Abstract
Purpose
RBP-7000 (PERSERIS™) is a once-monthly subcutaneous extended-release risperidone formulation approved by the United States Food and Drug Administration for the treatment of schizophrenia in adults. The objective of this study was to describe the long-term impact of RBP-7000 on health-related quality of life (HRQoL), subjective well-being, treatment satisfaction and medication preference in patients with schizophrenia.
Patients and methods
HRQoL was derived from a 52-week multicentre Phase III single-arm open-label outpatient study that assessed the safety and efficacy of RBP-7000 (120 mg) in patients with schizophrenia. HRQoL was measured using the EuroQol EQ-5D-5L and Short-Form Survey SF-36 version 2; well-being using the Subjective Well-being Under Neuroleptic Treatment – Short Version (SWN-S); satisfaction using the Medication Satisfaction Questionnaire and medication preference using the Preference of Medication questionnaire.
Results
Of 482 participants at baseline, 234 remained through the end of study (EOS; week 52). Mean HRQoL and well-being scores remained stable between baseline (EQ-5D-5L index: 0.83; SF-36v2 Physical Component Score: 50; SF-36v2 Mental Component Score: 46; total SWN-S score: 89) and EOS (EQ-5D-5L index: 0.86; SF-36v2 Physical Component Score: 49; SF-36v2 Mental Component Score: 47; total SWN-S score: 90). The proportion of participants reporting satisfaction increased between week 4 (66%) and EOS (81%), with a similar trend for the preference of RBP-7000 over previous treatment (week 4: 66%; EOS: 72%). Sensitivity analyses suggested a minor effect of dropout on characterization of change over time in patient-reported outcomes (PRO) measures.
Conclusion
Study participants attained mean HRQoL scores near that of the general US population. Over two-thirds reported high satisfaction with and preference for RBP-7000 across the study period. Additional research is needed to confirm whether these PRO translate into improved outcomes such as adherence and ultimately fewer relapses in patients with schizophrenia.
Keywords: antipsychotics, quality of life, medication satisfaction, medication preference, clinical trial
Introduction
Discontinuation or disruption of antipsychotic therapy in the treatment of schizophrenia is one of the primary causes of relapse among patients with schizophrenia or schizoaffective disorder.1 Administration of long-acting injectable (LAI) medications to patients with schizophrenia has the potential to improve medication adherence and lower discontinuation rates compared to patients on daily oral therapy.2,3 Previous research has found that switching patients with schizophrenia or schizoaffective disorder from shorter-acting oral to LAI formulations improved health-related quality of life (HRQoL)4,5 and reduced hospitalization rates.4,6
The safety and efficacy of risperidone in the management of positive symptoms of schizophrenia is well established.7–9 Risperidone is marketed in a number of countries, including the US, in various formulations including tablets, orally disintegrating tablets, oral solutions and intramuscular injections.10,11
After a 3-week initiation period during which patients have to be concurrently treated with oral risperidone to ensure adequate therapeutic plasma concentrations, the LAI intramuscular formulation of risperidone is administered every 2 weeks as deep deltoid or gluteal injections.12 In contrast, RBP-7000 (PERSERIS™, Indivior, Inc., Richmond, VA, USA), provides a once-monthly subcutaneous (SC) extended-release risperidone formulation that does not require any oral supplementation for the treatment of schizophrenia in adults.13 An 8-week, randomized, double-blind, placebo-controlled Phase III study of RBP-7000 (NCT02109562) demonstrated the clinical safety and efficacy of RBP-700014 and significant improvements in HRQoL and overall well-being.15 To build upon this evidence, the aim of the present analysis was to further describe the effects of RBP-7000 on patient-reported HRQoL, subjective well-being, treatment satisfaction and preference to medication over 52 weeks.
Methods
This analysis used data from a 52-week multicentre Phase III open-label outpatient study (NCT02203838) that was conducted at 50 sites in the US between June 2014 and September 2016 to assess the long-term safety and tolerability of RBP-7000 (120 mg) in patients with schizophrenia. The study methods and results for clinical efficacy and safety (eg, Positive and Negative Syndrome Scale [PANSS], Clinical Global Impression-Severity of Illness, Abnormal Involuntary Movement Scale) are reported elsewhere.16
The study was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines, Food and Drug Administration regulations governing clinical study conduct and the Declaration of Helsinki (2013). The study protocol, all protocol amendments, written study patient information, the written informed consent form and all other appropriate study-related information were reviewed and approved by the Copernicus Group Independent Review Board ® (CGIRB).
Participants
Two cohorts provided participants for the clinical study: a rollover cohort and a de novo cohort. The rollover cohort consisted of participants with schizophrenia, aged 18–55 years, who had completed an earlier 8-week, randomized double-blind, placebo-controlled RBP-7000 study (NCT02109562)14,15 during which they had received 2 monthly SC injections of either placebo, RBP-7000 90 mg or RBP-7000 120 mg and provided that treatment continuation was clinically warranted and participants were judged stable enough to enroll by the investigator. After the end of the placebo-controlled study (EOS), participants entered the open-label outpatient study directly with their third injection on day 1. Participants received up to an additional 11 SC injections of RBP-7000 over the course of 40 weeks in this study.
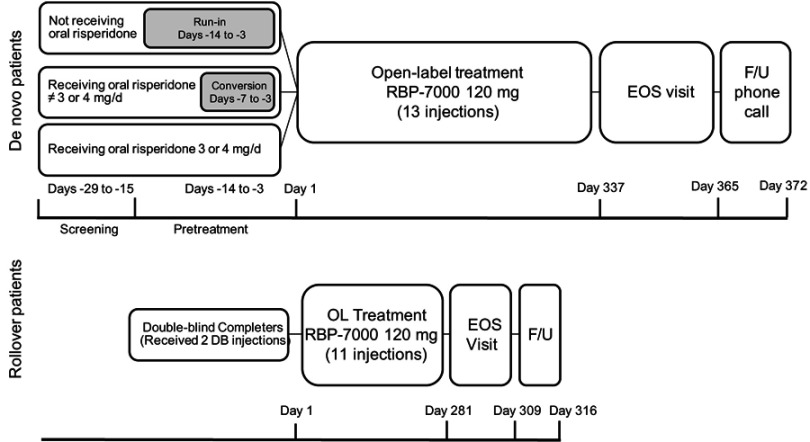
The de novo cohort consisted of participants who were diagnosed with schizophrenia, aged 18–65 years, who were not previously exposed to RBP-7000, were considered clinically stable with a PANSS total score ≤70 at screening and were otherwise healthy based on physical examination; those with suicidal ideation (within 6 months) or behavior (within 1 year) based on Columbia-Suicide Severity Rating Scale assessment, or with a significant risk of suicide in the opinion of the investigator, were excluded. De novo participants not on oral risperidone or on doses other than 3or 4 mg entered a run-in or conversion phase where they were tapered off their current antipsychotic medications (Figure 1) and started on a 7- to 14-day regimen of oral risperidone, whereas de novo participants already receiving oral risperidone did not have to complete the run-in or conversion phase and entered the study directly. De novo participants received up to 13 SC injections of RBP-7000 over the course of 52 weeks.
