Abstract
Background and Aims
Secondary growth is a process related to the formation of new cells that increase in size and wall thickness during xylogenesis. Temporal dynamics of wood formation influence cell traits, in turn affecting cell patterns across the tree ring. We verified the hypothesis that cell diameter and cell wall thickness are positively correlated with the duration of their differentiation phases.
Methods
Histological sections were produced by microcores to assess the periods of cell differentiation in black spruce [Picea mariana (Mill.) B.S.P.]. Samples were collected weekly between 2002 and 2016 from a total of 50 trees in five sites along a latitudinal gradient in Quebec (Canada). The intra-annual temporal dynamics of cell differentiation were estimated at a daily scale, and the relationships between cell traits and duration of differentiation were fitted using a modified von Bertalanffy growth equation.
Key Results
At all sites, larger cell diameters and cell wall thicknesses were observed in cells that experienced a longer period of differentiation. The relationship was a non-linear, decreasing trend that occasionally resulted in a clear asymptote. Overall, secondary wall deposition lasted longer than cell enlargement. Earlywood cells underwent an enlargement phase that lasted for 12 d on average, while secondary wall thickness lasted 15 d. Enlargement in latewood cells averaged 7 d and secondary wall deposition occurred over an average of 27 d.
Conclusions
Cell size across the tree ring is closely connected to the temporal dynamics of cell formation. Similar relationships were observed among the five study sites, indicating shared xylem formation dynamics across the entire latitudinal distribution of the species.The duration of cell differentiation is a key factor involved in cell growth and wall thickening of xylem, thereby determining the spatial variation of cell traits across the tree ring.
Keywords: Cell differentiation, cell diameter, cell wall thickness, cell enlargement, modelling, growth, Picea mariana, temporal dynamics, timing, wall thickening, xylogenesis
INTRODUCTION
Secondary growth in trees is at the base of biomass production and forest productivity. In extratropical ecosystems, tree growth is the result of an annual process of xylem formation that engenders the production of new cells, and whose traits influence volume quantity and quality of the resulting wood. In conifers, cell traits change across the tree ring, leading to the formation of larger thin-walled cells, followed by smaller thick-walled cells (Schweingruber, 2012). This transition can occur more or less gradually, depending on the species and cell origin – as either earlywood or latewood (Denne, 1989). Despite this arbitrary and simplistic categorization, the spatial pattern across the tree ring involves substantial changes in wood properties and the hydraulic capacity of the cells (Domec and Gartner, 2002; Chave et al., 2009; Fonti et al., 2010). The mechanism underlying the gradual transition between earlywood and latewood can be strongly endogenous, involving complex developmental dynamics (Cuny and Rathgeber, 2016; Cartenì et al., 2018).
During xylem formation, auxins are a major driver of vessel differentiation along the tree trunk and across its radial section (Aloni and Zimmermann, 1983; Aloni, 2013). However, recent experimental and modelling studies have questioned the major role of auxins in earlywood–latewood transition, as initially hypothesized by Larson (1960). In these studies, earlywood–latewood transition could not be explained by major changes in auxin concentrations; the concentrations were less than the threshold of detectability during latewood formation, and auxins failed to rebuild the decreasing latewood cell sizes in model simulations (Uggla et al., 2001; Hartmann et al., 2017; Fajstavr et al., 2018). Developmental dynamics are thus suggested as the most direct drivers of changes to tracheid size across the tree ring, whereas auxins maintain a fundamental role in regulating cambial growth and cell enlargement.
Apart from some recent meta-analyses (Rossi et al., 2013; Cuny et al., 2014), there have been few studies investigating changes in the intra-annual secondary growth of a given species across a wide geographical scale (Rossi et al., 2015). Cell enlargement and wall thickening, the two main phases of tracheid differentiation, involve different physiological needs; these needs are fulfilled by developmental or environmental factors that change over the growing season (Balducci et al., 2016; Cuny and Rathgeber, 2016; Deslauriers et al., 2016). The temporal dynamics of these two phases have long been known to be key factors in xylem formation (Skene, 1969; Denne, 1971; Wodzicki, 1971) and, more recently, multiple studies have shown that the duration of these two differentiation phases influences cell size across the tree ring (see Deslauriers et al., 2017 for a review).
Anfodillo et al. (2011) observed a linear relationship between a cell trait and the duration of cell formation processes by noting that larger initial earlywood cells along the stem were coupled with a longer duration of cell enlargement. Cells growing at the base of the stem were larger than those at the treetop, as cells lower in the stem had a longer duration of formation. In a 3 year study, Cuny et al. (2014) found a linear relationship between the duration of enlargement and cell diameter for all cells in a tree ring; however, they did not find any relationship between duration and cell wall thickness. Using a single-cell growth model, Cartenì et al. (2018) simulated the relationship between cell traits and the duration of their differentiation phases. They modelled a non-linear trend where cell trait size increased linearly with the duration of different phases but then reached a plateau beyond a certain point. This new insight vis à vis the temporal dynamics of cell trait development illustrates that our understanding of wood formation remains incomplete. It also confirms that xylogenesis is a complex process depending on many factors that may result in non-linear patterns, as has been observed for other biological processes (Cushing et al., 2002).
The non-linear pattern observed by Cartenì et al. (2018) was, however, limited to single cells. Ideally, confirming this relationship between cell traits and the length of the differentiation phases requires a large number of observations at an intra-annual resolution. Since the monitoring of xylogenesis requires weekly sampling, longer time series are rare and difficult to obtain. Furthermore, a mathematical relationship explaining univocally the relationship between cell traits (i.e. dimension of the lumen, cell wall, etc.) across the entire tree ring and their temporal dynamics has never been quantified. More specifically, the duration of cell enlargement and wall formation varies over the growing season (Deslauriers et al., 2003; Rossi et al., 2006a; Moser et al. 2009), yet this relationship remains unclear (Cuny et al., 2014).
This study aims to determine the relationship between cell traits and the duration of their differentiation phases. We test the hypothesis of a positive linear correlation between cell traits (i.e. cell diameter and cell wall thickness) and the duration of cell differentiation (i.e. enlargement and wall formation). For this, we monitored xylogenesis in individuals of black spruce (Picea mariana Mill. B.S.P.) from 2002 to 2016, sampled from five sites located across the species’ entire latitudinal distribution across Quebec (Canada). To incorporate the complexity of xylogenesis and its influence on cell traits, we applied a novel approach to the study of xylem formation (Cuny et al., 2013; Balducci et al., 2016). Our unique and lengthy data set permits a robust analysis and allows for a deeper understanding of the influences of temporal dynamics on cell traits.
