| Grass: 500–25009 | |||||
| Forb: 0–6509 |
Peak bud bank densities were used in this table and were estimated from graphs in the literature.
1 Fidelis et al., 2014*; 2Benson et al., 2004; 3VanderWeide and Hartnett, 2015; 4VanderWeide et al., 2014; 5Dalgleish and Hartnett, 2009; 6Dalgleish and Hartnett, 2006; 7Rogers and Hartnett, 2001; 8Zhang et al., 2009; 9Qian et al., 2017.
*Did not include buds of tussock grasses in community estimates.
Stage and age structure of bud banks
Buds can also exhibit different size or development classes, which are often correlated with their location on a bud-bearing organ (e.g. Mueller and Richards, 1986). Apical buds typically are larger than other buds and preferentially serve as the source for renewal of above-ground shoots after a dormant season, while other, smaller buds remain dormant under correlative inhibition until shoot flowering or death. Buds may even be characterized by preformation of flowering parts of the future shoot (Diggle, 1997) or may develop as juvenile below-ground stems (budlings; Ott and Hartnett, 2015b). The preformed buds are prepared for quick growth (Schnáblová et al., Institute of Botany, Czech Academy of Sciences, Průhonice, Czech Republic, unpubl. res.) and budlings are readily prepared to respond to disturbance or changes in resources or environmental conditions (Ott and Hartnett, 2015b).
Buds of different morphological origin (i.e. apical, axillary and adventitious buds) also have slightly different functions in the bud bank (see Bud Outgrowth section above). Shoot size, architecture, flowering and demography are affected by both the type of bud and the position of the bud from which the shoot developed (Carlsson and Callaghan, 1990; Martínková and Klimešová, 2017). For example, in the herb Rorippa palustris, Bartušková and Klimešová (2010) showed that shoots originating from axillary buds were more branched and produced more seeds than those originating from adventitious buds on roots. Considering the proportion of the bud bank that is at each developmental stage, from each morphological origin or belonging to each bud production cohort enables the stage, origin or age structure of the bud bank to be described.
Bud bank seasonality (phenology)
In addition to examining bud bank structure at key life history points (e.g. before seasonal renewal), examining the fluctuations of bud bank structure or size throughout one annual cycle will provide further insight into when the species will be most resilient and susceptible to disturbance. The seasonal bud bank dynamics of species coexisting in one community may differ substantially. For example, dominant C4 grasses in North American prairies tend to show strict synchrony in bud dormancy and tiller recruitment timing (Hendrickson and Briske, 1997; Ott and Hartnett, 2012a), whereas dominant C3 grasses maintain a relatively constant bud bank size throughout the year and asynchrony with buds in various stages of development (Fig. 5) (Ott and Hartnett, 2012a, 2015b). Timing of bud bank development may differ intraspecifically in relation to growing conditions or when found in different systems. For example, a sedge from seasonally flooded systems demonstrated that populations experiencing long-term inundation showed more damped changes in bud bank densities compared with populations experiencing short-term inundation (Chen et al., 2015a). A C4 grass delayed its bud natality when located in a cooler, more arid habitat with a shorter growing season at the edge of its range (Ott and Hartnett, 2015c).
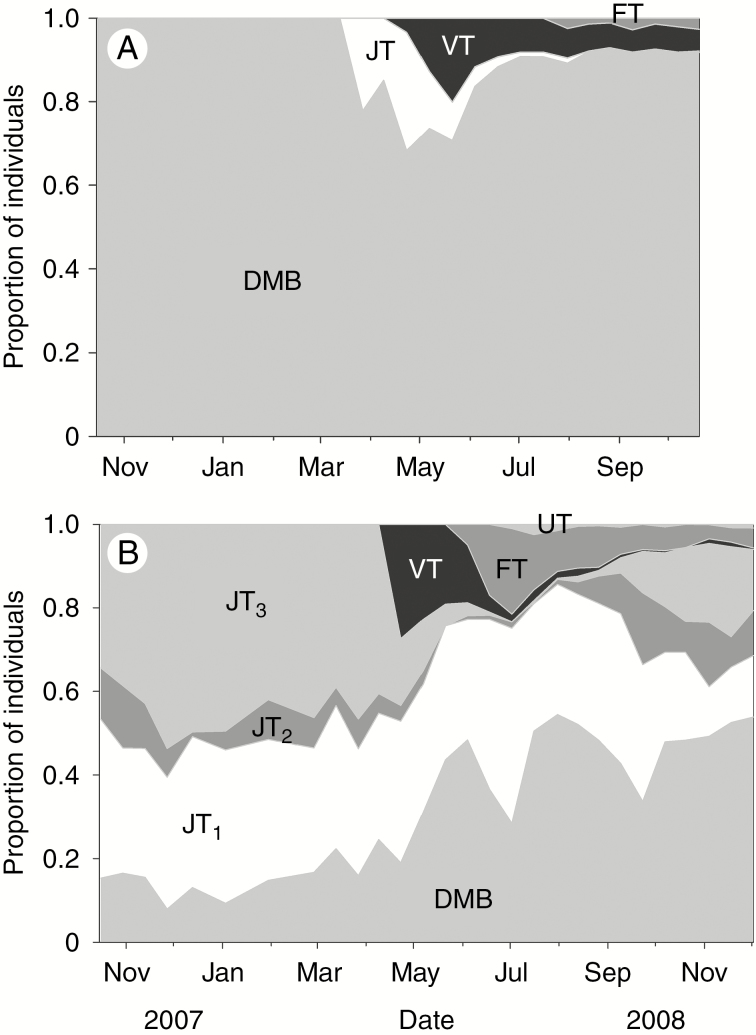
Fig. 5.
Proportion of individuals in different bud and tiller stages of (A) Andropogon gerardii and (B) Dichanthelium oligosanthes. DMB, developing or mature buds; JT, juvenile tillers (three size classes for D. oligosanthes: 1, no extended leaf; no photosynthesis; 2, no extended leaf, photosynthesis; 3, extended photosynthesizing leaf); VT, vegetative tillers; FT, flowering tillers; UT, unknown due to culm damage. Juvenile tillers are defined as having the primary shoot axis emerged past the bud’s prophyll but are typically still below ground. A. gerardii showed a high degree of synchrony in bud transitions, whereas D. oligosanthes showed asynchrony, with buds and tillers in various stages of development present at any given time. (Figure adapted from Ott and Hartnett, 2012a.)
The combined bud bank seasonal phenology of a community can provide a broader picture of points in the community that are vulnerable to disturbance or climate shifts. In herbaceous communities, seasonal changes in bud bank densities are closely tied to the timing of rhizome growth and above-ground stem recruitment. In temperate grasslands, grass bud bank densities are lowest in summer to winter and reach peak densities in early spring, coinciding with a pulse in tiller recruitment, whereas forb bud bank densities reach a peak in autumn and decrease by June during peak shoot recruitment (Dalgleish and Hartnett, 2006). Arid grasslands exhibit different patterns, with grass bud bank densities remaining very low over the winter while stem densities are still high. By the time stems senesce, bud densities reach a peak and are available for the next season of tiller renewal (Dalgleish and Hartnett, 2006). On the other hand, forbs in arid grasslands exhibit low and fairly constant bud bank densities, with variable shoot recruitment patterns throughout the year (Dalgleish and Hartnett, 2006; Qian et al., 2015).
Bud bank spatial structure
The bud bank is a crucial part of plant architecture as variation in the spatial patterns of bud production and outgrowth results in different plant growth forms (Briske, 1991; Perreta et al., 2011). Outgrowth of a bud can be upward (in grasses this is called intravaginal outgrowth, i.e. within the subtending leaf sheath), resulting in a new shoot immediately adjacent to the parent (Fig. 6), or horizontal (in grasses this is called extravaginal outgrowth, i.e. through the subtending leaf sheath). A horizontally growing bud may produce a horizontal stem, stolon or rhizome of varying length (Perreta et al., 2011). The species-specific pattern of bud outgrowth results in different growth forms, such as caespitose (bunchgrass), intermediate or spreading rhizomatous/stoloniferous growth forms (Briske, 1991), and at the same time influences the spatial structure of the bud bank. For example, long rhizomes typically bear larger below-ground bud banks than short ones (Zhang et al., 2009; Ott and Hartnett, 2012a, 2015a). Axillary buds along a rhizome, however, are more likely to remain dormant than apical rhizome buds or buds at the base of the parent plant (Zhang et al., 2009; Ott and Hartnett, 2015a). Therefore, most bud outgrowth occurs either directly at the base of the parent plant or at the most distal location along the rhizome. The timing of bud outgrowth may also influence plant architecture. Monocot buds that become rhizomes typically grow out before buds that become new tillers near the base of the parent shoot (Zhang et al., 2009; Ott and Hartnett, 2015a; Chen et al., 2015b). The initial investment in producing more distal rhizome buds could be part of a bet-hedging strategy ensuring local persistence while providing a means for dispersal. Rhizomes could remain short if necessary, placing buds close to the parent shoot, or could elongate as resources are available, placing buds further away (Ott and Hartnett, 2015a).
Fig. 6.