Figure 1.
Study design for the open-label phase of de novo and rollover participants. Rollover participants received 2 doses of RBP-7000 during the previous 8-week trial and up to an additional 11 SC injections over the course of 40 weeks in this study. De novo participants received up to 13 SC injections of RBP-7000 over the course of 52 weeks. If they were not on oral risperidone, or on doses other than 3 or 4 mg, they were tapered off their current antipsychotic medications and initiated on a 7- to 14-day regimen of oral risperidone. If they were already receiving oral risperidone, they did not have to complete the run-in or conversion phase and entered the study directly.
Abbreviations: C, conversion; DB; double-blind; EOS, end of study; F/U, follow-up; OL, open-label; RI, run-in; SC, subcutaneous.
Exposure
All participants received RBP-7000 120 mg at study entry. A single dose modification for tolerability to RBP-7000 90 mg was allowed at the investigator’s discretion. Participants who were down-titrated to the RBP-7000 90-mg dose who exhibited a worsening in psychiatric symptoms (ie, total PANSS score >70 or a 20% increase in PANSS score from previous assessment [at the 120-mg dose prior to the dose reduction]) received a subsequent single dose modification to RBP-7000 120 mg at the investigator’s discretion.
Patient-reported outcomes
Key patient-reported outcome (PRO) measures included the EuroQoL 5D 5-Level (EQ-5D-5L); Short-Form 36-item Questionnaire, Version 2 (SF-36v2); Subjective Well-being Under Neuroleptic Treatment-Short Version (SWN-S); Medication Satisfaction Questionnaire (MSQ) and the Preference of Medication (POM) Questionnaire. EQ-5D-5L, SF-36v2 and SWN-S assessments were administered at screening and weeks 0 (injection 1), 12 (injection 3), 24 (injection 6), 36 (injection 9) and 52 (EOS); MSQ and POM were administered at weeks 4, 12, 24, 36 and 52 with MSQ also administered at screening.
Depending on the measure and its inclusion in the double-blind trial, observations were compared to active baseline, open-label baseline or the first measurement within this clinical study. Active baseline was defined as the last non-missing measurement taken prior to the first dose of RBP-7000 regardless of whether received during the double-blind or open-label studies (ie, the measurement taken on the same day as the first dose). Open-label baseline was defined for all participants as the last non-missing measurement taken prior to the date of first dose of RBP-7000 in this clinical study.
Health-related quality of life
HRQoL was measured using the EQ-5D-5L as well as the SF-36v2. The EQ-5D-5L is a standardized, patient-reported, generic instrument for measuring health outcomes17,18 that has been validated in schizophrenic patients.19 It provides a simple descriptive profile and a single index value for health status. The instrument consists of the EQ-5D-5L descriptive system and the VAS. The descriptive system consists of 5 dimensions (mobility, self-care, usual activities, pain/discomfort, anxiety/depression) with 5 levels of severity (no problems, slight problems, moderate problems, severe problems, extreme problems) within each dimension, which are used to generate the index score anchored at 0 for death and 1 for no problems reported in any dimension. The VAS records the respondent’s self-rated health on a 20-cm, 100-point vertical VAS with endpoints labeled “The worst health you can imagine” at 0 and “The best health you can imagine” at 100.
The SF-36v2 is a self-reported, multipurpose, 36-item survey that measures 8 domains of health: bodily pain, general health, general mental health, physical functioning, role-emotional, role-physical, social functioning and vitality.20,21 The reliability and validity of this instrument have been demonstrated in a schizophrenic population.22 Scores range from 0 to 100, with higher scores indicating better HRQoL. The SF-36v2 yields scale scores for each of the 8 health domains and 2 summary measures derived from the domain scores: physical component summary (PCS) and mental component summary (MCS). The PCS and MCS are constructed to have a mean of 50 and standard deviation of 10 in the general US population. The SF-36v2 was scored per convention using proprietary software.
Subjective well-being
The SWN-S scale is a 20-item (10 positive and 10 negative) patient-rated instrument designed to capture subjective well-being during antipsychotic treatment.23 The reliability of this instrument has been demonstrated in patients with schizophrenia.24 Each item is scored on a Likert scale with 6 response categories ranging from “Not at all” to “Very much.” Each item is scored from 1 to 6, with a minimum total score of 20 (low subjective well-being) and a maximum total score of 120 (good subjective well-being). The SWN-S has 5 subscales (mental functioning, self-control, emotional regulation, physical functioning and social integration) from which a total score is derived.
Treatment satisfaction
Medication satisfaction was measured using the MSQ. The MSQ is a single-item questionnaire that evaluates satisfaction with current antipsychotic medication and was found to be reliable in patients with schizophrenia.25 The single item is scored from 1 (Extremely dissatisfied) to 7 (Extremely satisfied). The MSQ was assessed dichotomously as “Dissatisfied” (scores 1–4) and “Satisfied” (scores 5–7).
Preference of medication
The POM is a 2-item questionnaire assessing the preference for the current antipsychotic compared with the most recent pre-study antipsychotic.26 Even though both the items ask the same question, one is addressed to the subject and the other to the subject’s caregiver. The item is scored from 1 (Much better; I prefer this medication) to 5 (Much worse; I prefer my previous medication). The POM was evaluated dichotomously as “Better” (scores 1–2) and “Same or worse” (scores 3–5).
Statistical analysis
All items and/or summary scales were descriptively summarized by cohort (de novo and rollover) and combined and presented as an “All Participants” cohort. Categorical variables were summarized using frequencies and percentages, and continuous measures were summarized using descriptive statistics (mean, SD).
Sensitivity analyses
To check for withdrawal bias, baseline characteristics were compared between completers and non-completers. Completers were defined as participants who reached EOS, while non-completers were those who stopped treatment or dropped out before reaching EOS, in which case an end of treatment measurement had been collected.
Patterns of missingness were explored to determine the type of missingness. Mixed-effect models with repeated measures (MMRM) and joint process models were used to investigate the impact of informative censoring of longitudinal PRO outcomes due to dropout/discontinuation of the treatment regimen. Joint process models were used to simultaneously model longitudinal PRO and time to dropout/discontinuation.
Results
Of the 482 participants with a baseline PRO assessment within the clinical study (395 de novo and 87 rollover) (Table 1), 234 participants (48.5%) completed the study. The most common reasons for study discontinuation among study non-completers were participants withdrawing consent (21%) and dropout due to clinical reasons (20%), including lack of efficacy, adverse events and withdrawn by investigator (Table 2). The majority of participants in the PRO cohort were male (67.6%) and African American (71.0%) with a mean age of 45.1 years.