MATERIALS AND METHODS
Study sites and tree selection
The study area covers a latitudinal gradient stretching from 48 to 53°N across the boreal forest of Quebec, Canada (Table 1). All sample sites are in even-aged, uniform, adult stands dominated by black spruce. Two sites (SIM and BER) are located in the balsam fir (Abies balsamea L. Mill.)–white birch (Betula papyrifera Marsh.) bioclimatic domain. Two others (MIS and DAN) lie in the black spruce–moss bioclimatic domain. The most northern site (MIR) falls in the spruce–lichen domain and is characterized by a lower tree density (Rossi et al., 2015). Mean annual temperature along the gradient ranges between 1.6 and 4.1 °C; the sites are progressively cooler moving northward (Table 1). In summer, mean temperatures range from 11.1 to 14.6 °C, with a progressively shorter growing season toward the most northern site (Table 1). Annual precipitation decreases northward along the gradient from 1162 to 827 mm (Rossi et al., 2015).
Table 1.
Location, environmental conditions and average characteristics of the sampled trees at the five study sites; sites ordered in terms of latitude
| Site | Latitude | Longitude | Altitude (m asl) | Annual temperature (°C) | May–September temperature | Tree height (m) | Tree DBH (cm) | Annual precipitation (mm) |
|---|---|---|---|---|---|---|---|---|
| SIM | 48°13′ | 71°15′ | 338 | 1.9 | 13.3 | 16.1 ± 1.2 | 20.4 ± 2.4 | 1162 |
| BER | 48°51′ | 70°20′ | 611 | 0.2 | 11.4 | 17.3 ± 1.8 | 21.1 ± 3.7 | 1109 |
| MIS | 49°43′ | 71°56′ | 342 | 0.7 | 12.8 | 18.3 ± 1.1 | 19.6 ± 2.8 | 1009 |
| DAN | 50°41′ | 72°11′ | 487 | −1.2 | 11.0 | 16.6 ± 2.2 | 18.5 ± 2.9 | 1006 |
| MIR | 53°47′ | 72°52′ | 384 | 1.6 | 11.1 | 13.1 ± 1.2 | 19.6 ± 3.0 | 827 |
DBH: diameter at breast height.
Timing and duration
At each site, we selected ten dominant or co-dominant trees having upright stems and relatively larger diameters (Table 1). Trees with polycormic stems, partially dead crowns, reaction wood or evident damage due to parasites were avoided. In the four lower latitude sites of the latitudinal gradient, we collected one microcore per tree weekly, occasionally fortnightly, from April to October (2002–2016). Microcore sampling was done with surgical bone needles (2002–2007) or Trephor (2007–2016) (Rossi et al., 2006b). The northernmost site (MIR) was sampled following the same protocol but was sampled only from 2012 to 2016.
To avoid the development of resin ducts, samples were collected at least 10 cm apart from each other (Deslauriers et al., 2003). The microcores were dehydrated through successive series of immersions in ethanol and d-limonene. The microcores were embedded in paraffin, cut into 8 μm cross-sections (i.e. transversal sections) and stained with cresyl violet acetate (0.16 % in water) (Rossi et al., 2006b). We discriminated between developing and mature tracheids under visible and polarized light at magnifications of ×400–×500. Cells were classified as (1) enlarging, (2) thickening and lignifying or (3) mature. Cells were counted across three radial rows (Deslauriers et al., 2003). The enlargement zone was characterized by the absence of glistening under polarized light; this indicates the presence of only primary cell walls. Cells undergoing secondary cell wall formation glistened under polarized light. Cresyl violet acetate reacts with lignin, turning from violet to blue in mature cells. Maturation was reached when the cell walls were entirely blue (Rossi et al., 2006a).
Xylem cell anatomy
Two additional microcores per tree were collected in the summer of 2017. These samples were prepared following the above-mentioned protocol, stained with safranin (1 % in water) and fixed on slides with a mounting medium. Digital images of the tree ring cross-sections were collected using a camera fixed on an optical microscope at a magnification of ×20. We measured lumen area, lumen diameter and cell wall thickness (single wall) along at least 30 radial cell rows per tree ring using Wincell (Regent instruments, Canada). Tracheids having lumen smaller than twice the cell wall thickness were considered as latewood (Filion and Cournoyer, 1995). A summary of the measurements performed for the xylogenesis and wood anatomy data sets is provided in Supplementary Data Table S1.
Statistical analysis
We applied generalized additive models (GAMs) for each site to assess the temporal dynamics of the differentiation phases and to produce the tracheidograms (Supplementary Data Fig. S1). The assessment of the temporal dynamics was based on Cuny et al. (2014) and Balducci et al. (2016). The raw data provided the number of cells produced at each differentiation phase at the sampling times (Supplementary Data Fig. S1). These data, computed for ten individuals per site, were averaged by year and by site before modelling the temporal dynamics.
We fitted GAMs with splines to the xylogenesis data to obtain three curves that describe the daily sequence of cell production for the three differentiation phases, i.e. (1) enlargement, (2) secondary wall deposition and (3) maturation. The timing (the onset for each phase) was extracted from the three curves as the day when each cell position underwent a differentiation phase. The duration of enlargement and wall formation was defined as the difference between the onset of one phase and the onset of the successive phase (Supplementary Data Fig. S1).
The tracheidograms of lumen area, lumen diameter and cell wall thickness were obtained based on the percentile position of the cells across the tree ring. Using the GAMs, we measured cell traits along multiple radial cell rows. We then averaged the standardized percentiles of their horizontal position to provide average sizes (Supplementary Data Fig. S1). We computed cell diameter as the sum of the lumen diameter and 2× single cell wall thicknesses.
The relationship between dynamics (duration of enlargement and cell wall formation) and cell traits (cell diameter and cell wall thickness) was described as a yearly average by applying a modified von Bertalanffy growth equation according to the formula:
where a, k and b represent the asymptote, growth rate and horizontal intercept, respectively. The fit was validated visually using the distribution of the residuals, and, when required, parameters were adjusted with lower or upper bounds to improve the overall fit. Statistics were performed running the mgcv (Wood, 2017), nls and stats packages in R (R Core Team, 2017).