A bud of the perennial grass Schizachyrium scoparium is growing out into an intravaginal tiller (i.e. stem) within the subtending leaf sheath of the parent plant. Here, the bud has recently separated from its protecting prophyll. A bud growing extravaginally would have penetrated laterally through the subtending leaf sheath on the right.
In addition to this variation in horizontal growth, there are also ecological implications of the vertical distribution of buds within the soil. Although buds may be located within the first 15 cm of soil, most below-ground buds occur between 0 and 5 cm from the soil surface (Vesk et al., 2004; Klimešová and Klimeš, 2007). More deeply positioned buds are likely favoured in environments subject to frost, drought, fire or soil disturbance (e.g. ploughing, digging by animals) but they are at a disadvantage with slow growth and reduced competitive ability. For example, fire suppression results in excluding species with deep below-ground bud banks (Fidelis et al., 2014). The positioning of below-ground buds in herbs and shrubs usually provides sufficient protection of the bud bank. Therefore, different protective tissues and morphologies are usually found in the above-ground bud bank (Pausas et al., 2018).
BUD BANKS AND DISTURBANCE
The bud bank is predicted to play a major role in population and community regeneration when disturbance frequency is intermediate (Bellingham and Sparrow, 2000; Clarke et al., 2015). This can be observed on a large scale as many severely and frequently disturbed communities are dominated by annuals, while communities disturbed very rarely are dominated by trees, both growth forms that usually lack a bud bank. Communities between those extremes, as well as communities undergoing succession, show a higher abundance of perennial herbs and shrubs that regenerate from bud banks (Latzel et al., 2008; Herben et al., 2018). The pattern and rate of regeneration following disturbance are determined by interactions among the rate of depletion of the bud bank, the rate of new bud production, and the amount of resources available to support the regeneration process. An increasing number of studies suggest that the capacity for regeneration may be constrained more by bud bank size than by the amount of stored reserves (Cruz et al., 2003). For example, age-related declines in regenerative capacity are linked to declining bud banks due to bud senescence in some species (e.g. Bond and Midgley, 2001; Vesk, 2006). Similarly, due to an undeveloped bud bank, very young plants may be more vulnerable to disturbance than mature ones (Martínková et al., 2004; Klimešová et al., 2009).
Generalizations about the bud bank’s role in the response of plants to disturbance is challenging as not only bud bank parameters must be standardized for this purpose but also disturbance parameters need to be comparable. The response of a species to disturbance depends on the frequency, timing, intensity and type of disturbance and there is so far insufficient standardization for different disturbance regimes (Herben et al., 2016). For the flora of Central Europe, species optima (indicator values) were calculated along disturbance severity and disturbance frequency gradients (Herben et al., 2016). These disturbance indices provided researchers with a tool for studying bud banks in relation to disturbance in Europe. Other regions, however, await similar assessments. Another reason that precludes proper comparison across broad ecological gradients is that disturbance regimes interact with climate and they mutually affect the growth form composition of vegetation and hence bud banks of resident species. The effects of all these factors is difficult to disentangle.
The ecosystems and disturbance regimes most often studied are fire-prone woodlands and shrublands (Bell and Ojeda, 1999; Lloret et al., 1999; Bellingham and Sparrow, 2000; Clarke et al., 2013, 2015), grasslands across several continents subjected to fire, grazing, frost or drought (Busso et al., 1989; Brando and Durigan, 2004; Wang et al., 2004; Russell et al., 2015), forests damaged by wind throw or by anthropogenic activities such as slash-and-burn agriculture or logging (e.g. Bellingham et al., 1994; Kammesheidt, 1999; Guero-Campo et al., 2006), aquatic and wetland habitats with water currents and fluctuations (Barrat-Segretain et al., 1998), and arable land (Leakey, 1991).
Drought and nutrient enrichment
The bud bank response of plant populations and communities to drought as well as nutrient enrichment has been primarily studied in temperate grasslands. Bud banks can offer stability during drought conditions. For example, even though the species composition of the bud bank shifted, the overall bud densities of forb and grasses remained steady, enabling shoot densities to also remain stable during a 2-year drought (VanderWeide et al., 2014). However, bud banks of recently established communities without sufficient species diversity or communities experiencing severe drought can decrease in size, which could inhibit or prolong the time it takes for the community to recover (Carter et al., 2012; VanderWeide and Hartnett, 2015). Drought affects the bud bank by either reducing the number of shoots per plant or the number of buds produced per shoot (e.g. Reichmann and Sala, 2014). Drought can delay or reduce tiller growth, which affects bud production (Busso et al., 1989), or halt bud production prematurely (e.g. Reichmann and Sala, 2014). Therefore, a drought that occurs during the peak period of bud production and outgrowth would have the greatest effect on bud bank size (VanderWeide et al., 2014). Although bud bank densities appear to be positively correlated with annual precipitation, previous-year rainfall conditions can produce lag effects on current-year bud outgrowth (Reichmann and Sala, 2014) (Fig. 7). As droughts become more severe, increase in duration, and occur at times of peak bud production, community resiliency may decline as drought forces buds into long-term dormancy that exceeds their natural longevity. Thus, extreme long-term drought may exceed the buffering capacity of bud banks and lead to declines in perennial plant abundance (Qian et al., 2017; Bolles and Forman, 2018).
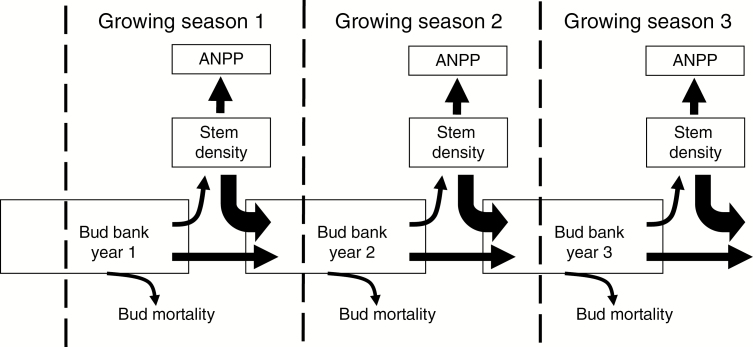
Fig. 7.
Conceptual diagram of the relationship between the bud bank, above-ground stem density and above-ground net primary production (ANPP). Modified from Ott and Hartnett (2012a) and Reichmann and Sala (2014). Not all species have buds that transition from year to year. Species that lack long lived buds (i.e. the horizontal arrow) are unable to buffer against inter-annual climatic variability. Long-lived bud banks serve as a mechanism by which lag effects on stem densities and productivity are produced.
Bud bank responses to nutrients and soil types (e.g. saline soils) have not been examined extensively. Experimentally increasing nitrogen availability to C3 grasses mitigated the reduction in bud numbers per tiller caused by fire, increased the number of active buds per tiller and increased bud outgrowth (Ye et al., 2006; Williamson et al., 2012; Russell and Vermeire, 2015). In C4 grasses, nitrogen seems to have a negligible effect on bud outgrowth or bud and tiller population growth rates (Dalgleish et al., 2008; Williamson et al., 2012). Therefore, nutrients may alter bud bank size and dormancy, thereby causing bud bank responses to differ between plant functional groups.
Regional or global environmental change may significantly alter both disturbance regimes and bud bank dynamics in many plant communities. Bud bank seasonality (i.e. phenology) will shift in response to changes in water availability and temperatures. In addition to altering drought and flood frequency and intensity in many regions, climate change will affect fire regimes globally and will also likely change patterns of resource availability and bud bank structure and dynamics in ways that significantly change woody and herbaceous plant species’ responses to fire, herbivory and other disturbances.
Herbivory
Herbivory can affect the bud bank by removing the source of buds, reducing photosynthates needed for bud development and/or by stimulating the outgrowth of buds. Increased shoot production following defoliation could be due to internal changes such as the release of apical dominance and increased growth rates in residual or re-growth foliage leading to reduced dormancy in existing and newly formed buds (e.g. Mueller and Richards, 1986) and/or the production of accessory buds that quickly grow out. Large herbivores also can influence bud outgrowth by increasing light and nutrient availability through reducing canopy density, dung and urine deposition, and more rapid decomposition of higher-quality litter. Because the bud bank is below ground, direct mortality of buds by grazing is minimal (Briske, 1991). However, small herbivores such as lepidopteran larvae can burrow into the centre of herbaceous stems and selectively consume below-ground buds (Ott, 2009).
The response of bud production and bud bank size to herbivory varies with plant species, plant ontogeny and defoliation frequency and intensity. Herbivores that defoliate an above-ground stem may cause a small amount of bud mortality on the defoliated stem and temporarily increase bud dormancy until adequate photosynthetic tissue has been regrown to support bud outgrowth (Ott et al., 2017). In a number of bunchgrasses, a single defoliation event appears to have little influence on bud natality, size or activity, but repeated defoliation may reduce the overall bud bank size (e.g. Busso et al., 1989; Hendrickson and Briske, 1997; Pelaez et al., 2009; N’Guessan and Hartnett, 2011). Herbivory reduces bud density by stimulating the recruitment of new shoots from the bud bank and increasing shoot mortality, which may hinder bud replenishment. As a result, long-term grazing can deplete the bud bank (Dalgleish and Hartnett, 2009). The decline in bud bank size in heavily grazed North American grasslands suggests that bud limitation may constrain the capacity of grasslands to recover from grazing and may drive declines in the relative abundance of some species and overall grassland productivity.