Table 1.
Baseline characteristics by cohort
| Participants, n (%) | |||
|---|---|---|---|
| De Novo | Rollover | All participants | |
| (N=395) | (N=87) | (N=482) | |
| Sex | |||
| Male | 266 (67.3) | 60 (69.0) | 326 (67.6) |
| Female | 129 (32.7) | 27 (31.0) | 156 (32.4) |
| Age, years | |||
| ≤20 | 2 (0.5) | 1 (1.1) | 3 (0.6) |
| 21–30 | 38 (9.6) | 10 (11.5) | 48 (10.0) |
| 31–40 | 78 (19.7) | 24 (27.6) | 102 (21.2) |
| 41–50 | 124 (31.4) | 32 (36.8) | 156 (32.4) |
| 51–55 | 83 (21.0) | 19 (21.8) | 102 (21.2) |
| 56–65 | 70 (17.7) | 1 (1.1) | 71 (14.7) |
| Race | |||
| Caucasian | 105 (26.6) | 25 (28.7) | 130 (27.0) |
| African American | 283 (71.6) | 59 (67.8) | 342 (71.0) |
| Asian | 3 (0.8) | 1 (1.1) | 4 (0.8) |
| American Indian or Alaska Native | 2 (0.5) | 0 | 2 (0.4) |
| Native Hawaiian or Other Pacific Islander | 1 (0.3) | 2 (2.3) | 3 (0.6) |
| Other | 1 (0.3) | 0 | 1 (0.2) |
Abbreviations: EOS, end of study; SWN-S, subjective well-being under neuroleptic scale.
Table 2.
Reasons for dropout by cohort
| De novo | Rollover | All participants | |
|---|---|---|---|
| Number of participants in the PRO analyses | 395 | 87 | 482 |
| Completed the study, n (%)a | 198 (50.1) | 36 (41.4) | 234 (48.5) |
| Discontinued from the study, n (%)a | 197 (49.9) | 51 (58.6) | 248 (51.5) |
| Clinical reasons for discontinuation | 80 (20.3) | 18 (20.7) | 98 (20.3) |
| Lack of efficacy | 7 (1.8) | 2 (2.3) | 9 (1.9) |
| Adverse event | 46 (11.6) | 11 (12.6) | 57 (11.8) |
| Withdrawn by investigator | 27 (6.8) | 5 (5.7) | 32 (6.6) |
| Non-clinical reasons for discontinuation | 117 (29.6) | 33 (37.9) | 150 (31.1) |
| Lost to follow-up | 33 (8.4) | 5 (5.7) | 38 (7.9) |
| Protocol deviation | 7 (1.8) | 3 (3.4) | 10 (2.1) |
| Withdrew consent | 76 (19.2) | 25 (28.7) | 101 (21.0) |
| Sponsor discontinued study | 0 | 0 | 0 |
| Unknown | 1 (0.3) | 0 | 1 (0.2) |
Notes: aBecause of rounding, percentages may not total 100.
Abbreviation: PRO, patient-reported outcomes.
Impact of RBP-7000 on HRQoL and subjective well-being
EQ-5D-5L index and VAS scores remained stable from active baseline to EOS (Table 3). At active baseline and EOS, participants reported the most problems in the EQ-5D-5L anxiety/depression dimension and the least problems in the self-care dimension.
Table 3.
Mean (SD) HRQoL scores by cohort at baseline and EOS
| Measure | Baselinea | End of study | ||||
|---|---|---|---|---|---|---|
| De novo (N=395) | Rollover (N=87) | All participants (N=482) | De Novo (N=198) | Rollover (N=36) | All participants (N=234) | |
| EQ-5D-5L | ||||||
| Index | 0.86 (0.15) | 0.84 (0.18) | 0.85 (0.16) | 0.85 (0.15) | 0.83 (0.18) | 0.85 (0.15) |
| VAS | 81.0 (17.9) | 83.1 (18.9) | 81.4 (18.1) | 82.9 (15.1) | 82.3 (16.8) | 82.8 (15.4) |
| SF-36v2 | ||||||
| Bodily pain | 73.2 (26.3) | 73.2 (27.0) | 73.2 (26.4) | 75.4 (27.1) | 69.3 (25.9) | 74.5 (26.9) |
| General health | 67.8 (21.7) | 67.0 (19.4) | 67.7 (21.3) | 67.5 (21.2) | 63.0 (22.9) | 66.8 (21.5) |
| Mental health | 67.3 (22.5) | 67.0 (19.3) | 67.3 (21.9) | 70.3 (20.6) | 63.1 (22.4) | 69.2 (21.0) |
| Physical functioning | 71.5 (28.1) | 73.8 (28.7) | 71.9 (28.2) | 70.7 (27.0)b | 65.7 (33.6) | 69.9 (28.1)b |
| Role-emotional | 67.8 (29.3) | 65.3 (26.9) | 67.3 (28.9) | 68.9 (28.5)b | 65.5 (29.7) | 68.4 (28.7)b |
| Role-physical | 68.1 (28.9) | 69.6 (27.3) | 68.4 (28.6) | 65.5 (28.9)b | 64.6 (29.1) | 65.4 (28.9)b |
| Social functioning | 69.8 (25.9) | 65.8 (23.5) | 69.0 (25.5) | 71.1 (25.4)b | 72.2 (23.5) | 71.3 (25.1)b |
| Vitality | 62.5 (21.2) | 62.1 (18.2) | 62.5 (20.6) | 63.1 (22.5)b | 60.2 (25.5) | 62.6 (23.0)b |
| PCS | 50.0 (9.1) | 50.7 (9.2) | 50.1 (9.1) | 49.4 (8.8) | 48.1 (10.0) | 49.2 (9.0) |
| MCS | 45.8 (11.5) | 44.5 (9.6) | 45.6 (11.2) | 47.0 (10.7) | 45.2 (10.1) | 46.7 (10.6) |
Notes: aOpen-label baseline. bFor these domains, the number of participants responding were 1 less than the overall N reported.
Abbreviations: EQ-5D-5L, EuroQol 5-Dimensions 5-Level; EOS, end of study; HRQoL, health-related quality of life; MCS, mental component summary; PCS, physical component summary; SF-36v2, Short-Form 36-item Questionnaire Version 2.
Among all SF-36v2 dimensions and across all cohorts, participants reported the lowest scores in the vitality dimension and the highest scores in the bodily pain dimension at both open-label baseline and EOS assessments. Scores across all SF-36v2 dimensions remained stable from open-label baseline to EOS in the all participants cohort. Within the same cohort, mean PCS and MCS scores also remained stable from open-label baseline to EOS (Table 3).