RESULTS
Cell traits
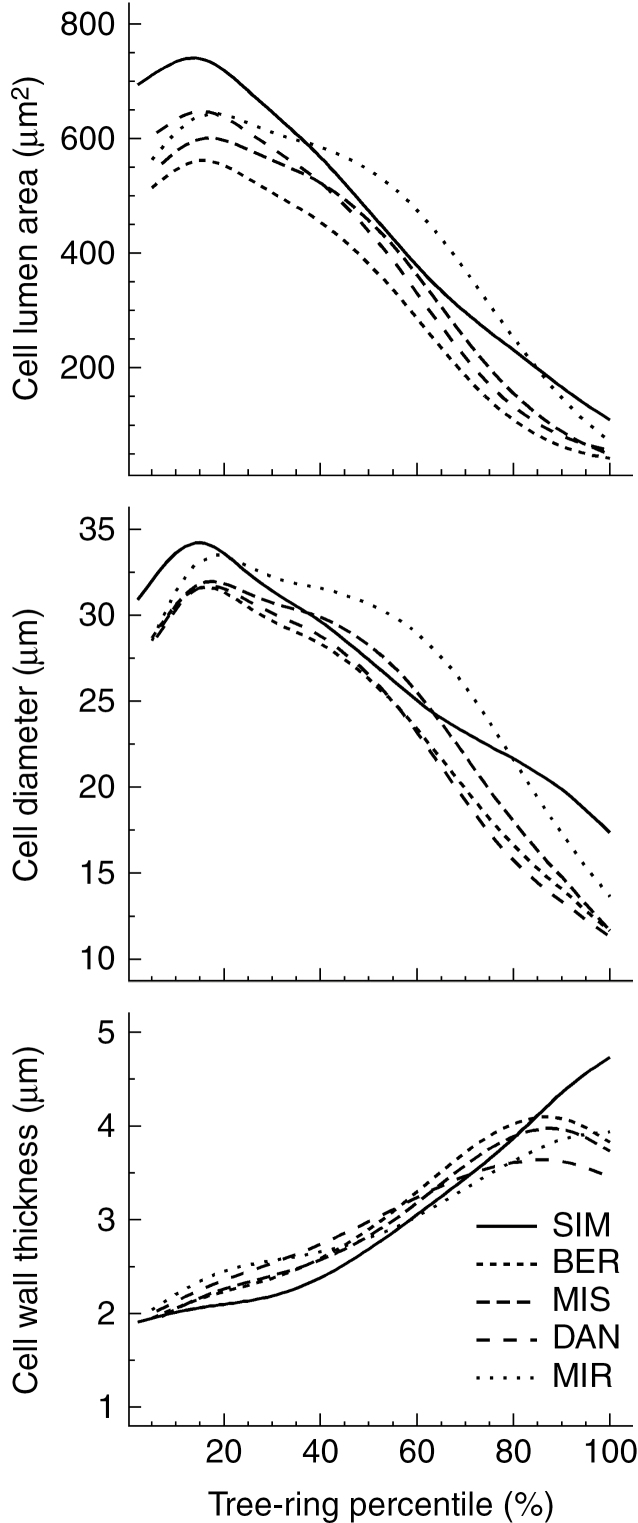
Generalized additive models described very well the changes in anatomical traits across the tree ring, summarizing the inter-annual variability in a single curve per site (Supplementary Data Fig. S2). The lowest variability was observed at MIR due to fewer years of sampling (Supplementary Data Fig. S2). Overall, lumen area ranged from 42 to 741 μm2, gradually decreasing in size from 20 % of the tree ring (Fig. 1). The largest and smallest lumen areas were measured at SIM and BER, the two southernmost sites. Cell diameter ranged from a minimum of 11 μm to a maximum of 34 μm. Cell diameter showed a similar pattern to lumen area; at most sites, maximum cell diameter occurred at about one-fifth of the way across the tree ring. Cell wall thickness increased through the tree ring, starting at an initial thickness of 1.91 μm and reaching a maximum thickness of 4.73 μm at about four-fifths of the way across the tree ring. SIM showed a different pattern, as cell wall thickness increased gradually throughout the tree ring.
Fig. 1.
Tracheidograms of black spruce from five sites distributed along a latitudinal gradient across the closed boreal forest of Quebec, Canada. Each tracheidogram represents the average intra-annual variation of cell traits over the years of study for each site as described in Table 1.
Timing and duration of xylogenesis
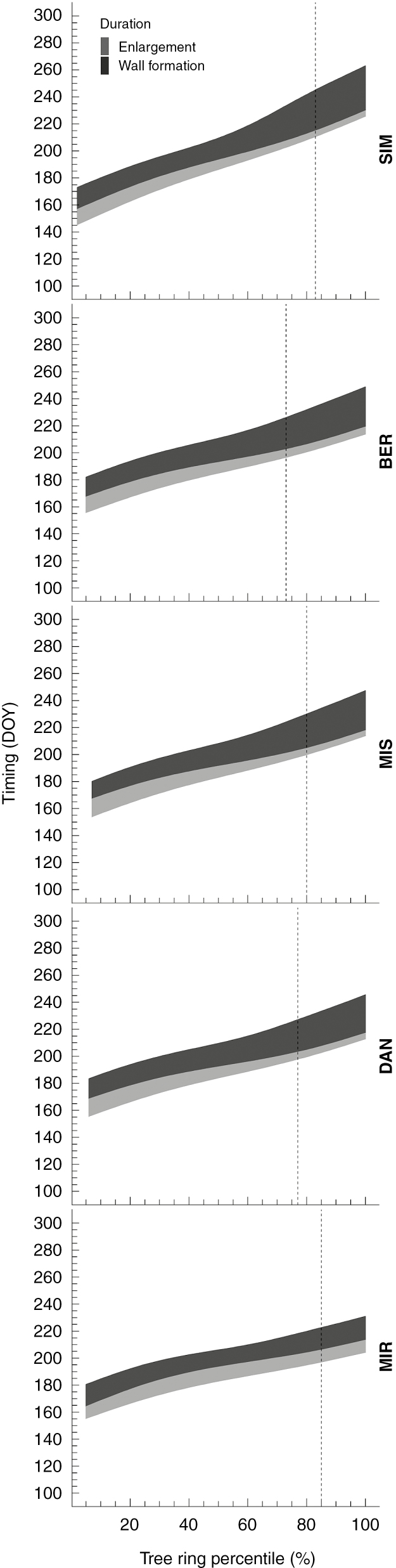
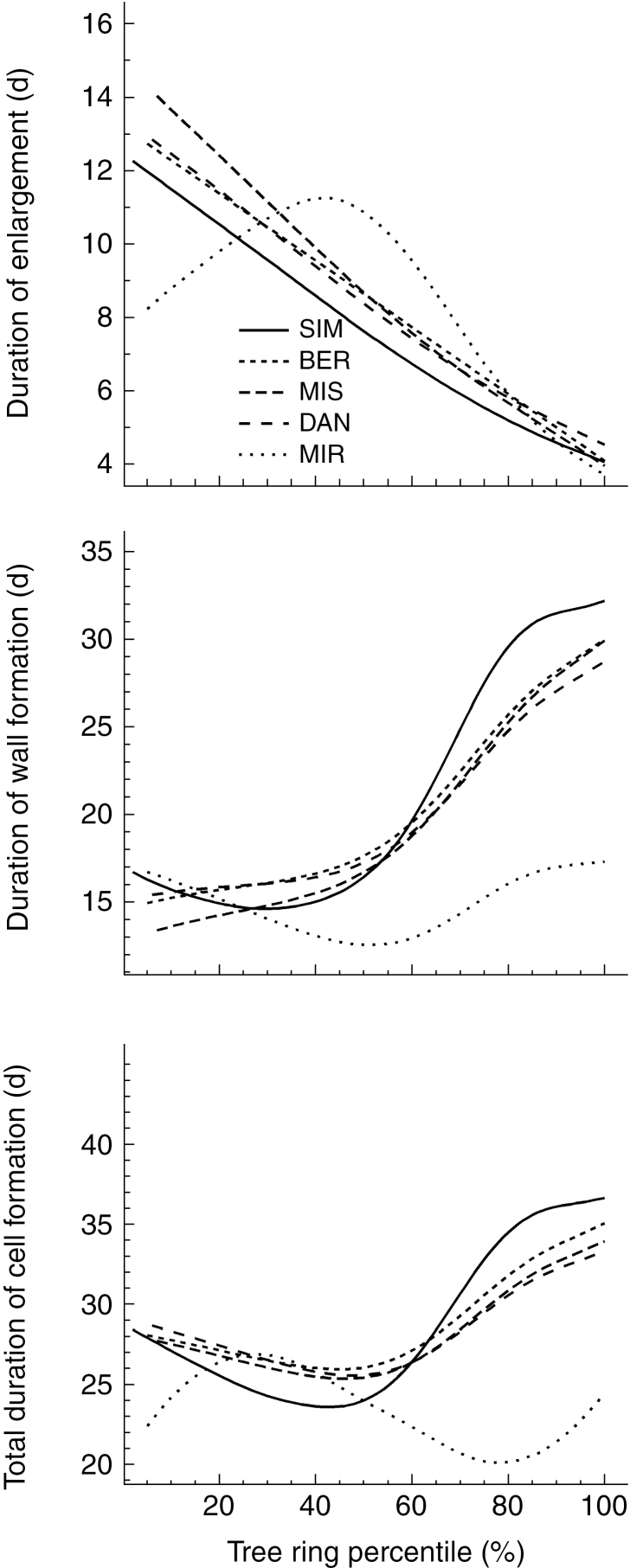
The timing of the onset of the differentiation phases varied along the gradient. The earliest activities were in the southernmost site, although the delays did not follow a linear pattern along the latitudinal gradient in relation to the latitude of the sample sites (Fig. 2). The earliest onset of cell enlargement was observed at SIM at the end of May [day of the year (DOY) 145] and then 10 d later in BER, DAN and MIR. At MIS, cells began enlarging on DOY 153. Similarly, cell wall formation began earlier in SIM (DOY 157) and 7 d later in MIR (DOY 164), with DAN being the last site to begin cell wall deposition (DOY 169). Mature tracheids were also observed earlier in SIM (DOY 173) compared with DAN (DOY 184), which was the last site to achieve