Defoliation or injury can also alter the spatial distribution of buds within the plant. For example, in the grass Schyzachrium scoparium repeated grazing causes a shift in architecture from strong vertical growth of tillers to more prostrate, lateral growth due to a reduction in tiller height and a change from primarily intravaginal to extravaginal bud outgrowth (N’Guessan and Hartnett, 2011). Similar architectural changes, including reduced bud number and altered branching patterns, have recently been observed in other prairie grasses (M. Shaffer and D. C. Hartnett, unpubl. res.). Long-term grazing or exclusion of grazing can lead to changes in species composition and hence also in bud banks. In inner Mongolian grasslands, 82–92 % of new stem recruitment on heavily grazed sites occurred from buds at the base of tillers rather than buds on rhizomes, bulbs or roots, which is the typical source of outgrowth at ungrazed sites (Qian et al., 2015). These differences in bud bank architecture contribute to positive plant–grazer feedbacks that maintain grazing lawns by concentrating forage biomass and increasing foraging efficiency (Shaffer and D. C. Hartnett, unpubl. res.).
Fire
Fire can alter bud bank dynamics via a variety of mechanisms, including direct bud mortality, alterations in the timing or frequency of bud transitions, and reductions in bud production. Most bud bank studies involving fire have focused on grasslands and to a lesser degree shrublands. In grasslands, immediate fire effects are primarily mediated through changes in bud natality and/or bud-to-tiller transition rates. Due to the high thermal resistance of soil and the very brief heat pulse associated with grassland fires, lethal heating is limited to the soil surface. Thus, there is negligible direct mortality of live buds, accounting for the high resilience of grasslands to fire (Scott et al., 2010). A wide diversity of below-ground bud-bearing organs exist in fire-prone systems (Pausas et al., 2018) and their position and bud position on them can determine survival after fire (e.g. Gonzalez et al., 2015). Among grasses, fire causes higher mortality in caespitose than in rhizomatous species as their buds are typically closer to the soil surface, where fuel is concentrated (Busso et al., 1993; Russell et al., 2015).
In fire-prone grasslands, the main effect of fire is the removal of accumulated litter and standing dead shoots, which greatly increases light at the soil surface. With adequate precipitation, this often stimulates bud outgrowth, resulting in higher stem densities and net primary production (Russell et al., 2015). Fire affects all plants within the community, unlike grazing, which selectively impacts only the palatable species. Therefore, fires may synchronize bud outgrowth and stem recruitment among species and potentially the timing of bud production (Benson and Hartnett, 2006). However, functional groups, such as forbs and grasses, may exhibit opposite responses to fire (e.g. Hartnett, 1991). In the long term, fire shifts the relative abundances of species within a community through its effects on bud bank dynamics (Hartnett, 1991; Russell and Vermeire, 2015).
The impact of fire on the bud bank varies according to fire frequency, seasonality and intensity. Total community bud bank density increases with increasing fire frequency in both North American prairies and South American Campos grasslands (Benson et al., 2004; Fidelis et al., 2014). The reduction of the bud bank experiencing low fire frequency is likely caused by a combination of reduced bud and stem densities due to low nutrient availability, dense litter and associated low light conditions, and/or shifts in species composition. In the subtropical grasslands of Brazil, fire results in a reduction in the size of the deep below-ground grass bud bank and an increase in the forb bud bank, exactly opposite to the patterns found in North American prairies (Fidelis et al., 2014). However, North American studies included grass buds at or near the soil surface. The number of successful grass stem recruits emerging from buds varied with fire frequency, but stem survivorship did not, further indicating that the principal effect of fire frequency was on bud outgrowth rather than on bud or stem mortality (Benson and Hartnett, 2006). More work is needed to look at bud bank community responses to fire seasonality and intensity.
In shrub communities, resprouting from below-ground buds following fire is a key life-history trait of many woody species that has large effects on post-fire survivorship, population dynamics, plant community composition, and productivity. For many woody plants, resprouting from the bud bank is a key component of the persistence niche and contributes to the high resilience to fire in many woodland, savannah and shrubland ecosystems (Bond and Midgley, 2001). However, traits other than bud bank size, such as bud protection by bark and the position of buds relative to the flame zone, may also determine woody plant responses to fire. Finally, bud demography also influences resprouting capacity after fire. Bud longevity and mortality differ among species, and resprouting ability in woody plants often declines with age due to bud senescence (Sands and Abrams, 2009). Keeley et al. (2012) suggest that greater bud bank size at the juvenile stage in woody plants is adaptive because juvenile plants cannot escape fire. A lot of literature focuses on the above-ground resprouting of woody shrubs and herbaceous dicots and their trade-off with seed production (e.g. Richards and Lamont, 1996; Groom and Lamont, 2011). This represents a field closely related to below-ground bud banks and further investigation into the relationship between above-ground versus below-ground resprouting capacities in relation to seed production following fire is beyond the scope of this review (but see Clarke et al., 2013; Pausas et al., 2018). The low number of bud bank studies in woody plants may be due to the complexity of examining and counting buds protected by bark and embedded in woody structures (Burrows, 2007).
Anthropogenic disturbance
On arable lands, annual plants without bud banks are dominant; however, perennial weeds relying on a bud bank also exist (Leakey, 1991). These perennials are characterized by lateral clonal spread and often have the capacity to sprout from adventitious buds on roots (Klimešová et al., 2017b; Herben et al., 2018). Fragmentation of the plant body by ploughing or by management aimed at eradication of invasive plants supports species that can regenerate from roots which are often located deeper in the soil profile and are not bud-limited (e.g. Richards and Burningham, 2011). Sprouting from roots can also be found and represents a tenable strategy in short-lived monocarps, along with regeneration from seed (Klimešová et al., 2008, 2014). Because of the filtering effect of continued anthropogenic disturbance, perennial plants within arable systems are more likely to be able to regenerate quickly in response to these deep soil disturbances than the suite of perennial species in a system that has received little previous anthropogenic disturbance.
In addition to their role in responses to natural disturbances, bud banks play a key role in the restoration of communities disturbed and/or degraded by human activities. The restoration of herbaceous communities often begins with recruitment from sown seed, but further development of the community occurs primarily through subsequent clonal growth and recruitment from the bud bank (Mudrák et al., 2018). As occurs during natural succession, species composition in the early stages of many restorations is largely determined by traits such as seed dispersal, the available species pool, the local seed rain and seed banks, while bud bank traits are more important at later stages (Latzel et al., 2011). The restoration itself, however, can be conducted by directly introducing a bud bank (e.g. through transplantation or topsoil translocation), although such a possibility is restricted to situations when the donor community providing buds is already planned to be disturbed (Ferreira et al., 2015).
Multiple disturbances
Many plant populations rarely experience a single disturbance type. For example, grasslands are shaped jointly by fire, grazing and climatic variability, which all occur in different spatial and temporal patterns. Examination of the interacting effects of drought and grazing on bud bank dynamics in tallgrass prairie demonstrated that grass bud banks were quite insensitive to drought, and remained constant across drought and grazing treatments (VanderWeide et al., 2014). Under these conditions, grazing did not interact with drought to reduce the bud bank size, which indicates a rapid recovery of the above-ground plant community after the drought regardless of grazing during the drought. In addition to looking at multiple concurrent disturbances, it should be borne in mind that bud bank traits may shift more when experiencing multiple disturbances sequentially or in a specific sequence.
Biological invasions
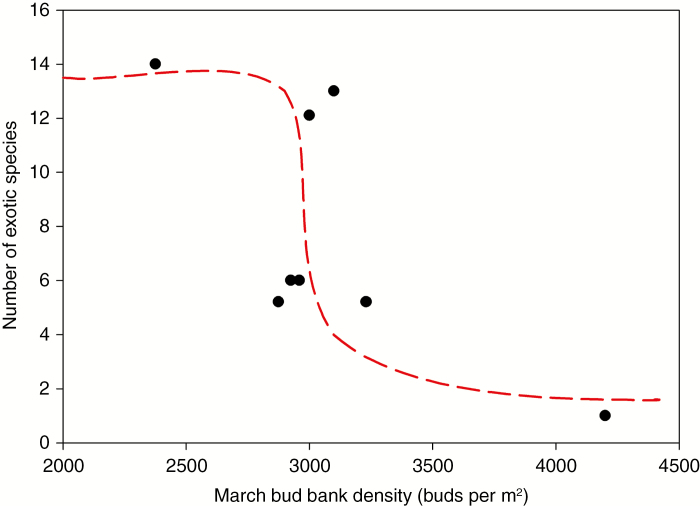
The ability conferred by bud banks to pre-empt available resources may also influence the invasibility of plant communities by exotic species. Resources are often made available in a community following disturbance. Davis et al. (2000) proposed that, given an adequate propagule supply, exotic species periodically successfully invade a plant community by pre-empting pulses of newly available resources. This hypothesis could be extended to argue that resident native species possessing a large bud bank confer resistance to invasion by allowing the resident species to rapidly pre-empt newly available resources before they can be captured by an exotic invader. Data from tallgrass prairies supporting this hypothesis show that the frequency of exotic species decreases significantly with increasing bud bank densities of resident species (Fig. 8). Conversely, for exotic species large bud banks may be a key trait that enables them to successfully invade novel communities (Klimešová, 2011). Woods et al. (2009) showed that the exotic invader Lespedeza cuneata maintained a significantly larger bud bank size than several congeneric native species in grasslands, suggesting that this trait contributed to its invasion success. Similarly, the invasive perennial grass Bromus inermis produced 2-fold the number of buds as the native perennial grass Pascopyrum smithii in a northern mixed-grass prairie (Ott et al., 2017). Classical examples of the bud bank’s role in invasion come from aquatic habitats where plants readily spread by detached buds or plant fragments that can easily regenerate in water or moist conditions. Rivers serve as spreading corridors for the terrestrial plant Fallopia bohemica, as well as for aquatic invaders such as Elodea canadensis or Eichhornia crassipes (Villamagna and Murphy, 2010; Zehnsdorf et al., 2015). The importance of the bud bank to invader survival becomes readily apparent during eradication management actions. For example, Rosa rugosa, which occurs on sand dunes in Denmark, resists mechanical removal due to its unlimited bud bank of adventitious buds located on deep roots (Kollmann et al., 2011).
