Abstract
Background and Aims
Ant–plant associations are widely diverse and distributed throughout the world, leading to complex ecological networks. Regarding ant–plant mutualism, ant pollination is a very rare interaction and few studies have shown the role of ants as pollinators. Therefore, we aimed to evaluate the role of ants as effective pollinators of Paepalanthus lundii (Eriocaulaceae) in a Brazilian savanna.
Methods
Fieldwork with experimental manipulation was conducted to evaluate the fitness of P. lundii, considering potential pollinators. For this, we mainly observed the number of seeds produced in different conditions: control, ant exclusion, exclusion of flying insects, and exclusion (entomophily test) of both ants and flying insects. Furthermore, we evaluated all floral visitors throughout the day, stigma receptivity, the numbers of male and female flowers, and patterns of species co-occurrence, which can indicate the presence of different pollinators in the plants at the same time.
Key Results
We observed a relation between seed production and ant visits; Camponotus crassus was the most frequent floral visitor and the most effective pollinator. Also, we observed a statistical difference between the numbers of male and female flowers produced, with a greater number of male flowers. Furthermore, P. lundii presented flowering asynchrony, with 12 different types of maturation sequence, which indicates a cross-pollination system. Lastly, we observed an overlap of the greatest abundance of C. crassus and the time of plant stigmatic receptivity, and a pattern of non co-occurrence of ants, which shows the pollinator role of this ant.
Conclusions
Our data provide evidence that previous generalizations neglecting the importance of ants as pollinators are wrong. Brazilian savanna can reveal a lot about the ant-pollination syndrome, since this environment presents peculiar characteristics related to this association. Thus, this study has great significance for the understanding of the ant-pollination syndrome, and for the understanding of the complex ecological networks present in these dry arid systems.
Keywords: Entomophily, cerrado, Brazil, ant-pollination syndrome, ant–plant interaction, Camponotus crassus, mutualism, pollen, vereda, myrmecophily
INTRODUCTION
Ants are omnipresent creatures in almost all terrestrial ecosystems. In the tropics, for example, they can represent >85 % of the arthropod biomass (Tobin, 1995). They have interacted with plants, possibly since the Cretaceous, in a continuum from antagonism to mutualism (Rico-Gray and Oliveira, 2007), and ant–plant systems form structurally complex ecological networks (Del-Claro et al., 2018). These systems are mostly based on the biotic defence that ants can offer to plants, mainly in exchange for housing and/or food provided by plants via extrafloral nectaries (Heil, 2015; Del-Claro et al., 2016; Calixto et al., 2018). Nectar is a food source that increases ant colony size and survivorship (Byk and Del-Claro, 2011). Extrafloral nectaries may be present in almost all above-ground plant parts, including inflorescences, where ants also make use of floral nectar. However, interactions between ants and flowers are generally assumed to be antagonistic.
Although nectar is an important floral resource to attract pollinators, ant pollination is assumed to be rare and restricted to a few species within angiosperms (Peakall and Beattie, 1991; Domingos-Melo et al., 2017; Kuriakose et al., 2018). The reasons why ants are considered poor agents of cross-pollination are related to three peculiar characteristics of these insects: (1) their small size, generally smaller than the floral reproductive structures; (2) grooming or self-cleaning behaviour, removing pollen before transport; and (3) limited displacement, as foragers cannot fly and thus only visit resources near the nest (Faegri and Pijl, 1979; de Vega et al., 2014; Domingos-Melo et al., 2017). Other important additional factors are the interference in pollen viability caused by substances acting antibiotically against fungal and bacterial attack, produced by the metapleural glands and spread over the surface of the ant’s body (the ‘antibiotic hypothesis’; Beattie, 1985; Peakall et al., 1991) and the consumption of pollen by some ants (Byk and Del-Claro, 2010). They can also repel other floral visitors, mainly due to their aggressive behaviour (Assunção et al., 2014; Cembrowski et al., 2014; Li et al., 2014; Sinu et al., 2017).
On the other hand, ant pollination may be an advantageous system with a low energetic cost, and could be favoured in habitats where ant frequency is high and plants are short, with near-ground-level inflorescences, small, sessile flowers that produce low amounts of pollen amount to avoid ant self-grooming behaviour, and nectar is the main reward; these features together characterize the ‘ant-pollination syndrome’ (Hickman, 1974). In fact, in recent years we have seen an increase in the recognition of ants as possible or effective pollinators, and they have been implicated as the main pollinator in at least 57 plant species (de Vega et al., 2009). Ants generally use volatile organic compounds as cues to find food sources and host plants (Blatrix and Mayer, 2010). Surprisingly, de Vega et al. (2014) suggested that an ant-pollinated plant can attract its ant pollinators also by producing floral scents, as commonly occurs in entomophilous pollination. Thus, recent studies (e.g. de Vega et al., 2014; Ibarra-Isassi and Sendoya, 2016; Domingos-Melo et al., 2017) highlight the need to reassess the ecological significance of ant pollination.