this stage. Xylem cells completed their maturation earliest on DOY 231 at MIR and 32 d later at SIM (DOY 263). The percentage of latewood also varied along the gradient, from 15 to 27 %. The lowest and highest latewood percentages were calculated for MIR and BER, respectively. The duration of cell differentiation showed a similar pattern between sites, with the exception of MIR (Fig. 3). Cell enlargement lasted 12–14 d for the first xylem cells, and it decreased to 4–5 d for the final cells of the tree ring. The duration of enlargement at MIR was strongly affected by the shorter period of observation (2012–2016) that amplified the effect of a different dynamic observed for 1 year, 2014, for which we had only 9 d of observations. The duration of enlargement in MIR was 8 d for the first cells. This peaked at 40 % of the tree ring, when cells enlarged for 12 d, then reached the minimum of 4 d over the last percentile of the tree ring. Cell wall formation showed an opposite trend; its duration increased across the tree ring. The duration of wall formation varied across the tree ring, starting from a minimum of 17 d in earlywood and peaking at 32 d in latewood. Minimum and maximum total duration of xylem formation occurred at 50 and 100 % of the tree ring, respectively. The length of xylem formation in MIR was different, however, as the duration gradually decreased across the tree ring (Fig. 3). On average, the length of the growing season decreased with increasing latitude, ranging from 118 d at SIM to 76 d at MIR.
Fig. 2.
Cell trait timings across the tree rings of black spruce collected from five sites distributed along a latitudinal gradient through the closed boreal forest of Quebec, Canada. Timings represent the day (DOY) at which a certain percentile of the tree ring was enlarging (light grey) or forming secondary walls (dark grey). The dashed line marks the percentage of latewood at each site.
Fig. 3.
Variation in the duration of the various cell differentiation phases across a black spruce tree ring at five sites along a latitudinal gradient in Quebec, Canada. The modelling protocol proposed by Cuny et al. (2014) and Balducci et al. (2016) was used to assess the duration of each differentiation phase.