Across all cohorts, stable SWN-S scores were reported from active baseline to EOS (Table 4).
Table 4.
Mean (SD) SWN-S scores by cohort at active baseline and end of study
| Measure | Active baseline | End of study | ||||
|---|---|---|---|---|---|---|
| De novo (N=395) | Rollover (N=87) | All participants (N=482) | De novo (N=197) | Rollover (N=36) | All participants (N=233) | |
| Emotional regulation | 18.6 (4.3) | 17.8 (3.9) | 18.4 (4.2) | 18.6 (4.0) | 18.1 (4.3) | 18.5 (4.0) |
| Mental functioning | 17.4 (4.5) | 16.7 (4.3) | 17.3 (4.5) | 17.8 (4.5) | 17.1 (4.1) | 17.7 (4.4) |
| Physical functioning | 18.7 (4.2) | 18.2 (4.4) | 18.7 (4.2) | 18.5 (4.3) | 17.9 (4.6) | 18.4 (4.4) |
| Self-control | 18.0 (3.7) | 18.4 (3.7) | 18.1 (3.7) | 18.6 (3.8) | 17.3 (3.6) | 18.4 (3.8) |
| Social integration | 16.9 (4.2) | 16.6 (4.0) | 16.8 (4.2) | 17.2 (4.0) | 16.7 (4.4) | 17.2 (4.0) |
| Total | 89.6 (17.2) | 87.7 (16.2) | 89.3 (17.0) | 90.7 (17.0) | 87.1 (17.8) | 90.1 (17.2) |
Impact of RBP-7000 on treatment satisfaction and preference
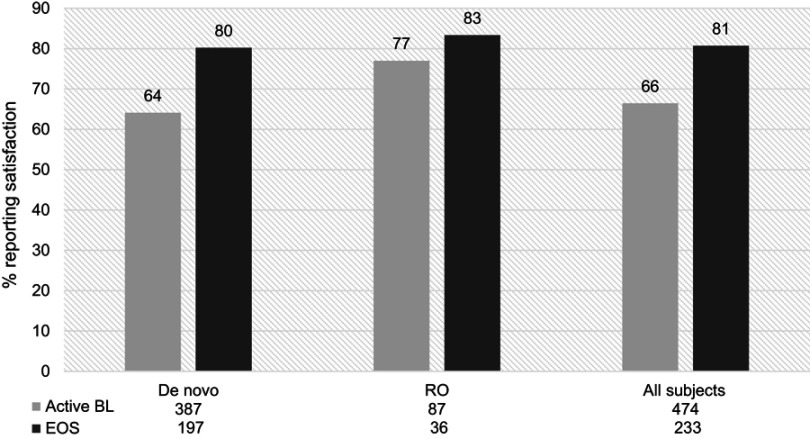
The proportion of satisfied participants based on the MSQ increased by 15 percentage points from first measurement after active RBP-7000 treatment to EOS (Figure 2). An increase in satisfaction with treatment was observed in the de novo treatment cohort with 80% reporting satisfaction at EOS compared to 64% at active baseline.
Figure 2.
Proportion of de novo and rollover (RO) participants reporting satisfaction with RBP-7000, at active baseline (BL) and end of study (EOS). There was a 15% increase in satisfaction among all participants from baseline to end of study.
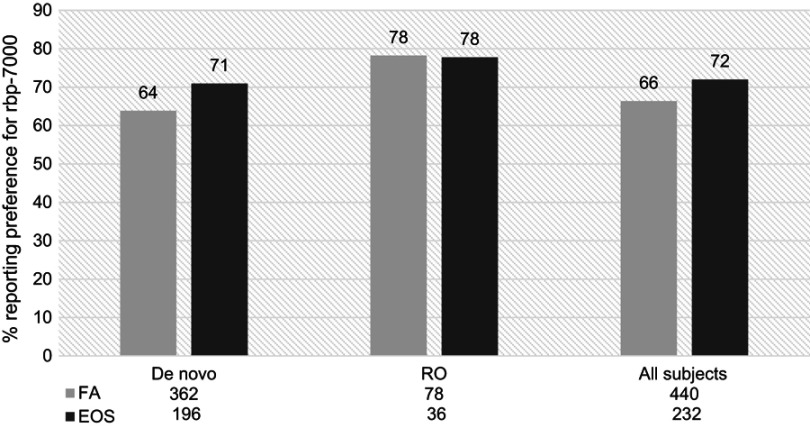
From first measurement (week 4) to EOS, the proportion of participants reporting preference on the POM for RBP-7000 as “better” compared to current antipsychotic medication increased among the all participants cohort (66% vs 72%) (Figure 3). By EOS, the proportion of participants reporting the preference for RBP-7000 on the POM in the rollover cohort remained stable, while the de novo cohort showed an increase in preference from first assessment (64–71%).
Figure 3.
Proportion of de novo and rollover (RO) participants reporting preference for RBP-7000 as compared with the most recent pre-study antipsychotic medication, at first assessment (FA,week 4) and end of study (EOS). There was a 6% increase in the proportion of patients reporting preference for current antipsychotic medication among all participants.
Sensitivity analysis
A comparison of open-label baseline characteristics between completers and non-completers revealed no significant differences in age, sex, race or body mass index. There were some minor differences in the prevalence of certain comorbid conditions (hypertension and insomnia), but overall there were no major differences between completers and non-completers at baseline.
Upon exploration, it was determined that the pattern of missingness was non-ignorable, ie, missing not at random, and a joint process modeling approach was considered appropriate (longitudinal modeling of PRO and time to discontinuation/dropout). Withdrawal bias due to dropouts for key PRO measures (SF-36v2 PCS, SF-36v2 MCS, SWN-S, MSQ) was assessed by comparing the estimates obtained from 3 models: MMRM (completers only), MMRM (all participants including both completers and non-completers) and joint process survival model (all participants including both completers and non-completers). The results from these models suggested a minor effect of dropout on characterization of change over time in PRO measures (Table S1).
Table S1.