The Brazilian cerrado savanna is shaped by a set of distinct landscapes and vegetal physiognomies growing over a poor-quality soil, very exposed to sun incidence, a biome dominated by small trees and shrubs, marked by a rainy season (September to March) and a very strong dry season (April to August), with frequent fires (Oliveira and Marquis, 2002). We have been working in these savannas for the last 27 years and observing intense ant visitation to the inflorescences of some plant species, particularly Eriocaulaceae shrubs, possessing several of the main features of the ant-pollination syndrome (Hickman, 1974). Thus, we suspect that ant pollination in the Brazilian savanna could be an underestimated ecological interaction. In this study, we evaluated the role of ants as the effective pollinators of Paepalanthus lundii (Eriocaulaceae) in a cerrado from Minas Gerais state, Brazil. We assessed all plant floral visitors and performed field experimental manipulations to achieve our aim. Also we asked the following questions: (1) Are ants the most abundant floral visitors of P. lundii? (2) Do plants visited exclusively by ants have a significantly higher fruit set than plants visited by other animals or restricted to self-pollination? (3) If P. lundii is ant-pollinated, is there some specific ant or group of ant species that are the most effective pollinators? If results support our hypothesis, they will be a further evidence of an underestimation of ant-pollination syndrome in the Brazilian savanna.
MATERIALS AND METHODS
Study area and plant species
Fieldwork was carried out from October to December 2014 in the Ecological Reserve of the Clube Caça e Pesca Itororó de Uberlândia (CCPIU; 18°59′00″ S, 48°17′45″ W), Minas Gerais, Brazil. In this area the dominant vegetation is savanna, cerrado sensu stricto (Oliveira and Marquis, 2002), with trees 2–8 m tall and an understorey of shrubs and grasses. Furthermore, palm swamp communities extend throughout the reserve, the veredas. This is an environment associated with freshwater bodies that naturally occur throughout most of the South American cerrado (Araújo et al., 2002). These swamps are characterized by the occurrence of the palm tree Mauritia flexuosa (Arecales: Arecaceae) (Araújo et al., 2002; Oliveira and Marquis, 2002). Between the veredas and the cerrado there is a strip that acts as a transition or boundary between these two vegetal physiognomies, a line of open field, 5–15 m wide, with grasses and shrubs <1 m tall (Fig. 1A). Plants of Asteraceae, Melastomataceae, Poaceae and Eriocaulaceae, such as Paepalanthus lundii, the object of the present study (Figs 1B, C and 2A), are common in this area. This sand soil strip receives intense solar radiation year-round. The climate in the reserve is humid and rainy from September to March and dry from April to August (for more details of the study area see Ferreira and Torezan-Silingardi, 2013; Velasque and Del-Claro, 2016).
Fig. 1.
View of a vereda (A), the humid area with palm trees on the left, separated from the cerrado (right) by a line of open field 5–15 m wide, with a domain of grasses and shrubs <1 m tall. (B) In this open field P. lundii (C) flourishes abundantly, mainly after fire occurrence.
Fig. 2.
Experimental groups of P. lundii in open-field cerrado. (A) Control. No treatment was done and all floral visitors had free access to the capitulum and flowers. (B) Exclusion (anemophily test). We isolated the capitulum with a voile bag so no floral visitor could access the capitulum. (C) Ants. We built pyramidal shacks that prevented access by flying visitors and allowed only access to ground visitors, especially ants. (D) No ants. A band of sticky Tanglefoot® resin (Grand Rapids, MI, USA) was applied to a plastic ring (10 cm) that was buried around the plant, preventing ant access to the plant, and all aerial bridges were clipped, allowing only flying visitors to reach the capitulum.
Paepalanthus lundii is an endemic species of southeast Brazil that was described by Koernicke (1863) and is considered synonymous with Paepalanthus macrotrichus (A. Loefgren 1485). The species is herbaceous (20–30 cm tall), perennial, grows in groups, and is characterized by the presence of hirsute linear leaves, long basal trichomes and linear floral bracts (Trovó and Sano, 2010). The inflorescences (15–20 cm tall) are arranged in racemous capitula of tiny unisexual white flowers (Figs 1C and 2A). Paepalanthus (‘everlasting flowers’) is the largest genus of Eriocaulaceae, with ~550 species mainly distributed in the Neotropics on rocky outcrops in the Brazilian savanna. Several species are endemic to the cerrado, usually being restricted to one locality (Stützel, 1998; Trovó and Sano, 2010). The Brazilian cerrado savanna is frequently disturbed by fire (Oliveira and Marquis, 2002) and P. lundii flourishes abundantly only after fire events (K.D.C., pers. obs) (Fig. 1B, C). In September of 2014 a fire occurred in the study area and 2 weeks later the rainy season began. Following these events between the last week of September and the second week of October, P. lundii flowered abundantly at the study site (Fig. 1B, C). At this time we initiated the fieldwork.