Fig. 8.
The relationship between exotic plant species richness and total below-ground bud bank density across multiple watershed units in the tallgrass prairie landscape at Konza Prairie Biological Station, KS, USA (D. C. Hartnett and H. J. Dalgleish, unpubl. res.). Bud bank densities were measured at the end of the winter dormant season (March) and plant species composition was measured during the growing season (May to August).
FUTURE RESEARCH NEEDS
Our assessment of the current understanding of the structure and dynamics of below-ground bud banks in plants indicates that progress has been made in identifying bud bank traits of several species; however, many questions remain unanswered. Below we summarize several suggestions for future research.
Data about bud banks are still scarce. The largest bud bank dataset is focused on the flora of Central Europe and is deposited in the CLO-PLA database (Klimešová and Klimeš, 2008; Klimešová et al., 2017a). Bud bank characteristics stored in this database are not based on exact counts of buds but represent rough categories that were obtained from plant descriptions in the literature or herbarium specimens, or by morphological study in the field, and they only partly capture intraspecific variability. Exact counts of buds for several individuals belonging to one species are available for only tens of species, have focused on grasslands in North and South America and China (e.g. Table 3), and are scattered in the literature. To more fully understand the roles of bud banks, additional detailed studies of below-ground bud bank traits are needed for many more species in a variety of plant community types. Studies that provide detailed descriptions of bud demography, dormancy patterns and transitions and their link to above-ground shoot population dynamics will be valuable. Field studies of more plant species are also needed to better understand the influence of bud type, age, status and spatial position on plant population dynamics. We also need more data on understudied bud bank traits such as preformation, carbohydrate storage in bud-bearing organs and bud protection. By using bud bank traits that can be gleaned from both herbarium specimens and field studies, we can obtain a better understanding of their dynamics and the ecological implications of maintaining a bud bank, especially if these data can be centrally housed in a comprehensive database, which makes it easier for larger inferences to be made.
As research on bud bank structure and dynamics continues, it will be necessary to standardize methodologies for bud classification and protocols in order to facilitate comparisons among studies (Ottaviani et al., 2017). Klimešová and Klimeš (2007) have helped clarify how the bud bank of herbaceous species is defined by extending Harper’s definition to include (1) renewal and regenerative buds, (2) aerial and below-ground buds, (3) buds on plant fragments, and (4) adventitious buds. Although clonal growth organ biomass (VanderWeide and Hartnett, 2015) and number of nodes or leaves on shoots (Klimešová and Klimeš, 2007) can be used as indirect means of estimating bud numbers, studies of herbaceous bud bank dynamics will still require direct counts of bud numbers until indirect means can be calibrated across a wide variety of taxa. To facilitate comparisons among studies, the methodology of bud counting needs to be exact as the literature contains a wide range of ways in which buds are being counted. Some studies only count buds that are deeply buried, others only count buds if they are visible at or above a specific magnification, while other studies only count buds on some of the bud-bearing organs of a species and not others (e.g. buds counted on bases of vertical stems but not along horizontal rhizomes).
Bud counting also requires a strict definition of the stage at which a bud transitions into a shoot that can be objectively identified across or within taxa with negligible investigator bias. In grasses, buds are enclosed in their first leaf, called a prophyll. Extension of a bud past its prophyll can be used as the discrete developmental transition distinguishing buds from tillers (e.g. Ott and Hartnett, 2015a, b). In most population studies, bud activity is determined metabolically or developmentally. Metabolic activity detects bud respiration, whereas bud development is detected by increasing bud size within the prophyll. Both have their limitations as metabolically active buds could still be dormant due to external constraints and developmentally active buds may only occur briefly before transition to tillers, especially if their prophyll is senesced. There is a need to link bud outgrowth and preformation to bud metabolic activity.
Thus far, much research on below-ground bud banks has been descriptive, and only a few studies have examined the response of bud banks to environmental variation and/or disturbance. To our knowledge, no studies have explicitly assessed the population consequences of the maintenance of a persistent bud bank. Thus, many of the hypotheses regarding the ecological implications of bud dormancy and the maintenance of a bud bank discussed in this review remain untested. For example, within a community, do plant species with larger bud banks show dynamics different from those with little or no bud bank? How does bud bank dormancy interact with bud bank size to impact meristem limitation? What role does plant ontogeny have in determining bud bank size and the capability of a plant to respond to disturbance? Additional studies of bud bank structure and dynamics in communities recovering from major disturbance and in restorations will provide greater insight into the changing role of bud banks during succession and the consequences of maintaining a bud bank. Similarly, considering costs of bud bank formation might bring a better understanding of various strategies of plant response to disturbance. Although costs of individual buds are negligible (Vesk and Westoby, 2004), building bud-bearing organs and carbohydrate reserves accompanying buds may incur costs that should be taken into account.
For many terrestrial plant species, the below-ground bud bank is a critical feature determining the resilience of their populations to environmental change. Patterns of dormancy are key to the ecological functioning of a bud bank, and the fraction of buds maintained in a dormant versus active stage is a major determinant of bud and above-ground plant population dynamics. We hypothesize that where a plant species falls along the continuum from negligible dormancy and bud accumulation to a very high dormant fraction and high bud bank size will be a significant predictor of its sensitivity or resilience to environmental change. Given that the renewal, growth rate and dynamics of many perennial plant populations are strongly influenced by their bud banks (e.g. they could be meristem-limited due to low bud number or high bud dormancy), predicting their responses to environmental change will require a greater understanding of the environmental sensitivity or resilience of different bud life history stages. At the community level, responses to disturbance depend on the predominant bud bank traits and the differential response of the various species’ bud banks to disturbance. Detailed study of how bud banks mediate plant population responses to short-term resource pulses (e.g. disturbance events of varying intensity and frequency) or long-term presses (e.g. nutrient enrichment, warming) as well as multiple simultaneous or sequential disturbances of different types (e.g. grazing following fire) will be important to further our understanding not only of the mechanisms operating below ground that shape plant population and community dynamics, but also the differential sensitivity of different plant populations, communities and ecosystems to environmental change.
Below-ground buds are important stages in the life histories of many plants, and the various stages and transitions that determine bud bank and above-ground growth dynamics are suitably modelled by stage-structured matrix models or integral projection models (e.g. Ott and Hartnett 2015c). Most demographic models of clonal species do not explicitly incorporate the bud bank (H. J. Dalgleish, R. Salguero-Gomez, J. P. Ott, unpubl. res.). Like seed banks, the bud bank has dynamic properties of its own. Cohen (1966) and others modelled the parameters affecting the size of the seed bank to calculate the optimal strategies for different theoretical environments. Using additional empirical studies aimed at understanding optimal bud production, dormancy and outgrowth strategies under different conditions, similar models could be developed to calculate the optimal bud bank strategies (e.g. size of the bud bank and proportion of dormant buds) for different environments. The development of such models can provide insight into the consequences of bud bank traits for overall population dynamics and predicting how bud banks might mediate plant population responses to changing environments. But building such models will require detailed quantitative study of the numbers of buds and their fates and transitions, and how various environmental factors affect bud production, dormancy and outgrowth. Contributions of models that explicitly incorporate the bud bank can be gathered as part of larger efforts, such as COMPADRE (Salguero-Gomez et al., 2015), that will help synthesize data from model studies.
CONCLUSIONS
Below-ground plant bud banks serve two primary ecological functions. They can serve as vegetative propagules for above-ground population renewal and growth and/or they can function in regeneration following plant damage due to disturbance. Bud bank demography (i.e. dynamics) is driven by inputs (bud natality), bud availability for outgrowth (dormancy), and outputs (bud mortality and outgrowth). By considering the characteristics of both individual buds and their collective dynamics as a bud bank, bud bank traits can be identified and used to compare plant species and communities. The pattern and rate of regeneration following disturbance are determined by interactions among the rate of depletion of the bud bank, the rate of new bud production and the amount of resources available to support the regeneration process. In general, differential bud bank responses to disturbance among growth forms and among species underlie disturbance effects on plant species composition and diversity in many ecosystems. Although the ecological implications of below-ground bud banks have not all been extensively tested, this synthesis demonstrates that bud banks can provide a demographic storage effect and stabilize population dynamics under various disturbance types, affect invasion success, and alter above-ground plant species composition and productivity through meristem limitation.