Relationship between cell traits and the duration of development
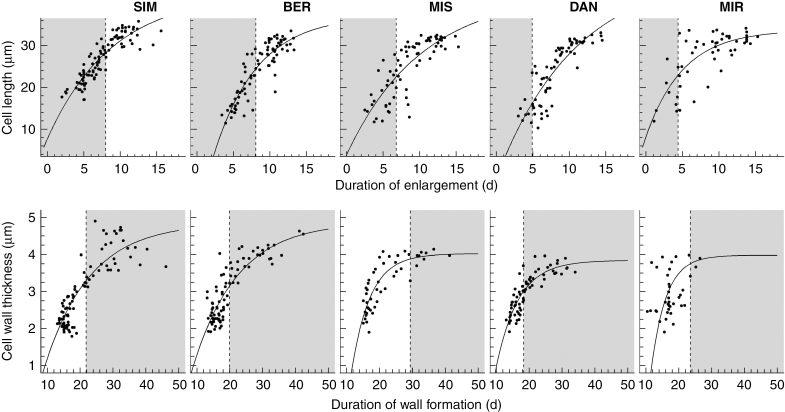
The modified von Bertalanffy equation represented very well the relationship between cell traits and the duration of cell differentiation at each site (Fig. 4). The pattern was clear: both cell traits increased along with a longer duration of development. Decreasing rates of growth were observed, which resulted in an asymptote. The standardized residuals were symmetrically distributed; this confirmed the good fit of the regressions. Overall, 97 % of the standardized residuals were located within the –2 to 2 range, demonstrating that the model suitably represented the data (Supplementary Data Fig. S3).
Fig. 4.
Cell traits vs. the duration of their developmental phases plotted using the modified von Bertalanffy equation. The top row presents cell diameter vs. the duration of enlargement. Cell wall thickness vs. the duration of lignification is presented in the bottom row. The dashed line represents the threshold between earlywood and latewood; the light grey background represents the latewood portion. Each plot represents one of the five sites along a latitudinal gradient across the closed boreal forest of Quebec, Canada.
Cell diameter vs. the duration of enlargement
The asymptote a ranged from 33.37 to 47.63, with the lowest and highest values being estimated for MIR and DAN, respectively (Table 2). This last value was slightly overestimated, as shown by the visual fitting and the larger error of the resulting parameter (Table 2). The growth rate k ranged from 0.09, detected at MIS and DAN, to 0.19 at MIR. The intercept b ranged from –1.77 to 1.64, for SIM and BER, respectively (Table 2). Overall, cells enlarged at 3 μm d–1. Earlywood cells averaged 29 μm in diameter; such a size was achieved in 11 d. Latewood cells required 6 d to reach a mean diameter of 24 μm (Fig. 4). On average, the enlargement of earlywood cells required a further 3 d compared with the enlargement of latewood cells. No latitudinal pattern was observed in the estimated parameters of the enlargement phase.
Table 2.
Parameters of the von Bertalanffy-modified equation for cell diameter vs. duration of cell enlargement and cell wall thickness vs. duration of wall formation
| Cell traits | Sites | a | k | b |
|---|---|---|---|---|
| Cell diameter | SIM | 42.36 ± 4.28 | 0.11 ± 0.03 | –1.77 ± 0.94 |
| BER | 37.18 ± 3.79 | 0.17 ± 0.05 | 1.64 ± 0.65 | |
| MIS | 42.58 ± 9.78 | 0.09±0.05 | –0.99 ± 1.57 | |
| DAN | 47.63 ± 11.81 | 0.09 ± 0.05 | 0.37 ± 1.03 | |
| MIR | 33.37 ± 2.45 | 0.19 ± 0.07 | –1.43 ± 1.34 | |
| Cell wall thickness | SIM | 4.85 ± 0.38 | 0.07 ± 0.01 | 6.08 ± 1.59 |
| BER | 4.87 ± 0.43 | 0.07 ± 0.02 | 5.78 ± 1.88 | |
| MIS | 4.02 ± 0.17 | 0.17 ± 0.05 | 10.16 ± 1.64 | |
| DAN | 3.84 ± 0.15 | 0.15 ± 0.03 | 8.01 ± 1.25 | |
| MIR | 3.98 ± 0.61 | 0.21 ± 0.10 | 10.40 ± 0.82 |
The parameters a, k and b represent the asymptote, growth rate and horizontal intercept, respectively.
Cell wall thickness vs. the duration of wall formation
The asymptote a ranged from 3.98 for MIR to 4.87 for BER (Table 2). This parameter followed the latitudinal gradient, decreasing toward the north; BER, which showed the highest asymptote, was the exception to this pattern. In general, the growth rate k was lower than that estimated for cell diameter, with values ranging between 0.07 – detected at BER and SIM – and 0.21, calculated for MIR. Compared with cell diameter, the horizontal intercept b was higher for cell wall thickness, ranging from 5.78 to 10.40 for BER and MIR, respectively (Table 2). In the two southern sites, a cell wall thickness of 3 μm was reached after 20 d, a longer duration than in the northern sites. For MIR, the model was adapted by using a higher limit for a. This limit improved the fit of the regression, but it generated a modest underestimation of cell wall thicknesses, as revealed by the residuals (Supplementary Data Fig. S2). Earlywood cell walls had an average thickness of 2 μm after 15 d, while latewood cell walls averaged 3 μm thick after 22 d.
DISCUSSION
This study assessed the intra-annual dynamics of xylem formation in adult black spruce trees across a wide latitudinal range. Based on 15 years of observations, our data set tested the hypothesis that cell traits are positively and linearly correlated with the duration of their differentiation phases. The initial hypothesis was only partially accepted; longer differentiation phases (i.e. cell enlargement and cell wall formation) resulted in larger sizes (i.e. cell diameter and cell wall thickness). However, we found a non-linear relationship.
The influence of the duration of enlargement on cell size changes across the tree ring
From the observed non-linear relationship (i.e. modified von Bertalanffy), our analysis shows that the maximum size of growing tracheids is limited at longer durations. Until this study, there was only a partial understanding of the relationship between cell size and duration of cell developmental stages across a radial section of a tree trunk. Anfodillo et al. (2011) demonstrated that different durations of enlargement could explain the size decrease of the first earlywood cells – produced by cambial reactivation – downwards along the stem. Indeed, the larger cells located at the base of the tree stem grew for a longer period than the smaller treetop cells, to form a tapering pattern. In addition, based on 3 years of observation, Cuny et al. (2014) assessed a species-specific linear relationship between cell size and the duration of their enlargement across the radial pattern. Cells across the tree ring increased linearly in size as the duration of enlargement increased (i.e. a linear relationship) (Cuny et al., 2014). Our data set, however, that involved cells that were heterogeneous in size allowed us to better describe this relationship. Indeed, by modelling the cell growth of a single cell, Cartenì et al. (2018) found a non-linear relationship over which longer durations did not necessarily correspond to increasing cell sizes. Therefore, by considering all the cells that form a tree ring and relying on a data set covering several years, it was possible to describe a non-linear relationship between cell diameter and the duration of enlargement.
