Abstract
Objective
Repetitive transcranial magnetic stimulation (rTMS) is beneficial for treatment-resistant patients with obsessive-compulsive disorder (OCD). The serotonin transporter gene (SLC6A4) may be associated with OCD. We aimed to determine whether SLC6A4 impacts the beneficial effects of rTMS in patients with OCD treated with selective serotonin reuptake inhibitors (SSRIs).
Methods
Fifty-seven untreated patients with OCD were randomly assigned to receive active or sham rTMS in a 4-week double-blind study. The participants received 1-Hz rTMS over the supplementary motor area once per day, for 5 days a week, for 4 weeks. One of the widely employed SSRIs was utilized at the initiation of active or sham rTMS. Yale–Brown obsessive–compulsive scale (Y-BOCS) scores were used for assessing the symptoms. The most-researched polymorphism of SLC6A4, 5-HTTLPR (L/S), was also examined.
Results
Y-BOCS scores in the active group at the completion of the treatment were significantly lower than those in the sham group. Interestingly, the improvement in Y-BOCS scores in patients with the LL genotype treated with active rTMS was significantly (p<0.05) greater than in those treated with sham rTMS. Conversely, rTMS did not produce significant improvements in S allele carriers.
Conclusions
The findings of this study suggest that rTMS can augment the beneficial effects of SSRIs in OCD patients with the LL genotype of 5-HTTLPR. Therefore, the presence of 5-HTTLPR (L/S) in SLC6A4 may be a predictable biomarker for the beneficial effects of rTMS, although more studies using larger sample sizes are warranted for confirming the results.
Keywords: obsessive-compulsive disorder, repetitive transcranial magnetic stimulation, 5-HTTLPR, polymorphism
Introduction
Obsessive-compulsive disorder (OCD) is characterized by persistent obsessions and/or compulsions and has a high prevalence, ranging from 1% to 3% worldwide.1,2 OCD is also the fourth-most prevalent psychiatric disorder in China, with a lifetime prevalence of 2.5%.3 Furthermore, OCD can seriously disrupt normal daily routines, leading to a low quality of life, social impairment, and continuous mental distress.4–6 Selective serotonin reuptake inhibitors (SSRIs), especially when combined with cognitive behavioral therapy (CBT), can be effective in the management of OCD.7,8 Although meta-analyses have shown that higher doses of SSRIs are marginally more effective than lower doses,9 approximately 60% of patients with OCD do not have satisfactory outcomes with SSRIs.10 On the contrary, numerous patients have experienced unpleasant adverse effects, including difficulty in urination, decreased blood pressure, dry mouth, drowsiness, nausea, headache, and dizziness.11 Therefore, a more effective and safer treatment remains an unmet medical need for OCD.
Repetitive transcranial magnetic stimulation (rTMS) is a novel and non-invasive treatment option for numerous psychiatric disorders.12–15 Several randomized controlled trials using rTMS in treatment-resistant patients with OCD have been published, but the results from meta-analyses have been inconclusive.15–18 However, the findings of a recent meta-analysis demonstrated that stimulation, specifically of the supplementary motor area (SMA), yielded the greatest reduction in the Yale-Brown obsessive-compulsive scale (Y-BOCS) scores, relative to that of other cortical regions.19 These reports regarding the effectiveness of rTMS involved patients who were unresponsive to earlier OCD treatments; the effectiveness of this approach when applied to previously untreated patients with OCD remains unknown.
The serotonin (or 5-hydroxytryptamine) transporter (5-HTT) gene (SLC6A4) contains a functional polymorphism (5-HTTLPR, 44 bp insertion/deletion), which results in a long (L)/short(S) variant in the promoter region, upstream from the transcription starting site.20 The S allele determines decreased transcriptional activity and is associated with poorer outcomes following both antidepressant pharmacological and non-pharmacological treatments.21 While meta-analyses have clearly demonstrated the association between 5-HTTLPR polymorphism and OCD,22–24 no report has yet shown the association between 5-HTTLPR polymorphism, specifically in SLC6A4, and how patients with OCD respond to rTMS.
Therefore, the aim of the present study was to investigate whether the 5-HTTLPR polymorphism in SLC6A4 can affect the influence of an rTMS adjunction to a standard medication of SSRIs in untreated patients with OCD. This was a 4-week double-blind, active versus sham rTMS controlled clinical trial, where the main outcome variables were the Y-BOCS scores and the response rates. Additionally, we also measured the changes in the severity of depression and anxiety using the 17-item Hamilton Depression Rating Scale (HAMD) and the Hamilton Anxiety Rating Scale (HAMA).
Methods and materials
Study participants
The participants comprised 65 outpatients who had been diagnosed with OCD in accordance with the Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM-IV) criteria. Patients were fully informed regarding the aims and procedures of this study as well as the confidential nature of the data selection handling involved, and they all provided their written informed consent. This investigation was performed at the Wuxi Mental Health Center (Wuxi, China) with the approval of the Review Board, in accordance with the principles of the Declaration of Helsinki. Ethics approval was granted by the Ethics Committee of Wuxi Mental Health Center. This study was registered at http://www.chictr.org.cn (ChiCTR1900023641), and the trial protocol has been published. No additional unpublished data are available.