Flower composition and maturation
To determine whether there was a difference between the numbers of male and female flowers on each plant, 20 capitula were marked, one capitulum per plant, and we counted all types of flowers in each capitulum. All plants had a similar phenological state, i.e. size and number of inflorescences (with a developed bud, but still without opened flowers), and were at least 5 m apart. Furthermore, to evaluate whether there were different types of flower maturation sequence and subsequently to observe flowering synchrony, we selected six flowers in another 16 capitula, one capitulum per plant, and observed the sequence of flower opening. These analyses are important because if P. lundii is in fact pollinated by ants, we expect that plants will produce a higher quantity of male flowers and will present flowering asynchrony, which can increase the probability of cross-pollination.
Stigma receptivity
We randomly selected and bagged with voile 23 capitula in different individuals (one per plant) until the time of analysis to evaluate stigma receptivity (n = 23). Stigmas were evaluated every 30 min, starting at 0700 h (when flowers start to open) and ending at 1800 h, using the hydrogen peroxide method (Dafni and Maués, 1998). The stigma was carefully inserted into a clear tube with 3 % hydrogen peroxide, and if there was blistering the stigma was considered receptive.
Floral visitors
Flower visitors and possible pollinators of P. lundii were observed in 35 different plants from 0800 to 1700 h with 30 min of observation per plant and one observer, ad libitum (Altmann, 1974). Each plant was observed twice, once before and once after 1200 h. We collected one voucher specimen from each floral visitor for further identification.
Plant fitness
To evaluate the plant fitness, we selected 40 individuals of P. lundii with similar phenological state, as described above (see Flower composition and maturation section). The plants were divided by the flip of a coin into four groups of ten plants, and one capitulum was randomly tagged in each individual. Thus, the groups were as follows: (1) Control, in which no treatment was done and all floral visitors had free access to the capitulum and flowers (Fig. 2A); (2) Exclusion (anemophily test), in which we isolated the capitulum with a voile bag and no floral visitor had access to the capitulum (Fig. 2B); (3) Ants, in which we built pyramidal shacks that prevented access by flying visitors and allowed only access by ground visitors, especially ants (Fig. 2C); (4) No ants, in which a band of sticky Tanglefoot® resin (Grand Rapids, MI, USA) was applied to a plastic ring (10 cm) that was buried around the plant, preventing ant access to the plant, and all aerial bridges were clipped, allowing only flying visitors to access the capitulum (Fig. 2D). To show the similarity between individuals, for each P. lundii individual of all experimental groups we measured capitulum height, base and canopy diameter and numbers of flowers and buds produced, and inspected the individuals weekly to collect and count the number of total seeds produced.
Climatic variables
Climatic variables, such as temperature and humidity, were evaluated at hourly intervals, starting at 0800 h and ending at 1700 h, using a digital thermo-hygrometer (Equitherm; maximum/minimum, internal/external). This device cannot measure relative humidity values <10 % and therefore we used this value (10 %) as the minimum value.
Data analysis
To determine whether there was a difference between the numbers of male and female flowers produced in each plant, we used a paired t-test. We used six flowers from one capitulum from each plant to observe the variation in maturation sequence.
In order to analyse whether stigmatic receptivity is dependent on ant abundance (our main hypothesis) we used simple logistic regression in which stigmatic receptivity was considered the binary dependent variable and Camponotus crassus (the main potential pollinator; see Results section) abundance the independent variable. We determined the total abundance of this ant species on plants at intervals of 30 min, the same time schedule as that used for stigmatic receptivity analysis.
To verify whether there was a difference in the total abundance of individuals per plant among the floral visitor groups, we used a generalized linear model (GLM) with negative binomial distribution error using the MASS package (Venables and Ripley, 2002). We also performed a Tukey test as a multiple comparison test using the multcomp package (Hothorn et al., 2008). In this analysis, we separated the ant species C. crassus from other ants to show that this species is the most abundant ant and the main floral visitor to P. lundii flowers. Also, we clustered all other floral visitors into three groups: (1) other ants (all ants, except C. crassus); (2) bees; and (3) flies. We did not use other visitors because they were not frequent on plants and did not represent a potential pollinator. To verify whether C. crassus is a dominant ant and consequently the main pollinator, we used a co-occurrence analysis using the C-score index with the EcoSimR package (Gotelli et al., 2015). We observed non-random patterns of species co-occurrence in a presence/absence matrix and created a specific number of random matrices (5000) by randomizing the original matrix (for more details of co-occurrence analysis see Gotelli, 2000; Ribas and Schoereder, 2002; Sanders et al., 2007).
To analyse whether there was a difference in plant fitness among treatments, we used a GLM with negative binomial distribution error followed by a Tukey test. In this case, the total number of seeds was the response variable and treatment group was the fixed factor. To show whether capitulum height, base and canopy diameters and the numbers of flowers and buds influenced the number of seeds produced in the different treatments, we used an ANOVA for each of those factors.
All analyses and graphs were made using the software R Studio 3.5.1 and Graphpad Prism 7.0 at a 5 % significance level, respectively.