Our data show that cells increase initially in size as the duration of enlargement is greater. After a given period, cell growth gradually slows down and then ceases. Cell diameter then remains unchanged even if cells could continue to grow. We hypothesize that cell size growth is constrained by physiological and biomechanical constraints. Cell enlargement results from a combination of DNA replication, i.e. endoreplication and cell expansion (Perrot-Rechenmann, 2010). The latter process occurs when enough water is absorbed to exceed the wall-yielding threshold pressure (Genard et al., 2001). During cell differentiation, the deposition of several layers of secondary walls stiffens cell walls and progressively reduces the cell’s capability to enlarge (Dünser and Kleine-Vehn, 2015). The loss of flexibility, due to the deposition of secondary walls, stops cell expansion.
This ceasing of cell expansion arrives later in earlywood than in latewood, thereby explaining their different cell sizes (Cartenì et al., 2018). During the initial phase of the growing season, the priority of primary (leaf) growth over secondary (wood) growth along the stem results in a low availability of sugars for xylogenesis (Cartenì et al., 2018). In our model, cell size increases as the period of differentiation lengthens; however, secondary walls are deposited slowly, eventually constraining cell enlargement after about 15 d. During the second part of the growing season, the deposition of secondary walls is faster as there is a higher quantity of sugars available for radial growth; this results in a more rapid loss of cell flexibility in latewood. For smaller cell sizes, the growth of the primary wall will constrain size only after 5 d of differentiation, resulting in latewood cells that are smaller than the earlywood cells. According to our model, the maximum duration of cell expansion, which corresponds to the mean theoretical asymptote, is 35 d (combining all sites together).
The relationship between the duration of enlargement and cell size may also play an important role in maintaining hydraulic safety. A shorter period of enlargement produces smaller cells that avoid cavitation more easily. We estimate that a maximum duration of enlargement of 16 d in black spruce will produce cells 30–36 μm in diameter. For temperate climates, Cuny et al. (2014) calculated that the largest cells of Norway spruce, Scots pine and silver fir had diameters of 50 μm after an average of 18 d. Therefore, two additional days of cell enlargement for these temperate species added 20 μm in cell diameter relative to our colder sites. We speculate that the duration of enlargement and the resulting cell diameter is species specific given that this trait is highly important in the trade-off between water transport efficiency and hydraulic safety. Larger conduits have a higher hydraulic efficiency, but they are more vulnerable to cavitation (Pitterman et al., 2006). In cold climates, bubbles generated by freezing-induced embolism are also more difficult to eliminate when conduits are larger (Pittermann and Sperry, 2003). Consequently, as we observed in the boreal forest, early spring cells grow for less time than cells growing in a temperate climate, thereby avoiding the increased risk of cavitation linked to larger cell sizes. The smaller early spring cells of the cooler boreal forest are also less prone to damage from freeze–thaw events.
In our study, we assessed a relationship that fully represents the dynamics of both earlywood and latewood cells and that applies to a wide geographical area. Nevertheless, the latitudinal gradient did not appear to affect the distribution of cell sizes. Indeed, even if the northernmost site had the lowest asymptote along the latitudinal gradient (33 μm at MIR), the other sites showed similar asymptotic values (42 μm on average). Water availability should directly drive cell enlargement, but it affects cell growth significantly only when water becomes a limiting factor (Cuny and Rathgeber, 2016; Prislan et al., 2018). We may not have observed climatic determinism in cell enlargement as water is not a limiting factor along our study’s latitudinal gradient (Table 1). However, evidence provided by rain exclusion experiments performed at our study sites (except MIR) highlight a limited influence of water stress on black spruce growth and tracheid anatomy (Belien et al., 2012). When comparing xylogenesis in black spruce growing in parcels having or lacking rain, Belien et al. (2012) observed that timing and xylem growth were not affected by induced water stress; this stress produced only slightly smaller cells. The high resilience of this cell trait is caused by adjustments in the duration and rate of cell enlargement and secondary wall deposition and lignification that mostly counterbalances any effect of water stress (Balducci et al., 2016).
The relationship we observed confirms many previous findings related to the quantitative aspects of xylogenesis in conifers. In Scots pine, the duration of enlargement was estimated at 21 d for the initial tree ring cells, decreasing to 10 d for the final cells (Wodzicki, 1971). Deslauriers et al. (2003) observed that cell enlargement in balsam fir lasted less than a week for earlywood cells, whereas it was more than a week for latewood cells. In three European species of the alpine timberline, European larch, stone pine and Norway spruce, Rossi et al. (2006a) found that cell enlargement occurred over an average of 20 d for the first earlywood cells, decreasing to a few days for the final latewood cells. More recently, a model of Cartenì et al. (2018) – calibrated using stone pine, Norway spruce, European larch and black spruce – estimated an average duration of 18.8 d for the first earlywood cells and 5.9 d for the final latewood cells.
Cell wall size is linked to the duration of cell wall deposition
In general, cell wall deposition depends linearly on the duration of earlywood formation. However, during latewood cell formation, cell wall thickness does not increase proportionally with duration. Across our latitudinal gradient, latewood cell differentiation begins when cell wall deposition reaches 20–40 d. Uggla et al. (2001) observed that in latewood, cell formation was tightly linked to a longer period of wall material deposition, not its rate. Since carbohydrate availability did not change significantly during the growing season, Uggla et al. (2001) deduced that this could not be a trigger for latewood formation and proposed that latewood formation was under developmental control. Further studies found that the increase in cell wall thickness was synchronous with the increasing availability of polysaccharides over the growing season, reassessing the role of sugars in the earlywood–latewood transition (Deslauriers et al., 2016; Cartenì et al., 2018). In our results, the duration of wall formation severely affects cell wall thickness in the first part of the tree ring. This influence decreases across the tree ring, where other factors slow down cell wall thickness deposition before bringing it to a complete halt.
The more abrupt decrease in cell wall thickness at longer durations could be linked to cell maturation and cell death. Groover and Jones (1999) indicate that cell death is activated when a critical amount of secondary wall is attained. The joint action of secondary wall precursors and protease induces calcium accumulation within a cell and generates vacuole collapse, occurring after about 6 h (Groover and Jones, 1999). As secondary cell wall formation exerts a control on cell death, the deposition of cell wall material abruptly slows down at longer durations. Interestingly, the maximum duration of secondary cell wall formation predicted by our model averaged 45 d, a value that was always observed along our latitudinal gradient. We thus propose that programmed cell death is involved in the faster attainment of the asymptote, in contrast to cell enlargement, where it is reached more slowly.