Inclusion criteria
Patients were enrolled in the study if the following seven inclusion criteria were met: (1) diagnosis of current OCD by a psychiatrist in accordance with DSM-IV on the basis of structured clinical interview for DSM (SCID); (2) patients were not on medication; (3) patients were willing and able to consent to the study on the basis of their ability to provide a spontaneous narrative description of its key elements; (4) after a careful neurological interview and the inspection of medical records, no seizures or further neurological disorders or major medical issues were reported or recorded; (5) absence of comorbid psychiatric disorders; (6) no current alcohol and other drug use; (7) ages between 18 and 65 years.
Exclusion criteria
Patients were not included in the study if (1) inclusion criteria mentioned above were not met; (2) patient had metal implants; (3) female participants were pregnant, breast-feeding, or intended to become pregnant during the period of the study; (4) there was a history of DSM-IV substance-dependence in the past 6 months; (5) acute suicidality; (6) patients experienced severe adverse effects during or after the treatment or if the patient withdrew from the study for any reason.
Since this is the first controlled trial focus on the relationship between 5-HTTLPR genotype and rTMS treatment for OCD patients and there are few data about the effect size of the intervention, the sample size could not be estimated on the basis of statistical considerations. Based on the results obtained from our preliminary study, we assume that a total sample size of 20 in each group was considered adequate for this exploratory study.
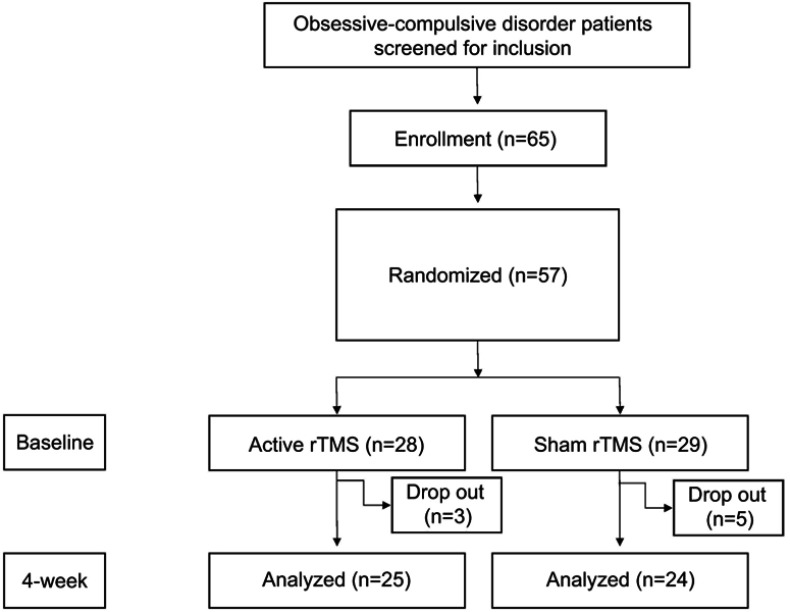
Of the 65 patients who were initially approached, 57 (87.69%) were enrolled, assessed, and randomly assigned to the study conditions (Figure 1).
Figure 1.
CONSORT diagram for a sham-controlled study of rTMS for patients with OCD.
Abbreviations: rTMS, repetitive transcranial magnetic stimulation; OCD, obsessive-compulsive disorder.
Study design
This study was a 4-week, randomized, double-blind, and sham-controlled clinical trial. All patients were randomly classified into the following two groups using a computer-generated schedule: those receiving active rTMS treatments and those receiving sham rTMS treatments. Twenty-eight patients were randomly assigned to the active rTMS group and 29 were assigned to the sham rTMS group. During the 4 weeks of treatments, three active rTMS patients and five from the sham group dropped out of the study; therefore, only 25 patients from the active rTMS group and 24 patients from the sham rTMS group were analyzed in this study (Figure 1).
rTMS therapy
rTMS treatments were administered using a MAGSTIM super-rapid stimulator (Magstim Company, Ltd., Whitland, UK) with a focal 8-shaped, 70-mm coil. Stimulation parameters were 1-Hz, 20 min trains (1,200 pulses/day) at 100% of the resting motor threshold (MT), once per day, 5 days per week, for 4 weeks. For determining the MT level, we used the thumb-movement visualization method by stimulating the left primary motor cortex. The coil was positioned over the pre-SMA, which we targeted using the International 10–20 EEG System. The pre-SMA was defined as 15% of the distance between the inion and nasion on the anterior plane to the Cz (vertex) on the sagittal midline. The coil was placed with the handle along the sagittal midline, pointing toward the occiput for bilaterally and simultaneously stimulating the pre-SMA. The sham treatment targeted using the International 10–20 EEG System. The sham treatment was performed using the Neurosoft sham coil, wherein a metal plate placed inside the coil prevents the magnetic field from stimulating the cortex. This coil looks and sounds like an active one; however, it does not feel the same when receiving active rTMS, which generates a tapping sensation on the scalp. Therefore, for maintaining patient blinding we excluded all those who had, for any reason, experienced active rTMS.)