FUNDING
This work was supported by the National Science Foundation Long Term Ecological Research program (DEB 1440484) and the Rocky Mountain Research Station.
ACKNOWLEDGEMENTS
We thank David Briske and an anonymous reviewer for helpful comments that improved the manuscript. We thank Harmony Dalgleish for assistance with initial grass bud bank data compilation of Tables 3 and 4, and Alena Bartušková for redrawing Figs 1 and 3. The views expressed are strictly those of the authors and do not necessarily represent the positions or policy of their respective institutions.
LITERATURE CITED
- Abernathy VJ, Willby NJ. 1999. Changes along a disturbance gradient in the density and composition of propagule banks in floodplain aquatic habitats. Plant Ecology 140: 177–190. [Google Scholar]
- Ahn J, Franklin SB, Douhovnikoff V. 2017. Epigenetic variation in clonal stands of aspen. Folia Geobotanica 52: 443–449. [Google Scholar]
- Alfonso-Corrado C, Clark-Tapia R, Mendoza A. 2007. Demography and management of two clonal oaks: Quercus eduardii and Q. potosina (Fagaceae) in central Mexico. Forest Ecology and Management 251: 129–141. [Google Scholar]
- Anderson JE, Inouye RS. 2001. Landscape-scale changes in plant species abundances and biodiversity of a sagebrush steppe over 45 years. Ecological Monographs 71: 531–556. [Google Scholar]
- Ayukawa E, Imura S, Kanda H. 2001. Bryophyte propagule bank in the Yukidori Valley, Langhovde, Antarctica. Antarctic Record 45: 320–328. [Google Scholar]
- Barrat-Segretain MH, Bornette G, Hering-Vilas-Bôas A. 1998. Comparative abilities of vegetative regeneration among aquatic plants growing in disturbed habitats. Aquatic Botany 60: 201–211. [Google Scholar]
- Bartušková A, Klimešová J. 2010. Reiteration in the short lived root-sprouting herb Rorippa palustris: does the origin of buds matter? Botany 88: 630–638. [Google Scholar]
- Bartušková A, Malíková L, Klimešová J. 2017. Checklist of root-sprouters in the Czech flora: mapping the gaps in our knowledge. Folia Geobotanica 52: 337–343. [Google Scholar]
- Becker GF, Busso CA, Montani T. 1997. Effects of defoliating Stipa tenuis and Piptochaetium napostaense at different phenological stages: axillary bud viability and growth. Journal of Arid Environments 35: 233–250. [Google Scholar]
- Bell TL, Ojeda F. 1999. Underground starch storage in Erica species of the Cape Floristic Region—differences between seeders and resprouters. New Phytologist 144: 143–152. [Google Scholar]
- Bellingham PJ, Sparrow AD. 2000. Resprouting as a life history strategy in woody plant communities. Oikos 89: 409–416. [Google Scholar]
- Bellingham PJ, Tanner EVJ, Healy JR. 1994. Sprouting of trees in Jamaican montane forests after a hurricane. Journal of Ecology 82: 747–758. [Google Scholar]
- Benson E, Hartnett DC. 2006. The role of seed and vegetative reproduction in plant recruitment and demography in tallgrass prairie. Plant Ecology 187: 163–177. [Google Scholar]
- Benson E, Hartnett DC, Mann KH. 2004. Belowground bud banks and meristem limitation in tallgrass prairie plant populations. American Journal of Botany 91: 416–421. [DOI] [PubMed] [Google Scholar]
- Bidartondo MI, Burghart B, Gebauer G, Bruns TD, Read DJ. 2004. Changing partners in the dark: isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proceedings of the Royal Society of London B, Biological Sciences 271: 1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles KC, Forman SL. 2018. Evaluating landscape degradation along climatic gradients during the 1930s dust bowl drought from panchromatic historical aerial photographs, United States Great Plains. Frontiers in Earth Science 6: 153. [Google Scholar]
- Bond WJ, Midgley JJ. 2001. Ecology of sprouting in woody plants: the persistence niche. Trends in Ecology and Evolution 16: 45–51. [DOI] [PubMed] [Google Scholar]
- Bonser SP, Aarssen LW. 1996. Meristem allocation: a new classification theory for adaptive strategies in herbaceous plants. Oikos 77: 347–352. [Google Scholar]
- Bonser SP, Aarssen LW. 2006. Meristem allocation and life-history evolution in herbaceous plants. Canadian Journal of Botany 84: 143–150. [Google Scholar]
- Brando PM, Durigan G. 2004. Changes in cerrado vegetation after disturbance by frost (Sao Paulo State, Brazil). Plant Ecology 175: 205–215. [Google Scholar]
- Briske DD. 1991. Developmental morphology and physiology of grasses. In: Heitschmidt RK, Stuth JW, eds. Grazing management: an ecological perspective. Portland: Timber Press, 85–108. [Google Scholar]
- Burrows GE. 2007. Epicormic strand structure in Angophora, Eucalyptus and Lophostemon (Myrtaceae) – implications for fire resistance and recovery. New Phytologist 153: 111–131. [Google Scholar]
- Busso CA, Mueller RJ, Richards JH. 1989. Effects of drought and defoliation on bud viability in two caespitose grasses. Annals of Botany 63: 477–485. [Google Scholar]
- Busso CA, Boo RM, Pelaez DV. 1993. Fire effects on bud viability and growth on Stipa tenuis in semiarid Argentina. Annals of Botany 71: 377–381. [Google Scholar]
- Busso CA, Gittins C, Becker GF, Ghermandi L. 2011. Tiller hierarchy and defoliation frequency determine bud viability in the grass Poa ligularis. Ecological Research 26: 985–997. [Google Scholar]
- Cable DR. 1971. Growth and development of Arizona cottontop (Trichachne californica [Benth.] Chase). Botanical Gazette 132: 119–145. [Google Scholar]
- Carlsson BA, Callaghan TV. 1990. Programmed tiller differentiation, intraclonal density regulation and nutrient dynamics in Carex bigelowii. Oikos 58: 219–230. [Google Scholar]
- Carter DL, VanderWeide BL. 2014. Belowground bud production is linked to population establishment in Sorghastrum nutans (Poaceae). Plant Ecology 215: 977–986. [Google Scholar]
- Carter DL, VanderWeide BL, Blair JM. 2012. Drought-mediated stem and below-ground bud dynamics in restored grasslands. Applied Vegetation Science 15: 470–478. [Google Scholar]
- Cellot B, Mouillot F, Henry CP. 1998. Flood drift and propagule bank of aquatic macrophytes in a riverine wetland. Journal of Vegetation Science 9: 631–640. [Google Scholar]
- Chen X, Deng Z, Xie Y, Li F, Hou Z, Li X. 2015a Belowground bud banks of four dominant macrophytes along a small-scale elevational gradient in Dongting Lake wetlands, China. Aquatic Botany 122: 9–14. [Google Scholar]
- Chen X, Li Y, Xie Y, Deng Z, Li X, Li F, Hou Z. 2015b Trade-off between allocation to reproductive ramets and rhizome buds in Carex brevicuspis populations along a small-scale elevational gradient. Scientific Reports 5: 12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PJ, Lawes MJ, Midgley JJ, et al. 2013. Resprouting as a key functional trait: how buds, protection and resources drive persistence after fire. New Phytologist 197: 19–35. [DOI] [PubMed] [Google Scholar]
- Clarke PJ, Lawes MJ, Murphy BP, et al. 2015. A synthesis of post-fire recovery traits of woody plants in Australian ecosystems. Science of the Total Environment 534: 31–42. [DOI] [PubMed] [Google Scholar]
- Cline MG. 1997. Concepts and terminology of apical dominance. American Journal of Botany 84: 1064–1069. [PubMed] [Google Scholar]
- Cohen D. 1966. Optimizing reproduction in a randomly varying environment. Journal of Theoretical Biology 12: 119–129. [DOI] [PubMed] [Google Scholar]
- Combroux ICS, Bornette G, Amoros C. 2002. Plant regenerative strategies after a major disturbance: the case of a riverine wetland restoration. Wetlands 22: 234–246. [Google Scholar]
- Cruz A, Perez B, Moreno JM. 2003. Resprouting of the Mediterranean-type shrub Erica australis with modified lignotuber carbohydrate content. Journal of Ecology 91: 348–356. [Google Scholar]
- Dalgleish HJ, Hartnett DC. 2006. Belowground bud banks increase along a precipitation gradient of the North American Great Plains: a test of the meristem limitation hypothesis. New Phytologist 171: 81–89. [DOI] [PubMed] [Google Scholar]
- Dalgleish HJ, Hartnett DC. 2009. The effects of fire frequency and grazing on tallgrass prairie plant composition and productivity are mediated through bud bank demography. Plant Ecology 201: 411–420. [Google Scholar]
- Dalgleish HJ, Kula AR, Hartnett DC, Sandercock BK. 2008. Responses of two bunchgrasses to nitrogen addition in tallgrass prairie: the role of bud bank demography. American Journal of Botany 95: 672–680. [DOI] [PubMed] [Google Scholar]