We observed that maximum cell wall thickness, which is reached in latewood, follows a temperature gradient. Latewood cells, generally constituting the final portion of the annual tree ring, are more sensitive to temperature than earlywood cells (Cuny and Rathgeber, 2016). Warmer conditions induce a thicker cell wall because they are linked to a longer growing season during which trees have more time to assimilate carbon (Fonti et al., 2013). Furthermore, warmer temperatures correspond to larger earlywood cells that allow a better assimilation of carbon due to their higher hydraulic efficiency (Fonti et al., 2013). Evidence of the link between temperature and cell traits was also found by Deslauriers et al. (2008) who observed that the high temperatures experienced by trees at treeline in 2003 induced a longer period of secondary wall deposition, which resulted in thicker cell walls. In our results, the values achieved by the asymptotes suggest that the maximum cell wall thickness reached at each site followed the latitudinal gradient. Trees growing at the southern sites produced thicker cell walls in latewood than trees growing at the more northern sites.
The observed duration for cell wall deposition in our study also agrees with previous estimates for boreal species, with latewood requiring a longer period of cell wall formation than earlywood (Wodzicki, 1971; Deslauriers et al., 2008; Lupi et al., 2011). In three conifer species in France, the duration of secondary wall formation increased from 20 to 55 d across the tree ring (Cuny et al., 2014). Compared with earlywood, Deslauriers et al. (2003) observed that the time required by balsam fir to complete cell wall formation was 10–15 d longer in latewood. Cartenì et al. (2018) estimated an average duration of cell wall formation ranging from 16 to 35 d, depending on the position of the cell within the tree ring. The duration of wall formation increases during the growing season, with the final latewood cells requiring up to 40 d to complete this differentiation phase (Deslauriers et al., 2008; Lupi et al., 2011).
CONCLUSION
In this study, we demonstrated the relationship between temporal dynamics of cell differentiation and cell traits. We tested and confirmed the hypothesis that the intra-annual growth in cell traits increases in a non-linear fashion with the duration of differentiation. Despite the wide geographical scale analysed, involving the broad latitudinal distribution of black spruce in Quebec, Canada, we were able to assess a general pattern that occurs independently of the variable site conditions. We found that cell growth and cell wall thickening reach a plateau, beyond which cell traits remain stable, independent of the duration of differentiation. Even if the existence of this non-linear pattern confirms the complexity of xylogenesis, we found a relationship that emphasizes the different biological mechanisms between earlywood and latewood cells. These findings provide a more integrated knowledge of xylogenesis and its developmental dynamics. In particular, we demonstrated that the duration of cell differentiation is a key factor that has a major role in establishing the final traits of xylem cells.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: schematic drawing summarizing the statistical analysis performed on the two data sets (xylogenesis and wood anatomy). Figure S2: tracheidograms of black spruce from five sites distributed along a latitudinal gradient across the closed boreal forest of Quebec, Canada. Figure S3: distribution of residuals obtained by the fitting of the modified von Bertalanffy equation.
FUNDING
This study was funded by the NSERC Industrial Research Chair, the Canada Foundation for Innovation, le Consortium de Recherche sur la Forêt Boréale Commerciale, les Fonds de Recherche sur la Nature et les Technologies du Québec and la Forêt d’Enseignement et de Recherche Simoncouche.
ACKNOWLEDGEMENTS
The authors thank M. Boulianne, J. Boulouf, B. Dufour, G. Dumont-Frenette, F. Gionest, M.-J. Girard, A. Lemay, C. Lupi, V. Nèron, S. Pedneault, P.-Y. Plourde, G. Savard, M. Thibeault-Martel and M.-J. Tremblay for technical support. Murray Hay verified the English.
LITERATURE CITED
- Aloni R. 2013. Role of hormones in controlling vascular differentiation and the mechanism of lateral root initiation. Planta 238: 819–830. [DOI] [PubMed] [Google Scholar]
- Aloni R, Zimmermann MH. 1983. The control of vessel size and density along the plant axis: a new hypothesis. Differentiation 24: 203–208. [Google Scholar]
- Anfodillo T, Deslauriers A, Menardi R, Tedoldi L, Petit G, Rossi S. 2011. Widening of xylem conduits in a conifer tree depends on the longer time of cell expansion downwards along the stem. Journal of Experimental Botany 63: 837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balducci L, Cuny HE, Rathgeber CBK, Deslauriers A, Giovannelli A, Rossi S. 2016. Compensatory mechanisms mitigate the effect of warming and drought on wood formation. Plant, Cell & Environment 39: 1338–1352. [DOI] [PubMed] [Google Scholar]
- Belien E, Rossi S, Morin H, Deslauriers A. 2012. Xylogenesis in black spruce subjected to rain exclusion in the field. Canadian Journal of Forest Research 42: 1306–1315. [Google Scholar]
- Carteni F, Deslauriers A, Rossi S, et al. 2018. The physiological mechanisms behind the earlywood-to-latewood transition: a process-based modeling approach. Frontiers in Plant Science 9: 1053. doi: 10.3389/fpls.2018.01053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE. 2009. Towards a worldwide wood economics spectrum. Ecology Letters 12: 351–366. [DOI] [PubMed] [Google Scholar]
- Cuny HE, Rathgeber CBK. 2016. Xylogenesis: coniferous trees of temperate forests are listening to the climate tale during the growing season but only remember the last words! Plant Physiology 171: 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuny HE, Rathgeber CBK, Kiessé TS, Hartmann FP, Barbeito I, Fournier M. 2013. Generalized additive models reveal the intrinsic complexity of wood formation dynamics. Journal of Experimental Botany 64: 1983–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuny HE, Rathgeber CBK, Frank D, Fonti P, Fournier M. 2014. Kinetics of tracheid development explain conifer tree-ring structure. New Phytologist 203: 1231–1241. [DOI] [PubMed] [Google Scholar]