Drug therapy
Patients received the drugs at the first day of study, and then gradually increase the dosage as directed by psychiatrists. All patients were treated using adequate dosages of SSRIs (sertraline, maximum 200 mg; citalopram, maximum 80 mg; fluoxetine, maximum 80 mg; and paroxetine maximum 80 mg) for at least 4 weeks, and these pharmacological treatments remained unchanged over the course of the study.
Assessment
Clinical evaluation was performed by a research psychiatrist at the baseline, at 2 weeks, and at 4 weeks following the first active or sham rTMS treatments. The interviewing psychiatrist and the study subjects were all blinded to the treatment conditions. The instruments used for the assessment included the Y-BOCS, the 17-item Hamilton Depression Rating Scale (HAMD), and the Hamilton Anxiety Rating Scale (HAMA). The Y-BOCS is a self-rating instrument for assessing OCD symptoms. The Chinese version of the Y-BOCS is a repeatable and sensitive computerized cognitive test with good validity and reliability. The assessment using the Y-BOCS was performed as reported previously.25 A positive response to treatment was defined as a 25% decrease in the Y-BOCS total score. Previous studies have indicated that the Chinese versions of the HAMD and HAMA are valid and sensitive measures of clinical severity in patients, which supports their continued use as research instruments.26
Genotyping
Genotyping of the 5-HTTLPR (serotonin-transporter-linked promoter region; 43bp Ins = L allele/Del = S allele) was performed at the Genetics department of Wuxi Mental Health Center, Nanjing Medical University. Approximately 5 ml of peripheral blood was collected from each patient into tubes coated with EDTA. Genomic DNA was isolated from whole blood using Tiangen DNA isolation kit (Tiangen Biotech, Beijing, China). Genotyping of the intron 2 (variable number tandem repeat, VNTR) polymorphism in SLC6A4 (5-HTT, SERT) was performed by simple sequence length analysis. The polymerase chain reaction (PCR) was performed on 50 ng genomic DNA using 10 pmol of forward primer (5ʹ-GGCGTTGCCGCTCTGAATGC-3ʹ) and 10 pmol of reverse primer (5ʹ-GAGGGACTGAGCTGGACAACCAC-3ʹ), 0.25 mM dNTPs, 0.5 U Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA) in a PCR buffer containing 0.3 M Tris-HCl (pH 8.5), 75 mM ammonium sulfate and 7.5 mM MgCl2. The cycling conditions for the PCR started with 5 min at 92 °C, followed by 35 cycles of 1 min at 92 °C, 1 min at the optimized annealing temperature (57.5 °C), and 1 min at 72 °C, followed by an extra 5 min at 72 °C. PCR products were analyzed on a 2.5% agarose gel. The amplification yielded distinct bands at 484 bp (short “S” allele) and 528 bp (long “L” allele).
Statistical analysis
The data were expressed as means ± standard deviations. Chi-square test and Student t-test were used for comparing demographic and baseline clinical measures (Y-BOCS, HAMD, and HAMA) between the active and sham groups. Repeated-measures analysis of variance (ANOVA) was applied for evaluating the group and time-dependent effects of the therapy method on the Y-BOCS, HAMD, and HAMA scores, followed by post hoc Tukey tests. All tests were conducted with two-sided significance levels (α =0.05) without corrections for multiple comparisons due to the small sample size and to the exploratory nature of the study. We conducted the analyses using statistical software (PASW Statistics 18, Chicago, IL, USA).
Results
Demographic data of all subjects enrolled in this study
Descriptive and statistical comparisons of the active and sham groups are shown in Table 1. At baseline, the active and sham groups did not differ significantly in demographics or in baseline clinical ratings (Table 1).
Table 1.
Demographic and clinical data of the OCD patients at baseline
| Active rTMS group (n=25) | Sham rTMS group (n=24) | t | p-value | |
|---|---|---|---|---|
| Gender (male/female) | 15/10 | 14/10 | 0.014a | 0.906 |
| Age (years) | 32.20±13.25 | 39.38±17.04 | −1.644 | 0.107 |
| Education (years) | 13.04±2.59 | 12.08±3.24 | 1.144 | 0.259 |
| Y-BOCS | 17.24±4.27 | 18.08±4.43 | −1.264 | 0.213 |
| HAMA | 12.76±9.34 | 10.13±6.30 | 1.153 | 0.255 |
| HAMD | 16.16±9.54 | 11.88±7.82 | 1.715 | 0.093 |
Notes: aChi-square test. Data presented as mean±SD.
Abbreviations: OCD, obsessive-compulsive disorder; Y-BOCS, Yale-Brown obsessive compulsive scale; HAMA, Hamilton anxiety rating scale; HAMD, Hamilton depression rating scale; rTMS, repetitive transcranial magnetic stimulation.