- Dalgleish HJ, Ott JP, Setshogo MP, Hartnett DC. 2012. Inter-specific variation in bud banks and flowering effort among semi-arid African savanna grasses. South African Journal of Botany 83: 127–133. [Google Scholar]
- Davis MA, Grime JP, Thompson K. 2000. Fluctuating resources in plant communities: a general theory of invasibility. Journal of Ecology 88: 528–534. [Google Scholar]
- Derner JD, Briske DD. 1999. Intraclonal regulation in a perennial caespitose grass: a field evaluation of aboveground and belowground resource availability. Journal of Ecology 87: 737–747. [Google Scholar]
- Diggle PK. 1997. Extreme preformation in alpine Polygonum viviparum: an architectural and developmental analysis. American Journal of Botany 84: 154–169. [PubMed] [Google Scholar]
- Ellstrand NC, Roose ML. 1987. Patterns of genotypic diversity in clonal plant species. American Journal of Botany 74: 123–131. [Google Scholar]
- Evert RF. 2006. Esau’s Plant anatomy: meristems, cells, and tissues of the plant body – their structure, function, and development. Hoboken: John Wiley. [Google Scholar]
- Fair J, Lauenroth WK, Coffin D. 1999. Demography of Bouteloua gracilis in a mixed prairie: analysis of genets and individuals. Journal of Ecology 87: 233–243. [Google Scholar]
- Ferreira MC, Walter BMT, Vieira DLM. 2015. Topsoil translocation for Brazilian savanna restoration: propagation of herbs, shrubs, and trees. Restoration Ecology 23: 723–728. [Google Scholar]
- Fidelis A, Appezzato-da-Gloria B, Pillar VD, Pfadenhauer J. 2014. Does disturbance affect bud bank size and belowground structures diversity in Brazilian subtropical grasslands? Flora 209: 110–116. [Google Scholar]
- Filartiga AL, Klimešová J, Appezzato-da-Glória B. 2017. Underground organs of Brazilian Asteraceae: testing the CLO-PLA database traits. Folia Geobotanica 52: 367–385. [Google Scholar]
- Geber MA, Watson MA, de Kroon H. 1997. Organ preformation, development, and resource allocation in perennials. In: Bazzaz FA, Grace J, eds. Plant resource allocation. San Diego: Academic Press, 113–141. [Google Scholar]
- Gonzalez SL, Ghermandi L, Pelaez DV. 2015. Growth and reproductive post-fire responses of two shrubs in semiarid Patagonian grasslands. International Journal of Wildland Fire 24: 809–818. [Google Scholar]
- Gremer JR, Sala A, Crone EE. 2010. Disappearing plants: why they hide and how they return. Ecology 91: 3407–3413. [DOI] [PubMed] [Google Scholar]
- Groom PK, Lamont BB. 2011. Regional and local effects on reproduction allocation in epicormics and lignotuberous populations of Banksia menziesii. Plant Ecology 212: 2003–2011. [Google Scholar]
- Guero-Campo J, Palacio S, Perez-Rontome C, Monserrat-Martí G. 2006. Effect of root system morphology on root-sprouting and shoot-rooting abilities in 123 plant species from eroded lands in north-east Spain. Annals of Botany 98: 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JL. 1977. Population biology of plants. London: Academic Press. [Google Scholar]
- Hartnett DC. 1991. Effects of fire in tallgrass prairie on growth and reproduction of prairie coneflower (Ratibida columnifera: Asteraceae). American Journal of Botany 78: 429–435. [Google Scholar]
- Hartnett DC, Setshogo MP, Dalgleish HJ. 2006. Bud banks of perennial savanna grasses in Botswana. African Journal of Ecology 44: 256–263. [Google Scholar]
- Hendrickson JR, Briske DD. 1997. Axillary bud banks of two semiarid perennial grasses: occurrence, longevity, and contribution to population persistence. Oecologia 110: 584–591. [DOI] [PubMed] [Google Scholar]
- Herben T, Nováková Z, Klimešová J, Hrouda L. 2012. Species traits and plant performance: functional trade-offs in a large set of species in a botanical garden. Journal of Ecology 100: 1522–1533. [Google Scholar]
- Herben T, Šerá B, Klimešová J. 2015. Clonal growth and sexual reproduction: tradeoffs and environmental constraints. Oikos 124: 469–476. [Google Scholar]
- Herben T, Chytrý M, Klimešová J. 2016. A quest for species-level indicator values for disturbance. Journal of Vegetation Science 27: 628–636. [Google Scholar]
- Herben T, Klimešová J, Chytrý M. 2018. Effects of disturbance frequency and severity on plant traits: an assessment across a temperate flora. Functional Ecology 32: 799–808. [Google Scholar]
- Horvath DP, Anderson JV, Chao WS, Foley ME. 2003. Knowing when to grow: signals regulating bud dormancy. Trends in Plant Science 8: 534–540. [DOI] [PubMed] [Google Scholar]
- Hughes EG. 1974. Coolibah trees return to life after 68 years ‘dead’. Arid Zone Newsletter, CSIRO, Perth 166. [Google Scholar]
- Hutchings MJ, Bradbury P. 1986. Ecological perspectives on clonal perennial herbs. Bioscience 36: 178–182. [Google Scholar]
- Jackson JBC, Buss LW, Cook RE. 1985. Population biology and evolution of clonal organisms. New Haven: Yale University Press. [Google Scholar]
- Kammesheidt L. 1999. Forest recovery by root suckers and above-ground sprouts after slash-and-burn agriculture, fire and logging in Paraguay and Venezuela. Journal of Tropical Ecology 15: 143–157. [Google Scholar]
- Keeley JE, Bond WJ, Bradstock RA, Pausas JG, Rundel PW. 2012. Fire in Mediterranean ecosystems: ecology, evolution and management. Cambridge: Cambridge University Press. [Google Scholar]
- Klimeš L. 2008. Clonal splitters and integrators in harsh environments of the Trans-Himalaya. Evolutionary Ecology 22: 351–367. [Google Scholar]
- Klimeš L, Klimešová J, Hendriks R, van Groenendael J. 1997. Clonal plant architecture: comparative analysis of form and function. In: de Kroon H, van Groenendael J, eds. The ecology and evolution of clonal plants. Leiden: Backhuys, 1–29. [Google Scholar]
- Klimešová J. 2011. Vegetative propagation. In: Simberloff D, Rejmánek M, eds. Encyclopedia of invasive introduced species. Berkeley: University of California Press, 678–679. [Google Scholar]
- Klimešová J, Klimeš L. 2007. Bud banks and their role in vegetative regeneration – a literature review and proposal for simple classification and assessment. Perspectives in Plant Ecology, Evolution and Systematics 8: 115–129. [Google Scholar]
- Klimešová J, Klimeš L. 2008. Clonal growth diversity and bud banks of plants in the Czech flora: an evaluation using the CLO-PLA3 database. Preslia 80: 255–275. [Google Scholar]
- Klimešová J, Martínková J. 2004. Intermediate growth forms as a model for the study of plant clonality functioning: an example with root sprouters. Evolutionary Ecology 18: 669–681. [Google Scholar]
- Klimešová J, Kociánová A, Martínková J. 2008. Weeds that can do both tricks: vegetative versus generative regeneration of the short-lived root-sprouting herbs Rorippa palustris and Barbarea vulgaris. Weed Research 48: 131–135. [Google Scholar]
- Klimešová J, Pokorná A, Klimeš L. 2009. Establishment growth and bud bank formation in Epilobium angustifolium: the effects of nutrient availability, plant injury and environmental heterogeneity. Botany 87: 195–201. [Google Scholar]
- Klimešová J, Maliková L, Rosenthal J, Šmilauer P. 2014. Potential bud bank responses to apical meristem damage and environmental variables: matching or complementing axillary meristems? PLoS One 9: e88093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimešová J, Nobis M, Herben T. 2015. Senescence, ageing and death of the whole plant: morphological prerequisites and constraints of plant immortality. New Phytologist 206: 14–18. [DOI] [PubMed] [Google Scholar]
- Klimešová J, Danihelka J, Chrtek J, de Bello F, Herben T. 2017a CLO-PLA: a database of clonal and bud bank traits of Central European flora. Ecology 98: 1179. [DOI] [PubMed] [Google Scholar]
- Klimešová J, Herben T, Martínková J. 2017b Disturbance is an important factor in the evolution and distribution of root-sprouting species. Evolutionary Ecology 31: 387–399. [Google Scholar]
- Knapp AK, Smith MD. 2001. Variation among biomes in temporal dynamics of aboveground primary production. Science 291: 481–484. [DOI] [PubMed] [Google Scholar]
- Kollmann J, Brink-Jensen K, Frandsen SI, Hansen MK. 2011. Uprooting and burial of invasive alien plants: A new tool in coastal restoration? Restoration Ecology 19: 371–378. [Google Scholar]
- de Kroon H, van Groenendael J, eds. 1997. The ecology and evolution of clonal plants. Leiden: Backhuys. [Google Scholar]
- Krumbiegel A. 2001. Vegetative reproduction strategies of pseudoannual plants in central Europe. Beiträge zur Biologie der Pflanzen 72: 287–313. [Google Scholar]