RESULTS
Flower composition and maturation
We found a significant difference between the numbers of male and female flowers produced (paired t-test = 10.86, d.f. = 19, P < 0.001), where capitula produced more male flowers (25.35 ± 8.48; mean ± s.d.) than females (7.1 ± 2.71). We observed 12 types of flower maturation sequence (Table 1), indicating flowering asynchrony. This asynchrony occurred because there were capitula presenting male and female flowers in anthesis simultaneously, and also there was no pattern of opening flowers within the capitulum.
Table 1.
Types of flower maturation sequence in P. lundii (n = 16)
| Sequence type | Opening flower sequence | Number of capitula | |||||
|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 6th | ||
| A | f | f | m | m | m | m | 1 |
| B | m | m | m | f | f | f | 1 |
| C | m | f | f | m | m | +++m | 3 |
| D | m | f | m | m | m | f | 1 |
| E | m | m | f | f | m | m | 2 |
| F | m | m | f | m | m | m | 1 |
| G | m | m | m | f | m | m | 1 |
| H | f | m | m | m | m | f | 1 |
| I | f | m | m | m | f | m | 2 |
| J | m | m | m | m | f | m | 1 |
| K | m | f | m | m | m | m | 1 |
| L | m | m | f | m | m | f | 1 |
| Total | 16 | ||||||
m, male flowers; f, female flowers.
Stigma receptivity
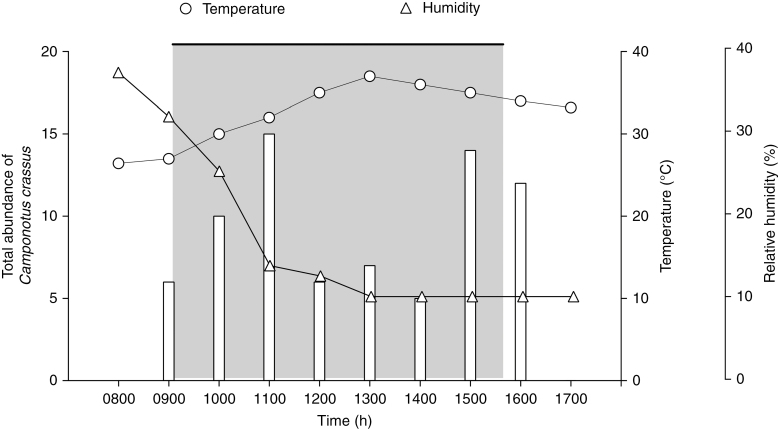
Stigmatic receptivity occurred at 0900 h, the same time that C. crassus began to visit the plant, and ended at 1530 h (Fig. 3). There was a clear overlap between C. crassus abundance on plants and the time of plant stigmatic receptivity (z = 2.481, P < 0.05, n = 23). The odds ratio was 1.67, which indicates that the probability of the stigma being receptive was 1.67 times higher when there was greater abundance of C. crassus.
Fig. 3.
Stigmatic receptivity of P. lundii (grey area), which started at 0900 h and ended by 1530 h, total abundance of C. crassus in individuals of P. lundii (columns), and temperature and relative humidity by observation time.
Floral visitors
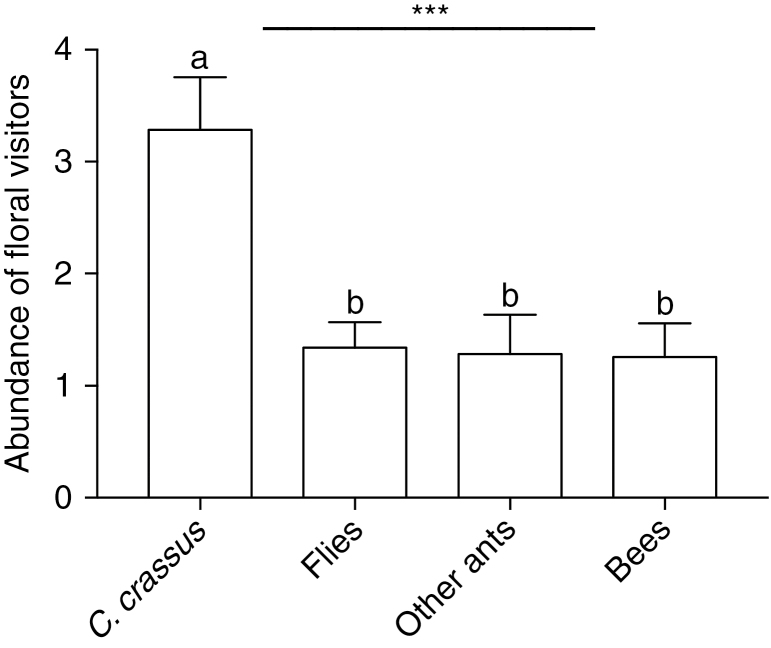
The field observations also showed that the ant C. crassus was the main floral visitor of P. lundii, with relative abundance 43.56 % (Table 2), followed by Melipona sp.1 with relative abundance 10.61 % and Muscidae sp.1 with 9.47 % (for other species see Table 2). Also, the groups of floral visitors presented a statistically significant difference among their abundances per plant (GLM: χ2 = 19.652, d.f. = 3, P < 0.001; Fig. 4). In sum, these results suggest that the ant C. crassus is the most effective pollinator of P. lundii in the studied savanna. This ant species was the floral visitor group with the highest average number of individuals per plant (3.28 ± 0.47, mean ± s.e.), followed by flies (1.34 ± 0.22), other ants (1.28 ± 0.34) and bees (1.25 ± 0.29) (Fig. 4). Field observations showed that the same individual of C. crassus could climb on to distinct capitula of the same or a different plant of P. lundii in <30 min, contacting several flowers (Fig. 5A), and that pollen grains attached to the head, ventral thorax and antennae of the ants abundantly (Fig. 5A).