Model parameter estimates for select PRO measures among study completers and all participants
| Model parameter | Completers (MMRM) | All participants (MMRM) | All participants (JPSM) | |||
|---|---|---|---|---|---|---|
| Estimate | p-value | Estimate | p-value | Estimate | p-value | |
| SF-36v2 PCS | ||||||
| Intercept | 49.540 | <0.001 | 50.009 | <0.001 | 50.036 | 0.001 |
| Visit | 0.041 | 0.764 | 0.013 | 0.917 | 0.020 | 0.911 |
| RO120 vs DN | −3.654 | 0.100 | −0.797 | 0.619 | −0.810 | 0.611 |
| RO90 vs DN | 0.877 | 0.693 | 2.488 | 0.127 | 2.507 | 0.122 |
| ROPL vs DN | 1.846 | 0.591 | −0.409 | 0.815 | −0.670 | 0.702 |
| Visit*RO120 vs DN | 0.845 | 0.099 | 0.668 | 0.159 | 0.674 | 0.147 |
| Visit*RO90 vs DN | −1.091 | 0.033 | −1.136 | 0.018 | −1.146 | 0.014 |
| Visit*ROPL vs DN | −0.294 | 0.709 | −0.664 | 0.303 | −0.519 | 0.420 |
| SF-36v2 MCS | ||||||
| Intercept | 47.187 | <0.001 | 46.203 | <0.001 | 46.264 | <0.001 |
| Visit | −0.029 | 0.868 | 0.113 | 0.487 | 0.054 | 0.826 |
| RO120 vs DN | −0.723 | 0.783 | −2.542 | 0.201 | −2.804 | 0.160 |
| RO90 vs DN | −4.016 | 0.128 | −0.748 | 0.711 | −0.728 | 0.720 |
| ROPL vs DN | −0.326 | 0.936 | −0.556 | 0.798 | −0.286 | 0.897 |
| Visit*RO120 vs DN | 0.432 | 0.515 | 0.388 | 0.535 | 0.513 | 0.400 |
| Visit*RO90 vs DN | −0.135 | 0.839 | −0.478 | 0.450 | −0.501 | 0.412 |
| Visit*ROPL vs DN | −0.281 | 0.784 | −0.662 | 0.437 | −0.921 | 0.277 |
| SWN-S | ||||||
| Intercept | 90.239 | <0.001 | 89.862 | <0.001 | 90.014 | <0.001 |
| Visit | 0.222 | 0.351 | 0.243 | 0.273 | 0.072 | 0.799 |
| RO120 vs DN | 1.801 | 0.662 | −1.000 | 0.743 | −1.157 | 0.704 |
| RO90 vs DN | −8.265 | 0.046 | −4.568 | 0.141 | −4.621 | 0.136 |
| ROPL vs DN | −6.439 | 0.313 | −3.018 | 0.366 | −3.515 | 0.295 |
| Visit*RO120 vs DN | −0.196 | 0.827 | −0.389 | 0.649 | −0.336 | 0.688 |
| Visit*RO90 vs DN | −0.302 | 0.735 | −0.302 | 0.726 | −0.300 | 0.722 |
| Visit*ROPL vs DN | 1.494 | 0.281 | −0.632 | 0.591 | −0.502 | 0.671 |
| MSQ | ||||||
| Intercept | a | NA | 0.922 | <0.001 | 0.696 | <0.001 |
| Visit | 0.655 | <0.001 | 0.408 | <0.001 | 0.020 | 0.851 |
| RO120 vs DN | 2.183 | 0.003 | 0.468 | 0.256 | 0.524 | 0.213 |
| RO90 vs DN | 1.731 | 0.010 | 0.654 | 0.128 | 0.711 | 0.104 |
| ROPL vs DN | 0.967 | 0.285 | 0.220 | 0.604 | 0.261 | 0.548 |
| Visit*RO120 vs DN | −0.398 | 0.180 | −0.004 | 0.987 | −0.052 | 0.812 |
| Visit*RO90 vs DN | −0.311 | 0.284 | −0.158 | 0.499 | −0.208 | 0.341 |
| Visit*ROPL vs DN | −0.406 | 0.290 | −0.321 | 0.217 | −0.412 | 0.095 |
Notes: aThe model (MSQ, completers) including the intercept failed to converge.
Abbreviations: DN, de novo; JPSM, joint process survival model; MCS, mental component score; MMRM, mixed model for repeated measures; PCS, physical component score; MSQ, medication satisfaction questionnaire; NA, not available; PRO, patient-reported outcomes; RO120, rollover 120 mg; RO90, rollover 90 mg; ROPL, rollover placebo; SF-36v2, Short-Form 36-item Questionnaire Version 2; SWN-S, Subjective Well-being Under Neuroleptic Scale – Short Version.
Discussion
Few LAI treatment studies conducted in patients with schizophrenia report on PRO measures even though the patient’s perception of medication benefit is a predictor of improved compliance.5,27,28 The results of this study demonstrate the long-term sustainability of improvements seen in HRQoL and overall well-being associated with RBP-7000 treatment. This builds on the evidence generated within the previously completed double-blind, randomized, placebo-controlled study, which reported significant improvements in HRQoL as measured by the EQ-5D-5L questionnaire and overall well-being as measured by the SWN-S questionnaire.15
Participants receiving RBP-7000 for 12 months reported mean SF-36v2 PCS and MCS scores at their EOS visit that were within one standard deviation of the norm-referenced scores of the SF-36v2 (as these scores are norm-referenced against a US population with a mean of 50 and SD of 10).21 Compared to previous studies of risperidone for the treatment of schizophrenia or other psychiatric disorders, RBP-7000 participants reported higher PCS and MCS.29,30 Similarly, EQ-5D-5L index scores for participants treated with RBP-7000 were higher than or similar to the mean EQ-5D-5L index scores reported by other studies conducted in non-US participants with schizophrenia.31–33 The high HRQoL observed within this analysis is particularly important considering that HRQoL (as assessed by SF-36 PCS and MCS scores) has been identified as an independent predictor of relapse in schizophrenia.34
Subjective well-being (as measured by SWN-S scores) remained stable in participants treated with RBP-7000 and overall reported higher total scores compared to a previous study with risperidone and paliperidone palmitate LAI users.35 Previous work has indicated that SWN-S scores in a schizophrenic population are positively correlated to compliance to neuroleptic treatment.23,36,37 Given that adherence is critical to the stability and positive outcomes in patients with schizophrenia,38 the SWN-S scores reported by RBP-7000 users in the current study are important to consider.
Within this study, we found that over two-thirds of participants reported high satisfaction with and preference for RBP-7000 as early as week 4. Satisfaction with and preference for RBP-7000 are important considerations since treatment satisfaction is a crucial component of long-term outcomes. For example, a panel of 12 psychiatrists discussed treatment satisfaction as an important domain for treatment effectiveness and retention in patients with schizophrenia39 and patient satisfaction has been associated with improvement in long-term outcomes in schizophrenia.6 Patient satisfaction has also been shown to be correlated with treatment retention in a non-schizophrenic population.40
It is important to interpret the study results within the context of limitations. The overall dropout rate observed in this study (51.5%) was high, but remains consistent with clinical trials of similar duration with second-generation antipsychotics.41–44 Our sensitivity analysis regarding potential withdrawal bias revealed no significant differences between study completers and non-completers (dropouts), ie, no bias was observed due to dropout. This study was not powered to detect differences in PRO endpoints as these endpoints were collected as tertiary endpoints in the study.