- Cushing JM, Costantino RF, Dennis B, Desharnais R, Henson SM. 2002. Chaos in ecology: experimental nonlinear dynamics. Amsterdam: Elsevier. [Google Scholar]
- Denne MP. 1971. Temperature and tracheid development in Pinus sylvestris seedlings. Journal of Experimental Botany 22: 362–370. [Google Scholar]
- Denne MP. 1989. Definition of latewood according to Mork (1928). IAWA Journal 10: 59–62. [Google Scholar]
- Deslauriers A, Morin H, Begin Y. 2003. Cellular phenology of annual ring formation of Abies balsamea in the Quebec boreal forest (Canada). Canadian Journal of Forest Research 33: 190–200. [Google Scholar]
- Deslauriers A, Rossi S, Anfodillo T, Saracino A. 2008. Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy. Tree Physiology 28: 863–871. [DOI] [PubMed] [Google Scholar]
- Deslauriers A, Huang J-G, Balducci L, Beaulieu M, Rossi S. 2016. The contribution of carbon and water in modulating wood formation in black spruce saplings. Plant Physiology 170: 2072–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslauriers A, Fonti P, Rossi S, Rathgeber CBK, Gričar J. 2017. Ecophysiology and plasticity of wood and phloem formation. In: Amoroso M, Daniels L, Baker P, Camarero J, eds. Dendroecology. Ecological Studies (Analysis and Synthesis), Vol. 231 Cham: Springer, 13–33. [Google Scholar]
- Domec J-C, Gartner BL. 2002. How do water transport and water storage differ in coniferous earlywood and latewood? Journal of Experimental Botany 53: 2369–2379. [DOI] [PubMed] [Google Scholar]
- Dünser K, Kleine-Vehn J. 2015. Differential growth regulation in plants – the acid growth balloon theory. Current Opinion in Plant Biology 28: 55–59. [DOI] [PubMed] [Google Scholar]
- Fajstavr M, Paschová Z, Giagli K, Vavrčik H, Gryc V, Urban J. 2018. Auxin (IAA) and soluble carbohydrate seasonal dynamics monitored during xylogenesis and phloemogenesis in Scots pine. iForest-Biogeosciences and Forestry 11: 553–562. [Google Scholar]
- Filion L, Cournoyer L. 1995. Variation in wood structure of eastern larch defoliated by the larch sawfly in subarctic Quebec, Canada. Canadian Journal of Forest Research 25: 1263–1268. [Google Scholar]
- Fonti P, Von Arx G, Garcia-González I, et al. 2010. Studying global change through investigation of the plastic responses of xylem anatomy in tree rings. New Phytologist 185: 42–53. [DOI] [PubMed] [Google Scholar]
- Fonti P, Bryukhanova MV, Myglan VS, Kirdyanov AV, Naumova OV, Vaganov EA. 2013. Temperature-induced responses of xylem structure of Larix sibirica (Pinaceae) from the Russian Altay. American Journal of Botany 100: 1332–1343. [DOI] [PubMed] [Google Scholar]
- Genard M, Fishman S, Vercambre G, et al. 2001. A biophysical analysis of stem and root diameter variations in woody plants. Plant Physiology 126: 188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groover A, Jones AM. 1999. Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary cell wall synthesis. Plant Physiology 119: 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann FP, Rathgeber CBK, Fournier M, Moulia B. 2017. Modelling wood formation and structure: power and limits of a morphogenetic gradient in controlling xylem cell proliferation and growth. Annals of Forest Science 74: 14. doi: 10.1007/s13595-016-0613-y. [DOI] [Google Scholar]
- Larson PR. 1960. A physiological consideration of the springwood summerwood transition in Red Pine [Pinus resinosa]. Forest Science 6: 110–122. [Google Scholar]
- Lupi C, Morin H, Deslauriers A, Rossi S. 2011. Xylogenesis in black spruce: does soil temperature matter? Tree Physiology 32: 74–82. [DOI] [PubMed] [Google Scholar]
- Moser L, Fonti P, Büntgen U, et al. 2009. Timing and duration of European larch growing season along altitudinal gradients in the Swiss Alps. Tree Physiology 30: 225–233. [DOI] [PubMed] [Google Scholar]
- Perrot-Rechenmann C. 2010. Cellular responses to auxin: division versus expansion. Cold Spring Harbor Perspectives in Biology 2: a001446. doi: 10.1101/cshperspect.a001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitterman J, Sperry JS, Wheeler J, Hacke UG, Sikkema EH. 2006. Mechanical reinforcement of tracheids compromises the hydraulic efficiency of conifer xylem. Plant, Cell & Environment 29: 1618–1628. [DOI] [PubMed] [Google Scholar]
- Pittermann J, Sperry J. 2003. Tracheid diameter is the key trait determining the extent of freezing-induced embolism in conifers. Tree Physiology 23: 907–914. [DOI] [PubMed] [Google Scholar]
- Prislan P, Čufar K, De Luis M, Gričar J. 2018. Precipitation is not limiting for xylem formation dynamics and vessel development in European beech from two temperate forest sites. Tree Physiology 38: 186–197. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, http://www.R-project.org/ [Google Scholar]
- Rossi S, Anfodillo T, Deslauriers A. 2006a Assessment of cambial activity and xylogenesis by microsampling tree species: an example at the alpine timberline. IAWA Journal 27: 383–394. [Google Scholar]
- Rossi S, Anfodillo T, Menardi R. 2006b Trephor: a new tool for sampling microcores from tree stems. IAWA Journal 27: 89–97. [Google Scholar]
- Rossi S, Anfodillo T, Čufar K, et al. 2013. A meta-analysis of cambium phenology and growth: linear and non-linear patterns in conifers of the northern hemisphere. Annals of Botany 112: 1911–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Cairo E, Krause C, Deslauriers A. 2015. Growth and basic wood properties of black spruce along an alti-latitudinal gradient in Quebec, Canada. Annals of Forest Science 72: 77–87. [Google Scholar]
- Schweingruber FH. 2012. Tree rings: basics and applications of dendrochronology. Dordrecht: Springer Science & Business Media. [Google Scholar]
- Skene DS. 1969. The period of time taken by cambial derivatives to grow and differentiate into tracheids in Pinus radiata: D. Don. Annals of Botany 33: 253–262. [Google Scholar]
- Uggla C, Magel E, Moritz T, Sundberg B. 2001. Function and dynamics of auxin and carbohydrates during earlywood/latewood transition in Scots pine. Plant Physiology 125: 2029–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodzicki TJ. 1971. Mechanism of xylem differentiation in Pinus silvestris L. Journal of Experimental Botany 22: 670–687. [Google Scholar]
- Wood SN. 2017. Generalized additive models: an introduction with R. Chapman and Hall/CRC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.