Y-BOCS, HAMA, and HAMD
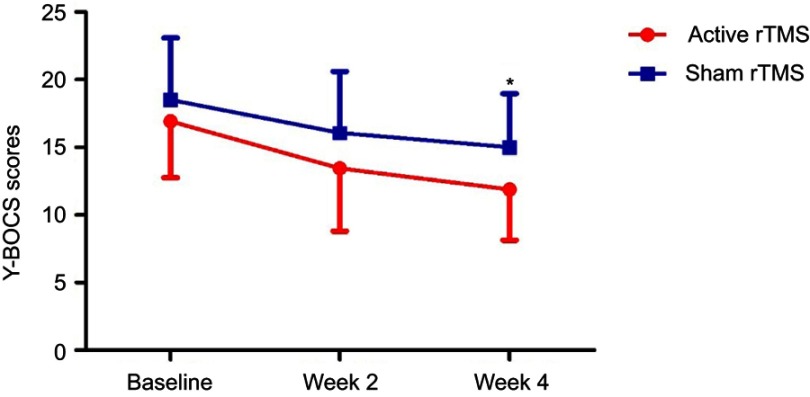
Y-BOCS scores decreased significantly over time, but more in the active rTMS group than in the sham rTMS group (Table 2); furthermore, there was a significant difference between the two groups at week 4 (Figure 2). Compared with the patients in the sham group, the patients in the active rTMS group had significantly (p<0.05) lower Y-BOCS scores at week 4 (Figure 2). Within-group comparisons revealed the following two patterns: for the active rTMS group, Y-BOCS scores significantly (p<0.01) decreased from baseline to week 4 (Figure 2). Likewise, for the sham rTMS group, Y-BOCS scores also significantly decreased from baseline to week 4 (Table 2).
Table 2.
Assessment scores of OCD patients after treatment
| Baseline | Week 2 | Week 4 | Analyses (p-values) | ||||
|---|---|---|---|---|---|---|---|
| ME time | ME rTMS | Interaction | |||||
| Y-BOCS | Active | 17.24±4.27 | 13.44±4.64 | 11.72±3.78** | 0.00 | 0.04 | 0.16 |
| Sham | 18.08±4.43 | 16.08±4.54 | 14.58±3.72** | ||||
| HAMA | Active | 12.76±9.34 | 9.32±7.99 | 8.12±7.79 | 0.00 | 0.73 | 0.06 |
| Sham | 10.13±6.30 | 9.50±5.42 | 8.58±5.54 | ||||
| HAMD | Active | 16.16±9.54 | 12.52±8.79 | 8.36±8.19** | 0.00 | 0.95 | 0.06 |
| Sham | 13.96±6.31 | 12.17±4.55 | 10.58±4.81* | ||||
Notes: **p<0.01 compared with the baseline *p<0.05 compared with the baseline. Data presented as mean±SD. The analysis method used was repeated measures ANOVA. The bold values are statistically significant.
Abbreviations: OCD, obsessive-compulsive disorder; Y-BOCS, Yale-Brown obsessive compulsive scale; HAMA, Hamilton anxiety rating scale; HAMD, Hamilton depression rating scale; ME time, main effect of time (baseline and follow-up); ME rTMS, main effect of rTMS status (active and sham).
Figure 2.
Change in Y-BOCS scores in patients with OCD during the study. Data are shown at the time of inclusion in the study (baseline) and after the period of active or sham stimulation (weeks 2 and 4). *p<0.05 compared with sham rTMS group.
Abbreviaions: Y-BOCS, Yale-Brown obsessive compulsive scale; OCD, obsessive-compulsive disorder; rTMS, repetitive transcranial magnetic stimulation.
HAMA scores did not significantly change following 2 or 4 weeks of treatment in either the active rTMS or the sham rTMS groups (Table 2). HAMD scores significantly decreased over time, but more in the active rTMS group than in the sham rTMS group (Table 2).
Effects of genotype on rTMS efficacy
Of the 25 patients who completed the experimental procedures in the active rTMS group, 4 had the LL genotype, 12 had the S/S genotype, and 9 had the S/L genotype. Of the 24 patients who completed the experimental procedures in the sham rTMS group, 3 had the LL genotype, 13 had the S/S genotype and 8 had the S/L genotype. Therefore, all these patients were classified into the following four groups: the active rTMS group with LL genotype, active rTMS S allele carrier group, sham rTMS group with LL genotype, and sham rTMS S allele carrier group. No significant differences were noted in general baseline characteristics among these four groups (data not shown).