Table 2.
Absolute and relative abundance of floral visitors observed on P. lundii in a Brazilian savanna
| Visitor | Absolute abundance | Relative abundance (%) |
|---|---|---|
| Hymenoptera | ||
| Ants | ||
| Camponotus crassus | 115 | 43.56 |
| Crematogaster erecta | 11 | 4.17 |
| Ectatomma sp.1 | 1 | 0.38 |
| Pseudomyrmex gracilis | 15 | 5.68 |
| Dorymyrmex sp.1 | 13 | 4.92 |
| Pseudomyrmex pallidus | 4 | 1.52 |
| Pseudomyrmex flavidulus | 1 | 0.38 |
| Bees | 0.00 | |
| Augochloropsis sp.1 | 16 | 6.06 |
| Melipona sp.1 | 28 | 10.61 |
| Diptera | ||
| Drosophila sp.1 | 3 | 1.14 |
| Muscidae sp.1 | 25 | 9.47 |
| Muscidae sp.2 | 7 | 2.65 |
| Syrphidae sp.1 | 3 | 1.14 |
| Syrphidae sp.2 | 9 | 3.41 |
| Hemiptera | ||
| Coreidae sp.1 | 8 | 3.03 |
| Lepidoptera | ||
| Sp.1 | 2 | 0.76 |
| Coleoptera | ||
| Scarabaeidae sp.1 | 1 | 0.38 |
| Scarabaeidae sp.2 | 2 | 0.76 |
| Total | 264 | 100 |
Fig. 4.
Number of floral visitors per group per plant of P. lundii. Bars represent mean and s.e.m. ***χ2 = 19.652, d.f. = 3, P < 0.001 (GLM). Different letters represent differences by Tukey’s test at 5 % probability.
Fig. 5.
(A) A C. crassus (Formicidae, Formicinae) ant visiting a capitulum of P. lundii. (B) Detail of the ant’s head and antennae showing pollen grains.
The results of co-occurrence analysis showed that ant species co-occurrence was higher than expected by chance (simulated C-score average 23.065, observed C-score 33.619, P < 0.001). Therefore, we nullify the existence of ant species co-occurrence and we can state that the community is competitively structured and there is a predominance of C. crassus in P. lundii.
Plant fitness
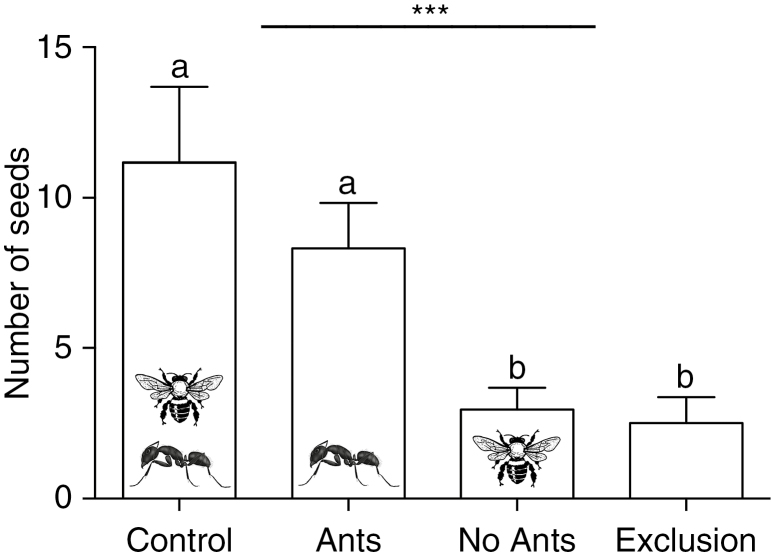
The results showed that ants improved seed production in P. lundii shrubs. Indeed, the plants that had free ant access (Control and Ants groups) did not differ from each other, but differed and produced significantly more seeds than the ant-excluded groups (No ants and Exclusion groups) (GLM: χ2 = 18.654, d.f. = 3, P < 0.001; Fig. 6). The Control group produced the highest number of seeds (11.17 ± 12.35, mean ± s.d.), followed by the Ants group (8.32 ± 7.9), the No ants group (2.96 ± 3.80) and the Exclusion group (2.52 ± 3.90). Considering plant characteristics, there was no significant difference among plants in the different treatments for all factors analysed (capitulum height, F3,39 = 0.756, P = 0.526; capitulum base diameter, F3,39 = 1.130, P = 0.349; capitulum canopy diameter, F3,39 = 1.985, P = 0.133; number of flowers, F3,39 = 1.512, P = 0.229; and number of flower buds, F3,39 = 1.819, P = 0.161), which shows that none of these factors influenced seed productivity.