These results will be most generalizable to a population similar to our study participants and cannot be generalized to a larger population. As this analysis was conducted on patients treated within a clinical trial setting, further research will be needed to assess the real-world effectiveness of RBP-7000.
Conclusion
A large portion of participants were satisfied with and preferred RBP-7000 within this study, attaining HRQoL scores close to those of a general US population. Even though risperidone is a well-known atypical antipsychotic, additional research is needed to confirm whether HRQoL, satisfaction and preference for this new once-monthly risperidone SC formulation translate into improved outcomes such as adherence and ultimately fewer relapses in patients with schizophrenia.
Acknowledgments
Indivior, Inc. thanks all the investigators and their patients who participated in the trial. The authors thank Dr Anne Andorn (Indivior, Inc.) for her review and insights on the manuscript, Daniel Serrano (Pharmerit International) for providing analytic support for missing data analyses and Beth Lesher (Pharmerit International) for providing medical writing support of the manuscript. This work was sponsored and supported by Indivior, Inc.
Ethics approval
The trial was approved by relevant independent ethics committees, institutional review boards, regulatory authorities and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. The trial was registered in ClinicalTrials.gov (NCT02203838).
Data sharing statement
The authors will not make data collected for the study available to others.
Supplementary material
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
MF reports research support from Abbott Laboratories; Acadia Pharmaceuticals; Alkermes, Inc.; American Cyanamid; Aspect Medical Systems; AstraZeneca; Avanir Pharmaceuticals; AXSOME Therapeutics; Biohaven; BioResearch; BrainCells Inc.; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon; Cerecor; Clarus Funds; Clexio Biosciences; Clintara, LLC; Covance; Covidien; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Forest Pharmaceuticals, Inc.; FORUM Pharmaceuticals; Ganeden Biotech, Inc.; GlaxoSmithKline; Harvard Clinical Research Institute; Hoffman-LaRoche; Icon Clinical Research; Indivior; i3 Innovus/Ingenix; Janssen R&D, LLC; Jed Foundation; Johnson & Johnson Pharmaceutical Research & Development; Lichtwer Pharma GmbH; Lorex Pharmaceuticals; Lundbeck Inc.; Marinus Pharmaceuticals; MedAvante; Methylation Sciences Inc; National Alliance for Research on Schizophrenia & Depression (NARSAD); National Center for Complementary and Alternative Medicine (NCCAM); National Coordinating Center for Integrated Medicine (NiiCM); National Institute of Drug Abuse (NIDA); National Institute of Mental Health (NIMH); Neuralstem, Inc.; NeuroRx; Novartis AG; Organon Pharmaceuticals; Otsuka Pharmaceutical Development, Inc.; PamLab, LLC.; Pfizer Inc.; Pharmacia-Upjohn; Pharmaceutical Research Associates., Inc.; Pharmavite® LLC; PharmoRx Therapeutics; Photothera; Reckitt Benckiser; Roche Pharmaceuticals; RCT Logic, LLC (formerly Clinical Trials Solutions, LLC); Sanofi-Aventis US LLC; Shenox Pharmaceuticals, LLC; Shire; Solvay Pharmaceuticals, Inc.; Stanley Medical Research Institute (SMRI); Synthelabo; Taisho Pharmaceuticals; Takeda Pharmaceuticals; Tal Medical; VistaGen; Wyeth-Ayerst Laboratories. MF reports advisory board/consultant fees from: Abbott Laboratories; Acadia; Affectis Pharmaceuticals AG; Alkermes, Inc.; Amarin Pharma Inc.; Aspect Medical Systems; AstraZeneca; Auspex Pharmaceuticals; Avanir Pharmaceuticals; AXSOME Therapeutics; Bayer AG; Best Practice Project Management, Inc.; Biogen; BioMarin Pharmaceuticals, Inc.; BioXcel Therapeutics; Biovail Corporation; Boehringer Ingelheim; Boston Pharmaceuticals; BrainCells Inc; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon, Inc.; Cerecor; Clexio Biosciences; CNS Response, Inc.; Compellis Pharmaceuticals; Cypress Pharmaceutical, Inc.; DiagnoSearch Life Sciences (P) Ltd.; Dinippon Sumitomo Pharma Co. Inc.; Dov Pharmaceuticals, Inc.; Edgemont Pharmaceuticals, Inc.; Eisai Inc.; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; ePharmaSolutions; EPIX Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Fabre-Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals, Inc.; Forum Pharmaceuticals; GenOmind, LLC; GlaxoSmithKline; Grunenthal GmbH; Indivior; i3 Innovus/Ingenis; Intracellular; Janssen Pharmaceutica; Jazz Pharmaceuticals, Inc.; Johnson & Johnson Pharmaceutical Research & Development, LLC; Knoll Pharmaceuticals Corp.; Labopharm Inc.; Lorex Pharmaceuticals; Lundbeck Inc.; Marinus Pharmaceuticals; MedAvante, Inc.; Merck & Co., Inc.; MSI Methylation Sciences, Inc.; Naurex, Inc.; Navitor Pharmaceuticals, Inc.; Nestle Health Sciences; Neuralstem, Inc.; Neuronetics, Inc.; NextWave Pharmaceuticals; Novartis AG; Nutrition 21; Orexigen Therapeutics, Inc.; Organon Pharmaceuticals; Osmotica; Otsuka Pharmaceuticals; Pamlab, LLC.; Perception Neuroscience; Pfizer Inc.; PharmaStar; Pharmavite® LLC.; PharmoRx Therapeutics; Polaris Partners; Praxis Precision Medicines; Precision Human Biolaboratory; Prexa Pharmaceuticals, Inc.; PPD; PThera, LLC; Purdue Pharma; Puretech Ventures; PsychoGenics; Psylin Neurosciences, Inc.; RCT Logic, LLC ( formerly Clinical Trials Solutions, LLC); Relmada Therapeutics, Inc.; Rexahn Pharmaceuticals, Inc.; Ridge Diagnostics, Inc.; Roche; Sanofi-Aventis US LLC.; Sepracor Inc.; Servier Laboratories; Schering-Plough Corporation; Shenox Pharmaceuticals, LLC; Solvay Pharmaceuticals, Inc.; Somaxon Pharmaceuticals, Inc.; Somerset Pharmaceuticals, Inc.; Sunovion Pharmaceuticals; Supernus Pharmaceuticals, Inc.; Synthelabo; Taisho Pharmaceuticals; Takeda Pharmaceutical Company Limited; Tal Medical, Inc.; Tetragenex; Teva Pharmaceuticals; TransForm Pharmaceuticals, Inc.; Transcept Pharmaceuticals, Inc.; Usona Institute,Inc.; Vanda Pharmaceuticals, Inc.; Versant Venture Management, LLC; VistaGen. MF reports speaking/publishing fees from: Adamed, Co; Advanced Meeting Partners; American Psychiatric Association; American Society of Clinical Psychopharmacology; AstraZeneca; Belvoir Media Group; Boehringer Ingelheim GmbH; Bristol-Myers Squibb; Cephalon, Inc.; CME Institute/Physicians Postgraduate Press, Inc.; Eli Lilly and Company; Forest Pharmaceuticals, Inc.; GlaxoSmithKline; Imedex, LLC; MGH Psychiatry Academy/Primedia; MGH Psychiatry Academy/Reed Elsevier; Novartis AG; Organon Pharmaceuticals; Pfizer Inc.; PharmaStar; United BioSource,Corp.; Wyeth-Ayerst Laboratories. MF reports equity holdings from: Compellis; PsyBrain, Inc. MF reports royalty/patent, other incomes from: Patents for Sequential Parallel Comparison Design (SPCD), licensed by MGH to Pharmaceutical Product Development, LLC (PPD) (US_7840419, US_7647235, US_7983936, US_8145504, US_8145505); and patent application for a combination of Ketamine plus Scopolamine in Major Depressive Disorder (MDD), licensed by MGH to Biohaven. Patents for pharmacogenomics of Depression Treatment with Folate (US_9546401, US_9540691). Copyright for the MGH Cognitive & Physical Functioning Questionnaire (CPFQ), Sexual Functioning Inventory (SFI), Antidepressant Treatment Response Questionnaire (ATRQ), Discontinuation-Emergent Signs & Symptoms (DESS), Symptoms of Depression Questionnaire (SDQ), and SAFER; Lippincott, Williams & Wilkins; Wolkers Kluwer; World Scientific Publishing Co. Pte. Ltd. NJ, CTS, and DV are employees of Pharmerit International. CTS reports grants, personal fees from Indivior Inc, during the conduct of the study. JAG, SML, CH, and VRN are employees of Indivior Inc. RD was an employee of Indivior Inc at the time of the study and manuscript development. The authors report no other conflicts of interest in this work.