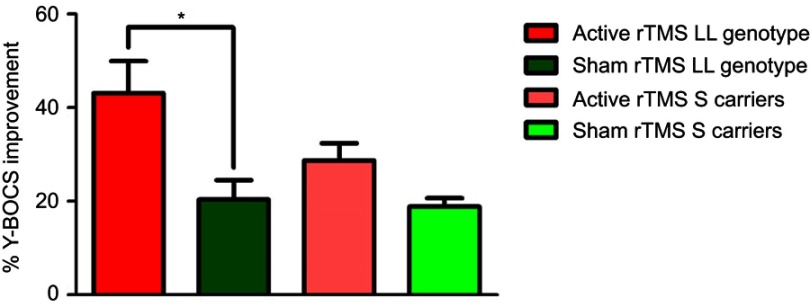
The intervention of low-frequency rTMS showed significantly greater improvement in the Y-BOCS score in the active group with the LL genotype compared with that in the sham group with the LL genotype (Figure 3). Conversely, rTMS did not decrease Y-BOCS scores in S allele carriers (Figure 3).
Figure 3.
Impacts of 5-HTTLPR on the beneficial effects of rTMS. OCD patients with the LL genotype in the 5-HTTLPR gene (n=4) showed greater improvement in obsessive and compulsive symptoms from low-frequency rTMS than from sham rTMS in patients (n=3) with the LL genotype. Conversely, no significance difference was noted between the active rTMS group in S allele carriers compared with the sham rTMS S allele carrier group (*p<0.05).
Abbreviations: Y-BOCS, Yale-Brown obsessive compulsive scale; OCD, obsessive-compulsive disorder; rTMS, repetitive transcranial magnetic stimulation.
Discussion
The findings of the present study showed that low-frequency rTMS over the SMA significantly augmented the beneficial effects of SSRIs in untreated patients with OCD. Interestingly, low-frequency rTMS produced beneficial effects in OCD patients with the LL genotype of 5-HTTLPR, although it did not improve the symptoms in S allele carriers with OCD. Thus, 5-HTTLPR polymorphism might predict a better response to rTMS in patients with OCD, since the two groups did not differ in demographic or clinical features at baseline. Collectively, the 5-HTTLPR (L/S) in the SLC6A4 may be a predictable biomarker for beneficial effects of rTMS in patients with OCD.
Although the first-line treatments for OCD include SSRIs or CBT,27 40–60% of patients with OCD fail to respond to these approaches, or are unable to tolerate their side effects.10 In this study, after 4 weeks of adequate dosages of SSRIs treatment, only 33.3% (8/24) patients showed an adequate response, usually defined by a reduction in Y-BOCS score ≥25% with respect to baseline, consistent with previous reports.28 Thus, modern neuromodulatory techniques such as rTMS, deep brain stimulation, and transcranial direct current stimulation potentially offer alternative forms of treatment for patients with OCD who either do not respond to, or are unable or unwilling to take SSRIs, or undergo CBT.29
Although the etiology of OCD is not completely understood, it is associated with dysfunctions in some special circuitry, such as the SMA, the dorsolateral prefrontal cortex (DLPFC), the anterior cingulate gyrus (ACC), and the orbitofrontal cortex (OFC).30 Neurophysiological studies have demonstrated that brain regions such as the SMA, DLPFC, and OFC are hyperactive in patients with OCD.31–35 Hyperactivity in the SMA may explain deficient inhibitory control over behavior in patients with OCD, since the SMA has extensive connections with subcortical striatal areas involved in response control.36,37 Several meta-analyses have demonstrated that the SMA is a more appropriate target for rTMS than the other regions, such as the DLPFC.16,19 For example, Hawken and colleague investigated six weeks of low frequency rTMS, applied bilaterally and simultaneously over the SMA.38 At the end of the six weeks of rTMS treatment, the patients in the active group showed a clinically significant decrease in Y-BOCS scores compared to both the baseline and the sham group. They declared that rTMS appeared to significantly improve the OCD symptoms of the treated patients beyond the treatment window. It has also been reported that active low-frequency (≤1-Hz) rTMS showed greater improvements in OCD symptoms than high-frequency (≥5-Hz) rTMS.15 In our current study we found that 4-week low-frequency rTMS stimulation of the SMA significantly improved the symptoms in patients with OCD.
5-HTTLPR polymorphism in SLC6A4 appears to be a key candidate for OCD, although the findings regarding this have been inconsistent.22,24,39,40 5-HTTLPR has two common alleles, which correspond with a 44-base pair insertion (L allele) or deletion (S allele). The S allele is reportedly associated with reduced transcription efficiency of the 5-HTT gene, and thus also with decreased 5-HTT expression and serotonin reuptake.41–43 Meta-analyses have shown that the L allele is only associated with OCD status in Caucasians,40 and that there was a significant time per genotype interaction between the 5-HTTLPR (L/S) and the Y-BOCS subtotal compulsion scores in patients with OCD.44 In the present study, we found that OCD patients with the LL genotype showed greater improvement in obsessive and compulsive symptoms following active rTMS treatment compared with the S allele carriers. To the best of our knowledge, this is the first investigation showing the association between 5-HTTLPR (L/S) and the rTMS augmentation of SSRIs in untreated patients with OCD. Previous research performed on treatment-resistant depressed patients showed that rTMS treatment significantly improved depression symptomatology and that this response was significantly greater in the 5-HTTLPR LL genotype compared with the S allele carriers.45 The precise mechanisms underlying the effects of the 5-HTTLPR on the beneficial effects of rTMS are presently unknown. rTMS stimulation of the SMA may potentiate serotonergic functions of SSRIs in OCD patients with the LL genotype, although further detailed research is required.