Fig. 6.
Comparison of seed number produced by P. lundii between treatments. Bars represent mean and s.e. ***χ2 = 18.654, d.f. = 3, P < 0.001 (GLM). Different letters represent differences by Tukey’s test at 5 % probability.
Lastly, our results showed that the highest temperature values occurred between 1200 and 1400 h, corresponding to the period of lower abundance of C. crassus. Between 0800 and 1100 h we observed a strong decrease in humidity, which remained at its minimum value until the end of the daily period of data collection (Fig. 3).
DISCUSSION
The everlasting flower P. lundii is ant-pollinated in the Brazilian tropical savanna, and the most effective pollinator in the study site is the Formicinae C. crassus. The P. lundii–C. crassus system fits all the main classical assumptions of ant-pollination syndrome (sensuHickman, 1974). The interaction occurs in an arid habitat, which is as expected in this syndrome (Gómez et al., 1996; de Vega et al., 2014). The plants are short, with near-ground-level inflorescences, and occur in groups; nectar is the main reward and blooming occurs over a short period (a few weeks) (Hickman, 1974; de Vega et al., 2014). Ants are extremely abundant in their habitat and their size enables contact with all floral structures (Hickman, 1974; Gómez et al., 1996; Ibarra-Isassi and Sendoya, 2016). An important characteristic in this case is that C. crassus does not have metapleural glands, so the antibiotic hypothesis (Beattie et al., 1984) is not applicable. The Camponotus genus is also commonly associated with effective or possible ant pollination in other tropical shrubs and herbs with white flowers (de Vega et al., 2014).
Some natural history and behavioural observations reinforce our hypothesis. Beyond the fact that this member of the Eriocaulaceae flourishes abundantly and in a synchronous manner, each capitulum presents asynchrony in the production of male and female flowers. The ants visiting several capitula of the same and neighbouring plants before returning to the nest. Thus, the ants will contact stamens and receptive stigmas of different plants in a single foraging event, favouring cross-pollination (Faegri and Pijl, 1979; Real, 1983). Pollen remains attached to the ant’s ventral body, face and antennae when the worker returns to the nest, showing that self-grooming is not enough in this case to prevent pollen transference among flowers. Finally, C. crassus has its peak of abundance in plants exactly when most stigmas are receptive.
Despite the fact that C. crassus is indeed the main and most abundant visitor of P. lundii flowers, as statistically proved, it is also true that ants are not the exclusive pollinators. The experimental pollination tests showed that other insects can complement ant services. The data showed that plants where any floral visitor had free access to the capitulum and flowers produced a few more seeds than plants exclusively visited by ants, though the difference was not statistically significant. On the other hand, in the absence of ant visits, plants with other visitors were able to produce a very small fruit set, with great statistical difference. Complementary pollination services may be provided by secondary visitors in most pollination syndromes (e.g. Real, 1983; Torezan-Silingardi, 2012; Rosas-Guerrero et al., 2014). While pollination syndromes are expected to reflect adaptation to primary pollinators, syndrome traits may not preclude visits by less efficient floral visitors, which could correspond to ancestral pollinators (Rosas-Guerrero et al., 2014). Thus, P. lundii is clearly an ant-pollinated plant that receives visits of secondary pollinators that can complement ant services.
If the ant-pollination syndrome is favoured by dry, arid environments where ants like Camponotus are abundant and where there are many herbs and shrubs growing in groups and presenting near-ground-level inflorescences formed by small whitish flowers having nectar as the main reward (Hickman, 1974; Gómez et al., 1996; de Vega et al., 2014), then the Brazilian cerrados and mountain rupestre fields can reveal a lot about the ant-pollination syndrome. Ant–plant interactions are extremely common and have been studied extensively in the South American savannas, and a few have been explored in other dry and very seasonal habitats, like rupestre fields and caatinga (Rico-Gray and Oliveira, 2007; Del-Claro et al., 2016). Only just Minas Gerais alone >700 species of Eriocaulaceae occur, all of them with characteristics very similar to those of P. lundii (Ferreira et al., 2011), including those related to the ant-pollination syndrome. These species have been particularly well studied with regard to their taxonomic aspects (e.g. Trovó and Sano, 2010), but in the last 10 years there have been a few papers related to pollination that have begun to evidence entomophily instead of anemophily in the group (Ramos et al., 2005). Indeed, there is a scarcity of experimental evidence of the importance of ants like pollinators in a country that hosts most of the world’s biodiversity (e.g. Ibarra-Isassi and Sendoya, 2016; Domingos-Melo et al., 2017) and we suspect that we could be surprised. We agree with colleagues who recently pointed out that ‘our understanding of ant–flower systems is still in its infancy’ (de Vega et al., 2014).