References
- 1.Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241–247. [DOI] [PubMed] [Google Scholar]
- 2.Greene M, Yan T, Chang E, Hartry A, Touya M, Broder MS. Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J Med Econ. 2018;21(2):127–134. doi: 10.1080/13696998.2017.1379412 [DOI] [PubMed] [Google Scholar]
- 3.Citrome L. Patient perspectives in the development and use of long-acting antipsychotics in schizophrenia: focus on olanzapine long-acting injection. Patient Prefer Adherence. 2009;3:345–355. doi: 10.2147/PPA.S5734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macfadden W, DeSouza C, Crivera C, et al. Assessment of effectiveness measures in patients with schizophrenia initiated on risperidone long-acting therapy: the SOURCE study results. BMC Psychiatry. 2011;11:167. doi: 10.1186/1471-244X-11-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasrallah HA, Duchesne I, Mehnert A, Janagap C, Eerdekens M. Health-related quality of life in patients with schizophrenia during treatment with long-acting, injectable risperidone. J Clin Psychiatry. 2004;65(4):531–536. [DOI] [PubMed] [Google Scholar]
- 6.Chue P, Llorca PM, Duchesne I, Leal A, Rosillon D, Mehnert A. Hospitalization rates in patients during long-term treatment with long-acting risperidone injection. J Appl Res. 2005;5:266–274. [Google Scholar]
- 7.Borison RL, Pathiraja AP, Diamond BI, Meibach RC. Risperidone: clinical safety and efficacy in schizophrenia. Psychopharmacol Bull. 1992;28(2):213–218. [PubMed] [Google Scholar]
- 8.Kane JM, Eerdekens M, Lindenmayer J-P, Keith SJ, Lesem M, Karcher K. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry. 2003;160(6):1125–1132. doi: 10.1176/appi.ajp.160.6.1125 [DOI] [PubMed] [Google Scholar]
- 9.Lindenmayer J-P, Eerdekens E, Berry SA, Eerdekens M. Safety and efficacy of long-acting risperidone in schizophrenia: a 12-week, multicenter, open-label study in stable patients switched from typical and atypical oral antipsychotics. J Clin Psychiatry. 2004;65(8):1084–1089. [PubMed] [Google Scholar]
- 10.Moller HJ. Risperidone: a review. Expert Opin Pharmacother. 2005;6(5):803–818. doi: 10.1517/14656566.6.5.803 [DOI] [PubMed] [Google Scholar]
- 11.Risperdal tablets, Risperdal oral solution, Risperdal M-tab orally disintegrating tablets (risperidone) [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019. [Google Scholar]
- 12.Risperdal Consta (risperidone) [package insert]. Titusville, NJ: Janssen Pharmaceutical Companies; 2019. [Google Scholar]
- 13.Perseris (risperidone) [package insert]. Richmond, VA: Indivior Inc; 2018. [Google Scholar]
- 14.Nasser AF, Henderson DC, Fava M, et al. Efficacy, safety, and tolerability of RBP-7000 once-monthly risperidone for the treatment of acute schizophrenia: an 8-week, randomized, double-blind, placebo-controlled, multicenter phase 3 study. J Clin Psychopharmacol. 2016;36(2):130–140. doi: 10.1097/JCP.0000000000000479 [DOI] [PubMed] [Google Scholar]
- 15.Isitt JJ, Nadipelli VR, Kouassi A, Fava M, Heidbreder C. Health-related quality of life in acute schizophrenia patients treated with RBP-7000 once monthly risperidone: an 8-week, randomized, double-blind, placebo-controlled, multicenter phase 3 study. Schizophr Res. 2016;174(1–3):126–131. doi: 10.1016/j.schres.2016.03.020 [DOI] [PubMed] [Google Scholar]
- 16.Andorn A, Graham J, Csernansky J, et al. Monthly Extended-release Risperidone (RBP-7000) in the Treatment of Schizophrenia: Results From The Phase 3 Program. J Clin Psychopharmacol In press 2019. [DOI] [PMC free article] [PubMed]
- 17.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.EuroQol. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. [DOI] [PubMed] [Google Scholar]
- 19.Prieto L, Sacristan JA, Hormaechea JA, Casado A, Badia X, Gomez JC. Psychometric validation of a generic health-related quality of life measure (EQ-5D) in a sample of schizophrenic patients. Curr Med Res Opin. 2004;20(6):827–835. doi: 10.1185/030079904125003674 [DOI] [PubMed] [Google Scholar]
- 20.Ware JE. SF-36 Health Survey: Manual and Interpretation Guide. Boston (MA): The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 21.Maruish ME. User's Manual for the SF-36v2 Health Survey. 3rd ed. Lincoln, RI: Quality Metric; 2011. [Google Scholar]
- 22.Tunis SL, Croghan TW, Heilman DK, Johnstone BM, Obenchain RL. Reliability, validity, and application of the medical outcomes study 36-item short-form health survey (SF-36) in schizophrenic patients treated with olanzapine versus haloperidol. Med Care. 1999;37(7):678–691. [DOI] [PubMed] [Google Scholar]
- 23.Naber D, Moritz S, Lambert M, et al. Improvement of schizophrenic patients’ subjective well-being under atypical antipsychotic drugs. Schizophr Res. 2001;50(1–2):79–88. [DOI] [PubMed] [Google Scholar]
- 24.Vothknecht S, Meijer C, Zwinderman A, et al. Psychometric evaluation of the Subjective Well-being Under Neuroleptic Treatment Scale (SWN) in patients with schizophrenia, their relatives and controls. Psychiatry Res. 2013;206(1):62–67. doi: 10.1016/j.psychres.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 25.Vernon MK, Revicki DA, Awad AG, et al. Psychometric evaluation of the Medication Satisfaction Questionnaire (MSQ) to assess satisfaction with antipsychotic medication among schizophrenia patients. Schizophr Res. 2010;118(1–3):271–278. doi: 10.1016/j.schres.2010.01.021 [DOI] [PubMed] [Google Scholar]