Limitations
The present study has two limitations. First, we investigated the 5-HTTLPR gene polymorphism in only 49 patients. Because of this small sample size, the results should be considered as preliminary. For confirming the present results, a future study using larger samples is required. Second, we did not measure the side effects following 4 weeks of low-frequency rTMS treatment, even though the most frequently reported adverse effects were headache, localized scalp pain, and dizziness. Third, the participants selected in this study had a relatively low Y-BOCS, and the effects of treatment were very modest compared to the sham. In the future study, we will include the OCD patients with relative severe symptoms to validate the results of this study. Fourth, the patients in our study lack psychoeducation or psychotherapy, and it is the standard treatment in combination with medication which is recommended for the treatment of OCD.
Conclusion
In conclusion, the results of this study show that 4 weeks of low-frequency rTMS stimulation significantly potentiated the beneficial effects of SSRIs in untreated patients with OCD. Furthermore, OCD patients with the LL genotype in the 5-HTTLPR of the SLC6A4 showed greater improvements in obsessive and compulsive symptoms than the S allele carriers following rTMS treatment. Therefore, the 5-HTTLPR (L/S) in the SLC6A4 appears to be a reliable biomarker for the beneficial effects of rTMS, although further studies using a large sample size are required.
Acknowledgments
We thank all patients who volunteered to participate in the study. This study was supported by the National Natural Science Foundation of China (81801341), the Medical and Public Health Technology Research Projects of Wuxi Technology Bureau (CSE31N1723 and CSE31N1613) and the Wuxi Municipal Health and Family Planning Commission research project (MS201704).
Significant Outcomes
The present results show that 4 weeks low-frequency rTMS stimulation significantly potentiated the beneficial effects of SSRIs in untreated OCD patients. Furthermore, OCD patients with LL genotype in the 5-HTTLPR of SLC6A4 gene showed greater improvements of obsessive and compulsive symptoms by rTMS than patients with the S allele carrier. Therefore, SLC6A4 gene would be predictable biomarker for beneficial effects of rTMS although further studies using a large sample size are needed.
Author contributions
KZ and GW conceived and designed the randomized controlled trial and JYu, JYi and HS contributed to the conception and design of the present study. KZ and XF recruited the patients and runned the study together with KH undertook the statistical analyses and wrote the first manuscript draft. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
KH received research supports from Otsuka, Sumitomo-Dainippon, and Taisho. The authors report no other conflicts of interest in this work.
References
- 1.Pauls DL. The genetics of obsessive-compulsive disorder: a review. Dialogues Clin Neurosci. 2010;12(2):149–163. doi: 10.1002/ajmg.c.30168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53–63. doi: 10.1038/mp.2008.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo X, Meng Z, Huang G, et al. Meta-analysis of the prevalence of anxiety disorders in mainland China from 2000 to 2015. Sci Rep. 2016;6:28033. doi: 10.1038/srep28033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lochner C, Mogotsi M, Du Toit PL, Kaminer D, Niehaus DJ, Stein DJ. Quality of life in anxiety disorders: a comparison of obsessive-compulsive disorder, social anxiety disorder, and panic disorder. Psychopathology. 2003;36(5):255–262. doi: 10.1159/000073451 [DOI] [PubMed] [Google Scholar]
- 5.Rapaport MH, Clary C, Fayyad R, Endicott J. Quality-of-life impairment in depressive and anxiety disorders. Am J Psychiatry. 2005;162(6):1171–1178. doi: 10.1176/appi.ajp.162.6.1171 [DOI] [PubMed] [Google Scholar]
- 6.Huppert JD, Simpson HB, Nissenson KJ, Liebowitz MR, Foa EB. Quality of life and functional impairment in obsessive-compulsive disorder: a comparison of patients with and without comorbidity, patients in remission, and healthy controls. Depress Anxiety. 2009;26(1):39–45. doi: 10.1002/da.20506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geller DA, Biederman J, Stewart SE, et al. Which SSRI? A meta-analysis of pharmacotherapy trials in pediatric obsessive-compulsive disorder. Am J Psychiatry. 2003;160(11):1919–1928. doi: 10.1176/appi.ajp.160.11.1919 [DOI] [PubMed] [Google Scholar]