ACKNOWLEDGEMENTS
We thank Eva Colberg for comments, suggestions and English review of the final version of the manuscript, Clube de Caça e Pesca Itororó de Uberlândia for providing the study area, and Universidade Federal de Uberlândia, Universidade de São Paulo and Instituto de Ecología A.C. for the use of laboratories and infrastructure. This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) - Finance Code 001 (ESC), the Brazilian National Council for Scientific and Technological Development (CNPq) – (KDC, HMTS), and Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG) – (KDC).
LITERATURE CITED
- Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour 48: 227–265. [DOI] [PubMed] [Google Scholar]
- Araújo G, Barbosa AAA, Arantes A, Amaral A. 2002. Composição florística deveredas no Município de Uberlândia, MG. Revista Brasileira de Botânica 25: 475–493. [Google Scholar]
- Assunção MA, Torezan-Silingardi HM, Del-Claro K. 2014. Do ant visitors to extrafloral nectaries of plants repel pollinators and cause an indirect cost of mutualism? Flora 209: 244–249. [Google Scholar]
- Beattie AJ. 1985. The evolutionary ecology of ant-plant mutualisms. New York: Cambridge University Press. [Google Scholar]
- Beattie AJ, Turnbull C, Knox RB, Williams EG. 1984. Ant inhibition of pollen function: a possible reason why ant pollination is rare. American Journal of Botany 71: 421–426. [Google Scholar]
- Blatrix R, Mayer V. 2010. Communication in ant–plant symbioses. In: Baluska F, Ninkovic V, eds. Plant communication from an ecological perspective. Berlin: Springer, 127–158. [Google Scholar]
- Byk J, Del-Claro K. 2010. Nectar- and pollen-gathering Cephalotes ants provide no protection against herbivory: a new manipulative experiment to test ant protective capabilities. Acta Ethologica 13: 33–38. [Google Scholar]
- Byk J, Del-Claro K. 2011. Ant-plant interaction in the Neotropical savanna: direct beneficial effects of extrafloral nectar on ant colony fitness. Population Ecology 53: 327–332. [Google Scholar]
- Calixto ES, Lange D, Del-Claro K. 2018. Protection mutualism: an overview of ant-plant interactions mediated by extrafloral nectaries. Oecologia Australis 22: 410–425. [Google Scholar]
- Cembrowski AR, Tan MG, Thomson JD, Frederickson ME. 2014. Ants and ant scent reduce bumblebee pollination of artificial flowers. American Naturalist 183: 133–139. [DOI] [PubMed] [Google Scholar]
- Dafni A, Maués MM. 1998. A rapid and simple procedure to determine stigma receptivity. Sexual Plant Reproduction 11: 177–180. [Google Scholar]
- Del-Claro K, Rico-Gray V, Torezan-Silingardi HM, et al. . 2016. Loss and gains in ant–plant interactions mediated by extrafloral nectar: fidelity, cheats, and lies. Insectes Sociaux 63: 207–221. [Google Scholar]
- Del-Claro K, Lange D, Torezan-Silingardi HM, et al. . 2018. The complex ant-plant relationship within tropical ecological networks. In: Dáttilo W, Rico-Gray V, eds. Ecological networks in the tropics: an integrative overview of species interactions from some of the most species-rich habitats on Earth. Cham: Springer, 59–71. [Google Scholar]
- de Vega C, Arista M, Ortiz PL, Herrera CM, Talavera S. 2009. The ant-pollination system of Cytinus hypocistis (Cytinaceae), a Mediterranean root holoparasite. Annals of Botany 103: 1065–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vega C, Herrera CM, Dötterl S. 2014. Floral volatiles play a key role in specialized ant pollination. Perspectives in Plant Ecology, Evolution and Systematics 16: 32–42. [Google Scholar]
- Domingos-Melo A, Nadia TL, Machado IC. 2017. Complex flowers and rare pollinators: does ant pollination in Ditassa show a stable system in Asclepiadoideae (Apocynaceae)? Arthropod-Plant Interactions 11: 339–349. [Google Scholar]
- Faegri K, van der Pijl L. 1979. The principles of pollination ecology. Oxford: Pergamon. [Google Scholar]
- Ferreira CA, Torezan-Silingardi HM. 2013. Implications of the floral herbivory on Malpighiacea plant fitness: visual aspect of the flower affects the attractiveness to pollinators. Sociobiology 60: 323–328. [Google Scholar]
- Ferreira C, Trovó M, Forzza R. 2011. A família Eriocaulaceae no Parque Estadual do Ibitipoca, Minas Gerais, Brasil. Boletim de Botânica 29: 19–36. [Google Scholar]
- Gómez JM, Zamora R, Hódar JA, García D. 1996. Experimental study of pollination by ants in Mediterranean high mountain and arid habitats. Oecologia 105: 236–242. [DOI] [PubMed] [Google Scholar]
- Gotelli N. 2000. Null model analysis of species co-occurrence patterns. Ecology 81: 2606–2621. [Google Scholar]
- Gotelli NJ, Hart EM, Ellison AM.EcoSimR: null model analysis for ecological data. R package version 0.1.0.http://github.com/gotellilab/EcoSimR 2015.