- Lang GA, Early JD, Martin GC, Darnell RL. 1987. Endo-, para-, and ecodormancy: physiological terminology and classification for dormancy research. HortScience 22: 371–377. [Google Scholar]
- Latzel V, Klimešová J. 2010. Transgenerational plasticity in clonal plants. Evolutionary Ecology 24: 1537–1543. [Google Scholar]
- Latzel V, Mihulka S, Klimešová J. 2008. Plant traits and regeneration of urban plant communities after disturbance: does the bud bank play any role? Applied Vegetation Science 11: 387–394. [Google Scholar]
- Latzel V, Klimešová J, Doležal J, Pyšek P, Tackenberg O, Prach K. 2011. The association of dispersal and persistence traits of plants with different stages of succession in Central European man-made habitats. Folia Geobotanica 46: 289–302. [Google Scholar]
- Leakey RRB. 1991. Adaptive biology of vegetatively regenerating weeds. Advances in Applied Biology 6: 57–90. [Google Scholar]
- Lee P. 2004. The impact of burn intensity from wildfires on seed and vegetative banks, and emergent understory in aspen-dominated boreal forests. Canadian Journal of Botany 82: 1468–1480. [Google Scholar]
- Li H, Yang Y. 2011. Bud banks of two perennial grasses: composition, size, dynamics and contribution to population maintenance during the flooded restoration succession on the Songnen Meadow, China. African Journal of Agricultural Research 6: 2198–2203. [Google Scholar]
- Liew J, Andersson L, Bostrom U, Forkman J, Hakman I, Magnuski E. 2013. Regeneration capacity from buds on roots and rhizomes in five herbaceous perennials as affected by time of fragmentation. Plant Ecology 214: 1199–1209. [Google Scholar]
- Lloret F, Verdu M, Flores-Hernandez N, Valiente-Banuet A. 1999. Fire and resprouting in Mediterranean ecosystems: insights from an external biogeographical region, the Mexical Shrubland. American Journal of Botany 86: 1655–1661. [PubMed] [Google Scholar]
- Mandák B, Pyšek P. 2001. Fruit dispersal and seed banks in Atriplex sagittata: the role of heterocarpy. Journal of Ecology 89: 159–165. [Google Scholar]
- Martínková J, Klimešová J. 2017. Position of tillers in a clone determines their fate and growth: an example of clonal grass Phalaris arundinacea. Folia Geobotanica 52: 317–325. [Google Scholar]
- Martínková J, Kočvarová M, Klimešová J. 2004. Resprouting after disturbance in the short-lived herb Rorippa palustris (Brassicaceae): an experiment with juveniles. Acta Oecologica 25: 143–150. [Google Scholar]
- McIntyre GI. 1970. Studies on bud development in rhizome of Agropyron repens. 1. Influence of temperature, light intensity, and bud position on pattern of development. Canadian Journal of Botany 48: 1903–1909. [Google Scholar]
- McSteen P. 2009. Hormonal regulation of branching in grasses. Plant Physiology 149: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley JJ. 1996. Why the world’s vegetation is not totally dominated by resprouting plants: because resprouters are shorter than reseeders. Ecography 19: 92–95. [Google Scholar]
- Mitchell KJ. 1953. Influence of light and temperature on the growth of ryegrass (Lolium spp.). II. The control of lateral bud development. Physiologia Plantarum 6: 425–443. [Google Scholar]
- Moles AT, Westoby M. 2004a.Seedling survival and seed size: a synthesis of the literature. Journal of Ecology 92: 372–383. [Google Scholar]
- Moles AT, Westoby M. 2004b What do seedlings die from and what are the implications for evolution of seed size? Oikos 106: 193–199. [Google Scholar]
- Mudrák O, Fajmon K, Jongepierová I, Prach K. 2018. Mass effects, clonality, and phenology but not seed traits predict species success in colonizing restored grasslands. Restoration Ecology 26: 489–496. [Google Scholar]
- Mueller RJ, Richards JH. 1986. Morphological analysis of tillering in Agropyron spicatum and Agropyron desertorum. Annals of Botany 58: 911–921. [Google Scholar]
- Mullahey JJ, Waller SS, Moser LE. 1991. Defoliation effects on yield and bud and tiller numbers of two Sandhills grasses. Journal of Range Management 44: 241–245. [Google Scholar]
- Murphy JS, Briske DD. 1994. Density-dependent regulation of ramet recruitment by the red:far red ratio of solar radiation – a field evaluation with the bunchgrass Schizachyrium scoparium. Oecologia 97: 462–469. [DOI] [PubMed] [Google Scholar]
- N’Guessan M. 2007. Effects of grazing on growth and morphology of rhizomatous and caespitose grasses in tallgrass prairie. MS Thesis, Kansas State University, USA. [Google Scholar]
- N’Guessan M, Hartnett DC. 2011. Differential responses to defoliation frequency in little bluestem (Schizachyrium scoparium) in tallgrass prairie: implications for herbivory tolerance and avoidance. Plant Ecology 212: 1275–1285. [Google Scholar]
- Ott JP. 2009. Bud bank morphology, dynamics, and production in perennial grasses. MS Thesis, Kansas State University, USA. [Google Scholar]
- Ott JP, Hartnett DC. 2011. Bud production and dynamics of flowering and vegetative tillers in Andropogon gerardii (Poaceae): The role of developmental constraints. American Journal of Botany 98: 1293–1298. [DOI] [PubMed] [Google Scholar]
- Ott JP, Hartnett DC. 2012a Contrasting bud bank dynamics of two co-occurring grasses in tallgrass prairie: implications for grassland dynamics. Plant Ecology 213: 1437–1448. [Google Scholar]
- Ott JP, Hartnett DC. 2012b Higher-order bud production increases tillering capacity in the perennial caespitose grass Scribner’s panicum (Dichanthelium oligosanthes). Botany 90: 884–890. [Google Scholar]
- Ott JP, Hartnett DC. 2015a Bud bank dynamics and clonal growth strategy in the rhizomatous grass, Pascopyrum smithii. Plant Ecology 216: 395–405. [Google Scholar]
- Ott JP, Hartnett DC. 2015b Bud-bank and tiller dynamics of co-occurring C3 caespitose grasses in mixed-grass prairie. American Journal of Botany 102: 1462–1471. [DOI] [PubMed] [Google Scholar]
- Ott JP, Hartnett DC. 2015c Vegetative reproduction and bud bank dynamics of the perennial grass Andropogon gerardii in mixed grass and tallgrass prairie. American Midland Naturalist 174: 14–32. [Google Scholar]
- Ott JP, Butler JL, Rong Y, Xu L. 2017. Greater bud outgrowth of Bromus inermis than Pascopyrum smithii under multiple environmental conditions. Journal of Plant Ecology 10: 518–527. [Google Scholar]
- Ottaviani G, Martínková J, Herben T, Pausas JG, Klimešová J. 2017. On plant modularity traits: functions and challenges. Trends in Plant Science 22: 648–651. [DOI] [PubMed] [Google Scholar]
- Paula S, Ojeda F. 2011. Response to recurrent disturbance in two co-occurring re-sprouter heath species: the ecological consequences of withstanding herbivores. Plant Ecology 212: 2035–2045. [Google Scholar]
- Paula S, Naulin P, Arce C, Galaz C, Pausas JG. 2016. Lignotubers in Mediterranean basin plants. Plant Ecology 217: 661–676. [Google Scholar]
- Pausas JG, Lamont BB, Paula S, Appezzato-da-Glória B, Fidelis A. 2018. Unearthing belowground bud banks in fire-prone ecosystems. New Phytologist 217: 1435–1448. [DOI] [PubMed] [Google Scholar]
- Pelaez DV, Boo RM, Elia OR, Mayor MD. 1997. Effect of fire intensity on bud viability of three grass species native to central semi-arid Argentina. Journal of Arid Environments 37: 309–317. [Google Scholar]
- Pelaez DV, Boo RM, Mayor MD, Elia OR, Cardona NM. 2009. Effect of post-fire defoliation on bud viability and plant mortality of Piptochaetium napostaense (Speg.) Hack. and Poa ligularis Ness. Journal of Arid Environments 73: 708–712. [Google Scholar]
- Perreta M, Ramos J, Tivano JC, Vegetti A. 2011. Descriptive characters of growth form in Poaceae – an overview. Flora 206: 283–293. [Google Scholar]
- Peters DPC. 2000. Climatic variation and simulated patterns in seedling establishment of two dominant grasses at a semi-arid-arid grassland ecotone. Journal of Vegetation Science 11: 493–504. [Google Scholar]
- Primack R, Stacy E. 1998. Cost of reproduction in the pink lady’s slipper orchid (Cypripedium acaule, Orchidaceae): an eleven-year experimental study of three populations. American Journal of Botany 85: 1672–1679. [PubMed] [Google Scholar]
- Putz N, Sukkau I. 2002. Seedling establishment, bud movement, and subterranean diversity of geophilous systems in Apiaceae. Flora 197: 385–393 [Google Scholar]
- Qian JQ, Busso CA, Wang ZW, Liu ZM. 2015. Ramet recruitment from different bud types along a grassland degradation gradient in Inner Mongolia, China. Polish Journal of Ecology 63: 38–52. [Google Scholar]