- 8.Skapinakis P, Caldwell D, Hollingworth W, et al. A systematic review of the clinical effectiveness and cost-effectiveness of pharmacological and psychological interventions for the management of obsessive-compulsive disorder in children/adolescents and adults. Health Technol Assess. 2016;20(43):1–392. doi: 10.3310/hta20430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloch MH, McGuire J, Landeros-Weisenberger A, Leckman JF, Pittenger C. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850–855. doi: 10.1038/mp.2009.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683–1696. doi: 10.1001/jama.297.15.1683 [DOI] [PubMed] [Google Scholar]
- 11.Soomro GM, Altman DG, Rajagopal S, Oakley-Browne M. Selective serotonin re-uptake inhibitors (SSRIs) versus placebo for obsessive compulsive disorder (OCD). Cochrane Database Syst Rev. 2008;(1):CD001765. doi: 10.1002/14651858.CD001765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunoni AR, Valiengo L, Baccaro A, et al. The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry. 2013;70(4):383–391. doi: 10.1001/2013.jamapsychiatry.32 [DOI] [PubMed] [Google Scholar]
- 13.Guo Q, Li C, Wang J. Updated review on the clinical use of repetitive transcranial magnetic stimulation in psychiatric disorders. Neurosci Bull. 2017;33(6):747–756. doi: 10.1007/s12264-017-0185-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin DM, McClintock SM, Forster JJ, Lo TY, Loo CK. Cognitive enhancing effects of rTMS administered to the prefrontal cortex in patients with depression: a systematic review and meta-analysis of individual task effects. Depress Anxiety. 2017;34(11):1029–1039. doi: 10.1002/da.22658 [DOI] [PubMed] [Google Scholar]
- 15.Zhou DD, Wang W, Wang GM, Li DQ, Kuang L. An updated meta-analysis: short-term therapeutic effects of repeated transcranial magnetic stimulation in treating obsessive-compulsive disorder. J Affect Disord. 2017;215:187–196. doi: 10.1016/j.jad.2017.03.033 [DOI] [PubMed] [Google Scholar]
- 16.Berlim MT, Neufeld NH, Van Den Eynde F. Repetitive transcranial magnetic stimulation (rTMS) for obsessive-compulsive disorder (OCD): an exploratory meta-analysis of randomized and sham-controlled trials. J Psychiatr Res. 2013;47(8):999–1006. doi: 10.1016/j.jpsychires.2013.03.022 [DOI] [PubMed] [Google Scholar]
- 17.Ma ZR, Shi LJ. Repetitive transcranial magnetic stimulation (rTMS) augmentation of selective serotonin reuptake inhibitors (SSRIs) for SSRI-resistant obsessive-compulsive disorder (OCD): a meta-analysis of randomized controlled trials. Int J Clin Exp Med. 2014;7(12):4897–4905. [PMC free article] [PubMed] [Google Scholar]
- 18.Trevizol AP, Shiozawa P, Cook IA, et al. Transcranial magnetic stimulation for obsessive-compulsive disorder: an updated systematic review and meta-analysis. J Ect. 2016;32(4):262–266. doi: 10.1097/YCT.0000000000000335 [DOI] [PubMed] [Google Scholar]
- 19.Rehn S, Eslick GD, Brakoulias V. A meta-analysis of the effectiveness of different cortical targets used in repetitive Transcranial Magnetic Stimulation (rTMS) for the Treatment of Obsessive-Compulsive Disorder (OCD). Psychiatr Q. 2018;89(3):645–665. doi: 10.1007/s11126-018-9566-7 [DOI] [PubMed] [Google Scholar]
- 20.Schürks M, Rist PM, Kurth T. 5-HTTLPR polymorphism in the serotonin transporter gene and migraine: a systematic review and meta-analysis. Cephalalgia. 2010;30(11):1296–1305. doi: 10.1177/0333102410362929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Provenzi L, Giorda R, Beri S, Montirosso R. SLC6A4 methylation as an epigenetic marker of life adversity exposures in humans: a systematic review of literature. Neurosci Biobehav Rev. 2016;71:7–20. doi: 10.1016/j.neubiorev.2016.08.021 [DOI] [PubMed] [Google Scholar]
- 22.Taylor S. Disorder-specific genetic factors in obsessive-compulsive disorder: a comprehensive meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2016;171B(3):325–332. doi: 10.1002/ajmg.b.32407 [DOI] [PubMed] [Google Scholar]
- 23.Mak L, Streiner DL, Steiner M. Is serotonin transporter polymorphism (5-HTTLPR) allele status a predictor for obsessive-compulsive disorder? A meta-analysis. Arch Womens Ment Health. 2015;18(3):435–445. doi: 10.1007/s00737-015-0526-z [DOI] [PubMed] [Google Scholar]
- 24.Sinopoli VM, Burton CL, Kronenberg S, Arnold PD. A review of the role of serotonin system genes in obsessive-compulsive disorder. Neurosci Biobehav Rev. 2017;80:372–381. doi: 10.1016/j.neubiorev.2017.05.029 [DOI] [PubMed] [Google Scholar]
- 25.Zhang K, Cao L, Zhu W, et al. Association between the efficacy of fluoxetine treatment in obsessive-compulsive disorder patients and SLC1A1 in a Han Chinese population. Psychiatry Res. 2015;229(1–2):631–632. doi: 10.1016/j.psychres.2015.06.039 [DOI] [PubMed] [Google Scholar]