- Heil M. 2015. Extrafloral nectar at the plant-insect interface: a spotlight on chemical ecology, phenotypic plasticity, and food webs. Annual Review of Entomology 60: 213–232. [DOI] [PubMed] [Google Scholar]
- Hickman JC. 1974. Pollination by ants: a low-energy system. Science 184: 1290–1292. [DOI] [PubMed] [Google Scholar]
- Hothorn T, Bretz F, Westfall P, Heiberger RM. 2008. multcomp: simultaneous inference in general parametric models http://cran.r-project.org. [DOI] [PubMed]
- Ibarra-Isassi J, Sendoya SF. 2016. Ants as floral visitors of Blutaparon portulacoides (A. St-Hil.) Mears (Amaranthaceae): an ant pollination system in the Atlantic Rainforest. Arthropod-Plant Interactions 10: 221–227. [Google Scholar]
- Koernicke F. 1863. Eriocaulaceae. In: Martius CFP von, Eichler AW, eds. Flora Brasiliensis 3. Leipzig: Fleischer, 273–307. [Google Scholar]
- Kuriakose G, Sinu PA, Shivanna KR. 2018. Ant pollination of Syzygium occidentale, an endemic tree species of tropical rain forests of the Western Ghats, India. Arthropod-Plant Interactions 12: 647–655. [Google Scholar]
- Li J, Wang Z, Tan K, Qu Y, Nieh JC. 2014. Giant Asian honeybees use olfactory eavesdropping to detect and avoid ant predators. Animal Behaviour 97: 69–76. [Google Scholar]
- Oliveira P, Marquis R. 2002. The cerrados of Brazil: ecology and natural history of a neotropical savanna. New York: Columbia University Press. [Google Scholar]
- Peakall R, Beattie AJ. 1991. The genetic consequences of worker ant pollination in a self-compatible, clonal orchid. Evolution 45: 1837–1848. [DOI] [PubMed] [Google Scholar]
- Peakall R, Handel SN, Beattie AJ. 1991. The evidence for, and importance of ant pollination. In: Huxley CR, Cutler DF, eds. Ant–plant interactions. Oxford: Oxford University Press, 421–429. [Google Scholar]
- Ramos COC, Borba EL, Funch LS. 2005. Pollination in Brazilian Syngonanthus (Eriocaulaceae) species: evidence for entomophily instead of anemophily. Annals of Botany 96: 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real L. 1983. Pollination biology. Orlando: AcademicPress. [Google Scholar]
- Ribas CR, Schoereder JH. 2002. Are all ant mosaics caused by competition? Oecologia 131: 606–611. [DOI] [PubMed] [Google Scholar]
- Rico-Gray V, Oliveira PS. 2007. The ecology and evolution of ant-plant interactions. Chicago: University of Chicago Press. [Google Scholar]
- Rosas-Guerrero V, Aguilar R, Martén-Rodríguez S, et al. . 2014. A quantitative review of pollination syndromes: do floral traits predict effective pollinators? Ecology Letters 17: 388–400. [DOI] [PubMed] [Google Scholar]
- Sanders NJ, Crutsinger GM, Dunn RR, Majer JD, Delabie JHC. 2007. An ant mosaic revisited: dominant ant species disassemble arboreal ant communities but co-occur randomly. Biotropica 39: 422–427. [Google Scholar]
- Sinu PA, Sibisha VC, Nikhila Reshmi MV, et al. . 2017. Invasive ant (Anoplolepis gracilipes) disrupts pollination in pumpkin. Biological Invasions 19: 2599–2607. [Google Scholar]
- Stützel T. 1998. Eriocaulaceae. In: Kubitzki K, ed. The families and genera of vascular plants — flowering plants: Monocotyledons — Alismatanae and Comelinanae (except Gramineae). Berlin: Springer, 197–207. [Google Scholar]
- Tobin JE. 1995. Ecology and diversity of tropical forest canopy ants. In: Lowman MD, Nadkarni NM, eds. Forest canopies. New York: Academic Press, 129–147. [Google Scholar]
- Torezan-Silingardi HM. 2012. Flores e animais: uma introdução à história natural da polinização. In: Del-Claro K, Torezan-Silingardi HM, eds. Ecologia das interações plantas-animais: uma abordagem ecológico-evolutiva. Rio de Janeiro: Technical Books, 111–139. [Google Scholar]
- Trovó M, Sano PT. 2010. Nomenclatural and taxonomic changes in Paepalanthus (Eriocaulaceae) from São Paulo and Minas Gerais, Brazil. Kew Bulletin 65: 275–278. [Google Scholar]
- Velasque M, Del-Claro K. 2016. Host plant phenology may determine the abundance of an ecosystem engineering herbivore in a tropical savanna. Ecological Entomology 41: 421–430. [Google Scholar]
- Venables WN, Ripley BD. 2002. Modern applied statistics with S. New York: Springer. [Google Scholar]