- 26.Tandon R, Marcus RN, Stock EG, et al. A prospective, multicenter, randomized, parallel-group, open-label study of aripiprazole in the management of patients with schizophrenia or schizoaffective disorder in general psychiatric practice: Broad Effectiveness Trial With Aripiprazole (BETA). Schizophr Res. 2006;84(1):77–89. doi: 10.1016/j.schres.2005.12.857 [DOI] [PubMed] [Google Scholar]
- 27.Canuso CM, Grinspan A, Kalali A, et al. Medication satisfaction in schizophrenia: a blinded-initiation study of paliperidone extended release in patients suboptimally responsive to risperidone. Int Clin Psychopharmacol. 2010;25(3):155–164. doi: 10.1097/YIC.0b013e3283372977 [DOI] [PubMed] [Google Scholar]
- 28.Fleischhacker WW, Rabinowitz J, Kemmler G, Eerdekens M, Mehnert A. Perceived functioning, well-being and psychiatric symptoms in patients with stable schizophrenia treated with long-acting risperidone for 1 year. Br J Psychiatry. 2005;187:131–136. doi: 10.1192/bjp.187.2.131 [DOI] [PubMed] [Google Scholar]
- 29.Gastpar M, Masiak M, Latif MA, Frazzingaro S, Medori R, Lombertie ER. Sustained improvement of clinical outcome with risperidone long-acting injectable in psychotic patients previously treated with olanzapine. J Psychopharmacol. 2005;19(5 Suppl):32–38. doi: 10.1177/0269881105056598 [DOI] [PubMed] [Google Scholar]
- 30.Marinis TD, Saleem PT, Glue P, et al. Switching to long-acting injectable risperidone is beneficial with regard to clinical outcomes, regardless of previous conventional medication in patients with schizophrenia. Pharmacopsychiatry. 2007;40(6):257–263. doi: 10.1055/s-2007-992140 [DOI] [PubMed] [Google Scholar]
- 31.Davies LM, Barnes TR, Jones PB, et al. A randomized controlled trial of the cost-utility of second-generation antipsychotics in people with psychosis and eligible for clozapine. Value Health. 2008;11(4):549–562. doi: 10.1111/j.1524-4733.2007.00280.x [DOI] [PubMed] [Google Scholar]
- 32.Konig HH, Roick C, Angermeyer MC. Validity of the EQ-5D in assessing and valuing health status in patients with schizophrenic, schizotypal or delusional disorders. Eur Psychiatry. 2007;22(3):177–187. doi: 10.1016/j.eurpsy.2006.08.004 [DOI] [PubMed] [Google Scholar]
- 33.Saarni SI, Viertio S, Perala J, Koskinen S, Lonnqvist J, Suvisaari J. Quality of life of people with schizophrenia, bipolar disorder and other psychotic disorders. Br J Psychiatry. 2010;197(5):386–394. doi: 10.1192/bjp.bp.109.076489 [DOI] [PubMed] [Google Scholar]
- 34.Boyer L, Millier A, Perthame E, Aballea S, Auquier P, Toumi M. Quality of life is predictive of relapse in schizophrenia. BMC Psychiatry. 2013;13:15. doi: 10.1186/1471-244X-13-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takekita Y, Koshikawa Y, Fabbri C, et al. Cognitive function and risperidone long-acting injection vs. paliperidone palmitate in schizophrenia: a 6-month, open-label, randomized, pilot trial. BMC Psychiatry. 2016;16:172. doi: 10.1186/s12888-016-0883-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karow A, Czekalla J, Dittmann RW, et al. Association of subjective well-being, symptoms, and side effects with compliance after 12 months of treatment in schizophrenia. J Clin Psychiatry. 2007;68(1):75–80. doi: 10.4088/JCP.v68n0110 [DOI] [PubMed] [Google Scholar]
- 37.Naber D. A self-rating to measure subjective effects of neuroleptic drugs, relationships to objective psychopathology, quality of life, compliance and other clinical variables. Int Clin Psychopharmacol. 1995;10(Suppl 3):133–138. [PubMed] [Google Scholar]
- 38.Masand PS, Roca M, Turner MS, Kane JM. Partial adherence to antipsychotic medication impacts the course of illness in patients with schizophrenia: a review. Prim Care Companion CNS Disord. 2009;11(4):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juckel G, de Bartolomeis A, Gorwood P, et al. Towards a framework for treatment effectiveness in schizophrenia. Neuropsychiatr Dis Treat. 2014;10:1867. doi: 10.2147/NDT.S61672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly SM, O’Grady KE, Brown BS, Mitchell SG, Schwartz RP. The role of patient satisfaction in methadone treatment. Am J Drug Alcohol Abuse. 2010;36(3):150–154. doi: 10.3109/00952991003736371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arato M, O’Connor R, Meltzer HY. A 1-year, double-blind, placebo-controlled trial of ziprasidone 40, 80 and 160 mg/day in chronic schizophrenia: the Ziprasidone Extended Use in Schizophrenia (ZEUS) study. Int Clin Psychopharmacol. 2002;17(5):207–215. [DOI] [PubMed] [Google Scholar]
- 42.Csernansky JG, Mahmoud R, Brenner R. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med. 2002;346(1):16–22. doi: 10.1056/NEJMoa002028 [DOI] [PubMed] [Google Scholar]
- 43.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688 [DOI] [PubMed] [Google Scholar]
- 44.Rosenheck R, Perlick D, Bingham S, et al. Effectiveness and cost of olanzapine and haloperidol in the treatment of schizophrenia: a randomized controlled trial. J Am Med Assoc. 2003;290(20):2693–2702. doi: 10.1001/jama.290.20.2693 [DOI] [PubMed] [Google Scholar]