- 26.Zheng Y, Zhao J, Phillips M, et al. Validity and reliability of the Chinese Hamilton depression rating scale. Br J Psychiatry. 1988;152:660–664. [DOI] [PubMed] [Google Scholar]
- 27.Pallanti S, Hollander E, Bienstock C, et al. Treatment non-response in OCD: methodological issue and operational definitions. Int J Neuropsychopharmacol. 2002;5(2):181–191. doi: 10.1017/S1461145702002900 [DOI] [PubMed] [Google Scholar]
- 28.Eddy KT, Dutra L, Bradley R, Westen D. A multidimensional meta-analysis of psychotherapy and pharmacotherapy for obsessive-compulsive disorder. Clin Psychol Rev. 2004;24(8):1011–1030. doi: 10.1016/j.cpr.2004.08.004 [DOI] [PubMed] [Google Scholar]
- 29.Zaman R, Robbins TW. Is there potential for repetitive Transcranial Magnetic Stimulation (rTMS) as a treatment of OCD? Psychiatr Danub. 2017;29(Suppl 3):672–678. [PubMed] [Google Scholar]
- 30.Norman LJ, Carlisi C, Lukito S, et al. Structural and functional brain abnormalities in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder: a comparative meta-analysis. JAMA Psychiatry. 2016;73(8):815–825. doi: 10.1001/jamapsychiatry.2016.0700 [DOI] [PubMed] [Google Scholar]
- 31.Abramovitch A, Abramowitz JS, Mittelman A, Stark A, Ramsey K, Geller DA. Research review: neuropsychological test performance in pediatric obsessive-compulsive disorder-a meta-analysis. J Child Psychol Psychiatry. 2015;56(8):837–847. doi: 10.1111/jcpp.12414 [DOI] [PubMed] [Google Scholar]
- 32.Purcell R, Maruff P, Kyrios M, Pantelis C. Neuropsychological deficits in obsessive-compulsive disorder: a comparison with unipolar depression, panic disorder, and normal controls. Arch Gen Psychiatry. 1998;55(5):415–423. [DOI] [PubMed] [Google Scholar]
- 33.Rao NP, Reddy YCJ, Kumar KJ, Kandavel T, Chandrashekar CR. Are neuropsychological deficits trait markers in OCD? Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1574–1579. doi: 10.1016/j.pnpbp.2008.05.026 [DOI] [PubMed] [Google Scholar]
- 34.Mantovani A, Rossi S, Bassi BD, Simpson HB, Fallon BA, Lisanby SH. Modulation of motor cortex excitability in obsessive-compulsive disorder: an exploratory study on the relations of neurophysiology measures with clinical outcome. Psychiatry Res. 2013;210(3):1026–1032. doi: 10.1016/j.psychres.2013.08.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haber SN, Heilbronner SR. Translational research in OCD: circuitry and mechanisms. Neuropsychopharmacology. 2013;38(1):252–253. doi: 10.1038/npp.2012.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 37.de Wit SJ, de Vries FE, van der Werf YD, et al. Presupplementary motor area hyperactivity during response inhibition: a candidate endophenotype of obsessive-compulsive disorder. Am J Psychiatry. 2012;169(10):1100–1108. doi: 10.1176/appi.ajp.2012.12010073 [DOI] [PubMed] [Google Scholar]
- 38.Hawken E, Dilkov D, Kaludiev E, Simek S, Zhang F, Milev R. Transcranial magnetic stimulation of the supplementary motor area in the treatment of obsessive-compulsive disorder: a multi-site study. Int J Mol Sci. 2016;17:420. doi: 10.3390/ijms17030420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin PY. Meta-analysis of the association of serotonin transporter gene polymorphism with obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):683–689. doi: 10.1016/j.pnpbp.2006.12.024 [DOI] [PubMed] [Google Scholar]
- 40.Bloch MH, Landeros-Weisenberger A, Sen S, et al. Association of the serotonin transporter polymorphism and obsessive-compulsive disorder: systematic review. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(6):850–858. doi: 10.1002/ajmg.b.30699 [DOI] [PubMed] [Google Scholar]
- 41.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968 [DOI] [PubMed] [Google Scholar]
- 42.Risch N, Herrell R, Lehner T, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301(23):2462–2471. doi: 10.1001/jama.2009.878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68(5):444–454. doi: 10.1001/archgenpsychiatry.2010.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Bella D, Erzegovesi S, Cavallini MC, Bellodi L. Obsessive-compulsive disorder, 5-HTTLPR polymorphism and treatment response. Pharmacogenomics J. 2002;2(3):176–181. doi: 10.1038/sj.tpj.6500090 [DOI] [PubMed] [Google Scholar]
- 45.Bocchio-Chiavetto L, Miniussi C, Zanardini R, et al. 5-HTTLPR and BDNF Val66Met polymorphisms and response to rTMS treatment in drug resistant depression. Neurosci Lett. 2008;437(2):130–134. doi: 10.1016/j.neulet.2008.04.005 [DOI] [PubMed] [Google Scholar]