- Qian J, Wang Z, Klimešová J, Lü X, Kuang W, Liu Z, Han X. 2017. Differences in below-ground bud bank density and composition along a climatic gradient in the temperate steppe of northern China. Annals of Botany 120: 755–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raunkiaer C. 1934. The life forms of plants and statistical plant geography. London: Clarendon Press. [Google Scholar]
- Reichmann LG, Sala OE. 2014. Differential sensitivities of grassland structural components to changes in precipitation mediate productivity response in a desert ecosystem. Functional Ecology 28: 1292–1298. [Google Scholar]
- Reichmann LG, Sala OE, Peters DPC. 2013. Precipitation legacies in desert grassland primary production occur through previous-year tiller density. Ecology 94: 435–443. [DOI] [PubMed] [Google Scholar]
- Richards EG, Burningham H. 2011. Hippophae rhamnoides on a coastal dune system: a thorny issue? Journal of Coastal Conservation 15: 73–85. [Google Scholar]
- Richards MB, Lamont BB. 1996. Post-fire mortality and water relations of three congeneric shrub species under extreme water stress – a trade-off with fecundity? Oecologia 107: 53–60. [DOI] [PubMed] [Google Scholar]
- Rogers WE, Hartnett DC. 2001. Temporal vegetation dynamics and recolonization mechanisms on different-sized soil disturbances in tallgrass prairie. American Journal of Botany 88: 1634–1642. [PubMed] [Google Scholar]
- Rusch GM, Wilmann B, Klimesova J, Evju M. 2011. Do clonal and bud bank traits vary in correspondence with soil properties and resource acquisition strategies? Patterns in alpine communities in the Scandian Mountains. Folia Geobotanica 46: 237–254. [Google Scholar]
- Russell ML, Vermeire LT. 2015. Fire and nitrogen alter axillary bud number and activity in purple threeawn. Rangeland Ecology and Management 68: 65–70. [Google Scholar]
- Russell ML, Vermeire LT, Ganguli AC, Hendrickson JR. 2015. Season of fire manipulates bud bank dynamics in northern mixed-grass prairie. Plant Ecology 216: 835–846. [Google Scholar]
- Salguero-Gomez R, Jones OR, Archer CR, et al. 2015. The COMPADRE Plant Matrix Database: an open online repository for plant demography. Journal of Ecology 103: 202–218. [Google Scholar]
- Sands BA, Abrams M. 2009. Effects of stump diameter on sprout number and size for three oak species in a Pennsylvania clear-cut. Northern Journal of Applied Forestry 26: 122–125. [Google Scholar]
- Scott KA, Setterfield SA, Douglas MM, Andersen AN. 2010. Fire tolerance of perennial grass tussocks in a savanna woodland. Austral Ecology 35: 858–861. [Google Scholar]
- Shefferson RP. 2009. The evolutionary ecology of vegetative dormancy in mature herbaceous perennial plants. Journal of Ecology 97: 1000–1009. [Google Scholar]
- Shefferson RP, Warren RJ II, Pulliam HR. 2014. Life-history costs make perfect sprouting maladaptive in two herbaceous perennials. Journal of Ecology 102: 1318–1328. [Google Scholar]
- Shimizu-Sato S, Mori H. 2001. Control of outgrowth and dormancy in axillary buds. Plant Physiology 127: 1405–1413. [PMC free article] [PubMed] [Google Scholar]
- Silvertown J, Franco M, Pisanty I, Mendoza A. 1993. Comparative plant demography—relative importance of life-cycle components to the finite rate of increase in woody and herbaceous perennials. Journal of Ecology 81: 465–476. [Google Scholar]
- Skálová H, Krahulec F. 1992. The response of 3 Festuca rubra clones to changes in light quality and plant density. Functional Ecology 6: 282–290 [Google Scholar]
- Sosnová M, van Diggelen R, Klimešová J. 2010. Distribution of clonal growth forms in wetlands. Aquatic Botany 92: 33–39. [Google Scholar]
- Stafstrom JP, Ripley BD, Devitt ML, Drake B. 1998. Dormancy-associated gene expression in pea axillary buds. Planta 205: 547–552. [DOI] [PubMed] [Google Scholar]
- Šťastná P, Klimešová J, Doležal J. 2012. Altitudinal changes in growth and allometry of Rumex alpinus. Alpine Botany 122: 35–44. [Google Scholar]
- Suzuki J, Mutchings MJ. 1997. Interactions between shoots in clonal plants and the effects of stored resources on the structure of shoot populations. In: de Kroon H, van Groenendael J, eds. The ecology and evolution of clonal plants. Leiden: Backhuys, 311–330. [Google Scholar]
- Tomlinson KW, O’Connor TG. 2004. Control of tiller recruitment in bunchgrasses: uniting physiology and ecology. Functional Ecology 18: 489–496. [Google Scholar]
- VanderWeide BL, Hartnett DC. 2015. Belowground bud bank response to grazing under severe, short-term drought. Oecologia 178: 795–806. [DOI] [PubMed] [Google Scholar]
- VanderWeide BL, Hartnett DC, Carter DL. 2014. Belowground bud banks of tallgrass prairie are insensitive to multi-year, growing-season drought. Ecosphere 5: 103. [Google Scholar]
- Vesk PA. 2006. Plant size and resprouting ability: trading tolerance and avoidance of damage? Journal of Ecology 94: 1027–1034. [Google Scholar]
- Vesk PA, Westoby M. 2004. Funding the bud bank: a review of the costs of buds. Oikos 106: 200–208. [Google Scholar]
- Vesk PA, Warton DI, Westoby M. 2004. Sprouting by semi-arid plants: testing a dichotomy and predictive traits. Oikos 107: 72–89. [Google Scholar]
- Villamagna AM, Murphy BR. 2010. Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes): a review. Freshwater Biology 55: 282–298. [Google Scholar]
- Vítová A, Macek P, Leps J. 2017. Disentangling the interplay of generative and vegetative propagation among different functional groups during gap colonization in meadows. Functional Ecology 31: 458–468. [Google Scholar]
- Waldie T, Hayward A, Beveridge CA. 2010. Axillary bud outgrowth in herbaceous shoots: how do strigolactones fit into the picture? Plant Molecular Biology 73: 27–36. [DOI] [PubMed] [Google Scholar]
- Wang Z, Li L, Han XG, Dong M. 2004. Does rhizome severing and shoot defoliation affect clonal growth of Leymus chinensis at ramet population level? Acta Oecologia 26: 255–260. [Google Scholar]
- Watkinson AR, Powell JC. 1993. Seedling recruitment and the maintenance of clonal diversity in plant populations – a computer simulation of Ranunculus repens. Journal of Ecology 81: 707–717. [Google Scholar]
- Watson MA, Casper BB. 1984. Morphogenetic constraints on patterns of carbon distribution in plants. Annual Review of Ecology and Systematics 15: 233–258. [Google Scholar]
- Widén B, Cronberg N, Widén M. 1994. Genotypic diversity, molecular markers and spatial distribution of genets in clonal plants, a literature survey. Folia Geobotanica 29: 245–263 [Google Scholar]
- Wiegand T, Snyman HA, Kellner K, Paruelo JM. 2004. Do grasslands have a memory: modeling phytomass production of a semiarid South African grassland. Ecosystems 7: 243–258. [Google Scholar]
- Wise MJ, Abrahamson WG. 2007. Effects of resource availability on tolerance of herbivory: a review and assessment of three opposing models. American Naturalist 169: 443–454. [DOI] [PubMed] [Google Scholar]
- Williams RF, Langer RHM. 1975. Growth and development of wheat tiller. 2. Dynamics of tiller growth. Australian Journal of Botany 23: 745–759. [Google Scholar]
- Williamson M, Wilson GWT, Hartnett DC. 2012. Controls on bud activation and tiller initiation in C3 and C4 tallgrass prairie grasses: the role of light and nitrogen. Botany 90: 1221–1228. [Google Scholar]
- Woods TM, Hartnett DC, Ferguson CJ. 2009. High propagule production and reproductive fitness homeostasis contribute to the invasiveness of Lespedeza cuneata (Fabaceae). Biological Invasions 11: 1913–1927. [Google Scholar]
- Xie X, Song Y, Zhang Y, Xu P, Dong M. 2014. Phylogenetic meta-analysis of the functional traits of clonal plants foraging in changing environments. PLoS ONE 9: e107114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye XH, Yu FH, Dong M. 2006. A trade-off between guerrilla and phalanx growth forms in Leymus secalinus under different nutrient supplies. Annals of Botany 98: 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnsdorf A, Hussner A, Eismann F, Ronicke H, Melzer A. 2015. Management options of invasive Elodea nuttallii and Elodea canadensis. Limnologica 51: 110–117. [Google Scholar]
- Zhang J, Mu C, Wang D, Wang J, Chen G. 2009. Shoot population recruitment from a bud bank over two seasons of undisturbed growth of Leymus chinensis. Botany 87: 1241–1249. [Google Scholar]